Abstract
Background
Systemic inflammatory response syndrome (SIRS) is an inflammatory process associated with poor outcomes in acute ischemic stroke (AIS) patients. However, no study to date has investigated predictors of SIRS in AIS patients treated with intravenous (IV) tissue plasminogen activator (tPA).
Methods
Consecutive patients were retrospectively reviewed for evidence of SIRS during their acute hospitalization. SIRS was defined as the presence of 2 or more of the following: (1) body temperature less than 36°C or greater than 38°C, (2) heart rate greater than 90, (3) respiratory rate greater than 20, or (4) white blood cell count less than 4000/mm or greater than 12,000/mm or more than 10% bands for more than 24 hours. Those diagnosed with an infection were excluded. A scoring system was created to predict SIRS based on patient characteristics available at the time of admission. Logistic regression was used to evaluate potential predictors of SIRS using a sensitivity cutoff of ≥65% or area under the curve of .6 or more.
Results
Of 212 patients, 44 had evidence of SIRS (21%). Patients with SIRS were more likely to be black (61% versus 54%; P = .011), have lower median total cholesterol at baseline (143 versus 167 mg/dL; P = .0207), and have history of previous stroke (51% versus 35%; P = .0810). Ranging from 0 to 6, the SIRS prediction score consists of African American (2 points), history of hypertension (1 point), history of previous stroke (1 point), and admission total cholesterol less than 200 (2 points). Patients with an SIRS score of 4 or more were 3 times as likely to develop SIRS when compared with patients with a score of ≤3 (odds ratio = 2.815, 95% confidence interval 1.43–5.56, P = .0029).
Conclusions
In our sample of IV tPA-treated AIS patients, clinical and laboratory characteristics available on presentation were able to identify patients likely to develop SIRS during their acute hospitalization. Validation is required in other populations. If validated, this score could assist providers in predicting who will develop SIRS after treatment with IV tPA.
Keywords: Thrombolysis, systemic inflammatory response syndrome, stroke outcome, inflammation
Introduction
Systemic inflammatory response syndrome (SIRS) is an inflammatory process in the absence of infection that is characterized by 2 of the following: body temperature changes (<36°C or >38°C), leukocytosis or leukopenia, elevated heart rate, or elevated respiratory rate.1 Inflammation plays a role in the pathophysiology of tissue damage through ischemia–reperfusion injury.2–5 Audebert et al6 showed that inflammatory reactions after stroke result from the activation of cellular, humoral, and metabolic mechanisms, which can lead to an increase in necrotic tissue in the ischemic penumbra.
Similar to increased body temperature, leukocytosis is independently associated with poor functional outcome in acute stroke patients.7,8 Previous research has shown that acute ischemic stroke (AIS) patients with more severe strokes are at higher odds of having SIRS but that successful thrombolytic therapy attenuates this process.6 A study of tissue plasminogen activator (tPA)–treated patients illustrated how SIRS is associated with poor short-term functional outcome and increased length of hospital stay.9
We aimed to identify predictors of SIRS in patients with acute ischemic stroke who were treated with tPA and, subsequently, develop a prediction score to aide clinicians in assessing which stroke patients are at risk for SIRS development.
Methods
Study Population and Variable Definition
Consecutive patients presenting with acute ischemic stroke to a single academic center from 2009 to 2011 who were treated with intravenous (IV) tPA were identified using an existing prospective stroke registry. Admission demographic and clinical data and outcome measures were extracted. Clinical characteristics included vital signs, physical exam findings, stroke severity (as measured by the National Institutes of Health Stroke Scale [NIHSS]), and laboratory and imaging results. Retrospective chart review was used to identify patients who developed SIRS during their hospital stay. SIRS was defined as the presence of 2 or more of the following: (1) body temperature less than 36°C or greater than 38°C, (2) heart rate greater than 90, (3) respiratory rate greater than 20, or (4) white blood cell count less than 4000/mm or greater than 12, 000/mm or more than 10% bands for more than 24 hours. Patients who were diagnosed with an infection were excluded because the focus of the study was an uninfected inflammatory response after acute ischemic stroke, not sepsis.1,10
The outcome of interest was the presence of SIRS during the acute hospitalization period. We compared admission, clinical, and discharge information between patients who developed SIRS and patients who did not develop SIRS. This information was used to determine which features were predictive of a patient developing SIRS.
Statistics
Demographic and clinical data during the inpatient stay was compared across patients with SIRS and those without SIRS using chi-square and t tests, with nonparametric equivalents when appropriate. A prediction model was designed to estimate which patients would develop SIRS. The prediction models were built using a random sample of 55% of the data set (build group) and subsequently tested on the remaining random 45% (test group). Additionally, the scores were tested on the entire population after score development. All available demographic, clinical, and laboratory variables available at the time of admission were examined, using logistic regression models where development of SIRS was equal to 1. Variables with P values of .2 or less were retained in the final model. ROC curves were used to evaluate continuous variables. In addition, sensitivities were calculated to investigate grouping continuous variables. After the variables were assessed individually using the .2 or less cut point for the P value, we then placed variables that met this requirement in the multivariable model. The points assigned to the variables in the score were determined using the beta coefficients from the final multivariable logistic regression model. Once in the multivariable model, we then maximized the area under the curve (AUC) of the ROC curve by weighting variables from the multivariable models in an effort to develop the most predictive scoring algorithm. Spearman correlation and ROC curves were used to evaluate the final score. Additional logistic regression analyses were used to test the SIRS prediction score as a predictor of those with 2 SIRS components, those with 3 SIRS components, and those with 4 SIRS components. As this was an exploratory analysis, no adjustments were made for multiple comparisons.11 An alpha of .05 was set as the level of significance.
Results
Baseline Results and Prevalence of SIRS
In the 241 IV tPA-treated patients who met study inclusion criteria, there were 44 who had evidence of SIRS (18.2%). The median age of the 241 participants was 63 (range 20–99), with 107 females (44%), and a median admission NIHSS score of 7 (range 0–32). Table 1 demonstrates the differences in baseline characteristics between patients who developed SIRS during their inpatient stay and patients who did not develop SIRS. Patients with SIRS were more likely to be black (48% versus 25%; P = .0117), had lower median total cholesterol at baseline (143 versus 168 mg/dL; P = .0207), and more frequently reported a history of previous stroke (52% versus 35%; P = .0810) and hypertension (82% versus 70%, P = .1019). In the unadjusted models, black race (odds ratio [OR] = 2.7, 95% confidence interval [CI] 1.37–5.26, P = .0040) was a significant independent predictor of SIRS, whereas previous stroke (OR = 1.98, 95% CI .91–4.29, P = .0839) and history of hypertension (OR = 1.97, 95% CI .86–4.49, P = .1066) failed to be significant independent predictors of SIRS. When divided into 3 categories (0–7, 8–14, and >14),12 admission stroke severity was not found to be a significant independent predictor of SIRS (OR = 1.19, 95% CI .79–1.81, P = .3898). The SIRS frequency data and patient characteristics were further used to develop a score to aid prediction of which patients would develop SIRS. Of those with SIRS, 4 patients had 4 of the SIRS components, 14 patients had 3 of the SIRS components, and 26 patients had 2 of the SIRS components. Table 2 shows the breakdown of SIRS components for each of these SIRS categories.
Table 1.
Baseline characteristics of patients with SIRS compared to patients without SIRS
| No SIRS (N = 197) | SIRS (N = 44) | P value | |
|---|---|---|---|
| Age | 63 (20–99) | 64 (21–96) | .7704 |
| Gender, % male | 107 (54.3%) | 27 (61.4%) | .3949 |
| Black race | 50 (25.4%) | 21 (47.7%) | .0117 |
| Medical history, % | |||
| Stroke | 44 (34.9%) | 17 (51.5%) | .0810 |
| Atrial fibrillation | 30 (15.2%) | 8 (18.2%) | .6270 |
| Diabetes | 49 (24.9%) | 9 (20.5%) | .5353 |
| Hypertension | 137 (69.5%) | 36 (81.8%) | .1019 |
| Dyslipidemia | 67 (34.0%) | 13 (29.6%) | .5696 |
| Heart failure | 22 (11.2%) | 9 (20.5%) | .0962 |
| ESRD/CKD | 10 (5.1%) | 3 (6.8%) | .6437 |
| Active smoker | 54 (27.4%) | 11 (25%) | .7446 |
| On BP meds at home | 112 (56.8%) | 26 (59.1%) | .7861 |
| On DM meds at home | 32 (16.2%) | 7 (15.9%) | .9566 |
| On AP meds at home | 69 (35.0%) | 18 (40.9%) | .4625 |
| Transfer | 71 (36.0%) | 11 (25%) | .1623 |
| NIHSS on admission | 7 (0–31) | 9 (0–32) | .1015 |
| Length of stay | 3 (0–52) | 5 (1–41) | <.0001 |
| Glucose | 111 (73–536) | 114 (65–450) | .4761 |
| PT | 14 (11–36) | 14 (13–20) | .0043 |
| INR | 1.07 (.83–3.48) | 1.11 (.96–1.63) | .0088 |
| PTT | 28 (19–63) | 27.5 (20–52) | .7848 |
| HCT | 40 (15–89) | 38 (27–50) | .1200 |
| RBC | 4.4 (2.1–15.1) | 4.2 (2.4–12.6) | .1169 |
| A1C | 5.8 (4.8–16.5) | 5.7 (4.9–12.2) | .6143 |
| Total cholesterol | 167.5 (60–439) | 143.5 (88–245) | .0207 |
| Transfusion | 2 (1.0%) | 1 (2.3%) | .3687 |
| Follow-up imaging | 164 (83.3%) | 39 (88.6%) | .3753 |
| Hemorrhagic transformation | 17 (8.6%) | 4 (9.3%) | .8875 |
| SIRS criteria | |||
| Temperature | 0 | 16 (36.4%) | |
| RR | 0 | 37 (84.1%) | |
| HR | 0 | 37 (84.1%) | |
| WBC | 0 | 20 (45.5%) | |
| On Nicardipine | 15 (7.6%) | 8 (18.6%) | .0265 |
| mRS on admission | 0 (0–5) | 0 (0–4) | .1486 |
| mRS on discharge | 3 (0–6) | 4 (1–6) | .0003 |
| mRS score 0–2 on discharge | 92 (48.2%) | 9 (20.9%) | .0011 |
| Favorable discharge disposition | 146 (76.4 %) | 31 (73.8%) | .7180 |
| Death | 21 (10.7%) | 8 (18.2%) | .1656 |
Abbreviations: AP, antiplatelet; BP, blood pressure; DM, diabetes mellitus; HCT, hematocrit; HR, heart rate; INR, international normalized ratio; meds, medications; NIHSS, National Institutes of Health Stroke Scale; PT, prothrombin time; PTT, partial thromboplastin time; RBC, red blood cell; RR, respiratory rate; SIRS, systemic inflammatory response syndrome; WBC, white blood cell.
Table 2.
Proportion of each SIRS criteria seen in patients with 2, 3, and 4 SIRS criteria
| SIRS 4 (N = 4) | SIRS 3 (N = 14) | SIRS 2 (N = 26) | P value | |
|---|---|---|---|---|
| Heart rate>90 | 4 (100%) | 13 (92.8%) | 20 (76.9%) | .0841 |
| Respiratory rate > 20 | 4 (100%) | 12 (85.7%) | 21 (80.8%) | .1562 |
| Temperature < 36°C or >38°C | 4 (100%) | 9 (64.3%) | 3 (11.5%) | <.0001 |
| White blood cells < 4000/mm or >12,000/mm or >10% bands for >24 h | 4 (100%) | 8 (57.1%) | 8 (30.8%) | .0027 |
SIRS Prediction Score
Black race, total cholesterol, history of hypertension, history of previous stroke, on nicardipine, and history of heart failure met the <.2 univariable P value cutoff (Table 3). The variables such as treatment with nicardipine and heart failure were not included in the final prediction model because of low predictive value as measured by AUC of the ROC (.5550 and .5464, respectively). The cutoff for total cholesterol was determined by testing different cut points in the final multivariable model to assess which cutoff was more predictive through the total model AUC.
Table 3.
Variables included in the prediction model
| Sensitivity/specificity or area under the curve | Univariable P value | Univariable beta coefficient (SE) | |
|---|---|---|---|
| Black race | 47.8/74.6 | .0040 | .987 (.34) |
| Hypertension | 81.8/30.5 | .1066 | .6784 (.42) |
| Total cholesterol | .6238 | .0328 | −.01 (.004) |
| History of previous stroke | 51.5/65.1 | .0839 | −.34 (.20) |
| Heart failure | 20.4/88.8 | .1014 | .7156 (.44) |
| On Nicardipine | 18.6/92.4 | .0318 | 1.02 (.48) |
| PT | .6349 | .4243 | .0560 (.06) |
| INR | .6294 | .4442 | .4929 (.64) |
Abbreviations: INR, international normalized ratio; PT, prothrombin time.
The final score allotted 2 points for black race, 2 points for total cholesterol being lower than 200, 1 point for history of hypertension, and 1 point for history of previous stroke. The SIRS prediction score ranged from 0 to 6 (Table 4) and produced an AUC of .7430 (Fig 1). Using the entire cohort, 80% of patients with an SIRS prediction score of 4 or more developed SIRS. Figure 2 illustrates the distribution of the SIRS prediction score compared with patients who develop SIRS. Additionally, the odds of a patient with an SIRS prediction score of 4 or more developing SIRS was nearly 3 times that of patients with SIRS prediction scores of 0–3 (OR = 2.82, 95% CI 1.43–5.56, P = .0029). Figure 3 illustrates the distribution of the SIRS prediction score stratified by those with 2, 3, and 4 SIRS components.
Table 4.
SIRS prediction score (0–6 points)
| Race | Total cholesterol |
| Black/African American = 2 points | <200 = 2 points |
| Other race = 0 point | ≥200 = 0 point |
| Hypertension | History of previous stroke |
| Hypertension = 1 point | History of previous stroke = 1 |
| No hypertension = 0 point | No evidence = 0 point |
Figure 1.
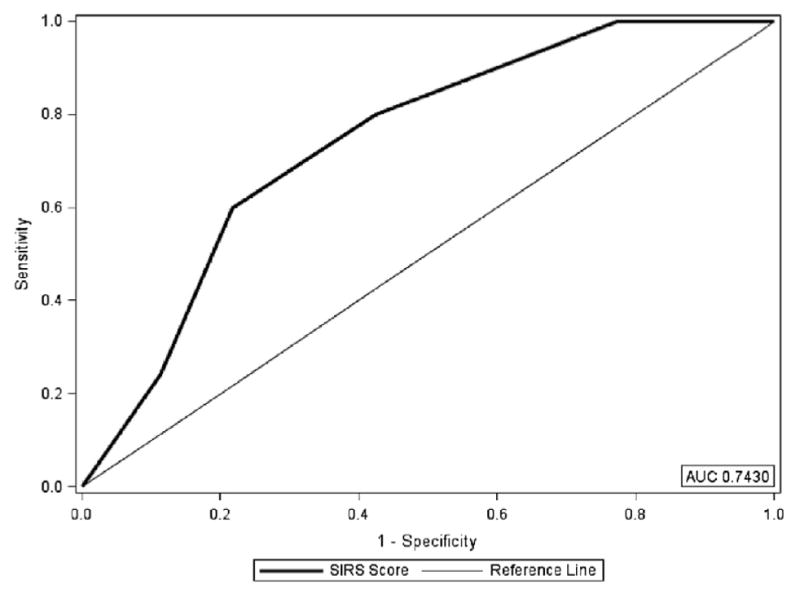
SIRS prediction score as a predictor for SIRS. Abbreviation: SIRS, systemic inflammatory response syndrome.
Figure 2.
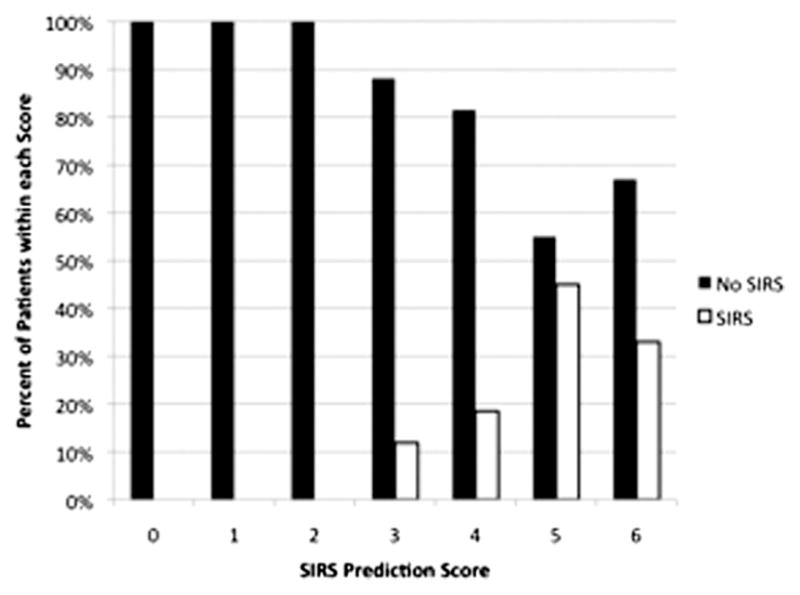
Distribution of patients with SIRS based on SIRS prediction score. Abbreviation: SIRS, systemic inflammatory response syndrome.
Figure 3.
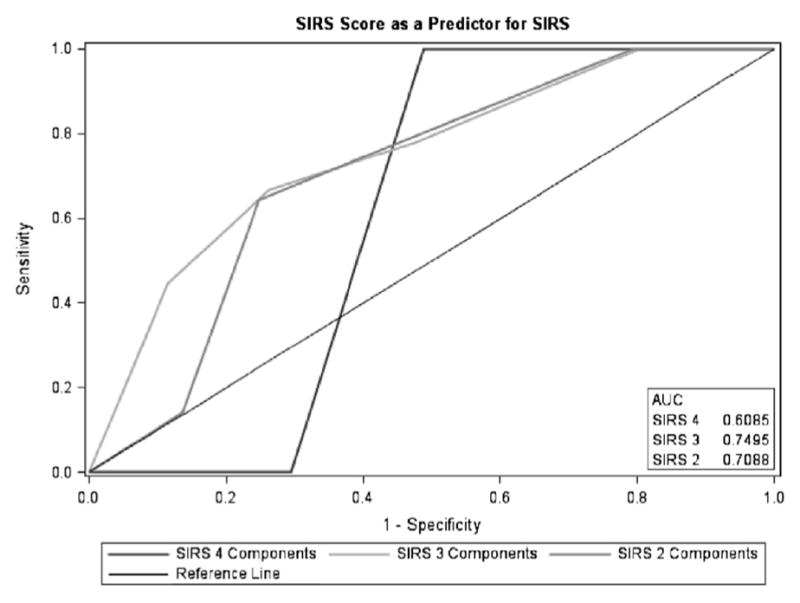
SIRS prediction score as a predictor for SIRS stratified by number of SIRS components. Abbreviation: SIRS, systemic inflammatory response syndrome.
Discussion
Our study showed that a novel predictive score can identify patients who are at 3-fold higher odds to develop SIRS after ischemic stroke and treatment with tPA. To our knowledge, this is the first study to investigate the predictors of SIRS in tPA-treated acute ischemic stroke patients using the definition and diagnostic workup that rules out sepsis and infection during the hospital stay. Using information available at the time of admission, we developed an SIRS prediction score for tPA-treated ischemic stroke patients. The SIRS prediction score is a simple, easy to use score that can provide clinicians with the probability that a tPA-treated patient will develop SIRS. It is highly predictive in those with 2 and 3 of the SIRS components, and it is technically predictive in those with 4 of SIRS components, but the total number of those with 4 SIRS components is very small and the predictive ability could be stronger in a larger sample size. We acknowledge that by setting the SIRS prediction score threshold relatively high (ie, 4), some patients with low SIRS prediction scores (and possibly a milder form of SIRS) will be missed.
Little research has been done on SIRS in acute ischemic stroke patients, but despite this, the specific components of the SIRS prediction score are comparable with work done in inflammation in stroke and SIRS in other conditions. The finding of history of previous stroke as a significant predictor of SIRS could be a function of stroke severity and greater overall risk factor burden before stroke recurrence. Audebert et al6 showed that patients with higher admission NIHSS were more likely to experience SIRS. Work performed by Dhar et al13 in subarachnoid hemorrhage patients showed that patients with SIRS were more likely to have a history of hypertension than those without SIRS (50% versus 34%). Furthermore, lower total cholesterol has been shown to be associated with critical illness and correlated with increased concentrations of cytokines.14–17 Bonville et al18 found that decreased serum cholesterol is an independent predictor of mortality in SIRS patients. The frequency of SIRS in AIS could be a function of chronic disease burden. Wang et al19,20 found that incident sepsis episodes were associated with older age, dyslipidemia, hypertension, atrial fibrillation, stroke and peripheral artery disease to name a few, and the risk of sepsis increases with the number of chronic medical conditions. Interestingly, previous research has shown that black trauma victims have lower rates of SIRS than whites and lower rates of sepsis as well.19,21 The finding that black patients have higher rates of SIRS may be a function of the chronic disease burden seen in blacks.
Our study is limited by the retrospective nature and small sample size involving only 1 academic center. We also are only examining AIS patients treated with IV tPA. Validation in additional cohorts is needed to determine the generalizability of our SIRS prediction score to AIS patients in general and other patient populations. Additionally, we did not have inflammation biomarkers. Despite our limitations, this is the first study to develop an SIRS prediction score for tPA-treated acute ischemic stroke patients.
Conclusions
This study provides a scoring system that predicts the development of SIRS in tPA-treated patients, using information available at the time of admission. SIRS is an important clinical event that affects prognosis. Prospective studies with a larger sample size are needed to determine if early identification of patients at risk for SIRS, followed by swift treatment for those who develop SIRS, can decrease the risk of poor functional outcome in this susceptible group.
Acknowledgments
The project described was supported by award numbers 5 T32 HS013852-10 from the Agency for Healthcare Research and Quality, 3 P60 MD000502-08S1 from the National Institute on Minority Health and Health Disparities, National Institutes of Health and 13PRE13830003 from the American Heart Association.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality, American Heart Association, or the National Institutes of Health.
Disclosures: None.
References
- 1.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 2.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 3.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 4.D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Mol Med. 2001;7:367–382. [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen ED, Waje-Andreassen U, Vedeler CA, et al. Systemic complement activation following human acute ischaemic stroke. Clin Exp Immunol. 2004;137:117–122. doi: 10.1111/j.1365-2249.2004.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Audebert HJ, Rott MM, Eck T, et al. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. 2004;35:2128–2133. doi: 10.1161/01.STR.0000137607.61697.77. [DOI] [PubMed] [Google Scholar]
- 7.Kumar AD, Boehme AK, Siegler JE, et al. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis. 2013;22:e111–e117. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boysen G, Christensen H. Stroke severity determines body temperature in acute stroke. Stroke. 2001;32:413–417. doi: 10.1161/01.str.32.2.413. [DOI] [PubMed] [Google Scholar]
- 9.Boehme AK, Kapoor N, Albright KC, et al. Systemic inflammatory response syndrome in tissue-type plasminogen activator-treated patients is associated with worse short-term functional outcome. Stroke. 2013;44:2321–2323. doi: 10.1161/STROKEAHA.113.001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25:1789–1795. doi: 10.1097/00003246-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Yafeh B. Confirmation of tPA treatment effect by baseline severity-adjusted end point reanalysis of the NINDS-tPA stroke trials. Stroke. 2007;38:414–416. doi: 10.1161/01.STR.0000254580.39297.3c. [DOI] [PubMed] [Google Scholar]
- 13.Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008;8:404–412. doi: 10.1007/s12028-008-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon BR, Parker TS, Levine DM, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med. 2001;29:1563–1568. doi: 10.1097/00003246-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Gordon BR, Parker TS, Levine DM, et al. Low lipid concentrations in critical illness: implications for preventing and treating endotoxemia. Crit Care Med. 1996;24:584–589. doi: 10.1097/00003246-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ruan XZ, Varghese Z, Powis SH, et al. Dysregulation of LDL receptor under the influence of inflammatory cytokines: a new pathway for foam cell formation. Kidney Int. 2001;60:1716–1725. doi: 10.1046/j.1523-1755.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 17.Gierens H, Nauck M, Roth M, et al. Interleukin-6 stimulates LDL receptor gene expression via activation of sterol-responsive and Sp1 binding elements. Arterioscler Thromb Vasc Biol. 2000;20:1777–1783. doi: 10.1161/01.atv.20.7.1777. [DOI] [PubMed] [Google Scholar]
- 18.Bonville DA, Parker TS, Levine DM, et al. The relationships of hypocholesterolemia to cytokine concentrations and mortality in critically ill patients with systemic inflammatory response syndrome. Surg Infect (Larchmt) 2004;5:39–49. doi: 10.1089/109629604773860291. [DOI] [PubMed] [Google Scholar]
- 19.Wang HE, Shapiro NI, Griffin R, et al. Chronic medical conditions and risk of sepsis. PLoS One. 2012;7:e48307. doi: 10.1371/journal.pone.0048307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HE, Griffin R, Judd S, et al. Obesity and risk of sepsis: a population-based cohort study. Obesity (Silver Spring) 2013;21:E762–E769. doi: 10.1002/oby.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NeSmith EG, Weinrich SP, Andrews JO, et al. Demographic differences in systemic inflammatory response syndrome score after trauma. Am J Crit Care. 2012;21:35–41. doi: 10.4037/ajcc2012852. quiz 2. [DOI] [PubMed] [Google Scholar]


