Abstract
Dichlorodiphenyltrichloroethane (DDT) is still used in certain areas of tropics and subtropics to control malaria and other insect-transmitted diseases. DDT and its metabolites have been extensively studied for their toxicity and carcinogenicity in animals and humans and shown to have an endocrine disrupting potential affecting reproductive system although the effects may vary among animal species in correlation with exposure levels. Epidemiologic studies revealed either positive or negative associations between exposure to DDT and tumor development, but there has been no clear evidence that DDT causes cancer in humans. In experimental animals, tumor induction by DDT has been shown in the liver, lung, and adrenals. The mechanisms of hepatic tumor development by DDT have been studied in rats and mice. DDT is known as a non-genotoxic hepatocarcinogen and has been shown to induce microsomal enzymes through activation of constitutive androstane receptor (CAR) and to inhibit gap junctional intercellular communication (GJIC) in the rodent liver. The results from our previously conducted 4-week and 2-year feeding studies of p,p′-DDT in F344 rats indicate that DDT may induce hepatocellular eosinophilic foci as a result of oxidative DNA damage and leads them to hepatic neoplasia in combination with its mitogenic activity and inhibitory effect on GJIC. Oxidative stress could be a key factor in hepatocarcinogenesis by DDT.
Keywords: Enzyme induction, CAR activation, Oxidative stress, Cell proliferation, Intercellular communication, Eosinophilic foci, DDT
INTRODUCTION
DDT (dichlorodiphenyltrichloroethane) was first synthesized in 1874 and its insecticidal properties were discovered in 1939. Since then DDT was widely used in the world to control insects on agricultural crops and those that carry diseases such as malaria and typhus. However, the use of this compound has been banned in many countries since 1970s because of its chemical characteristics such as accumulation and bio-concentration in lipid systems of all animal species which may result in occurrence of potential adverse effects on humans and wild animals (1). DDT has been suggested to be toxic to a range of wildlife including birds and marine animals, and its metabolite DDE (dichlorodiphenyldichloroethylene) causes eggshell thinning of certain bird species such as bald eagle and brown pelican, leading to declines of their populations (1,2). It is considered that DDE inhibits calcium adenosine triphosphatase (ATPase) in the membrane of the shell gland and reduces the transport of calcium carbonate from blood into the eggshell gland (1). Despite these circumstances, DDT is still used in the certain areas of tropics and subtropics for the control of malaria and other insect-transmitted diseases causing high death rates (3). In 2006, World Health Organization (WHO) permitted the use of DDT in those areas to reduce the rate of deaths caused by malaria. DDT and its metabolites have been extensively studied for their toxicity and carcinogenicity in experimental animals and humans and the results of their investigations are well documented (1,4). It has been shown that DDT and its metabolites may have adverse effects on various organs/tissues of mammals including nervous, liver, kidney, reproductive, endocrine, and immune systems (1,4). The present review paper describes the overview of neurotoxicity, reproductive toxicity with endocrine effects, hepatotoxicity and carcinogenicity of DDT and its metabolites. In addition, potential factors including microsomal enzymes, cell proliferation, intercellular communication, oxidative stress which might be involved in hepatocarcinogenesis by DDT are discussed based on the results of our previously conducted 4-week and 2-year feeding studies of p,p′-DDT in F344 rats (5). Furthermore, a potential effect of DDT or its metabolites on mitochondria is also addressed, since the mitochondrion plays a crucial role in maintaining hepatocyte integrity and functions, and the mitochondrial dysfunction is considered one of the important mechanisms for the chemical-mediated hepatic toxicity and/or carcinogenicity (6–9).
Neurotoxicity
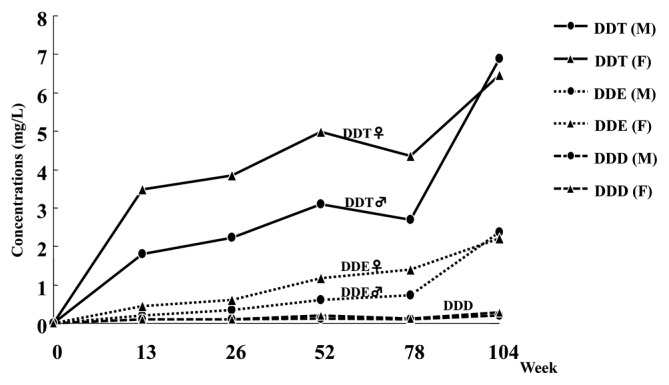
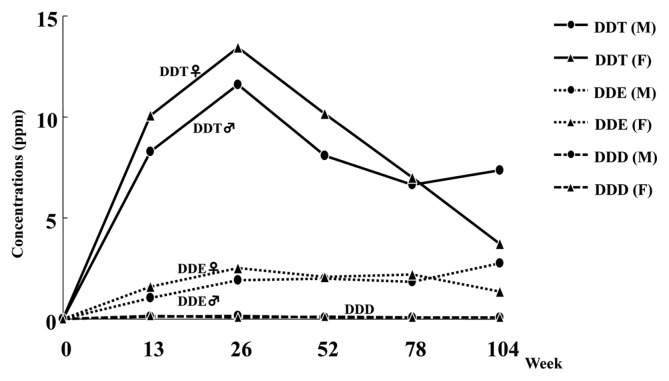
It is known that DDT delays the closing of the sodium ion channel and prevents the opening of the potassium gates and also targets a specific neuronal ATPase considered to be involved in the control of the rate of sodium, potassium, and calcium fluxes through the nerve membrane (1). In addition, DDT has been suggested to inhibit the ability to transport calcium ions which are essential to the release of neurotransmitters. These actions combine to effectively maintain the depolarization of the nerve membrane, potentiating the release of transmitters and leading to central nervous system excitation manifested as hyperexcitability, tremors, and convulusions. It was reported that occupational exposure to DDT in retired workers from Costa Rica was associated with neurobehabioral symptoms in a dose-response pattern (10). In our 2-year rat feeding study of p,p′-DDT, a whole body tremor was observed in the high dose (500 ppm) group of both sexes that was more evident in females. This sex difference was consistent with the toxicokinetics data obtained from the rat 2-year study in that plasma and brain concentrations of DDT and its metabolite DDE tended to be higher in females than males (Figs. 1 and 2).
Fig. 1.
Plasma concentration of DDT, DDE, and DDD in F344 rats at high-dose (500 ppm) in a 2-year feeding study. (M) male; (F) female.
Fig. 2.
Brain concentration of DDT, DDE, and DDD in F344 rats at high-dose (500 ppm) in a 2-year feeding study. (M) male; (F) female.
Reproductive toxicity with endocrine effects
It has been suggested that DDT and its metabolites may have an endocrine disrupting potential to affect reproductive system through their estrogenic or androgenic activity (1). Receptor-binding assays indicate that o,p′-DDT has week estrogenic activity and p,p′-DDE is an androgen receptor antagonist, while the main DDT’s component p,p′-DDT has little estrogenic or androgenic activity (11). In our institute, a 2-generation reproduction toxicity study of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (p,p′-DDT) in Sprague Dawley (SD) rats was conducted in accordance with the current test guidelines of MAFF (Japan), EPA (USA) and OECD with some modifications and additions (12). In the reproduction study, p,p′-DDT was given to parental rats at dietary levels of 0, 5, 50 or 350 ppm. As a result, parental animals in the mid- and high-dose groups showed tremors (both sexes), subsequent deaths (females only), and/or pathological changes in the liver (both sexes). However, reproductive and postnatal developmental toxicities were not evident up to 350 ppm (high-dose) except for the decreased pup viability index on postnatal day 21 in the high-dose group. Although changes in serum estradiol and progesterone levels and/or a delay in male sexual maturation were noted in the mid- and high-dose groups, no substantial reproductive disorders occurred. Based on these results, it was concluded that the in vivo effects of p,p′-DDT associated with its endocrine activities are not adverse at least under the experimental condition and thus p,p′-DDT is not considered to exert overt endocrine disrupting effects in SD rats at levels up to 350 ppm. The reproductive toxicity with endocrine effects of DDT may differ among animal species/ strains, depending on their susceptibility and dose levels.
Hepatotoxicity and carcinogenicity
Hepatotoxicity and carcinogenicity of DDT have been demonstrated in animals to show increased liver weights, hepatocellular hypertrophy, microsomal enzyme induction similar to phenobarbital, and hepatocellular tumor induction (1). Many epidemiologic studies have been conducted and the results indicate either positive or negative associations between exposure to DDT and tumor development in humans (1,13). The International Agency for Research on Cancer (IARC) classifies DDT into Group 2B (possibly carcinogenic to humans). However, there has been no clear evidence that exposure to DDT causes cancer in humans. The toxic changes caused by DDT in the liver and potential factors involved in hepatocarcinogenesis by DDT are discussed in the following sections on the basis of the results obtained from our previously conducted 4-week feeding study of p,p′-DDT in male F344 rats at 0, 50, 160, 500 ppm and 2-year feeding study of p,p′-DDT in F344 rats of both sexes at 0, 5, 50, 500 ppm (5).
Hepatic microsomal enzyme induction
Analyses of hepatic microsomal enzymes revealed significant increases in pentoxyresorufin O-dealkylase (PROD) activity and P450 isozyme contents of CYP2B1 and CYP3A2 in the DDT-treated rats (Tables 1~3). The results are generally consistent with previous works (14–17,23–25) and considered due to activation of constitutive androstane receptor (CAR) (24). As shown in Tables 1~3, the increases in CYP2B1 and CYP3A2 were dose-dependent, whereas the elevation of PROD activity was most evident in the mid-dose group and not significant in males of the high-dose group after 52, 78, and 104 weeks. This suggests that microsomal enzyme activity is not always consistent with its associated protein content and there seems to be an almost inverse correlation between the increase in PROD at different time points and the concurrent incidence of pre-neoplastic hepatocellular eosinophilic foci and hepatocellular tumors. As to P450 isozyme contents, the increases in CYP2B1 and CYP3A2 tended to be more evident in females than males. A similar result (preferential induction of CYP3A2 in females) also has been reported in Wistar rats treated with the technical grade DDT, a mixture of p,p′-DDT (85%), o,p′-DDT (15%) and o,o′-DDT (trace amount) (17). Since CYP3A2 is androgen-dependent and not normally expressed in adult female rats (25), the induction of CYP3A2 by DDT in female rats suggests that DDT is able to modulate sexual metabolic dimorphism by affecting regulatory sites of hepatic metabolism (17). The preferential induction of CYP2B1 and CYP3A2 by DDT in female rats indicates an endocrine disrupting potential of DDT because these CYPs are involved in steroid metabolism. With respect to other P450 isozyme contents, statistically significant increases or decreases in CYP1A2 and CYP4A1 were noted in the DDT-treated groups of both sexes, but those changes were not consistent during the study and their toxicological significance remains obscure.
Table 1.
Hepatic microsomal P450 isozyme content in male F344 rats from a 4-week feeding study of p,p′-DDT
| Dose (ppm) | CYP2B1 content (pmol/mg protein) on days: | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 2 | 3 | 7 | 14 | 28 | |
| 0 | 20.01 ± 5.41 | 12.95 ± 1.23 | 11.93 ± 2.93 | 12.97 ± 2.77 | 12.54 ± 1.73 | 9.47 ± 2.41 |
| 50 | 96.13 ± 12.70** | 187.10 ± 17.52** | 181.06 ± 52.39** | 210.41 ± 25.13** | 185.19 ± 36.17** | 273.32 ± 48.78** |
| 160 | 162.45 ± 19.27** | 321.44 ± 33.67** | 248.15 ± 27.01** | 244.15 ± 33.03** | 329.74 ± 63.64** | 252.10 ± 31.65** |
| 500 | 166.65 ± 53.76** | 274.82 ± 21.13** | 281.66 ± 45.10** | 280.96 ± 53.66** | 303.18 ± 21.92** | 348.87 ± 80.59** |
Values represent mean ± S.D. (n = 5).
Significantly different from control at p < 0.01 (Dunnett’s multiple comparison test).
Table 3.
Hepatic microsomal enzyme activity and P450 isozyme contents in female F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | PROD (pmol/min/mg) | P450 isozyme contents (pmol/mg protein) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CYP1A2 | CYP2B1 | CYP3A2 | CYP4A1 | ||||||||
| 0 | 26 | 12 ± 1 | (6) | 16.8 ± 6.5 | (6) | 6.48 ± 0.94 | (6) | 10.7 ± 1.7 | (6) | 18.8 ± 2.5 | (6) |
| 52 | 8 ± 2 | (6) | 21.6 ± 6.2 | (6) | 3.81 ± 1.11 | (6) | 12.7 ± 2.1 | (6) | 14.4 ± 2.0 | (6) | |
| 78 | 7 ± 1 | (6) | 10.9 ± 2.9 | (6) | 4.48 ± 0.93 | (6) | 12.1 ± 2.7 | (6) | 20.7 ± 5.1 | (6) | |
| 104 | 4 ± 3 | (6) | 17.9 ± 8.2 | (6) | 5.86 ± 0.77 | (6) | 7.98 ± 5.28 | (6) | 17.9 ± 5.4 | (6) | |
|
| |||||||||||
| 5 | 26 | 92 ± 16 | (6) | 22.6 ± 4.3 | (6) | 32.5 ± 3.6 | (6) | 16.4 ± 1.7 | (6) | 15.8 ± 2.2 | (6) |
| 52 | 95 ± 26 | (6) | 29.6 ± 9.9 | (6) | 16.9 ± 4.6 | (6) | 25.8 ± 4.4 | (6) | 20.1 ± 1.3** | (6) | |
| 78 | 51 ± 13 | (6) | 12.0 ± 3.5 | (6) | 37.4 ± 3.7 | (6) | 29.2 ± 5.2 | (6) | 19.2 ± 4.7 | (6) | |
| 104 | 50 ± 18 | (6) | 17.9 ± 4.8 | (6) | 23.8 ± 6.3 | (6) | 28.6 ± 8.4 | (6) | 22.2 ± 6.5 | (6) | |
|
| |||||||||||
| 50 | 26 | 402 ± 74** | (6) | 23.6 ± 2.4 | (6) | 263 ± 39** | (6) | 53.5 ± 9.8** | (6) | 19.5 ± 2.9 | (6) |
| 52 | 393 ± 44** | (6) | 31.3 ± 6.2 | (6) | 157 ± 17** | (6) | 82.3 ± 8.9** | (6) | 26.1 ± 3.0** | (6) | |
| 78 | 237 ± 23** | (6) | 11.5 ± 4.5 | (6) | 183 ± 39** | (6) | 80.8 ± 12.4** | (6) | 21.4 ± 0.5 | (6) | |
| 104 | 171 ± 39** | (6) | 14.5 ± 4.0 | (6) | 104 ± 25** | (6) | 63.1 ± 11.3** | (6) | 21.4 ± 1.7 | (6) | |
|
| |||||||||||
| 500 | 26 | 245 ± 44** | (6) | 20.6 ± 4.4 | (6) | 371 ± 46** | (6) | 171 ± 25** | (6) | 14.8 ± 4.3 | (6) |
| 52 | 241 ± 30** | (6) | 31.0 ± 7.2 | (6) | 350 ± 29** | (6) | 155 ± 21** | (6) | 20.3 ± 2.9** | (6) | |
| 78 | 106 ± 10** | (6) | 18.8 ± 3.5** | (6) | 218 ± 29** | (6) | 171 ± 35** | (6) | 15.7 ± 3.5 | (6) | |
| 104 | 90 ± 25* | (5) | 20.6 ± 1.5 | (5) | 210 ± 34** | (5) | 189 ± 28** | (5) | 15.6 ± 4.8 | (5) | |
Values represent mean ± S.D.
(n): Number of animals examined.
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Oxidative stress
Measurements of hepatic oxidative stress markers in our 2-year rat study disclosed significant increases in lipid peroxide (LPO) and 8-hydroxydeoxyguanosine (8-OHdG) in the mid- and high-dose groups that developed hepatocellular tumors, which were more evident in males than females (Tables 4 and 5). These results indicate that hepatocytes in the DDT-treated livers are exposed to oxidative stress and could have cellular and DNA damages. It is postulated that the metabolic activation with enzyme induction of P450 by DDT may result in formation of reactive oxygen radicals (23,26). In addition, it is conceivable that DDT or its metabolites may affect mitochondria and cause mitochondrial dysfunction which results in formation of deleterious reactive oxygen species (ROS) leading to lipid peroxidation since mitochondria are prominent target sites of many hepatotoxic chemicals and also the main source of ROS in the hepatocytes (6–9). In the DDT-treated groups that developed hepatocellular carcinomas, 8-OHdG levels were significantly increased. The increase in 8-OHdG levels (an evidence of oxidative DNA damage) could play an important role in hepatocarcinogenesis by DDT. It has been shown that 8-OHdG leads to base mis-pairing (mutation) on DNA replication (27). In our 2-year rat study, the appearance of eosinophilic altered hepatocellular foci (AHF) in the high-dose group was significantly earlier than that in controls. This result indicates that the occurrence of initiated cells could be accelerated by DDT in the high-dose group, which is considered to be due to hepatocellular DNA damage caused by oxidative stress. It is possible that the accelerated occurrence of initiated cells may result in the early appearance and increased incidence of eosinophilic AHF described later.
Table 4.
Hepatic lipid peroxide (LPO) contents in F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Sex | No. of rats examined | LPO (nmol/g tissue) at weeks | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 26 | 52 | 78 | 104 | |||
| 0 | M | 6 | 130 ± 13 | 152 ± 37 | 157 ± 37 | 138 ± 29 |
| F | 6 | 168 ± 30 | 202 ± 15 | 150 ± 21 | 173 ± 53 | |
| 5 | M | 6 | 210 ± 113 | 140 ± ± 30 | 217 ± 71 | 257 ± 122 |
| F | 6 | 177 ± 20 | 188 ± 8 | 157 ± 43 | 188 ± 77 | |
| 50 | M | 6 | 1005 ± 324** | 537 ± 74** | 530 ± 132** | 523 ± 332* |
| F | 6 | 247 ± 62* | 248 ± 75 | 212 ± 48 | 307 ± 78* | |
| 500 | M | 6 | 838 ± 286** | 393 ± 54** | 360 ± 172* | 423 ± 68** |
| F | 6 | 267 ± 69* | 178 ± 24 | 185 ± 53 | 267 ± 101 | |
Values represent mean ± S.D.
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Table 5.
Hepatic 8-OHdG levels in F344 rats from a 2-year feeding study of p,p′-DDT\
| Dose (ppm) | Sex | No. of rats examined | 8-OHdG (mg/mg DNA) at weeks | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 26 | 52 | 78 | 104 | |||
| 0 | M | 6 | 4.4 ± 1.0 | 3.0 ± 0.9 | 2.7 ± 1.1 | 3.2 ± 0.8 |
| F | 6 | 1.9 ± 0.5 | 2.7 ± 0.5 | 2.5 ± 0.5 | 3.0 ± 0.8 | |
| 5 | M | 6 | 3.7 ± 1.3 | 3.8 ± 0.8 | 2.2 ± 0.7 | 3.5 ± 0.7 |
| F | 6 | 2.0 ± 0.2 | 4.0 ± 1.9 | 2.7 ± 0.3 | 2.9 ± 0.6 | |
| 50 | M | 6 | 5.9 ± 1.6 | 6.1 ± 2.8 | 2.9 ± 1.2 | 5.0 ± 1.0 |
| F | 6 | 3.0 ± 1.6 | 5.2 ± 1.4 | 3.1 ± 0.4 | 2.7 ± 0.5 | |
| 500 | M | 6 | 15.4 ± 4.5** | 13.8 ± 4.3** | 7.5 ± 2.2** | 15.9 ± 9.9** |
| F | 6 | 2.6 ± 1.1 | 14.1 ± 5.2** | 6.2 ± 2.9** | 5.8 ± 0.9** | |
Values represent mean ± S.D.
Significantly different from control at p < 0.01 (Dunnett’s multiple comparison test).
Cell proliferation
PCNA labeling index (LI) in the liver from rats treated with DDT showed that cell proliferation was enhanced within 3 days of treatment but returned to normal thereafter (Tables 6 and 7). This cell proliferation pattern is consistent with that by non-genotoxic mitogenic hepatocarcinogens (28–30). It is generally known that the hepatic cell proliferation response to non-genotoxic mitogenic hepatocarcinogens such as phenobarbital typically occurs through an initial burst of enhanced DNA synthesis followed by enhanced mitosis (29–31). The enhanced cell proliferation, however, ceases after a few days even if treatment is continued. It is considered that an effective feedback mechanism (checkpoint function such as G1 or G2 arrest in cell cycle) may prevent excessive cell multiplication in the normal liver even if the growth stimulatory signals are steadily present due to continuous chemical treatment (30). It has been postulated that non-genotoxic chemicals with mitogenic activity may provide a selective growth advantage to spontaneously initiated precancerous cells over normal hepatocytes and lead them to neoplasms (28,29). The increases in the number and size of eosinophilic AHF in our 2-year study could be a reflection of the mitogenic activity of DDT that contributes to the growth of initiated cells.
Table 6.
PCNA LI (%) in the liver of male F344 rats from a 4-week feeding study of p,p′-DDT
| Dose (ppm) | PCNA LI (mean ± SD) on days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 7 | 14 | 28 | |||||||
| 0 | 2.00 ± 0.80 | (5) | 1.58 ± 0.68 | (5) | 3.19 ± 1.44 | (5) | 4.46 ± 1.41 | (5) | 1.75 ± 0.77 | (5) | 0.96 ± 0.50 | (5) |
| 50 | 2.06 ± 0.46 | (5) | 3.22 ± 0.51* | (5) | 4.84 ± 1.60 | (5) | 5.37 ± 0.36 | (5) | 0.96 ± 0.31 | (5) | 0.58 ± 0.18 | (5) |
| 160 | 2.81 ± 0.67 | (5) | 5.91 ± 1.49** | (5) | 6.85 ± 1.71** | (5) | 4.13 ± 0.72 | (5) | 0.62 ± 0.54 | (5) | 0.60 ± 0.34 | (5) |
| 500 | 4.62 ± 1.01** | (5) | 5.33 ± 0.59** | (5) | 6.31 ± 1.57* | (5) | 4.52 ± 1.27 | (5) | 1.39 ± 1.19 | (5) | 0.94 ± 0.48 | (5) |
(n): Number of animals examined.
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Table 7.
PCNA LI (%) in the liver of F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Sex | PCNA LI (mean ± SD) at weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 26 | 52 | 78 | 104 | ||||||
| 0 | M | 0.70 ± 0.34 | (6) | 0.43 ± 0.11 | (6) | 0.82 ± 0.63 | (6) | 0.80 ± 1.03 | (5) |
| F | 0.25 ± 0.22 | (6) | 0.17 ± 0.11 | (6) | 0.93 ± 0.52 | (6) | 0.45 ± 0.32 | (5) | |
| 5 | M | 0.67 ± 0.38 | (6) | 0.40 ± 0.22 | (6) | 0.69 ± 0.25 | (6) | 0.43 ± 0.19 | (5) |
| F | 0.13 ± 0.05 | (6) | 0.14 ± 0.08 | (6) | 1.13 ± 1.17 | (6) | 0.28 ± 0.07 | (6) | |
| 50 | M | 0.77 ± 0.19 | (6) | 0.28 ± 0.13 | (6) | 0.75 ± 0.31 | (6) | 0.42 ± 0.21 | (4) |
| F | 0.87 ± 0.69 | (6) | 0.36 ± 0.16 | (6) | 1.10 ± 0.59 | (6) | 0.46 ± 0.25 | (6) | |
| 500 | M | 0.32 ± 0.22 | (6) | 0.29 ± 0.16 | (6) | 0.78 ± 0.24 | (6) | 0.73 ± 0.48 | (6) |
| F | 0.45 ± 0.18 | (6) | 0.34 ± 0.31 | (6) | 0.62 ± 0.20 | (6) | 0.55 ± 0.06 | (4) | |
(n): Number of animals examined.
Gap junctional intercellular communication (GJIC)
Quantitative analysis of gap junctional intercellular communication (GJIC) in the liver from rats treated with DDT demonstrated a persistent decrease in GJIC protein Cx32 from the beginning to the end of treatment (Tables 8 and 9). GJIC in the liver has been shown to be inhibited by various non-genotoxic tumor-promoting agents including phenobarbital and DDT in vivo and in vitro (14,18–20,22,32–34). The inhibition of GJIC by tumor promoters may be produced in several ways (21). Since DDT is highly lipophilic and accumulates in cell membranes, it could interfere directly with the function of GJIC, whereas phenobarbital which is not highly lipophilic may inhibit GJIC in a different way. It is known that GJIC involves the passage of low molecular weight substances between adjacent cells via gap junctions and its function includes the possible regulation of cellular division through cell-to-cell exchange of replication signal molecules (19,34,35). Therefore, inhibition of GJIC may isolate initiated cells from the growth regulatory signals of neighboring cells and permit the clonal expansion of initiated cells. This suggests an important role for GJIC in the process of tumor formation.
Table 8.
Number of GJIC protein Cx32 spots in the liver of male F344 rats from a 4-week feeding study of p,p′-DDT
| Dose (ppm) | No. of Cx32 spots (mean ± SD) on days: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 7 | 14 | 28 | |||||||
| 0 | 8.29 ± 0.36 | (5) | 7.81 ± 0.75 | (5) | 7.86 ± 0.81 | (5) | 7.24 ± 0.38 | (5) | 7.64 ± 0.52 | (5) | 8.11 ± 0.22 | (5) |
| 50 | 5.59 ± 0.77** | (5) | 4.69 ± 0.33** | (5) | 5.41 ± 0.40** | (5) | 3.14 ± 0.33** | (5) | 2.99 ± 0.53** | (5) | 3.33 ± 0.74** | (5) |
| 160 | 4.90 ± 0.63** | (5) | 4.37 ± 0.64** | (5) | 3.94 ± 0.64** | (5) | 3.06 ± 0.46** | (5) | 2.71 ± 0.68** | (5) | 2.77 ± 0.43** | (5) |
| 500 | 5.35 ± 1.03** | (5) | 3.98 ± 0.41** | (5) | 3.12 ± 0.33** | (5) | 2.62 ± 0.70** | (5) | 1.75 ± 0.51** | (5) | 1.68 ± 0.79** | (5) |
(n): Number of animals examined.
Significantly different from control at p < 0.01 (Dunnett’s multiple comparison test).
Table 9.
Number of GJIC protein Cx32 spots in the liver of F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Sex | No. of Cx32 spots (mean ± SD) at weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 26 | 52 | 78 | 104 | ||||||
| 0 | M | 7.07 ± 0.94 | (6) | 6.49 ± 1.46 | (6) | 3.05 ± 0.19 | (6) | 3.11 ± 1.15 | (5) |
| F | 6.08 ± 0.97 | (6) | 6.48 ± 0.66 | (6) | 3.26 ± 0.74 | (6) | 3.73 ± 0.23 | (5) | |
| 5 | M | 6.87 ± 0.54 | (6) | 6.95 ± 0.88 | (6) | 2.51 ± 0.50* | (6) | 2.13 ± 0.37 | (5) |
| F | 5.67 ± 0.93 | (6) | 5.51 ± 1.62 | (6) | 2.90 ± 0.41 | (6) | 3.59 ± 0.42 | (6) | |
| 50 | M | 5.53 ± 0.83* | (6) | 6.28 ± 1.88 | (6) | 2.47 ± 0.44* | (6) | 2.13 ± 0.35 | (4) |
| F | 4.80 ± 0.86 | (6) | 4.20 ± 0.56** | (6) | 1.76 ± 0.69* | (6) | 2.65 ± 0.33** | (6) | |
| 500 | M | 5.39 ± 1.30* | (6) | 4.64 ± 0.91 | (6) | 1.14 ± 0.22** | (6) | 1.52 ± 0.28* | (6) |
| F | 4.20 ± 1.41* | (6) | 3.28 ± 0.72** | (6) | 1.03 ± 0.16** | (6) | 1.75 ± 0.47** | (4) | |
(n): Number of animals examined.
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Changes in liver weights
Time-related changes in liver weights in F344 rats treated with DDT are shown in Tables 10~12. Absolute and/or relative (ratio to body weight) liver weights significantly increased in a dose-dependent manner from the beginning of treatment throughout the study. The liver weight also increased in correlation with duration of exposure but tended to reach a plateau after certain time. It is known that administration of mitogenic agents such as phenobarbital and buthylhydroxytoluene causes an increase in liver weight through mitogenic stimulation of cell proliferation (28). Upon continued administration, the increased liver weight is maintained, even though the rate of cell turnover returns to normal levels (28). The increased liver weights in the DDT-treated rats might be due to mitogenic stimulation of cell proliferation to some extent, but the major cause seems to be proliferation of smooth-surfaced endoplasmic reticulum (SER) associated with microsomal enzyme induction since the time of increase in liver weight and enzyme induction was consistent with each other during the study.
Table 10.
Liver weights of male F344 rats from a 4-week feeding study of p,p′-DDT
| Dose (ppm) | Liver weights | Days on study | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 7 | 14 | 28 | ||
| 50 | Absolute | 109 (5) | 107 (5) | 106 (5) | 112 (5)* | 126 (5)* | 112 (5)** |
| Relative | 108 (5)** | 107 (5) | 107 (5)* | 111 (5)* | 121 (5)** | 110 (5)** | |
| 160 | Absolute | 105 (5) | 114 (5) | 121 (5)** | 127 (5)** | 142 (5)** | 124 (5)** |
| Relative | 107 (5)* | 113 (5)** | 119 (5)** | 123 (5)** | 136 (5)** | 122 (5)** | |
| 500 | Absolute | 110 (5) | 125 (5)** | 127 (5)** | 153 (5)** | 148 (5)** | 142 (5)** |
| Relative | 111 (5)** | 125 (5)** | 129 (5)** | 147 (5)** | 148 (5)** | 145 (5)** | |
Values represent percentages (%) to control values.
(n): Number of animals examined.
Relative: Ratio to body weight (%)
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Table 12.
Liver weights of female F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Liver weights | Weeks on study | |||
|---|---|---|---|---|---|
|
| |||||
| 26 | 52 | 78 | 104 | ||
| 5 | Absolute | 109 (6) | 106 (6) | 104 (8) | 102 (10) |
| Relative | 106 (6) | 103 (6) | 103 (8) | 101 (10) | |
| 50 | Absolute | 111 (6)* | 123 (6) | 110 (7) | 111 (10) |
| Relative | 114 (6)* | 118 (6)** | 109 (7) | 110 (10) | |
| 500 | Absolute | 146 (6)** | 151 (6)** | 143 (8)** | 144 (10)** |
| Relative | 163 (6)** | 170 (6)** | 165 (8)** | 190 (10)** | |
Values represent percentages (%) to control values.
(n): Number of animals examined.
Relative: Ratio to body weight (%)
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Histopathology of the liver with morphometry
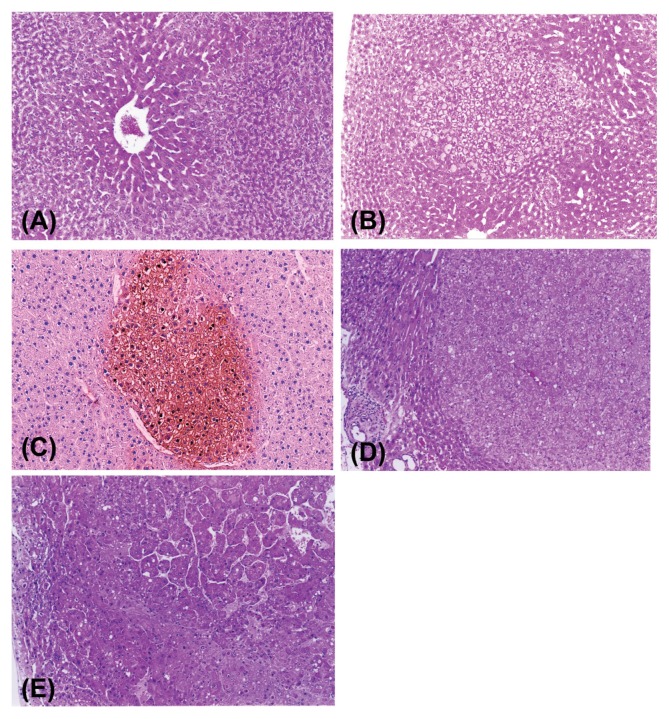
In our 2-year rat study, treatment with DDT induced centrilobular hepatocellular hypertrophy and increased eosinophilic AHF, hepatocellular adenomas and carcinomas (Table 13). The severity of hepatocellular hypertrophy (Fig. 4A) was dose-dependent and the hypertrophic alteration could be due to SER proliferation associated with microsomal enzyme induction. The eosinophilic AHF noted in the liver treated with DDT typically contained hepatocytes with pale pink or ground glass appearance cytoplasm (Fig. 4B) and sometimes had cytoplasmic clear spaces which may represent glycogen deposits. The eosinophilic AHF was positive for glutathione S-transferase placental form (GST-P) (Fig. 4C). The eosinophilic AHF tended to be located in the region close to or within the hypertrophic area. Such a spatial relationship was also reported in rats after continuous administration of low doses of N-nitrosomorpholine, which resulted in centrilobular perivenular hepatocellular hypertrophy, being closely related to the later development of pre-neoplastic hepatic foci including clear/acidophilic foci as well as hepatocellular neoplasms (36).
Table 13.
Incidences of hepatocellular hypertrophy and altered hepatocellular foci (AHF) in F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | Hypertrophy | Eosinophilic AHF | Tigroid basophilic AHF | Clear cell AHF | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| 0 | 26 | 0/6 | 0/6 | 0/6 | 0/6 | 2/6 | 2/6 | 0/6 | 0/6 |
| 52 | 0/6 | 0/6 | 0/6 | 0/6 | 3/6 | 4/6 | 5/6 | 0/6 | |
| 78 | 0/8 | 0/8 | 1/8 | 0/8 | 8/8 | 8/8 | 5/8 | 3/8 | |
| 104 | 0/35 | 0/33 | 23/35 | 6/33 | 34/35 | 30/33 | 30/35 | 8/33 | |
| Totala | 0/40 | 0/40 | 24/40 | 8/40 | 37/40 | 37/40 | 30/40 | 9/40 | |
|
| |||||||||
| 5 | 26 | 2/6 | 0/6 | 0/6 | 0/6 | 1/6 | 1/6 | 1/6 | 0/6 |
| 52 | 6/6** | 0/6 | 0/6 | 0/6 | 1/6 | 6/6 | 4/6 | 0/6 | |
| 78 | 4/8* | 1/8 | 2/8 | 1/8 | 8/8 | 8/8 | 6/8 | 2/8 | |
| 104 | 15/30** | 1/27 | 18/30 | 8/27 | 29/30 | 26/27 | 24/30 | 0/27** | |
| Total | 15/40** | 1/40 | 21/40 | 10/40 | 35/40 | 38/40 | 24/40 | 0/40** | |
|
| |||||||||
| 50 | 26 | 6/6** | 6/6** | 0/6 | 0/6 | 1/6 | 3/6 | 1/6 | 0/6 |
| 52 | 6/6** | 6/6** | 0/6 | 0/6 | 0/6 | 6/6 | 4/6 | 0/6 | |
| 78 | 8/8** | 7/7** | 8/8** | 1/7 | 8/8 | 7/7 | 8/8 | 0/7 | |
| 104 | 33/36** | 31/32** | 30/36 | 16/32** | 34/36 | 30/32 | 27/36 | 8/32 | |
| Total | 35/40** | 34/40** | 34/40** | 19/40** | 38/40 | 38/40 | 27/40 | 8/40 | |
|
| |||||||||
| 500 | 26 | 6/6** | 6/6** | 4/6* | 0/6 | 0/6 | 2/6 | 0/6 | 0/6 |
| 52 | 6/6** | 6/6** | 6/6** | 2/6 | 0/6 | 0/6* | 0/6** | 0/6 | |
| 78 | 7/7** | 8/8** | 7/7** | 8/8** | 3/7* | 6/8 | 0/7** | 0/8 | |
| 104 | 31/33** | 31/33** | 33/33** | 31/33** | 29/33 | 23/33* | 13/33** | 11/33 | |
| Total | 38/40** | 37/40** | 38/40** | 37/40** | 34/40 | 27/40** | 14/40* | 11/40 | |
Total number of animals examined (No. of scheduled deaths after 104 weeks + No. of unscheduled deaths).
Significantly different from control at p < 0.05 and p < 0.01, respectively (Fisher’s exact test).
Fig. 4.
Centrilobular hepatocellular hypertrophy in a male F344 rat treated with DDT at 500 ppm for 26 weeks (A), H&E stain, ×112; An eosinophilic focus with ground glass appearance in a male F344 rat treated with DDT at 500 ppm for 78 weeks (B), H&E stain, ×54; GST-P positive focus in a male F344 rat treated with DDT at 500 ppm for 52 weeks, which was eosinophilic type of focus in H&E-stained section (C), GST-P stain, ×112; Hepato-cellular adenoma comprising eosinophilic hepatocytes with pale pink or ground glass appearance cytoplasm in a male F344 rat treated with DDT at 500 ppm for 104 weeks (D), H&E stain, ×85; Hepatocellular carcinoma containing both eosinophilic and basophilic phenotypes of hepatocytes with trabecular pattern in a male F344 rat treated with DDT at 500 ppm for 104 weeks (E), H&E stain, ×85.
Quantitative morphometric analysis revealed that the number and size of eosinophilic AHF were increased in correlation with duration of exposure and dose levels and its appearance was earlier in males than females (Tables 14~ 16). The incidence of eosinophilic AHF in the high-dose group after 104 weeks of treatment reached nearly 100% for both sexes. Since the first appearance of eosinophilic AHF in the high-dose group (at week 26) was much earlier than that in the controls (at week 78), the eosinophilic pre-neoplastic lesions could be induced by DDT as a result of DNA damage of hepatocytes exposed to oxidative stress as described before. It is conceivable that DDT may accelerate the occurrence of initiated cells by oxidative stress, leading to the early appearance of eosinophilic AHF and promotes the growth of eosinophilic preneoplastic lesions through its mitogenic activity in combination with the inhibitory effect on GJIC as mentioned before. In other types of AHF, tigroid basophilic AHF decresed in number and size in females in the high dose group. A similar result was also reported in rats treated with phenobarbital (33).
Table 14.
Number of altered hepatocellular foci (AHF) in F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | No. of rats examined | No. of AHF (No./cm2): Mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Eosinophilic | Tigroid basophilic | Clear cell | |||||||
|
|
|
|
|
||||||
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| 0 | 26 | 6 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.6 | 0.6 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 52 | 6 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 1.3 | 1.6 ± 1.6 | 1.6 ± 1.1 | 0.0 ± 0.0 | |
| 78 | 8 | 8 | 0.1 ± 0.4 | 0.0 ± 0.0 | 2.2 ± 1.3 | 9.3 ± 3.7 | 0.8 ± 0.8 | 0.5 ± 0.7 | |
| 104 | 10 | 10 | 1.5 ± 1.5 | 0.6 ± 1.0 | 6.5 ± 4.8 | 11.0 ± 5.3 | 1.9 ± 1.7 | 0.8 ± 0.9 | |
|
| |||||||||
| 5 | 26 | 6 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.4 | 0.3 ± 0.7 | 0.2 ± 0.4 | 0.0 ± 0.0 |
| 52 | 6 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.8 | 4.7 ± 1.7 | 1.3 ± 1.4 | 0.0 ± 0.0 | |
| 78 | 8 | 8 | 0.5 ± 0.9 | 0.2 ± 0.5 | 2.8 ± 1.7 | 10.0 ± 5.3 | 1.4 ± 1.2 | 0.6 ± 1.1 | |
| 104 | 10 | 10 | 2.0 ± 1.8 | 0.6 ± ± 0.7 | 5.1 ± 4.6 | 14.2 ± 4.0 | 2.4 ± 2.0 | 0.0 ± 0.0 | |
|
| |||||||||
| 50 | 26 | 6 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.9 | 0.8 ± 0.8 | 0.2 ± 0.4 | 0.0 ± 0.0 |
| 52 | 6 | 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.5 ± 1.6* | 1.1 ± 1.1 | 0.0 ± 0.0 | |
| 78 | 8 | 7 | 3.1 ± 1.5* | 0.6 ± 1.5 | 2.0 ± 1.9 | 8.7 ± 4.1 | 1.2 ± 0.5 | 0.0 ± 0.0 | |
| 104 | 10 | 10 | 8.9 ± 5.1** | 1.0 ± 2.2 | 3.8 ± 2.3 | 17.6 ± 4.5 | 2.9 ± 1.2 | 0.3 ± 0.7 | |
|
| |||||||||
| 500 | 26 | 6 | 6 | 0.8 ± 0.7** | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 52 | 6 | 6 | 3.7 ± 1.6** | 1.4 ± 2.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0* | 0.0 ± 0.0 | |
| 78 | 7 | 8 | 9.2 ± 3.1** | 12.1 ± 3.5** | 0.5 ± 0.6 | 1.0 ± 0.7** | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| 104 | 10 | 10 | 10.4 ± 3.1** | 9.8 ± 3.8** | 2.1 ± 1.0 | 1.3 ± 1.3* | 0.4 ± 0.6* | 1.0 ± 1.3 | |
Significantly different from control at p < 0.05 and p < 0.01, respectively (Fisher’s exact test).
Table 16.
Area fraction of liver occupied by altered hepatocellular foci (AHF) in F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | No. of rats examined | Area fraction occupied by AHF (%): Mean ± SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Eosinophilic | Tigroid basophilic | Clear cell | ||||||||
|
|
|
|
|
|||||||
| Male | Female | Male | Female | Male | Female | Male | Female | |||
| 0 | 26 | 6 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 52 | 6 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.16 | 0.26 ± 0.42 | 0.04 ± 0.04 | 0.00 ± 0.00 | ||
| 78 | 8 | 8 | 0.02 ± 0.05 | 0.00 ± 0.00 | 0.18 ± 0.10 | 1.06 ± 0.54 | 0.04 ± 0.05 | 0.02 ± 0.03 | ||
| 104 | 10 | 10 | 0.51 ± 0.41 | 0.25 ± 0.44 | 0.98 ± 0.70 | 1.82 ± 1.16 | 0.16 ± 0.18 | 0.08 ± 0.13 | ||
|
| ||||||||||
| 5 | 26 | 6 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.005 ± 0.012 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.00 ± 0.00 | |
| 52 | 6 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.02 | 0.41 ± 0.26 | 0.04 ± 0.04 | 0.00 ± 0.00 | ||
| 78 | 8 | 8 | 0.31 ± 0.77 | 0.01 ± 0.02 | 0.21 ± 0.11 | 0.85 ± 0.52 | 0.06 ± 0.07 | 0.02 ± 0.04 | ||
| 104 | 10 | 10 | 1.07 ± 1.85 | 0.20 ± 0.38 | 0.54 ± 0.42 | 1.93 ± 0.45 | 0.19 ± 0.16 | 0.00 ± 0.00* | ||
|
| ||||||||||
| 50 | 26 | 6 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.05 | 0.03 ± 0.03 | 0.005 ± 0.011 | 0.00 ± 0.00 | |
| 52 | 6 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.47 ± 0.12 | 0.06 ± 0.09 | 0.00 ± 0.00 | ||
| 78 | 8 | 7 | 0.60 ± 0.39* | 0.18 ± 0.48 | 0.34 ± 0.48 | 0.89 ± 0.53 | 0.12 ± 0.08 | 0.00 ± 0.00 | ||
| 104 | 10 | 10 | 5.10 ± 4.74* | 0.32 ± 0.71 | 0.41 ± 0.32 | 2.54 ± 0.76 | 0.42 ± 0.52 | 0.02 ± 0.04 | ||
|
| ||||||||||
| 500 | 26 | 6 | 6 | 0.04 ± 0.05** | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.07 | 0.00 ± | 0.00 | 0.00 ± 0.00 |
| 52 | 6 | 6 | 0.23 ± 0.09** | 0.08 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± | 0.00 | 0.00 ± 0.00 | |
| 78 | 7 | 8 | 2.72 ± 1.05** | 3.01 ± 1.84** | 0.03 ± 0.05* | 0.10 ± 0.10** | 0.00 ± | 0.00 | 0.00 ± 0.00 | |
| 104 | 10 | 10 | 9.25 ± 6.28** | 8.25 ± 4.53** | 0.31 ± 0.19 | 0.10 ± 0.09** | 0.14 ± | 0.30 | 0.03 ± 0.03 | |
Significantly different from control at p < 0.05 and p < 0.01, respectively (Fisher’s exact test).
As to neoplasia, the overall incidences of hepatocellular adenomas and carcinomas in the high-dose group during the study were 55% and 35% for males and 40% and 5% for females, respectively (Table 17). Morphologically, the hepatocellular adenomas contained typically eosinophilic hepatocytes (Fig. 4D) which were similar to those of eosinophilc AHF described above and often had basophilic phenotypes in small numbers. Basophilic cell type of adenomas was also noted but only in a few animals. On the other hand, the hepatocellular carcinomas contained hepatocytes with eosinophilic or basophilic cytoplasm and typically had admixture of both phenotypes (Fig. 4E). The population of basophilic cells in the carcinomas was much higher than that in adenomas or large eosinophilic AHF. Since the hepatocytes within the majority of adenomas were morphologically similar to those of eosinophilic AHF and there was no increase in other types of AHF, it was suggested that the eosinophilic AHF could develop into neoplasms without passing an intermediate stage (37). However, large eosinophic AHF and adenomas sometimes contained small number of basophilic cells within the lesions. The presence of basophilic cells might be an indication of malignant transformation of hepatocytes within the lesions since the population of basophilic phenotypes is largest in the hepatocellular carcinomas (37). A similar type of nongenotoxic hepatocarcinogen, phenobarbital (PB), also has been shown to induce eosinophilic altered foci and hepatocellular tumors containing eosinophilic cells (GGT positive) (33). However, it should be recognized that the occurrence of eosinophilic AHF is not limited to PB and DDT because many other chemicals including genotoxic agents also induced such eosinophilic preneoplastic lesions (37,38). In addition, it has been suggested that the development of hepatocellular tumors in rats and mice after long-term exposure to CAR activators such as PB and DDT seems to be rodent-specific and not relevant to human (24).
Table 17.
Incidence of hepatocellular tumors in F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | Adenoma | Carcinoma | ||
|---|---|---|---|---|---|
|
|
|
||||
| Male | Female | Male | Female | ||
| 0 | 26 | 0/6 | 0/6 | 0/6 | 0/6 |
| 52 | 0/6 | 0/6 | 0/6 | 0/6 | |
| 78 | 0/8 | 0/8 | 0/8 | 0/8 | |
| 104 | 0/35 | 0/33 | 0/35 | 0/33 | |
| Totala | 0/40 | 0/40 | 0/40 | 0/40 | |
|
| |||||
| 5 | 26 | 0/6 | 0/6 | 0/6 | 0/6 |
| 52 | 0/6 | 0/6 | 0/6 | 0/6 | |
| 78 | 0/8 | 0/8 | 0/8 | 0/8 | |
| 104 | 0/30 | 0/27 | 0/30 | 0/27 | |
| Total | 0/40 | 0/40 | 0/40 | 0/40 | |
|
| |||||
| 50 | 26 | 0/6 | 0/6 | 0/6 | 0/6 |
| 52 | 0/6 | 0/6 | 0/6 | 0/6 | |
| 78 | 0/8 | 0/7 | 0/8 | 0/7 | |
| 104 | 5/36* | 0/32 | 0/36 | 0/32 | |
| Total | 5/40* | 0/40 | 0/40 | 0/40 | |
|
| |||||
| 500 | 26 | 0/6 | 0/6 | 0/6 | 0/6 |
| 52 | 0/6 | 0/6 | 0/6 | 0/6 | |
| 78 | 6/7** | 1/8 | 0/7 | 0/8 | |
| 104 | 20/33** | 16/33** | 13/33** | 2/33 | |
| Total | 22/40** | 16/40** | 14/40** | 2/40 | |
Total number of animals examined (No. of scheduled deaths after 104 weeks + No. of unscheduled deaths).
Significantly different from control at p < 0.05 and p < 0.01, respectively (Fisher’s exact test).
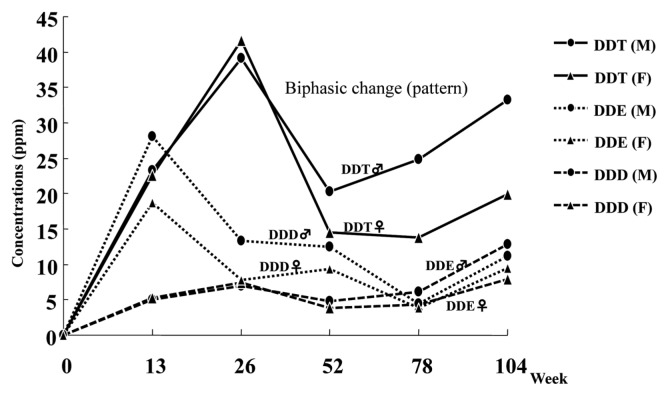
With respect to sex difference in tumor development, it is generally believed that males have a higher incidence of hepatic tumors than females in rodents as well as in humans (39). Factors contributing to this sex difference have not been clearly demonstrated, but it may be due to the difference of hormonal pattern which is of primary importance for determining the metabolic activation of carcinogens and also due to the sex chromosome in its role as a carrier of genetic messages (39). In our 2-year rat study, the incidence of hepatocellular tumors, especially carcinomas, was much higher in males than females. This sex difference might be partly due to the higher concentrations of DDT and its metabolites (DDE and DDD) in the liver of males (Fig. 3) which may result in higher production of oxidative stress through metabolic activation. In fact, the levels of LPO and 8-OHdG were much higher in males than females. In addition, the higher incidence of spontaneous eosinophilic foci in male F344 rats (40) may contribute to the sex difference. In other words, the male liver may have a preferable microenvironment for occurrence of eosinophilic AHF. The reduced body weight gain in the high-dose group which was more evident in females than males (5) was also considered as an influential factor for tumor development, but it seems to have no correlation with the occurrence of hepatocellular tumors in F344 rats (41).
Fig. 3.
Liver concentrations of DDT, DDE, and DDD in F344 rats at high-dose (500 ppm) in a 2-year feeding study. (M) male; (F) female.
Gene expression in target sites
Analysis of gene expression by microarray following laser-captured micro-dissection was performed on the target tissues including hepatocellular hypertrophy, eosinophilic AHF, and hepatocellular adenoma observed in the high-dose and/or control rats. The results are summarized in Table 18. As shown in Table 18, various genes relating to cell proliferation, apoptosis or anti-oxidative function were up-regulated or down-regulated in the target sites and the most prominent changes were noted in the eosinophilic AHF from the high-dose rat. These gene expressions might be corresponding changes to the mitogenic activity of DDT and/or oxidative stress generated though the metabolic activation. However, we need further accumulation of gene expression data to arrive at conclusion since the information from our results is quite limited.
Table 18.
Microarray assay of hepatoproliferative lesions in male F344 rats from a 2-year feeding study of p,p′-DDT
| Gene/protein name | Function | Fold change (ratio to normal control value)* | |||
|---|---|---|---|---|---|
|
| |||||
| Hypertorophy | Eosinophilic AHF | Adenoma | |||
|
|
|
|
|||
| 500 ppm | 0 ppm | 500 ppm | 500 ppm | ||
| Protein kinase C-eta | Cell proliferation (CP) | 3.6 | 3.2 | 3.1 | 2.5 |
| Uncouplingprotein 2, mitochondrial | CP | 2.0 | - | 2.0 | - |
| Phosphorylase B kinase catalytic subunit | CP | - | - | 2.2 | - |
| Guanine nucleotide-binding protein G-s, α subunit | CP | 2.5 | - | 2.6 | 2.3 |
| G protein γ-5 subunit | CP | 2.0 | - | 3.5 | - |
| CDK106 | CP (Downerg. TGF-β) | - | - | 2.0 | 2.0 |
| Follistatin | CP (Downerg. TGF-β) | - | - | 0.5 | - |
| Lipoprotein lipase | CP (TGF-β) | - | - | 0.5 | - |
| Ubiquitin-conjugating enzyme E2D3 | CP (Cell cycle) | - | - | 0.5 | - |
| p53-activated gene 608 | Apoptosis (Apo) | - | - | 2.3 | 2.0 |
| Sodium channel β2 | Apo (suppre) | 2.9 | 2.2 | 3.2 | 2.4 |
| Protein disulfide isomerase A6 precursor | Apo (suppre) | - | - | 0.5 | - |
| Protein phosphatase type 1α, catalytic subunit | Apo (suppre) | 0.5 | - | 0.4 | - |
| Potassium channel gene 1 (Kir6) | Anti-oxidative | 3.2 | 2.7 | 3.1 | 2.5 |
| Dopamine receptor D2 | Anti-oxidative | 2.7 | 3.0 | 2.1 | 2.2 |
Values of 2.0 or more and 0.5 or less represent up-regulation and down-regulation, respectively.
–: Within normal range.
CONCLUSIONS
DDT and its metabolites may have an endocrine disrupting potential affecting reproductive system although the effects may vary among animal species in correlation with exposure levels. Epidemiologic studies revealed either positive or negative associations between exposure to DDT and tumor development, but there has been no clear evidence that DDT causes cancer in humans. In experimental animals, tumor induction by DDT has been shown in the liver, lung, and adrenal. The mechanisms of hepatic tumor development by DDT has been studied in rats and mice. DDT is known as a non-genotoxic hepatocarcinogen and has been shown to induce microsomal enzymes through activation of constitutive androstane receptor (CAR) and to inhibit gap junctional intercellular communication (GJIC) in the rodent liver. The results from our previously conducted 4-week and 2-year feeding studies of p,p′-DDT in F344 rats indicate that DDT may induce hepatocellular eosinophilic foci as a result of oxidative DNA damage and leads them to hepatic neoplasia in combination with its mitogenic activity and inhibitory effect on GJIC. Oxidative stress could be a key factor in hepatocarcinogenesis by DDT.
Table 2.
Hepatic microsomal enzyme activity and P450 isozyme contents in male F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | PROD (pmol/min/mg) | P450 isozyme contents (pmol/mg protein) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CYP1A2 | CYP2B1 | CYP3A2 | CYP4A1 | ||||||||
| 0 | 26 | 18 ± 2 | (6) | 4.24 ± 0.97 | (6) | 10.4 ± 1.0 | (6) | 72.3 ± 15.3 | (6) | 21.9 ± 2.1 | (6) |
| 52 | 15 ± 2 | (6) | 3.22 ± 0.97 | (6) | 9.75 ± 1.02 | (6) | 83.4 ± 6.9 | (6) | 35.4 ± 2.8 | (6) | |
| 78 | 5 ± 2 | (6) | 6.97 ± 1.42 | (6) | 11.4 ± 1.7 | (6) | 69.6 ± 16.3 | (6) | 40.9 ± 7.5 | (6) | |
| 104 | 3 ± 2 | (6) | 6.37 ± 1.90 | (6) | 12.4 ± 2.7 | (6) | 10.1 ± 5.9 | (6) | 17.5 ± 4.7 | (6) | |
|
| |||||||||||
| 5 | 26 | 155 ± 9 | (6) | 6.88 ± 1.39** | (6) | 66.7 ± 8.9 | (6) | 175 ± 23** | (6) | 19.4 ± 1.6 | (6) |
| 52 | 205 ± 18** | (6) | 7.05 ± 1.15** | (6) | 61.9 ± 8.0 | (6) | 175 ± 18 | (6) | 36.3 ± 5.4 | (6) | |
| 78 | 118 ± 15* | (6) | 5.55 ± 1.50 | (6) | 74.9 ± 9.0 | (6) | 94.0 ± 36.0 | (6) | 39.1 ± 12.4 | (6) | |
| 104 | 81 ± 29* | (6) | 8.99 ± 4.39 | (6) | 76.2 ± 17.8 | (6) | 56.6 ± 31.6 | (6) | 18.4 ± 7.7 | (6) | |
|
| |||||||||||
| 50 | 26 | 456 ± 25** | (6) | 3.25 ± 0.70 | (6) | 366 ± 34** | (6) | 368 ± 34** | (6) | 35.8 ± 2.4** | (6) |
| 52 | 466 ± 42** | (6) | 2.65 ± 1.19 | (6) | 398 ± 50** | (6) | 364 ± 56** | (6) | 44.4 ± 7.0* | (6) | |
| 78 | 293 ± 95** | (6) | 3.44 ± 3.06* | (6) | 172 ± 56** | (6) | 150 ± 82 | (6) | 42.0 ± 15.8 | (6) | |
| 104 | 143 ± 45** | (6) | 7.79 ± 4.04 | (6) | 179 ± 33** | (6) | 60.4 ± 16.4 | (6) | 20.4 ± 8.7 | (6) | |
|
| |||||||||||
| 500 | 26 | 173 ± 26* | (6) | 1.78 ± 0.36** | (6) | 607 ± 71** | (6) | 534 ± 50** | (6) | 36.5 ± 3.9** | (6) |
| 52 | 143 ± 15 | (6) | 3.96 ± 0.87 | (6) | 476 ± 70** | (6) | 540 ± 92** | (6) | 33.4 ± 3.3 | (6) | |
| 78 | 81 ± 15 | (6) | 3.73 ± 2.64 | (6) | 222 ± 38** | (6) | 189 ± 74** | (6) | 30.4 ± 6.2 | (6) | |
| 104 | 64 ± 26 | (6) | 5.04 ± 1.62 | (6) | 271 ± 65** | (6) | 176 ± 42** | (6) | 18.8 ± 9.1 | (6) | |
Values represent mean ± S.D. (n): Number of animals examined.
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Table 11.
Liver weights of male F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Liver weights | Weeks on study | |||
|---|---|---|---|---|---|
|
| |||||
| 26 | 52 | 78 | 104 | ||
| 5 | Absolute | 106 (6) | 108 (6) | 108 (8) | 110 (10) |
| Relative | 102 (6) | 108 (6) | 105 (8) | 105 (10) | |
| 50 | Absolute | 114 (6)** | 117 (6)* | 126 (8)** | 125 (10)** |
| Relative | 112 (6)* | 118 (6)* | 125 (8)** | 123 (10)* | |
| 500 | Absolute | 166 (6)** | 154 (6)** | 153 (7)** | 149 (10)** |
| Relative | 170 (6)** | 168 (6)** | 166 (7)** | 171 (10)** | |
Values represent percentages (%) to control values.
(n): Number of animals examined.
Relative: Ratio to body weight (%)
Significantly different from control at p < 0.05 and p < 0.01, respectively (Dunnett’s multiple comparison test).
Table 15.
Mean area of altered hepatocellular foci (AHF) in F344 rats from a 2-year feeding study of p,p′-DDT
| Dose (ppm) | Duration (weeks) | No. of rats examined | Mean area of AHF (mm2): Mean ± SD (No. of rats with AHF) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Eosinophilic | Tigroid basophilic | Clear cell | |||||||
|
|
|
|
|
||||||
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| 0 | 26 | 6 | 6 | - | - | 0.02 ± 0.004 (2) | 0.03 ± 0.004 (2) | - | - |
| 52 | 6 | 6 | - | - | 0.06 ± 0.05 (3) | 0.13 ± 0.09 (4) | 0.02 ± 0.02 (5) | - | |
| 78 | 8 | 8 | 0.16 (1) | - | 0.09 ± 0.03 (8) | 0.11 ± 0.03 (8) | 0.05 ± 0.02 (5) | 0.04 ± 0.01 (3) | |
| 104 | 10 | 10 | 0.33 ± 0.16 (8) | 0.46 ± 0.18 (3) | 0.16 ± 0.04 (9) | 0.17 ± 0.08 (10) | 0.08 ± 0.04 (9) | 0.09 ± 0.08 (5) | |
|
| |||||||||
| 5 | 26 | 6 | 6 | - | - | 0.03 (1) | 0.02 (1) | 0.03 (1) | - |
| 52 | 6 | 6 | - | - | 0.03 (1) | 0.09 ± 0.03 (6) | 0.03 ± 0.01 (4) | - | |
| 78 | 8 | 8 | 0.60 ± 0.63 (2) | 0.04 (1) | 0.09 ± 0.05 (8) | 0.08 ± 0.03 (8)* | 0.05 ± 0.03 (6) | 0.05 ± 0.02 (2) | |
| 104 | 10 | 10 | 0.44 ± 0.57 (7) | 0.38 ± 0.39 (4) | 0.11 ± 0.04 (9)** | 0.14 ± 0.03 (10) | 0.08 ± 0.04 (10) | - | |
|
| |||||||||
| 50 | 26 | 6 | 6 | - | - | 0.06 (1) | 0.03 ± 0.003 (3) | 0.03 (1) | - |
| 52 | 6 | 6 | - | - | - | 0.09 ± 0.02 (6) | 0.05 ± 0.02 (4) | - | |
| 78 | 8 | 7 | 0.21 ± 0.17 (8) | 0.32 (1) | 0.25 ± 0.49 (8) | 0.10 ± 0.04 (7) | 0.09 ± 0.06 (8) | - | |
| 104 | 10 | 10 | 0.58 ± 0.54 (9) | 0.35 ± 0.21 (2) | 0.11 ± 0.06 (9)* | 0.15 ± 0.03 (10) | 0.18 ± 0.24 (10) | 0.05 ± 0.03 (2) | |
|
| |||||||||
| 500 | 26 | 6 | 6 | 0.05 ± 0.03 (4) | - | - | 0.07 ± 0.06 (2) | - | - |
| 52 | 6 | 6 | 0.06 ± 0.03 (6) | 0.06 ± 0.02 (2) | - | - | - | - | |
| 78 | 7 | 8 | 0.32 ± 0.18 (7) | 0.26 ± 0.13 (8) | 0.07 ± 0.02 (3) | 0.09 ± 0.06 (6) | - | - | |
| 104 | 10 | 10 | 0.90 ± 0.43 (10)** | 1.02 ± 0.70 (10) | 0.17 ± 0.10 (10) | 0.08 ± 0.02 (8)** | 0.41 ± 0.53 (4) | 0.03 ± 0.01 (4) | |
-: Not available because of no AHF.
Significantly different from control at p < 0.05 and p < 0.01, respectively (Fisher’s exact test).
ACKNOWLEDGMENTS
Our previously conducted 4-week and 2-year feeding studies of p,p′-DDT in F344 rats were supported by a grant from the Ministry of Agriculture, Forestry and Fisheries. We are grateful to our colleagues in the Toxicology and Chemictry Divisions of the Institute of Environmental Toxicology for their valuable suggestions, cooperation and/or technical support in this work.
REFERENCES
- 1.ATSDR. Toxicological profile for DDT, DDE and DDD. U.S. department of health and human services. Public health service. Agency for toxic substances and disease registry; 2002. [PubMed] [Google Scholar]
- 2.Vos JG, Dybing E, Greim HA, Ladefoged O, Lambré C, Tarazona JV, Brandt I, Vethaak AD. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to European situation. Crit Rev Toxicol. 2000;30:71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- 3.WHO. World malaria report 2008 Global malaria program. World health organization; 2008. [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) Occupational exposures in insecticide application, and some pesticides. IARC monographs on the evaluation of carcinogenic risks to humans. 1991;53:179–249. [PMC free article] [PubMed] [Google Scholar]
- 5.Harada T, Yamaguchi S, Ohtsuka R, Takeda M, Fujisawa H, Yoshida T, Enomoto A, Chiba Y, Fukumori J, Kojima S, Tomiyama N, Saka M, Ozaki M, Maita K. Mechanisms of promotion and progression of preneoplastic lesions in hepatocarcinogenesis by DDT in F344 rats. Toxicol Pathol. 2003;31:87–98. doi: 10.1080/01926230390173941. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kim JS. Mitophagy: Therapeutic potentials for liver disease and beyond. Toxicol Res. 2014;30:243–250. doi: 10.5487/TR.2014.30.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auger C, Alhasawi A, Contravadoo M, Appanna VD. Dysfunctional bioenergetics and the pathogenesis of hepatic disorders. Front Cell Dev Biol. 2015;3:40. doi: 10.3389/fcell.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 9.Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol. 1999;15:367–373. doi: 10.1023/A:1007649815992. [DOI] [PubMed] [Google Scholar]
- 10.Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT) Lancet. 2005;366:763–773. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- 11.Kelce WR, Stone CR, Laws SC, Gray LE, Kemp-painen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 12.Hojo H, Aoyama H, Takahashi KL, Shimizu N, Araki M, Takizawa Y, Sakasai K, Kuwahara M, Saka M, Teramoto S. Two-generation reproduction toxicity study in rats with 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (p,p′-DDT) Congenital Anomalies. 2006;46:105–114. doi: 10.1111/j.1741-4520.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 13.Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110:125–128. doi: 10.1289/ehp.02110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flodström S, Hemming H, Wärngård L, Ahlborg UG. Promotion of altered hepatic foci development in rat liver, cytochrome P450 enzyme induction and inhibition of cell-cell communication by DDT and some structurally related organohalogen pesticides. Carcinogenesis. 1990;11:1413–1417. doi: 10.1093/carcin/11.8.1413. [DOI] [PubMed] [Google Scholar]
- 15.Lubet RA, Dragnev KH, Chauhan DP, Nims RW, Diwan BA, Ward JM, Jones CR, Rice JM, Miller MS. A pleiotropic response to phenobarbital-type enzyme inducers in the F344/NCr rat. Biochem Pharmacol. 1992;43:1067–1078. doi: 10.1016/0006-2952(92)90614-O. [DOI] [PubMed] [Google Scholar]
- 16.Nims RW, Lubet RA, Fox SD, Jones CR, Thomas PE, Reddy AB, Kocarek TA. Comparative pharmacodynamics of CYP2B induction by DDT, DDE, and DDD in male rat liver and cultured rat hepatocytes. J Toxicol Environ Health Part A. 1998;53:455–477. doi: 10.1080/009841098159187. [DOI] [PubMed] [Google Scholar]
- 17.Sierra-Santoyo A, Hernández M, Albores A, Cebrián ME. Sex-dependent regulation of hepatic cytochrome P-450 by DDT. Toxicol Sci. 2000;54:81–87. doi: 10.1093/toxsci/54.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Jansen LA, Jongen WM. The use of initiated cells as a test system for detection of inhibitors of gap junctional intercellular communication. Carcinogenesis. 1996;17:333–339. doi: 10.1093/carcin/17.2.333. [DOI] [PubMed] [Google Scholar]
- 19.Klaunig JE, Ruch RJ. Strain and species effects on the inhibition of hepatocyte intercellular communication by liver tumor promoters. Cancer Lett. 1987;36:161–168. doi: 10.1016/0304-3835(87)90087-5. [DOI] [PubMed] [Google Scholar]
- 20.Krutovskikh VA, Mesnil M, Mazzoleni G, Yamasaki H. Inhibition of rat liver gap junction intercellular communication by tumor-promoting agents in vivo. Lab Invest. 1995;72:571–577. [PubMed] [Google Scholar]
- 21.Williams GM. In: Epigenetic mechanisms of liver tumor promotion In: mouse liver carcinogenesis: mechanisms and species comparisons. Stevenson DE, McClain RM, Popp JA, Slaga TJ, Ward JM, Pitot HC, editors. Wiley-Liss; New York: 1990. pp. 131–145. [Google Scholar]
- 22.Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr Drug Targets. 2002;3:465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- 23.Parke DV, Ioannides C. Role of cytochromes P-450 in mouse liver tumor production. In: Stevenson DE, McClain RM, Popp JA, Slaga TJ, Ward JM, Pitot HC, editors. mouse liver carcinogenesis: Mechanisms and species Comparisons. Wiley-Liss; New York: 1990. pp. 215–230. [PubMed] [Google Scholar]
- 24.Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, Kuttler K, Malarkey DE, Maronpot RR, Nishikawa A, Nolte T, Schulte A, Strauss V, York MJ. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes- conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol. 2012;40:971–994. doi: 10.1177/0192623312448935. [DOI] [PubMed] [Google Scholar]
- 25.Parkinson A. An overview of current cytochrome P450 technology for assessing the safety and efficacy of new materials. Toxicol Pathol. 1996;24:48–57. doi: 10.1177/019262339602400107. [DOI] [PubMed] [Google Scholar]
- 26.Simic MG. Mechanisms of inhibition of free-radical processes in mutagenesis and carcinogenesis. Mutat Res. 1988;202:377–386. doi: 10.1016/0027-5107(88)90199-6. [DOI] [PubMed] [Google Scholar]
- 27.Clayson DB, Mehta R, Iverson F. Oxidative DNA damage - The effects of certain genotoxic and operationally non-genotoxic carcinogens. Mutat Res. 1994;317:25–42. doi: 10.1016/0165-1110(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 28.Butterworth BE, Conolly RB, Morgan KT. A strategy for establishing mode of action of chemical carcinogens as a guide for approaches to risk assessments. Cancer Lett. 1995;93:129–146. doi: 10.1016/0304-3835(95)03794-W. [DOI] [PubMed] [Google Scholar]
- 29.Schulte-Hermann R, Schuppler J, Timmermann-Trosiener I, Ohde G, Bursch W, Berger H. The role of growth of normal and preneoplastic cell populations for tumor promotion in rat liver. Environ Health Perspect. 1983;50:185–194. doi: 10.1289/ehp.8350185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte-Hermann R, Bursch W, Grasl-Kranpp B, Huber W, Parzefall W. Nongenotoxic carcinogenesis in the liver. In: Cockburn A, Smith L, editors. nongenotoxic carcinogenesis. Springer-Verlag; Berlin: 1994. pp. 109–120. [DOI] [Google Scholar]
- 31.Büsser MT, Lutz WK. Stimulation of DNA synthesis in rat and mouse liver by various tumor promoters. Carcinogenesis. 1987;8:1433–1437. doi: 10.1093/carcin/8.10.1433. [DOI] [PubMed] [Google Scholar]
- 32.Beer DG, Neveu MJ. Proto-oncogene and gap-junction protein expression in rodent liver neoplasms. In: Stevenson DE, McClain RM, Popp JA, Slaga TJ, Ward JM, Pitot HC, editors. mouse liver carcinogenesis: mechanisms and species comparisons. Wiley-Liss; New York: 1990. pp. 293–309. [PubMed] [Google Scholar]
- 33.Whysner J, Ross PM, Williams GM. Phenobarbital mechanistic data and risk assessment: enzyme induction, enhanced cell proliferation, and tumor promotion. Phamacol Ther. 1996;71:153–191. doi: 10.1016/0163-7258(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki H. Role of disrupted gap junctional intercellular communication in detection and characterization of carcinogens. Mutat Res. 1996;365:91–105. doi: 10.1016/S0165-1110(96)90014-7. [DOI] [PubMed] [Google Scholar]
- 35.Loewenstein WR. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979;560:1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 36.Ströbel P, Klimek F, Zerban H, Kopp-Schneider A, Bannasch P. Xenomorphic hepatocellular precursors and neoplastic progression of tigroid cell foci induced in rats with low doses of N-nitrosomorpholine. Carcinogenesis. 1998;19:2069–2080. doi: 10.1093/carcin/19.12.2069. [DOI] [PubMed] [Google Scholar]
- 37.Bannasch P, Zerban H, Hacker HJ. Foci of altered hepatocytes, rat. In: Jones TC, Mohr U, Hunt RD, editors. monographs on pathology of laboratory animals, digestive system. Springer-Verlag; Berlin: 1985. pp. 10–30. [DOI] [Google Scholar]
- 38.Harada T, Maronpot RR, Morris RW, Boorman GA. Observations on altered hepatocellular foci in National Toxicology Program two-year carcinogenicity studies in rats. Toxicol Pathol. 1989;17:690–706. doi: 10.1177/0192623389017004114. [DOI] [PubMed] [Google Scholar]
- 39.Toh YC. Physiological and biochemical reviews of sex differences and carcinogenesis with particular reference to the liver. Adv Cancer Res. 1973;18:155–209. doi: 10.1016/S0065-230X(08)60752-6. [DOI] [PubMed] [Google Scholar]
- 40.Harada T, Maronpot RR, Morris RW, Stitzel KA, Boorman GA. Morphological and stereological characterization of hepatic foci of cellular alteration in control Fischer 344 rats. Toxicol Pathol. 1989;17:579–593. doi: 10.1177/0192623389017004104. [DOI] [PubMed] [Google Scholar]
- 41.Thurman JD, Bucci TJ, Hart RW, Turturro A. Survival, body weight, and spontaneous neoplasms in ad libitum-fed and food-restricted Fischer-344 rats. Toxicol Pathol. 1994;22:1–9. doi: 10.1177/019262339402200101. [DOI] [PubMed] [Google Scholar]