Abstract
Metastatic cancer cells are lethal. Understanding the molecular mechanisms that bolster the conversion from benign to malignant progression is key to treating these heterogeneous and resistant neoplasms. The epithelial-mesenchymal transition (EMT) is a conserved cellular program that alters cell shape, adhesion, and movement. The shift to a more mesenchymal-like phenotype can promote tumor cell intravasation of surrounding blood vessels and emigration to a new organ, yet may not be necessary for extravasation or colonization into that environment. Lymphatic dissemination, on the other hand, may not require EMT. This review presents emerging data on the modes by which tumor cells promote EMT/MET via microRNA and prepare the pre-metastatic niche via exosomes.
Keywords: epithelial-mesenchymal transition, hematogenous metastasis, lymphogenous metastasis, lymphatics, exosomes, microRNA
Routes of Cancer Dissemination
Cancer mortality is primarily due to metastasis and the resulting compromise of organs secondary to the initial tumor. Metastasis is a complex multi-step process in which the primary tumor spreads to distant organs. Cells must exit the primary tumor and invade through the surrounding tissue, enter a blood or lymphatic vessel (intravasation), be carried to a distant site, exit the vessel (extravasation) and re-establish a tumor mass in a new organ environment (1, 2). The net process involves the interaction of numerous different cell types and various extracellular environments. Each step in the metastatic cascade has been intensely studied, and some of the molecular mechanisms have been dissected in order to establish targets for clinical prevention and treatment.
Tumor cells can disseminate using two different conduits – the hematogenous (blood) vascular system and/or the lymphogenous (lymphatic) system. The process of metastasis through blood vessels has been much more widely studied than through lymphatic vessels despite the fact that the presence of lymphatic metastasis (or “node positive” disease) is a key indicator of a less favorable prognosis in most epithelial cancers. While many of the critical metastatic steps are similar in both forms of metastasis, there are distinct differences in the matrices, cellular interactions and microenvironments that should be considered in order to better understand the overall metastatic process.
The functions of the lymphatic and vascular systems are quite different. The lymphatic system is an “open” system while the vascular system is a “closed” circulatory system. In either case, a disseminating tumor cell first encounters a capillary vessel, whose structure varies depending on whether it is lymphatic or blood vascular. Lymphatic capillaries are blind-ended vessels that drain fluid from the interstitial space. They are lined by thin-walled lymphatic endothelial cells lacking basal lamina and lacking pericyte coverage (3). Initial lymphatics drain into larger collecting vessels covered on the abluminal side with a basement membrane and smooth muscle cells. Lymphatic channels, beyond the capillary level, contain valves that prevent fluid backflow. Collecting lymphatic ducts funnel fluid (and cells) toward the lymph node. After being filtered through one or more lymph nodes the lymphatic system recycles the fluid back to the blood vascular system through the right or left thoracic ducts into the subclavian veins.
Fluid entry into the lymphatic capillary is regulated by hydrostatic pressure (pressure from inside the vessel pushing out into the tissue) and oncotic pressure (pressure in the tissue pushing in toward the vessel lumen). As interstitial fluid increases from the plasma leakage from blood vessels, neighboring lymphatic endothelial cells are pulled apart at specialized junctions called “button junctions” via anchoring filaments (4). These openings allow for the free flow of fluid and cells into the lymphatic capillary lumen. Using this escape route, tumor cells do not need to invade through a thick basement membrane or secrete proteases, although they may need to alter their expression of cellular adhesion molecules, integrins, or chemokine receptors to gain access to the node (5, 6).
In contrast, the blood circulatory system is tightly sealed in most tissues to prevent bleeding or the leakage of red blood cells. Blood is pumped by the heart through arteries to capillaries where nutrient and gas exchange occurs with neighboring cells (or tumor cells), and waste products are returned through veins. Vascular capillaries can be very small in diameter – sometimes only a single endothelial cell comprising the entire circumference of the capillary – allowing only enough space for red blood cells to pass through single-file. These capillaries are surrounded by a basement membrane and are intermittently covered by smooth muscle cells called pericytes. Larger arterioles and venules are covered by a continuous smooth muscle layer. In order to escape the primary tumor site, cancer cells must first degrade the matrix proteins in the basement membrane of a capillary and squeeze by pericytes and between (or through) endothelial cells to enter the blood stream.
This potential network of escape routes for the invading tumor cell is further enhanced by tumor angiogenesis and lymphangiogenesis. As tumor cells multiply, they eventually outgrow their blood supply—meaning that the center of the tumor mass is too far from a vessel to receive nutrients or oxygen by diffusion. This hypoxic environment drives the tumor cells to upregulate growth factors and chemokines, which recruit endothelial cells and stimulate nearby capillaries to sprout toward the tumor (angiogenesis). Newly formed tumor blood vessels are often tortuous, malformed and leaky (7). High levels of tumor cell-secreted angiogenic factors cause vascular permeability, plasma leakage, and increased interstitial fluid pressure within the tumor nodule. Tumor-associated angiogenesis may dramatically increase the microvessel density within the tumor, thereby decreasing the distance a tumor cell must travel to invade a vessel and increasing the potential for metastasis. In fact, microvessel density may predict metastatic potential in a number of human cancers (8, 9). Many growth factors stimulate the proliferation of lymphatic endothelial cells in addition to vascular endothelial cells, and tumor-associated lymphatic capillaries may be 10-50 times the luminal diameter of normal lymphatic capillaries (10). In melanoma, the area of peritumoral lymphatic vessels has been shown to correlate with metastasis better than tumor thickness (11).
Overview of Epithelial-Mesenchymal Transition
Epithelial cells line all organs exposed to the outside world, including the skin, respiratory tract, gastrointestinal tract, and urogenital tract, and also including all glands such as in the prostate, breast, and pancreas. During development, epithelial cells undergo massive changes in their characteristics and increase their motility in order to migrate, branch, or form tubes. This process of functional and phenotypic changes in cell polarity and differentiation status is termed the Epithelial-Mesenchymal Transition (EMT). In addition to embryonic development, EMT is fundamental in the normal physiological processes of stem cell formation and wound healing (12). During EMT basal epithelial cells lose their “epithelial phenotype,” including lateral cell-cell adhesions (adherens junctions, desmosomes and tight junctions) and cell-matrix adhesions (hemidesmosomes) to the basement membrane, leading to a loss of apical-basal polarity. The cells downregulate epithelial markers, e.g. E-cadherin, and upregulate expression of mesenchymal markers, e.g. vimentin (see Table 1, discussed below). Cells undergoing EMT become migratory and invasive, gain a resistance to apoptosis, increase secretion of degradative enzymes, and digest underlying extracellular matrix (ECM) together with increased secretion of new ECM components. These characteristic changes during development have been termed “Type I EMT” and are nearly complete in their transition to the mesenchymal phenotype yet transient in time and often followed by a reversion to the epithelial phenotype to create a secondary epithelium (13). “Type II EMT” describes changes that occur during inflammation and fibrosis and can be partial or complete in its mesenchymal transition and occur over prolonged periods of time, often with pathological consequences (14).
Table 1.
Markers of EMT
| Phenotype |
||||
|---|---|---|---|---|
| Epithelial | Mesenchymal | |||
| Markers | E-cadherin | ZO-1 | N-cadherin | TCFC4 |
| P-cadherin | EpCAM | Vimentin | SIX1 | |
| Epithelial Membrane | Occludin | Fibronectin | Twist | |
| Antigen/MUC-1 | Claudins 3,4,7 | FSP1/S100A4 | Snail | |
| Cytokeratins 8,18,19 | Laminin 1 | Goosecoid | Slug | |
| Desmoplakin | Entactin | FOXC2 | ZEB1 | |
| Desmocollin 2,3 | Syndecan 1 | MMP2,3,9 | Integrins α5β1,αvβ6 | |
| γ-catenin | miR-200 | Nuclear β-catenin | α-smooth muscle actin | |
| Integrin β4 | LEF-1 | miR-21 | ||
| SOX1 | ||||
EMT in Carcinoma Cells
Carcinomas are tumors of epithelial origin. Since carcinoma cells are genetically unstable, they do not start from a purely normal epithelial phenotype but rather a somewhat activated phenotype. While EMT in the cancer context bears many parallels to classical developmental EMT, the transition is often heterogeneous and/or incomplete. Tumor cells have been characterized as undergoing “Type III EMT.” Some distinctions of Type III result from the abnormal expression of oncogenes and lack of tumor suppressor genes as a backdrop in the neoplastic cells, and the discord this causes subsequent to EMT activation (13). Considering the heterogeneity and unstable genetic background of tumor cells, it is perhaps not surprising that in Type III EMT the cellular plasticity is often accompanied by a change in a varying cohort of EMT markers, which can differ among tumor cell types and EMT pathways. Tumor EMT is not an on/off binary switch, but rather a graded series of interrelated and overlapping events that can be quite variable. While the expression of key markers such as E-cadherin and vimentin are altered in most cell types, there are different profiles of many other EMT markers in different cancer cells, as shown in Table 1. The process of EMT is now recognized to involve interplay between several different levels of regulation. While many structural proteins represent the characteristic “marker profile” of EMT, the expression of these molecules is mediated by additional layers of control that include regulators of transcription and translation, protein stability and alternative splicing. We briefly review some of these key EMT signs below, and refer the reader to more extensive reviews on each topic (15-19).
Markers of Tumor EMT/MET
The EMT molecular machinery has been classified into three categories: inducers, regulators and effectors (20). Briefly, inducers are the upstream growth factors and receptors that initially signal the transition; regulators are the transcription factors or drivers of the process (downstream of the growth factors and upstream of the effectors); and effectors are the proteins responsible for eliciting a resultant cellular shape change or an invasive advantage (20). This EMT molecular program is plastic and can be reversed to return to the epithelial phenotype, called mesenchymal-epithelial transition (MET).
EMT inducers
Transformed epithelial cells (often genetically mutated) secrete autocrine growth factors, such as EGF, HGF, FGF, and TGFβ, that sustain their continual proliferation. These growth factors all bind to respective receptor tyrosine kinases to induce EMT and to elicit an invasive and migratory state. TGFβ is the most well-studied and potent inducer of EMT (21). As the tumor grows in size, the center becomes hypoxic and induces the upregulation of multiple angiogenic mediators, including VEGF, IGF, TGFβ, HGF, FGF, Wnt and Notch, that induce tumor EMT (22). These mesenchymal changes in tumor morphology are presumably an adaptive strategy to escape the hostile hypoxic environment of the tumor. Tumor-induced inflammation brings into the milieu immune cells that secrete cytokines like TNFα, IFNγ, IL6, and IL1β. Chronic inflammatory mediators induce the expression of transcription factors such as snail and zinc finger E-box-binding homeobox (ZEB) proteins that repress the epithelial phenotype and promote fibrosis, tumor EMT, and metastasis (23).
EMT regulators
In carcinoma cells, the default state is epithelial in nature. A number of transcription factors have been described which drive the transition to the mesenchymal condition, and many of them act by repressing epithelial-related genes (Table 1). Snail 1 (SNAI1), Slug (SNAI2), ZEB1 and ZEB2 bind directly to the E-cadherin promotor to repress transcription. Other transcription factors, such as Twist, can repress E-cadherin indirectly (24). In TGFβ-driven EMT, transcription factors such as SMAD and BMP can initiate many of the EMT-related changes (12). HIF1α is a potent driver of EMT during hypoxia (25). Besides these classic EMT regulators, novel transcription modulator families including GATA, SOX, and forkhead box (FOX) proteins regulate cell fate, differentiation, stemness, and cell polarity to drive EMT decisions (26-28). Lastly, microRNAs, while not transcription factors, can function to silence gene expression and thereby regulate changes in EMT and MET (discussed in depth below).
EMT effectors
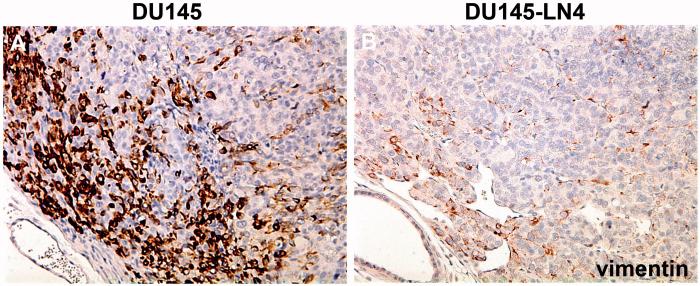
The EMT process involves changes in the levels and localization of many structural protein components of cell-cell and cell-ECM adhesions, such as tight junctions, gap junctions, adherens junctions, desmosomes, and hemidesmosomes. Cadherin switches, such as epithelial-cadherin (E-cadherin) to neural-cadherin (N-cadherin) are classic representative markers of the EMT process (Table 1). Other cell junction-related changes include catenins, claudins, desmocollins, and JAMs (junctional adhesion molecules). The alterations in cell surface proteins twinned with changes in the dynamic cytoskeleton intermediate filament proteins, such as vimentin and keratins, act to shift the overall cell shape and behavior from stable interconnected cell sheets to separate, spindle-shaped motile mesenchymal cells. Cells at the leading edge of the tumor are often found to express the mesenchymal marker, vimentin (Figure 1A), while tumor cells selected for lymphatic dissemination lost or downregulated vimentin expression (Figure 1B).
Figure 1. Vimentin is decreased in metastatic tumor cells cycled through the lymphatic system.
Human prostate tumor lines, DU145 (A) or DU145-LN4 (B), were grown orthotopically in the prostates of immunodeficient nude mice. Tumor sections were stained for the mesenchymal marker vimentin (brown color) and counterstained with hematoxylin (blue color). DU145LN4 tumors show fewer cells expressing vimentin and reduced vimentin expression per cell, compared to parental DU145. In both tumors, vimentin staining was strongest on the leading edge (upper left corner).
Besides losing adhesions and becoming motile, a mesenchymal tumor cell must also be able to invade through the ECM and the basement membrane in a blood capillary in order to escape the primary tumor site and gain access to other organs. Therefore, the EMT process also upregulates many enzymes, including collagenases (MMP2, MMP9) and stromelysin1 (MMP3) (29). Typically, when epithelial cells (which are anchorage-dependent) lose adhesion to the matrix and enter the blood stream, they become susceptible to apoptosis via a process called anoikis. Mesenchymal-like cells are protected from anoikis via several signaling mechanisms, including the PI3K/AKT, NF-κB, Wnt/β-catenin, and p53/p63 pathways (reviewed by (30)). EMT also increases drug resistance and blocks immune surveillance—processes that promote metastasis (31, 32).
Tumor Migration and Metastasis
Tumor cells display considerable plasticity and heterogeneity. The EMT switch may not be enough to describe all of the kinds of migratory phenotypes observed within carcinoma cells, but may represent one subtype of invasive behavior. Friedl and Wolf have further classified cell migration into categories of “single cell movement” or “collective cell movement” (33). Single cell movement describes how mesenchymal-like tumor cells move with one side of the cell leading the movement, forming focal adhesions between integrins and the ECM, releasing proteolytic enzymes in a directional manner, contracting using actomyosin bundles, and ending with the tail of the cell detaching (33). However, other invasive single tumor cells are found to be less mesenchymal in morphology and marker profile. These single cell migration patterns are termed “amoeboid” and can include cells that are more epithelial in their characteristics or are at least incomplete in the EMT. In fact, tumor cells are incredibly diverse and can transition quickly between mesenchymal and amoeboid migration (34).
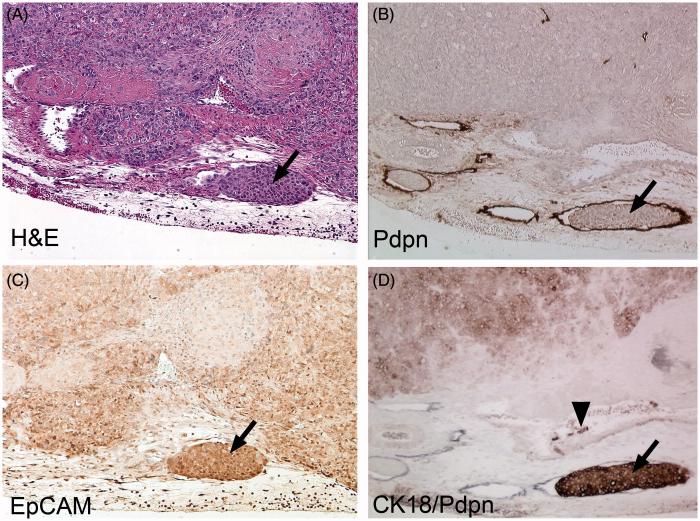
Histological studies have shown that metastatic carcinomas have rough, ‘infiltrative’ edges to the tumor, meaning that the leading edge of the tumor has a zigzag pattern with cells invading the surrounding stroma in strands and sheets, whereas benign tumors tend to have smooth ‘expanding’ borders (35). Invasive yet differentiated carcinomas (found especially in breast and prostate cancer) tend to relocate using collective cell migration. These tumor cells lose some of their adhesion molecules yet keep their homotypic cell-to-cell adhesions (such as E-Cadherin); therefore they are more epithelial-like in appearance and may be classified as partially converted by EMT or incomplete EMT. Collective cell migration can also be in the form of tumor cell clusters that break off from the whole tumor and are often found to enter lymphatic vessels (3, 36, 37). Our studies using cycled human prostate cancer cells (four times from prostate to lymph node) showed that the highly invasive DU145-LN4 cell line displayed an epithelial morphology and invaded in a collective migration pattern into lymphatic vessels (38). Tumor cell clusters inside peritumoral lymphatic vessels expressed E-Cadherin and Cytokeratin 18 (Figure 2). Tumor cell collective migration can convert to single cell mesenchymal migration via TGFβ signaling (39). Further, inhibition of TGFβ signaling can shift cells back toward collective movement. Importantly, tumor cells restricted to collective invasion were capable of lymphatic dissemination but not blood-borne metastasis (39). Taken together, these results suggest that lymphatic metastasis does not require the EMT.
Figure 2. Tumor cells in peritumoral lymphatic vessels are epithelial in phenotype.
DU145-LN4 tumor sections from mouse prostate were stained with: (A) H&E; (B,D) podoplanin (Pdpn); (C) EpCAM; or (D) Cytokeratin 18. Serial sections show tumor cells inside lymphatic vessels at the tumor periphery (arrows). Lymphatic vessels were detected with Pdpn staining (B, brown color; D, black color). Tumor cells inside lymphatic vessels appeared as “plugs” and stained positive for the epithelial markers, EpCAM (C, brown color) and Cytokeratin 18 (D, brown color). Sections (B-C) were counterstained with hematoxylin (blue color). This data suggests that lymphatic metastasis does not require EMT. Panels B, D are modified from (38).
EMT and metastasis
MET
The epithelial-to-mesenchymal transition has often been considered essential for metastasis. Many studies have shown that overexpression of individual EMT-associated transcription factors (e.g., twist, snail) into benign cells can increase their invasive potential (40-42), but confirming that human cancers actually undergo EMT in patients is more difficult. An overwhelming problem with the EMT concept is that human metastases examined histologically appear epithelial in phenotype and resemble the primary tumor (43). In other words, the metastatic nodules are not mesenchymal. Several theories have been put forth to explain this issue. The most popular theory is the Mesenchymal-Epithelial Transition (MET) (44). In this model, tumor cells that have undergone EMT can intravasate blood capillaries at the primary tumor site and extravasate into the distant organ, but they must revert back to the epithelial phenotype in order to grow in the secondary site and become a clinically relevant and detectable mass. This premise is based upon strong evidence in embryonic systems but confirmation in human cancer patients as to when the MET event occurs, if at all, is unclear. Some suggest that there is cooperation between epithelial and mesenchymal cells such that mesenchymal cells “pave the way” for the escape of epithelial cells, while epithelial cells have a proliferative advantage at the secondary location and therefore make up the majority of the second mass (45). Evidence from blood-borne circulating tumor cells (CTC) from human patients shows a largely epithelial profile but these data are confounded by the technical aspects used to isolate these cells, which is largely based on receptors common to epithelial cells (46). More recently, CTC populations have been separated into both mesenchymal and epithelial status (47, 48).
There are several key studies which show the importance of an in vivo MET to establish metastases. Chaffer and colleagues showed that bladder cancer cells with increased metastatic ability had undergone MET after in vivo selection (49). They used intracardiac injection as an experimental model, which skips the intravasation steps of the metastatic cascade where EMT may play a role. When the cancer cells were injected in an orthotopic location, where intravasation was required for escape, the same cells metastasized poorly (49). Our laboratory used a different in vivo selection model to cycle the human DU145 prostate cancer cell line multiple times from the prostate to sentinel lymph node. The selection criteria was without individual marker-bias since cells were cultured directly from lymph nodes. Similarly, we observed a strong and progressive shift toward the epithelial phenotype with each in vivo lymphatic passage (Figure 1) (38, 50). This MET occurred spontaneously without ectopic overexpression or silencing of EMT-related transcription factors or microRNAs. Ocaṅa and coworkers induced MET by silencing the paired-related homeobox transcription factor Prrx1 (51). Prrx1-silenced BT-549 breast cancer cells gained metastatic ability following intravenous injection in the experimental lung metastasis model. Prrx1-silencing did not affect primary tumor growth but did inhibit vascular invasion and spontaneous lung metastasis (51).
Metastatic cells have also been associated with a high miR-200, epithelial phenotype in several breast cancer models, including the widely-used and aggressive 4T1 cell line. Ectopic expression of miR-200 reduced the number of breast cancer CTCs but increased metastasis following intravenous injection (52). The role of the mesenchymal phenotype was demonstrated using Twist1-induced EMT in a spontaneous squamous cell carcinoma model. Twist1 induction resulted in increased tumor growth and invasion, accompanied by increased circulating tumor cells and extravasation. However, Twist1 inactivation and MET was required for effective metastatic colonization (53).
CSC
There is also evidence that EMT is strongly linked to the cancer stem cell (CSC) theory—which supposes that most tumor cells lack tumor-initiating ability and that only a rare subpopulation of “stem-like” cells can lead to metastatic disease. Cancer stem cells show a plasticity that allows them to transition between EMT and MET-like states. Many pathways affecting CSCs also induce EMT, including TGFβ. CSCs are resistant to chemotherapies, likely due to their ability to actively pump out xenobiotics. CSCs and their daughter cells often take up a niche around capillaries in a cuff-shape where there is an ample supply of oxygen and nutrients. Evidence suggests that breast CSCs can exist in an EpCAM-negative mesenchymal-like state with CD29−/CD44+ or a highly proliferative EpCAM-positive state (54). Other studies indicate that the CSC or tumor initiating cell (TIC) populations exhibit a hybrid phenotype (55). Clearly, TIC capacity can exist within an epithelial phenotype (56, 57). Barriere and colleagues have suggested the following CSC markers to identify stem-like cells in the blood, regardless of whether they are epithelial or mesenchymal: Aldehyde dehydrogenase-1 (ALDH1), CD44, Gangliosides (GD2, GD3 and GD1a), and ATP-binding cassette transporters (ABC extrusion pump proteins) (58). A recent review presents an “EMT gradient model” or histogram in which there is a biphasic distribution such that a partial EMT would stimulate stem cell qualities and initial metastasis formation and full EMT would lose stem cell properties (59). Other researchers suggest that EMT-positive CTCs extravasate into distant organs and remain dormant for long periods before converting into CSCs that are resistant to therapy (60).
Lymphatic Metastasis Does Not Require EMT
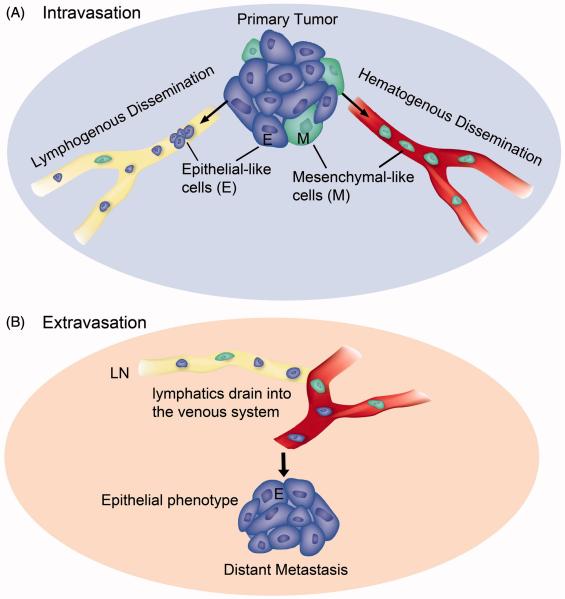
A third possible scheme to describe the clinical data is the possibility that lymphatic dissemination does not require EMT. Our data strongly suggest that prostate cancer isolated from sentinel lymph nodes increases in epithelial status with each passing cycle (50). Although the tumor cells increased their invasive potential, no traditional mesenchymal markers were upregulated (38). Additionally, tumor cells found inside lymphatic vessels expressed epithelial markers (Figure 2). Interestingly, Prrx1/Twist1-silenced breast cancer cells metastasized to axillary lymph nodes but not the lung, consistent with the concept that lymph node metastasis does not require EMT (51). Tsuji and colleagues reported a series of in vivo studies using fluorescently-labeled carcinoma cells that were stably epithelial-like (E-like; red, RFP) or mesenchymal-like (M-like; green, GFP) (61). Both the cancer cell types formed subcutaneous tumors, but PCR analysis of the blood indicated that only M-like cancer cells could intravasate. Surprisingly, when injected into the tail vein of the mouse, only red E-like cancer cells formed experimental metastases in the lung (38). When E- and M-like cancer cells were mixed and then implanted subcutaneously, both types could be found in the blood; and only red E-like spontaneous metastases formed in the lung (in 100% of the mice). Taken together, this data suggests that mesenchymal cells are more successful at intravasation while extravasation does not require EMT (illustrated in Figure 3). Tsuji and colleagues suggested that in the mixed tumor, mesenchymal cells could facilitate intravasation for epithelial-like cancer cells (38).
Figure 3. Illustration of EMT-status for lymphogenous and hematogenous metastasis.
(A) Intravasation. Carcinoma cells that transition to the mesenchymal phenotype (M, green color) are able to digest the capillary basement membrane and invade the blood vessel (hematogenous dissemination). Tumor cells of either epithelial phenotype (E, blue color), partial EMT phenotype (not shown), or mesenchymal phenotype can flow into the lymphatic capillary (lymphogenous dissemination). (B) Extravasation. Lymphatic ducts eventually empty into the venous system. Exit from blood capillaries into a secondary organ does not require EMT (at least in the lung, liver or bone marrow where capillary basement membrane is minimal). Metastases at the secondary site resemble primary tumors in that they are mainly epithelial in phenotype. This illustration is based on evidence from this review and (38, 50, 61).
An alternative explanation is that both epithelial and mesenchymal cancer cells can disseminate via the lymphatic system, which requires less invasive potential since the lymphatic capillaries lack a basement membrane and have open gaps (5). Mesenchymal-like cancer cells, known to express lymphangiogenic factors, may promote tumor-associated lymphangiogenesis and therefore increase the lymphatic vessel density within and around the tumor. For instance, overexpression of VEGF-C in cancer cells results in an increased volumetric flow rate in lymphatics, which increases the cancer cell accumulation in the sentinel node by 200-fold (62). Lymphovascular invasion of epithelial-like cancer cells may eventually reach the blood system. To date, CTC have not been isolated strictly from the lymphatic system prior to draining into the blood. The phenotype of the lymphatic-CTC (L-CTC) may shed light on the requirement of EMT for lymphatic metastasis.
MicroRNA and EMT/MET
MicroRNAs (miRNA or miR) can influence multiple steps in cancer cell metastasis and are well established as key regulators of the EMT program in epithelial cells (63, 64). EMT is a complex process that can be influenced by different pathways and thus has many potential mechanisms of regulation by miRNA. MiRNA can directly bind and suppress transcription factors, directly suppress transcription of key EMT molecules, such as vimentin and E-cadherin, or affect epigenetic regulators of EMT (64, 65). MiRNAs can promote EMT (therefore suppressing MET) or promote MET (thus inhibiting EMT) depending on the target molecules that they bind and suppress. We will briefly review these different groups and their mechanisms.
MicroRNA EMT Suppressors
EMT suppression (or MET promotion) can be achieved through miRNA suppression of EMT-related transcription factors. A group of EMT-inducing transcription factors, such as Snail1 (SNAI1), Snail2/Slug (SNAI2), ZEB (zinc finger E-box-binding homeobox)-1, and ZEB2 directly bind and repress E-cadherin transcription. Other transcription factors, such as Twist, Goosecoid, TCF4 and FOXC2, indirectly repress E-cadherin. miRNA inhibition of these transcription factors results in higher E-cadherin expression and suppression of EMT pathways.
One of the best studied pathways of miRNA-mediated EMT inhibition is that of the miR-200 family. Members of the miR-200 family (including miR-200b, miR-200c and miR-141) bind directly to the transcriptional repressors ZEB1 and ZEB2 (66-68). Conversely, miR-200 inhibition reduces E-cadherin and promotes vimentin expression, and, thus, EMT pathways. miR-200 and ZEB form a reciprocal repression loop (69). TGFβ, a key growth factor in EMT, induces ZEB expression; and miR-200 expression is adequate to block TGF-β-induced EMT (70).
Other EMT-inducing transcription factors affected by miRNAs include Snail1, which is repressed by miR-30a (71), miR-34a (72), and miR-203 (73). Similarly, miR-186 binding and suppression of Twist1 resulted in a MET in ovarian cancer cell lines (74).
MicroRNA EMT Promoters
MiRNA can also promote an EMT (or suppress a MET). MiRNA shown to possess this ability include miR-9, which binds and suppresses E-cadherin (75). Our laboratory demonstrated that miR-424 expression drives EMT in prostate tumor cells (50). A recent elegant study has supported and extended our observations; Drasin and colleagues showed that miR-424 promotes EMT and is upregulated by Twist1 or Snail in breast cancer cells (76). Other examples include miR-10a (77), miR-221/22 (78), miR-29a (79), miR-103/107 (80), and miR-21, which binds to PTEN to mediate EMT and CSC phenotypes (81).
MicroRNA and Metastasis
MicroRNA influence many other cell behaviors impacting dissemination, including cell migration and invasion, which likely control processes such as intravasation and extravasation. A number of miRNAs have been shown to directly influence metastasis in vivo, including miR-9, miR-10b, miR-31, and miR-103/107.
MiR-9 directly targets CDH1, the mRNA coding for E-cadherin in breast cancer cells. E-cadherin repression leads to increased cell motility and invasion and increased β-catenin signaling. As a consequence, VEGF expression is upregulated, which results in increased angiogenesis. Importantly, expression of miR-9 in non-metastatic breast cancer cells enabled the formation of lung micrometastases, while metastasis of highly aggressive cells could be reduced using a ‘miRNA sponge.’ MYC and MYCN induce miR-9 expression, tying traditional oncogenes to previously unknown miRNA-mediated mechanisms (75).
MiR-10b induces invasion and distant metastasis in breast cancer cells through the transcriptional repressor HOXD10. HOXD10 modulates genes including RhoC, uPAR, MMP14 and integrin α3 (82). The well established EMT-promoting transcription factor, Twist, was also shown to increase miR-10b expression (83).
MiR-31 suppresses metastasis through effects at multiple points in the metastatic cascade. MiR-31 expression impedes local invasion at the primary site, extravasation, and metastatic colonization. Although metastatic tumor cells could lodge in the lung, their attachment and colonization at this distant site was inhibited (84). MiR-31 effects are mediated through a cohort of targets, including Fzd3, RhoA, ITGA5 and RDX.
The miRNA-103/107 family (miR-103.1, miR103.2 and miR-107) induces an EMT by down-regulating miR-200 but it also generally downregulates miRNA synthesis by targeting Dicer, an important enzyme for miRNA processing. Cancers are often accompanied by a global miRNA downregulation, and this may represent a mechanism through which this is achieved. miR-107 expression enabled the formation of lung metastases in non-metastatic breast cancer cells, and miR-103/107 silencing inhibited metastatic colonization by aggressive 4T1 breast cancer cells (80).
Clearly, microRNA have emerged as key regulators of numerous biological processes, including EMT, tumor progression, and metastasis. In this summary, we have focused on the influence of endogenous tumor cell miRNA expression on EMT and metastasis, but it should also be mentioned that miRNA may influence the tumor-associated blood and lymphatic vessels to facilitate tumor cell dissemination. Furthermore, in addition to transcriptional control, epigenetic control mechanisms such as CpG island methylation and histone modifications can also modulate miRNA expression and EMT (85, 86).
Exosomes and EMT
All epithelial cells contain bilayered membrane-bound vesicles in the cytoplasm of the cell called multivesicular bodies (MVB) or endosomes. These vesicles typically carry cargo to the lysosome to be degraded or recycled. Alternately, endosomes can fuse with the plasma membrane and be released, after which they are called exosomes. In contrast to larger microvesicles (100-1000 nm) that bud from the plasma membrane, exosomes are smaller (30-100 nm) and are derived from the endosomal pathway (87). They are extracellular chaperones that are abundant in tetraspanins and their biogenesis is governed by endosomal sorting complexes including the Rab proteins (27).
Exosomes were once thought to be merely cellular garbage, but their importance in many physiological systems and in tumor progression, in particular, is emerging. The contents of exosomes can vary but generally reflect the origin of the vesicle. For instance, exosomes from tumor cells can contain oncogenic proteins such as receptor tyrosine kinases, oncoproteins like Ras, or phosphorylated downstream signaling molecules such as activated AKT. They may also contain microRNA that can then affect the transcription/translation of new proteins in the target cell (88).
Exosomes are analogous to a Postal Service—they deliver a package from one location to another and keep the contents safe during delivery. Exosomes can act in autocrine, paracrine, or endocrine fashion. They act as independent entities trafficking to either proximal neighboring cells (tumor or stromal) or to distant lymph nodes or organs (89). Once at a target site, they can either release their contents, such as growth factors, to influence the cells in the new site or fuse with a new cell membrane and deliver their contents to the cytosol of a new cell, essentially transfecting that new cell with exogenous RNA, microRNA, or proteins. Exosomes have been found in nearly every bodily fluid including saliva, urine, and blood. Their systemic nature has now been exploited as a biomarker for many diseases (90).
In the “Seed and Soil” hypothesis (originally proposed by Stephen Paget but popularized by Isaiah J. Fidler), the tumor cell is a seed seeking the most fertile soil (distant organ) for it to grow in (91, 92). In this scenario, we may consider exosomes as the “fertilizer.” Tumor cells release exosomes that travel to distant organs and release growth factors to acclimate the “foreign soil” or to prepare the metastatic niche (93). Lymph nodes can undergo lymphangiogenesis prior to the appearance of lymph node metastasis (94). This may be due to the release of systemic growth factors such as VEGF-C or the release of exosomes that carry lymphangiogenic proteins to the lymph node to prepare the microenvironment. In theory, due to its primary function of draining interstitial fluid which includes cellular debris and exosomes, the lymphatic system or the lymph node should contain the highest concentration of exosomes in the body.
The cargo in exosomes can induce EMT and promote tumor growth, invasion, and metastasis (95). EMT inducers (TGFβ, TNFα, HDGF) and EMT effectors (MMPs) have been found in exosomes (89). Exosomes derived from mesenchymal tumor cells (CD105+) can stimulate angiogenesis in a Matrigel model and promote a premetastatic niche in the lung when injected intravenously prior to tumor cells (96). Prostate cancer cell-derived exosomes (via TGFβ1) can influence normal stromal cells to differentiate into specialized myofibroblasts that promote angiogenesis and accelerate tumor growth (97).
Exosomes can carry viral-encoded oncogenic proteins to latent cells. Nasopharyngeal carcinoma (NPC) can be caused by Epstein-Barr virus (EBV). LMP1 is the primary oncogenic protein associated with EBV infection, and LMP1 is found in NPC-secreted exosomes (98). LMP1 delivered to uninfected cells induces HIF1a and EMT. Targeted cells shift from E- to N-Cadherin expression. HIF1a also induces VEGF and the angiogenic shift in recipient cells (99). This cascade is self-promoting because hypoxia itself (and HIF1a) promotes the release of more exosomes from tumor cells that enhance invasiveness and stemness (100).
Exosomes can promote target cell invasion by transferring proteins such as CD147 and CD133 (95). Melanoma-derived exosomes promoted lung endothelial cell leakiness, a hallmark of pre-metastatic niche formation. Additionally, when bone marrow-derived cells were co-cultured with melanoma exosomes prior to engraftment, the “educated” pro-angiogenic bone marrow progenitor cells promoted tumor growth and metastasis. This response was due to the horizontal transfer of the c-MET tyrosine kinase receptor from melanoma exosomes to the bone marrow cells (101).
Exosomes deliver bioactive molecules such as microRNA to distant cells. These tumor-derived exosomes can transform benign cells or promote metastasis. Specifically, metastatic breast cancer-derived exosomal miR-105 could be transferred to vascular endothelial cells to cause disruption in barrier function via the downregulation of the tight junction protein ZO-1 (102). When mice were pretreated with miR-105 containing exosomes, metastasis to the lung and brain was enhanced. In another study, miR-200 family members were found in exosomes from metastatic breast cancer and not in exosomes from benign breast cancer (88). Additionally, miR-200 containing exosomes could promote lung colonization of benign cells. The transfer of exosomal microRNA, and therefore the aggressive phenotype, could occur at the primary tumor location, in the circulation, or at the metastatic site (88).
Summary
Metastasis is a complex process with each step subject to influence by multiple layers of regulation. As many of the metastatic steps are further dissected their role becomes more complex and additional questions arise. The relative contribution of cells disseminating via the lymphatic system versus the vascular system to the overall burden of metastasis is unclear. We are clearly starting to understand more about EMT and where it may be beneficial and where it needs to be reversed. Parallel to the EMT/MET-mediated mechanism of metastasis, evidence suggests that this may not always be relevant. Is there a subset of cells with a distinct epithelial phenotype that can circumvent this process and escape via lymphatics? Have tumor cells been sneaking unnoticed out the side door? Considering the importance of lymph node metastasis in clinical staging and outcome in many epithelial cancers, we suggest that this compartment has been understudied and may open new therapeutic opportunities.
Acknowledgments
We acknowledge the contribution of Matthew Migliozzi for histological staining. We thank Melissa Anderson for editing and administrative assistance and Ricardo Sanchez (Ricasan Rowley Histology Consulting, LLC) for tissue sections.
Funding
This publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers P50CA09381, R21CA155728, and K01CA118732 and The Vascular Biology Program at Boston Children’s Hospital. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interests
The authors declare no conflicts of interest.
References
- 1.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Research. 2010;70(14):5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Migliozzi MT, Mucka P, Bielenberg DR. Lymphangiogenesis and metastasis-A closer look at the neuropilin/semaphorin3 axis. Microvascular Research. 2014;96C:68–76. doi: 10.1016/j.mvr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. The Journal of Experimental Medicine. 2007;204(10):2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5(8):812–7. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. The Journal of Experimental Medicine. 2013;210(8):1509–28. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36(3):321–31. doi: 10.1055/s-0030-1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol. 1993;10(4):302–13. [PubMed] [Google Scholar]
- 9.Nico B, Benagiano V, Mangieri D, Maruotti N, Vacca A, Ribatti D. Evaluation of microvascular density in tumors: pro and contra. Histol Histopathol. 2008;23(5):601–7. doi: 10.14670/HH-23.601. [DOI] [PubMed] [Google Scholar]
- 10.Zwaans BM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvascular Research. 2007;74(2-3):145–58. doi: 10.1016/j.mvr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18(9):1232–42. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 12.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21(8):1247–53. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 15.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 16.Samatov TR, Tonevitsky AG, Schumacher U. Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer. 2013;12(1):107. doi: 10.1186/1476-4598-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu CY, Tsai YP, Wu MZ, Teng SC, Wu KJ. Epigenetic reprogramming and post-transcriptional regulation during the epithelial-mesenchymal transition. Trends Genet. 2012;28(9):454–63. doi: 10.1016/j.tig.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Warzecha CC, Carstens RP. Complex changes in alternative pre-mRNA splicing play a central role in the epithelial-to-mesenchymal transition (EMT) Semin Cancer Biol. 2012;22(5-6):417–27. doi: 10.1016/j.semcancer.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33(14):1755–63. doi: 10.1038/onc.2013.128. [DOI] [PubMed] [Google Scholar]
- 20.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes & Development. 2013;27(20):2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papageorgis P. TGFbeta Signaling in Tumor Initiation, Epithelial-to-Mesenchymal Transition, and Metastasis. J Oncol. 2015;2015:587193. doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11(8):714–23. doi: 10.4161/cbt.11.8.15274. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6-7):303–14. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumour Biol. 2013;34(5):2497–506. doi: 10.1007/s13277-013-1002-x. [DOI] [PubMed] [Google Scholar]
- 25.Philip B, Ito K, Moreno-Sanchez R, Ralph SJ. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34(8):1699–707. doi: 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 26.Su J, Yin X, Zhou X, Wei W, Wang Z. The functions of F-box proteins in regulating the epithelial to mesenchymal transition. Curr Pharm Des. 2015;21(10):1311–7. doi: 10.2174/1381612821666141211144203. [DOI] [PubMed] [Google Scholar]
- 27.Greening DW, Gopal SK, Mathias RA, Liu L, Sheng J, Zhu HJ, et al. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin Cell Dev Biol. 2015;40:60–71. doi: 10.1016/j.semcdb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. The Journal of Cell Biology. 2006;172(7):973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nistico P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2) doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22(3):194–207. doi: 10.1016/j.semcancer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chouaib S, Janji B, Tittarelli A, Eggermont A, Thiery JP. Tumor plasticity interferes with anti-tumor immunity. Crit Rev Immunol. 2014;34(2):91–102. doi: 10.1615/critrevimmunol.2014010183. [DOI] [PubMed] [Google Scholar]
- 33.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 34.Pankova K, Rosel D, Novotny M, Brabek J. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell Mol Life Sci. 2010;67(1):63–71. doi: 10.1007/s00018-009-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KJ, Hong SW, Lee YS, Kim BW, Lee SC, Chang HS, et al. Tumor margin histology predicts tumor aggressiveness in papillary thyroid carcinoma: a study of 514 consecutive patients. J Korean Med Sci. 2011;26(3):346–51. doi: 10.3346/jkms.2011.26.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JA, Bae JW, Woo SU, Kim H, Kim CH. D2-40, Podoplanin, and CD31 as a Prognostic Predictor in Invasive Ductal Carcinomas of the Breast. Journal of Breast Cancer. 2011;14(2):104–11. doi: 10.4048/jbc.2011.14.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasebe T, Tamura N, Iwasaki M, Okada N, Akashi-Tanaka S, Hojo T, et al. Grading system for lymph vessel tumor emboli: significant outcome predictor for patients with invasive ductal carcinoma of the breast who received neoadjuvant therapy. Modern Pathology. 2010;23(4):581–92. doi: 10.1038/modpathol.2010.3. [DOI] [PubMed] [Google Scholar]
- 38.Banyard J, Chung I, Migliozzi M, Phan DT, Wilson AM, Zetter BR, et al. Identification of genes regulating migration and invasion using a new model of metastatic prostate cancer. BMC Cancer. 2014;14:387. doi: 10.1186/1471-2407-14-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14(1):79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Ozawa M, Kobayashi W. Reversibility of the Snail-induced epithelial-mesenchymal transition revealed by the Cre-loxP system. Biochem Biophys Res Commun. 2015;458(3):608–13. doi: 10.1016/j.bbrc.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Chui MH. Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. International Journal of Cancer. 2013;132(7):1487–95. doi: 10.1002/ijc.27745. [DOI] [PubMed] [Google Scholar]
- 44.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 45.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22(5-6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorges TM, Tinhofer I, Drosch M, Rose L, Zollner TM, Krahn T, et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, et al. Classification of Circulating Tumor Cells by Epithelial-Mesenchymal Transition Markers. PloS One. 2015;10(4):e0123976. doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular Cancer Research. 2011;9(8):997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Research. 2006;66(23):11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 50.Banyard J, Chung I, Wilson AM, Vetter G, Le Bechec A, Bielenberg DR, et al. Regulation of epithelial plasticity by miR-424 and miR-200 in a new prostate cancer metastasis model. Sci Rep. 2013;3:3151. doi: 10.1038/srep03151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709–24. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature Medicine. 2011;17(9):1101–8. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–36. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Q, Wang G, Chen Y, Li G, Yang D, Kang J. A miR-590/Acvr2a/Rad51b axis regulates DNA damage repair during mESC proliferation. Stem Cell Reports. 2014;3(6):1103–17. doi: 10.1016/j.stemcr.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. British Journal of Cancer. 2011;105(9):1338–41. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie G, Ji A, Yuan Q, Jin Z, Yuan Y, Ren C, et al. Tumour-initiating capacity is independent of epithelial-mesenchymal transition status in breast cancer cell lines. British Journal of Cancer. 2014;110(10):2514–23. doi: 10.1038/bjc.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Celia-Terrassa T, Meca-Cortes O, Mateo F, de Paz AM, Rubio N, Arnal-Estape A, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. The Journal of Clinical Investigation. 2012;122(5):1849–68. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barriere G, Fici P, Gallerani G, Fabbri F, Zoli W, Rigaud M. Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Ann Transl Med. 2014;2(11):109. doi: 10.3978/j.issn.2305-5839.2014.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ombrato L, Malanchi I. The EMT universe: space between cancer cell dissemination and metastasis initiation. Critical Reviews in Oncogenesis. 2014;19(5):349–61. doi: 10.1615/critrevoncog.2014011802. [DOI] [PubMed] [Google Scholar]
- 60.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697–711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Research. 2008;68(24):10377–86. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Research. 2006;66(16):8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Ma L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Reviews. 2012;31(3-4):653–62. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamouille S, Subramanyam D, Blelloch R, Derynck R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol. 2013;25(2):200–7. doi: 10.1016/j.ceb.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaravinos A. The Regulatory Role of MicroRNAs in EMT and Cancer. J Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 67.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & Development. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9(6):582–9. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Research. 2008;68(19):7846–54. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 70.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22(10):1686–98. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumarswamy R, Mudduluru G, Ceppi P, Muppala S, Kozlowski M, Niklinski J, et al. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer. 2012;130(9):2044–53. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- 72.Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10(24):4256–71. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 73.Moes M, Le Bechec A, Crespo I, Laurini C, Halavatyi A, Vetter G, et al. A novel network integrating a miRNA-203/SNAI1 feedback loop which regulates epithelial to mesenchymal transition. PloS One. 2012;7(4):e35440. doi: 10.1371/journal.pone.0035440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu X, Shen H, Yin X, Long L, Xie C, Liu Y, et al. miR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin. Oncogene. 2015 doi: 10.1038/onc.2015.84. in press. [DOI] [PubMed] [Google Scholar]
- 75.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–56. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drasin DJ, Guarnieri AL, Neelakantan D, Kim J, Cabrera JH, Wang CA, et al. TWIST1-Induced miR-424 Reversibly Drives Mesenchymal Programming while Inhibiting Tumor Initiation. Cancer Research. 2015;75(9):1908–21. doi: 10.1158/0008-5472.CAN-14-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan Y, Wang Q, Yan XL, Zhang Y, Li W, Tang F, et al. miR-10a controls glioma migration and invasion through regulating epithelial-mesenchymal transition via EphA8. FEBS Lett. 2015;589(6):756–65. doi: 10.1016/j.febslet.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Stinson S, Lackner MR, Adai AT, Yu N, Kim HJ, O'Brien C, et al. TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci Signal. 2011;4(177):ra41. doi: 10.1126/scisignal.2001538. [DOI] [PubMed] [Google Scholar]
- 79.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10(4):400–5. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141(7):1195–207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, et al. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PloS One. 2012;7(6):e39520. doi: 10.1371/journal.pone.0039520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nature Biotechnology. 2010;28(4):341–7. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–8. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 84.Valastyan S, Benaich N, Chang A, Reinhardt F, Weinberg RA. Concomitant suppression of three target genes can explain the impact of a microRNA on metastasis. Genes & Development. 2009;23(22):2592–7. doi: 10.1101/gad.1832709. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Wang Y, Shang Y. Epigenetic control of epithelial-to-mesenchymal transition and cancer metastasis. Experimental Cell Research. 2013;319(2):160–9. doi: 10.1016/j.yexcr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 86.Nickel A, Stadler SC. Role of epigenetic mechanisms in epithelial-to-mesenchymal transition of breast cancer cells. Transl Res. 2015;165(1):126–42. doi: 10.1016/j.trsl.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. The Journal of Clinical Investigation. 2014;124(12):5109–28. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vella LJ. The emerging role of exosomes in epithelial-mesenchymal-transition in cancer. Front Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9(3-4):358–67. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paget S. The Distribution of Secondary Growths in Cancer of the Breast. The Lancet. 1889;133(3421):571–3. [PubMed] [Google Scholar]
- 92.Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9(8):808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 93.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. Journal of Cellular Physiology. 2008;216(2):347–54. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 95.Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C, et al. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PloS One. 2013;8(9):e75054. doi: 10.1371/journal.pone.0075054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Research. 2011;71(15):5346–56. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 97.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 98.Yoshizaki T, Kondo S, Wakisaka N, Murono S, Endo K, Sugimoto H, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337(1):1–7. doi: 10.1016/j.canlet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 99.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–22. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog. 2013 doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Medicine. 2012;18(6):883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. The Journal of Clinical Investigation. 2009;119(6):1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]