Abstract
Impaired social interaction is a hallmark symptom of many psychiatric diseases, including dependence syndromes (substance use disorders). Helping the addict reorient her/his behavior away from the drug of abuse toward social interaction would be of considerable therapeutic benefit. To study the neural basis of such a reorientation, we have developed several animal models in which the attractiveness of a dyadic (i.e. one-to-one) social interaction (DSI) can be compared directly with that of cocaine as a prototypical drug of abuse. Our models are based on the conditioned place preference (CPP) paradigm. In an ongoing effort to validate our experimental paradigms in C57BL/6 mice to make use of the plethora of transgenic models available in this genus, we found the following: (a) DSI with a live mouse produced CPP, whereas an interaction with an inanimate mouse-like object (i.e. a ‘toy mouse’; toy mouse interaction) led to conditioned place aversion – but only in the Jackson substrain (C57BL/6J). (b) In the NIH substrain (C57BL/6N), both DSI and toy mouse interaction produced individual aversion in more than 50% of the tested mice. (c) Four 15 min DSI episodes did not result in the development of an observable hierarchy, that is, dominance/subordination behavior in the overwhelming majority (i.e. 30 of 32) of the tested Jackson mouse pairs. Therefore, dominance/subordination does not seem to be a confounding variable in our paradigm, at least not in C57BL/6J mice. Respective data for NIH mice were too limited to allow any conclusion. The present findings indicate that (a) DSI with a live mouse produces CPP to a greater degree than an interaction with an inanimate object resembling a mouse and that (b) certain substrain differences with respect to CPP/aversion to DSI do exist between the Jax and NIH substrain of C57BL/6 mice. These differences have to be considered when choosing a proper mouse substrain model for investigating the neural basis of DSI reward versus drug reward.
Keywords: C57BL/6J substrain, C57BL/6N substrain, conditioned place avoidance, conditioned place preference, dyadic social interaction, interspecies comparison, mouse, Sprague–Dawley rat, substrain comparison
Introduction
Converging evidence from three independent laboratories using conditioned place preference (CPP) to determine the attractiveness of a stimulus indicates that dyadic (i.e. one-to-one) social interaction (DSI) is preferred by sex-matched and weight-matched young adult rats over psychostimulant drugs of abuse, that is, cocaine (Fritz et al., 2011; Prast et al., 2014a,2014b; Zernig and Pinheiro, 2015) or amphetamine (Yates et al., 2013), and that direct physical contact is rewarding for them (Kummer et al., 2011; Peartree et al., 2012). Only four 15 min episodes of DSI were found to be able to countercondition previously cocaine-preferring rats and prevent the subsequent reacquisition/re-expression of CPP for cocaine [reviewed in Zernig et al. (2013) and Zernig and Pinheiro (2015)]. All these findings indicate that DSI is a powerful alternative (i.e. nondrug) stimulus in rodents. Helping the recovering addict to reorient her/his behavior away from the drug of abuse toward DSI would be of considerable therapeutic interest. We have subsequently validated most of our experimental models in C57BL/6N mice, thus allowing researchers to make use of the plethora of transgenic models in this genus to study the neural basis of the attractiveness of DSI, although DSI may be more rewarding for Sprague–Dawley rats than for C57BL/6N mice as rats spend more time in direct physical contact than mice (Kummer et al., 2014). A number of questions have been addressed, such as what the most attractive component of the composite stimulus ‘social interaction’ is (i.e., touch, Kummer et al., 2011), at what age DSI seems most attractive, that is, in early adulthood (Yates et al., 2013) [see Zernig and Pinheiro (2015) for a detailed discussion], and which other experimental conditions decrease the attractiveness of DSI, that is, crowding and weight (size) difference (Kummer et al., 2011). The following questions, however, still needed to be answered. They are the focus of the present study:
Besides DSI, environmental enrichment (EE) has been shown to be of considerable therapeutic promise for the treatment of substance use disorders (Solinas et al., 2008; Zakharova et al., 2009; Thiel et al., 2010; Chauvet et al., 2011). Although a standard for EE has not been established yet (Olsson and Dahlborn, 2002; Wolfensohn and Lloyd, 2013) with respect to the nature, number, and density of objects added that constitute EE or the nature and dimensions of the control environment, all the EE studies mentioned above used the presence of – and, hence, social interaction with – conspecifics as part of EE. However, in a previous study (Kummer et al., 2014), we had found that C57BL/6N mice spent surprisingly little time (i.e. 17% of the 15 min session duration) in direct physical contact with each other, and yet developed predominantly CPP for DSI. The rats in our previous experiments had, in comparison, spent at least 76% in direct physical contact and had shown a number of easily observable prosocial behaviors (Kummer et al., 2014). In the present study, we therefore directly compared the ability of DSI with a live mouse with that of an interaction with an inanimate object resembling a mouse (i.e. a ‘toy mouse’) to produce CPP or conditioned place aversion (CPA). The toy mouse can also be considered a minimal form of EE. Our hypothesis at the outset of the present study was that in mice, this minimal form of EE would not produce CPP. This direct comparison of the interaction with a live mouse (DSI) with a toy mouse interaction (TMI) also allows to address the impact of novelty as a confounding variable in our paradigm. By strict definition, ‘novelty’ can be ruled out upon the second interaction episode for both DSI and TMI and should have disappeared upon the fourth interaction with the same object. A toy mouse does not emit behavior that is inherently variable if the interaction partner is a live mouse. Thus, the last of four 15 min interactions with a toy mouse should be mostly devoid of any ‘novelty’ and may thus be considered as ‘non-novel’. As reported (Fritz et al., 2011) and discussed (Zernig and Pinheiro, 2015) previously for rats, our hypothesis at the outset of the present study was that in mice, also, the attractiveness of the interaction with a live mouse (DSI) would, after the fourth episode, override the waning novelty of such an interaction.
A growing number of studies (discussed in detail below) indicate that in the C57BL/6 mouse strain, mice from the NIH substrain (C57BL/6N, abbreviated ‘NIH mice’ in the following) are behaviorally different from the Jackson Laboratories substrain (C57BL/6J, abbreviated ‘Jax mice’ in the following). We therefore investigated all of the above behaviors in both Jax and NIH mice to provide information for the field as to which substrain to choose for subsequent studies of a similar nature. As NIH mice seem to be more anxious (Matsuo et al., 2010; Simon et al., 2013) than Jax mice, we expected that NIH mice would find DSI less attractive than Jax mice.
A previous experiment in rats (Kummer et al., 2011) had suggested that dominance/subordination may be detrimental to the attractiveness of DSI. In that study, we had simply varied the weight difference between the DSI partners, without assessing dominance/subordination behavior. In the present study, we scored each weight-matched pair for dominance/subordination (Veyrac et al., 2011) and correlated these scores to the degree of subsequently tested CPP or CPA for DSI. We expected hierarchy to negatively affect the attractiveness of DSI.
Finally, we systematically studied the experimenter effect that has to be considered in our behavioral paradigms (Zernig and Pinheiro, 2015). We expected less experienced experimenters, who, in our experience, handle the animals more roughly, to be able to show less DSI CPP because the stress of the experimenter–mouse interaction would impact negatively on the attractiveness of the subsequent interaction of the mice with each other.
Methods
Subjects
Male C57BL/6 mice of the Jackson (Jax) or the NIH (NIH) substrain (8 weeks old, weighing 22–23 g) were obtained from Charles River Laboratories (Sulzfeld, Germany). All animals were housed at a constant room temperature of 22°C and had free access to tap water and pelleted chow (Tagger, Innsbruck, Austria). Experiments were conducted during the light phase of a continuous 12 h light/dark cycle with the lights on from 08.00 to 20.00 h. Before the start of the CPP/CPA experiments, animals were singly housed 5–7 days and experienced a total of seven 2 min handling episodes with their allocated experimenter (at least one handling episode per day). The present experiments were approved by the Austrian National Animal Experiment Ethics Committee.
Conditioned place preference apparatus
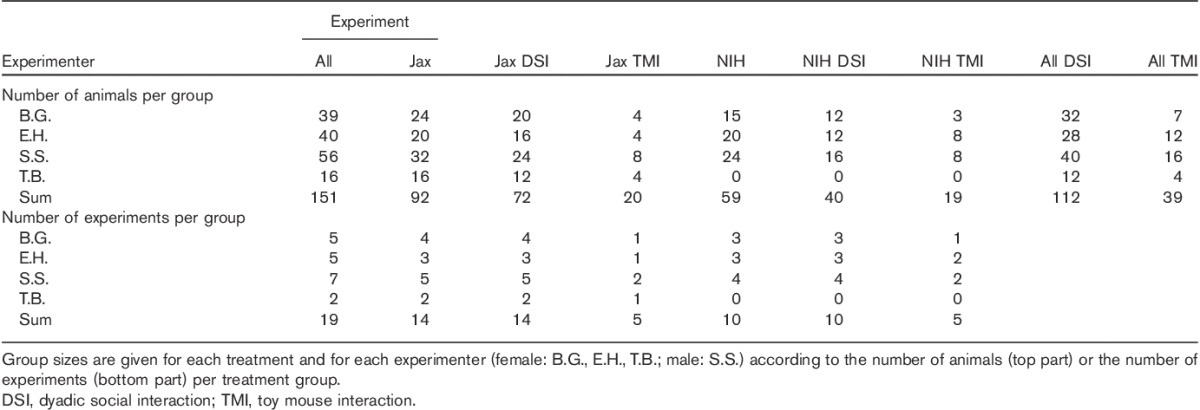
Conditioning was conducted in a custom-made three-chamber CPP apparatus (64 cm wide×32 cm deep×31 cm high) made of unplasticized polyvinyl chloride. The middle (neutral) compartment (10×30×30 cm) had white walls and a white floor. Two doorways led to the two conditioning compartments (25×30×30 cm each) with walls showing either vertical or horizontal black-and-white stripes of the same overall brightness (Zernig and Pinheiro, 2015) and with stainless-steel floors containing either 168 holes (diameter 0.5 cm) or 56 slits (4.2×0.2 cm each). A systematic investigation of the time spent in each conditioning compartment in a pretest session did not indicate any compartment bias (i.e. we used a nonbiased apparatus; data not shown). Time spent in each compartment was digitally recorded with a video camera and analyzed offline with hand timers. The CPP apparatus was cleaned with a 70% camphorated ethanol solution after each session. All experiments were conducted under neon ceiling light (58 W, 1 m distance) and white noise from continuously running allergen filter boxes. Three of the four experimenters (B.G., E.H., S.S.) tested both Jax and NIH mice, whereas experimenter T.B. tested only Jax mice (Table 1).
Table 1.
Group sizes
Acquisition and expression of conditioned place preference or avoidance for dyadic social interaction or toy mouse interaction
Our conditioning procedure has been described and discussed in detail previously (Fritz et al., 2011; Zernig et al., 2013; Prast et al., 2014a,2014b; Zernig and Pinheiro, 2015). For the acquisition of CPP/CPA for DSI or TMI, the conditioning procedure comprised a pretest session on day 1, followed by eight consecutive training days in an alternate-day-design of the pattern DSI-sal-DSI-sal-DSI-sal-DSI-sal or TMI-sal-TMI-sal-TMI-sal-TMI-sal, respectively (one training session/day). CPP/CPA was tested on day 10. In the DSI group, the stimuli were either (a) a 15 min DSI session with a sex-matched and weight-matched male conspecific preceded by an intraperitoneal injection of 10 ml/kg saline or (b) only a saline injection as the comparator stimulus. In the TMI group, the test mouse was either (a) injected intraperitoneally with saline and immediately placed in the same compartment with a black mouse-shaped object with a fur-like texture (‘toy mouse’) or (b) received only a saline injection. The toy mouse weighed 2.9 g, had a body length (excluding the tail) of 5 cm (i.e. was roughly 50% the size of the test mouse), and contained a bell that emitted a clicking sound when shaken. The toy mouse was obtained from a pet shop (http://www.zoowelt.cc), which offered it as a toy/training object for cats. The toy mouse was washed in a washing machine for about 70 min at 30°C with an apparently nonscented detergent for wool (‘Lana/Wolle’, http://www.glenfield.it) before each conditioning experiment. Each test mouse was allocated one and the same toy mouse during all four TMI training sessions. Consequently, the toy mouse should have absorbed only the smell of its allocated test mouse. To emphasize, pretest, training, and CPP test sessions were of equal duration, that is, 15 min. Pretest bias for any of the two conditioning chambers was declared if during pretest the animal spent more time in one of the conditioning chambers. The initially nonpreferred chamber was subsequently paired with the stimulus of interest (noncounterbalanced compartment allocation; see [Zernig et al. (2013); Zernig and Pinheiro (2015) for a detailed discussion].
Hierarchy analysis: scoring of dominance versus subordination
The last of the four DSI episodes during CPP training was video-recorded and evaluated offline for signs of dominance/subordination in each mouse pair strictly according to the scoring system of Bakker and colleagues (Veyrac et al., 2011): aggressive dominance (a hierarchy score of h3) was defined as three consecutive attacks by one mouse (aggressive grooming, biting, and chasing); passive dominance (a score of h2) was defined as consistent threatening displacement by one mouse including upright or sideways postures; and subordinate behavior (score of h0) was defined as retreat or fleeing by one mouse including ‘on back’ position and crouching, and a draw (a score of h1) was defined as no attacks or consistent displacement occurring on the part of either mouse. Although the scoring experimenters were instructed to ignore all previously collected information on the individual mice, the offline hierarchy analysis was carried out by the same experimenter who had previously quantified the time spent by the respective mice in the subsequent CPP test; thus, blinding to the behavior in the subsequent CPP was not absolute. However, considering the large number of video recordings analyzed by each experimenter, actual blinding seems plausible in most of the cases.
Statistical analyses
Data were first analyzed for normality using the D’Agostino and Pearson omnibus normality test. As values showed a normal distribution, a two-sided unpaired t-test was used as a parametric test used to assess the statistical significance of the treatment effects. Experimenter effects were first analyzed by analysis of variance, and then individual experimenters’ data were compared using two-sided unpaired t-tests. For the contingency analysis of the number of individuals in each sample showing CPP or CPA, only Fisher’s exact test was used. All statistical analyses were carried out using Prism 6 (http://www.graphpad.com).
Results
Conditioned place preference/aversion by dyadic social interaction with a live mouse versus interaction with an inanimate mouse-shaped object (toy mouse)
For the sake of maximal data transparency, the left part of Fig. 1 first gives the raw data, that is, the time spent during the CPP test in a conditioning compartment associated previously with DSI, that is, interaction with a live conspecific, for each individual C57BL/6 mouse of the Jax (green symbols) or the NIH (red symbols) substrain, and compares it with the time spent by mice in the conditioning compartment if this compartment had previously contained only an inanimate mouse-shaped object, that is, a toy mouse. Following field convention, CPP/avoidance scores were analyzed in two different ways, that is, (a) as the time spent in the DSI-associated or TMI-associated compartment minus the time spent in the saline injection-associated compartment during the CPP test (shown in the right panel of Fig. 1) or (b) as the time spent in the DSI-associated or TMI-associated compartment during the CPP test minus the time spent in the same compartment during pretest (not shown in Fig. 1; see discussion for a comparison of the two methods of calculation).
Fig.1.
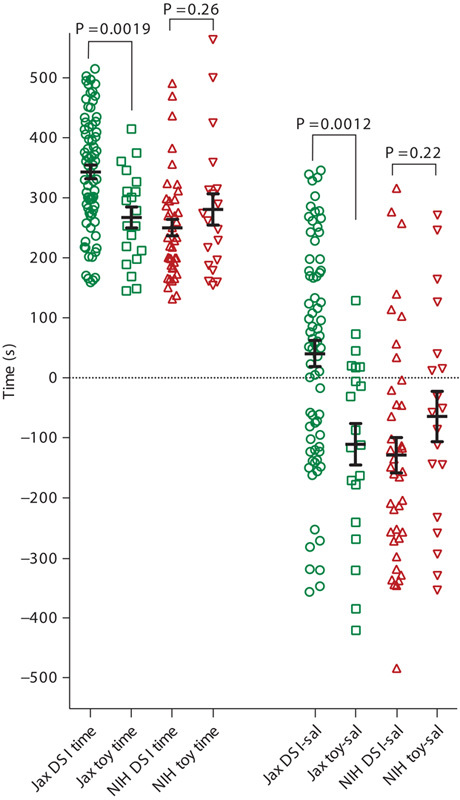
Conditioned place preference or aversion produced by interaction with a live mouse or with a toy mouse: Raw data and preference scores for C57BL/6 mice from the Jackson or the NIH substrain. Raw data, that is, the times spent by each individual animal in the compartment associated with dyadic social interaction (DSI) with a live mouse or in the compartment associated with a toy mouse interaction (TMI), are shown in the left part of the figure both for each individual mouse (symbols) and as the mean±SEM for each group (black lines). Interaction with a live (DSI) or a toy (TMI) mouse was preceded by an i.p. injection of saline (which served as the comparator unconditioned stimulus for the alternative conditioning compartment). Times are given as seconds (total test session duration, 900 s) for C57BL/6 mice from either the Jackson (green) or the NIH (red) substrain. In the right part of the figure, preference or avoidance is expressed as the time spent in the DSI-associated or TMI-associated compartment minus the time spent in the saline injection-associated compartment during the CPP test. Group sizes are shown in Table 1. i.p., intraperitoneal.
At the level of the raw data group mean (left part of Fig. 1), C57BL/6 mice of the Jax substrain (green symbols) spent more time in the DSI-associated compartment than in the TMI-associated compartment (P<0.002). If CPP/CPA was quantified as the time spent in the DSI-associated or TMI-associated compartment minus the time spent in the saline injection-associated compartment (right part of Fig. 1), DSI produced CPP whereas TMI produced CPA in the Jax substrain (P<0.002). As we detected an experimenter effect (see below), we reanalyzed the data of Jax mice after excluding the data obtained by experimenter T.B. The respective P value then decreased to 0.0001. If preference scores were calculated by subtracting the time spent in the same compartment at pretest from the time spent in the same compartment at the CPP test, the statistical significance remained (P<0.01 for all four experimenters, P<0.001 if data by experimenter T.B. were excluded; not shown).
In contrast to the data obtained in the Jax substrain, C57BL/6 mice from the NIH substrain did not spend more time in the DSI-associated compartment than in the TMI-associated compartment (P=0.26; Fig. 1, left part, red symbols). NIH mice developed CPA to both DSI and TMI if preference scores (Fig. 1, right part, red symbols) were calculated as: (time in the DSI or the TMI compartment)−(time in the saline compartment). NIH mice, at the level of the group mean, developed CPA for DSI and a slight CPP for TMI (not shown). However, none of the differences between any DSI versus the TMI group means of the NIH mice reached statistical significance at the P value less than 0.05 level (P=0.22 for DSI-sal vs. TMI-sal and P=0.064 for the DSI-test-minus-pretest vs. the TMI-test-minus-pretest).
Table 2 shows the effects of DSI and TMI on conditioned preference/avoidance at the level of the individual mouse and compares them with previously published data by our group obtained in C57BL/6 mice of the NIH substrain and in Sprague–Dawley rats. To emphasize and distinguish the following results from the group mean data, individual preference (IP) was declared for each rodent if the time spent by a rodent in the DSI-associated or TMI-associated compartment was longer than the time spent by the same rodent in the saline compartment at the time of the CPP test. Individual avoidance was declared if the opposite was true.
Table 2.
Statistical results for dyadic social interaction versus toy mouse interaction for various experiments in the NIH versus Jackson substrains of C57BL/6 mice versus DSI in Sprague–Dawley rats
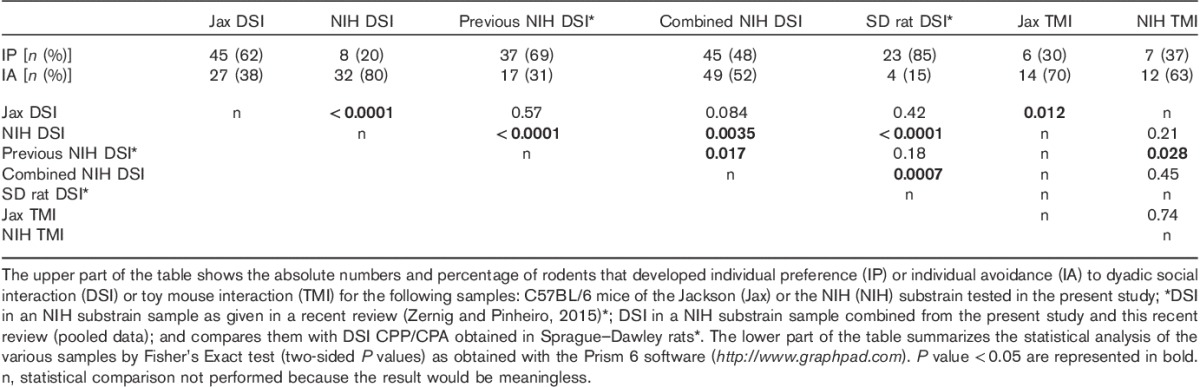
Contingency analyses at the level of the individual mouse (carried out using Fisher’s Exact test, which uses the absolute numbers of individuals and is more stringent than the often-used χ2-test, which is sometimes used wrongly on percentages of groups smaller than 100 individuals) yielded the same results (Table 2) as the group mean analysis: 62% of the Jax mice developed IP for DSI, whereas only 30% developed IP for TMI (P<0.02; see Table 2 for a summary of all statistical comparisons). In contrast, only 20% of the NIH mice showed IP for DSI and only 37% of the NIH mice showed IP for TMI (P=0.21).
Hierarchy (dominance/subordination) effect
We also investigated whether hierarchy, that is, dominance or subordination in a C57BL/6 mouse pair (of either the Jax or NIH substrain), which was scored in the last of four DSI episodes, had an effect on subsequent CPP or CPA developed for DSI. Our testable hypothesis was that dominance should produce greater conditioned preference than subordination. Surprisingly, in the overwhelming majority of Jax mice pairs, that is, 30 of 32 pairs, no visible signs of a hierarchy emerged within the four 15 min episodes of DSI, the hierarchy scores being h1 (i.e. a draw) for a total of 60 mice (Fig. 2, top panel) that showed a large variation in their preference/avoidance of DSI-associated contextual cues. In only two of 32 Jax mice pairs had a visible hierarchy emerged by the fourth DSI episode. These two pairs (Fig. 2, top panel) did not show any systematic relationship between hierarchical status and subsequent CPP for DSI (Fig. 2, top panel). Thus, our data suggest that dominance/subordination does not seem to be a confounding variable in our paradigm. The respective data for NIH mice were too limited to allow any conclusion: only one of six pairs showed a hierarchy (h0 and h3).
Fig. 2.
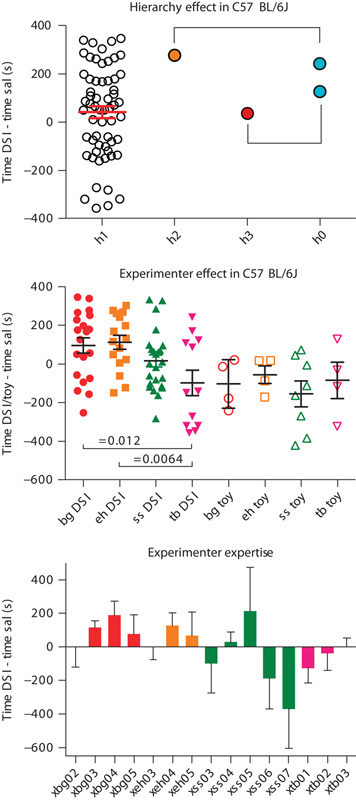
Effects of hierarchy or experimenter on CPP/CPA by dyadic social interaction. The top panel shows the CPP preference scores, that is, the time spent by each individual animal in the compartment associated with dyadic social interaction (DSI) minus the time spent in the saline injection-associated compartment for those experiments in which hierarchy scores during the preceding fourth DSI episode had been obtained. In 30 of 32 pairs, that is, 60 animals, no dominance/subordination pattern could be observed (hierarchy score h1; open circles). Red lines show the mean±SEM for this group. In one of 32 pairs, one mouse was found to be passive dominant (hierarchy score h2; tangerine circle) during the fourth DSI episode, whereas his partner was subordinate (hierarchy score h0; turquoise circle). In another one of 32 pairs, one mouse was fully dominant, that is, aggressive dominant (h3; deep maraschino circle). Each pair is identified by a bracket. The middle panel shows the effect that each individual experimenter had on the CPP preference score of each individual mouse for dyadic social interaction with a live mouse (DSI, filled symbols) or a TMI (toy, open symbols). Three experimenters (B.G., maraschino red; E.H., tangerine red; T.B.; strawberry red) were women and one (S.S., green) was a man. Black lines show the mean±SEM for each group. Group sizes are shown in Table 1. The bottom panel shows the effect of increasing experimenter expertise by numbering experiments conducted by each experimenter consecutively (e.g. xbg02 is the second experiment conducted by experimenter B.G.). For the sake of group homogeneity, only data for DSI in Jax mice (C57BL/6J) are shown. The identification of each experimenter by color follows the convention of the middle panel. Means±SEM for 4 or 8 Jax mice per experiment are shown.
Experimenter effect
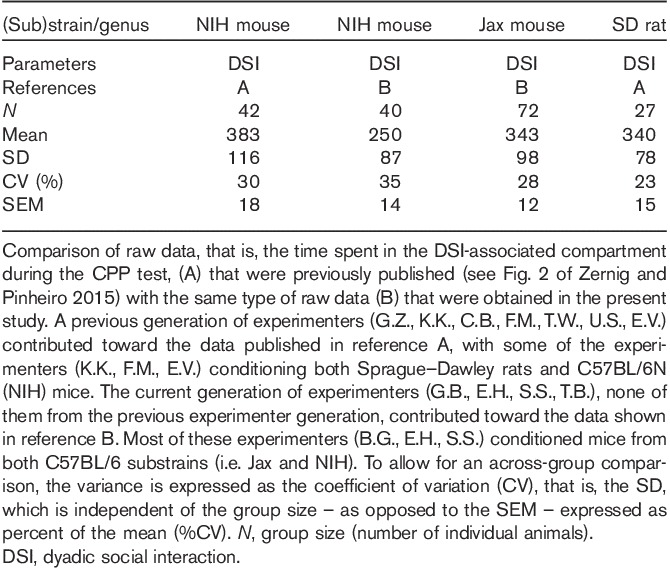
Finally, we investigated whether an experimenter effect in our CPP-based paradigms (Kummer et al., 2014; Zernig and Pinheiro, 2015) had also occurred in the present study. To ensure homogeneity of the experimental group, we focused on the larger substrain sample, that is, the Jax mice, and the more frequently tested CPP stimulus, that is, DSI. The middle panel of Fig. 2 shows each individual mouse’s CPP or CPA scores (expressed as the time spent in the DSI-associated or toy mouse-associated compartment minus the time spent in the saline-associated compartment) for four different experimenters, three of whom were women (B.G., E.H., T.B.) and one of whom was a man (S.S.). The DSI CPP data generated by experimenter T.B. were significantly different from those of experimenter B.G. (P<0.02; two-sided t-test) and experimenter E.H. (P<0.01), all of the same (i.e. female) sex. As the data obtained by experimenter T.B. were generated in her first and second experiment, whereas experimenters B.G. and E.H. were slightly more experienced, we proceeded to investigate whether experimenter expertise had a systematic effect on the CPP test outcome, that is, whether greater expertise in handling and injecting the mice produced greater CPP for DSI in the Jax substrain. To this end, we also included a third experiment by T.B. (xtb03, bottom panel of Fig. 2), conducted by her after the data freeze and analysis of the present study. Inspection of the bottom panel of Fig. 2 suggests that there may have been an initial learning/training effect in that DSI CPP systematically increased in the initial experiments by experimenter B.G., S.S., and T.B. Overall, however, the mean DSI CPP/CPA values varied nonsystematically for each experimenter once four to five experiments had been conducted by him/her. Finally, Table 3 presents a comparison of previously published data [compiled and reviewed in Fig. 2 of Zernig and Pinheiro (2015)] that had been obtained by a previous generation of experimenters in a different C57BL/6 substrain (i.e. NIH mice) and in a different genus, that is, Sprague–Dawley rats with the data obtained in the present study in NIH and Jax mice by the present generation of experimenters (all identified in the legend to Table 3). The coefficient of variation, that is, the SD expressed as % of the mean (%CV), a measure of variance that allows across-group comparison, did not show any discernable or systematic variation with respect to experimenter generation or C57BL/6 mouse substrain, whereas Sprague–Dawley rats showed less variance than all mouse groups, both in comparison with the mouse groups conditioned by the same experimenters as the rats and mouse groups conditioned by a subsequent generation of experimenters (Table 3).
Table 3.
Within-laboratory comparison of the variance of previously published and present data on conditioned place preference to dyadic social interaction with respect to rodent genus, C57BL/6 substrain, and experimenter generation
Discussion
Dyadic social interaction is more rewarding for C57BL/6J mice than toy mouse interaction
The present findings indicate that DSI, that is, interaction with a live partner over four consecutive 15 min sessions, produces CPP of a more pronounced degree (Fig. 1) and in a higher proportion (Table 2) of C57BL/6J mice than being in the same conditioning compartment with an inanimate mouse-shaped object (TMI). When designing the experiment and taking into account that the size of the partner rodent had adversely affected CPP for DSI in Sprague–Dawley rats (Kummer et al., 2011), we took care to render the toy mouse as nonthreatening as possible by decreasing its size to only about 50% of the size of the test mouse. Limited observation of TMIs indicated that the test mouse was initially attentive to the toy mouse and then disregarded it, even carelessly walking over it in some cases. Only if touching the toy mouse produced some sort of movement in the toy mouse did the test mouse show a startle response in some instances.
If one considers the presence of one toy mouse in an otherwise empty compartment a minimal form of EE, the toy mouse seemed to provide very little observable EE. Accordingly, the presence of and the very limited interaction with the toy mouse produced CPA in both the Jax and the NIH substrains (i.e. caused the mice to spend even less time in the toy mouse-associated compartment than in a previously empty compartment, after being injected with intraperitoneal saline before being placed in either compartment). It caused the Jax test mice to spend even less time in the initially nonpreferred compartment (i.e. the nonpreferred compartment during the pretest session). Accordingly, a much higher percentage of Jax mice developed an IP for DSI than for TMI (62 vs. 30%, Table 2). As emphasized in the introduction, experimental models of EE (Olsson and Dahlborn, 2002; Solinas et al., 2008; Zakharova et al., 2009; Thiel et al., 2010; Chauvet et al., 2011) use the presence of conspecifics and, hence, social interaction with these conspecifics as part of EE, although in all the above-mentioned studies on the effects of EE on measures of drug abuse/dependence, the conspecific was placed into the home cage and not the test environment as in the present study. Our data suggest that social interaction with the conspecific is a major determinant of EE. Clearly, extensive parametric EE studies would be necessary to quantify the contribution of social interaction versus interaction with inanimate objects toward the beneficial effects of EE on measures of drug abuse and drug dependence.
With respect to novelty, our TMI data show that after the four 15 min training sessions of our CPP procedure, no appreciable novelty reward (Bardo et al., 2013) could be detected.
Substrain differences between C57BL/6J and C57BL/6N mice
In contrast to the C57BL/6J mice, the NIH substrain (C57BL/6N) showed essentially the same overall CPA for both DSI with a live mouse and TMI with the toy mouse (Fig. 1). Only 20% of the NIH mice developed an IP for DSI and 37% developed IP for TMI, the difference being nonsignificant (Table 2). These data suggest that the majority of individuals from the NIH substrain of C57BL/6 mice do not find interaction with either a live mouse or a toy mouse preferable to being alone in a CPP box compartment. Therefore, C57BL/6N mice may be less suitable than C57BL/J mice for studying the beneficial effects of DSI as an alternative stimulus to drugs of abuse. The present findings and conclusion may explain previous results obtained by us in a concurrent CPP paradigm in which DSI was directly pitched against cocaine as the prototypical drug of abuse [see Figure 4 of (Kummer et al. (2014)]: the tested NIH mice showed a relative CPP for cocaine starting at a dose as low as 0.017 mg/kg intraperitoneal, whereas Sprague–Dawley rats had shown no preference for cocaine over DSI at a cocaine dose as high as 15 mg/kg intraperitoneal (Fritz et al., 2011). Thus, the much higher relative preference for cocaine versus DSI by NIH mice – which cannot be explained by pharmacokinetic differences between SD rats and C57BL/6 mice [see evidence reviewed in Kummer et al. (2014)] – may very well be because of the fact that DSI is such a weak reward for the NIH substrain of C57BL/6 mice.
Our findings on the differential effects on DSI CPP/CPA and TMI CPP/CPA in Jackson versus NIH substrains of C57BL/6 mice are in accordance with previously published data (see below) and anecdotal reports indicating that there are a number of behavioral differences between the Jackson and NIH substrains of C57BL/6 mice. NIH mice seem to be more anxious (Matsuo et al., 2010; Simon et al., 2013), which could explain why NIH mice, on average, find DSI aversive, in contrast to the Jax substrain. Interestingly, Matsuo and colleagues had found that Jax mice emitted 16–25% more DSI contacts than NIH mice, a difference that became highly significant (P<0.001) if the group sizes exceeded 10. These data indicate that Jax mice find DSI more attractive than NIH mice, thus supporting the CPP data of the present study. Of particular interest for the present investigation, Kirkpatrick and Bryant (2014) had shown that opioid-naive mice of the C57BL/6J and C57BL/6NJ substrains differ with respect to their CPA to naloxone, indicating differences in endorphin levels. Considering that nucleus accumbens µ-opioid receptors, that is, endorphin targets, have been found to mediate social reward (Trezza et al., 2011), that is, the reward brought by play, which is a specific form of DSI, substrain differences in the endorphin responsiveness to the DSI stimulus may well impact on its attractiveness.
Essentially the same effects were observed in Jax and NIH mice if, following the heterogeneous field convention, we expressed CPP/CPA scores as the time spent in the DSI-associated or TMI-associated compartment during the CPP test minus the time spent in the same compartment during pretest. The only resulting difference was a slight CPP (as opposed to a CPA) for TMI by NIH mice that, however, was not significantly different from the CPA that NIH mice had shown for DSI when using the pretest-versus-test-calculation. NIH mice showed CPA for both DSI and TMI when using the interaction-versus-saline-injection-alone comparison (detailed above). The pretest-versus-test-calculation is based on the argument that the investigation of conditioning in its strict sense would require a comparison between the behavior before and after the experimental intervention. A number of laboratories, however, do not use this comparison to define CPP or CPA, but express either by a comparison between the time spent in the compartment associated with the unconditioned stimulus (US) of interest (in the present study, the presence of a live conspecific or a toy mouse within the confines of the CPP compartment, both following an intraperitoneal injection of saline) versus the time spent in the compartment associated with the US that is not of interest, but most closely resembles the US of interest (in the present study, only the intraperitoneal injection of saline without the subsequent interaction within the confines of the CPP compartment) at the time of the CPP test. We would argue that expressing CPP as the difference between those two conditioning procedures (i.e. conditioning to the US ‘saline injection alone’ vs. conditioning to the US ‘saline injection, followed by social interaction’) enables a better comparison of the relative conditioning strength and direction of conditioning (appetitive or aversive) than a side-by-side comparison of each US separately (expressed as a pretest-vs.-test preference score). Furthermore, we do not believe that expressing conditioned preference as the difference between the time spent in a compartment at pretest and the time spent in the same compartment during the CPP test is of translational value for the human situation, especially when investigating DSI as an alternative stimulus to a drug of abuse stimulus (see the detailed discussion in (Zernig and Pinheiro, 2015).
To re-emphasize, our findings suggest that the Jackson substrain of C57BL/6 mice may be better suited than the NIH substrain to study the neural basis of the reorientation from drugs of abuse to DSI because the Jackson substrain seems to find DSI more attractive.
Genus differences in DSI CPP/CPA: C57BL/6 mice versus Sprague–Dawley rats
In a recent review (Zernig and Pinheiro, 2015), we had pointed out that a higher percentage of mice than rats may find DSI aversive. At that time, we had only tested C57BL/6 mice of the NIH substrain and the sample size was too small to reach a definitive conclusion (i.e. statistical significance; P=0.18; Table 2). With the present study, we have enlarged the sample size for C57BL/6N mice and have added the Jackson substrain as a further comparator group (Table 2). When pooling all NIH mouse data ever generated in our laboratory, the higher rate of DSI CPA developed by C57BL/6N mice (49 of 94 mice, i.e. 54%) compared with Sprague–Dawley rats (four of 27 rats, i.e. 15%) became statistically significantly different (P<0.001; Table 2). Thus, as predicted in our previous review (Zernig and Pinheiro, 2015), the percentage of C57BL/6N mice that developed CPA to DSI has shifted toward 50%, having become 54% in fact. This finding also supports the conclusion that the NIH substrain of C57BL/6 mice (i.e. C57BL/6N) may be less suitable than its Jackson counterpart (i.e. C57BL/6J) to study the differential neural basis of DSI versus cocaine reward.
Absence of a visible hierarchy (dominance/subordination)
Similar to rats, mice mark (i.e. declare) and defend territories and form hierarchies, albeit at a more rudimentary level than rats (Zernig and Pinheiro, 2015). In our DSI CPP paradigm, however, no visible hierarchy had developed by the fourth and final dyadic social encounter in the overwhelming majority of the tested mouse pairs (i.e. in 30 of 32 pairs; Fig. 2). In the two pairs in which a visible hierarchy had developed, it did not systematically influence DSI reward. Therefore, dominance/subordination – which is an important factor when studying negative aspects of social interaction (Bardo et al., 2013) – does not seem to be a confounding variable in our paradigm that focuses on the positive aspects of DSI, at least not in C57BL/6 mice (but see Kummer et al., 2011 for the effect of weight differences on DSI CPP in Sprague–Dawley rats).
Experimenter effect
Our experimental models, which are based on place preference conditioning for DSI, are prone to an experimenter effect (Zernig et al., 2013; Kummer et al., 2014; Zernig and Pinheiro, 2015), plausibly because the quality of the DSI of the rodent with the experimenter (a human primate and, thus, most likely threatening to the much smaller rodent) impacts on its subsequent DSI with a rodent from the same genus. Finally, we compared the previously published raw data, that is, the time spent in the DSI-associated compartment during the CPP test (Zernig and Pinheiro, 2015) that had been obtained by a previous generation of experimenters in a different C57BL/6 substrain (i.e. NIH mice) and in a different genus (i.e. Sprague–Dawley rats) with the raw data obtained in the present study in NIH and Jax mice by the present generation of experimenters. The coefficient of variation (%CV), as a measure of variance that allows across-group comparison, did not show any discernable or systematic variation with respect to experimenter generation or the C57BL/6 mouse substrain, whereas Sprague–Dawley rats showed less variance than all mouse groups, both in comparison with the mouse groups conditioned by the same experimenters who tested the rats and in comparison with the mouse groups conditioned by a subsequent generation of experimenters (Table 3). Our comparison thus indicates that Sprague–Dawley rats may show less variance than C57BL/6 mice with respect to CPP engendered by DSI. An exhaustive analysis of this phenomenon, however, is beyond the scope of the present investigation.
Conclusion
The present findings clearly indicate that (a) DSI with a live mouse – as opposed to an interaction with an inanimate object resembling a mouse produces CPP and that (b) substrain differences with respect to CPP/aversion to DSI do exist between the Jackson and NIH substrain of C57BL/6 mice. These differences have to be considered when choosing a proper mouse substrain model for investigating the differential neural basis of DSI reward versus drug of abuse reward.
Acknowledgements
Austrian Science Fund grants W1206-B18 (PhD program ‘Signal Processing in Neurons’, http://www.neurospin.at, B.S.P. and G.Z.) and P26248-B24 (G.Z.).
The authors would like to thank the two anonymous reviewers and the editor for their time and effort in improving the quality of the article.
Conflicts of interest
There are no conflicts of interest.
References
- Bardo MT, Neisewander JL, Kelly TH. (2013). Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev 65:255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Jaber M, Solinas M. (2011). Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment. Neuroscience 184:88–96. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, et al. (2011). Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol 16:273–284. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick SL, Bryant CD. (2014). Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Front Behav Neurosci 8:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. (2011). Conditioned place preference for social interaction in rats: contribution of sensory components. Front Behav Neurosci 5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer KK, Hofhansel L, Barwitz CM, Schardl A, Prast JM, Salti A, et al. (2014). Differences in social interaction- vs. cocaine reward in mouse vs. rat. Front Behav Neurosci 8:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. (2010). Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson IA, Dahlborn K. (2002). Improving housing conditions for laboratory mice: a review of ‘environmental enrichment’. Lab Anim 36:243–270. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. (2012). Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol Behav 105:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prast JM, Schardl A, Sartori SB, Singewald N, Saria A, Zernig G. (2014a). Increased conditioned place preference for cocaine in high anxiety related behavior (HAB) mice is associated with an increased activation in the accumbens corridor. Front Behav Neurosci 8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prast JM, Schardl A, Schwarzer C, Dechant G, Saria A, Zernig G. (2014b). Reacquisition of cocaine conditioned place preference and its inhibition by previous social interaction preferentially affect D1-medium spiny neurons in the accumbens corridor. Front Behav Neurosci 8:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, et al. (2013). A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol 14:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. (2008). Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci USA 105:17145–17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. (2010). Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and FOS protein expression. Neuroscience 171:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. (2011). Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci 31:6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Wang G, Baum MJ, Bakker J. (2011). The main and accessory olfactory systems of female mice are activated differentially by dominant versus subordinate male urinary odors. Brain Res 1402:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensohn S, Lloyd M. (2013). Handbook of laboratory animal management and welfare. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT. (2013). Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alcohol Depend 129:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. (2009). Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience 163:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Pinheiro BS. (2015). Dyadic social interaction inhibits cocaine-conditioned place preference and the associated activation of the accumbens corridor. Behav Pharmacol 26:580–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, Prast JM. (2013). Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry 4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]


