Abstract
PURPOSE
Patients with recurrence after complete resection of colorectal liver metastases (CLM) are considered for re-resection as a potential salvage therapy (PST). However, outcomes for this approach are not well defined. We sought to analyze the natural history of recurrence and PST in a large cohort of patients with long-term follow-up.
METHODS
Recurrence patterns, treatments, and outcomes in consecutive patients undergoing resection for CLM were analyzed retrospectively. PST was defined as re-resection of all recurrent disease and effective salvage therapy (EST) as free of disease for 36 months after last PST. Factors associated with PST, EST, and outcomes were analyzed.
RESULTS
Of 952 patients who underwent resection, 594 (62%) recurred (median interval=13 months). Initial recurrences involved liver (n=157,26%), lung (n=167,28%), multiple sites (n=171,29%), and other single sites (n=99,17%). PST was performed in 160/594 (27%), most commonly with a single site of recurrence (n=149). Young age (p=0.01), negative initial resection margin (p=0.003), initial tumor size <5 cm (p=0.006), and recurrence pattern (p<0.001) were independently associated with PST. Thirty-six patients achieved EST (25% of PSTs). Overall median survival was 61 months and 43 months in those that recurred. Median survival of patients undergoing PST was 87 months as compared to 34 months for those who did not.
CONCLUSIONS
Recurrence is common after CLM resection, but 27% of patients were able to undergo PST. Approximately one-quarter of these achieved EST and may be cured. PST is associated with long-term survival and possible cure and therefore active surveillance after CLM resection is justified.
Keywords: colorectal metastases, recurrence, salvage therapy, outcomes
INTRODUCTION
Liver is the organ to which colorectal cancer most frequently metastasizes.1 In 15-25% of colorectal cancer patients, liver metastases are present at initial presentation, and an additional 40-50% will ultimately develop colorectal liver metastases (CLM).1-4 Hepatic resection is the best treatment for patients with resectable tumors and is associated with long-term survival and cure. Unfortunately, in at least 70% of resected patients, disease will recur.3, 5, 6 Previous studies on specific patterns of post-hepatectomy recurrence showed that selected patients can undergo complete resection with long-term survival rates better than those reported with palliative treatment.7, 8 However, these reported outcomes may be related as much to underlying tumor biology and selection bias as to treatment. Importantly, these series have not addressed the complete denominator of patients that have recurred and lack long-term follow-up. However, there are documented cases of cure in patients who have undergone resection of recurrent disease.9-11 Consequently, in selected patients, complete resection of recurrent disease might be considered a potential salvage therapy (PST).12-15
The indications and outcomes for resection of recurrent disease after hepatectomy for CLM have not been well-studied or defined. Resection of recurrence has usually been performed in patients with limited liver or lung-only disease; however, some patients with multiple sites of recurrence have also been re-resected.16 Improved ablative therapies have broadened treatment options for patients with recurrence.15, 17 Little is known, however, about the natural history, success rates, and factors predictive of outcome in patients who undergo PST. Previously published studies are mostly small series with limited follow-up that have analyzed re-resection in single-organ sites.18-21 No prior study has comprehensively reviewed all recurrences after hepatectomy for CLM and the long-term outcomes of recurrence-specific treatments.
The aim of this study was to analyze the natural history of recurrence and PST after complete resection for CLM in a large cohort with long-term follow-up. We also sought to identify factors associated with the performance and outcome of a PST.
METHODS
Subjects and data collection
Following Institutional Review Board approval at Memorial Sloan Kettering Cancer Center (MSKCC), records of all patients who underwent liver resection for CLM between January 1, 1994, and March 31, 2004, were identified from a prospective database. Patients with incomplete information or resection (R2) were excluded. Data were supplemented with retrospective medical record review to document follow-up, patterns and treatment of recurrence, and survival. A clinical risk score (CRS) before liver resection was calculated, as previously published,22 for each patient. Scores of 0, 1, or 2 points were classified as “low,” and 3, 4, or 5 points as “high.”
Selection criteria for the initial liver resection required fitness for a major resection, and an adequate future remnant. Extrahepatic disease was not an absolute contraindication.23 Preoperative evaluation included physical examination; colonoscopy within the last year; and computed tomography (CT) of chest, abdomen, and pelvis. Magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose positron emission tomography computed tomography (18FDG PETCT) were utilized selectively. Postoperative follow-up was performed every 4–6 months.
Operative details
The type of initial resection selected was based on the extent of disease and surgical margins. Major hepatectomy was defined as a right or left hemi-hepatectomy, central hepatectomy (Couinaud segments 4, 5, and 8), or extended hepatectomy. Minor hepatectomy was defined as resection of less than a hemi-liver. Hepatic arterial infusion pumps were placed at time of surgery selectively and frequently on prospective trials. An R1 resection was defined as a microscopic margin less than 1 mm.
Follow-up, survival, and recurrence
Surgical mortality was defined as death from postoperative complications within 90 days. At last follow-up, patients were categorized as no evidence of disease (NED), alive with disease (AWD), dead of disease (DOD), dead of other causes (DOC), or dead of unknown causes (DOU). Disease-specific survival (DSS) was defined as the interval between the date of index liver resection or PST (depending on the specific analysis) and the date of last follow-up or death from cancer. Patients who were DOC or DOU were censored. Recurrence-free survival (RFS) was defined as the interval between the date of index liver resection and the date of documented recurrence or last follow-up in patients without recurrence. Recurrences were documented histologically or by definitive radiologic evidence of progression.
Potential salvage therapy and effective salvage therapy
PST was defined as a complete resection of all recurrent disease after initial hepatectomy. Rarely, thermal ablative therapies were used as PST. Treatments considered palliative included chemotherapy, radiation therapy, or incomplete resection. Recurrences and new treatments after a PST were recorded, as were multiple PSTs undertaken in a single patient. Effective salvage therapy (EST) was defined as free of recurrent disease with at least 36 months of follow-up after last PST. Thirty-six months was chosen as the cut-off to define EST since recurrence after this interval is uncommon.5
Statistical analysis
Categorical and continuous variables were summarized using proportions, mean (± standard deviation), and median (range). Chi-square and Kruskal-Wallis tests were used to compare categorical and continuous variables, respectively. Univariate and multivariate Cox proportional hazards regression was used to identify factors independently associated with PST. Factors associated with EST were not assessed in multivariate analysis due to insufficient number of events. All variables significant at the 10% level in univariate analysis were considered for multivariate analysis. P-values from the univariate and multivariate models were from the Wald test. CRS was not included in the multivariate analysis to avoid problems of collinearity with its components included in the analysis. Fifteen of 160 patients undergoing a PST were not included in the EST analysis because they had less than 36 months of follow-up after last PST. Survival curves were constructed by the Kaplan-Meier method and compared using the log-rank test. All tests were two-sided (p<0.05). Statistical analysis was performed with S.A.S. (v.9.2) and S.P.S.S (v.19.0).
RESULTS
Clinical presentation
A total of 1,034 patients underwent liver resection for CLM during the study period. Eighty-two (7.9%) patients were excluded, leaving 952 for analysis. Reasons for exclusion were incomplete information (n=48), R2 resection (n=23), or initial liver resection for CLM prior to 1994 (n=11). Tables 1 and 2 summarize clinical and therapeutic characteristics of the primary tumor and CLM for the cohort (N=952).
Table 1.
Clinical characteristics of 952 patients undergoing complete resection for CLM
| Total N=952 | |
|---|---|
| Gender | |
| Male | 544 (57.1) |
| Female | 408 (42.9) |
| Age | |
| Median (range) | 62.5 (23-89) |
| Mean ± SD | 61.1 ± 12 |
| Simultaneous diagnosis | |
| No | 827 (86.9) |
| Yes | 125 (13.1) |
| Disease-free interval < 12 months | |
| No | 507 (53.3) |
| Yes | 445 (46.7) |
| Disease-free interval [months] | |
| Median (range) | (13 (0-307) |
| Mean ± SD | 18.6 ± 24 |
| PRIMARY TUMOR | |
| Primary | |
| Colon | 689 (72.4) |
| Rectum | 263 (27.6) |
| T (n=887) | |
| 1 | 28 (3.2) |
| 2 | 119 (13.4) |
| 3 | 693 (78.1) |
| 4 | 47 (5.3) |
| Differentiation (n=848) | |
| Well | 25 (2.9) |
| Moderately | 740 (87.3) |
| Poorly | 83 (9.8) |
| LVI (n=643) | |
| No | 396 (61.6) |
| Yes | 247 (38.4) |
| PNI (n=556) | |
| No | 444 (79.9) |
| Yes | 112 (20.1) |
| Lymph node status | |
| Negative | 371 (39.0) |
| Positive | 581 (61.0) |
| Total number of lymph nodes resected (n=809) | |
| Median (range) | 11 (0-62) |
| Mean ± SD | 13 ± 9.2 |
| Total positive number (n=883) | |
| Median (range) | 1 (0-38) |
| Mean ± SD | 2.19 ± 3.3 |
CLM=colorectal liver metastases, SD=standard deviation, LVI=lymphovascular invasion, PNI=perineural invasion.
Table 2.
Clinical and therapeutic characteristics of liver metastases in 952 patients undergoing complete liver resection for colorectal liver metastases
| LIVER METASTASES | Total N=952 |
|---|---|
| Extrahepatic disease | |
| No | 794 (83.4) |
| Yes | 158 (16.6) |
| Major Resection | |
| No | 371 (39.0) |
| Yes | 581 (61.0) |
| Margin | |
| Negative | 808 (84.9) |
| Positive | 144 (15.1) |
| CEA > 200 ng/mL (n=850) | |
| No | 755 (88.8) |
| Yes | 95 (11.2) |
| Preoperative CEA ng/mL (n=847) | |
| Median (range) | 15 (0-16348) |
| Mean ± SD | 154 ± 868 |
| >1 tumor | |
| No | 438 (46.0) |
| Yes | 514 (54.0) |
| Number of tumors | |
| Median (range) | 2 (1-17) |
| Mean ± SD | 2.6 ± 2.4 |
| Tumor size > 5 cm | |
| No | 637 (66.9) |
| Yes | 315 (33.1) |
| Tumor size (largest) [cm] | |
| Median (range) | 4 (0-40) |
| Mean ± SD | 4.9 ± 3.4 |
| Clinical risk score | |
| 0 | 59 (6.2) |
| 1 | 259 (27.2) |
| 2 | 316 (33.2) |
| 3 | 230 (24.2) |
| 4 | 71 (7.5) |
| 5 | 17 (1.8) |
| Clinical risk score | |
| Low | 634 (66.6) |
| High | 318 (33.4) |
| PERIOPERATIVE TREATMENTS | |
| Neoadjuvant chemotherapy (n=936) | |
| No | 709 (75.7) |
| Yes | 227 (24.3) |
| Adjuvant chemotherapy (n=893) | |
| No | 124 (13.9) |
| Yes | 769 (86.1) |
| HAIP chemotherapy (n=929) | |
| No | 676 (72.8) |
| Yes | 253 (27.2) |
CEA=carcinoembryonic antigen, SD=standard deviation, HAIP=hepatic arterial infusion pump.
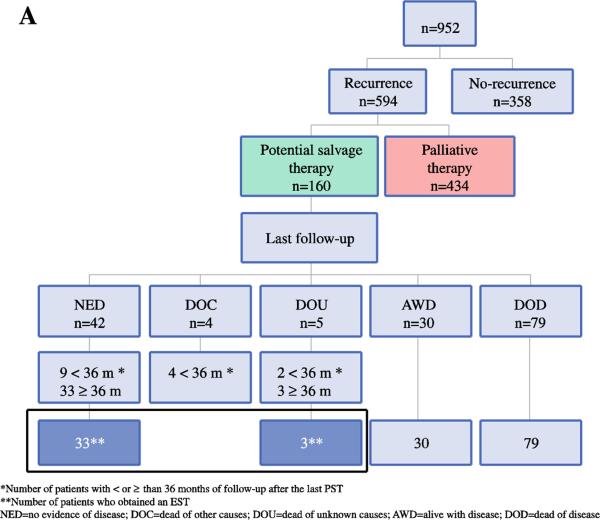
Five hundred ninety-four (62.4%) patients recurred at a median interval of 13 months (range=1-127). Initial recurrence involved the liver as sole site (n=157,26.4%), the lung as sole site (n=167,28.1%), multiple sites (n=171,28.8%), and other single sites (n=99,16.7%). Other single sites of recurrence were retroperitoneum (n=18), bone (n=17), peritoneum (n=14), pelvis (n=11), colonic anastomosis (n=9), ovaries (n=8), portocaval lymph nodes (n=6), brain (n=5), pancreas (n=2), abdominal wall (n=2), adrenal (n=2), mediastinum (n=2), celiac lymph nodes (n=1), inguinal lymph nodes (n=1), and mesentery (n=1).
Treatment of patients undergoing PST
Of the 594 patients with recurrence, 160 (26.9%) underwent at least one PST and 434 (73.1%) received palliative therapy (Figure 1A). Of these 160 patients, 96 (60.0%) underwent one PST, 46 (28.8%) underwent two, 15 (9.4%) underwent three, and 3 (1.9%) underwent four for subsequent recurrences. One hundred forty-nine (93.1%) of 160 patients who underwent a PST had recurrence at a single site and most (n=94) had a single tumor at the time of initial recurrence. The great majority of patients (156/160, 97.5%) underwent resection as their first PST; R0 resection in 134 and R1 in 22. Of the 156 patients that underwent resection as first PST, 100 received adjuvant chemotherapy, 10 received adjuvant external beam radiation therapy, and 3 underwent thermal ablation for additional tumors. The remaining 4 patients (2.6%) received radiofrequency ablation as their principal PST, 2 of whom received adjuvant chemotherapy.
Figure 1A.
Natural history of patients with CLM who underwent complete resection, presented with recurrence, and received a PST.
Uni- and multivariate analyses of factors predictive of PST
Young age, absence of lymphovascular invasion and metastatic lymph nodes from the primary tumor, negative margin of initial liver resection, CEA levels <200 ng/mL, tumor size <5 cm, low CRS, and the pattern of first recurrence were identified on univariate analysis as factors associated with performance of a PST (Table 3). Use of hepatic arterial infusion chemotherapy (HAIC) was not associated with PST. On multivariate analysis, young age (p=0.01), negative margin of liver resection (p=0.003), tumor size <5 cm (p=0.006), and pattern of recurrence (p<0.001) were independently associated with a PST (Table 3).
Table 3.
Uni- and multivariate analysis of factors associated with the performance of a potential salvage therapy
| Total n=594 | PST YES n=160 (%) | PST NO n=434 (%) | P Univariate Multivariate | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| Gender | 0.1 | |||||
| Male | 337 | 99 (29.4) | 238 (70.6) | |||
| Female | 257 | 61 (23.7) | 196 (76.3) | |||
| Age | 0.009 | 0.01 | 1.02 (1.00-1.05) | |||
| Median (range) | 62 (23-89) | 59 (31-81) | 62.5 (23-89) | |||
| Mean ± SD | 60.2 ± 11.9 | 58.1 ± 10.9 | 61 ± 12.2 | |||
| Simultaneous diagnosis | 0.2 | |||||
| No | 505 | 131 (25.9) | 374 (74.1) | |||
| Yes | 89 | 29 (32.6) | 60 (67.4) | |||
| Disease-free interval <12 months | 0.6 | |||||
| No | 296 | 77 (26.0) | 219 (74.0) | |||
| Yes | 298 | 83 (27.9) | 215 (72.1) | |||
| Disease-free interval [months] | 0.8 | |||||
| Median (range) | 11 (0-307) | 10 (0-151) | 12 (0-307) | |||
| Mean ± SD | 17.3 ± 24 | 16.9 ± 22.4 | 17.5 ± 24.6 | |||
| PRIMARY TUMOR | ||||||
| Primary | 0.4 | |||||
| Colon | 425 | 111 (26.1) | 314 (73.9) | |||
| Rectum | 169 | 49 (29.0) | 120 (71.0) | |||
| T (n=563) | 0.3 | |||||
| 1 | 16 | 5 (31.2) | 11 (68.8) | |||
| 2 | 65 | 13 (20.0) | 52 (80.0) | |||
| 3 | 448 | 127 (28.3) | 321 (71.7) | |||
| 4 | 34 | 6 (17.6) | 28 (82.4) | |||
| Differentiation (n=543) | 0.8 | |||||
| Well | 17 | 5 (29.4) | 12 (70.6) | |||
| Moderately | 474 | 129 (27.2) | 345 (72.8) | |||
| Poorly | 52 | 12 (23.1) | 40 (76.9) | |||
| LVI (n=394) | 0.03 | |||||
| No | 222 | 75 (33.8) | 147 (66.2) | |||
| Yes | 172 | 41 (23.8) | 131 (76.2) | |||
| PNI (n=342) | 0.6 | |||||
| No | 256 | 75 (29.3) | 181 (70.7) | |||
| Yes | 86 | 22 (25.6) | 64 (74.4) | |||
| Lymph node status | 0.009 | |||||
| Negative | 216 | 72 (33.3) | 144 (66.7) | |||
| Positive | 378 | 88 (23.3) | 290 (76.7) | |||
| Total number of lymph nodes resected (n=518) | 0.2 | |||||
| Median (range) | 11 (0-62) | 12 (0-52) | 11 (0-62) | |||
| Mean ± SD | 13.3 ± 9.3 | 14.2 ± 10 | 13 ± 8.9 | |||
| Total positive number (n=558) | 0.034 | |||||
| Median (range) | 1 (0-38) | 1 (0-14) | 1 (0-38) | |||
| Mean ± SD | 2.5 ± 3.5 | 1.9 ± 2.8 | 2.7 ± 3.7 | |||
| LIVER METASTASES | ||||||
| Extrahepatic disease | 1 | |||||
| No | 454 (76.4) | 131 (28.9) | 323 (71.1) | |||
| Yes | 140 (23.6) | 29 (20.7) | 111 (79.3) | |||
| Major Resection | 0.1 | |||||
| No | 217 | 66 (30.4) | 151 (69.6) | |||
| Yes | 377 | 94 (24.9) | 283 (75.1) | |||
| Margin | 0.001 | 0.003 | ||||
| Negative | 485 | 144 (29.7) | 341 (70.3) | 4.13 (1.6-10.6) | ||
| Positive | 109 | 16 (14.7) | 93 (85.3) | 1 | ||
| CEA < 200 ng/mL (n=542) | 0.001 | |||||
| No | 78 | 10 (12.8) | 68 (87.2) | |||
| Yes | 464 | 138 (29.7) | 326 (70.3) | |||
| Preoperative CEA ng/mL (n=538) | 0.5 | |||||
| Median (range) | 16.4 (0-16348 | 10 (0.5-16348) | 24.9 (0-6870) | |||
| Mean ± SD | 203 ± 1048 | 253 ± 1689 | 183 ± 660 | |||
| >1 tumor | 0.7 | |||||
| No | 237 | 66 (27.8) | 171 (72.2) | |||
| Yes | 357 | 94 (26.3) | 263 (73.7) | |||
| Number of tumors | 0.1 | |||||
| Median (range) | 2 (1-17) | 2 (1-11) | 2 (1-17) | |||
| Mean ± SD | 2.9 ± 2.7 | 2.6 ± 2.4 | 3 ± 2.7 | |||
| Tumor size < 5 cm | 0.001 | 0.006 | ||||
| No | 209 | 39 (18.7) | 170 (81.3) | 2.42 (1.27-4.59) | ||
| Yes | 385 | 121 (31.4) | 264 (68.6) | 1 | ||
| Tumor size (largest) [cm] | 0.002 | |||||
| Median (range) | 4.2 (0.6-40) | 3.5 (0.6-15) | 4.5 (0.7-40) | |||
| Mean ± SD | 5 ± 3.6 | 4.3 ± 2.8 | 5.3 ± 3.8 | |||
| Clinical risk score | 0.003 | N/A | ||||
| 0 | 33 | 15 (45.5) | 18 (54.5) | |||
| 1 | 131 | 35 (26.7) | 96 (73.3) | |||
| 2 | 189 | 60 (31.7) | 129 (68.3) | |||
| 3 | 168 | 41 (24.4) | 127 (75.6) | |||
| 4 | 58 | 9 (15.5) | 49 (84.5) | |||
| 5 | 15 | 0 | 15 (100.0) | |||
| Clinical risk score | 0.006 | |||||
| Low | 353 | 110 (31.2) | 243 (68.8) | |||
| High | 241 | 50 (20.7) | 191 (79.3) | |||
| Neoadjuvant chemotherapy (n=590) | 0.8 | |||||
| No | 420 | 112 (26.7) | 308 (73.3) | |||
| Yes | 170 | 47 (27.6) | 123 (72.4) | |||
| Adjuvant chemotherapy (n=569) | 0.06 | |||||
| No | 57 | 22 (38.6) | 35 (61.4) | |||
| Yes | 512 | 133 (26.0) | 379 (64.0) | |||
| HAIP chemotherapy (n=586) | 0.9 | |||||
| No | 419 | 113 (27.0) | 306 (73.0) | |||
| Yes | 167 | 46 (27.5) | 121 (72.5) | |||
| Pattern of first recurrence | <0.0001 | <0.001 | ||||
| Lung only recurrence | 167 | 70 (41.9) | 97 (58.1) | 10.8 (4.1-28.3) | ||
| Liver only recurrence | 157 | 49 (31.2) | 108 (68.8) | 8.6 (3.2-22.9) | ||
| Other single sites only recurrence | 99 | 12 (12.1) | 87 (87.9) | 5.5 (1.9-15.5) | ||
| Multiple sites recurrence | 171 | 29 (17.0) | 142 (83.0) | 1 | ||
* Results of the multivariate analysis shown only for significant variables. The probability of getting a PST decreased as the age increased. Clinical risk score was not included in the multivariate analysis to avoid problems of collinearity with their components that were included in the analysis. PST=potential salvage therapy, SD=standard deviation, LVI=lymphovascular invasion, PNI=perineural invasion, CEA=carcinoembryonic antigen, HAIP=hepatic arterial infusion pump.
Effective salvage therapy
Of the 160 patients who underwent a PST, 15 (9.4%) had less than 36 months of follow-up after their last PST, leaving 145 patients for analysis. Thirty-six of these 145 patients (6.1% of all patients with recurrence after index liver resection and 24.8% of 145 patients undergoing PST) met the criteria for EST. The median follow-up of these 36 patients was 84 months (range=36-172) (Figure 1A). EST was obtained after one PST in 23 (63.9%) patients, two in 8 (22.2%), three in 3 (8.3%), and four in 2 (5.6%). Table 4 summarizes the characteristics of the 145 analyzable PST patients, including the 36 with EST. Negative lymph nodes in the primary (p=0.03), single initial liver metastasis (p=0.03), and low CRS (p=0.03) were significantly associated with EST. Use of HAIC at the time of the index liver resection was not associated with EST. If we limit the analysis to patients without extrahepatic disease or positive margins at their index liver resection (n=647), the EST rate was 7.3% (27/369) of all recurrences and 27.8% (27/97) of those undergoing PST.
Table 4.
Comparison of clinical and therapeutic features between patients who obtained and did not obtain effective salvage therapy after potential salvage therapy
| Total 145 | EST YES n=36 (%) | EST NO n=109 (%) | p Univariate | |
|---|---|---|---|---|
| Gender | 0.3 | |||
| Male | 91 | 20 (22.0) | 71 (78.0) | |
| Female | 54 | 16 (29.6) | 38 (70.4) | |
| Age | 0.9 | |||
| Median (range) | 59 (31-81) | 61.5 (31-81) | 59 (37-78) | |
| Mean ± SD | 58.4 ± 10.7 | 58.3 ± 14.2 | 58.4 ± 9.4 | |
| Simultaneous diagnosis | 0.6 | |||
| No | 119 | 31 (26.1) | 88 (73.9) | |
| Yes | 26 | 5 (19.2) | 21 (80.8) | |
| Disease-free interval < 12 months | 0.7 | |||
| No | 71 | 19 (26.8) | 52 (73.2) | |
| Yes | 74 | 17 (23.0) | 57 (77.0) | |
| Disease-free interval [months] | 0.7 | |||
| Median (range) | 10 (0-151) | 13 (0-128) | 9 (0-151) | |
| Mean ± SD | 17.4 ± 23.3 | 18.6 ± 24.5 | 17 ± 22 | |
| PRIMARY TUMOR | ||||
| Primary | 0.5 | |||
| Colon | 99 | 23 (23.2) | 76 (76.8) | |
| Rectum | 46 | 13 (28.3) | 33 (71.7) | |
| T (n=138) | 0.8 | |||
| 1 | 4 | 1 (25.0) | 3 (75.0) | |
| 2 | 12 | 2 (16.7) | 10 (83.3) | |
| 3 | 116 | 31 (26.7) | 85 (73.3) | |
| 4 | 6 | 1 (16.7) | 5 (83.3) | |
| Differentiation (n=135) | 0.9 | |||
| Well | 3 | 1 (33.3) | 2 (66.7) | |
| Moderately | 122 | 31 (25.4) | 91 (74.6) | |
| Poorly | 10 | 2 (20.0) | 8 (80.0) | |
| LVI (n=107) | 0.8 | |||
| No | 70 | 18 (25.7) | 52 (74.3) | |
| Yes | 37 | 8 (21.6) | 29 (78.4) | |
| PNI (n=89) | 1 | |||
| No | 69 | 19 (27.5) | 50 (72.5) | |
| Yes | 20 | 5 (25.0) | 15 (75.0) | |
| Lymph nodes (primary) | 0.03 | |||
| Negative | 66 | 22 (33.3) | 44 (66.7) | |
| Positive | 79 | 14 (17.7) | 65 (82.3) | |
| Total number of lymph nodes resected (n=122) | 0.6 | |||
| Median (range) | 12 (0-52) | 10 (0-39) | 12 (0-52) | |
| Mean ± SD | 14.5 ± 10.5 | 13.7 ± 10.4 | 14.7 ± 10.6 | |
| Total positive number (n=138) | 0.4 | |||
| Median (range) | 1 (0-14) | 0 (0-12) | 1 (0-14) | |
| Mean ± SD | 2 ± 2.9 | 1.7 ± 3.3 | 2.1 ± 2.7 | |
| LIVER METASTASES | ||||
| Extrahepatic disease | 0.8 | |||
| No | 106 | 27 (25.5) | 79 (74.5) | |
| Yes | 39 | 9 (23.1) | 30 (76.9) | |
| Major Resection | 1 | |||
| No | 57 | 14 (24.6) | 43 (75.4) | |
| Yes | 88 | 22 (25.0) | 66 (75.0) | |
| Margin | 0.3 | |||
| Negative | 133 | 35 (26.3) | 98 (73.7) | |
| Positive | 12 | 1 (8.3) | 11 (91.7) | |
| CEA > 200 ng/mL (n=134) | 0.7 | |||
| No | 125 | 31 (24.8) | 94 (75.2) | |
| Yes | 9 | 1 (111) | 8 (88.9) | |
| Preoperative CEA ng/mL (n=134) | 0.6 | |||
| Median (range) | 10.2 (0.5-16348) | 10.2 (0.5-12325) | 10.3 (0.5-16348) | |
| Mean ± SD | 275 ± 1774 | 403 ± 2176 | 235 ± 1639 | |
| >1 tumor | 0.03 | |||
| No | 57 | 20 (35.1) | 37 (64.9) | |
| Yes | 88 | 16 (18.2) | 72 (81.8) | |
| Number of tumors | 0.1 | |||
| Median (range) | 2 (1-11) | 1 (1-11) | 2 (1-11) | |
| Mean ± SD | 2.8 ± 2.5 | 2.3 ± 2.1 | 2.9 ± 2.6 | |
| Tumor size > 5cm | 0.5 | |||
| No | 109 | 29 (26.6) | 80 (73.4) | |
| Yes | 36 | 7 (19.4) | 29 (80.6) | |
| Tumor size (largest) [cm] | 0.1 | |||
| Median (range) | 3.5 (0.6-14.5) | 3.1 (1.2-9.5) | 4 (0.6-14.5) | |
| Mean ± SD | 4.3 ± 2.7 | 3.7 ± 2.2 | 4.4 ± 2.9 | |
| Clinical risk score | 0.03 | |||
| 0 | 13 | 7 (53.8) | 6 (46.2) | |
| 1 | 32 | 11 (34.4) | 21 (65.6) | |
| 2 | 54 | 11 (20.4) | 43 (79.6) | |
| 3 | 38 | 6 (15.8) | 32 (84.2) | |
| 4 | 8 | 1 (12.5) | 7 (87.5) | |
| 5 | 0 | 0 | 0 | |
| Clinical risk score | 0.09 | |||
| Low | 99 | 29 (29.3) | 70 (70.7) | |
| High | 46 | 7 (15.2) | 39 (84.8) | |
| Neoadjuvant chemotherapy (n=144) | 0.7 | |||
| No | 100 | 24 (24.0) | 76 (76.0) | |
| Yes | 44 | 12 (27.3) | 32 (72.7) | |
| Adjuvant chemotherapy (n=140) | 0.8 | |||
| No | 17 | 3 (17.6) | 14 (82.4) | |
| Yes | 123 | 31 (25.2) | 92 (74.8) | |
| HAIP chemotherapy (n=144) | 0.8 | |||
| No | 102 | 25 (24.5) | 77 (75.5) | |
| Yes | 42 | 11(26.2) | 31 (73.8) | |
| Pattern of first recurrence | 0.8 | |||
| Liver only recurrence | 44 | 10 (22.7) | 34 (77.3) | |
| Lung only recurrence | 64 | 18 (28.1) | 46 (71.9) | |
| Other single sites only recurrence | 26 | 6 (23.1) | 20 (76.9) | |
| Multiple sites recurrence | 11 | 2 (18.2) | 9 (81.8) | |
| Number of tumors after recurrence | 0.78 | |||
| Solitary | 85 | 26 (30.6) | 59 (69.4) | |
| Multiple | 60 | 10 (16.7) | 50 (83.3) | |
| Type of resection after 1st recurrence (n=141) | 0.026 | |||
| R0 resection | 121 | 34 (28.1) | 87 (71.9) | |
| R1 resection | 20 | 1 (5.0) | 19 (95.0) | |
EST=effective salvage therapy, SD=standard deviation, LVI=lymphovascular invasion, PNI=perineural invasion, CEA=carcinoembryonic antigen, HAIP=hepatic arterial infusion pump.
Of the 434 patients treated with palliative therapy, 47 (10.8%) were alive at last follow-up. Most of these patients were AWD (n=44) and 3 (0.7%) were NED. Thirty of these 47 patients had at least 3 years of follow-up after last recurrence.
Survival analysis
The median follow-up period was 59 months (range=0-189) for survivors. Median DSS for the cohort (N=952) was 61 months (95% CI, 54.8-67.2), with actuarial DSS of 50.1% at 5 years. Median RFS of the cohort was 21 months (95% CI, 18.5-23.5), with actuarial RFS of 31.9% at 5 years.
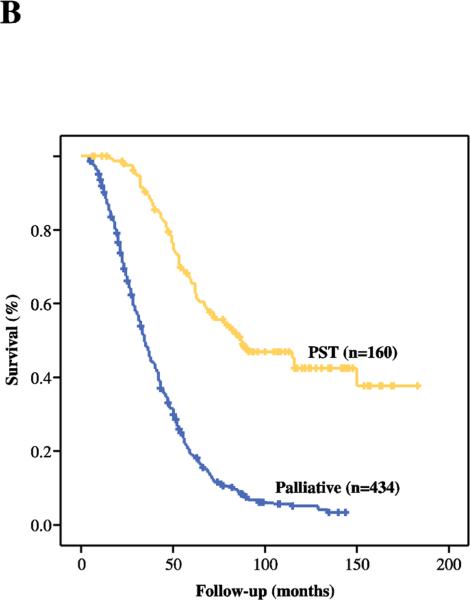
Median DSS of those patients who recurred (n=594) was 43 months (95% CI, 39.6-46.4), with actuarial DSS of 31.3% at 5 years. Median DSS, measured from date of initial liver resection, was 87 months (95% CI, 59.6-114.4) in patients who underwent a PST (n=160) and 34 months (95% CI, 31-37) in patients who received palliative treatment (n=434). The actuarial 5-year DSS was 65.4% for patients undergoing PST and 19% for palliative treatment (Figure 1B).
Figure 1B.
Disease-specific survival of patients treated with a PST (n=160) and those who underwent a palliative treatment (n=434).
DISCUSSION
The treatment of choice for patients with resectable CLM is complete resection2 because it is associated with long-term survival and cure.10 Nevertheless, the majority of patients will develop recurrence, most commonly involving liver and/or lungs.5 Resection as PST has not been well studied. Previous publications suggest that resection of recurrent disease in selected patients is as safe and effective as the initial operation in terms of survival and postoperative complications.16 However, the denominator from which patients are chosen for re-resection is typically ill-defined and long-term outcomes poorly documented.18, 24, 25 Also, previous analyses are limited by small cohorts and short follow-up, and lack definition of the complete denominator of patients with recurrence. Additionally, most studies evaluate treatment of the first recurrence or limit their analyses to single organ sites.19, 26, 27
de Jong et al6 evaluated curative intent surgery for liver recurrence in a multicenter study. Six hundred forty-five of 1706 patients presented with liver recurrence and re-resection was performed in 38%. However, the analysis did not include patients with extrahepatic recurrence. A similar study by Jones et al28 analyzed surgical treatment of liver recurrence in 150 patients. Survival was significantly better in patients who underwent complete resection than those treated without resection. However, this analysis did not include the 65 patients who presented with extrahepatic recurrence and had short follow-up time (19 months).
The present study differs from previous studies in several aspects. It includes a complete assessment of all types of recurrences and provides long-term follow-up to evaluate the durability of a PST. This is the first study that comprehensively defines long-term survival and possible cure after a PST in post-hepatectomy patients with recurrent CLM. Since most patients develop recurrence within 24 months, EST was defined as free of recurrent disease with at least 36 months of follow-up after last PST. While some patients NED at 36 months might ultimately fail, the great majority are likely cured of their disease. We also described the outcomes of patients not selected for a PST, demonstrating their relatively poor survival. These results help to understand the natural history of this clinical scenario and provide definitions of PST and EST. Also, this information can be used to guide further studies and develop guidelines for follow-up and treatment.
This study confirmed that at least 60% of patients with CLM will recur after complete resection. The minority selected for PST presented with a single recurrent tumor in a single organ. The majority of patients received palliative systemic chemotherapy or supportive treatment for their recurrence. In comparison with patients who were able to undergo PST, those who received palliative therapy had a shorter survival. Palliative systemic chemotherapy was rarely associated with cure; only 3 patients were NED at last follow-up.
Prognostic factors such as number and size of liver metastases, lymph node status of the primary tumor, CEA level, presence of extrahepatic disease, and margin-negative resection have been associated with survival after hepatic resection for CLM. However, these factors have not been shown to predict possibility of a PST after recurrence. This study showed that young age, margin-negative liver resection, tumor size <5 cm, and pattern of recurrence were associated with PST.5, 29 The CRS was excluded from the multivariate analysis because its components were individually included.
This study also evaluated patients who achieved long-term DFS after PST (EST). It is important that 1 in 4 patients who underwent a PST were definitively salvaged and may be cured of their disease. Unfortunately, it is a small minority (6.06%) of all patients with recurrence that achieve EST. Nonetheless, we find this rate along with the associated long-term survival among patients undergoing PST sufficient to justify active surveillance since the outcome of these patients may include cure. This study also identified the CRS and the margin of the initial liver resection as factors associated with EST, factors that may be helpful prognostic indicators. Due to limited events, these factors could not be analyzed in a multivariate analysis.
This study, as with all retrospective studies, has limitations that should temper interpretation of the results. Intrinsic to the nature of the data used in this retrospective analysis is that the choice to proceed with a PST was not mandated by a specific protocol. Many factors may have gone into the decision to proceed with PST, introducing bias into the results. Nonetheless, all our patients were under active surveillance and PST was considered in the great majority of situations.
In summary, this is the first study that evaluates re-resection, including all recurrences and their treatments, as a PST for recurrence following initial complete resection of CLM. The results demonstrate that 26.9% of patients were amenable to a PST after recurrence and that 6.06% of all patients with recurrence and 25% of patients who underwent PST may be cured by re-resection. Active follow-up is recommended to select patients who may be amenable to PST.
Salvage surgery for recurrence after hepatic resection for metastatic colorectal cancer has not been well-studied. In this study, 27% of patients underwent salvage surgery, which was associated with prolonged survival and cure. Active surveillance after initial hepatic resection is justified.
Acknowledgments
Source of Funding: This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11(8):1057–77. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 2.House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744–52. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 3.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16(1):3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Mantke R, Schmidt U, Wolff S, Kube R, Lippert H. Incidence of synchronous liver metastases in patients with colorectal cancer in relationship to clinico-pathologic characteristics. Results of a German prospective multicentre observational study. Eur J Surg Oncol. 2012;38(3):259–65. doi: 10.1016/j.ejso.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 6.de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–51. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 7.Nordlinger B, Vaillant JC, Guiguet M, et al. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. Association Francaise de Chirurgie. J Clin Oncol. 1994;12(7):1491–6. doi: 10.1200/JCO.1994.12.7.1491. [DOI] [PubMed] [Google Scholar]
- 8.Pinson CW, Wright JK, Chapman WC, Garrard CL, Blair TK, Sawyers JL. Repeat hepatic surgery for colorectal cancer metastasis to the liver. Ann Surg. 1996;223(6):765–73. doi: 10.1097/00000658-199606000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogata Y, Matono K, Hayashi A, et al. Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonary resection in colorectal cancer. World J Surg. 2005;29(3):363–8. doi: 10.1007/s00268-004-7537-7. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 11.Miller G, Biernacki P, Kemeny NE, et al. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007;205(2):231–8. doi: 10.1016/j.jamcollsurg.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225(1):51–60. doi: 10.1097/00000658-199701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govindarajan A, Arnaoutakis D, D'Angelica M, et al. Use of intraoperative ablation as an adjunct to surgical resection in the treatment of recurrent colorectal liver metastases. J Gastrointest Surg. 2011;15(7):1168–72. doi: 10.1007/s11605-011-1470-5. [DOI] [PubMed] [Google Scholar]
- 14.Leung EY, Roxburgh CS, Leen E, Horgan PG. Combined resection and radiofrequency ablation for bilobar colorectal cancer liver metastases. Hepatogastroenterology. 2010;57(97):41–6. [PubMed] [Google Scholar]
- 15.Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–61. doi: 10.1016/j.jvir.2011.01.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mise Y, Imamura H, Hashimoto T, et al. Cohort study of the survival benefit of resection for recurrent hepatic and/or pulmonary metastases after primary hepatectomy for colorectal metastases. Ann Surg. 2010;251(5):902–9. doi: 10.1097/SLA.0b013e3181c9868a. [DOI] [PubMed] [Google Scholar]
- 17.Elias D, Baton O, Sideris L, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90(1):36–42. doi: 10.1002/jso.20237. [DOI] [PubMed] [Google Scholar]
- 18.Brouquet A, Vauthey JN, Badgwell BD, et al. Hepatectomy for recurrent colorectal liver metastases after radiofrequency ablation. Br J Surg. 2011;98(7):1003–9. doi: 10.1002/bjs.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S, Sakaguchi T, Yokoi Y, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129(4):421–8. doi: 10.1067/msy.2001.112486. [DOI] [PubMed] [Google Scholar]
- 20.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238(6):871–83. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Pool AE, Lalmahomed ZS, de Wilt JH, Eggermont AM, Ijzermans JM, Verhoef C. Local treatment for recurrent colorectal hepatic metastases after partial hepatectomy. J Gastrointest Surg. 2009;13(5):890–5. doi: 10.1007/s11605-008-0794-2. [DOI] [PubMed] [Google Scholar]
- 22.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpizo DR, Are C, Jarnagin W, et al. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16(8):2138–46. doi: 10.1245/s10434-009-0521-6. [DOI] [PubMed] [Google Scholar]
- 24.Yan TD, Lian KQ, Chang D, Morris DL. Management of intrahepatic recurrence after curative treatment of colorectal liver metastases. Br J Surg. 2006;93(7):854–9. doi: 10.1002/bjs.5359. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou A, Lovegrove RE, Tilney HS, et al. Meta-analysis of clinical outcome after first and second liver resection for colorectal metastases. Surgery. 2007;141(1):9–18. doi: 10.1016/j.surg.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura S, Sakaguchi S, Nishiyama R, et al. Aggressive repeat liver resection for hepatic metastases of colorectal carcinoma. Surg Today. 1992;22(3):260–4. doi: 10.1007/BF00308832. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S, Inoue K, Konishi M, Nakagouri T, Kinoshita T. Prognostic factors for poor survival after repeat hepatectomy in patients with colorectal liver metastases. Surgery. 2003;133(6):627–34. doi: 10.1067/msy.2003.151. [DOI] [PubMed] [Google Scholar]
- 28.Jones NB, McNally ME, Malhotra L, et al. Repeat Hepatectomy for Metastatic Colorectal Cancer Is Safe but Marginally Effective. Ann Surg Oncol. 2011;30:30. doi: 10.1245/s10434-011-2179-0. [DOI] [PubMed] [Google Scholar]
- 29.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246(2):295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]