Summary
Sickle cell disease (SCD) is associated with vascular complications including premature stroke. The role of atherothrombosis in these vascular complications is unclear. To determine the effect of SCD on atherosclerosis and thrombosis, mice with SCD along with controls were generated by transplantation of bone marrow from mice carrying the homozygous sickle cell mutation (Hbbhβs/hβs) or wild-type mice (Hbb+/+) into C57BL6/J or apolipoprotein E deficient (Apoe−/−) recipient mice. At the time of sacrifice, 23–28 weeks following bone marrow transplantation, anaemia, reticulocytosis, and splenomegaly were present in mice receiving Hbbhβs/hβs bone marrow compared with control mice. Analysis of atherosclerosis involving the aortic root revealed reduced atherosclerotic lesion area with reduced macrophage content and increased collagen content in Apoe−/−, Hbbhβs/hβs mice compared to Apoe−/−, Hbb+/+ mice. In a carotid thrombosis model, the time to thrombosis was prolonged in Hbbhβs/hβs mice compared to Hbb+/+ mice. This apparent protective effect of SCD on atherosclerosis and thrombosis was diminished by inhibition of heme oxygenase-1 (HMOX1) using zinc protoporphyrin IX.
We conclude that SCD in mice is paradoxically protective against atherosclerosis and thrombosis, highlighting the complexity of vascular events in SCD. This protective effect is at least partially mediated by induction of HMOX1.
Keywords: atherosclerosis, bone marrow transplant, haem oxygenase, thrombosis
Sickle cell disease (SCD) is a haemoglobinopathy caused by a missense mutation in the beta globin gene, predisposing haemoglobin to abnormal polymerization in response to stressors (Rees et al, 2010). This change in haemoglobin polymerization leads to stiff red blood cells that form aggregates, often resulting in impairment of microcirculatory flow, tissue ischaemia, and infarction. While SCD is generally associated with a proinflammatory state, protective mechanisms have also been shown to be activated in SCD that may lessen the severity of disease complications (Rees et al, 2010). Some patients experience stroke at an early age, although the underlying mechanisms are not fully understood (Adams et al, 1997; Morris, 2011). Myocardical infarction (MI) has been reported although the true incidence is unclear. In one review of 108 patients with SCD, MI occurred in 2·7% of patients (Barrett et al, 1984). However, the diagnosis is often missed and made only at autopsy (Barrett et al, 1984; Martin et al, 1996). The effect of SCD on atherosclerosis, the underlying substrate for most MI and stroke, is also unclear because of small study populations. However, some studies have demonstrated normal coronary arteries in SCD patients with MI, suggesting underlying atherosclerosis is not the culprit (Gerry et al, 1978; McCormick, 1988; Mansi & Rosner, 2002). Given that vascular changes similar to premature atherosclerosis and/or thrombophilia could contribute to stroke in SCD, we studied the effect of sickle cell anaemia on the progression of atherosclerosis and on arterial thrombosis in a mouse model of SCD. To study atherosclerosis, we used the atherosclerotic-prone apolipoprotein E deficient mouse model because these mice develop hyperlipidaemia and accelerated atherosclerosis (Plump et al, 1992; Nakashima et al, 1994).
Methods
Animals
Male apolipoprotein E deficient (Apoe−/−) and C57BL6/J (wild-type, WT) mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Donor mice carrying homozygous sickle cell mutation (Hbbhβs/hβs) were originally from University of Alabama at Birmingham and these mice have subsequently been bred to C57BL6/J mice to generate heterozygous mice, which were then intercrossed to produce the homozygous Hbbhβs/hβs donors (Campbell et al, 2011). SCD and control experimental mice were then generated by bone marrow transplantation (BMT) from Hbbhβs/hβs mice or wild-type (Hbb+/+) donors to wild-type or Apoe−/− recipients. Mice were housed under specific pathogen-free conditions in static microisolator cages with tap water ad libitum in a temperature-controlled room with a 12:12-h light/dark cycle. The bone marrow chimeric mice with Apoe−/− background were fed a Western diet (TD88137, Harlan, WI, USA) following BMT. Bone marrow chimeras with Apoe+/+ background were fed a standard laboratory rodent diet (No. 5001, TestDiet, Richmond, IN, USA). All animal use protocols complied with the Principle of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Bone marrow transplantation
At 8 weeks of age, WT or Apoe−/− mice were irradiated and BMT was performed as previously described (Bodary et al, 2002). Each recipient mouse was irradiated (2 × 650 rad [0·02 × 6·5 Gy]) and injected with 4 × 106 bone marrow cells via the tail vein. For atherosclerosis studies, Apoe−/− mice were used as recipients for Hbb+/+ and Hbbhβs/hβs donor marrow, and they were fed a Western diet for 14 weeks beginning at 6 weeks following the BMT, after which mice were euthanized. For thrombosis studies, WT mice were used as recipients for Hbb+/+ and Hbbhβs/hβs donor marrow, and they were fed a standard diet for 23 weeks following BMT, after which they were euthanized.
Haemoglobin analysis
Blood haemoglobin content was determined by high-performance liquid chromatography (HPLC) 10 weeks after BMT as previously described (Campbell et al, 2011).
Atherosclerosis analysis
For quantification of atherosclerotic lesions at aortic root, mice were euthanized under intraperitoneal pentobarbital anesthesia (100 mg/kg) and the hearts, including aortic root, were fixed in 10% buffered formalin solution, followed by paraffin embedding. A series of 5 μm sections were obtained at the level of aortic valve and four cross sections were analysed from each mouse. To quantitate lesion area and collagen content of aortic root, the sections were stained with haematoxylin and eosin, and Sirius red (Sigma, St. Louis, MO, USA) respectively. Macrophage content of lesion at the aortic root was studied as previously described (Ohman et al, 2008) using immunohistochemistry with antibody to Mac-3 (1:100; BD Biosciences, San Jose, CA, USA), although some reports have shown that this antibody also reacts with fibroblasts (Inoue et al, 2005). The lesion area was defined as the area between the endothelial cell layer and internal elastic lamina. Sirius red-positive area was expressed as percentage of the total lesion area. All images of atherosclerotic lesions were analysed using National Institutes of Health (NIH, Bethesda, MD, USA) IMAGE software (ImageJ).
Carotid arterial thrombosis
To induce thrombosis, photochemical injury was performed on carotid arteries from WT mice receiving WT or Hbbhβs/hβs bone marrow as previously described (Eitzman et al, 2000). Briefly, mice were anesthetized and secured in the supine position under a dissecting microscope (Nikon SMZ-2T, Mager Scientific, Inc., Dexter, MI, USA). The right common carotid artery was isolated and blood flow was monitored with a Doppler flow probe (Transonic, Ithaca, NY, USA). A 1·5-mW green light laser (540 nm) (Melles Griot, Carlsbad, CA, USA) was applied to the mid common carotid artery before injection of Rose Bengal (50 mg/kg in phosphate-buffered saline; PBS) (Fisher, Fair Lawn, NJ, USA) via the tail vein. Arterial thrombosis was defined as flow cessation for at least 10 min. Flow in the carotid artery was monitored for 90 min.
Leucocyte infusion
Leucocytes were isolated from whole blood obtained from WT mice that had been transplanted with either WT or Hbbhβs/hβs bone marrow using ACK lysis buffer (Lanza Inc., Walkersville, MD, USA). 10-week-old WT mice were used as recipients for leucocyte infusion. Each recipient mouse was injected with 0·5 × 106 leucocytes via the tail vein. Following leucocyte infusion, the carotid arterial thrombosis study was performed as described above.
Real-Time Polymerase Chain Reaction (RT-PCR)
RNA from frozen liver (20 mg) was isolated using a QIAGEN RNeasy Mini Kit (QIAGEN Inc., Valencia, CA, USA). The primer sets for specific amplification of heme oxygenase-1 (HMOX1) and GAPDH were purchased from Applied Biosystems (Carlsbad, CA, USA). RT-PCR was performed using an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA). 100 ng of RNA and 1 μl of primer were used per reaction. 7000 System SDS Software and the 2-ΔΔCT method (Livak & Schmittgen, 2001) were used to analyse the results. Results were presented as fold change of transcripts for target normalized to internal control (GAPDH).
HMOX1 inhibition
A specific HMOX1 inhibitor, zinc protoporphyrin IX (ZnPPIX, Sigma, St. Louis, MO, USA), was prepared in 0·1 N NaOH, and then titrated to pH 7·4 using 0·1 N HCl. At 14 weeks of age (6 weeks after BMT), ZnPPIX (5 mg/kg in 0·9% NaCl) was given to BMT Apoe−/−, Hbbhβs/hβs mice intraperitoneally twice a week for 14 weeks. Control BMT Apoe−/−, Hbbhβs/hβs mice received vehicle control.
For thrombosis studies, ZnPPIX (25 mg/kg) was injected intraperitoneally to BMT Hbbhβs/hβs mice for total of two doses every other day. Control BMT Hbbhβs/hβs mice received vehicle control.
Heme oxygenase activity assay
The enzyme activity of heme oxygenase (HMOX) was measured as previously described (Motterlini et al, 1995). Briefly, 50 mg frozen liver tissue was homogenized in 250 μl PBS, and centrifuged at 18 000 g for 10 min at 4°C. The source of biliverdin reductase for the assay was liver cytosol prepared from the 105 000 g supernatant fraction of 2 mg homogenized WT liver tissue. To initiate the reaction, 200 μl supernatant of liver sample was added to reaction system containing 0·8 mmol/l NADPH, 2 mmol/l glucose-6-phosphate, 0·2 units glucose-6-phosphate dehydrogenase, 0·2 mmol/l MgCl2, 0·02 mmol/l haemin, and 200 μl liver cytosol in a final volume of 300 μl. The reaction occurred for 1 h in the dark at 37°C and was then stopped by mixing 1:1 with chloroform. The extracted bilirubin in the chloroform layer was measured at 464 nm subtracted by background absorption at 530 nm. The HMOX activity was expressed as formation of bilirubin (pmol) per milligram of sample in 1 h.
Plasma measurements
Plasma samples were collected via ventricular puncture at the time of euthanasia. Circulating levels of HMOX1 were measured with a commercially available murine enzyme-linked immunsorbent assay kit (Clontech Laboratories, Inc., Mountain View, CA, USA) according to the manufacturer’s instructions. Lipids were measured in the Chemistry Core of the Michigan Diabetes Research and Training Center using Enzymatic-Colorimetric, HDL-Direct, or Equal Diagnostics kits (Roche, Indianapolis, IN, USA).
Statistical analysis
All data are presented as mean ± standard error. Statistical analysis was carried out using GraphPad Prism. Results were analysed using unpaired t-test for comparison between two groups. For multiple comparisons, results were analysed using one-way ANOVA followed by Tukey post-test analysis. Probability values <0·05 were considered statistically significant.
Results
Characteristics of BMT sickle mice
Mice receiving Hbbhβs/hβs BMT were anaemic and displayed reticulocytosis and splenomegaly compared to mice receiving WT BMT, indicating the BMT was successful in producing a model of SCD (Table I). Human sickle haemoglobin content was 89·8 ± 0·8% in Apoe−/− SCD mice (n = 9), similar to previous reports of SCD mice without BMT (Ryan et al, 1997; Campbell et al, 2011). Spleen weights were greater in Apoe−/− recipients compared to Apoe+/+ recipients and were markedly increased in Apoe−/− SCD mice compared to Apoe+/+ SCD mice. The increased spleen size correlated with increased reticulocytosis in the Apoe−/− SCD mice with a trend towards more severe anaemia (Table I). White blood cell counts were significantly increased in Apoe−/− SCD mice compared with Apoe−/− control and Apoe+/+SCD mice (Table I).
Table I.
Characteristics of BMT SCD mice.
| Apoe−/− | Apoe−/− SCD | WT | SCD | |
|---|---|---|---|---|
| Body weight (g) | 29·2 ± 1·6 | 29·3 ± 0·5 | 25·4 ± 0·6☆ | 24·8 ± 0·2☆☆ |
| Spleen weight (mg) | 157·5 ± 13·6 | 654·2 ± 39·2† | 71·5 ± 0·002☆ | 356·1 ± 0·01☆☆,† |
| Reticulocyte (%) | 6·9 ± 0·7 | 31·7 ± 1·1† | 3·04 ± 0·8☆ | 19·4 ± 1·2☆☆,† |
| Haemoglobin (g/l) | 125 ± 5·0 | 92 ± 6·0‡ | 117 ± 2·0 | 96 ± 4·0† |
| Haematocrit (%) | 37·1 ± 1·5 | 26·7 ± 1·9† | 35·6 ± 0·6 | 29·1 ± 1·7‡ |
| WBC (x 109/l) | 9·5 ± 1·2 | 24·8 ± 2·9‡ | 10·1 ± 1·3 | 13·9 ± 1·2☆☆ |
| Platelets (x 109/l) | 732·0 ± 100·5 | 728·0 ± 77·0 | 855·6 ± 104·5 | 717·5 ± 32·8 |
SCD, sickle cell disease; WT, wild-type; WBC, white blood cells.
P < 0·05 compared with Apoe−/− or Apoe−/− SCD.
P < 0·01 compared with corresponding control.
P < 0·05 compared with corresponding control.
P < 0·01 compared with Apoe−/− or Apoe−/− SCD.
Effect of SCD on atherosclerosis and lipids
To study the effect of SCD on atherosclerosis, Apoe−/− chimeric mice with either Hbbhβs/hβs or WT bone marrow on a Western diet were analysed. Atherosclerotic lesion area involving the aortic root was significantly reduced in Apoe−/− SCD mice compared with Apoe−/− control mice (Fig 1A–C). Reduced lesion area in Apoe−/− SCD mice was associated with reduced macrophage content of atherosclerotic plaques, as demonstrated by reduced immunostaining for Mac-3 (Fig 1D–F) along with increased collagen content as determined by Sirius red staining (Fig 1G–I). Atherosclerotic lesions involving the carotid arteries were not observed in either group at this age in this model. Cholesterol levels were reduced in Apoe−/− SCD mice compared to Apoe−/− control mice (Table II). In particular, low-density lipoprotein (LDL) levels were lower in Apoe−/− SCD mice while high-density lipoprotein (HDL) and triglyceride levels were not significantly different (Table II).
Fig 1.
Effect of SCD on atherosclerosis in Apoe−/− mice. A, B: Representative photomicrographs of aortic lesions in (A) Apoe−/− control (n = 7) and (B) Apoe−/− sickle cell disease (SCD) mice (n = 9). C: Quantification of lesion area at aortic root. D, E: Representative photomicrographs of Mac3 staining of aortic lesion in (D) Apoe−/− control and (E) Apoe−/− SCD mice. F: Quantification of Mac3 positive area in aortic lesions. G, H: Representative photomicrographs of Sirius red staining of aortic lesion in (G) Apoe−/− control and (H) Apoe−/− SCD mice. I: Quantification of the ratio of Sirius red positive area over lesion area in aortic lesions. Scale bar: 50 μm. *P < 0.01.
Table II.
Lipid profile of Apoe−/− SCD mice.
| Apoe−/− | Apoe−/− SCD | |
|---|---|---|
| Cholesterol (mmol/l) | 4·9 ± 0·4 | 2·9 ± 0·1☆ |
| Triglyceride (mmol/l) | 1·1 ± 0·2 | 1·2 ± 0·2 |
| HDL (mmol/l) | 0·8 ± 0·1 | 0·6 ± 0·1 |
| LDL (mmol/l) | 1·6 ± 0·1 | 0·6 ± 0·1† |
P < 0·05 compared with Apoe−/− control.
P < 0·01 compared with Apoe−/− control.
Effect of SCD on carotid arterial thrombosis
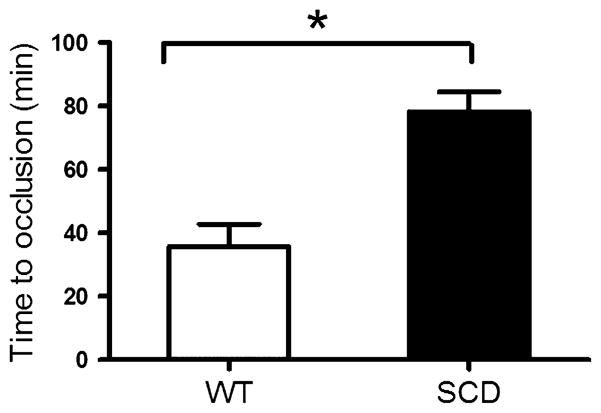
To determine if carotid thrombosis following endothelial injury was affected in SCD mice, WT or SCD mice were subjected to photochemical injury at the mid common carotid artery with rose bengal. Time to thrombosis was prolonged in SCD mice compared to WT mice (Fig 2), indicating that SCD confers protection against arterial thrombosis in this model. As leucocytes have been shown to be a potential source of HMOX1 in SCD (Jison et al, 2004), we infused leucocytes isolated from either SCD or WT mice and determined the time to thrombosis in WT mice. Consistent with a vasculo-protective effect of SCD leucocytes, WT mice that received infusion of SCD leucocytes exhibited prolonged time to thrombosis compared to WT mice that received WT leucocytes (Fig 3).
Fig 2.
Effect of SCD on carotid thrombosis. Time to occlusive carotid thrombosis in WT mice (n = 9) and SCD mice (n = 9). *P < 0·01. WT, wild type; SCD, sickle cell disease.
Fig 3.
Effect of leucocyte infusion on carotid thrombosis. Time to occlusive carotid thrombosis in WT mice (n = 9) infused with WT leucocytes and WT mice (n = 9) infused with SCD leucocytes. *P < 0·05. WT, wild type; SCD, sickle cell disease.
Effect of HMOX1 inhibition on atherosclerosis and thrombosis in SCD mice
To specifically address the role of HMOX1 towards protection from atherosclerosis and thrombosis in SCD mice, we first measured expression and activity of HMOX1. HMOX activity (Fig 4A) and HMOX1 expression (Fig 4B) in the liver were both increased in SCD mice compared to control mice. Circulating levels of HMOX1 were also significantly higher in SCD mice compared with control mice (Fig 4C).
Fig 4.
Expression and activity of heme oxygenase (HMOX) and effect of HMOX1 inhibition by ZnPPIX. A: Activity of HMOX in liver from WT (n = 6) and SCD mice (n = 5) with or without HMOX1 inhibition. B: Expression levels of Hmox1 transcript in liver from WT and SCD mice with or without HMOX1 inhibition. C: Plasma HMOX1 levels in WT and SCD mice with or without HMOX1 inhibition. *P < 0·01. **P < 0·05. WT, wild type; SCD, sickle cell disease.
To determine the causal role of HMOX1 in mediating vascular protection in SCD mice, a specific inhibitor of HMOX1 activity, ZnPPIX, was administered to SCD mice. Following treatment, HMOX activity was inhibited by ZnPPIX in livers of SCD mice compared with saline-injected control SCD mice (Fig 4A). Hmox1 mRNA expression levels of in ZnPPIX-injected SCD mice were similar compared to those in saline-injected control SCD mice (Fig 4B). Circulating levels of HMOX1 were significantly increased after inhibition in WT control mice, but not in SCD mice (Fig 4C).
Following ZnPPIX treatment of Apoe−/− SCD mice, the atherosclerotic lesion area involving the aortic root was compared to lesion areas in saline-injected Apoe−/− SCD mice. The lesion area was significantly increased after chronic inhibition of HMOX1 in Apoe−/− SCD mice (Fig 5A-C). The collagen content of aortic atherosclerotic lesions was significantly decreased after HMOX1 inhibition in Apoe−/− SCD mice compared with saline-injected SCD mice (Fig 5D–F). Cholesterol levels were not affected in Apoe−/− SCD mice by HMOX1 inhibition compared with saline-injected Apoe−/− SCD mice (3·7 ± 0·3 vs. 4·1 ± 0·3 mmol/l, n = 5 per group). Similarly, HMOX1 inhibition had no significant effect on LDL levels compared with saline-injected Apoe−/− SCD mice (0·7 ± 0·1 vs. 0·8 ± 0·1 mmol/l, n = 5 per group). Splenomegaly was not affected by HMOX1 inhibition in Apoe−/− SCD mice compared with saline-injected Apoe−/− SCD mice (464·6 ± 44·9 vs. 488·9 ± 45·0 mg, n = 5 per group). To determine if HMOX1 was involved in the protective effect of SCD on carotid artery thrombosis, SCD mice were treated with ZnPPIX for 3 d prior to thrombosis studies. Thrombosis times in ZnPPIX-treated SCD mice were significantly reduced compared to times from vehicle-injected control SCD mice (Fig 6).
Fig 5.
Effect of ZnPPIX on atherosclerosis in SCD mice. A, B: Representative photomicrographs of aortic lesions in saline-injected (n = 5) (A) and ZnPPIX-injected (n = 5) (B) Apoe−/− SCD mice. C: Quantification of lesion area at aortic root in Apoe−/− SCD mice after saline or ZnPPIX injection. D, E: Representative photomicrographs of Sirius red staining of aortic lesion in vehicle-injected (D) and ZnPPIX-injected (E) Apoe−/− SCD mice. F: Quantification of Sirius red positive in aortic lesions from Apoe−/− SCD mice after saline or ZnPPIX injection. Scale bar: 50 μm. *P < 0·01. **P < 0·05. SCD, sickle cell disease.
Fig 6.
Effect of SCD and HMOX1 inhibition on carotid thrombosis. Time to occlusive carotid thrombosis in SCD mice with vehicle injection (SCD+vehicle) (n = 9), WT mice with ZnPPIX injection (WT+ZnPPIX) (n = 3), and SCD mice with ZnPPIX injection (SCD+ZnPPIX) (n = 3). *P < 0·01. **P < 0·05. WT, wild type; SCD, sickle cell disease.
Discussion
Sickle cell disease is characterized by rigid sickled erythrocytes that undergo premature destruction, resulting in haemolytic anaemia and microvascular occlusions (Rees et al, 2010). In addition to long term consequences of severe anaemia, multiple vascular complications are associated with SCD (Platt et al, 1991). The vascular complications associated with SCD are complex and diverse with variable incidence rate: pulmonary hypertension (10·5%) (Mehari et al, 2012), left ventricular dysfunction (50%) (Anthi et al, 2007), dysrhythmia during crisis (80%) (Maisel et al, 1983) and sudden death (40%) (Manci et al, 2003). Pathophysiological studies have shown that sickling of erythrocytes in capillaries and small blood vessels lead to vaso-occlusion in multiple organs including lung, spleen, and brain (Elsharawy et al, 2009). Stroke is considered one of the most common vascular complications in patients with SCD, occurring in 24% of individuals by 45 years of age (Rothman et al, 1986; Platt et al, 1991). In addition to erythrocyte-mediated microvascular occlusions, activation of endothelial cells, platelets, coagulation factors, inflammatory cytokines and leucocytes have been shown to occur in SCD (Rees et al, 2010; De Franceschi et al, 2011). Increased blood flow velocity measured by transcranial Doppler has also been shown to be a risk factor for stroke in SCD (Adams et al, 1997). However, the mechanisms responsible for macrovascular events in SCD are largely unknown, although both protective and deleterious effects of SCD towards the vasculature have been described (Kato et al, 2007; Rees et al, 2010).
Atherosclerosis is the most common underlying substrate for stroke in the general population (Viles-Gonzalez et al, 2004). Early events in atherosclerosis involve lipid and macrophage influx into the subendothelial space of arteries (Ross, 1995). Even though stroke occurs even in very young patients with SCD, the pathophysiological events observed in early atherosclerosis could be similarly involved in SCD as increases in markers and mediators of atherosclerosis, such as soluble vascular cell adhesion molecule 1, intercellular adhesion molecule, E-selectin, P-selectin, C-reactive protein, tumour necrosis factor-α, tissue factor and leucocyte activation, have been described in patients with SCD (Kato & Gladwin, 2008; Hoppe et al, 2011). This would be important to know because therapies useful for patients with atherosclerosis may be of benefit to patients with SCD.
To determine the effect of SCD on atherosclerosis, we used a mouse model of atherosclerosis and generated superimposed SCD by transplanting bone marrow from SCD mice. Because of the complex genetics (both strain-related background and induced mutations), the BMT approach was more efficient than crossing the different mutant strains. Drawbacks of the BMT approach include the irradiation required for the transplant procedure, which may affect vascular endpoints, and the presence of apolipoprotein E (APOE) in the donor marrow, which also affects atherosclerosis (Boisvert et al, 1995; Linton et al, 1995). Despite these caveats, the mice still developed sufficient atherosclerosis for quantitation and therefore constituted a reasonable model to address the effect of SCD on atherosclerosis. For these experiments, mice were placed on a Western diet following the BMT to accelerate the formation of atherosclerotic lesions. Apoe−/− SCD mice developed anaemia, reticulocytosis and marked splenomegaly compared to Apoe−/− control mice. Of considerable interest, anaemia, splenomegaly and reticulocytosis were also greater in the Apoe−/− SCD mice compared to the Apoe+/+ SCD mice, indicating that either hypercholesterolaemia or some other function of non-haematopoietic apolipoprotein E status may affect haemolytic anaemia in SCD. Apoe−/− mice have previously been reported to have larger spleens than Apoe+/+ mice, however this difference was greatly exaggerated in the setting of SCD. It could be that splenic apoptosis is deficient in Apoe−/− mice (Wang et al, 2012) and that this effect is exaggerated in the setting of a chronic haemolytic anaemic state. The degree to which defective splenic apoptosis versus splenic hypertrophy due to hyperlipidemia-related increased haemolysis accounts for splenomegaly is not clear from this study. APOE has also been shown to regulate leucocytosis (Murphy et al, 2011) and Apoe−/− SCD mice displayed exaggerated leucocytosis, which is particularly interesting because these findings suggest a synergistic effect of non-haematopoietic apolipoprotein E with SCD on leucocytosis.
Cholesterol and LDL levels were lower in Apoe−/− SCD mice compared to Apoe−/− control mice while no differences were observed in body weight. This indicates that despite similar dietary intake, SCD has an effect on lipid metabolism. These results are consistent with previous reports that cholesterol and LDL levels are lower in SCD patients (Oztas et al, 2012). The effect of SCD on lipid metabolism may be due to increased erythropoietic activity or a consequence of the haemolytic anaemia in SCD (Zorca et al, 2010; Oztas et al, 2012).
Contrary to our hypotheses that SCD would accelerate atherosclerosis, Apoe−/− SCD mice developed less atherosclerosis compared to Apoe−/− control mice. This could be due to the reduced LDL or to another vasculo-protective effect of SCD. Other potential vasculo-protective mechanisms in SCD include upregulation of HMOX1. HMOX1 is the inducible rate-limiting enzyme involved in haem catabolism (Paine et al, 2010). It is a cytoprotective enzyme, degrading the oxidant haem into equimolar amounts of carbon monoxide, biliverdin, and ferrous iron (Durante, 2010). The protective effect of HMOX1 and its products are mediated by regulation of oxidative stress/mediators, such as nuclear factor kappaB (NFKB1), activating protein (AP-1), c-Jun-NH2-terminal kinase (JNK; MAPK8), or mitogen-activated protein kinase (MAPK1) (Tongers et al, 2004; Kim et al, 2006; Jadhav & Ndisang, 2009). HMOX1 has previously been shown to be upregulated in SCD and defective induction of HMOX1 may play a causal role in some inflammatory endpoints (Hanson et al, 2011). This is a promising candidate for protection in these models given that induction of HMOX1 is protective against development of vascular diseases, including endothelial dysfunction, atherosclerosis, and thrombosis (Juan et al, 2001; Durante, 2010). Haemin induction of HMOX1 has also been shown to be protective against vascular disease in preclinical studies (Ishikawa et al, 2001; Liu et al, 2002; Li et al, 2011). Consistent with other studies of SCD (Belcher et al, 2006), we found that HMOX activity and levels were increased in SCD mice. The mechanism by which HMOX1 is induced is presumably due to haemolysis/haem products; however, we cannot exclude other factors.
To determine the causal role of HMOX activity in contributing to the protective role of SCD on atherosclerosis, mice were treated chronically with ZnPPIX. Similar to previous studies (Yamashita et al, 2006), treatment with ZnPPIX reduced HMOX activity while increasing HMOX1 protein levels (Labbe et al, 1999). This intervention has been previously shown to also inhibit HMOX activity in SCD mice and worsen inflammation in the lung (Belcher et al, 2006). In the current study, reduction in HMOX activity reversed some, but not all, of the atheroprotection observed in SCD mice. This indicates that HMOX1 upregulation in SCD contributes to atheroprotection. This effect was not related to changes in cholesterol because ZnPPIX treatment did not increase the levels of cholesterol or LDL. The incomplete reversal of protection could be due to incomplete inhibition of HMOX1or to additional protective mechanisms, such as the SCD-induced cholesterol lowering. A particularly striking difference noted in the composition of atherosclerotic plaques between the groups was the fibrotic nature, as evidenced by collagen and lack of foam cells, in the SCD-related lesions. As previously suggested (Kato & Gladwin, 2008), it is possible that a low-LDL profibrotic response in SCD mice could lead to enhanced vascular proliferative lesions. These may be particularly relevant at other vascular sites, such as pulmonary and cerebral vasculature, where complications are relatively common in SCD patients.
Given that an arterial prothrombotic state could also contribute to vascular complications of SCD, we studied this murine model of SCD in a photochemical thrombosis model. Previous studies that have demonstrated that SCD is associated with multiple perturbations that would be expected to enhance arterial thrombosis, including increase in coagulation-related factors (Rees et al, 2010), platelet activation and aggregation (Ataga & Orringer, 2003), impaired nitric oxide activity (Villagra et al, 2007), and endothelial inflammation (Ataga et al, 2008). However, as in the case with the atherosclerosis endpoint, SCD mice exhibited prolongation in the time to thrombosis following carotid injury. In this model, time to thrombosis was shown to be sensitive to alteration in coagulation factors, platelets, and endothelial function (Westrick et al, 2007). The mechanism by which SCD protects against early occlusive thrombus formation is unclear but could also be related to HMOX1, because deficiency of HMOX1 is prothrombotic in this model (Johns et al, 2009; Fei et al, 2012) while overexpression of HMOX1 is protective against thrombosis (Lindenblatt et al, 2004). As HMOX1 has been shown to be upregulated in SCD leucocytes and proposed to mediate a compensatory response to repetitive vascular injury in SCD (Jison et al, 2004), we tested the effect of SCD or WT leucocyte infusions into recipient WT mice. Remarkably, acute infusion of leucocytes from SCD mice was sufficient to prolong the time to thrombosis in WT mice, implicating leucocytes as possible mediators of the SCD protective effect.
To determine if HMOX1 was specifically responsible for the protection of SCD against thrombosis in this model, mice were treated with ZnPPIX. This treatment attenuated the protective effect of SCD on thrombosis, suggesting that HMOX1 upregulation is mediating protection in SCD.
While these studies were originally intended to study adverse macrovascular effects of SCD, they may provide unique insight into vascular disease associated with SCD. Paradoxically, we found that SCD mice are actually protected in models of atherosclerosis and thrombosis. It may be that protective mechanisms against vascular disease are upregulated in patients with SCD and that deficient upregulation of these protective responses lead to vascular events. Studies in humans with SCD vascular complications will be necessary to test this hypothesis in regards to HMOX1. If this is the case, then targeted therapies designed to enhance HMOX1 activity in SCD patients identified to be at risk of macrovascular complications could be beneficial. Additionally, the vasculoprotective findings in this study could have potential therapeutic applications to the non-SCD population at risk of vascular disease.
Acknowledgments
This work was supported by the National Institutes of Health (HL073150 to D.T.E.) and a VA Merit Award (BX000353 to D.T.E).
Footnotes
Author contributions
Hui Wang: performed research, analysed data, and wrote paper, Wei Luo, Chiao Guo, Stephanie Wolffe, Julia Wang, Eddy Sun: performed research, Jintao Wang: performed research and analysed data, Kori Bradley: genotyping and phenotyping mice with sickle cell aneamia, Andrew Campbell: contributed essential reagents, genotyping and phenotyping SS mice, Daniel Eitzman – designed study, analysed data, and wrote paper.
Disclosures
None.
References
- Adams RJ, McKie VC, Carl EM, Nichols FT, Perry R, Brock K, McKie K, Figueroa R, Litaker M, Weiner S, Brambilla D. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Annals of Neurology. 1997;42:699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- Anthi A, Machado RF, Jison ML, Taveira-Dasilva AM, Rubin LJ, Hunter L, Hunter CJ, Coles W, Nichols J, Avila NA, Sachdev V, Chen CC, Gladwin MT. Hemo-dynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2007;175:1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI, Orringer EP. Hypercoagulability in sickle cell disease: a curious paradox. American Journal of Medicine. 2003;115:721–728. doi: 10.1016/j.amjmed.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, Sohier C, Hinderliter A, Parise LV, Orringer EP. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93:20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- Barrett O, Jr, Saunders DE, Jr, McFarland DE, Humphries JO. Myocardial infarction in sickle cell anemia. American Journal of Hematology. 1984;16:139–147. doi: 10.1002/ajh.2830160206. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. The Journal of Clinical Investigation. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- Boisvert WA, Spangenberg J, Curtiss LK. Treatment of severe hypercholesterolemia in apolipoprotein E-deficient mice by bone marrow transplantation. The Journal of Clinical Investigation. 1995;96:1118–1124. doi: 10.1172/JCI118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AD, Cui S, Shi L, Urbonya R, Mathias A, Bradley K, Bonsu KO, Douglas RR, Halford B, Schmidt L, Harro D, Giacherio D, Tanimoto K, Tanabe O, Engel JD. Forced TR2/TR4 expression in sickle cell disease mice confers enhanced fetal hemoglobin synthesis and alleviated disease phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18808–18813. doi: 10.1073/pnas.1104964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Seminars in Thrombosis and Hemostasis. 2011;37:226–236. doi: 10.1055/s-0031-1273087. [DOI] [PubMed] [Google Scholar]
- Durante W. Targeting heme oxygenase-1 in vascular disease. Current Drug Targets. 2010;11:1504–1516. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzman DT, Westrick RJ, Nabel EG, Ginsburg D. Plasminogen activator inhibitor-1 and vitronectin promote vascular thrombosis in mice. Blood. 2000;95:577–580. [PubMed] [Google Scholar]
- Elsharawy MA, Moghazy KM, Shawarby MA. Atherosclerosis in sickle cell disease - a review. International Journal of Angiology. 2009;18:62–66. doi: 10.1055/s-0031-1278326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei D, Meng X, Zhao M, Kang K, Tan G, Pan S, Luo Y, Liu W, Nan C, Jiang H, Krissansen GW, Sun X. Enhanced induction of heme oxygenase-1 suppresses thrombus formation and affects the protein C system in sepsis. Translation Research. 2012;159:99–109. doi: 10.1016/j.trsl.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Gerry JL, Bulkley BH, Hutchins GM. Clinicopathologic analysis of cardiac dysfunction in 52 patients with sickle cell anemia. American Journal of Cardiology. 1978;42:211–216. doi: 10.1016/0002-9149(78)90902-5. [DOI] [PubMed] [Google Scholar]
- Hanson MS, Piknova B, Keszler A, Diers AR, Wang X, Gladwin MT, Hillery CA, Hogg N. Methaemalbumin formation in sickle cell disease: effect on oxidative protein modification and HO-1 induction. British Journal of Haematology. 2011;154:502–511. doi: 10.1111/j.1365-2141.2011.08738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe C, Kuypers F, Larkin S, Hagar W, Vichinsky E, Styles L. A pilot study of the short-term use of simvastatin in sickle cell disease: effects on markers of vascular dysfunction. British Journal of Haematology. 2011;153:655–663. doi: 10.1111/j.1365-2141.2010.08480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney International. 2005;67:2488–2493. doi: 10.1111/j.1523-1755.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circulation Research. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- Jadhav A, Ndisang JF. Heme arginate suppresses cardiac lesions and hypertrophy in deoxycorticosterone acetate-salt hypertension. Experimental Biology and Medicine (Maywood) 2009;234:764–778. doi: 10.3181/0810-RM-302. [DOI] [PubMed] [Google Scholar]
- Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Zelent D, Ao Z, Bradley BT, Cooke A, Contino L, Hu E, Douglas SA, Jaye MC. Heme-oxygenase induction inhibits arteriolar thrombosis in vivo: effect of the non-substrate inducer cobalt protoporphyrin. European Journal of Pharmacology. 2009;606:109–114. doi: 10.1016/j.ejphar.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT. Evolution of novel small-molecule therapeutics targeting sickle cell vasculopathy. JAMA. 2008;300:2638–2646. doi: 10.1001/jama.2008.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Tsoy I, Park MK, Lee YS, Lee JH, Seo HG, Chang KC. Iron released by sodium nitroprusside contributes to heme oxygenase-1 induction via the cAMP-protein kinase A-mitogen-activated protein kinase pathway in RAW 264.7 cells. Molecular Pharmacology. 2006;69:1633–1640. doi: 10.1124/mol.105.020487. [DOI] [PubMed] [Google Scholar]
- Labbe RF, Vreman HJ, Stevenson DK. Zinc protoporphyrin: a metabolite with a mission. Clinical Chemistry. 1999;45:2060–2072. [PubMed] [Google Scholar]
- Li T, Tian H, Zhao Y, An F, Zhang L, Zhang J, Peng J, Zhang Y, Guo Y. Heme oxygenase-1 inhibits progression and destabilization of vulnerable plaques in a rabbit model of atherosclerosis. European Journal of Pharmacology. 2011;672:143–152. doi: 10.1016/j.ejphar.2011.09.188. [DOI] [PubMed] [Google Scholar]
- Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:601–606. doi: 10.1161/01.ATV.0000118279.74056.8a. [DOI] [PubMed] [Google Scholar]
- Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maisel A, Friedman H, Flint L, Koshy M, Prabhu R. Continuous electrocardiographic monitoring in patients with sickle-cell anemia during pain crisis. Clinical Cardiology. 1983;6:339–344. doi: 10.1002/clc.4960060707. [DOI] [PubMed] [Google Scholar]
- Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr, Shah AK, Mankad VN. Causes of death in sickle cell disease: an autopsy study. British Journal of Haematology. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- Mansi IA, Rosner F. Myocardial infarction in sickle cell disease. Journal of the National Medical Association. 2002;94:448–452. [PMC free article] [PubMed] [Google Scholar]
- Martin CR, Johnson CS, Cobb C, Tatter D, Haywood LJ. Myocardial infarction in sickle cell disease. Journal of the National Medical Association. 1996;88:428–432. [PMC free article] [PubMed] [Google Scholar]
- McCormick WF. Massive nonatherosclerotic myocardial infarction in sickle cell anemia. American Journal of Forensic Medicine and Pathology. 1988;9:151–154. doi: 10.1097/00000433-198806000-00012. [DOI] [PubMed] [Google Scholar]
- Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CR. Vascular risk assessment in patients with sickle cell disease. Haematologica. 2011;96:1–5. doi: 10.3324/haematol.2010.035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Vandegriff K, Intaglietta M, Winslow RM. Oxidative-stress response in vascular endothelial cells exposed to acellular hemoglobin solutions. American Journal of Physiology. 1995;269:H648–H655. doi: 10.1152/ajpheart.1995.269.2.H648. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. The Journal of Clinical Investigation. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis and Thrombosis. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztas Y, Durukan I, Unal S, Ozgunes N. Plasma protein oxidation is correlated positively with plasma iron levels and negatively with hemolysate zinc levels in sickle-cell anemia patients. International Journal of Laboratory Hematology. 2012;34:129–135. doi: 10.1111/j.1751-553X.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. New England Journal of Medicine. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Ross R. Cell biology of atherosclerosis. Annual Review of Physiology. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: a neuropathological study. Annals Neurology. 1986;20:684–690. doi: 10.1002/ana.410200606. [DOI] [PubMed] [Google Scholar]
- Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- Tongers J, Fiedler B, Konig D, Kempf T, Klein G, Heineke J, Kraft T, Gambaryan S, Lohmann SM, Drexler H, Wollert KC. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/ NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovascular Research. 2004;63:545–552. doi: 10.1016/j.cardiores.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. European Heart Journal. 2004;25:1197–1207. doi: 10.1016/j.ehj.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang Z, Lu H, Lin H, Wang Z, Chen X, Ouyang Q, Tang M, Hao P, Ni J, Xu D, Zhang M, Zhang Q, Lin L, Zhang Y. Apolipoprotein E-knockout mice show increased titers of serum anti-nuclear and anti-dsDNA antibodies. Biochemical and Biophysical Research Communications. 2012;423:805–812. doi: 10.1016/j.bbrc.2012.06.044. [DOI] [PubMed] [Google Scholar]
- Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series) Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Ollinger R, McDaid J, Sakahama H, Wang H, Tyagi S, Csizmadia E, Smith NR, Soares MP, Bach FH. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB Journal. 2006;20:776–778. doi: 10.1096/fj.05-4791fje. [DOI] [PubMed] [Google Scholar]
- Zorca S, Freeman L, Hildesheim M, Allen D, Remaley AT, Taylor JGt, Kato GJ. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. British Journal of Haematology. 2010;149:436–445. doi: 10.1111/j.1365-2141.2010.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]