Abstract
The objectives of this systematic literature review (SLR) were to identify domains and outcome measures used in psoriatic arthritis (PsA) studies in the past 5 years, and to compare the measurement of the Outcome Measures in Rheumatology (OMERACT) 2006 PsA Core Domain Set in studies published in 2010–2015 vs those published in 2006–2010. We performed a systematic literature search in two databases, PubMed and Embase, to identify randomised controlled trials (RCTs) in PsA. We also identified PsA longitudinal observational studies (LOS). Three patient research partners provided input into study conception, and data collection and interpretation. We identified 41 studies representing 22 unique RCTs, 27 LOS and 12 registries. Across all studies, we identified 24 domains and 169 outcome measures. In addition to the PsA Core Domain Set (6 domains), the following domains were also assessed in more than 30% of RCTs: acute phase reactants, dactylitis, enthesitis, fatigue and work productivity. We identified a range of 1–15 outcome measures per domain with a mean (SD) of 7 (4.7) per domain. The complete PsA Core Domain Set was assessed in 59% of RCTs in 2010–2015 compared to 23.5% RCTs in 2006–2010. There has been increased measurement of the PsA Core Domain Set in RCTs and LOS in the past 5 years. Numerous additional outcomes were also measured. The PsA Core Domain Set needs an update to standardise PsA outcome assessments. This SLR will inform the development of an updated PsA Core Domain Set with patient research partner input.
Keywords: Psoriatic Arthritis, Outcomes research, Health services research
Key messages.
There is great heterogeneity in PsA manifestations and assessment.
There is an ever increasing number of PsA outcomes and outcome measures as quantified in this review.
It is difficult to draw conclusions on comparative effectiveness with such heterogeneity across PsA clinical trial outcomes. This has direct implications for clinical practice by increasing the complexity of evidence based treatment decisions.
Introduction
Psoriatic arthritis (PsA) is a chronic form of immune-mediated inflammatory arthritis that can lead to permanent joint damage.1 The prevalence of PsA is 0.01–0.47% in the general population2 and, depending on classification methods, 11–34%2–5 among people with psoriasis. The heterogeneity of clinical manifestations (eg, oligoarthritis and polyarthritis, and spinal, ligamentous and tendon involvement) complicates assessment of PsA outcomes and broadens its impact on daily life.6 PsA has been shown to negatively impact health-related quality of life (HRQL) independently of psoriasis skin manifestations.7 Given the heterogeneity of the clinical manifestations, measuring disease activity and disease impact can be difficult. A Core Domain Set for the assessment of PsA was endorsed at the Outcome Measures in Rheumatology (OMERACT) meeting in 2006 and includes: pain, joints, physical function, skin, patient global and HRQL.8 To date, a PsA core outcome measurement set has not been endorsed by OMERACT due to lack of patient involvement in core set development.9 10
Recently, new therapeutic agents with novel mechanisms of action have been approved for PsA. The outcome measurement instruments used in PsA randomised controlled trials (RCTs) are based on instruments adopted from rheumatoid arthritis (RA), with the example of American College of Rheumatology (ACR) 20/50/70 responses as primary efficacy endpoints. PsA-specific manifestations (dactylitis, enthesitis, spondyloarthritis, skin and nail disease) were reported only as secondary outcomes in recent years. Furthermore, fatigue, skin symptoms, and aspects of psychological and social well-being, all prioritised by patient research partners (PRPs) as important for disease impact,11 have not been consistently measured in PsA RCTs.
The purpose of this review is twofold: (1) to review and summarise domains assessed in the past 5 years in PsA RCTs, as well as longitudinal observational studies (LOS) and studies of patient-generated domains, and (2) to compare domains assessed in recent clinical trials (published 2010–2015) to those published in 2006–2010.12 This systematic literature review will inform the development of an updated PsA core set of domains and outcome measures that will include patient input,9 13 and will integrate disease-specific aspects of clinical relevance to PsA for longitudinal care and interventional trials.
Methods
We searched two databases, PubMed and Embase, with the search terms ‘Psoriatic Arthritis’ combined with the Cochrane RCT sensitivity filter,14 15 using the Boolean operator ‘AND’, on 19 May 2015. We excluded paediatric studies (children age 0–18 years), and used the following limits: human studies, English language and time 1/2010 forward (box 1). We extended the search to the past 5 years to allow overlap with the most recent review of the topic which covered clinical trials from 2006 to 2010.12 We included, for full-text review, all RCTs that reported PsA patient outcomes.
Box 1. Search parameters and strategies of psoriatic arthritis literature review.
PubMed: ("Arthritis, Psoriatic"[Mesh] OR "Psoriatic arthritis" OR "psoriatic arthropathy" OR "arthritis psoriatica" OR "arthropathic psoriasis" OR "psoriasis arthropathica" OR "psoriatic arthropathy" OR "psoriatic polyarthritis" OR "psoriatic rheumatism") AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR "clinical trials as topic"[Mesh] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT ("Animals"[MeSH] NOT ("Animals"[MeSH] AND "Humans"[MeSH])) NOT ("Child"[Mesh] OR "Infant"[Mesh] OR "Infant, Newborn"[MH] OR "Adolescent"[Mesh] OR "Child, Preschool"[MH] OR "child"[all] OR "infant"[all] OR "adolescent"[all] OR "children"[all] OR "infants"[all] OR "adolescents"[all] OR "pediatric patient"[all] OR "pediatric patients"[all] OR "adolescence"[all] OR "youth"[all] OR "youths"[all] OR "juvenile"[all] OR "childhood"[all] OR "teenager"[all] OR "teenagers"[all] OR "teen"[all] OR "teens"[all] OR "preschool child"[all] OR "neonate"[all] OR "newborn"[all] OR "baby"[all]) AND English[lang] AND ("2010/01/01"[PDAT] : "3000/12/31"[PDAT])
Embase: (‘psoriatic arthritis'/exp OR "Psoriatic arthritis" OR "psoriatic arthropathy" OR "arthritis psoriatica" OR "arthropathic psoriasis" OR "psoriasis arthropathica" OR "psoriasis pustulosa arthropathica" OR "psoriatic arthropathy" OR "psoriatic polyarthritis" OR "psoriatic rheumatism") AND (random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEXT/1 blind* OR singl* NEXT/1 blind* OR assign* OR allocate* OR volunteer* OR 'crossover procedure'/exp OR 'double-blind procedure'/exp OR 'randomized controlled trials'/exp OR 'single-blind procedure'/exp) NOT ('animal'/exp NOT ('animal'/exp AND 'human'/exp)) NOT ('Child'/de OR ‘Infant'/de OR ‘Adolescent'/de OR 'preschool child'/exp OR 'child’:ti,ab OR ‘infant’:ti,ab OR ‘adolescent’ OR ‘children’ OR ‘infants’ OR ‘adolescents’ OR ‘pediatric patient’ OR ‘pediatric patients’ OR ‘adolescence’ OR ‘youth’ OR ‘youths’ OR ‘juvenile'/exp OR 'juvenile’ OR ‘childhood’ OR ‘teenager’ OR ‘teenagers’ OR ‘teen’ OR ‘teens’ OR ‘preschool child’ OR ‘neonate’ OR ‘newborn'/de OR 'newborn’:ti,ab OR ‘baby’ OR ‘babies’ OR ‘pediatric’:ti,ab OR ‘pediatrics’:ti,ab OR ‘paediatric’:ti,ab OR ‘paediatrics’:ti,ab OR ‘toddler'/exp OR 'toddler’ OR ‘toddlers’) AND [english]/lim AND [2010-2015]/py
We searched LOS, registries and patient-generated domain studies (eg, Psoriatis Arthritis Impact of Disease (PsAID)) by hand, after consultation with experts in the field and reference lists from review articles. Search terms were ‘psoriatic arthritis’ and ‘cohort’, ‘registry’, ‘observational study’, with a time limit from 1 January 2010 forward.
We used a standardised data collection form for all included studies and extracted the following data: population (adults with PsA), type of intervention and control, number of participants, age, sex, psoriasis/PsA duration, primary and secondary outcomes, and outcome measures (including composite indices). We illustrated the process as recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 Domains and corresponding outcome measures are organised in the order of the PsA core domain set, and also grouped domains according to the broad OMERACT Filter 2.0 core areas: pathophysiologic manifestations, life impact and resource use.17 18 We are also reviewing the European League Against Rheumatism–PsAID (EULAR–PsAID) outcome measure11 domains since it was recently developed (2014) with international PRP involvement. Beginning at the conception phase of this systematic literature review, we involved three PRPs, who provided input into the search strategy, data extraction form and interpretation of results.
Results
PsA RCTs reported 2010–2015
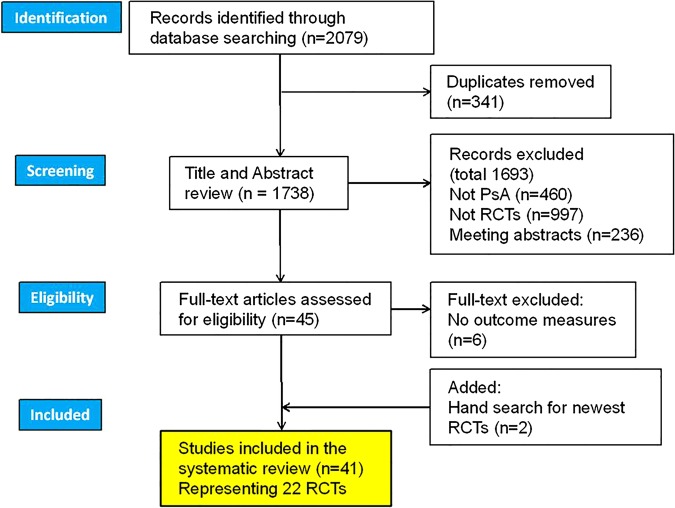
PubMed and EMBASE literature searches retrieved 1738 entries and, after exclusion of abstracts and off-topic items (figure 1), we included 41 full-text articles representing 22 distinct RCTs (see online supplementary references). The total number of PsA patients included within the studies reviewed was 5970, female 46.7%, mean (SD) age 47.8 (5.9) years, psoriasis duration 15 (2.6) years and PsA duration 6.5 (2.7) years. Eighteen (81.8%) trials used placebo as control group, two used standard therapy and two used active control arm. Interventions were: biological drugs (16), DMARDs (2), other drug (zolendronic acid) (1), tight control protocol (1), educational intervention (1) and mud-bath therapy (1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram, record identification, screening, eligibility and inclusion. PsA, psoriatic arthritis; RCT, randomised controlled trials.
rmdopen-2015-000217supp_references.pdf (124KB, pdf)
Composite indices were primary or secondary outcomes in a majority of PsA RCTs as follows: ACR responses (82%), DAS28 (73%), Psoriatic Arthritic Response Criteria (Psoriatic Arthritis Response Criteria; PsARC)/modified PsARC (no grading of joint tenderness) (36%), EULAR response (27%) and minimal disease activity (MDA) (18%). Other composite indices were calculated as secondary analyses of RCT data (see table 1 for complete list).
Table 1.
Composite indices assessed in PsA randomised controlled trials 2010–2015
| Patient reported domains |
Physician reported domains |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composite index | Number (%) RCTs assessing each index N=22 |
RCT Ref | Pain | PsA global | Joint | Skin | Physical function | Disability | Spine | Quality of life | General health | Joints | PsA global | Dactylitis | Enthesitis | Skin | ESR/CRP |
| ACR response | 18 (82) | (23–26, 29–34, 38–40, 43–47) | X | X | X | X | X | X | |||||||||
| DAS-28 | 16 (73) | (23, 24, 27, 28, 30, 31, 33, 36, 38, 40, 42–47) | X | X | X | ||||||||||||
| PsARC mPsARC |
8 (36) | (25, 32, 33, 38, 39, 45–47) | X | X | X | ||||||||||||
| EULAR response | 6 (27) | (27, 30, 33, 38, 46, 47) | X | X | X | ||||||||||||
| MDA | 4 (18) | (15, 30, 31, 34, 39) | X | X | X | X | X | X | |||||||||
| CDAI | 2 (9) | (40, 45) | X | X | X | ||||||||||||
| PASDAS | 2 (9) | (15, 31, 39) | X | X | X | X | X | X | X | ||||||||
| CPDAI | 2 (9) | (15, 31, 39) | X | X | XX | X | X | X | X | ||||||||
| DAPSA | 2 (9) | (15, 31) (11, 25) | X | X | X | X | X | ||||||||||
| AMDF | 2 (9) | (15, 31) (11, 25) | X | X | X | X | X | X | X | ||||||||
| GRACE | 1 (5) | (15, 31) | X | X | X | X | X | X | X | ||||||||
Patient Reported Domains: Joint and Skin is 100 mm visual analogue scale, Physical Function is Short Form-36 Physical Component Score, Disability is Health Assessment Questionnaire, Spine is Bath Ankylosing Spondylitis Disease Activity Index, Quality of Life is Psoriatic Arthritis Quality of Life Index in GRACE/AMDF; and Dermatology Life Quality Index and Ankylosing Spondylitis Quality of Life Index (both included) in CPDAI. Ref 15 is secondary data analysis of RCT ref 31 and ref 11 is secondary data analysis of RCT ref 25.
ACR, American College of Rheumatology; AMDF, Arithmetic Mean of the Desirability Function; CDAI, Clinical Disease Activity Index; CPDAI, Composite Psoriatic Disease Activity Index; CRP, C reactive protein; DAPSA, Disease Activity Index for Psoriatic Arthritis; DAS, Disease Activity Score; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; GRACE, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis Composite Exercise; MDA, Minimal Disease Activity; PASDAS, Psoriatic Arthritis Disease Activity Score; PsARC, Psoriatic Arthritis Response Criteria; RCTs, randomised controlled trials.
We collected outcome domains reported in PsA RCTs (22 domains in total) with corresponding outcome measures for each domain, and summarised these data in table 2. The 2006 PsA core domain set was reported in its entirety in 13 (59%) of 22 RCTs, which reported individual core domains as follows: pain 100%, peripheral joints 95%, physical function 91%, skin 86%, patient global assessment 82% and HRQL 77%. The majority of RCTs also reported domains not included in the current PsA core set: acute phase reactants 100%, physician global assessment 82%, enthesitis 59% and dactylitis 59%. Fatigue was reported in 35% of RCTs, productivity in 32%, radiographic outcomes in 27%, nail pathology in 23%, MRI/ultrasound outcomes in 20%, stiffness in 18%, depression/anxiety in 9%, and participation and coping in 5%. The number of outcome measures reported in PsA RCTs ranged from 1 to 9 with an average (SD) of 4.1 (2.4) outcome measures per domain. We observed the greatest heterogeneity in reporting for skin, with nine outcome measures, followed by enthesitis, fatigue, nail and productivity with seven measures each, and physician global and radiological outcomes with six measures each. Of note, tissue analysis, self-efficacy and sleep outcomes included in the ‘outer circle’ of the 2006 PsA Core Domain Set were not assessed in any RCT.
Table 2.
Domains and number of corresponding outcome measurement instruments in PsA randomised controlled trials, longitudinal observational studies and registries in 2010–2015
| Studies assessing each domain n (%) |
||||||
|---|---|---|---|---|---|---|
| 2006 PsA core domain set |
Domains | Outcome measures Number/domain |
Randomised controlled trials (N=22) | Longitudinal observational studies (N=27) | Registries (N=12) | Overlap with patient PsAID domains |
| Inner circle | Pain | 8 | 22 (100) | 18 (66.7) | 8 (66.7) | Yes |
| Peripheral joint activity | 14 | 21 (95.4) | 24 (88.9) | 10 (83.3) | No | |
| Physical function | 8 | 20 (90.9) | 21 (77.8) | 11 (91.7) | Yes | |
| Skin | 15 | 19 (86.4) | 19 (70.4) | 5 (41.7) | Yes | |
| Patient global | 9 | 18 (81.8) | 20 (74.1) | 10 (83.3) | No | |
| HRQL | 10 | 17 (77.3) | 14 (51.8) | 7 (58.3) | No | |
| Outer circle | ‘Acute phase reactants’ | 2 | 22 (100) | 20 (74.1) | 10 (83.3) | No |
| Physician global | 10 | 18 (81.8) | 13 (48.1) | 7 (58.3) | No | |
| Enthesitis | 10 | 13 (59.1) | 12 (44.4) | 3 (25.0) | No | |
| Dactylitis | 5 | 13 (59.1) | 12 (44.4) | 1 (8.3) | No | |
| Fatigue | 10 | 7 (35) | 12 (44.4) | 4 (33.3) | Yes | |
| Nail | 8 | 5 (22.7) | 8 (29.6) | 1 (8.3) | No | |
| Spine | 11 | 4 (20) | 9 (33.3) | 5 (41.7) | No | |
| ‘Radiology’ | 10 | 6 (27.3) | 6 (22.2) | 2 (16.7) | No | |
| Research agenda | ‘MRI/CT/US’ | 9 | 4 (20) | 4 (14.8) | 1 (8.3) | No |
| Participation | 3 | 1 (4.5) | 1 (3.7) | 0 | Yes | |
| ‘Tissue analysis’ | 0 | 0 | 0 | 0 | No | |
| Other domains not in the core set | Productivity | 15 | 7 (31.8) | 5 (18.5) | 4 (33.3) | Yes |
| Stiffness | 4 | 4 (18.2) | 4 (14.8) | 1 (8.3) | No | |
| Anxiety | 1 | 2 (9.1) | 1 (3.7) | 0 (0) | Yes | |
| Depression | 2 | 2 (9.1) | 2 (7.4) | 0 (0) | Yes | |
| Coping | 2 | 1 (4.5) | 0 | 0 | Yes | |
| Well-being | 1 | 1 (4.5) | 0 | 0 | No | |
| Self-efficacy | 1 | 0 | 1 (3.7) | 0 | No | |
| Sleep | 1 | 0 | 1 (3.7) | 0 | Yes | |
Domains are listed in decreasing order of frequency of their assessment in PsA randomised controlled trials. The original unabbreviated version of this table is listed as online supplementary table S2a and contains the complete list of all outcome measurement instruments used to assess each domain.
HRQL, health-related quality of life; PsA, psoriatic arthritis; PsAID, Psoriatis Arthritis Impact of Disease; US, ultrasound.
rmdopen-2015-000217supp_table.pdf (179.3KB, pdf)
PsA LOS and registries reported from 2010 to 2015
Searches and review of reference lists identified 27 LOS and 12 registries (see online supplementary references). PsA LOS reported a total of 23 domains (table 2). Nine of 27 (33.3%) LOS reported the complete 2006 PsA core domain set and individual domains as follows: pain 66.7%, peripheral joints 88.9%, physical function 77.8%, skin 70.4%, patient global assessment 74.1% and HRQL 51.8%. Acute phase reactants were reported in most LOS (74.1%). Physician global was reported in 48.1% and fatigue, enthesitis and dactylitis were each reported in 44.4%. One of the 12 (8.3%) registries reported the complete 2006 PsA core domain set. Fatigue (33.3%), skin (33.3%), enthesitis (25%) and dactylitis (8%) were reported at a lower frequency in registries than in LOS.
PsAID outcome measure domains
The EULAR-PsAID outcome measure11 developed in 2014 is unique among PsA outcome measures since its domains are entirely patient-generated. It contains domains prioritised by PRPs from 13 European countries after they were presented with results of a literature review of outcomes and measures assessed in PsA studies.12 At the end of the meeting, patients decided on 16 domains, which were further reduced through ranking by a larger number of patients (n=139 from 13 European countries) to 12 domains for longitudinal clinical care and 9 domains for use in RCTs. Selection of domains for the final PsAID questionnaire was in median order of importance to patients, from 1, most important, to 16, least important. The PsAID uses a weighted score; weights for each domain were based on patient preferences. There are three overlapping PsAID and 2006 PsA Core Set domains: pain, physical function and skin.
Domains and corresponding outcome measures assessed in PsA studies from 2010 to 2015
We identified 24 domains assessed across all PsA studies (table 2). These are listed in order of decreasing frequency of assessment in RCTs, and organised as follows: (1) 2006 PsA core domain set: pain, joints, physical function, skin, patient global assessment and HRQL, (2) 2006 PsA outer circle domains: acute phase reactants, physician global, enthesitis, dactylitis, fatigue, nail, spine and radiology, (3) 2006 PsA research agenda: MRI/CT/ultrasound, participation and tissue analysis; and (4) other domains assessed but outside of the 2006 domains: productivity, stiffness, anxiety, depression, coping, well-being, self-efficacy and sleep. At the extremes of the spectrum, pain and acute phase reactants were assessed in all RCTs, and tissue analysis was assessed in none.
At least 1 outcome measure was found for each domain with a mean (SD) number of outcome measures per domain of 7 (4.7) and range 1–15. Across all studies, there was great heterogeneity for outcome measure selection and reporting as follows (domain and corresponding number of outcome measures identified in order of listing in table 2): pain 8, joints 14, physical function 8, skin 15 (include 8 physician and 7 patient reported measures), patient global assessment 9, HRQL 10, acute phase reactants 2, physician global 10, enthesitis 10, dactylitis 5, fatigue 10, nail 8, spine 11 (symptoms and mobility), radiology 10 (peripheral joints, spine and hip), MRI 2 (1 MRI score, imaging of peripheral joints, large joints, pelvis and spine)/CT none/ultrasound 7 (2 synovitis scores, 3 enthesitis scores, ultrasound examination for enthesitis, dactylitis and joints), participation 3, tissue analysis (not assessed), work productivity 15, stiffness 4, anxiety 1, depression 2, coping 2, well-being 1, self-efficacy 1 and sleep 1. For most domains, there was a preferred outcome measure that was assessed/reported in a majority (>50%) of studies except for: joint count, physician global, fatigue, nail and productivity. Specifically for productivity, each of the seven RCTs assessing this domain used a different productivity outcome measure.
We then sorted all domains measured in RCTs into OMERACT-recommended core areas17 18 (table 3), including pathophysiologic manifestations, life impact and resource use. Pathophysiology was well measured in all RCTs with pain and acute phase reactants universally measured, however, disease-specific manifestations were measured in only 55% or less. Most common domains within life impact were physical function (91%), patient global (82%) and quality of life (77%), whereas fatigue and productivity were 32–35%, with participation, mood, coping and well-being not frequently measured (5–10%). Productivity was the only domain within the area of resource use, but this was only measured in 32% of the RCTs.
Table 3.
Domains assessed in PsA RCTs by OMERACT Recommended Core Areas
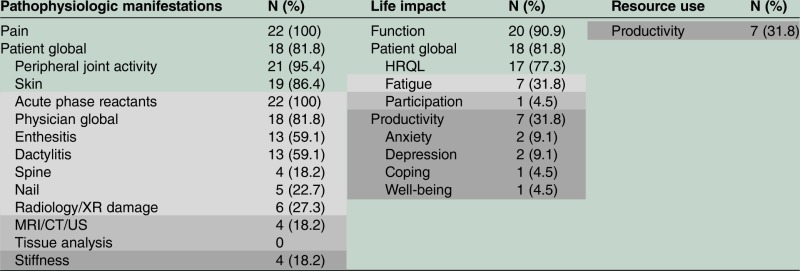 |
2006 PsA Core Domain Set: white background; Outer circle: light grey; Research agenda: dark grey; Domains assessed in RCTs not in the PsA core set: darkest grey; Domains in bold belong to more than one core area. HRQL health-related quality of life; PsA, psoriatic arthritis; OMERACT, Outcome Measures in Rheumatology; RCT, randomised controlled trials; US, ultrasound.
Discussion
From 2010 to 2015, a majority of PsA RCTs (59%) included measures that addressed the complete 2006 PsA core domain set. This is an increase compared to 23.5% of RCTs assessing the PsA core set in the prior assessment period from 2006 to 2010.12 The increased inclusion of the PsA core domain set in RCTs can be explained at least in part by its availability in published form since 2007, and supports the idea that such a core set is needed, and that it is widely accepted and used.
Of importance, many other domains (enthesitis, dactylitis, spine and nails) specific to PsA are now being assessed to supplement the 2006 PsA core set in a considerable proportion of PsA RCTs, but the selection of additional domains is heterogeneous across trials. Furthermore, the strategy of domain supplementation of the core domain set in PsA RCTs led to demonstrated PsA-specific effects of new therapeutic agents and US FDA approval of new medications for PsA as primary indication in the past 2 years, while prior medications in PsA were initially approved for RA. Enthesitis, dactylitis, spine and nail involvement, in addition to skin, are PsA-specific pathophysiological manifestations that distinguish it from other forms of inflammatory arthritis and should be included in regular PsA assessments to facilitate better understanding of disease risk factors, and prognosis and development of targeted treatments.19 However, disease specificity of the PsA core set would have to be reconciled with feasibility/simplicity. LOS and registries lag behind RCTs in terms of reporting the complete PsA core set (33.3% and 8.3% vs 59%). This may be due to lack of reporting of all data collected, the primary focus on peripheral arthritis rather than the other disease manifestations, collecting of PsA data as add-on in RA cohorts, or the difficulty of collecting large amounts of data in clinical practice. Another difference is the adoption of simpler outcome measures in longitudinal studies compared to RCTs. For example, composite indices, although developed and tested in PsA, include proprietary outcome measures (eg, SF-36, PsAQoL) and are not feasible to assess in regular clinical care.
An aspect that could be improved in the updated PsA Core Domain Set is clarity. Some items currently listed as core set domains actually represent methods of measurement (examples are Radiology, CT/MRI/US, acute phase reactants and tissue analysis). Domains are measurable disease concepts. Methods of measurement are techniques that may be applied to developing outcome measurement instruments for each domain. Corresponding domains for the above techniques may be PsA radiographic damage (vs Radiology), synovial/entheseal/digital/spinal tissue inflammation/activity (vs MRI/CT/US techniques), or PsA-specific biomarkers (vs acute phase reactants and tissue analysis). Precise definition of PsA Core Domains is necessary so that outcome measurement instrument selection and development can proceed.
We identified domains corresponding to all core areas recommended by Filter 2.0.17 18 The area of life impact is currently least represented in all studies. Physical function is the most highly assessed domain in the area of life impact (91% of RCTs), however, physical function represents only a fraction of how patients conceptualise the impact of PsA on their lives.11 20 21 In the PsAID study,11 patients from 13 European countries decided on and ranked PsA domains in order of decreasing importance as follows: pain; skin; fatigue; ability to work/leisure; functional capacity; feeling of discomfort; sleep disturbance; anxiety, fear and uncertainty; coping; embarrassment and/or shame due to appearance; social participation; and depression. This is important information regarding PsA patient priorities and three of the PsAID domains (pain, skin and physical function) are present in the 2006 PsA core set. In the case of skin assessments, measures used in PsA RCTs are almost exclusively physician measures (>88%) while PRPs prioritised the symptom aspect of the skin domain. Skin symptoms can only be captured with patient reported measures. Fatigue and ability to work, although ranked as third and fourth priority by PRPs, are missing from the current core set. The finding that patient treatment goals go beyond pathophysiological improvement and encompass being able to participate in specific life activities was replicated in qualitative studies in other chronic diseases.22–24
Heterogeneity of outcome measures per domain emphasises the need for uniform standards that can be adopted for outcome measure selection. For productivity, none of the seven RCTs assessed the same measure, which is problematic for comparison of results. Similarly for enthesitis, 13 RCTs used 7 enthesitis outcome measures, which is again problematic for comparative effectiveness analyses. Another consideration is heterogeneity in collecting and reporting the same measure: visual analogue scales for global assessment (patient and provider) or pain, fatigue, stiffness where it is uncertain if the question stem, anchors and recollection period are all the same across studies. Yet another consideration is representativeness or content validity of the outcome measure for the domain to be measured. For example, skin assessment in the PsAID is patient reported, whereas RCTs predominantly use physician assessments of psoriasis. Depending on how HRQL is assessed, fatigue, depression, anxiety and social functioning may be included in some outcome measures, and it should be noted that HRQL subdomain recommendations are not specified in the PsA core set. These findings reflect the need to precisely define each outcome to be measured in PsA so that a decision can be made on whether it is best measured using a patient or a physician reported measure, or if there is added value to use both. The evidence for each outcome measure in adequately capturing the construct is also needed before implementation, and this is the object of a continuation of this work.
Limitations of our review consist of a limited review period of 5 years, however, this is an update of a previous review.12 Searches for LOS are not exhaustive since there is no validated filter, and we were as inclusive as possible when screening for these publications. Additionally, we did not specifically contact the authors of the LOS and registries to inquire about the data collected as more data may have been collected than reported in the representative papers identified. Synthesising information on outcome measurement instrument properties and validation evidence in PsA is beyond the scope of this review and will be addressed in future work.
In conclusion, an updated PsA core set is much needed to increase its validity for PsA, to clarify domain definitions, to include patient priorities and to add disease-specific manifestations. Precise definition of constructs to be measured as PsA domains is a critical first step. This will facilitate the development of a core outcome measurement set that can be standardised for use in PsA research and clinical care. The consistent use of a standardised outcome measurement instrument set for PsA will then allow comparisons of the effects of various interventions, and improve the quality of the information available to patients and their doctors for making informed treatment decisions.
Footnotes
Acknowledgements: The authors acknowledge Dr Laure Gossec for kindly reviewing the manuscript and providing feedback.
Contributors: A-MO, UK, AO, WC, COB and IS were involved in the study design. UK, A-MO and VGR were involved in the data collection and analysis. A-MO, UK, AO, WC, COB, MdW, DDG, PM, IS, VS and VGR were involved in the data interpretation. UK and A-MO were involved in the writing the first version of the manuscript. UK, AO, WC, COB, MdW, DDG, PM, IS, VS, VGR and A-MO were involved in the revision for critical intellectual content and final approval of manuscript.
Funding: UK was supported by an exchange research fellowship grant to Hacettepe University from the Rheumatology Society of Turkey and the Association for Monitoring Rheumatic Diseases. A-MO was supported in part by a Scientist Development Award from the Rheumatology Research Foundation. AO was supported by research grant K23 AR063764 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. This work was supported in part by the Johns Hopkins Arthritis Center Discovery Fund.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data used in this publication are articles available in the public domain in the following two databases: Medline via PubMed and EMBASE. We provided a complete list of our references in the manuscript and online supplementary appendix. For additional data from our data extraction sheets, address questions to the corresponding author.
References
- 1.Kane D, Stafford L, Bresnihan B et al. . A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8. 10.1093/rheumatology/keg384 [DOI] [PubMed] [Google Scholar]
- 2.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun 2010;34:J314–21. 10.1016/j.jaut.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum 2009;61:1373–8. 10.1002/art.24608 [DOI] [PubMed] [Google Scholar]
- 4.Ogdie A, Langan S, Love T et al. . Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford) 2013;52:568–75. 10.1093/rheumatology/kes324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachariae H, Zachariae R, Blomqvist K et al. . Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108–13. 10.1080/00015550252948130 [DOI] [PubMed] [Google Scholar]
- 6.Taylor WJ, Mease PJ, Adebajo A et al. . Effect of psoriatic arthritis according to the affected categories of the international classification of functioning, disability and health. J Rheumatol 2010;37:1885–91. 10.3899/jrheum.091315 [DOI] [PubMed] [Google Scholar]
- 7.Rosen CF, Mussani F, Chandran V et al. . Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford) 2012;51:571–6. 10.1093/rheumatology/ker365 [DOI] [PubMed] [Google Scholar]
- 8.Gladman DD, Mease PJ, Strand V et al. . Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 2007;34:1167–70. [PubMed] [Google Scholar]
- 9.Tillett W, Adebajo A, Brooke M et al. . Patient involvement in outcome measures for psoriatic arthritis. Curr Rheumatol Rep 2014;16:418 10.1007/s11926-014-0418-7 [DOI] [PubMed] [Google Scholar]
- 10.Tillett W, Eder L, Goel N et al. . Enhanced patient involvement and the need to revise the core set—report from the psoriatic arthritis working group at OMERACT 2014. J Rheumatol 2015;42:2198–203. 10.3899/jrheum.141156 [DOI] [PubMed] [Google Scholar]
- 11.Gossec L, de Wit M, Kiltz U et al. . A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–19. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 12.Palominos PE, Gaujoux-Viala C, Fautrel B et al. . Clinical outcomes in psoriatic arthritis: a systematic literature review. Arthritis Care Res (Hoboken) 2012;64:397–406. 10.1002/acr.21552 [DOI] [PubMed] [Google Scholar]
- 13.de Wit M, Campbell W, FitzGerald O et al. . Patient participation in psoriasis and psoriatic arthritis outcome research: a report from the GRAPPA 2013 Annual Meeting. J Rheumatol 2014;41:1206–11. 10.3899/jrheum.140171 [DOI] [PubMed] [Google Scholar]
- 14.Glanville JM, Lefebvre C, Miles JN et al. . How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc 2006;94:130–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre C, Eisinga A, McDonald S et al. . Enhancing access to reports of randomized trials published world-wide—the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerg Themes Epidemiol 2008;5:13 10.1186/1742-7622-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Kirwan JR, Boers M, Tugwell P. Updating the OMERACT filter at OMERACT 11. J Rheumatol 2014;41:975–7. 10.3899/jrheum.131306 [DOI] [PubMed] [Google Scholar]
- 18.Boers M, Kirwan J, Tugwell P et al. . The OMERACT Handbook. OMERACT, 2014. [Google Scholar]
- 19.Gladman DD, Chandran V. Review of clinical registries of psoriatic arthritis: lessons learned?: value for the future? Curr Rheumatol Rep 2011;13:346–52. 10.1007/s11926-011-0182-x [DOI] [PubMed] [Google Scholar]
- 20.Moverley AR, Vinall-Collier KA, Helliwell PS. It's not just the joints, it's the whole thing: qualitative analysis of patients’ experience of flare in psoriatic arthritis. Rheumatology (Oxford) 2015;54:1448–53. 10.1093/rheumatology/kev009 [DOI] [PubMed] [Google Scholar]
- 21.Stamm TA, Nell V, Mathis M et al. . Concepts important to patients with psoriatic arthritis are not adequately covered by standard measures of functioning. Arthritis Rheum 2007;57:487–94. 10.1002/art.22605 [DOI] [PubMed] [Google Scholar]
- 22.Coylewright M, Palmer R, O'Neill ES et al. . Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect 2015. 10.1111/hex.12393. [Epub ahead of print 14 Aug 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer JP, Chen J, Katz PP et al. . Defining novel health-related quality of life domains in lung transplantation: a qualitative analysis. Qual Life Res 2015;24:1521–33. 10.1007/s11136-014-0875-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Tuyl LH, Hewlett S, Sadlonova M et al. . The patient perspective on remission in rheumatoid arthritis: ‘You've got limits, but you're back to being you again’. Ann Rheum Dis 2015;74:1004–10. 10.1136/annrheumdis-2013-204798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2015-000217supp_references.pdf (124KB, pdf)
rmdopen-2015-000217supp_table.pdf (179.3KB, pdf)