Abstract
Importance
Percutaneous ventricular assist devices (PVADs) provide robust hemodynamic support compared with intra-aortic balloon pumps (IABPs), but clinical use patterns are unknown.
Objective
To examine contemporary patterns in PVAD use in the United States and compare them with use of IABPs.
Design, Setting, and Participants
Retrospective study of adults older than 18 years who received a PVAD or IABP while hospitalized in the United States (2007-2012).
Main Outcomes and Measures
Temporal trends in utilization, patient and hospital characteristics, in-hospital mortality, and cost of PVAD use compared with IABP.
Results
During 2007 through 2012, utilization of PVADs increased 30-fold (4.6 per million discharges in 2007 to 138 per million discharges in 2012; P for trend < .001) while utilization of IABPs decreased from 1738 per million discharges in 2008 to 1608 per million discharges in 2012 (P for trend = .02). In 2007, an estimated 72 hospitals used PVADs, increasing to 477 in 2011 (P for trend < .001). The number of hospitals with an annual volume of 10 or more PVAD procedures per year increased from 0 in 2007 to 102 in 2011 (21.4% ofPVAD-using hospitals; P for trend < .001). Among PVAD recipients, 67.3% had a diagnosis of cardiogenic shock or acute myocardial infarction (AMI). There was a temporal increase in the use of PVADs in older patients and patients with AMI, hypertension, diabetes mellitus, and chronic kidney disease (P for trend < .001 for all). Overall, mortality in PVAD recipients was 28.8%, and mean (SE) hospitalization cost was $85 580 ($4165); both were significantly higher in PVAD recipients with cardiogenic shock (mortality, 47.5%; mean [SE] cost, $113 695 [$6260]; P < .001 for both). The PVAD recipients were less likely than IABP recipients to have cardiogenic shock (34.3% vs 41.2%; P = .001), AMI (48.0% vs68.6%; P < .001), and undergo coronary artery bypass graft surgery (6.2% vs 43.2%; P < .001), but more likely to undergo percutaneous coronary intervention (70.9% vs 40.4%; P < .001). In propensity-matched analysis, PVADs were associated with higher mortality compared with IABP (odds ratio, 1.23 [95% CI, 1.06-1.43]; P = .007).
Conclusions and Relevance
There has been a substantial increase in the use of PVADs in recent years with an accompanying decrease in the use of IABPs. Given the high mortality, associated cost, and uncertain evidence for a clear benefit, randomized clinical trials are needed to determine whether use of PVADs leads to improved patient outcomes.
Cardiogenic shock is characterized by severe myocardial dysfunction, impairment in organ perfusion, and high mortality.1 Until recently, options for rapid mechanical circulatory support were limited to intra-aortic balloon pumps (IABPs). Recently, percutaneous ventricular assist devices (PVADs) were approved by the Food and Drug Administration (FDA) for temporary mechanical circulatory support. The Impella (Abiomed Inc) device is a catheter-based pump that is inserted via the femoral artery, advanced over a wire, and positioned across the aortic valve. The inlet of the device, positioned in the left ventricle, draws blood from the left ventricle and pumps it into the aorta. Conversely, the TandemHeart (CardiacAssist Inc) is a continuous-flow external pump.2,3 The device withdraws oxygenated blood via a large cannula inserted into the left atrium via transseptal route and then pumps it into the arterial system using a cannula inserted in the femoral artery. Both these devices can be readily inserted in the cardiac catheterization laboratory and provide up to 5 L per minute of cardiac output.
Clinical practice guidelines support the use of PVADs in patients with (1) cardiogenic shock as a “bridge to recovery” (class IIa), (2) acute myocardial infarction (AMI) with cardiogenic shock (class IIb), and (3) high-risk percutaneous coronary intervention (PCI) (class IIb).4,5 This is based on data showing superior hemodynamic parameters (eg, mean arterial pressure) with PVADs compared with IABPs.6,7 However, a reduction in hard clinical end points with PVADs has not been established.8-11 The PROTECT II trial, which compared Impella 2.5 with IABP in elective high-risk PCI and was powered for clinical end points, was stopped early because of futility.11 Given the lack of high-quality evidence from randomized trials, we examined contemporary patterns in the use of PVADs in the United States and compared them with IABPs.
Methods
The study was approved by the University of Iowa Institutional Review Board, which waived the requirement for informed consent because the study used deidentified data.
Data Sources
The National Inpatient Sample (NIS) is the largest all-payer in-patient database in the United States.12 Developed by the Agency for Healthcare Research and Quality, it comprises a 20% sample of all inpatient discharges from US hospitals. The database contains deidentified information regarding each hospitalization, including demographic characteristics, admission status, comorbidities, discharge diagnoses, procedures, outcomes, and cost of hospitalization. Patients admitted under observation status and patients admitted to short-term rehabilitation hospitals, long-term non–acute care hospitals, psychiatric hospitals, and alcoholism or chemical dependency units are not included. In the present study, we used data for the years 2007 through 2012.
The design of the NIS changed during our study.13 Between 2007 and 2011, the NIS comprised all inpatient discharges (100%) from a random 20% sample of acute-care hospitals in the United States. Patient-level and hospital-level weights were provided to obtain national estimates. However, in 2012, instead of including all discharges from a 20% sample of hospitals, the database was constructed using a systematic sampling of 20% of discharges from all (100%) hospitals stratified by hospital, census division, ownership status, urban vs rural location, teaching status, and bed size, as well as patient diagnosis-related group and admission month. Moreover, the discharge universe was redefined using the state in-patient database. Prior to release of the 2012 data, the impact of these changes was closely studied (see Supplement).13 To facilitate patient-level trend analysis, a new set of weights called “trend weights” were developed for the 2012 data, as well as for data for previous years (1993-2011).12,14 The trend weights are meant to replace the original NIS discharge weights for trend analysis spanning 2012 and earlier NIS data, to facilitate comparison of estimates from previous years with the 2012 data. We used the newly provided trend weights for all patient-level analyses.14 For hospital-level analyses, the NIS report does not describe the impact of the 2012 sampling methodology on hospital-level trends. Whereas the NIS data include a 20% sample of all discharges in the United States and discharging hospital is included in the sampling frame, the report states that the fraction of patients included from each hospital may vary as a result of missing data.13 Such variability would affect estimates of hospital PVAD volume for 2012. Because of the uncertainty regarding the appropriate methods to perform hospital-level trend analyses with data spanning year 2012, we have restricted hospital-level trend analysis to 2007 through 2011, when the sampling methodology was constant.15
Study Population and Variables
We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to identify all hospitalized adults aged at least 18 years who underwent implantation of a PVAD (ICD-9-CM code 37.68) or IABP (ICD-9-CM code 37.61), during 2007 through 2012. Data elements in the NIS include demographic characteristics (age, sex, race), primary and secondary discharge diagnoses, and procedures. The discharge diagnoses and procedures were recoded using the clinical classification of diseases (CCS) software into broad categories, available as separate variables within the NIS data set. We used the CCS coded discharge diagnoses for comorbid conditions. When CCS codes were not available, we used ICD-9-CM codes. We were also interested in examining how use of circulatory support devices differed in important subgroups. Because the NIS data set does not include information on procedural indication for PVAD implantation, we indirectly inferred indication using the discharge diagnoses and procedure codes and created the following 4 hierarchical subgroups: (1) cardiogenic shock, (2) AMI without cardiogenic shock, (3) PCI without AMI or cardiogenic shock, and (4) other. Other study variables included insurance status (Medicare, Medicaid, private, other), admission status (elective or non-elective), length of stay, and hospital characteristics (rural vs urban, teaching status, bed size, and ownership). Cost of hospitalization was obtained by multiplying hospital charges with the cost-to-charge ratios for each hospital for a given year, and indexing to year 2011 to adjust for inflation.16 Cost-to-charge ratios were not available for 2012.
Statistical Analysis
We followed there commendations from the Agency for Health-care Research and Quality for analysis using survey data. Survey-specific statements (eg, SURVEYFREQ, SURVEYMEANS) were used to obtain descriptive statistics. Patient-specific and hospital-specific discharge weights were used to obtain national estimates. For analysis of subpopulations, we used a domain analysis to ensure that our estimated population statistics and measures of variance were accurate.17 For tests of trend, we used the Cochrane Armitage test of trend for categorical variables and survey-specific linear regression for continuous variables.
First, we examined temporal trends in PVAD use (number of PVADs divided by number of discharges per million) and PVAD volume both overall and within clinical subgroups. We compared trends in PVAD use with trends in IABP use over the same period. Next, we examined temporal trends in characteristics of hospitals that performed PVAD implantation during the study period. Next, we examined trends in patient characteristics, in-hospital mortality, length of stay, and cost of hospitalization in the PVAD cohort over time. Cost and length of stay were log-transformed because they were not normally distributed, and trends in geometric means were examined.18 For analysis of calendar year trends in mortality, we adjusted for trends in patient characteristics over time using a multivariable logistic regression model for survey data (SURVEYLOGISTIC) and also accounted for hospital-level clustering of patients and the sampling design in our models using CLUSTER and STRATA statements, respectively. We included calendar year as a categorical variable and adjusted for all patient-level variables listed in eTable 1 in the Supplement. We also examined whether risk-adjusted mortality differed within our defined subgroups using similar methods.
Next, we compared patient characteristics and clinical outcomes between PVAD and IABP recipients. For this analysis, we excluded patients who received both IABP and PVAD during the same hospital stay. Differences in patient characteristics were compared using the Rao-Scott χ2 test for categorical variables and survey-specific t test for continuous variables. Finally, we examined whether the use of PVAD compared with IABP was associated with lower mortality. Given that patients who receive PVAD may differ from those who receive IABP in terms of baseline risk and disease severity (confounding by indication), we used a matched propensity score design for survey data to account for indication bias. To minimize confounding due to between-hospital differences in patients who receive IABP at hospitals that do not use PVADs, for the propensity-matched analyses we restricted the IABP recipients to only PVAD-using hospitals. Details of the propensity score estimation model and matching algorithm are provided in the Supplement. Briefly, we used a nonparsimonious multivariable logistic regression model to determine each patient's propensity of receiving a PVAD. In addition to patient-level covariates, we also included patient-level NIS weights in the propensity estimation model as recommended for such analyses using survey data.19 We then performed 2:1 matching between IABP and PVAD recipients based on propensity scores with a caliper width of one-quarter of the standard deviation of the logit of the propensity score, as well as the nearest available Mahalanobis metric20 (eMethods in the Supplement).We used the Cochrane Mantel-Haenszel test for matched data to compare the effect of PVAD with that of IABP on in-hospital mortality. All analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
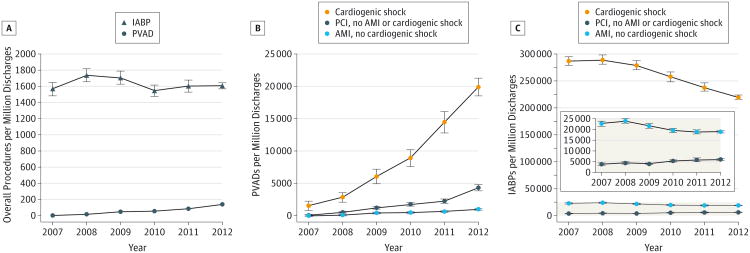
We found 2231 patients who received a PVAD, which translated to an estimated total of 10 793 patients in the 46 US states represented in the NIS. Between 2007 and 2012, there was a 30-fold increase in the estimated annual PVAD utilization (from 4.6 per million discharges in 2007 to 138 per million discharges in 2012) (Figure 1A) and a 25-fold increase in the estimated annual PVAD implantation volume (from 167 in 2007 to 4245 in 2012) (P for trend < .001 for both) (eFigure 2 in the Supplement). An increase in PVAD utilization was seen within all subgroups, with the greatest increase in patients with cardiogenic shock (from 1506 per million in 2007 to 19 913 per million in 2012; P for trend < .001) (Figure 1B). In contrast, the estimated annual IABP utilization increased slightly between 2007 and 2008 but decreased there after–both overall (P for trend = .02) (Figure 1A) and within the subgroups of patients with cardiogenic shock and AMI without cardiogenic shock (P for trend < .001) (Figure 1C). Utilization of IABP increased slightly in the subgroup of patients with PCI without cardiogenic shock or AMI (P for trend <.001) (Figure 1C).
Figure 1. Calendar Year Trends in the Use of Percutaneous Ventricular Assist Devices (PVADs) and Intra-aortic Balloon Pumps (IABPs) in the United States, 2007 Through 2012.
The figure shows estimated use of PVADs and IABPs per million discharges, and the error bars represent standard errors. A, Use of PVADs increased from 4.6 per million in 2007 to 138 per million in 2012 (P for trend < .001). In contrast, use of IABPs decreased from 1738 per million in 2008 to 1608 per million in 2012 (P for trend = .02). B, Use of PVADs increased in patients with cardiogenic shock, acute myocardial infarction (AMI) without cardiogenic shock, and percutaneous coronary intervention (PCI) without AMI or cardiogenic shock (P for trend < .001 for all). C, Use of IABPs decreased in patients with cardiogenic shock and AMI without cardiogenic shock but increased in patients who underwent PCI without cardiogenic shock or AMI (P for trend < .001 for all).
The estimated number of hospitals implanting PVADs increased from 72 in 2007 to 477 in 2011 (P for trend < .001) (Table 1). Median (range) hospital PVAD volume increased from 1 (1-6) to 2.9 (1-31), and the number of PVAD-using hospitals using more than 10 PVADs per year increased from 0 in 2007 to 102 in 2011 (21.4% of all PVAD-using hospitals; P for trend <.001) (Table 1). Whereas a majority of PVAD-using hospitals were teaching, had large bed size, and were not for profit, the proportion of small and medium-sized hospitals (P for trend <.001) as well as for-profit hospitals (P for trend = .01) implanting PVADs increased over time (Table 1).
Table 1. Hospital-Level Characteristics for Percutaneous Ventricular Assist Device (PVAD) Use.
| Characteristic | Hospitals With PVAD Use, All Indications | P value for Trend | ||||
|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | ||
| Hospitals using PVADs | ||||||
| Estimated No. (SE) | 72 (0.3) | 160 (0.4) | 346 (0.5) | 410 (0.6) | 477 (0.6) | <.001 |
| % (SE) | 1.4 (0.4) | 3.1 (0.5) | 6.7 (0.7) | 7.9 (0.7) | 9.3 (0.8) | <.001 |
| PVAD procedures per hospital, median (range) | 1 (1-6) | 1.4 (1-20) | 2.5 (1-19) | 2.2 (1-19) | 2.9 (1-31) | <.001 |
| Hospitals implanting ≥10 per year, % (SE) | 0 | 9.0 (4.9) | 9.8 (3.5) | 8.4 (3.0) | 21.4 (3.9) | <.001 |
| Bed size, % (SE)a | ||||||
| Small | 6.2 (6.0) | 3.1 (3.1) | 5.9 (2.7) | 7.1 (2.7) | 8.2 (2.7) | <.001b |
| Medium | 6.8 (6.5) | 12.1 (5.6) | 13.5 (3.9) | 17.8 (3.7) | 20.5 (3.5) | |
| Large | 87.0 (8.6) | 84.8 (6.2) | 80.5 (4.5) | 75.1 (4.2) | 71.2 (4.0) | |
| Teaching hospital, % (SE) | 66.1 (12.0) | 72.7 (7.5) | 66.6 (5.2) | 70.1 (4.5) | 62.2 (4.2) | .07 |
| Ownership, % (SE)c | ||||||
| Government | …c | 8.8 (4.9) | 7.5 (3.1) | 15.4 (3.9) | 6.1 (2.4) | .01d |
| Private | ||||||
| Nonprofit | …c | 81.9 (6.6) | 83.4 (4.5) | 68.0 (4.9) | 78.5 (4.0) | |
| For profit | …c | 9.3 (5.0) | 9.1 (3.5) | 16.6 (4.0) | 15.5 (3.5) | |
| Hospital in urban location, % (SE) | 93.6 (6.2) | 96.9 (3.0) | 87.9 (3.7) | 91.8 (2.9) | 95.9 (2.0) | .15 |
Bed size categorization using Agency for Healthcare Research and Quality methods based on number of hospital beds, hospital's location, and teaching status.
Large vs others, negative trend.
Hospital ownership information categories differently coded in 2007.
Trend for private (for profit) vs others.
The mean (SE) age of PVAD recipients was 65.0 (0.4) years. During the study period, there was a temporal increase in older age, comorbidities (eg, diabetes mellitus, hypertension, chronic kidney disease), use of PCI, and patients with Medicare insurance (Table 2). Overall, 45.4% had cardiogenic shock, 52.3% had AMI, 70.1% had congestive heart failure, and 66.9% received PCI (Table 2). Patient characteristics and outcomes according to the 4 subgroups are provided in eTable 2 in the Supplement. Patients with cardiogenic shock had a significantly higher prevalence of cardiac arrest, liver disease, and requirement of mechanical ventilation (P < .001 for all) (eTable 2 in the Supplement). Overall in-hospital mortality in PVAD recipients was 28.8% and remained unchanged over time in adjusted analyses (P for trend = .82) (Table 2). Mortality was highest in patients with cardiogenic shock (47.5%), which persisted even after differences in patient characteristics were accounted for (eTable 2 in the Supplement). The overall mean (SE) cost of hospitalization in PVAD recipients was $85 580 ($4165) and remained unchanged over time (Table 2). Mean (SE) cost was higher in PVAD recipients with cardiogenic shock ($113 695 [$6260]) compared with those with AMI without cardiogenic shock ($63 485 [$2458]) and PCI without cardiogenic shock or AMI ($48 900 [$1934]). In comparison, the mean (SE) cost of hospitalization in IABP recipients was $55 168 ($979).
Table 2. Patient-Level Characteristics for Percutaneous Ventricular Assist Device (PVAD) Use by Calendar Year.
| Characteristics | Overall | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | P Value for Trend |
|---|---|---|---|---|---|---|---|---|
| Total PVAD implants, weighted No., mean (SD) | 10793 (637) | 167 (57) | 559 (144) | 1409 (200) | 1705 (235) | 2709 (331) | 4245 (290) | <.001 |
| Patient Characteristics | ||||||||
| Age | ||||||||
| Mean (SE), y | 65.0 (0.4) | 55.1 (1.9) | 62.5 (2.1) | 64.9 (1.1) | 65.8 (0.9) | 64.8 (0.7) | 65.7 (0.5) | <.001 |
| ≥65 y, No. (%) | 54.5 (1.3) | 25.8 (9.3) | 45.5 (5.5) | 56.0 (3.8) | 57.4 (2.5) | 53.5 (2.5) | 55.7 (1.8) | <.001 |
| Male sex, % (SE) | 73.8 (0.9) | 71.8 (6.2) | 72.9 (3.0) | 76.3 (2.2) | 73.0 (2.6) | 73.2 (1.6) | 73.9 (1.6) | .65 |
| Race, % (SE) | ||||||||
| White | 62.4 (1.9) | 48.7 (9.3) | 56.9 (8.4) | 62.6 (4.8) | 58.3 (4.0) | 60.2 (4.5) | 66.5 (2.4) | .048a |
| Black | 10.3 (1.1) | 12.1 (6.7) | 6.8 (2.3) | 10.3 (2.9) | 11.8 (2.9) | 9.6 (2.0) | 10.6 (1.5) | |
| Others | 15.2 (1.2) | 8.5 (5.3) | 19.9 (9.2) | 12.8 (3.3) | 16.7 (2.6) | 16.0 (2.7) | 14.5 (1.5) | |
| Missing/unknown | 12.1 (1.9) | 30.7 (12.2) | 16.4 (5.8) | 14.3 (4.8) | 13.2 (3.5) | 14.2 (5.4) | 8.4 (1.9) | |
| Income quartiles, % (SE)b | ||||||||
| 0-25 | 29.8 (1.6) | 18.2 (7.7) | 27.5 (6.0) | 22.5 (3.9) | 34.6 (4.2) | 32.7 (3.3) | 29.3 (2.0) | <.001c |
| 26-50 | 26.1 (1.2) | 19.3 (6.5) | 22.1 (4.9) | 23.1 (3.5) | 24.5 (2.4) | 24.3 (2.1) | 29.8 (1.8) | |
| 51-75 | 23.1 (1.1) | 25.1 (7.7) | 29.0 (3.9) | 24.8 (3.2) | 22.7 (3.1) | 21.8 (1.8) | 22.5 (1.5) | |
| 76-100 | 21.0 (1.5) | 37.4 (10.0) | 21.4 (4.9) | 29.6 (4.9) | 18.2 (3.2) | 21.2 (3.0) | 18.4 (1.7) | |
| Discharge diagnoses, % (SE) | ||||||||
| Cardiogenic shock | 45.5 (1.5) | 56.2 (12.5) | 35.6 (5.1) | 35.0 (4.1) | 42.8 (3.4) | 52.0 (3.0) | 46.6 (2.2) | <.001 |
| Acute myocardial infarction | 52.3 (1.2) | 33.4 (8.7) | 38.0 (3.9) | 47.9 (2.8) | 52.6 (3.2) | 55.0 (2.2) | 54.6 (2.0) | <.001 |
| Congestive heart failure | 70.1 (1.2) | 83.7 (7.7) | 63.4 (7.2) | 64.2 (3.6) | 72.8 (2.7) | 74.0 (2.0) | 68.8 (1.9) | .17 |
| Coronary artery disease | 82.4 (1.1) | 47.2 (10.4) | 77.1 (5.2) | 81.7 (2.6) | 80.5 (2.8) | 82.3 (1.7) | 85.5 (1.5) | <.001 |
| Cardiac arrest | 21.0 (1.0) | 37.9 (10.0) | 12.8 (3.7) | 16.1 (2.1) | 18.8 (2.1) | 22.3 (1.9) | 23.1 (1.5) | <.001 |
| Valvular heart disease | 23.0 (1.0) | 35.0 (6.7) | 23.5 (3.8) | 24.9 (2.9) | 18.3 (2.2) | 25.4 (2.0) | 22.3 (1.6) | .11 |
| Peripheral artery disease | 13.4 (0.8) | 5.4 (5.4) | 11.0 (2.7) | 12.9 (1.9) | 11.7 (1.7) | 14.5 (1.4) | 14.2 (1.3) | <.001 |
| Arrhythmia | 48.1 (1.2) | 45.9 (4.5) | 38.9 (4.2) | 47.4 (3.7) | 42.8 (3.2) | 52.3 (2.5) | 49.1 (1.9) | <.001 |
| Comorbid conditions | ||||||||
| Hypertension | 60.4 (1.3) | 43.7 (10.2) | 43.6 (9.0) | 61.1 (2.7) | 60.0 (3.3) | 58.9 (2.2) | 64.3 (2.0) | <.001 |
| Diabetes mellitus | ||||||||
| Uncomplicated | 31.3 (1.1) | 25.2 (6.1) | 34.4 (5.1) | 29.1 (3.2) | 28.9 (2.5) | 32.2 (2.0) | 32.3 (1.9) | .04 |
| Complicated | 10.1 (0.6) | 2.9 (2.7) | 5.9 (1.9) | 7.5 (1.4) | 10.7 (1.9) | 11.3 (1.1) | 10.8 (1.1) | <.001 |
| Cancer | 8.2 (0.6) | 13.1 (3.9) | 8.2 (2.6) | 9.0 (1.5) | 6.9 (1.3) | 9.8 (1.0) | 7.2 (0.9) | .04d |
| Liver disease | 18.4 (1.1) | 33.4 (8.2) | 12.8 (3.4) | 16.5 (3.6) | 17.1 (2.2) | 21.5 (2.3) | 17.7 (1.4) | .48 |
| COPD | 16.6 (0.9) | 8.4 (5.3) | 16.6 (3.2) | 14.7 (1.6) | 15.2 (2.1) | 18.5 (1.8) | 17.0 (1.4) | .004 |
| Dyslipidemia | 47.4 (1.3) | 26.1 (8.0) | 34.1 (6.8) | 44.7 (3.6) | 45.7 (3.3) | 49.2 (2.2) | 50.4 (2.1) | <.001 |
| Chronic kidney disease | 25.9 (1.0) | 21.3 (10.0) | 21.0 (4.7) | 27.4 (2.8) | 25.1 (2.5) | 25.3 (2.0) | 27.0 (1.5) | .03 |
| Fluid/electrolyte disorder | 40.7 (1.4) | 36.1 (7.0) | 27.2 (4.7) | 35.9 (4.0) | 35.7 (3.2) | 44.6 (2.8) | 43.7 (2.0) | <.001 |
| Coagulation disorder | 20.0 (1.1) | 33.1 (11.6) | 16.0 (5.3) | 15.0 (3.0) | 19.0 (2.7) | 24.5 (2.4) | 19.3 (1.4) | .049 |
| Substance abuse | 1.9 (0.3) | 0 | 1.7 (1.0) | 2.2 (0.9) | 1.4 (0.6) | 1.7 (0.5) | 2.1 (0.5) | .19 |
| Procedures, % (SE) | ||||||||
| PCI | 66.9 (1.5) | 31.7 (12.2) | 72.0 (4.8) | 73.1 (4.2) | 72.8 (3.8) | 62.2 (2.8) | 66.3 (1.9) | .02d |
| CABG | 9.3 (0.8) | 13.8 (4.8) | 8.6 (3.4) | 8.1 (1.9) | 6.3 (1.2) | 10.8 (1.7) | 9.8 (1.2) | .03 |
| Mechanical ventilation | 30.8 (1.3) | 54.4 (11.6) | 26.5 (5.9) | 28.9 (3.5) | 30.2 (3.0) | 34.8 (2.7) | 28.7 (1.8) | |
| Administrative/Financial Details | ||||||||
| Payment source, % (SE) | ||||||||
| Medicare | 56.8 (1.3) | 34.5 (10.0) | 43.4 (4.8) | 56.3 (3.2) | 57.9 (2.8) | 54.3 (2.8) | 60.8 (1.8) | <.001e |
| Medicaid | 8.5 (0.7) | 4.2 (3.3) | 13.3 (5.5) | 10.0 (2.2) | 6.8 (1.7) | 10.2 (1.4) | 7.1 (0.9) | |
| Private insurance | 27.3 (1.2) | 46.0 (7.9) | 39.1 (5.0) | 27.7 (2.4) | 27.8 (3.1) | 27.8 (2.8) | 24.4 (1.6) | |
| Others | 7.4 (0.7) | 15.3 (7.3) | 4.2 (2.2) | 6.0 (1.3) | 7.5 (1.8) | 7.7 (1.3) | 7.7 (1.1) | |
| Elective admission | 24.7 (1.3) | 29.2 (11.7) | 36.6 (5.8) | 26.7 (3.7) | 24.6 (2.8) | 21.1 (2.6) | 24.7 (1.8) | <.001d |
| Hospitalization outcome, % (SE) | ||||||||
| Home or self-care | 38.0 (1.3) | 20.3 (9.3) | 45.6 (5.4) | 46.2 (2.8) | 38.7 (2.8) | 33.3 (2.7) | 37.5 (2.1) | |
| Short-term hospital | 4.4 (0.5) | 3.2 (3.3) | 4.5 (2.7) | 4.0 (1.0) | 4.7 (1.3) | 5.3 (1.2) | 3.8 (0.7) | |
| Skilled care facility | 16.7 (0.9) | 21.1 (8.8) | 11.7 (3.1) | 14.3 (2.4) | 17.8 (2.6) | 19.2 (1.7) | 16.0 (1.3) | <.001 |
| Home health care | 12.2 (0.8) | 25.8 (10.5) | 11.0 (3.6) | 12.6 (2.3) | 11.7 (1.6) | 12.3 (1.6) | 11.8 (1.1) | |
| Died | 28.8 (1.1) | 29.6 (10.3) | 27.2 (4.8) | 22.9 (2.7) | 27.1 (2.6) | 29.9 (2.1) | 31.0 (1.8) | |
| Length of stay, mean (SE), d | 12.1 (0.5) | 19.3 (3.6) | 10.4 (1.6) | 13.0 (1.6) | 10.8 (0.9) | 13.4 (1.1) | 11.4 (0.7) | .06 |
| Hospitalization cost, % (SE), US$ | 85 580 (4165) | 95 855 (15 112) | 79623 (10 948) | 79 292 (5610) | 82 815 (8100) | 91 086 (5862) | …f | .46 |
| Unadjusted mortality, % (SE) | 28.8 (1.1) | 29.6 (10.3) | 27.2 (4.8) | 22.9 (2.7) | 27.1 (2.6) | 29.9 (2.1) | 31.0 (1.8) | <.001 |
| Mortality, yearly risk-adjusted odds ratio (95% CI)g | … | 1 [Reference] | 0.90 (0.3-2.6) | 0.69 (0.2-1.8) | 0.85 (0.2-2.3) | 0.76 (0.3-2.0) | 0.90 (0.3-2.4) | .70 |
Abbreviations: CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention.
For trends between whites vs others.
Median household income quartiles based on patient zip code.
Trend for lowest quartile (0-25th percentile) vs others.
P value for negative trend.
For Medicare vs others.
Cost data not available for 2012.
Obtained using a multivariable logistic regression model for survey data. Full model in eTable 1 in the Supplement.
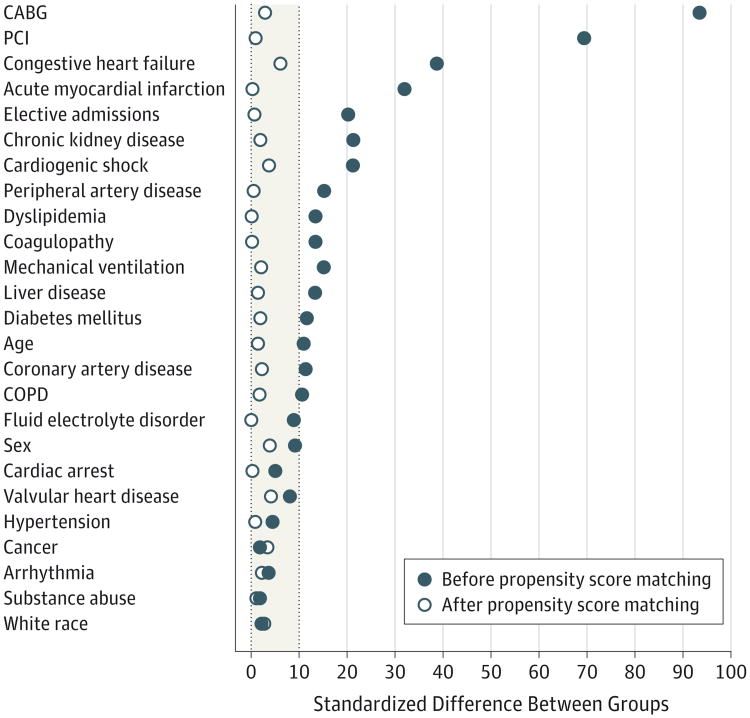
We also compared patient characteristics and outcomes in PVAD (n = 1675, unweighted) and IABP (n = 63 384, unweighted) recipients after excluding patients who received both devices during the same hospital stay (Table 3). Compared with IABP recipients, PVAD recipients were less likely to have cardiogenic shock, AMI, and undergo coronary artery bypass graft surgery but more likely to have congestive heart failure or chronic kidney disease and undergo PCI (P <.001 for all). We conducted a propensity score–matched analysis (eTable 3 in the Supplement) and successfully matched 1446 patients (86% of sample, unweighted) who received a PVAD with 2888 patients who received an IABP. We confirmed that matching was successful in achieving covariate balance between the PVAD and IABP groups as demonstrated by a standardized difference of less than 10% for all covariates after matching (Figure 2). In propensity score–matched analysis, use of PVAD was associated with higher mortality (odds ratio, 1.23 [95% CI, 1.06-1.43]; P = .007).
Table 3. Differences in Patient Characteristics Between Percutaneous Ventricular Assist Device (PVAD) and Intra-aortic Balloon Pump (IABP) Recipientsin the United States, 2007 Through 2012.
| Characteristic | % (SE) | P Value | |
|---|---|---|---|
| PVAD | IABP, All Programs | ||
| Estimated procedure volume, weighted No. (SD) | 8123 (499) | 304 729 (9612) | <.001 |
| Patient Characteristics | |||
| Age | |||
| Mean (SE), y | 66.1 (0.4) | 64.9 (0.1) | .007 |
| ≥65 y, No. (%) | 58.0 (1.4) | 52.5 (0.3) | <.001 |
| Male sex | 73.6 (1.1) | 69.0 (0.2) | <.001 |
| Race | |||
| White | 63.4 (1.9) | 64.2 (1.0) | .03 |
| Nonwhite | 24.7 (1.7) | 20.6 (0.7) | |
| Missing/unknown | 12.0 (1.9) | 15.2 (1.1) | |
| Income quartilea | |||
| 0-25 | 31.1 (1.7) | 26.4 (0.8) | .01 |
| 26-50 | 26.3 (1.2) | 26.7 (0.7) | |
| 51-75 | 22.2 (1.2) | 24.5 (0.5) | |
| 76-100 | 20.4 (1.6) | 22.4 (1.2) | |
| Discharge diagnoses | |||
| Cardiogenic shock | 34.3 (1.6) | 41.2 (0.6) | .001 |
| Acute myocardial infarction | 48.0 (1.3) | 68.6 (0.5) | <.001 |
| Congestive heart failure | 70.5 (1.3) | 47.9 (0.5) | <.001 |
| Coronary artery disease | 85.0 (1.0) | 82.5 (0.4) | .02 |
| Cardiac arrest | 17.4 (1.0) | 18.9 (0.3) | .16 |
| Valvular heart disease | 22.4 (1.1) | 23.5 (0.4) | .31 |
| Peripheral artery disease | 14.5 (0.9) | 8.5 (0.2) | <.001 |
| Arrhythmia | 45.5 (1.4) | 44.1 (0.4) | .29 |
| Comorbid conditions | |||
| Hypertension | 63.1 (1.5) | 60.0 (0.5) | .04 |
| Diabetes mellitus | 42.8 (1.3) | 36.5 (0.4) | <.001 |
| Cancer | 8.4 (0.6) | 7.5 (0.2) | .12 |
| Liver disease | 15.6 (1.2) | 9.3 (0.2) | <.001 |
| COPD | 18.4 (1.0) | 15.6 (0.3) | .003 |
| Dyslipidemia | 50.4 (1.4) | 47.4 (0.5) | .03 |
| Chronic kidney disease | 27.3 (1.1) | 16.1 (0.3) | <.001 |
| Fluid/electrolyte disorder | 35.5 (1.5) | 35.1 (0.6) | .81 |
| Coagulation disorder | 16.4 (1.1) | 18.0 (0.4) | .17 |
| Substance abuse | 2.0 (0.3) | 2.0 (0.1) | .85 |
| Procedures | |||
| PCI | 70.9 (1.5) | 40.4 (0.5) | <.001 |
| CABG | 6.2 (0.7) | 43.2 (0.6) | <.001 |
| Mechanical ventilation | 24.3 (1.3) | 29.8 (0.5) | <.001 |
| Administrative/Financial Details | |||
| Payment source | |||
| Medicare | 60.9 (1.5) | 51.6 (0.4) | <.001 |
| Medicaid | 7.5 (0.7) | 7.5 (0.2) | |
| Private insurance | 25.0 (1.4) | 31.3 (0.4) | |
| Others | 6.6 (0.7) | 9.6 (0.2) | |
| Elective admission | 27.8 (1.5) | 18.0 (0.5) | <.001 |
| Hospitalization outcome | |||
| Home or self-care | 45.8 (1.4) | 34.6 (0.5) | <.001 |
| Short-term hospital | 3.3 (0.5) | 7.5 (0.3) | |
| Skilled care facility | 16.3 (1.0) | 19.7 (0.3) | |
| Home health care | 12.7 (0.9) | 18.3 (0.4) | |
| Discharged alive, unknown | 0.2 (0.1) | 0.4 (0.0) | |
| Died | 21.7 (1.2) | 19.5 (0.3) | |
| Length of stay, mean (SE), d | 10.4 (0.5) | 11.3 (0.1) | <.001b |
| Unadjusted mortality | 21.7 (1.1) | 19.5 (0.3) | .05 |
Abbreviations: CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; PVAD, percutaneous ventricular assist device.
Median household income quartiles based on patient zip code.
For differences in geometric means of length of stay.
Figure 2. Standardized Differences Between Variables Before and After Propensity Matching for Intra-aortic Balloon Pumps vsPercutaneous Ventricular Assist Devices.
Vertical dotted lines indicate the acceptable range of standardized difference after propensity score matching (0-10%). CABG indicates coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention.
Discussion
We found a 30-fold increase in the utilization of PVADs in the United States over a 6-year period. The marked increase in PVAD utilization nationally has occurred because of both an increase in volume at each hospital, as well as introduction of PVAD procedures to new hospitals, and has been accompanied by a simultaneous decrease in the utilization of IABPs. We found that PVADs are being increasingly used in an older and sicker population, and most PVAD recipients have cardiogenic shock or AMI. Mortality among PVAD recipients is high (28.8%), especially in patients with cardiogenic shock (47.5%). Although substantially more expensive than IABPs, we did not find PVAD use to be associated with lower mortality in a matched propensity score analysis. A number of our findings are important and merit further consideration.
Several factors have likely contributed to the national trends in utilization of PVADs and IABPs observed in our study. First, before the introduction of PVADs into clinical practice, there was a relative dearth of temporary mechanical circulatory support devices, which were mostly limited to IABPs. Compared with IABPs, which only provide 0.5 L/min of cardiac output, PVADs provide more robust hemodynamic support (up to 5 L/min).10,21 Clinical studies have shown that PVADs are more effective in improving hemodynamic parameters in patients with cardiogenic shock such as improvement in cardiac index (mean difference, 0.35 L/min/m2), mean arterial pressure (mean difference, 12.8 mm Hg), and reduction in pulmonary capillary wedge pressure (mean difference, −5.3 mm Hg).8 Second, recent randomized clinical trials have questioned the benefit of IABP in reducing mortality for patients with AMI with cardiogenic shock undergoing early PCI or elective high-risk PCI.22,23 Both factors may partly explain the shift in enthusiasm away from IABP toward PVAD that we observed. Third, PVADs can be readily inserted in the cardiac catheterization laboratory using standard percutaneous techniques. As a result, a number of interventional cardiologists have become facile with implanting these devices. Finally, expedited regulatory approval, aggressive marketing from device manufacturers, physician training programs, and generous physician and hospital reimbursement for using PVADs may have also contributed to the growth in PVAD volume nationally.24
Whereas improvement in hemodynamic parameters with PVAD implantation has been documented, improvement in survival has not been proven. The PROTECT II trial was designed to evaluate whether use of the Impella 2.5 was superior to IABP in patients undergoing elective high-risk PCI. After less than 70% of enrollment was completed, the trial was stopped early because of futility. The primary end point–a combination of 10 intraprocedural and postprocedural events at 30 days–occurred in 35.1% in the Impella group and 40.1% in the IABP group (P = .23). In the per-protocol analyses, there was a lower incidence of the primary end point in the Impella group (40.0% [Impella] vs 51.0% [IABP]; P = .02) at 90 days; however, there was no difference in mortality (11.6% [Impella] vs 9.0% [IABP]; P = .38). Because this difference was not seen in the primary intention-to-treat analyses, further confirmation from future studies is required. We are not aware of any other randomized clinical trials that have examined clinical end points with the use of PVADs in other settings (eg, cardiogenic shock, AMI). The MINI-AMI trial designed to examine the efficacy of use of the Impella 2.5 device in reducing infarct size in patients with ST-segment elevation myocardial infarction was terminated after only 5 patients were enrolled because of undisclosed reasons, forgoing the opportunity to examine whether PVADs are effective in this setting.25
In contrast to randomized clinical trials, in our observational study, we found that PVAD implantation was associated with higher in-hospital mortality compared with IABP use (odds ratio,1.23[95% CI, 1.06-1.43]). Whereas we used a propensity score– matched analysis to explicitly account for confounding by indication, and our matching algorithm was successful in achieving covariate balance between PVAD and IABP recipients, it is still possible that PVAD recipients in our study were sicker compared with IABP recipients because of unmeasured confounders (eg, severity of underlying illness, comorbidities, or high-risk coronary anatomy). Nevertheless, our findings highlight the uncertainty regarding the clinical benefit of PVAD implantation in contemporary practice.
Although the use of PVADs has grown exponentially in recent years, mortality in PVAD recipients is high (28.8%) and has remained unchanged. Mortality was highest in patients with cardiogenic shock (47.5%), followed by AMI without cardiogenic shock (17.0%), whereas patients with PCI without cardiogenic shock or AMI had the lowest mortality (3.3%). The risk of bleeding, vascular injury, and limb ischemia can be substantial in PVAD recipients given the use of large-sized cannulae (Impella: 13F; TandemHeart: 15-17F for arterial, 21F for venous access).8
Moreover, the cost associated with PVADs is also substantial. The cost of the Impella 2.5 device alone is more than $20 000.26 We estimated that the mean cost of hospitalization in PVAD recipients was $85 580 per patient, which would translate into a total cost of approximately $1 billion during 2007 through 2012. In comparison, the mean hospitalization cost in all IABP recipients was $55 168 and that for all patients with cardiogenic shock was $48 097 during 2011 (data not shown). Whereas patients who receive mechanical circulatory support devices such as PVADs are, by definition, critically ill and therefore require resource-intensive care, it is likely that at least some portion of the cost is attributed to the PVAD procedure and post-procedure care. Given the high mortality, uncertain evidence for a clear benefit, risk of complications, and cost, there is a pressing need for carefully designed, adequately powered randomized clinical trials to determine the clinical effectiveness of PVADs in improving patient outcomes.
Our study findings should also spark a debate on the process of regulation of medical devices. The Impella devices and the TandemHeart device were approved by the FDA using the 510(K) mechanism, which is based on demonstration of “substantial equivalence” to a previously cleared device.27,28 For example, the Impella 2.5 received FDA approval on the basis of substantial equivalence to the TandemHeart, which in turn was approved on the basis of substantial equivalence to the Medtronic BP-80 Bio-Pump device, a non percutaneous temporary left ventricular support device,29 and so on. Although the 510(K) process is a faster and less stringent mechanism to obtain FDA clearance, demonstration of substantial equivalence hardly ensures that a device is effective in improving clinical outcomes and that its benefits outweigh the risks, at a cost that is acceptable. Moreover, premarket notification using the 510(K) pathway may hamper future trials because patients and physicians have ready access to the device outside a clinical trial. Therefore, in a recently commissioned report from the FDA, the Institute of Medicine has recommended that the 510(K) pathway be completely eliminated and be replaced with an integrated premarketing and postmarketing surveillance that provides reasonable assurance of safety and effectiveness throughout the device life cycle.30 Such measures would ensure ongoing scrutiny for high-risk medical devices to ensure that they are safe and effective in clinical practice.
Our study findings should be interpreted in light of the following limitations. First, because of reliance on ICD-9-CM codes within administrative data, we were unable to distinguish whether a patient received an Impella or TandemHeart because both devices have the same ICD-9-CM procedure code. However, in our experience, most of the growth in PVAD use in recent years has occurred with the use of Impella. In 2013, Abiomed announced the implantation of the 15 000th Impella device in the United States, which suggests continued growth in PVAD use even after 2012.31 Second, we lacked information regarding the clinical indication of PVAD implantation and indirectly inferred that information from the discharge diagnoses. Although there is potential for misclassification of patients based on this approach, our mortality estimates within each group are consistent with published literature.1,32,33 Third, data regarding cause of death and procedural complications are not consistently recorded in the NIS data, which makes it difficult to determine whether patients died as a result of underlying illness or a complication from the PVAD procedure. Fourth, although we used a matched propensity score design to account for indication bias and our matching algorithm was successful in achieving covariate balance, important clinical variables that may be predictors of outcomes, as well as receipt of PVAD (eg, ejection fraction, coronary anatomy, underlying disease severity), were not available. Fifth, because of the administrative nature of data, we were unable to distinguish comorbidities from complications of hospitalization.
Conclusions
We found a marked increase in the use of PVADs in recent years, and this increase was accompanied by a decrease in the use of IABPs. Given the increasing use, costs, and potential complications of PVADs, evidence from well-conducted randomized clinical trials is needed to ensure that use of PVADs leads to improved patient outcomes.
Supplementary Material
Acknowledgments
Funding/Support: This study is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award K08HL122527 (Dr Girotra) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases under award K24-AR062133 (Dr Cram).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Khera and Girotra had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Khera, Gerke, Horwitz, Girotra.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Khera, Cram, Girotra.
Critical revision of the manuscript for important intellectual content: Khera, Lu, Vyas, Gerke, Rosenthal, Horwitz, Girotra.
Statistical analysis: Khera, Lu, Girotra.
Obtained funding: Girotra.
Administrative, technical, or material support: Cram, Lu, Rosenthal, Girotra.
Study supervision: Cram, Girotra.
Conflict of Interest Disclosures: Dr Horwitz receives grant support from Edwards Lifesciences, St Jude Medical, and Biotronik. No other disclosures are reported.
Additional Contributions: We are immensely grateful to Mary S. Vaughan-Sarrazin, PhD, Department of Internal Medicine, University of Iowa Carver College of Medicine, for her critical input and guidance for the matched propensity score analysis for survey data. Dr Vaughan-Sarrazin did not receive any compensation for her effort.
Correction: This article was corrected on May 5, 2015. The footnotes for Table 2 were missing when the article posted online March 30, 2015.
Contributor Information
Rohan Khera, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
Peter Cram, Department of Medicine, University of Toronto, Toronto, Ontario, Canada; Division of General Internal Medicine, University Health Network/Mt Sinai Hospitals, Toronto, Ontario, Canada.
Xin Lu, Institute of Clinical and Translational Science, University of Iowa Carver College of Medicine, Iowa City.
Ankur Vyas, Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
Alicia Gerke, Division of Pulmonary, Critical Care, and Occupational Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
Gary E. Rosenthal, Institute of Clinical and Translational Science, University of Iowa Carver College of Medicine, Iowa City; Division of General Internal Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
Phillip A. Horwitz, Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
Saket Girotra, Institute of Clinical and Translational Science, University of Iowa Carver College of Medicine, Iowa City; Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
References
- 1.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 2.Kar B, Basra SS, Shah NR, Loyalka P. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation. 2012;125(14):1809–1817. doi: 10.1161/CIRCULATIONAHA.111.040220. [DOI] [PubMed] [Google Scholar]
- 3.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35(3):156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Blankenship JC, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 5.O'Gara PT, Kushner FG, Ascheim DD, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 6.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 7.Shah NR, Bieniarz MC, Basra SS, et al. Serum biomarkers in severe refractory cardiogenic shock. JACC Heart Fail. 2013;1(3):200–206. doi: 10.1016/j.jchf.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 9.Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.e1–469.e8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 12.Rockville, MD: Agency for Healthcare Research and Quality; Jul, 2014. [Accessed October 24. 2014]. Healthcare Cost and Utilization Project (HCUP) Overview of the National (Nationwide) Inpatient Sample (NIS) http://www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 13.Houchens RL, Ross DN, Elixhauser A, Jiang J. Nationwide Inpatient Sample Redesign: Final Report. [Accessed July 20, 2014];2014 Apr 4; https://www.hcup-us.ahrq.gov/db/nation/nis/reports/NISRedesignFinalReport040914.pdf.
- 14.HCUP. Rockville, MD: Agency for Healthcare Research and Quality; Sep, 2014. [Accessed July 20, 2014]. Trend Weights for HCUP NIS Data. http://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. [Google Scholar]
- 15.HCUP. Rockville MD: Agency for Healthcare Research and Quality; [Accessed October 24, 2014]. HCUP National Estimates. http://www.hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course.htm. [Google Scholar]
- 16.US Bureau of Labor Statistics. CPI inflation calculator. [Accessed May 30, 2014];2014 http://www.bls.gov/data/inflation_calculator.htm.
- 17.Houchens R, Elixhauser A. HCUP Method Series Report 2003-02. Rockville, MD: Agency for Healthcare Research and Quality; Jun 2005, [Accessed May 13, 2014]. Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. http://www.hcup-us.ahrq.gov/reports/methods/CalculatingNISVariances200106092005.pdf. [Google Scholar]
- 18.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312(7038):1079. doi: 10.1136/bmj.312.7038.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. doi: 10.1111/1475-6773.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng W, Jun Y, Xu R. A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study. Paper presented at: SAS PharmaSUG 2006 Conference; May 22, 2006; Florida: Bonita Springs; [Google Scholar]
- 21.Dixon SR, Henriques JP, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I Trial): initial US experience. JACC Cardiovasc Interv. 2009;2(2):91–96. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Thiele H, Zeymer U, Neumann FJ, et al. IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 23.Perera D, Stables R, Clayton T, et al. BCIS-1 Investigators. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013;127(2):207–212. doi: 10.1161/CIRCULATIONAHA.112.132209. [DOI] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Warning Letter: Abiomed. [Accessed July 20, 2014];2011 Jun 10; http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm260341.htm.
- 25.MINI-AMI: Minimizing Infarct Size With Impella 2.5 Following PCI for Acute Myocardial Infarction. [Accessed June 24, 2014]; http://clinicaltrials.gov/show/NCT01319760.
- 26.Yu D. The Advisory Board Company website; Nov 14, 2011. [Accessed October 23, 2014]. TCT 2011: Cost-effectiveness data and society guidelines shed new light on Abiomed's Impella 2.5. http://www.advisory.com/Research/Service-Line-Strategy-Advisor/the-pipeline/2011/11/tct-2011-cost-effectiveness-data-and-society-guidelines-shed-new-light-on-abiomeds-impella-25. [Google Scholar]
- 27.Curfman GD, Redberg RF. Medical devices–balancing regulation and innovation. N Engl J Med. 2011;365(11):975–977. doi: 10.1056/NEJMp1109094. [DOI] [PubMed] [Google Scholar]
- 28.Challoner DR, Vodra WW. Medical devices and health–creating a new regulatory framework for moderate-risk devices. N Engl J Med. 2011;365(11):977–979. doi: 10.1056/NEJMp1109150. [DOI] [PubMed] [Google Scholar]
- 29.Food and Drug Administration. 510(k) Premarket Notification: K991783. [Accessed October 20, 2014];2000 http://www.accessdata.fda.gov/cdrh_docs/pdf/K991783.pdf.
- 30.Medical Devices and the Public's Health: The FDA 510(k) Clearance Process at 35 Years. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 31.Abiomed website; Aug 2, 2013. [Accessed June 24,2014]. Abiomed Surpasses 15,000 Impella Patients in the United States: New Impella CP Reaches 1,000th Global Patient Milestone. press release. http://investors.abiomed.com/releasedetail.cfm?ReleaseID=782468. [Google Scholar]
- 32.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 33.Sjauw KD, Konorza T, Erbel R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device: the Europella registry. J Am Coll Cardiol. 2009;54(25):2430–2434. doi: 10.1016/j.jacc.2009.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.