Abstract
BACKGROUND
The perinucleolar compartment (PNC) is a subnuclear structure localized at the nucleolar periphery. Previous studies using breast cancer as a model system demonstrated that PNC prevalence (the percentage of cells with 1 or more PNC) increased with disease progression and was associated with poor patient outcomes.
METHODS
To evaluate the validity of developing PNC prevalence as a novel pan-cancer prognostic marker, the authors investigated whether PNC prevalence was correlated with malignancy in a spectrum of tissue types and evaluated its selective association with malignancy under various experimental conditions.
RESULTS
PNC prevalence was low in primary and immortalized cells and in cell lines derived from hematologic malignancies, but it was heterogeneous in cell lines derived from solid tumors, including those of epithelial and nonepithelial origins. Studies using human myometrial tissue and thyroid cancer cell lines with various levels of malignancy demonstrated a correlation between high PNC prevalence and malignant potential. Furthermore, PNC prevalence corresponded directly to metastatic capacities in a series of well characterized cell lines of the same origin that were selected for various levels of metastatic capacity in a mouse model. Conversely, PNC prevalence was reduced experimentally by over expressing an antimetastatic protein in breast cancer cells. However, PNC prevalence was not associated with traits that were shared by both cancer and normal cells, including proliferation, glycolysis, and differentiation.
CONCLUSIONS
Together, these observations helped to verify that PNC prevalence selectively represents malignancy in a broad spectrum of solid tissue tumors, demonstrating its potential to be developed as a pancancer prognostic marker of malignancy.
Keywords: perinucleolar compartment, metastasis, phenotypic marker, pancancer marker
Much effort has been made to increase the survival rate of cancer patients through the development of early disease detection and the improvement of prognoses to guide appropriate therapeutic interventions. Although these strategies have improved survival rates significantly in cancer patients, the current prognostic and predictive markers used to determine treatment strategy remain insufficiently accurate in forecasting the outcome of individual patients. Because cancers are highly heterogeneous diseases, there often are exceptions to the current prognostic criteria, leading to the under treatment or over treatment of patients.1,2
Oncogenesis and cancer progression are complex processes involving a combination of many genetic and epigenetic insults. Cells undergo extensive biochemical and structural alterations during the process of malignant transformation because of these insults. Recent advances in cellular and molecular techniques have fostered a better understanding of the specific genetic and epigenetic changes that take place during cancer development and progression. From these changes, a large number of molecular tumor markers have been identified; however, only a few them have been shown to be reproducible and clinically relevant.3,4 In addition, most molecular markers are developed for specific types of tumors, thus allowing only a small portion of cancer patients to benefit from each type of marker. Phenotypic markers, such as histologic grading and staging, have been very useful tools for indicating the progression of cancer across a broad range of tumor types; however, these markers have limited prognostic capabilities, because there are often exceptions to the generally accepted trends of disease progression and inconsistent scoring of samples.5,6 Therefore, an easily detectable phenotypic marker that can be scored accurately and precisely in a broad range of tumor types would be ideal to increase the accuracy of prognosis for many cancer patients. In the current study, we describe the potential for developing the perinucleolar compartment (PNC) into such a prognostic marker.
The PNC is a nuclear substructure that is associated with, but structurally distinct from, the nucleolus (Fig. 1A).7 The PNC is a generally heritable trait, and the structure is stable through interphase, disassembles during mitosis, and reassembles in early G1 phase.7 The PNC is concentrated with newly synthesized RNA polymerase III transcripts and RNA binding proteins,8–14 implicating the involvement of PNCs in RNA metabolism.15 However, the complete molecular composition and function of the PNC remain to be elucidated. Initial examination of the PNC in 13 human cell lines demonstrated that the PNC prevalence (the percentage of nonapoptotic and nonmitotic cells with 1 or more PNC) was invariably low in normal cell lines but was higher, yet hetrogenous, among cancerous and transformed cell lines,7 suggesting that the PNC forms preferentially in cancer cells. Investigations using human breast tissue samples demonstrated that PNC prevalence is 0% in normal breast epithelium, increases in parallel with disease progression (as determined by staging), and reaches nearly 100% in distant metastases, demonstrating that the PNC prevalence is associated with the malignancy of breast cancer. Multivariate analysis also demonstrated that PNC prevalence contains additional prognostic information over grading and tumor size for patients with stage I disease.16
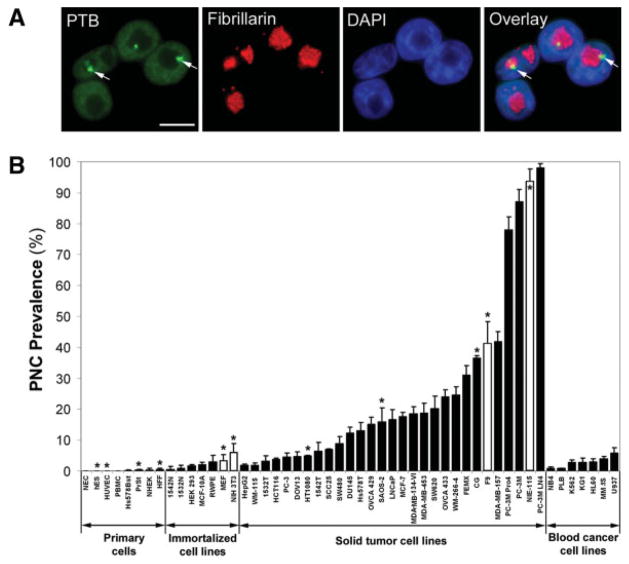
FIGURE 1.
Survey of perinucleolar compartment (PNC) prevalence (the percentage of cells with 1 or more PNC) in primary cells and cell lines. (A) Immunofluorescent staining of HeLa cells with a polypyrimide tract binding protein (PTB) antibody was used to mark the nucleoplasm and PNCs (exemplary PNCs are marked with arrows), a fibrillarin antibody was used to mark the nucleolus, and 4,6-diamidino-2-phenylindole (DAPI) was used to mark the nucleoplasm. Scale bar = 10 μm. (B) The PNC prevalence is illustrated in several primary cell types and in normal, immortalized, and cancerous cell lines. White bars indicate murine cell lines; black bars, human cells; asterisks, nonepithelial cell lines; error bars, standard deviation (n = 3 experiments). All cell line definitions are provided in the text.
Although PNC prevalence has potential to be developed clinically as a prognostic marker for breast cancer,16 it is not clear whether the association of PNC prevalence with malignancy can be extended to tumors other than those originating in the breast. An association with malignancy in a broad range of tissue types would indicate that PNC prevalence has the potential to be a pancancer biomarker. To explore this possibility and to determine the specificity of the association between PNC and malignant behavior, we examined a large number of cells lines, primary cells, and human tumor samples derived from a broad spectrum of tissues for PNC prevalence and evaluated the selective association of PNC prevalence with metastatic capacity under various experimental conditions.
MATERIALS AND METHODS
Cell Culture
All cell lines were maintained at 37 °C in a 5% carbon dioxide atmosphere, and all media were supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, Ga), 100 U/mL of penicillin, and 100 mg/mL streptomycin (Invitrogen, Carlsbad Calif) unless noted otherwise. Cells were grown in 100-mm dishes for time-dependent growth assays and were counted with hemacytometer and Trypan blue (Sigma Chemical Company, St. Louis, Mo). NIE-115 cells17 and F9 cells18 were treated as described previously for 5 days to induce differentiation. All reagents for differentiation were obtained from Sigma.
The following cells were grown in modified Dulbecco minimal essential media (DMEM) (Invitrogen): HeLa (human cervical carcinoma), HEK 293 (human embryonic kidney), MEF (mouse embryonic fibroblasts), NIH 3T3 (mouse fibroblasts), SW480 (human colorectal adenocarcinoma), Hs 578T (human breast carcinoma), SAOS-2 (human osteosarcoma), MDA-MB-453 (human breast carcinoma metastasis), MDA-MB-134-VI (human breast carcinoma), SW620 (human colorectal adenocarcinoma metastasis), WM-244-6, FEMX (human melanomas), CG (human neuroblastoma), F9 (mouse testicular teratoma), MDA-MB-157 (human breast carcinoma), NIE-115 (mouse neuroblastoma), K562 (human chronic myelogenous leukemia), KG1 (human acute myelogenous leukemia), HL60 (human acute promyelocytic leukemia), U937 (human histiocytic lymphoma) and mES murine embryonic stem cells (kindly provided by Dr. York Marahrens of the University of Minnesota). mES media was supplemented with 15% FBS, 0.1 mM nonessential amino acids (NEAAs), 0.1 mM β-mercaptoethanol, 1 mM glutamine, and 500 U/mL of leukemia inhibitory factor. Glucose- and pyruvate-free DMEM was obtained from Invitrogen.
The following cells were grown in RPMI 1640 media (Invitrogen): freshly isolated human peripheral blood mononuclear cells; human primary prostate stroma cells (PrSt) (kindly provided by Dr. Chung Lee of Northwestern Medical School); RWPE (normal human prostate epithelial cells; also from Dr. Lee); PC-3, DU145, LNCaP, PC-3M Pro4, PC-3M, and PC-3M LN4 (human prostate carcinomas); MCF-7 (human breast carcinoma); NB4 (human promyelocytic leukemia); PLB 985 (undifferentiated human myeloid cells); TPC, NPA, and BHP (human papillary thyroid carcinomas); WRO (human malignant follicular thyroid carcinoma); FRO and ARO (human malignant anaplastic carcinomas; thyroid cell lines kindly provided by Dr. Peter Kopp of Northwestern University Medical School); and MM.1S (human multiple myeloma; kindly provided by Dr. Steve Rosen of Northwestern Medical School).
The following cells were grown in minimal essential media (Invitrogen): HepG2 (human hepatocellular carcinoma), WM-115 (human melanoma; grown at 35°C), HT1080 (human fibrosarcoma), and OVCA 429 and OVCA 433 (human ovarian cancers). WM-115, HT1080, OVCA 429, and OVCA 433 were supplemented with 0.1 mM NEAAs (Invitrogen). Human foreskin fibroblasts (HFFs) were supplemented with 0.1 mM NEAAs, 15% FBS, and 1 × vitamin solution (Invitrogen).
The following cells were grown in keratinocyte serum-free media (Keratinocyte SFM; Invitrogen) along with the added supplements provided by the manufacturer: 1532N, 1542N (human normal prostate epithelial cells), 1532T, 1542T (human prostate carcinoma), and NHEK (normal human epidermal keratinocytes).
SCC25 (human head and neck squamous cell carcinoma); DOV13 (human ovarian carcinoma);MDA-MB-231, MDA-MB-231 BRMS, MDA-MB-435, and MDA-MB-435 BRMS (breast carcinomas; stable BRMS-expressing cell lines were grown in 500 μg/mL G-418 [Invtirogen] and kindly provided by Dr. Daniel Welch of the University of Alabama); and MCF-10A cells were grown in a 1:1 mixture of DMEM and Ham-F12 media (Invitrogen). MCF-10A cells were supplemented with 10 μg/mL insulin (Sigma), 100 ng/mL cholera toxin (Calbiochem, San Diego, Calif), 500 ng/mL hydrocortisone (Collaborative Research, Bedford, Mass), 20 ng/mL epidermal growth factor (EGF) (Collaborative Research), and 5% heat-inactivated equine serum (Invitrogen).
Human primary umbilical vein endothelial cells (HUVEC) were grown in endothelial growth media 2 (EGM-2; Lonza, Walersville, Md) supplemented with the Bullet Kit components from the manufacturer. Hs 578T cells were grown in ATCC Hybricare media (American Type Culture Collection, Manassas, Va) supplemented with 30 ng/mL of EGF and 1.5 g/L of bicarbonate. HCT116 (human colorectal carcinoma) cells were grown in McCoy 5A media (Sigma).
Human embryonic stem cells (hES) (National Institutes of Health [NIH] code WA07) were cultured in media according to WiCell (Madison, Wis) recommendations, except that Knockout DMEM (Invitrogen) was used as a base media instead of DMEM-F12 media.
Human Uterine Sample Obtainment
Leiomyosarcoma, leiomyoma, myometrial, and normal endometrial cells were obtained from premenopausal women who were not receiving any hormone treatments at the time of hysterectomy. Permission to use these human specimens was approved by the Northwestern University Institutional Review Board. The cells were isolated from the uterus immediately after the hysterectomy. Briefly, the tissue was minced into approximately 2-mm2 pieces in calcium- and magnesium-free Hank balanced salt solution. The minced tissue was placed in an enzyme solution that contained 0.5% collagenase and 0.02% DNase and was incubated for at least 2 hours at 37 °C in a shaking water bath. The supernatant fluid was recovered and centrifuged at ×1500g for 5 minutes. The pellet was resuspended in 1:1 DMEM-F12 supplemented with 0.1 mM sodium pyruvate, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% FBS. Cells were seeded at 1 × 105 cells/cm2 in 100-mm tissue culture plates and incubated overnight at 37 °C in 5% carbon dioxide. The medium was changed the next day and subsequently every 3 days. At confluence, cells were trypsinized (trypsin/ethylenediamine tetracetic acid; Invitrogen) and subcultured onto glass coverslips. After attaching to the coverslips, the cells were fixed in 4% (weight/volume) paraformaldehyde for 10 minutes and stored in phosphate-buffered saline (PBS) at 4 °C until they were stained.
Immunofluorescent Staining
To stain for PNC, cell lines were plated in 35-mm wells that contained sterile glass coverslips to give a confluency of 20% to 40% after 24 hours. Then, the cells were fixed with 4% (weight/volume) paraformaldehyde in PBS for 12 minutes (nonadherent cells were fixed while being spun at ×500g in a swing bucket centrifuge to attached the cells to the cover-slip), washed with PBS, solubilized in 0.5% (volume/volume) Triton X-100 (Sigma) in PBS for 5 minutes, washed, incubated in mouse antihuman polypyrimide tract binding protein (PTB) antibody (SH54)7 at a dilution of 1:200 or in rabbit antimouse PTB antibody at 1:500 (kind gift from Dr. Douglas Black of the University of California-Los Angeles) for 1 hour, washed, and finally incubated in antimouse or anti-rabbit fluorescein isothiocyanate-labeled secondary antibodies at 1:200 (Jackson ImmunoResearch, West Grove, Pa). Uterine samples were stained similarly but were fixed before staining and stored at 4 °C in PBS. In Figure 1A, the fibrillarin antibody (Santa Cruz Biotechnology, Santa Cruz, Calif) was used at 1:100 and detected with goat antirabbit Texas Red-labeled secondary antibody (Jackson ImmunoResearch). For each coverslip, >200 cells were scored to determine PNC prevalence (n = 3). For scoring, a PNC was defined as an easily visible (with a ×60 objective for solid tissue cells and a ×100 objective for hematologic cells) perinucleolar spot with an intensity of PTB labeling approximately 2-fold or more intense than the nucleoplasm. The PNC size in the PC-3 series of cell lines was measured in >100 cells by microscopic digital photography (SenSys cooled coupled device camera; Photometrics, Tucson, Ariz) and Metamorph software (Universal Imaging Corp., West Chester, Pa). All statistical analyses were performed using a 2-tailed Student t test with unequal variances.
RESULTS
The PNC Is Associated With Solid Tumor Cells Derived From a Broad Range of Tissues
To determine whether the PNC is associated with cancer cells of specific tissue types, we examined PNC prevalence in many primary cells and cell lines from different tissue origins by immunofluorescent staining for PTB, an RNA binding protein that is highly enriched in the PNC (Fig. 1A). In primary cells, the PNC prevalence is usually 0% but is consistently below 0.5%, demonstrating that the PNC does not usually form in normal cells. The PNC prevalence in immortalized cells is between 0% and 6%. The increased PNC prevalence in some immortal cell lines is most likely a consequence of these lines being transformed from long-term cell culture. PNC prevalence in solid tumor cells is heterogeneous but usually is higher than in normal cells. PNC prevalence can be high not only in cell lines derived from carcinomas but also in cell lines derived from blastomas (eg, CG) and sarcomas (eg, SAOS-2) (Fig. 1B), demonstrating that PNCs can form in cancer cells from nonepithelial origins, such as stroma and undifferentiated cells. In addition to human cells, PNCs also were observed in murine cell lines derived from solid tumors, such as NIE-115 (neuroblastoma) and F9 (testicular teratoma) (Fig. 1B). It is noteworthy that PNC prevalence in all cell lines derived from hematologic malignancies is below 6%, even in highly malignant lines such as MM.1S, which is an aggressive and fatal multiple myeloma isolated at the leukemic stage, suggesting that PNC prevalence is high only in solid tumor cells (Fig. 1B). Together, these findings demonstrate that the PNC preferentially forms in cancer cells derived from solid tissues and is present in a wide range of solid tumor types.
PNC Prevalence Is Associated With Malignancy in Myometrial and Thyroid Cells
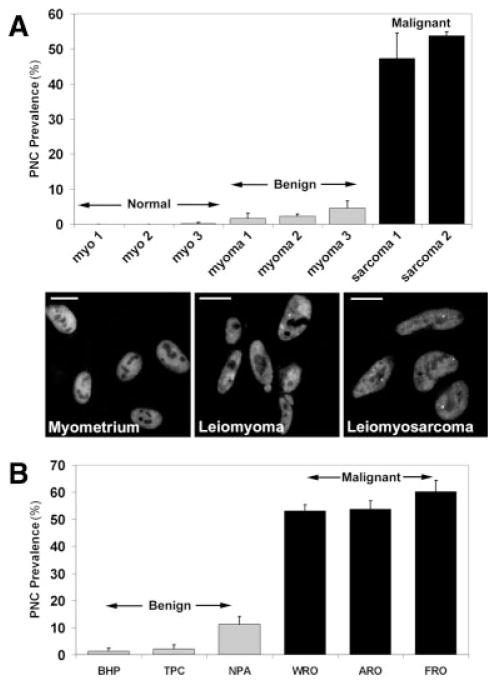
PNC prevalence in human breast cancer increases in parallel with disease progression and provides independent prognostic value.16 The heterogeneity of PNC prevalences in solid tumor cell lines observed in Figure 1B suggests that PNC prevalence may indicate the level of malignancy for several types of solid tumors other than breast carcinoma. To determine whether PNC prevalence may be developed as a pan-cancer prognostic marker for other solid tumors, we examined the correlation of PNC prevalence with malignancy in cells from various tissue origins. First, we examined normal, hyperplastic, and malignant uterine smooth muscle (myometrium) tissue samples. Myometrial fibroids (leiomyomas) are benign hyperplastic growths, whereas myometrial malignancies (leiomyosarcomas) are aggressive tumors and have a poor prognosis. Freshly excised tissues were trypsinized to release cells, which were then seeded into culture dishes for growth. Next, the cells were immunolabeled with the α-PTB antibody (SH54) for PNC detection. PNC prevalence in normal myometrial cells is at or near 0% in all samples, is <5% in the benign leiomyoma cells, and is significantly higher (>47%; P < .05) in leiomyosarcoma cells (Fig. 2A), demonstrating that PNC prevalence increases with malignancy in solid tumors, including those of nonepithelial origin.
FIGURE 2.
Perinucleolar compartment (PNC) prevalence (the percentage of cells with 1 or more PNC) was correlated with malignancy. (A) Top: PNC prevalence (n = 3 experiments; error bars indicate the standard deviation [SD]) is illustrated in normal myometrium (myo), benign leiomyomas (myoma), and malignant leiomyosarcoma (sarcoma) patient samples and in a leiomyosarcoma cell line (SK-UT-1; sarcoma 2). Bottom: Immunofluorescent images of patient samples (myometrium 1, leiomyoma 1, and leiomyosarcoma 1) were stained with a polypyrimide tract binding protein (PTB) antibody to show the PNCs. Scale bars = 10 μm. (B) PNC prevalence (n = 3 experiments; error bars indicate SD) is illustrated in thyroid cancer cell lines from relatively benign papillary carcinomas (BHP, TPC, and NPA), from the more malignant follicular (WRO) and anaplastic carcinomas (FRO and ARO).
Conclusions
In addition, we examined PNC prevalence in cell lines derived form thyroid tumors of various malignant capacities. Thyroid papillary carcinomas are relatively benign with generally good prognoses, whereas the follicular and anaplastic carcinomas are more malignant with worse prognoses.19 The results indicate that PNC prevalence is significantly lower (P<.01) in all of the cell lines derived from the papillary carcinoma (BHP, TPC, and NPA; <12%) than in all of the malignant cell lines (WRO, ARO, and FRO; >53%) (Fig. 2B). It is often difficult to distinguish the malignant thyroid adenocarcinomas clinically from the benign adenomas20; therefore PNC prevalence has potential to be developed as a prognostic marker for thyroid cancers. These findings further demonstrate that PNC prevalence reflects the degree of malignancy in solid tumors independent of tissue origin.
PNC Prevalence Indicates Metastatic Capacity
The ultimate malignant behavior of solid tumor cells is the ability to form distant metastatic lesions. Our previous study, in which PNC prevalence was found to be correlated with malignancy of breast cancer,16 and data from the myometrial and thyroid cancers (Fig. 2) together suggest that the PNC prevalence represents metastatic behavior. To further address this hypothesis in experimental systems, we examined the PNC prevalence of 2 pairs of cell lines, each consisting of a line created from primary tumor cells and another line from a metastatic lesion of the primary tumor in the same patient. WM-115 (PNC prevalence, 1.9%) is from a primary melanoma, and WM-266-4 (PNC prevalence, 24.8%) is from a distant metastasis of the primary tumor. SW480 (PNC prevalence, 8.9%) is derived from a primary colorectal tumor, and SW620 (PNC prevalence, 20.2%) is derived from a lymph node metastasis of the same tumor. In both cell line pairs, the PNC prevalence is significantly (P < .021) higher in the metastatic cell line (Fig. 1B), indicating that the PNC prevalence increases in association with metastatic behavior in tissues other than breast epithelium.
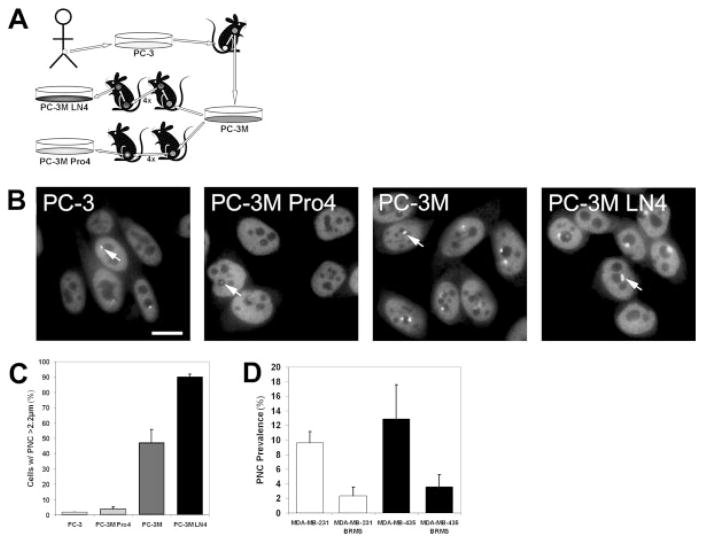
To further address whether PNC prevalence reflects metastatic behavior, we examined the PNC prevalence in cell lines that were created from the same origin but selected for various metastatic capabilities in a mouse model of a human prostate cancer. The PC-3 cell line, which was created from a human prostate tumor, was implanted into a nude mouse, and the PC-3M cell line was created by removing and culturing a metastatic lesion from the primary PC-3 tumor.21 The PC-3M LN4 cell line was generated through 4 rounds of in vivo serial enrichment for metastatic cells after inoculation of PC-3M into the mouse prostate. In contrast, the PC-3M Pro4 cell line was created through 4 rounds of in vivo serial enrichment for nonmetastatic prostate localized tumor cells22 (Fig. 3A). Therefore, the 4 cell lines represent a spectrum of metastatic capabilities; and, if PNC prevalence is an indicator of metastatic behavior, it should reflect these phenotypic differences. The results demonstrate that PNC prevalence jumps from 4% in PC-3 cells (the primary tumor) to 85% in PC-3M cells (1 round of enrichment for metastasis), reaches near 100% in highly malignant PC-3M LN4 cells (5 rounds of selection for metastasis), and is significantly (P <.01) lower in prostate-localized PC-3M Pro4 cells (78%) than in PC-3M LN4 cells (Fig. 1B). More important, the PNCs are atypically small (hardly detectable) in PC-3M Pro4 cells and are atypically large in PC-3M LN4 cells (Fig. 3B, arrows). When the data are adjusted to the percentage of cells with a PNC dimension >2.2 μm, the PNC prevalence correlates closely with the metastatic behavior of these cell lines (Fig. 3C). Therefore, in vivo selection of metastatic cells causes an increase in PNC prevalence and size; conversely, selection for nonmetastatic cells from a metastatic population significantly reduces PNC prevalence and size. These findings confirm that PNCs form in metastatic cells and suggest that PNC prevalence and size reflect the meta-static capacity of a cell population.
FIGURE 3.
Perinucleolar compartment (PNC) prevalence (the percentage of cells with 1 or more PNC) represents metastatic behavior. (A) Schematic of the creation of the PC-3 series of cell lines. (B) Immunofluorescent staining was used against polypyrimide tract binding protein (PTB) to show the PNCs in the PC-3 series of cell lines. Photomicrographs were captured with the same exposure parameters and are displayed with consistent contrast and brightness. Arrows indicate representative PNCs for each cell line. Scale bar = 10 μm. (C) PNC prevalence in the PC-3 series adjusted to the percentage of cells with a greatest PNC dimension >2.2 μm. (D) PNC prevalence in breast cancer cell lines that stably express breast cancer-related metastatic suppressor 1 (BRMS1) and in the parental lines.
To determine whether inhibition of metastatic behavior is able to reduce PNC prevalence, we examined breast cancer cells that over express the breast cancer-related metastatic suppressor 1 (BRMS1) protein. BRMS1 over expression can reduce the formation of distant metastasis of breast cancer cells in mice without decreasing proliferation in the primary tumor.23 Evaluation of 2 pairs of breast cancer cell lines, each consisting of the parental line and one line that stably expresses BRMS1, demonstrates that the PNC prevalence is significantly lower (P <.07) in 2 breast cancer cell lines that stably over express BRMS1 compared with the parental tumor cell lines (Fig. 3D). The reduction of PNC prevalence upon inhibition of metastatic capability further suggests that PNC prevalence directly reflects metastatic capacity in solid tumor cells from several tissue origins, indicating that PNC prevalence has potential to be developed as a pancancer marker of malignancy.
PNC Prevalence Does Not Represent Traits Shared by Normal and Cancer Cells
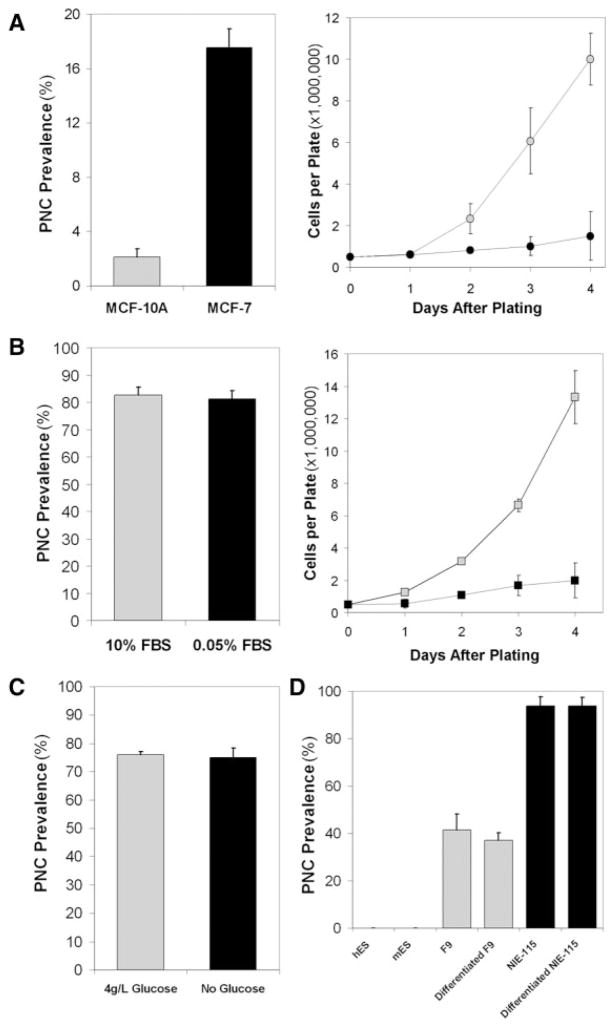
During the malignant transformation of normal cells, many cellular behaviors and characteristics are altered; however, some traits of transformed cells can be shared with normal cells, including rapid proliferation rate, increased glycolytic rate, and an undifferentiated status. To clarify whether PNC prevalence is associated with these shared traits or whether it is associated selectively with malignant behavior, we examined PNC prevalence in several experimental conditions. To determine the association of PNC prevalence with cell proliferation, normal (MCF-10A) and cancerous (MCF-7) breast epithelial cells were compared. The results demonstrate that PNC prevalence is much lower in normal MCF-10A cells than in cancerous MCF-7 cells despite the much higher proliferation rate of MCF-10A cells over MCF-7 cells (Fig. 4A). This dissociation is demonstrated further by serum starvation of HeLa cells, which significantly reduces the proliferation rate (but does not induce apoptosis; data not shown) without alteration of the PNC prevalence (Fig. 4B). In addition, the stimulation of cellular metabolism and proliferation in primary normal human cells did not induce PNC formation (data not shown). These observations demonstrate that the PNC prevalence is not indicative of the proliferation rate. To analyze the association of PNC prevalence with glycolysis, which generally is enhanced in cancer cells, HeLa cells were grown in glucose- and pyruvate-free media for 24 hours. Although inhibiting glycolysis reduces HeLa cell proliferation (data not shown), PNC prevalence remains unchanged (Fig. 4C). Furthermore, we evaluated the association between PNC prevalence and differentiation state, because cancer cells often are less differentiated than normal somatic cells. We examined PNC prevalence in both human and mouse embryonic stem cells and observed that the PNC prevalence was 0% in these cells (Fig. 4D). Consistent with this finding, PNC prevalence remained unchanged in 2 undifferentiated cancer cell lines, NIE-115 and F9, after chemically induced differentiation17,18 (Fig. 4D), demonstrating that the PNC is not a marker of an undifferentiated state. Together, these findings indicate that PNC prevalence is not associated with traits that are shared by normal cells and cancer cells but selectively reflects the malignant behavior of cancer cells.
FIGURE 4.
Perinucleolar compartment (PNC) prevalence (the percentage of cells with 1 or more PNC) does not correlate with traits that can be shared by normal cells and cancer cells. (A) The growth rate of normal breast epithelial cells (MCF-10A; gray) and breast carcinoma cells (MCF-7; black) are compared according to their PNC prevalence. (B) Serum starvation (black) of HeLa cells decreases cell proliferation compared with normal serum conditions (gray) but does not decrease PNC prevalence. FBS indicates fetal bovine serum. (C) Twenty-four hour glucose deprivation (black) of HeLa cells does not decrease PNC prevalence compared with normal glucose conditions (gray). (D) PNC prevalence in human and mouse embryonic stem cells (hES and mES, respectively), in undifferentiated murine teratoma (F9; gray), in murine blastoma cells (NIE-115; black), and in chemically differentiated F9 and NIE-115 cells.
CONCLUSIONS
In conclusion, the studies presented here demonstrate that the PNC is a unique subnuclear structure that forms in a broad spectrum of solid tumor cells and that PNC prevalence selectively and positively correlates with malignancy. Our current findings, together with our previous finding that PNC prevalence has prognostic value for breast cancer, render PNC prevalence an ideal candidate to be developed as a pancancer prognostic marker, especially for those cancers that currently lack effective prognostic markers and for those that affect a large portion of the population. Although PNC formation appears to be the consequence of transformation, it also may play a role in the maintenance of malignant behavior or in promoting further transformation. Studies are underway to investigate the function of the PNC at a molecular level and its role in malignancy, the understanding of which will help uncover novel cancer cell biology and potentially provide new therapeutic targets for the treatment of a range of cancers.
Acknowledgments
Dr. Huang was supported by National Institutes of Health Grant R01GM078555-01.
Drs. Kim and Schink were supported by a grant from Friends of Prentice.
Dr. Norton was supported in part by National Institutes of Health/National Cancer Institute Grant T32CA09560.
References
- 1.McGuire WL. Breast cancer prognostic factors: evaluation guidelines. J Natl Cancer Inst. 1991;83:154–155. doi: 10.1093/jnci/83.3.154. [DOI] [PubMed] [Google Scholar]
- 2.Cotran RS. The breast. In: Lester SC, editor. Robbins Pathologic Basis of Disease. Philadelphia, Pa: W.B. Saunders Company; 1999. pp. 1093–1120. [Google Scholar]
- 3.Faratian D, Bartlett J. Predictive markers in breast cancer—the future. Histopathology. 2008;52:91–98. doi: 10.1111/j.1365-2559.2007.02896.x. [DOI] [PubMed] [Google Scholar]
- 4.Ransohoff DF. How to improve reliability and efficiency of research about molecular markers: roles of phases, guidelines, and study design. J Clin Epidemiol. 2007;60:1205–1219. doi: 10.1016/j.jclinepi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Berney DM. Low Gleason score prostatic adenocarcinomas are no longer viable entities. Histopathology. 2007;50:683–690. doi: 10.1111/j.1365-2559.2007.02596.x. [DOI] [PubMed] [Google Scholar]
- 6.Komaki K, Sano N, Tangoku A. Problems in histological grading of malignancy and its clinical significance in patients with operable breast cancer. Breast Cancer. 2006;13:249–253. doi: 10.2325/jbcs.13.249. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Deerinck TJ, Ellisman MH, Spector DL. The dynamic organization of the perinucleolar compartment in the cell nucleus. J Cell Biol. 1997;137:965–974. doi: 10.1083/jcb.137.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Deerinck TJ, Ellisman MH, Spector DL. The perinucleolar compartment and transcription. J Cell Biol. 1998;143:35–47. doi: 10.1083/jcb.143.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matera AG, Frey MR, Margelot K, Wolin SL. A perinucleolar compartment contains several RNA polymerase III transcripts as well as the polypyrimidine tract-binding protein, hnRNP I. J Cell Biol. 1995;129:1181–1193. doi: 10.1083/jcb.129.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Politz JC, Pederson T, Huang S. RNA polymerase III transcripts and the PTB protein are essential for the integrity of the perinucleolar compartment. Mol Biol Cell. 2003;14:2425–2435. doi: 10.1091/mbc.E02-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timchenko LT, Miller JW, Timchenko NA, et al. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MP, Huang S, Black DL. Differentiation-induced colocalization of the KH-type splicing regulatory protein with polypyrimidine tract binding protein and the c-src pre-mRNA. Mol Biol Cell. 2004;15:774–786. doi: 10.1091/mbc.E03-09-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huttelmaier S, Illenberger S, Grosheva I, Rudiger M, Singer RH, Jockusch BM. Raver1, a dual compartment protein, is a ligand for PTB/hnRNPI and microfilament attachment proteins. J Cell Biol. 2001;155:775–786. doi: 10.1083/jcb.200105044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinhenz M, Fabienke S, Swiniarski N, et al. Raver2, a new member of the hnRNP family. FEBS Lett. 2005;579:4254–4258. doi: 10.1016/j.febslet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kopp K, Huang S. The perinucleolar compartment and transformation. J Cell Biochem. 2005;95:217–225. doi: 10.1002/jcb.20403. [DOI] [PubMed] [Google Scholar]
- 16.Kamath RV, Thor AD, Wang C, et al. Perinucleolar compartment prevalence has an independent prognostic value for breast cancer. Cancer Res. 2005;65:246–253. [PubMed] [Google Scholar]
- 17.Oh JE, Karlmark KR, Shin JH, et al. Differentiation of neuroblastoma cell line N1E-115 involves several signaling cascades. Neurochem Res. 2005;30:333–348. doi: 10.1007/s11064-005-2607-2. [DOI] [PubMed] [Google Scholar]
- 18.Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21:347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- 19.Gimm O. Thyroid cancer. Cancer Lett. 2001;26:143–156. doi: 10.1016/s0304-3835(00)00697-2. [DOI] [PubMed] [Google Scholar]
- 20.Suster S. Thyroid tumors with a follicular growth pattern: problems in differential diagnosis. Arch Pathol Lab Med. 2006;130:984–988. doi: 10.5858/2006-130-984-TTWAFG. [DOI] [PubMed] [Google Scholar]
- 21.Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522–3529. [PubMed] [Google Scholar]
- 22.Pettaway CA, Pathak S, Greene G, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 23.Samant RS, Seraj MJ, Saunders MM, et al. Analysis of mechanisms underlying BRMS1 suppression of metastasis. Clin Exp Metastasis. 2000;18:683–693. doi: 10.1023/a:1013124725690. [DOI] [PubMed] [Google Scholar]