Abstract
Background
Left atrial (LA) enlargement is associated with adverse events in heart failure with preserved ejection fraction (HFpEF). However, the role of LA mechanics (i.e., LA strain measures) in HFpEF has not been well studied. We hypothesized that in HFpEF, reduced (worse) LA strain is a key pathophysiologic abnormality and is a stronger correlate of adverse events than left ventricular (LV) or right ventricular (RV) longitudinal strain.
Methods and Results
We evaluated baseline LA function in 308 patients with HFpEF who were followed longitudinally for adverse outcomes. All patients underwent speckle-tracking echocardiography for measurement of LV longitudinal strain, RV free wall strain, and LA booster, conduit, and reservoir strains. The clinical and prognostic significance of LV, RV, and LA strain measures was assessed by regression analyses. The mean age was 65±13 years; 64% were female; 26% had atrial fibrillation; and LA enlargement was present in the majority (67%) of patients. Decreased LA reservoir strain was associated with increased pulmonary vascular resistance (P<0.0001) and decreased peak oxygen consumption (P=0.0001). Of the LV, RV, and LA strain measures, LA reservoir strain was the strongest correlate of adverse events, and was independently associated with the composite outcome of cardiovascular hospitalization or death (adjusted HR per 1-SD decrease in LA strain = 1.54; 95% CI = 1.15–2.07; P=0.006).
Conclusions
Abnormal indices of LA mechanics (particularly LA reservoir strain) are powerful clinical and prognostic factors in HFpEF. Unloading the LA and/or augmentation of LA function may be important future therapeutic targets in HFpEF.
Registration Information
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01030991.
Keywords: diastolic heart failure, left atrium, strain, outcome
The left atrium (LA) plays an integral role in the pathophysiology and prognosis of heart failure with preserved ejection fraction (HFpEF).1 As diastolic function worsens due to vascular and left ventricular (LV) stiffening, LV filling pressures increase, leading to LA pressure overload and enlargement.
More recently, LA mechanical dysfunction (above and beyond LA size) has gained a considerable amount of attention due to technological advances in non-invasive imaging and a better understanding of the pathophysiology of the HFpEF syndrome. LA function is comprised of reservoir, conduit, and booster phases, all of which can be accurately measured with high feasibility and reproducibility by two-dimensional (2D) speckle-tracking echocardiography analysis for the calculation of LA strain.2–4
With worsening LV diastolic function, both LA compliance and LA function decline.5–7 By exploiting this phenomenon with speckle-tracking strain analysis, investigators have found that indices of LA strain add incremental diagnostic value to conventional markers in HFpEF, above and beyond LA size.8, 9 However, the clinical significance and prognostic value of LA strain in patients with HFpEF is not known. Furthermore, no prior studies have compared the prognostic utility of LA strain versus LV and right ventricular (RV) strain in HFpEF.
We therefore sought to (1) determine the clinical, invasive hemodynamic, and cardiopulmonary exercise testing (CPET) correlates of LA strain in HFpEF; and (2) evaluate the prognostic utility of LA strain in HFpEF and determine its significance when compared to conventional echocardiographic and clinical factors, and indices of LV and RV mechanics. We hypothesized that in HFpEF, decreased LA strain (indicative of worse LA function) is associated with worse hemodynamics and decreased peak oxygen consumption (VO2); is associated with poor outcomes; and is a stronger correlate of adverse events than LV or RV strain.
METHODS
Study population
Between March 2008 and May 2011, consecutive patients were prospectively enrolled from the outpatient clinic of the Northwestern University HFpEF Program as part of a systematic observational study of HFpEF (ClinicalTrials.gov; NCT01030991). All patients were enrolled into the study in the outpatient setting after a hospitalization for heart failure. Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital (at the time of hospitalization) using the following search criteria: 1) diagnosis of heart failure or the term heart failure in hospital notes; or 2) B-type natriuretic peptide (BNP) >100 pg/mL; or 3) administration of ≥2 doses of intravenous diuretics. Patients were offered post-discharge follow-up in a specialized HFpEF outpatient program if they met the following 3 inclusion criteria: age ≥ 21 years, LV ejection fraction (LVEF) ≥ 50%, and presence of heart failure as defined by Framingham criteria.10
Post-hospitalization, the heart failure diagnosis was confirmed in the outpatient HFpEF clinic. All patients met the European Society of Cardiology criteria for the diagnosis of HFpEF.11 Patients were excluded if they had more than moderate valvular disease, previous cardiac transplantation, previous history of a reduced LVEF <40% (i.e., recovered EF), severe LV dilation (LV end-diastolic volume >97 mL/m2), or constrictive pericarditis. All study participants gave written informed consent, and the institutional review board at Northwestern University approved the study.
Clinical characteristics
We collected the following data in all study participants: demographics, New York Heart Association (NYHA) functional class, comorbidities, medications, vital signs, body mass index, and laboratory data, including creatinine, hemoglobin, and BNP. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation. Definitions of each of the individual comorbidities are listed in the Supplementary Data section.
Conventional echocardiography
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging (TDI) using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500; Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp, Waukesha, WI), as detailed in the Supplementary Data section.
Speckle tracking echocardiography
All images used for speckle-tracking echocardiographic analysis were obtained at a frame rate of 50–70 fps. Strain was analyzed by a single investigator using a customized software package (2D Cardiac Performance Analysis, TomTec v4.5, Munich, Germany). Three consecutive cardiac cycles were recorded and averaged. Speckle-tracking analysis was not performed in patients with unacceptable image quality, defined as >1 segment dropout, missing view, or significant foreshortening of the LV, RV, or LA.
We used the ventricular cycle as the reference point (i.e. zero baseline) to calculate LA strain.2 Therefore, the onset of the QRS complex is the zero reference, and all longitudinal LA strain values are positive. We defined the following components of LA function (strain): LA reservoir strain = peak (maximal) longitudinal LA strain; LA booster strain = longitudinal LA strain measured between onset of the P wave and onset of the QRS complex; and LA conduit strain = LA reservoir strain – LA booster strain. In patients who were in atrial fibrillation at the time of echocardiography, there is no LA booster function because of the loss of coordinated LA contraction; in these cases, LA conduit strain = LA reservoir strain.
In order to generate LA strain curves, the LA endocardial border was manually traced in the apical 4- and 2- chamber views. The region of interest generated was subsequently adjusted to include the full thickness of the LA myocardium. In each patient, the software divided the LA into six separate segments, longitudinal strain curves were generated, and tracking was evaluated. Any segment that did not sufficiently track well was excluded from final analysis, and the remaining segments were averaged for each view. LA reservoir, booster, and conduit strains were calculated by averaging the apical 4- and 2-chamber strain values. In patients with atrial fibrillation at the time of echocardiography, speckle-tracking of the apical 4- and 2- chamber views was performed on 3 different beats, and LA strain values from the 3 beats were averaged in each view. LA stiffness index was calculated as the ratio of E/e’ to LA reservoir strain as previously defined.5
In addition to LA strain, LV longitudinal strain and longitudinal RV free wall strain were also measured. The LV endocardial border was manually traced in the apical 4- and 2- chamber views, and the RV endocardial border traced in the apical 4-chamber RV-focused view. Similar to the LA, the software divided the LV and RV into six segments and regions with insufficient tracking were excluded. LV longitudinal strain was calculated by averaging the remaining segments for each view, and RV free wall strain was calculated by averaging the 3 RV free wall segments.
For ease of reporting and interpretation, all strain values were reported as absolute values (lower absolute strain values correspond to worse cardiac mechanics).
Invasive hemodynamic testing and cardiopulmonary exercise testing
In subsets of the study participants, right-sided heart catheterization and symptom-limited CPET were performed as described in the Supplementary Data section.
Outcomes
After enrollment, study participants were evaluated in the Northwestern HFpEF Program at least every 6 months. At each visit, inter-current hospitalizations were documented, reviewed, and categorized as due to cardiovascular or non-cardiovascular causes. Every 6 months, participants (or their proxy) who were not able to come into clinic were contacted to determine vital status with verification of deaths through query of the Social Security Death Index. Enrollment date was defined as the first visit to the outpatient HFpEF clinic. Date of last follow-up was defined as date of death or last HFpEF clinic visit. Follow-up was complete in all patients. The primary endpoint was a combined outcome of cardiovascular hospitalization and death, which included hospitalization for any cardiovascular cause (including heart failure) and death from any cause.
Statistical analysis
Intraobserver variability for LA strain was assessed in 15 randomly selected patients by having the same observer repeat the analysis 1 month apart. Interobserver variability for LA strain was assessed in 30 randomly selected patients by having the same observer and another experienced observer repeat the analysis. Reproducibility data were reported using interclass correlation coefficients and coefficient of variation.
Clinical characteristics, laboratory data, echocardiographic measures, invasive hemodynamics, and CPET data were summarized for the entire cohort, and univariable Cox regression analyses were used to determine the association between these variables and adverse outcomes (cardiovascular hospitalization [which included HF hospitalization], or death). Next, we examined the correlation between indices of cardiac mechanics (LV, RV, and LA strain measures) and both invasive hemodynamics and CPET variables. These analyses were performed using a Pearson pairwise correlation. For the dependent variables PA systolic pressure, thermodilution cardiac output, pulmonary vascular resistance (PVR), and peak VO2, we used unadjusted and multivariable-adjusted linear regression analyses with LA reservoir strain as the independent variable. Covariates included age, sex, obesity, atrial fibrillation, LA volume, LV mass, and E/e’ ratio. Formal interaction testing with multiplicative interaction terms and the likelihood ratio (LR) test was used to determine whether clinical characteristics (age, sex, and comorbidities) modified the associations between LA reservoir strain and the aforementioned hemodynamic and CPET indices.
For survival analyses, we used Cox proportional hazards regression to evaluate the unadjusted relationship between the measures of LA function and outcomes. Models were then adjusted for covariates chosen based on a combination of clinical relevance and association with adverse outcomes in HFpEF. We used a series of models for our Cox regression analyses. After performing unadjusted analyses, we performed the following multivariable-adjusted analyses: Model 1 included sex, atrial fibrillation, the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score, LV mass, and LA volume; Model 2 included Model 1 covariates + E/e’ ratio; and Model 3 included Model 2 covariates + LV longitudinal strain and RV free wall strain. The MAGGIC risk score12 is a mortality risk score for patients with heart failure, including those with HFpEF, and includes age, LV ejection fraction, creatinine, diabetes, chronic obstructive pulmonary disease, systolic blood pressure, body-mass index, heart rate, NYHA functional class, ACE-inhibitor use, beta-blocker use, heart failure duration, and current smoking.
To determine the relative utility of strain measures beyond conventional risk predictors, and to compare the prognostic and discriminative utility across strain measures, we used a combination of tests, including Harrell’s C-statistic, integrated discrimination improvement (IDI), net reclassification improvement (NRI), the LR test, and Bayes information criterion (BIC).
In sensitivity analyses, we repeated linear and Cox regression analyses after excluding participants who had atrial fibrillation or moderate mitral regurgitation at the time of echocardiography. A two-sided P-value < 0.05 was considered to indicate statistical significance. All analyses were performed using Stata v.12 (StataCorp, College Station, TX).
RESULTS
Baseline clinical characteristics
Of the 419 enrolled patients, echocardiographic images could not be retrieved in 56 patients. An additional 55 patients were excluded from the final analysis due to poor image quality for speckle-tracking strain analysis (feasibility = 85%). Table 1 summarizes the clinical characteristics and the association of these characteristics with adverse events for the remaining 308 patients. The majority of patients were women, nearly half of the study sample was non-white (48%), and most patients (84%) had NYHA functional class II or III symptoms.
Table 1.
Summary of Clinical Characteristics of the Study Cohort, and Association of Clinical Characteristics with Cardiovascular Hospitalization or Death on Cox Regression Analysis
| Clinical characteristic | Total cohort (N=308) |
Hazard ratio (95% CI) |
P-value |
|---|---|---|---|
| Age, years* | 65±13.0 | 1.27 (1.10–1.47) | 0.001 |
| Female, n(%) | 197(64) | 1.02 (0.69–1.49) | 0.94 |
| Race, n(%) | 0.29 | ||
| • Caucasian | 159 (52) | 1.00 (referent) | |
| • African American | 118 (38) | 1.26 (0.86–1.84) | |
| • Other | 31 (10) | 0.78 (0.37–1.64) | |
| NYHA class, n(%) | <0.001 | ||
| • I | 44 (14) | 1.00 (referent) | |
| • II | 118 (38) | 1.15 (0.61–2.18) | |
| • III | 141 (46) | 2.40 (1.32–4.38) | |
| • IV | 4 (1) | 2.78 (0.63–12.33) | |
| Comorbidities, n(%) | |||
| • Atrial fibrillation | 79(26) | 1.24 (0.83–1.86) | 0.30 |
| • Coronary artery disease | 153(50) | 1.32 (0.92–1.91) | 0.14 |
| • Hypertension | 232(75) | 1.61 (1.00–2.58) | 0.05 |
| • Diabetes | 91(30) | 1.46 (1.00–2.14) | 0.05 |
| • Cigarette smoker | 125(41) | 1.00 (0.69–1.45) | 0.99 |
| • Hyperlipidemia | 161(52) | 0.89 (0.62–1.29) | 0.54 |
| • Obesity | 154(50) | 1.02 (0.70–1.46) | 0.94 |
| • Chronic kidney disease | 94(31) | 1.89 (1.29–2.78) | 0.001 |
| • COPD | 106(34) | 1.33 (0.92–1.94) | 0.13 |
| • Obstructive sleep apnea | 105(34) | 1.40 (0.96–2.03) | 0.08 |
| Vital signs and laboratory data | |||
| • Systolic blood pressure, mmHg** | 125±13 | 0.88 (0.73–1.06) | 0.19 |
| • Diastolic blood pressure, mmHg** | 70±12 | 0.73 (0.59–0.89) | 0.002 |
| • Body mass index, kg/m2** | 31.5±8.6 | 1.02 (0.85–1.23) | 0.82 |
| • Hemoglobin, g/dL† | 11.9±1.8 | 1.26 (1.06–1.52) | 0.009 |
| • Estimated GFR, mL/min per 1.73 m2† | 60±28 | 1.45 (1.20–1.77) | <0.001 |
| • BNP, pg/mL (median, 25th–75th percentile)** | 230 (69–474) | 1.25 (1.10–1.41) | 0.001 |
| Medications, n(%) | |||
| • ACE-inhibitor or ARB | 166(54) | 0.90 (0.62–1.30) | 0.58 |
| • β-blocker | 206(67) | 1.55 (1.01–2.36) | 0.04 |
| • Calcium channel blocker | 101(33) | 1.39 (0.96–2.02) | 0.08 |
| • Nitrate | 40(13) | 2.84 (1.78–4.52) | <0.001 |
| • Loop diuretic | 168(55) | 2.28 (1.54–3.37) | <0.001 |
| • Thiazide diuretic | 67(22) | 1.05 (0.68–1.64) | 0.81 |
| • Mineralocorticoid receptor antagonist | 38(12) | 1.24 (0.74–2.09) | 0.41 |
| • Statin | 148(48) | 1.43 (0.99–2.06) | 0.06 |
| • Aspirin | 138(45) | 1.37 (0.95–1.98) | 0.09 |
| • Warfarin | 70(23) | 1.26 (0.83–1.93) | 0.28 |
| MAGGIC risk score** | 19.3±7.3 | 1.07 (1.04–1.10) | <0.001 |
Hazard ratio is per 10-year increase
Hazard ratio is per 1-SD increase
Hazard ratio is per 1-SD decrease
Categorical variables are presented as counts and percentages; continuous variables are presented as mean ± standard deviation unless otherwise specified.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; COPD = chronic obstructive pulmonary disease; GFR = glomerular filtration rate; NYHA = New York Heart Association; MAGGIC = Meta-Analysis Global Group in Chronic heart failure
The median follow-up time was 13.8 months (25th–75th percentile, 4.5–23.9 months). During the follow-up period, 94 patients (31%) were hospitalized for a cardiovascular reason, 66 (21%) were hospitalized for HF, 37 (12%) died, and 115 (37%) experienced the composite end point of cardiovascular hospitalization (including HF hospitalization) or death.
Several clinical and laboratory characteristics were associated with adverse outcomes on univariable Cox regression analyses. Older age, worse NYHA functional class, systemic hypertension, chronic kidney disease, certain medications (loop diuretics, beta-blockers, and nitrates), and higher BNP and lower hemoglobin were associated with adverse outcomes. In addition, a higher MAGGIC risk score was associated with adverse events.
Baseline echocardiographic characteristics, including speckle-tracking LV and RV longitudinal strain measures
Table 2 summarizes the conventional echocardiographic, tissue Doppler, and speckle-tracking measures of the study cohort and their association with adverse events. Overall, patients had evidence of structural heart disease with high prevalence of LV hypertrophy (43%) or concentric remodeling (38%), and moderate or greater LV diastolic dysfunction (75%). Although LVEF was preserved overall (≥ 50% in all patients, with a mean value of 61±6%), TDI s’ velocity and LV longitudinal strain were decreased in the study cohort.13 Of the 308 study patients, 230 (75%) had abnormal absolute LV longitudinal strain (defined as absolute LV longitudinal strain < 20%14). The prevalence of RV systolic dysfunction was relatively low when defined by conventional echocardiographic measures (19% of patients had an RV fractional area change < 35%; 26% of patients had a TAPSE < 1.6 cm). However, when defined by RV free wall strain (absolute RV free wall strain < 20%14), the prevalence of RV systolic dysfunction was higher (48% of patients).
Table 2.
Summary of Echocardiographic, Invasive Hemodynamic, Cardiopulmonary Exercise Test Characteristics of the Study Cohort, and Association of These Characteristics with Cardiovascular Hospitalization or Death on Cox Regression Analysis
| Parameter | Total Cohort (N=308) |
Hazard Ratio (95% CI) |
P-value |
|---|---|---|---|
| Echocardiography | |||
| • Septal wall thickness, cm* | 1.20±0.30 | 1.28 (1.11–1.48) | 0.001 |
| • Posterior wall thickness, cm* | 1.15±0.28 | 1.27 (1.11–1.46) | <0.001 |
| • Relative wall thickness* | 0.51±0.16 | 1.25 (1.09–1.43) | 0.001 |
| • LV mass index, g/m2* | 104.5±39.7 | 1.26 (1.10–1.44) | 0.001 |
| • LV end-diastolic volume index, mL/m2* | 41.6±12.3 | 0.92 (0.74–1.13) | 0.43 |
| • LV end-systolic volume index, mL/m2* | 16.7±7.5 | 0.93 (0.76–1.15) | 0.52 |
| • LV ejection fraction, %** | 61.0±6.4 | 0.94 (0.79–1.14) | 0.56 |
| • LA volume index, ml/m2* | 34.4±13.7 | 1.16 (0.99–1.35) | 0.06 |
| • E velocity, cm/s* | 104.6±35.8 | 1.27 (1.07–1.50) | 0.006 |
| • A velocity, cm/s* | 85.9±30.3 | 1.12 (0.90–1.40) | 0.30 |
| • E/A ratio* | 1.3±0.7 | 1.13 (0.94–1.36) | 0.19 |
| • RV fractional area change, %** | 44±7 | 1.20 (1.01–1,42) | 0.04 |
| • TAPSE, cm** | 2.0±0.6 | 1.19 (0.99–1.43) | 0.06 |
| • PA systolic pressure, mmHg* | 43.7±15.5 | 1.21 (0.98–1.49) | 0.08 |
| • Tissue Doppler measures† | |||
| ○ s’ velocity, cm/s** | 7.2±2.1 | 1.42 (1.13–1.77) | 0.002 |
| ○ e’ velocity, cm/s** | 7.0±2.7 | 1.19 (0.96–1.46) | 0.10 |
| ○ a’ velocity, cm/s** | 8.4±3.1 | 1.61 (1.31–1.98) | <0.001 |
| ○ E/e’ ratio** | 15.0±8.1 | 1.31 (1.14–1.50) | <0.001 |
| • Aortic stenosis, n(%) | |||
| ○ Mild | 1 (0.3) | — | — |
| ○ Moderate | 3 (1) | 0.97 (0.14–6.96) | 0.98 |
| • Aortic regurgitation, n(%) | |||
| ○ Mild | 5 (1.6) | 1.81 (0.57–5.69) | 0.31 |
| ○ Moderate | 2 (0.6) | — | — |
| • Moderate mitral regurgitation, n(%) | 44 (14) | 1.66 (1.01–2.73) | 0.04 |
| Speckle-tracking echocardiography‡ | |||
| • LV longitudinal strain, %** | 17.5±4.1 | 1.25 (1.03–1.52) | 0.02 |
| • RV free wall strain, %** | 21.1±8.1 | 1.30 (1.07–1.58) | 0.009 |
| • LA conduit strain, %** | 19.8±8.5 | 1.58 (1.25–2.00) | <0.001 |
| • LA booster strain, %** | 18.3±7.7 | 1.56 (1.23–1.99) | <0.001 |
| • LA reservoir strain, %** | 36.2±14.9 | 1.72 (1.37–2.15) | <0.001 |
| • LA stiffness index* | 0.49±0.43 | 1.44 (1.27–1.62) | <0.001 |
| Invasive hemodynamics (N=177) | |||
| • Right atrial pressure, mmHg* | 13±6 | 1.43 (1.14–1.80) | 0.002 |
| • Mean PA pressure, mmHg* | 33±10 | 1.37 (1.08–1.72) | 0.008 |
| • PCWP, mmHg * | 23±8 | 1.27 (1.00–1.62) | 0.05 |
| • Cardiac output, L/min** | 6.0±2.2 | 1.14 (0.87–1.50) | 0.35 |
| • Pulmonary vascular resistance, WU* | 1.9±1.4 | 1.22 (1.02–1.46) | 0.03 |
| Cardiopulmonary exercise testing (N=117) | |||
| • Respiratory exchange ratio** | 1.11±0.13 | 1.13 (0.82–1.56) | 0.44 |
| • Workload, watts** | 60.1±31.7 | 1.96 (1.29–2.98) | 0.002 |
| • Exercise time, seconds** | 391±252 | 2.06 (1.34–3.18) | 0.001 |
| • Anaerobic threshold** | 11.0±3.6 | 3.78 (1.72–8.30) | 0.001 |
| • Peak VO2, ml/kg/min** | 13.9±5.4 | 2.33 (1.44–3.76) | 0.001 |
| • Oxygen pulse, mL/beat** | 10.8±3.85 | 1.59 (1.10–2.30) | 0.01 |
| • VE/VCO2 ratio at anaerobic threshold* | 32.7±4.8 | 1.14 (0.81–1.59) | 0.46 |
Hazard ratio is per 1-SD increase
Hazard ratio is per 1-SD decrease
All tissue Doppler values represent average of septal and lateral indices.
All speckle-tracking measures are presented as absolute values.
Summary values represent mean ± SD. There were too few events to calculate hazard ratios for mild aortic stenosis or moderate aortic regurgitation.
LV = left ventricular; LA = left atrial; E = early mitral inflow; A = late (atrial) mitral inflow; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; PA = pulmonary arterial; PCWP = pulmonary capillary wedge pressure; WU = Wood units; VO2 = oxygen consumption; VE/VCO2 = ventilatory efficiency.
A higher LV mass index and increased E/e’ ratio, consistent with pathological LV hypertrophy and elevated LV filling pressures, respectively, were associated with adverse outcomes. Lower TDI s’ velocities, decreased (worse) LV longitudinal strain, and decreased (worse) RV free wall strain were also associated with adverse outcomes (Table 2).
Baseline LA size and function, including speckle-tracking LA strain measures
On average, LA size, as measured by LV volume index, was dilated in the study population, and 67% of the study patients had evidence of LA enlargement (using a cut-off of > 28 ml/m2). Figure 1 displays examples of LA strain curves in HFpEF patients with and without atrial fibrillation. Supplementary Table S1 displays the intra- and interobserver variability of LA strain measures. The normal range for LA reservoir strain using TomTec strain software is not defined. However, based on published normal values for LA reservoir strain15 using GE strain software, 26% of the patients had LA reservoir strain values < 22.7%, which is 2 standard deviations (SDs) below the mean in healthy controls (44.1%); and 56% of patients had LA reservoir strain values < 34.1%, which is 1 SD below the mean in healthy controls.
Figure 1. Representative left atrial strain images.
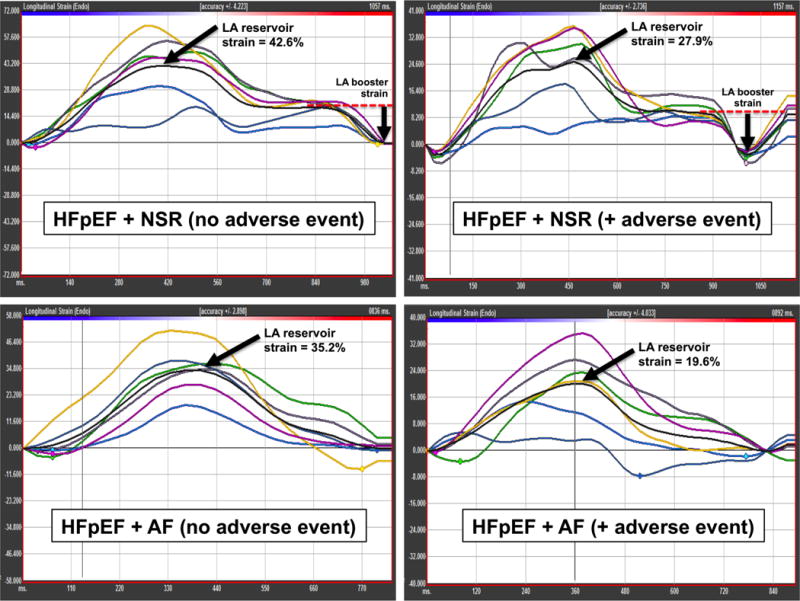
Y-axis = longitudinal strain (%). X-axis = time (ms). Note: each graph has different range for the Y-axis. LA booster strain is not present in the setting of AF. LA conduit strain = LA reservoir strain – LA booster strain. In AF, LA reservoir strain = LA conduit strain.
LA = left atrial; HFpEF = heart failure with preserved ejection fraction; NSR = normal sinus rhythm; AF = atrial fibrillation.
Beat-to-beat variability was evaluated in patients with atrial fibrillation. The mean value for LA reservoir strain in patients with atrial fibrillation at the time of echocardiography was 16.9%. The standard deviation of LA reservoir strain measured across multiple beats was 1.7 %-units. The coefficient of variation for LA reservoir strain measured across multiple beats was 9.2%.
LA volume index was not associated with adverse outcomes during follow-up. However, lower septal and lateral TDI a’ velocities (markers of the LA contribution to mitral annular motion at end-diastole) were associated with adverse events. In addition, as shown in Table 2, decreased (worse) LA booster, conduit, and reservoir strain were all associated with adverse events. Increased LA stiffness was also associated with poor outcomes in the study sample.
Comparison of the clinical and prognostic utility of LV, RV, and LA strain
Supplementary Table S2 displays the demographic, clinical, laboratory, and echocardiographic measures that were associated with LA booster, conduit, and reservoir strain. Several factors, including atrial fibrillation; increased BNP, MAGGIC risk score, LV mass, LA volume, and E/e’ ratio; and decreased GFR, tissue velocities were each associated with associated with worse LA strain values. LV longitudinal strain, and RV free wall strain were associated with decreased (worse) LA strain, particularly LA reservoir strain. To determine the clinical utility of LV, RV, and LA strain measures, we also examined the association between strain measures and (1) invasive hemodynamics and (2) CPET variables. Several invasive hemodynamic and CPET variables, including right atrial pressure, PA pressure, and peak VO2, known to carry prognostic value in heart failure, were also associated with adverse events in our study (Supplementary Table S3).
As shown in Supplementary Table S3, LV longitudinal strain was only marginally associated with PA pressure, cardiac index, exercise workload, and ventilatory efficiency. RV free wall strain was associated with cardiac index and PVR, but was not associated with any CPET variables. However, LA reservoir strain was significantly associated with several invasive hemodynamic indices and CPET variables. LA reservoir strain correlated with elevated PA pressures and PVR, decreased pulmonary artery compliance, and decreased resting thermodilution cardiac output, exercise workload, peak VO2, and ventilatory efficiency.
On linear regression analyses, LA reservoir strain remained associated with PA systolic pressure and PVR after multivariable adjustment (Table 3). We did not identify any interactions between clinical characteristics (e.g., comorbidities) and LA reservoir strain for the association with PA systolic pressure or PVR. Figure 2 displays the relationship between quartiles of LA reservoir strain and PVR.
Table 3.
Association of Left Atrial Reservoir Strain with Selected Invasive Hemodynamic and Cardiopulmonary Exercise Testing Measures on Linear Regression Analysis
| Measure (dependent variable) | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
| ||||
| Beta-coefficient** (95% CI) |
P-value | Beta-coefficient** (95% CI) |
P-value | |
| PA systolic pressure, mmHg | 4.0 (1.6, 6.4) | 0.001 | 3.3 (0.5–6.0) | 0.019 |
| Cardiac output, L/min | −0.64 (−0.99, −0.29) | <0.001 | −0.55 (−0.93, −0.17) | 0.005 |
| Pulmonary vascular resistance, WU | 0.47 (0.27, 0.67) | <0.001 | 0.41 (0.18, 0.64) | <0.001 |
| Peak VO2, ml/kg/min | −1.9 (−2.8, −0.9) | <0.001 | −1.8 (−2.8, −0.8) | 0.001 |
Adjusted for age, sex, obesity, atrial fibrillation, left atrial volume, left ventricular mass, and E/e’ ratio
Per 1-SD decrease (worsening) in left atrial reservoir strain
CI = confidence interval; PA = pulmonary artery; VO2 = oxygen consumption
Figure 2. Box-and-whisker plots of pulmonary vascular resistance by quartiles of left atrial reservoir strain.
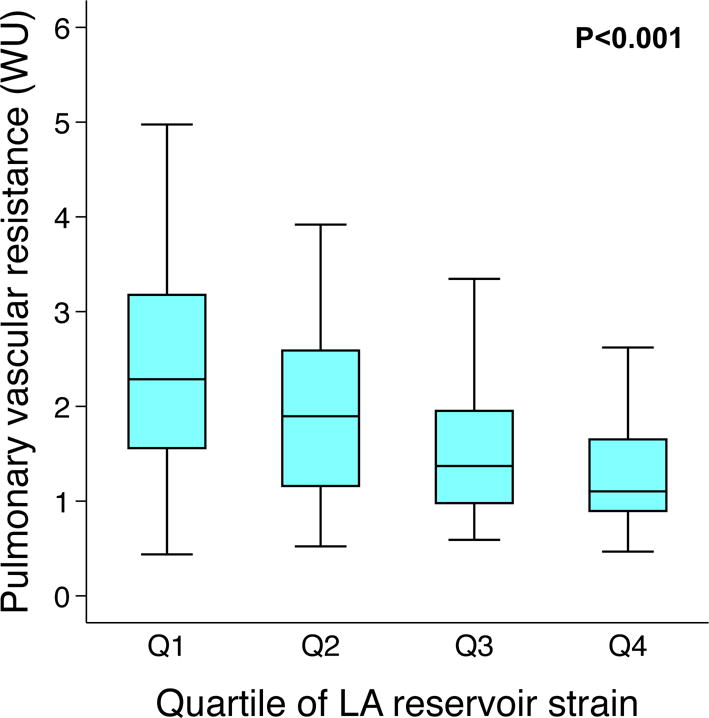
LA = left atrial; WU = Wood units. Quartiles of LA reservoir strain correspond to the following values: Q1 < 22.2%; Q2 22.2–31.1%; Q3 31.2–42.8%; Q4 > 42.9%.
On multivariable-adjusted linear regression analyses, LA reservoir strain also remained associated with thermodilution cardiac output (measured invasively) and peak VO2 (Table 3). We found multiple significant interactions (P<0.05) for the association between LA reservoir strain and peak VO2. The association between LA reservoir strain and peak VO2 was much stronger in those who were younger and in the absence of chronic obstructive pulmonary disease, obesity, chronic kidney disease, and/or diabetes. Figure 3 displays examples of the aforementioned interactions: Figure 3A shows the relationship between LA strain and peak VO2, stratified by median age (65 years); and Figure 3B shows the same relationship stratified by the presence or absence of obesity.
Figure 3. Relationship between left atrial reservoir strain and peak oxygen consumption stratified by (A) median age (65 years) and (B) presence or absence of obesity.
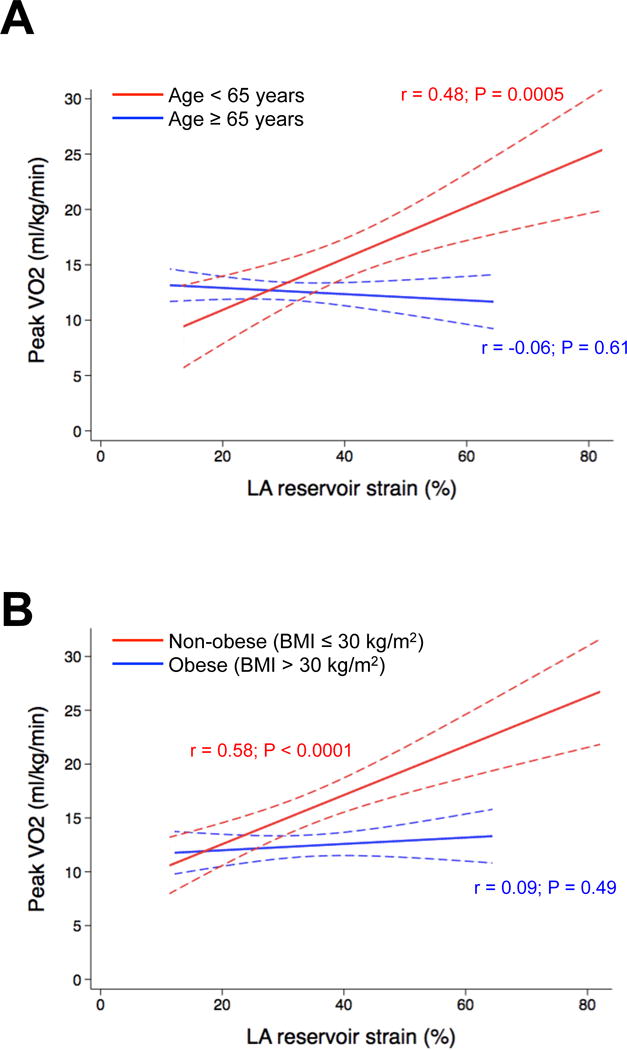
Lines represent linear fit of the relationship between LA reservoir strain and peak oxygen consumption (dotted lines represent 95% confidence intervals). LA = left atrial; VO2 = oxygen consumption.
Supplementary Table S4 shows that exclusion of patients with either atrial fibrillation (n=39) or moderate mitral regurgitation (n=36) at the time of echocardiography did not eliminate most of the associations between LA reservoir strain and invasive hemodynamic and CPET measures. LA reservoir strain was still associated with cardiac index, PVR, and peak VO2 after multivariable adjustment.
Association of indices of cardiac mechanics with outcomes on Cox regression analysis
In unadjusted Cox proportional hazards models all indices of cardiac mechanics were associated with adverse outcomes (Table 4). Based on the hazard ratios per 1-SD worsening of indices of cardiac mechanics, LA reservoir strain was most closely associated with adverse events, followed by LA conduit and booster strains. After multivariable adjustment for several demographic, clinical, and echocardiographic variables, LV and RV longitudinal strain, both LA reservoir and booster strains still remained associated with adverse outcomes. Further adjustment for the presence and severity of mitral regurgitation did not attenuate the association between LA strain and outcomes.
Table 4.
Association of Indices of Cardiac Mechanics with the Combined Outcome of Cardiovascular Hospitalization, Heart Failure Hospitalization, or Death in Heart Failure with Preserved Ejection Fraction
| Independent variable | Unadjusted | Adjusted (Model 1) | Adjusted (Model 2) | Adjusted (Model 3) | ||||
|---|---|---|---|---|---|---|---|---|
| HR* (95%CI) |
P-value | HR* (95%CI) |
P-value | HR* (95%CI) |
P-value | HR* (95%CI) |
P-value | |
| LV longitudinal strain | 1.25 (1.03–1.52) |
0.023 | 1.18 (0.97–1.45) |
0.10 | 1.17 (0.95–1.43) |
0.13 | — | — |
| RV free wall strain | 1.30 (1.07–1.58) |
0.009 | 1.20 (0.98–1.02) |
0.08 | 1.19 (0.97–1.47) |
0.10 | — | — |
| LA conduit strain | 1.74 (1.35–2.24) |
<0.001 | 1.42 (1.07–1.87) |
0.013 | 1.33 (1.00–1.76) |
0.05 | 1.22 (0.91–1.84) |
0.18 |
| LA booster strain | 1.56 (1.23–1.99) |
<0.001 | 1.45 (1.12–1.88) |
0.004 | 1.40 (1.08–1.82) |
0.01 | 1.33 (1.01–1.73) |
0.04 |
| LA reservoir strain | 1.72 (1.37–2.15) |
<0.001 | 1.63 (1.22–2.19) |
0.001 | 1.54 (1.15–2.07) |
0.006 | 1.43 (1.05–1.95) |
0.02 |
| LA stiffness index** | 1.44 (1.27–1.63) | <0.001 | 1.44 (1.22–1.70) |
<0.001 | — | — | 1.39 (1.17–1.66) |
<0.001 |
Per 1-SD decrease in each strain variable; all strain values are presented as absolute values (thus, a decrease in each independent variable indicates worse strain). For LA stiffness index, HRs are per 1-SD increase in the independent variable.
LA stiffness index = E/e’ ÷ LA reservoir strain; thus, Model 2 (which adjusts for E/e’ ratio) does not apply to LA stiffness index since E/e’ is used to calculate the LA stiffness index, and Model 3 for the LA stiffness index includes LV longitudinal strain and RV free wall strain but does not include E/e’.
Model 1 adjusts for sex; atrial fibrillation; MAGGIC risk score (which includes the following variables: age, ejection fraction, creatinine, diabetes, chronic obstructive pulmonary disease, systolic blood pressure, body-mass index, heart rate, New York Heart Association functional class, ACE-inhibitor use, beta-blocker use, heart failure duration, and current smoker); LV mass; and LA volume.
Model 2 adjusts for Model 1 variables + E/e’ ratio (average of septal and lateral E/e’ ratios).
Model 3 adjusts for Model 2 variables + LV longitudinal strain and RV free wall strain.
HR = hazard ratio; CI = confidence interval; LV = left ventricular; RV = right ventricular; LA = left atrial; MAGGIC = Meta-Analysis Global Group in Chronic heart failure.
The relationship between LA strain measures with the composite outcome of HF hospitalization, cardiovascular hospitalization, or death was relatively linear (as shown in Supplementary Figure S1), especially for LA reservoir strain. Figure 4A displays the Kaplan-Meier curves for LA reservoir strain, stratified by the median value (= 31.2%). Figure 4B demonstrates that the Kaplan-Meier curves appeared similar when considering only patients without atrial fibrillation. Supplementary Table S5 displays results from Cox regression analyses after excluding patients with either atrial fibrillation or moderate mitral regurgitation at the time of echocardiography.
Figure 4. Kaplan-Meier curves for survival free of cardiovascular hospitalization (including heart failure hospitalization or death), stratified by median left atrial reservoir strain in (A) all patients and (B) after excluding patients with a history of atrial fibrillation.
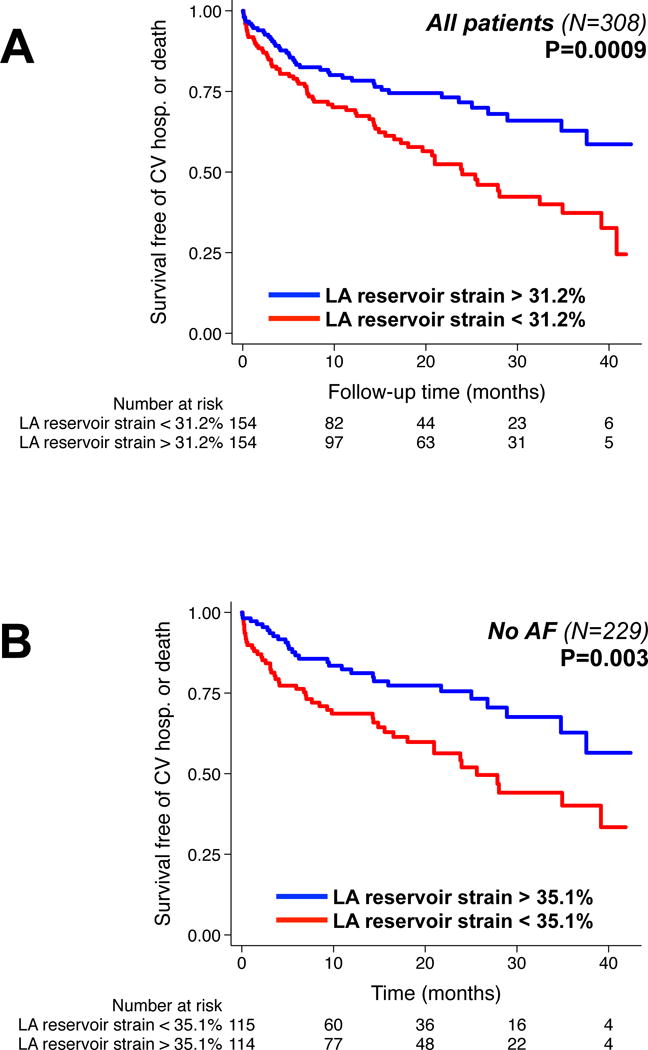
CV = cardiovascular; hosp. = hospitalization; LA = left atrial; AF = atrial fibrillation
Incremental prognostic utility of indices of cardiac mechanics
As shown in Supplementary Table S6, LA reservoir strain outperformed LV longitudinal strain and RV free wall strain in its prognostic and discriminative utility above and beyond traditional risk markers such as the MAGGIC risk score and LA volume. LA reservoir strain had the highest relative IDI and increase in the C-statistic.
DISCUSSION
In this study of a large, contemporary cohort of patients with HFpEF, we found that all components of LA strain (LA conduit, LA booster, and LA reservoir), as determined by speckle-tracking 2D echocardiography, were predictive of cardiovascular hospitalizations (including HF hospitalization) and death. In addition, LA reservoir strain remained strongly prognostic after adjustment for atrial fibrillation, LA volume, LV mass, and the MAGGIC risk score. Even after further adjustment for LV longitudinal strain and RV free wall strain, LA reservoir strain still retained its prognostic value. In addition, LA reservoir strain was more closely associated with PVR and VO2 than LV and RV strain. Our study is the first to our knowledge to clearly demonstrate the central clinical and prognostic importance of LA strain in HFpEF, above and beyond LV or LA structure, LV strain, and RV strain.
Prognostic value of LA function compared to LA size in HFpEF
While multiple studies have provided evidence of the value of LA function for the diagnosis of HFpEF5, 8, 9 and the role LA function plays in the pathophysiology of HFpEF,16, 17 our study is the first to show the powerful prognostic role of LA strain in this patient population. Earlier publications showed that indexed LA volume is a robust correlate of adverse events including incident HF in patients with normal LVEF18 and incident atrial arrhythmia in people ≥ 65 years old.19 However, these studies did not measure LA function using speckle-tracking analysis.
Our data are consistent with these recent publications and builds on it by showing the improved ability of LA strain to predict adverse outcomes in patients with HFpEF compared to LA size. This result underscores the idea that indexed LA volume is simply a surrogate of LV filling pressures and more accurately reflects an adaptive change to the increased pressure rather than the intrinsic LA myocardial abnormalities that occur with HFpEF. This is particularly true in our patient population in which the overall severity of symptoms (46% NYHA Class III) and enlarged LA in the majority of patients (67%) makes LA size alone a relatively poor marker for predicting adverse events.
Prognostic value of LA function compared to other strain measures
We also found that in patients with HFpEF, speckle-tracking strain of the LA is a more powerful correlate of adverse outcomes than LV and RV longitudinal strain. Using a variety of metrics, we show convincingly that of the speckle-tracking indices of longitudinal cardiac mechanics, LA strain is most associated with future risk of adverse events. Although exclusion of patients with moderate mitral regurgitation and/or atrial fibrillation attenuated the significant association between LA strain and adverse outcomes in the fully adjusted multivariable analysis (Model 3), LA booster and reservoir strains were still associated with adverse outcomes after adjustment for all variables except LV and RV longitudinal strains (Model 2) in this subgroup. Furthermore, LA reservoir strain in this subgroup retained significance in a model including only LA reservoir strain, LV longitudinal strain, and RV free wall strain (P<0.001 on Cox regression analyses).
Only one previous study compared the prognostic roles of LA and LV strain using speckle-tracking echocardiography20 This study demonstrated that LV longitudinal strain was a better correlate of death and cardiovascular hospitalization than LA function in patients with acute myocardial infarction. The different patient populations studied most likely explain the discrepancy in these results as ischemia affects LV longitudinal strain to a greater extent in patients with acute myocardial infarction compared to patients with HFpEF.
The finding that LA strain is a more powerful correlate of outcomes compared to LV and RV longitudinal strain is meaningful because multiple pathophysiologic factors contribute to the HFpEF syndrome.21 LA reservoir function is considerably influenced by LA relaxation and compliance. In HFpEF, myocardial fibrosis of the LA likely plays a significant role in disease progression as it does in patients with atrial fibrillation22 and severe mitral regurgitation.23 This is evident in our study by the significant higher LA stiffness index in patients who experienced adverse events during follow-up. The subsequent remodeling of the LA myocardium decreases LA compliance and blunts LA reservoir function in response to increases in preload.9
Abnormal LA function: a key stimulus for elevated PVR and reduced exercise capacity in HFpEF?
Several pathophysiologies exist in patients with HFpEF, and each of these may result in reduced exercise capacity and worse outcomes. HFpEF patients with elevated PVR and right heart failure are particularly vulnerable to worse outcomes, and the factors that lead to these pathophysiologic abnormalities are unclear. Our study indicates that abnormal LA mechanics, more so than E/e’ ratio or LA volume, may be indicative of significant chronic LA pressure and volume overload with subsequent chronic pulmonary venous congestion, ultimately resulting in pulmonary vasoconstriction and decreased pulmonary artery compliance. In a smaller study (N=101) that examined LA EF in HFpEF,24 Melenovsky and colleagues also found an association between LA function and PVR; however, this study did not control for E/e’ or LA volume, did not measure LA strain, and did not compare LA mechanics to LV mechanics in relationship to PVR. In addition, because of the smaller number of patients, this study was limited by an inability to perform multivariable adjustment for LA size or history of atrial fibrillation.
The association of LA reservoir strain and peak VO2 suggests that worse LA mechanics leads to poor augmentation of cardiac output with exertion and decreased exercise tolerance. As shown in Figure 3, the results of our statistical interaction testing analysis demonstrate abnormal LA mechanics is especially important in younger patients with less comorbidities because these individuals are less likely to have extracardiac reasons for exercise intolerance (such as aging-related musculoskeletal problems or obesity).
Potential therapeutic implications of LA dysfunction in HFpEF
The findings of our study point to a potential central role of abnormal LA mechanics in the HFpEF syndrome. Speckle-tracking LA strain measures, already known to be useful for the diagnosis of HFpEF, may also be useful in understanding responsiveness to pharmacological and device-based therapies. Indeed, in response to the growing recognition of the critical role the LA plays in the pathophysiology of HFpEF, new devices to unload and/or decompress the LA are increasingly becoming available. An interatrial shunt device to decrease LA pressure is currently being studied in controlled trials.25 Another potential therapeutic strategy in advanced HFpEF patients is a LA assist device.26 Further study is necessary to determine whether improvement in LA mechanics could lead to decreased PVR, increased cardiac output, and/or increased exercise capacity.
Strengths and limitations
The strengths of our study include the prospective and standardized recruitment of high-risk HFpEF patients, the large number of patients included in the final analysis (with high feasibility of speckle-tracking echocardiography), and the prognostic comparison of LA strain to LV and RV strain measures. Furthermore, in relatively large subsets of patients we were able to examine the associations between LA strain measures and invasive hemodynamics and CPET variables, thereby providing pathophysiological insight into the importance of LA strain in HFpEF. Finally, the sample size and number of events allowed us to perform comprehensive multivariable adjustment to clearly show the prognostic utility of LA mechanics in HFpEF.
Nevertheless, certain limitations should also be considered when interpreting our results. First, strain acquisition was not possible in 55 patients. However, we were able to perform speckle-tracking analysis on the majority (85%) of the study participants and reproducibility was excellent. Strain analysis was also performed by averaging all 6 segments of the LA endocardium in only 2 imaging planes. Other studies have used 3 imaging planes and some have consistently excluded the posterior LA. Currently, there is no standardization of LA strain acquisition. 3D speckle-tracking strain might have overcome some of the limitations in LA strain analysis but this technique is not widely used, and there is limited data to support the use of this method. Second, the cut-off values for abnormal strain values—particularly for LA strain using TomTec software—are not well defined, and our study did not include a control group. Thus, the prevalences of abnormal LV, RV, and LA strain in our cohort should be interpreted with caution. Third, patients with either atrial fibrillation or moderate mitral regurgitation, both of which can affect LA mechanics, were included in our primary analyses. However, we adjusted for these factors in our multivariable analyses, and the associations of LA strain with PVR, peak VO2, and adverse outcomes persisted. In addition, we performed sensitivity analyses after excluding patients with atrial fibrillation or moderate mitral regurgitation at the time of echocardiography. Finally, the associations identified in the present study cannot prove causation given our study design and the possibility of unmeasured confounders in regression analyses.
Conclusions
In patients with HFpEF, indices of LA mechanics—particularly LA reservoir strain—are independently associated with adverse outcomes. LA reservoir strain is the speckle-tracking measure most associated with elevated PVR, decreased cardiac output, reduced exercise capacity, and the increased risk of the combined endpoint of cardiovascular hospitalization or death. Given these findings, novel therapeutic options for unloading the LA and/or augmenting its function may be beneficial in HFpEF.
Supplementary Material
CLINICAL PERSPECTIVE.
Effective treatment for patients with heart failure with preserved ejection fraction (HFpEF) remains elusive due, in part, to the myriad pathophysiologies that define this syndrome. One common clinic characteristic is left atrial (LA) enlargement due to decreased left ventricular (LV) compliance and increased LV filling pressure. Although not required for the diagnosis of HFpEF, prior studies have demonstrated the prognostic utility of LA size in this patient population. However, less is known about the role of LA mechanics (which may be more important than LA size) in HFpEF. Using speckle-tracking echocardiography for the measurement of cardiac mechanics, our study shows that reduced LA strain (indicating worse left atrial mechanics) is a key pathophysiologic abnormality that is associated with a worse clinical profile, higher pulmonary vascular resistance, decreased peak oxygen consumption, and worse outcomes—above and beyond abnormalities in LV and right ventricular mechanics. These findings suggest that improving LA function may be an important therapeutic target in this challenging syndrome. Specifically, speckle-tracking LA strain parameters, already known to be useful for the diagnosis of HFpEF, may also be useful in understanding responsiveness to therapies that unload and/or decompress the LA.
Acknowledgments
SOURCES OF FUNDING
American Heart Association Scientist Development Grant (#0835488N) and National Institutes of Health (R01 HL107557, R01 HL127028) to S.J.S.
Footnotes
DISCLOSURES
S.J.S is a non-paid consultant to Corvia Medical and has received consulting fees from Novartis, AstraZeneca, Bayer, and Merck. S.J.S. has research grants from Actelion and Novartis, and B.H.F has a research grant from Bayer/ISHLT.
References
- 1.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 2.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 3.Saraiva RM, Demirkol S, Buakhamsri A, Greenberg N, Popovic ZB, Thomas JD, Klein AL. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–180. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 6.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 7.Morris DA, Gailani M, Vaz Perez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:651–662. doi: 10.1016/j.echo.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Khan UA, de Simone G, Hill J, Tighe DA, Aurigemma GP. Depressed atrial function in diastolic dysfunction: a speckle tracking imaging study. Echocardiography. 2013;30:309–316. doi: 10.1111/echo.12043. [DOI] [PubMed] [Google Scholar]
- 9.Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, Tange S, Arai M, Kurabayashi M. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–758. doi: 10.1016/j.jcmg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 10.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 12.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 13.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Morris DA, Takeuchi M, Krisper M, Kohncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Osmanoglou E, Kuhnle Y, Dungen HD, Nakatani S, Otsuji Y, Haverkamp W, Boldt LH. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:364–372. doi: 10.1093/ehjci/jeu219. [DOI] [PubMed] [Google Scholar]
- 16.Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD, Investigators P. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:1096–1103. doi: 10.1002/ejhf.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchis L, Gabrielli L, Andrea R, Falces C, Duchateau N, Perez-Villa F, Bijnens B, Sitges M. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16:62–67. doi: 10.1093/ehjci/jeu165. [DOI] [PubMed] [Google Scholar]
- 18.Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–836. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101:1626–1629. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 20.Ersboll M, Andersen MJ, Valeur N, Mogensen UM, Waziri H, Moller JE, Hassager C, Sogaard P, Kober L. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging. 2013;6:26–33. doi: 10.1161/CIRCIMAGING.112.978296. [DOI] [PubMed] [Google Scholar]
- 21.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 23.Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, Lisi G, Maccherini M, Chiavarelli M, Massetti M, Tanganelli P, Mondillo S. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 24.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 25.Sondergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, Franzen O, Neuzil P, Ihlemann N, Gustafsson F. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796–801. doi: 10.1002/ejhf.111. [DOI] [PubMed] [Google Scholar]
- 26.Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, Shah SJ. Left Atrial Decompression Pump for Severe Heart Failure With Preserved Ejection Fraction: Theoretical and Clinical Considerations. JACC Heart failure. 2015;3:275–282. doi: 10.1016/j.jchf.2014.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


