Abstract
Case reports describe significant norovirus gastroenteritis morbidity in immunocompromised patients. We evaluated norovirus pathogenesis in prospectively enrolled solid organ (SOT) and hematopoietic stem cell transplant (HSCT) patients with diarrhea who presented to Texas Children’s Hospital and submitted stool for enteric testing. Noroviruses were detected by real-time reverse transcription polymerase chain reaction. Clinical outcomes of norovirus diarrhea and non-norovirus diarrhea patients, matched by transplanted organ type, were compared. Norovirus infection was identified in 25 (22%) of 116 patients, more frequently than other enteropathogens. Fifty percent of norovirus patients experienced diarrhea lasting ≥14 days, with median duration of 12.5 days (range 1 – 324 days); 29% developed diarrhea recurrence. Fifty-five percent of norovirus patients were hospitalized for diarrhea, with 27% requiring intensive care unit (ICU) admission. One HSCT recipient developed pneumatosis intestinalis. Three HSCT patients expired ≤6 months of norovirus diarrhea onset. Compared to non-norovirus diarrhea patients, norovirus patients experienced significantly more frequent ICU admission (27% vs. 0%, p = 0.02), greater serum creatinine rise (median 0.3 vs. 0.2 mg/dL, p = 0.01), and more weight loss (median 1.6 vs. 0.6 kg, p < 0.01). Noroviruses are an important cause of diarrhea in pediatric transplant patients and are associated with significant clinical complications.
Keywords: norovirus, diarrhea, transplant, children, solid organ transplantation, hematopoietic stem cell transplantation
Introduction
Noroviruses (NoVs) are the most common cause of foodborne disease and acute non-bacterial gastroenteritis in the US and worldwide. In the US, approximately 19–21 million cases of NoV infection occur each year (1). NoV gastroenteritis contributes to ~70,000 hospitalizations (1) and is the second most common cause of US gastroenteritis-related mortality, leading to ~800 deaths annually, particularly among the elderly and infants (2). With the success of the rotavirus vaccine, NoVs have emerged as the leading cause of US healthcare visits for acute gastroenteritis in immunocompetent children (3). Genogroup I (GI) and II (GII) NoVs are the most common NoV genetic groups causing gastroenteritis in humans. The GII.4 genotype predominates worldwide (4); however, geographic and temporal variation in NoV genotype frequency may be observed (5).
Diarrhea is commonly experienced among both solid organ (SOT) and hematopoietic stem cell transplant (HSCT) patients, occurring in up to 90% of transplant patients (6–9). Mortality rates of up to 55% have been associated with infectious diarrhea in these immunocompromised patients (10). Up to 60% of diarrheal episodes in transplant recipients have no specific etiology identified (10, 11).
NoVs represent a potentially important, underestimated enteric pathogen in the immunocompromised transplant population. Lack of clinical suspicion of NoVs as a cause of diarrhea and of available, sensitive molecular diagnostics at healthcare institutions has likely led to a significant underestimation of NoV epidemiology, particularly among immunocompromised patients. However, increasing NoV case reports among SOT and HSCT patients suggest that NoV infection may cause significant morbidity in these patient populations (12–15). These studies highlight the need to examine NoV infection as a cause of diarrhea in immunocompromised patients. We conducted a prospectively enrolled, epidemiologic surveillance study to evaluate the prevalence and clinical significance of norovirus diarrhea among pediatric transplant recipients at Texas Children’s Hospital (TCH) in Houston, Texas. TCH is one of the largest US children’s hospitals and has one of the most active pediatric SOT and HSCT programs worldwide, performing approximately 100 SOTs and over 100 HSCTs each year. We have previously demonstrated that NoVs are the most commonly recognized enteric pathogen in the TCH general pediatric population (16).
Materials and Methods
Study Population
Pediatric SOT and HSCT recipients with diarrhea, presenting as outpatients or admitted to TCH and who submitted a stool specimen to the TCH microbiology laboratory for enteropathogen testing, were screened for enrollment. Subjects who met the definition of diarrhea were prospectively enrolled. Clinical information was collected by reviewing medical records. The Baylor College of Medicine Institutional Review Board approved the study protocol (protocol number: H-31513).
Definitions
Diarrhea was defined as a change in bowel habits with passage of ≥3 unformed stools within a 24-hour period or medical documentation of diarrhea by the treating healthcare provider. Diarrhea resolution was considered as a ≥48 hour period during which no unformed stools were passed (17). Diarrhea recurrence was defined as a recurrent diarrhea episode occurring >1 week after but ≤4 weeks of diarrhea resolution. Subjects who experienced diarrhea resolution, but developed a diarrhea relapse ≤1 week afterwards were considered to have continuation of their original diarrhea episode. Persistent diarrhea was defined as diarrhea duration ≥14 days (18). Diarrhea with onset in the community or ≤48 hours after hospital admission was described as community-associated diarrhea. Onset of diarrhea >48 hours after hospital admission was considered as healthcare-associated diarrhea (19).
Norovirus detection
Stool specimens from all subjects were tested for noroviruses upon enrollment. Subsequent stools collected at the discretion of the patient’s healthcare provider and received ≥14 days after a previously collected stool were also tested. Viral RNA was extracted from a 10% stool suspension in 0.01 M PBS using the QIAamp Viral RNA kit (QIAGEN) according to the manufacturer’s directions. GI and GII NoV-positive and NoV-negative control stools were included in each RNA extraction experiment. RNA extracts were kept at −80°C prior to use.
Viral RNA extracts were amplified in a duplex, real-time (TaqMan®) reverse transcription polymerase chain reaction (RT-PCR), using the AgPath-ID™ One-Step RT-PCR Kit (Applied Biosystems). Each 25 µL real-time RT-PCR reaction mixture consisted of 3 µL of RNA template, 400 nM of each primer (COG1F and COG1R for GI NoVs, COG2F and COG2R for GII NoVs), and 200 nM of probe (RING1c and RING2 for GI and GII NoVs, respectively; Table 1) (20). Real-time RT-PCR amplification was performed with a StepOne ABI Real Time PCR system (Applied Biosystems): cDNA synthesis at 45°C for 10 min, DNA denaturation at 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec to melt DNA and 60°C for 60 sec to anneal and extend primers. In each run, GI.1 Norwalk and GII.4 HOV 2009 RNA transcripts were used as controls and to generate GI and GII-specific standard curves by 10-fold serial dilutions (106 to 102 genome copies) of purified NoV GI and GII RNA transcripts, respectively. GI.1 Norwalk and GII.4 HOV 2009 RNA transcripts were in vitro transcribed by using pCR2.1 TOPO plasmid (Invitrogen) and pGEM-11z plasmid (Promega Corporation), respectively. RNA transcripts were aliquoted into 0.5 ml tubes and stored at −80°C prior to use. The detection limit for the real-time RT-PCR assay is 100 GI or GII NoV genomic copies, which has been established by testing 10-fold serial dilutions of the transcribed RNAs.
Table 1.
Primer and probe sequences for conventional (NoV genotyping) and real-time RT-PCR (NoV detection)
| Purpose | Primer/probe | Sequence (5′–3′) | Amplicon size (bp) |
|---|---|---|---|
| GI detection | COG IF | CGYTGGATGCGITTYCATGA | 85 |
| COG IR | CTTAGACGCCATCATCATTYAC | ||
| Ring 1C | FAM - AGATYGCGITCICCTGTCCA - BHQ | ||
| GII detection | COG 2F | CARGARBCNATGTTYAGRTGGATGAG | 98 |
| COG 2R | TCGACGCCATCTTCATTCACA | ||
| Ring 2 | JOE - TGGGAGGGCGATCGCAATCT - BHQ | ||
| GI genotyping | G1SKF | CTGCCCGAATTYGTAAATGA | 330 |
| G1SKR | CCAACCCARCCATTRTACA | ||
| GII genotyping | G2SKF | CNTGGGAGGGCGATCGCAA | 344 |
| G2SKR | CCRCCNGCATRHCCRTTRTACAT |
Note: FAM, 6-carboxyfluorescein; BHQ, Black Hole Quencher; JOE, 2,7 dimethoxy-4,5-dichloro-6-carboxyfluorescein.
Norovirus Genotyping
NoV genotypes were identified through sequence analysis of PCR amplicons from all NoV-positive stools. Conventional RT-PCR was performed to sequence real-time RT-PCR-positive specimens. Briefly, 3 µL of viral RNA was amplified using 400 nM of each oligonucleotide primer (G1SKF, G1SKR and G2SKF, G2SKR for GI and GII NoVs, respectively) and the AgPath-ID™ One-Step RT-PCR Kit (Applied Biosystems) in a final 25 µL reaction volume. After 10 min of reverse transcription at 45°C, followed by heat activation of Taq polymerase for 10 min at 95°C, PCR was carried out using 40 cycles at 94°C for 30 sec, 50°C for 30 sec, and 72°C for 60 sec followed by final extension for 5 min at 72°C. RT-PCR products of the expected size were extracted after gel electrophoresis and purified with the QIAquick Gel Extraction Kit (QIAGEN) according to the manufacturer’s protocols. PCR products were sequenced by Genewiz, Inc. Nucleotide sequences were compared with reference strain sequences to determine genotypes (21).
TCH Stool Microbiology Studies
Stool microbiology studies for other enteric pathogens were performed at the treating physician’s discretion. Clostridium difficile was detected by a real-time PCR assay amplifying toxin A (tcdA) and B (tcdB) genes (22). A membrane-based immunogold assay was used as the initial rotavirus assay with secondary electron microscopy (EM) analysis on rotavirus-negative samples from January 1st to May 31st of each year. EM was used alone for rotavirus and adenovirus identification throughout the remaining year. Adenovirus was also detected by quantitative real-time PCR. Cytomegalovirus (CMV) screening was performed by quantitative real-time PCR of plasma specimens and qualitative real-time PCR of fecal samples (Viracor-IBT Laboratories). Immunofluorescence assay for CMV antigenemia in peripheral blood leukocytes was available upon request. Stool samples were screened for enteric bacterial pathogens, including Salmonella sp., Shigella sp., Yersinia sp., Campylobacter jejuni, Aeromonas sp., and Plesiomonas sp. by selective media (23). Shiga-toxin producing Escherichia coli (STEC) were detected by inoculating the stool in MacConkey broth (Remel), followed by stx1 and stx2 toxin-specific enzyme immunoassay (Premier EHEC, Meridian Bioscience, Inc.). An EIA was performed for Cryptosporidium sp. and Giardia sp. detection (ImmunoCard STAT, Meridian Bioscience, Inc.). Stool examination for ova and parasites was performed by microscopy (ARUP Laboratories). Beginning in January 2013, a multiplex nucleic acid amplification assay using Luminex® xTag® technology became available for detecting Campylobacter sp., C.difficile toxins A and B, Cryptosporidium sp., E. coli O157, Enterotoxigenic E. coli (LT/ST), STEC stx1/stx2, Giardia lamblia, GI and GII NoVs, rotavirus A, Salmonella sp., and Shigella sp. (xTAG® Gastrointestinal Pathogen Panel, Viracor-IBT Laboratories, Inc.).
Statistical analysis
Clinical characteristics and outcomes of subjects experiencing first NoV diarrhea episodes were compared to non-NoV diarrhea subjects, who were systematically selected as the first NoV-negative subject with a matching organ transplant type enrolled after a NoV diarrhea subject was identified. Subjects receiving any tube feeding after enrollment, including those receiving chronic enteral nutrition, were included in the epidemiologic surveillance for NoV diarrhea in transplant patients, but were excluded from the matched case-control and viral load analyses because tube feeding can potentially cause diarrhea. NoV and non-NoV diarrhea patients identified with alternative enteropathogens were included in the matched case-control comparison. NoV viral load was correlated with continuous variable host characteristics and outcomes by Spearman correlation coefficient. Significant proportional differences were evaluated with chi-square analysis for categorical variables. Continuous variables were compared by Wilcoxon ranksum test. Statistical analyses were conducted using SAS (version 9.1) software.
Results
One hundred sixteen transplant recipients, including 61 SOT and 55 HSCT patients, were enrolled from December 1, 2012 to September 1, 2013. The mean age (± SD) of the transplant recipients was 9 ± 6.8 years, and 55% of subjects were male. Forty-percent of subjects were of Hispanic ethnicity, 78% of Caucasian race. The average duration from date of transplantation to enrollment (± SD) was 433 ± 879.4 days; 126 ± 302.2 days for HSCT patients and 734.3 ± 1,127.1 days for SOT patients (p<0.01).
Noroviruses were detected in 25 (22%) of the transplant patients with diarrhea (n=116), including 9 SOT and 16 HSCT patients (Tables S1 and S2). NoVs were identified more frequently than any other enteric pathogen, even when the number of tests performed for each enteropathogen was taken into consideration (Table 2). Other diarrheal pathogens included adenovirus (12%), C. difficile (7%), rotavirus (3%), Salmonella sp. (2%), and Cryptosporidium sp. (1%). Co-pathogens were identified among five NoV patients, including rotavirus (n=2), C. difficile (n=1), adenovirus (n=1), and both C. difficile and adenovirus (n=1). Alternative enteropathogens were detected in 22 NoV-negative patients. Three of 18 (17%) NoV patients screened positive for cytomegalovirus (CMV) viremia, but none had confirmation of gastrointestinal invasive disease by histopathology. The majority (76%) of NoV diarrhea cases were community-associated, while 24% were healthcare-associated.
Table 2.
Enteric Pathogen Prevalence in Transplant Recipients (n=116)
| Enteric Pathogen | Prevalence (% Total Subjects) |
No. Tests Performed (% Positive) |
|---|---|---|
| Norovirus | 25 (22) | 116 (22) |
| Adenovirus | 14 (12) | 71 (20) |
| Clostridium difficile | 8 (7) | 92 (9) |
| Rotavirus | 3 (3) | 60 (5) |
| Salmonella sp. | 2 (2) | 96 (2) |
| Cryptosporidium sp. | 1 (1) | 58 (2) |
| Giardia sp. | 0 | 58 (0) |
| Shigella sp. | 0 | 96 (0) |
| Campylobacter sp. | 0 | 96 (0) |
| STEC* | 0 | 96 (0) |
| Yersinia sp. | 0 | 96 (0) |
Note.
Abbreviation: STEC, Shiga-toxin producing Escherichia coli
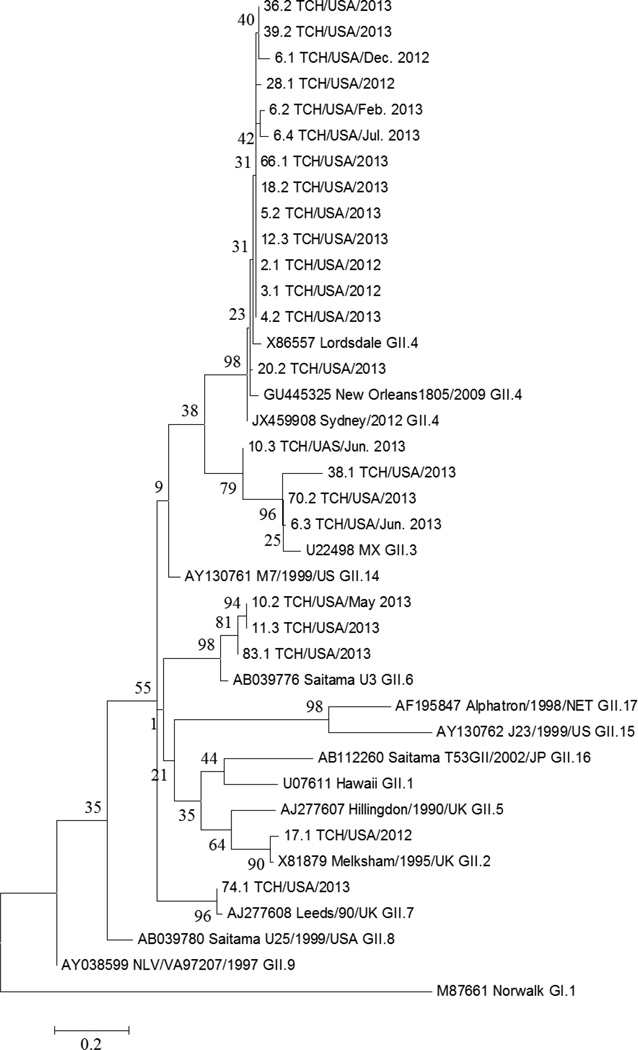
All NoV strains were identified as GII NoVs. NoV genotypes were successfully determined for 19 patients and included GII.4 (n=12), GII.3 (n=4), GII.6 (n=3), GII.7 (n=1), and GII.2 (n=1) strains (Figure). NoV genotypes, different from the original strain and confirmed by repeated sequencing, were detected in two transplant patients during their diarrheal episode. One GII.6 NoV patient was later infected with a GII.3 strain. The second patient was positive for a GII.4 NoV strain twice over two months, followed by a GII.3 strain ~four months later, and then the original GII.4 NoV strain one month afterwards. Based on sequence analysis, GII.4 co-infection of the GII.3 strain in this patient cannot be excluded, but further confirmation by cloning and NoV capsid sequencing is necessary (24).
Figure.
Norovirus Genotypes Identified
Note. Phylogenetic analysis of genogroup II (GII) norovirus (NoV) strains identified from pediatric transplant recipients based on partial nucleotide sequences of the NoV capsid gene. The dendogram was constructed by the maximum likelihood method using the software MEGA 6.0. A Kimura 2-parameter model was used for nucleotide substitution; substitution rates were assumed to be gamma distributed. Study samples are listed by subject number, sample number (e.g., 6.1); location (TCH, Texas Children’s Hospital), month (for patients with NoV detection on more than one occasion), and year of collection. Percentage bootstrap values are shown at the branch nodes. The scale indicates nucleotide substitutions per site. Subjects 6 and 10 were positive for different GII NoV genotypes during their diarrheal episode.
Fifty percent of transplant recipients with NoV diarrhea experienced persistent diarrhea lasting ≥14 days, and the median duration of NoV diarrhea was 12.5 days (range 1 – 324 days) (Table 3). The median maximum number of stools passed in a 24-hour period was 9 stools (range 3 – 16 stools). Approximately 30% of NoV diarrhea patients developed recurrent diarrhea within one month of NoV diarrhea resolution. Fifty-five percent of NoV patients were hospitalized for their diarrhea; 27% of these patients required intensive care unit (ICU) admission during their diarrhea. Precipitating events requiring ICU admission among NoV diarrhea patients included severe dehydration and hypovolemia from uncontrolled diarrhea (n=2), respiratory distress (n=2), septic shock (n=1), and cardiac arrhythmia (n=1). Three HSCT patients expired ≤6 months of NoV diarrhea onset. Two deaths were secondary to sepsis; one death was related to pulmonary hemorrhage. One patient suffered chronic diarrhea for >300 days, which continued until his death. Diarrhea persistence contributed to his steady physical decline and required prolonged parenteral nutrition, which ultimately led to fatal septic complications. NoV shedding was detected in this patient’s stools for 238 days, after which no further stools were available for testing. The patient died approximately 3 months after the last NoV-positive stool. Gastrointestinal graft-versus-host disease (GVHD) and tube feeding may have also contributed to his chronic diarrhea. Bloody stools were noted in 1 HSCT and 2 SOT patients and pneumatosis intestinalis in 1 HSCT patient; NoVs were the only enteropathogen identified in these patients. One additional HSCT patient developed bloody diarrhea, but was also positive for adenovirus.
Table 3.
Characteristics of Transplant Recipients with Norovirus (NoV) Diarrhea and non-NoV Diarrhea*
| NoV Diarrhea (n=22) |
non-NoV Diarrhea (n=22) |
P-value | |
|---|---|---|---|
| Age, mean years ± SD | 10.4 ± 6.2 | 9.3 ± 7.2 | 0.49 |
| Female patient (%) | 11 (50) | 7 (32) | 0.22 |
| Duration from transplantation to diarrhea onset (days) | |||
| Median | 136.5 | 93 | 0.77 |
| Range | −3 to 1962 | −5 to 4079 | |
| Hospital admission for diarrhea (%) | 12 (55) | 8 (36) | 0.23 |
| Intensive care unit admission during diarrhea episode (%) | 6 (27) | 0 | 0.02 |
| Median white blood cell count (cells/uL) (range)‡ | 4.3 (0–14.1) | 4.1 (0–11.8) | 0.72 |
| Median absolute lymphocyte count (cells/uL) (range)‡ | 777 (0–6453) | 392 (0–2068) | 0.59 |
| Presence of fever at diarrhea onset (%) | 7 (32) | 8 (36) | 0.75 |
| Median duration of diarrhea (range) | 12.5 (1–324) | 7 (1–34) | 0.16 |
| Persistent diarrhea** (%) | 11 (50) | 8 (36) | 0.36 |
| Recurrence of diarrhea† | 6 (29) | 7 (33) | 0.74 |
| Median maximum no. stool in 24 hours (range) | 9 (3–16) | 8 (3–14) | 0.16 |
| Median rise in serum creatinine (mg/dL) during diarrhea (range) | 0.3 (0–1.4) | 0.2 (0–0.9) | 0.01 |
| Median weight loss (kg) during diarrhea (range) | 1.6 (0–7) | 0.6 (0–3.8) | <0.01 |
| Required total parenteral nutrition during diarrhea (%) | 12 (55) | 8 (37) | 0.23 |
| Death during diarrhea episode (%) | 1 (5) | 0 | 1.00 |
| Death within 6 months of diarrhea onset (%) | 2 (9) | 1 (5) | 1.00 |
Note:
Transplant patients receiving tube feeding were excluded, including 3 NoV diarrhea subjects, one of whom expired ≤6 months of diarrhea onset. Five NoV diarrhea patients were co-infected with C. difficile (n=2), rotavirus (n=2), or adenovirus (n=2). Four non-NoV diarrhea patients were identified with C. difficile (n=2) or adenovirus (n=2).
Within 24 hours of diarrhea onset.
Persistent diarrhea was defined as diarrhea duration ≥14 days.
Recurrence of diarrhea >1 week but ≤4 weeks of diarrhea resolution. Data for diarrhea recurrence was not available for 1 NoV and 1 non-NoV patient.
To compare host characteristics and diarrhea severity between NoV diarrhea and non-NoV diarrhea transplant recipients, we performed a matched case-control analysis of NoV diarrhea subjects (n=22) and non-NoV diarrhea subjects (n=22), after excluding 3 NoV-positive subjects (2 HSCT and 1 SOT patients) who received tube feeding. NoV diarrhea patients were significantly more frequently admitted to the ICU during their diarrhea (27% vs. 0%, p = 0.02), experienced a greater rise in serum creatinine (median 0.3 vs. 0.2 mg/dL, p = 0.01), and lost more weight (median 1.6 vs. 0.6 kg, p < 0.01) than non-NoV diarrhea patients. NoV diarrhea patients were also more frequently admitted to the hospital for diarrhea, experienced greater diarrhea persistence, and passed more stools within a 24-hour period than non-NoV diarrhea patients; however, these differences were not statistically significant.
Three (14%; 3 HSCT) patients developed NoV diarrhea prior to transplantation (1–3 days), 2 (9%; 2 HSCT) <1 month after transplantation, 6 (27%; 5 HSCT, 1 SOT) 1–6 months after transplantation, and 11 (50%; 4 HSCT, 7 SOT) >6 months after transplantation. Onset of non-NoV diarrhea in relation to transplantation, including more frequent diarrhea in the late post-transplantation period for SOT than HSCT patients, was similarly distributed.
NoV diarrhea was more prevalent among HSCT patients (30%) than in SOT patients (13%, p = 0.03). NoV HSCT patients were significantly younger (mean ± SD, 8.3 ± 5.7 vs. 14.2 ± 5.5 years, p = 0.02) and more likely to experience diarrhea recurrence (46% vs. 0%, p <0.05) than NoV SOT patients. Graft-versus-host disease (GVHD) was present in 8 HSCT patients with NoV diarrhea, including 3 recipients with gastrointestinal GVHD. Two patients were diagnosed endoscopically with gut GVHD during their diarrheal episode and received increased immunosuppression; however, their diarrhea was refractory and persisted for 98 – 301 days. One patient with a past history of gastrointestinal GVHD developed diarrhea, which was attributed to NoV gastroenteritis. CMV viremia (range 200 – 5,200 copies/mL) was detected in 3 NoV HSCT patients. Two of these patients received either ganciclovir or valganciclovir for viremia; however, CMV infection was not deemed as contributing to the diarrhea by the treating physicians. No significant differences in NoV diarrhea severity, regarding duration, number of diarrheal stools, associated complications including hospital or ICU admission, or laboratory abnormalities between the two transplant groups were observed (data not shown).
The mean NoV viral load was 6.3 × 108 genomic copies per gram of stool among NoV diarrhea patients. NoV fecal load was similar between HSCT and SOT patients (mean 7.4 × 108 vs. 4.3 × 108 genomic copies per gram of stool, p = 0.68). Fifteen of the twenty-five NoV diarrhea subjects (60%) provided ≥1 additional stool samples collected ≥14 days apart. Nine (60%) of these NoV diarrhea patients were persistently positive, with median NoV shedding duration of 31 days (range 14 – 238 days). Three subjects continued to shed NoVs asymptomatically after diarrhea resolution, ranging from 10 – 91 days. Greater NoV load was significantly correlated with increased maximal number of stools in a 24-hour period (rs = 0.50, p = 0.03). No correlation was observed between NoV shedding and other measures of diarrhea severity (duration), complications (fever, renal insufficiency, weight loss, hospital or ICU requirement), or host absolute lymphocyte count at diarrhea onset (data not shown).
Discussion
SOT and HSCT recipients are at high risk for developing infections because of their significant immunosuppression related to their underlying disease, immunosuppressive therapy for organ engraftment and prevention of organ rejection, delayed immune recovery following transplantation, and complications such as graft-versus-host disease (25). Humoral and cell-mediated immunosuppression likely render SOT and HSCT recipients susceptible to NoV infection and severe disease and impair their ability to clear NoV infection.
Case reports and small, retrospective studies have described severe, prolonged NoV disease among immunocompromised hosts complicated by fever, recurrent hospitalizations for dehydration (26), chronic diarrhea persisting months to years (12), acute renal failure requiring hemodialysis (13, 14), weight loss, malnutrition requiring supplemental enteral or parenteral nutrition (13), enteritis (15), pneumatosis intestinalis (27), peritonitis (15), impaired mucosal barrier function with secondary bacteremia (15, 28), and death (12, 15). However, these studies were primarily anecdotal and included relatively few transplant patients. We conducted a large, prospectively enrolled surveillance study in an attempt to better define the epidemiologic and clinical significance of NoV diarrhea among pediatric transplant recipients.
Overall NoV prevalence was 22% among pediatric transplant recipients with diarrhea. NoVs were the most common enteric pathogen identified, almost as common as all other enteropathogens collectively. The majority of NoV diarrhea cases were associated with the community, but approximately 25% of patients were diagnosed with NoV infection in the healthcare setting. NoV diarrhea was often severe in these children, lasting on average >1 month, requiring frequent hospitalization for uncontrolled diarrhea, and was associated with dehydration and malnutrition complications and ICU admission. Four NoV diarrhea patients, including two patients excluded from the matched case-control analysis for receipt of tube feeding, required ICU admission for complications from severe diarrhea. One patient developed pneumatosis intestinalis. Three NoV patients died within 6 months of NoV diagnosis, including one death related to persistent diarrhea.
Establishing the etiology of diarrhea in immunocompromised hosts is challenging, with a myriad of potential causes including medications such as immunosuppressants and antibiotics, infection, chronic gastrointestinal disorders, immune phenomenon such as allograft rejection and GVHD, post-transplantation lymphoproliferative disease in SOT patients, and mucositis secondary to conditioning regimens in HSCT recipients (29–31). In addition, asymptomatic NoV infection has been described in up to one-third of healthy persons exposed to NoVs (32). Asymptomatic NoV prevalence among immunocompromised individuals is unknown. In our study, NoV diarrhea transplant patients required significantly more frequent ICU admission and experienced greater renal insufficiency and weight loss than non-NoV diarrhea patients. Worse renal insufficiency in NoV patients likely resulted from significant diarrhea-related dehydration. These adverse clinical outcomes associated with NoV diarrhea support the pathogenic role of NoV infection in these transplant recipients.
Distinguishing NoV disease from asymptomatic infection based on higher NoV loads has been proposed (33). However, the majority of studies evaluating the association between NoV fecal concentrations and clinical manifestations comprised immunocompetent hosts and reported conflicting results (17, 34, 35). Higher NoV fecal loads were associated with increased diarrheal stools in a 24-hour period in our transplant cohort, but did not correlate with other diarrhea severity markers. NoV shedding persisted a median of 31 days and up to 238 days. Future systematic collection and testing of stools from NoV-positive patients needs to be performed to more precisely assess NoV persistence in transplant patients. Chronic NoV shedding may have contributed to nosocomial acquisition of NoV infection, leading to the significant proportion of healthcare-associated NoV cases. However, additional epidemiologic and NoV sequencing studies are needed to confirm nosocomial transmission in our transplant patient cohort. Optimal infection control measures for persistent immunocompromised NoV shedders need to be established. These chronic NoV-infected hosts may also represent a reservoir for the emergence of new epidemic strains after accelerated NoV genomic evolution (36).
SOT patients usually experience maximal immunosuppression 1 – 6 months after transplantation. Allogeneic HSCT recipients may undergo even more profound immunosuppression with myeloablative regimens, which eradicate the recipient’s lymphohematopoietic system in preparation for donor stem cell transfer. In our study, HSCT patients presented with diarrhea earlier after transplantation than SOT patients, which likely reflects this severe immunosuppression prior to stem cell engraftment. In our matched case-control cohort, HSCT recipients also had significantly lower median white blood cell (2.6 vs. 9.1 cells/µL, p < 0.01) and median absolute lymphocyte counts (279 vs. 1,035 cells/µL, p < 0.01) than SOT patients. In addition, 8 HSCT patients had GVHD. Greater immunosuppression among HSCT patients likely led to significantly higher NoV diarrhea prevalence and recurrence in HSCT patients compared to SOT subjects.
Study limitations include the passive enrollment and surveillance of subjects. Only subjects who submitted stool samples to the TCH microbiology laboratory and experienced diarrhea were enrolled. Another study limitation was the collection of clinical information from medical records. Stool frequency and form were well-documented in hospital records for patients admitted to the hospital, but more variable for outpatients. Although this study only enrolled transplant patients with diarrhea, a minority of patients (n=9) began having diarrhea symptoms prior to their transplantation. These patients were included, as previously published case reports have described significant NoV morbidity in HSCT patients who acquired NoV infection prior to their HSCT (9, 37). An additional study limitation was the inclusion of NoV-positive and – negative subjects identified with other diarrheal pathogens in our matched case-control comparison of NoV-diarrhea and non-NoV diarrhea subjects. Subjects with co-pathogens were not excluded to avoid potentially underpowering our analysis, given the relatively small number of subjects. While these other diarrheal pathogens may have contributed to clinical outcomes attributed to NoVs, non-NoV diarrhea subjects with co-pathogens were also included. Finally, we cannot exclude that transplant patients with NoV infection may have developed diarrhea due to other causes. However, the worse clinical outcomes observed with NoV diarrhea support the pathogenic role of noroviruses in these immunocompromised patients.
Noroviruses are an important cause of diarrhea and are associated with significant complications in immunocompromised pediatric transplant recipients. However, studies are urgently needed to further define NoV pathogenesis in transplant recipients. Establishing NoVs as the causative diarrheal agent in a timely manner is critical to improving clinical management and limiting unwarranted adverse effects from antibiotics for possible bacterial diarrhea; invasive, endoscopic procedures to diagnose GVHD; and inappropriate escalation of immunosuppression for presumed gastrointestinal GVHD rather than reduction to control NoV infection. Immunocompromised transplant patients represent an important population susceptible to norovirus diarrhea, who may benefit from future novel therapeutic and preventative strategies such as immunoglobulin therapy (38), including monoclonal antibodies (39), or norovirus antivirals or vaccines (40).
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1K23DK084513 to HLK).
Abbreviations
- NoV
norovirus
- SOT
solid organ transplantation
- HSCT
hematopoietic stem cell transplantation
- GI
genogroup I
- GII
genogroup II
- ICU
intensive care unit
- TCH
Texas Children’s Hospital
- RT-PCR
reverse transcription polymerase chain reaction
- EM
electron microscopy
- STEC
Shiga-toxin producing Escherichia coli
- CMV
cytomegalovirus
- GVHD
graft-versus-host disease
Footnotes
A preliminary version of data included in this manuscript was presented as oral abstract presentations at the IDWeek 2014 Meeting, Philadelphia, October 10, 2014 and at the Fifth International Conference on Caliciviruses, Beijing, China, October 13, 2013.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Table S1. Hematopoietic Stem Cell Transplant Recipients with Norovirus (NoV) Diarrhea
Table S2. Solid Organ Transplant Recipients with Norovirus (NoV) Diarrhea
References
- 1.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 3.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. 2006;44:327–333. doi: 10.1128/JCM.44.2.327-333.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo HL, Ajami NJ, Jiang ZD, Neill FH, Atmar RL, Ericsson CD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010;48:1673–1676. doi: 10.1128/JCM.02072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsburg PM, Thuluvath PJ. Diarrhea in liver transplant recipients: etiology and management. Liver Transpl. 2005;11:881–890. doi: 10.1002/lt.20500. [DOI] [PubMed] [Google Scholar]
- 7.Cox GJ, Matsui SM, Lo RS, et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107:1398–1407. doi: 10.1016/0016-5085(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 8.van Kraaij MG, Dekker AW, Verdonck LF, et al. Infectious gastro-enteritis: an uncommon cause of diarrhoea in adult allogeneic and autologous stem cell transplant recipients. Bone Marrow Transplant. 2000;26:299–303. doi: 10.1038/sj.bmt.1702484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2012;18:1883–1889. doi: 10.1016/j.bbmt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Yolken RH, Bishop CA, Townsend TR, et al. Infectious gastroenteritis in bone-marrow-transplant recipients. N Engl J Med. 1982;306:1010–1012. doi: 10.1056/NEJM198204293061701. [DOI] [PubMed] [Google Scholar]
- 11.Kamboj M, Mihu CN, Sepkowitz K, Kernan NA, Papanicolaou GA. Work-up for infectious diarrhea after allogeneic hematopoietic stem cell transplantation: single specimen testing results in cost savings without compromising diagnostic yield. Transpl Infect Dis. 2007;9:265–269. doi: 10.1111/j.1399-3062.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Roddie C, Paul JP, Benjamin R, et al. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis. 2009;49:1061–1068. doi: 10.1086/605557. [DOI] [PubMed] [Google Scholar]
- 13.Ebdrup L, Bottiger B, Molgaard H, Laursen AL. Devastating diarrhoea in a heart-transplanted patient. J Clin Virol. 2011;50:263–265. doi: 10.1016/j.jcv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Roos-Weil D, Ambert-Balay K, Lanternier F, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92:61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz S, Vergoulidou M, Schreier E, et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117:5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo HL, Neill FH, Estes MK, Munoz FM, Cameron A, Dupont HL, et al. Noroviruses: The Most Common Pediatric Viral Enteric Pathogen at a Large University Hospital After Introduction of Rotavirus Vaccination. J Pediatric Infect Dis Soc. 2013;2:57–60. doi: 10.1093/jpids/pis070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, Goel-Apaza S, Espetia S, et al. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin Infect Dis. 2014;58:483–491. doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Diarrhoeal Disease Fact Sheet. [Accessed July 29, 2014]; Available at: http://www.who.int/mediacentre/factsheets/fs330/en/
- 19.Koo HL, Ajami NJ, Jiang ZD, Atmar RL, DuPont HL. Norovirus infection as a cause of sporadic healthcare-associated diarrhoea. J Hosp Infect. 2009;72:185–187. doi: 10.1016/j.jhin.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinje J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis. 2011;17:1389–1395. doi: 10.3201/eid1708.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroneman A, Vennema H, Deforche K, et al. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol. 2011;51 doi: 10.1016/j.jcv.2011.03.006. 121-5.23. [DOI] [PubMed] [Google Scholar]
- 22.Luna RA, Boyanton BL, Jr, Mehta S, et al. Rapid stool-based diagnosis of Clostridium difficile infection by real-time PCR in a children's hospital. J Clin Microbiol. 2011;49:851–857. doi: 10.1128/JCM.01983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang ZD, Lowe B, Verenkar MP, et al. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay) J Infect Dis. 2002;185:497–502. doi: 10.1086/338834. [DOI] [PubMed] [Google Scholar]
- 24.Kageyama T, Shinohara M, Uchida K, et al. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to Norovirus in Japan. J Clin Microbiol. 2004;42:2988–2995. doi: 10.1128/JCM.42.7.2988-2995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florescu DF, Hill LA, McCartan MA, Grant W. Two cases of Norwalk virus enteritis following small bowel transplantation treated with oral human serum immunoglobulin. Pediatr Transplant. 2008;12:372–375. doi: 10.1111/j.1399-3046.2007.00875.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim MJ, Kim YJ, Lee JH, et al. Norovirus: a possible cause of pneumatosis intestinalis. J Pediatr Gastroenterol Nutr. 2011;52:314–318. doi: 10.1097/MPG.0b013e3181ebfa01. [DOI] [PubMed] [Google Scholar]
- 28.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman SS, Chatterjee NK, Fuschino ME, et al. Characteristics of human calicivirus enteritis in intestinal transplant recipients. J Pediatr Gastroenterol Nutr. 2005;40:328–333. doi: 10.1097/01.mpg.0000155182.54001.48. [DOI] [PubMed] [Google Scholar]
- 31.Krones E, Hogenauer C. Diarrhea in the immunocompromised patient. Gastroenterol Clin North Am. 2012;41:677–701. doi: 10.1016/j.gtc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 33.Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. Diagnosing norovirus-associated infectious intestinal disease using viral load. BMC Infect Dis. 2009;9:63. doi: 10.1186/1471-2334-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajami N, Koo H, Darkoh C, et al. Characterization of norovirus-associated traveler's diarrhea. Clin Infect Dis. 2010;51:123–130. doi: 10.1086/653530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boon D, Mahar JE, Abente EJ, et al. Comparative evolution of GII.3 and GII.4 norovirus over a 31-year period. J Virol. 2011;85:8656–8666. doi: 10.1128/JVI.00472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, et al. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant. 2011;15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 38.Florescu DF, Hermsen ED, Kwon JY, et al. Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr Transplant. 2011;15:718–721. doi: 10.1111/j.1399-3046.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Sosnovtsev SV, Bok K, et al. Development of Norwalk virus-specific monoclonal antibodies with therapeutic potential for the treatment of Norwalk virus gastroenteritis. J Virol. 2013;87:9547–9557. doi: 10.1128/JVI.01376-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.