Abstract
Cardiac arrest is a leading cause of death in developed countries. Although a majority of cardiac arrest patients die during the acute event, a substantial proportion of cardiac arrest deaths occur in patients following successful resuscitation and can be attributed to the development of post-cardiac arrest syndrome. There is growing recognition that integrated post-resuscitation care, which encompasses targeted temperature management (TTM), early coronary angiography and comprehensive critical care, can improve patient outcomes. TTM has been shown to improve survival and neurological outcome in patients who remain comatose especially following out-of-hospital cardiac arrest due to ventricular arrhythmias. Early coronary angiography and revascularisation if needed may also be beneficial during the post-resuscitation phase, based on data from observational studies. In addition, resuscitated patients usually require intensive care, which includes mechanical ventilator, haemodynamic support and close monitoring of blood gases, glucose, electrolytes, seizures and other disease-specific intervention. Efforts should be taken to avoid premature withdrawal of life-supporting treatment, especially in patients treated with TTM. Given that resources and personnel needed to provide high-quality post-resuscitation care may not exist at all hospitals, professional societies have recommended regionalisation of post-resuscitation care in specialised ‘cardiac arrest centres’ as a strategy to improve cardiac arrest outcomes. Finally, evidence for post-resuscitation care following in-hospital cardiac arrest is largely extrapolated from studies in patients with out-of-hospital cardiac arrest. Future studies need to examine the effectiveness of different post-resuscitation strategies, such as TTM, in patients with in-hospital cardiac arrest.
INTRODUCTION
Cardiac arrest accounts for nearly 500 000 deaths annually in the USA and Europe.1–3 Survival in patients with out-of-hospital cardiac arrest is <15%,4 while survival with in-hospital cardiac arrest is approximately 22%.5 There is a significant risk of neurological disability among survivors in both groups. Although a majority of deaths due to cardiac arrest occur during the initial resuscitation, a substantial proportion of cardiac arrest deaths occur in patients who have been successfully resuscitated (post-resuscitation phase). High mortality during the post-resuscitation phase can be attributed to the combination of whole-body ischaemia, reperfusion-mediated damage and the underlying pathological process that led to cardiac arrest. There is growing recognition that optimal treatment strategies during the post-resuscitation phase may improve outcomes.6,7 In this article, we will review strategies for post-resuscitation care, with a particular focus on targeted temperature management (TTM), early coronary angiography, avoidance of early prognostication and regionalisation of cardiac arrest care.
PATHOPHYSIOLOGY OF POST-CARDIAC ARREST SYNDROME
Although cardiac arrest impacts all organ systems, its impact is most profound on the neurological system as brain tissue is exquisitely sensitive to lack of oxygen. Oxygen stores in the brain are depleted within 20 s, and glucose and ATP within 5 min of complete anoxia.8 This leads to a loss of membrane resting potential, influx of calcium and release of excitatory neurotransmitters, which further exacerbates neuronal injury due to tissue hypoxia.9 With return of spontaneous circulation, a phase of secondary injury due to formation of oxygen-free radicals ensues causing direct injury to cell membranes and promoting inflammation.10 Dysfunction of the cerebral autoregulation may also occur, contributing to persistent cerebral ischaemia.11 Patients with extensive anoxic brain injury may develop cerebral oedema and risk herniation of the brain with increased intracranial pressure. Besides neurological injury, myocardial dysfunction may also develop due to systemic ischaemia, which may manifest as hypotension, low cardiac output, increased filling pressures and a global decrease in myocardial contractility on echocardiography.12
TARGETED TEMPERATURE MANAGEMENT
TTM is a strategy of intentionally lowering body temperature with the goal of reducing ischaemia-mediated and reperfusion-mediated neurological injury. Evidence supporting the benefit of TTM in cardiac arrest patients is summarised below.
In 2012, two landmark trials found a significant benefit of TTM in comatose patients resuscitated from out-of-hospital cardiac arrest due to ventricular fibrillation (VF).13,14 Patients were cooled to a temperature of 32–34°C using external cooling blankets or ice packs, and hypothermia was maintained for 12–24 h. Rates of neurological function were improved in both studies, while survival was improved in one study. Based on these findings, TTM was adopted as a Class I recommendation by the 2005 American Heart Association (AHA)15 and the European Society of Cardiology16 guidelines, especially in out-of-hospital cardiac arrest due to VF or pulseless ventricular tachycardia (VT). However, questions remained regarding the optimal target temperature, the timing, method and duration of cooling, and whether cooling is beneficial for cardiac arrest due to non-shockable rhythms or in-hospital cardiac arrest.
Recent studies have examined whether a less intensive temperature target provides the same clinical benefit as traditional TTM. A large randomised trial compared a target temperature of 33 vs 36°C in 950 patients with out-of-hospital cardiac arrest across Europe and Australia. The study found no difference in in-hospital mortality (50% vs 48%, p=0.51) or survival with favourable neurological outcome at 6 months (46% vs 48%, p=0.78) between the groups.17 Although the overall risk of adverse events was also similar, hypokalaemia was less common in the 36°C group (13% vs 19%, p=0.02). It is important to note that the above findings do not suggest a lack of benefit of TTM since patients in the 36°C group also received active temperature management. A higher temperature target may be attractive due to a potentially lower risk of cooling-related adverse effects and avoidance of sedatives and paralytics. A possible limitation of clinical trials of TTM may be their reliance on crude measures of neurological function, such as the Cerebral Performance Category (CPC) score. Although CPC score is easy to use and strongly associated with long-term survival,18 it is less sensitive than formal, objective functional testing instruments such as the Health Utilities Index.19
In clinical practice, TTM can be provided using a number of different methods. These can be classified into surface-cooling methods (eg, use of ice packs around the body) or core-cooling methods (eg, intravenous catheters that circulate cold saline) or a combination approach. Once target temperature is achieved, it should be maintained for a period of 12–24 h. Sedatives and paralytic agents are usually necessary to ensure comfort and prevent shivering. Rewarming should be accomplished slowly at 0.25–0.5°C/h with special care to avoid hyperthermia. Once normothermia is achieved, sedation and paralytic agents may be discontinued to monitor recovery.
All of the aforementioned clinical trials used one of the surface-cooling methods. A recent trial of out-of-hospital cardiac arrest patients compared surface cooling using fans, a homemade tent and ice packs with the use of an intravascular cooling catheter. Hypothermia was achieved faster with the use of a cooling catheter compared with surface cooling. However, there was no statistically significant difference in overall survival (41.9% vs 38.1%, p=0.44) or survival with favourable neurological outcome (36.0% vs 28.4%, p=0.11) at day 28,20 although a trend for improved favourable neurological survival at 90 days (34.6% vs 26.0%, p=0.07) was observed in the cooling catheter group.
The best time to initiate TTM has also not been determined. Animal studies have suggested earlier initiation of cooling is associated with better survival.21 However, this finding was not borne out in a randomised controlled trial that included 1359 patients with out-of-hospital cardiac arrest due to both shockable and non-shockable rhythms and found similar rates of survival to discharge or neurological outcome.22 Moreover, a higher incidence of hypoxia and pulmonary oedema after cold saline infusion was noted in the prehospital cooling arm. It remains unclear whether the use of a surface cooling method, as used in the other hypothermia trials instead of cold saline infusion, would have altered the study's outcome.
The effectiveness of TTM in cardiac arrest due to nonshockable rhythms has not been evaluated in a randomised trial, and most of the data come from observational studies, which provided conflicting results.23–25 While current guidelines recommend TTM in patients with non-shockable rhythms, an ongoing study will likely address this question in the future.26
In patients with in-hospital cardiac arrest, the evidence is even less clear. Two small observational studies, which had significant limitations, did not find TTM to be associated with improved survival.27,28 There are several reasons why TTM may not provide the same benefit for in-hospital cardiac arrest. First, the prevalence of VF and pulseless VT—rhythms known to benefit from hypothermia—is much lower in patients with in-hospital cardiac.5 This is because cardiac arrest in hospitalised patients occurs commonly in the setting of acute respiratory failure, pneumonia and sepsis, rather than ischaemic heart disease. Second, risk of neurological injury may be lower in the in-hospital setting since response times are short. Third, hospitalised patients are generally sicker and have numerous comorbidities, which may influence their potential to benefit from hypothermia. Although current guidelines support the use of TTM for in-hospital cardiac arrest, the benefit remains uncertain.
EARLY CORONARY ANGIOGRAPHY AND PERCUTANEOUS CARDIOVASCULAR INTERVENTIONS
Myocardial ischaemia is a potent stimulus for VF and likely accounts for a significant proportion of cardiac arrest cases, especially in the out-of-hospital setting.29 Although randomised trials have shown improved outcome with early coronary angiography and revascularisation in acute myocardial infarction (AMI),30 there are several considerations regarding application of these findings to the cardiac arrest population. First, cardiac arrest patients were excluded from randomised trials of early revascularisation in AMI leading to clinical uncertainty. Second, AMI diagnosis in resuscitated patients can be difficult as 12-lead ECGs can have a high false-negative rate.31 Third, some physicians may prefer to withhold invasive procedures such as coronary angiography in cardiac arrest patients when the potential for neurological recovery may be unclear. Finally, in the current era of public reporting, interventional cardiologists may be reluctant to offer coronary angiography to cardiac arrest patients due to their high risk of mortality.32
In the absence of randomised trials, data regarding the benefit of early coronary angiography in cardiac arrest patients come from observational studies, some of which are summarised in table 1.33–44 A majority of these studies found early coronary angiography to be associated with survival to discharge, although the benefit was not consistent.34,39 Based on this evidence, the European Association for Percutaneous Cardiovascular Interventions /Stent for Life groups recently issued a consensus statement regarding interventional management of resuscitated out-of-hospital cardiac arrest patient.45 The committee recommended that conscious survivors of out-of-hospital cardiac arrest with suspected AMI should be treated similar to patients with ST segment elevation myocardial infarction (STEMI) and high-risk non-ST segment elevation myocardial infarction who do not have cardiac arrest. However, among comatose patients, the evidence is more limited. The committee recommends immediate coronary angiography and revascularisation as needed in comatose patients with STEMI. In contrast, a strategy of a short emergency department ‘stop’ is advised in comatose patients without STEMI to exclude non-coronary causes of cardiac arrest. In the absence of a non-coronary cause, and particularly in patients who are unstable, the committee recommends early coronary angiography.
Table 1.
Summary of selected studies examining the role of early coronary angiography and survival in patients successfully resuscitated from out-of-hospital cardiac arrest
| Author, year | Location | Sites, N | N | Age | Comatose, % | STEMI, % | Shockable, % | Timing of early catheterisation | Early catheterisation, % | Survival (early catheterisation vs no early catheterisation) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aurore, 2011 | Creteil, France | 1 centre | 445 | 61 | NA | 17.5 | 31.7 | <2 h | 29.9 | 23.0% vs 9.6% |
| Bro-Jeppeson, 2012 | Copenhagen, Denmark | 1 centre | 360 | 61 | 100 | 32.2 | 80 | <12 h | 55 | 66.0% vs 54.0% |
| Callaway, 2014 | Pittsburgh, PA, USA | 151 hospitals | 3981 | 67 | NA | 17.5 | 40.7 | <24 h | 19.2 | 64.7% vs 27.1% |
| Hollenbeck, 2014 | Nashville, TN, USA | 6 hospitals | 269 | 60.1 | 100 | 0 | 100 | Emergency | 45.4 | 65.6% vs 48.6% |
| Kern, 2015 | Tucson, AZ, USA | 34 hospitals in US & Europe | 746 | 61.0 | 100 | 26.0 | 58% | Immediate | 48.3 | STEMI: 54.7% vs 33.3% No STEMI: 57.9% vs 20.3% |
| Mooney, 2011 | Minneapolis, MN, USA | 1 centre | 140 | 62 | NA | 49 | 76 | Emergency | 72 | 62.4% vs 38.0% |
| Nanjayya, 2011 | Westmead, Australia | 1 centre | 70 | 60.7 | 100 | 64.3 | 100 | Immediate | 50 | 51.0% vs 34.0% |
| Nielsen, 2009 | Lund, Sweden | 38 centres, 7 countries | 986 | 63 | 100 | NA | 70 | Emergency | 49 | 63.0% vs 49.9% |
| Strote, 2012 | Seattle, WA, USA | 11 hospitals | 240 | 66.2 | NA | 34.2 | 100 | <6 h | 25 | 72.0% vs 49.0% |
| Tomte, 2011 | Oslo, Norway | 1 centre | 248 | 62 | 70 | NA | 92 | Emergency | 77 | 52.0% vs 31.0% |
| Vyas, 2015 | Iowa City, IA, USA | 374 hospitals | 4029 | 61 | NA | 19.9 | 100 | <1 calendar day | 48.5 | 76.0% vs 59.4% |
| Zanuttini, 2012 | Udine, Italy | 1 centre | 93 | 67 | 100% | 34 | 65 | Emergency | 51.6 | 60.0% vs 47.0% |
NA, not available; STEMI, ST segment elevation myocardial infarction.
The limitations of the observational studies of early coronary angiography in cardiac arrest must also be considered, particularly the potential for selection bias. It is likely that early coronary angiography is selectively offered to patients with good prognosis for neurological recovery. Most of the observational studies were small, and retrospective in nature, and therefore limited in their ability to use methods to account for selection bias. There are two ongoing randomised trials designed to determine whether early coronary angiography improves outcomes in out-of-hospital cardiac arrest patients without STEMI.46,47
For patients with in-hospital cardiac arrest, only a single study examined use of early coronary angiography in 110 patients successfully resuscitated from VF and pulseless VT.48 Only 27% patients in that study underwent coronary angiography within one day of cardiac arrest. Early coronary angiography was associated with increased survival to discharge (OR 3.8, 95% CI 1.35 to 10.90). However, the study was limited due to its small sample size, lack of information on key confounding variables (eg, neurological status post-arrest) and potential for selection bias. Additional studies are needed to determine whether early coronary angiography improves patient outcomes following in-hospital cardiac arrest.
GENERAL SUPPORTIVE MEASURES
In addition to TTM and early coronary angiography, patients resuscitated from a cardiac arrest are often critically ill and need intensive monitoring and treatment. Given the lack of randomised controlled trials, supportive care during post-resuscitation phase is largely based on evidence from strategies for general intensive care. The key elements of the general supportive care of post-cardiac arrest patients are summarised in table 2. Patients usually require airway protection, mechanical ventilator support and monitoring to ensure adequate oxygenation and ventilation.49,50 Haemodynamic support with fluids, vasopressors, inotropes or mechanical circulatory support devices may be necessary. Seizures are common post-arrest and can be very difficult to diagnose due to use of sedatives and paralytic agents in hypothermia-treated patients without EEG monitoring.51 Careful monitoring of blood gases, electrolytes, blood glucose, infection and other disease-specific intervention is also required.52,53 In addition, appropriate testing to identify and treat the underlying cause of arrest (eg, CT angiography to diagnose pulmonary embolism) should be initiated.
Table 2.
Summary of key elements of general supportive care for post-resuscitation care
| Current evidence and key message | Recommendation | |
|---|---|---|
| Oxygenation | ▶ Hyperoxia is associated with brain injury in animal studies due to free radical formation ▶ Conflicting results from studies of hyperoxia and post-cardiac arrest survival in humans ▶ No clear benefit of maintaining supra-normal oxygen levels post arrest |
Titrate oxygenation to maintain pulse oximetry around 95% |
| Ventilation | ▶ Hypocapnia may decrease cerebral blood flow and lead to worse neurological outcome ▶ Hypercapnia may be neuroprotective although the evidence is mixed |
Routine hyperventilation should be avoided. Maintain normal PaCo2 (35–45 mm Hg) |
| Blood pressure | ▶ Hypotension is common post arrest and is associated with worse outcomes ▶ Cerebral autoregulation may be impaired post arrest, making cerebral perfusion sensitive to hypotension ▶ Circulatory support post arrest may include intravenous fluids, vasopressors, inotropes and consideration for mechanical circulatory support ▶ Early haemodynamic optimisation similar to the approach used for sepsis has been advocated for post-cardiac arrest syndrome based on their shared pathophysiology. Although no randomised trials exist, outcomes were improved in a study that implemented haemodynamic optimisation as part of a bundled intervention compared to historical controls |
Haemodynamic management should be guided by clinical judgement and may include use of intravenous fluids, vasopressors and inotropes Mechanical circulatory support devices may be considered in patients with advanced myocardial dysfunction |
| Seizure control | ▶ Seizures may occur in 10–15% of patients post arrest and are associated with worse outcomes ▶ Seizures may be difficult to diagnose in post-arrest patients in absence of EEG monitoring especially in patients treated with hypothermia ▶ Randomised trials of seizure prophylaxis in improving survival have been negative |
Periodic or continuous EEG monitoring is recommended to diagnose and treat subclinical seizure Antiseizure prophylaxis is not recommended Aggressive treatment of first seizures is recommended |
| Glucose | ▶ Hyperglycaemia is common post cardiac arrest and is associated with increased risk of neurological injury ▶ Randomised trials comparing strict glucose control with moderate control post arrest showed no benefit of strict control in cardiac arrest as well as otherwise critically ill patients |
Avoid hypoglycaemia Glucose levels >10 mmol/L (180 mg/dL) may need treatment with insulin |
| Steroids | ▶ Adrenal insufficiency may develop post-cardiac arrest ▶ Steroids may provide haemodynamic stability and reduce inflammation ▶ One randomised trial from three centres in Greece showed that a combination of vasopressin, epinephrine intra-arrest and steroids post arrest led to improved rates of survival. Other studies of steroids post arrest have been negative |
Routine use of steroids post arrest is not recommended |
NEUROPROGNOSTICATION
The experience of a cardiac arrest can be overwhelming for patients’ families and their treating physicians. Accurate assessment of neurological prognosis can be valuable in communicating expectations, and ensuring that treatment intensity is in line with patient's preferences or advance directives. However, it is equally important to ensure that withdrawal of care does not occur prematurely when patients may have a chance of recovery.
In 2006, the American Academy of Neurology recommended that absence of pupillary response, corneal reflex and motor response by day 3 were highly predictive of poor neurological outcome with a 0% false-positive rate.54 However, these recommendations were based on studies conducted in an era prior to the routine use of TTM. Given that TTM can significantly impact normal body function and recovery, use of the above criteria can lead to premature withdrawal of care. Moreover, sedative and paralytic agents commonly used during TTM can accumulate due to a reduction in drug clearance making neurological assessment difficult. Therefore, the AHA and the International Liaison Committee on Resuscitation strongly recommend that assessment of neurological prognosis should be delayed for at least 72 h post rewarming.55
A number of methods to assess neurological prognosis in patients receiving hypothermia have been studied in the literature.56 These include findings on neurological examination (absence of pupillary and corneal reflex and motor response at 72 h), somatosensory evoked potential (absent bilateral cortical N20 response after stimulation of the median nerve at 48–72 h), EEG (discontinuous suppression burst pattern, status epilepticus), presence of myoclonus or status epilepticus, biomarkers (rising level of neuron-specific enolase, S100) and neuroimaging studies.57 However, none of the above modalities have been shown to predict lack of neurological recovery with absolute certainty. Therefore, a combination of criteria, rather than any single criterion, should be used in neurological assessment and prognosis should be delayed whenever uncertainty exists.
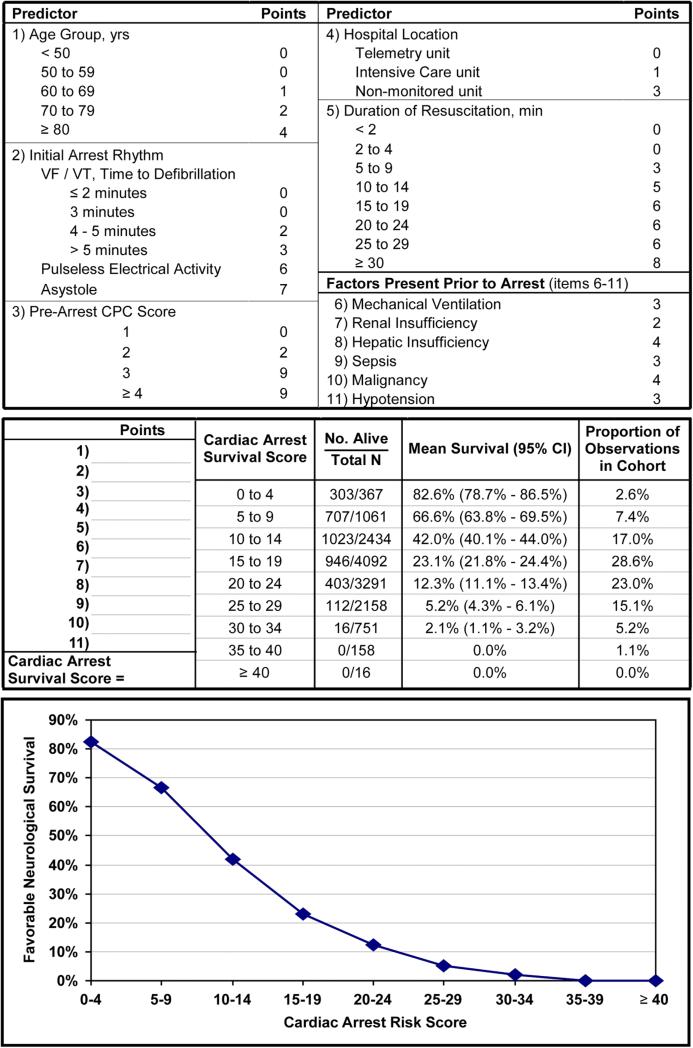
For patients with in-hospital cardiac arrest, a previous study developed and validated the Cardiac Arrest Survival Postresuscitation In-hospital (CASPRI) risk score to predict the likelihood of favourable neurological survival in successfully resuscitated patients.58 The score is comprised of 11 pre-arrest and intra-arrest variables, and ranges from 0 to 50 with higher scores, indicating a worse probability of favourable neurological survival (figure 1). Although the CASPRI score can be helpful in communicating prognosis and managing family expectations, it should not be solely used as a tool to make treatment decisions or advocate for early withdrawal of care given that the chance of neurological recovery is not zero at any score.
Figure 1.
The Cardiac Arrest Survival Postresuscitation In-hospital (CASPRI) scorecard and nomogram for favourable neurological survival. For this in-hospital cardiac arrest risk score, points for each variable are determined, and a summary score is obtained. The corresponding likelihood of surviving to hospital discharge without severe neurological disability is determined from the risk table or plot. Adapted with permission from Chan et al.58 CPC, cerebral performance score; VF/VT, ventricular fibrillation or ventricular tachycardia.
CARDIAC ARREST CENTRES OF EXCELLENCE
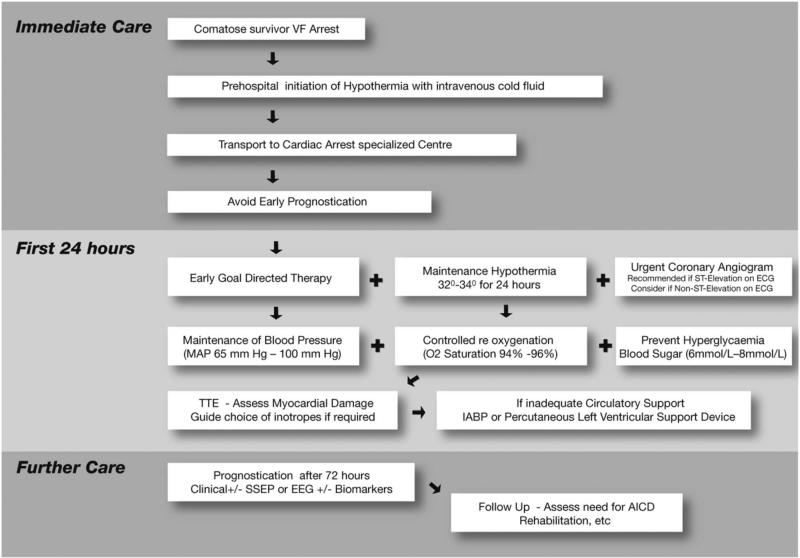
There is growing recognition that integrated post-resuscitation care, which encompasses provision of TTM, early coronary angiography and comprehensive critical care, is important for improving outcomes. Moreover, the resources and experience necessary to deliver high-quality post-resuscitation care may not be available uniformly across all hospitals. Studies have shown a marked variation in cardiac arrest survival across hospitals after adjusting for differences in patient characteristics, which highlights the importance of hospital quality.59,60 Based on such studies, the AHA has advocated for regionalisation of post-resuscitation care in specialised centres, similar to stroke or STEMI care.61 As a result, ‘cardiac arrest centres’ have developed in several hospitals with a goal of enhancing clinical care of resuscitated patients. Although centres may differ in structure, common features are (1) multidisciplinary collaboration (emergency medicine, cardiology, neurology, pulmonary and critical care), (2) a designated hospital unit for admitting all post-arrest patients, (3) a standardised treatment protocol to ensure that care processes are consistent, (4) a dedicated 24×7 cardiac arrest consult team, (5) avoidance of early neuroprognostication and (6) ongoing data collection to monitor and improve quality of care.62 A ‘centre’ approach allows institutions to concentrate resources and equipment in dedicated hospital locations (eg, cardiac intensive care unit), staffed by highly experienced physicians and nurses using structured treatment protocols to provide high-quality post-resuscitation care. An example of a structured approach to care is shown in figure 2 that outlines the key elements of post-resuscitation care discussed in this article.63
Figure 2.
Post-cardiac arrest treatment algorithm. Adapted with permission from Stub et al.63 AICD, automated internal cardioverter defibrillator; IABP, intra-aortic balloon pump; MAP, mean arterial blood pressure; SSEP, somatosensory evoked potentials; TTE, transthoracic echocardiogram.
The state of Arizona in the USA has moved towards regionalising cardiac arrest care at a state level. Between 2007 and 2010, a total of 31 hospitals throughout the state were designated as cardiac arrest centres. The participating emergency medical services agencies have been directed to transport resuscitated patients to cardiac arrest centres bypassing local hospitals. A before-and-after comparison of the statewide initiative showed an increase in provision of hypothermia from 0% to 44% and use of percutaneous cardiovascular intervention from 11.7% to 30.7%. These changes in care were accompanied by an increase in survival (8.9% to 14.4%), favourable neurological survival (5.9% to 8.9%) and survival in patients with witnessed shockable rhythms (21.4% to 39.2%).64 Future studies need to determine whether a similar approach would improve outcomes in other geographic regions.
POST-DISCHARGE CARE
Recent studies have showed that post-discharge survival among cardiac arrest survivors is not as dismal as previously assumed. In a study of 200 survivors of out-of-hospital cardiac arrest, 5-year survival was 79.6%, which was similar to an age-matched, sex-matched and disease-matched control group.65 Likewise, 2-year survival in a cohort of 6972 elderly Medicare patients was 49.6%, which was comparable to a cohort of heart failure patients.18 However, significant physical, psychological and intellectual deficits that may range from subtle to complete dependence on caregivers may be present. This may lead to impairment in quality of life, physical functioning, depression, as well as caregiver strain. A rehabilitation programme that is focused on addressing the specific cognitive, physical and psychological needs of patients could benefit patients and mitigate the deleterious consequences of neurological injury post arrest.
An implantable cardioverter defibrillator (ICD) should be considered in cardiac arrest survivors. Current guidelines recommend an ICD in such patients who survive an out-of-hospital cardiac arrest due to VF or pulseless VT provided reversible causes (eg, AMI) have been excluded.66 The guidelines are less clear regarding patients with in-hospital cardiac arrest, and the best evidence comes from an observational study that found a 24% reduction in 3-year mortality with the use of ICDs in survivors of in-hospital cardiac arrest due to a shockable rhythm.67
CONCLUSION
Patients resuscitated from a cardiac arrest have a significant risk of death and disability. A comprehensive treatment protocol that includes TTM, consideration for early coronary revascularisation, general supportive care and avoidance of early neuroprognostication can potentially improve outcomes in this high-risk population. Evidence is emerging that concentrating post-resuscitation care in centres of excellence with multidisciplinary teams could be an innovative strategy to improve cardiac arrest survival similar to other time-sensitive conditions.
Acknowledgments
Funding SG (K08HL122 527) and PSC (K23HL102224 and 1R01HL123980) are supported by the National Heart, Lung, and Blood Institute (NHLBI) at the National Institutes of Health. SB (HSR&D-CDA2 10-199) is supported by the Veterans Affairs Health Services Research and Development.
Footnotes
Contributors Study concept and design: SG and SMB; acquisition of data: SG; analysis and interpretation of data: SG, PSC and SMB; drafting of the manuscript: SG; critical revision of the manuscript for important intellectual content: SG, PSC and SMB; study supervision: SG; all authors have approved the manuscript prior to submission.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
REFERENCES
- 1.Atwood C, Eisenberg MS, Herlitz J, et al. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation. 2005;67:75–80. doi: 10.1016/j.resuscitation.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Sans S, Kesteloot H, Kromhout D. The burden of cardiovascular diseases mortality in Europe. Task Force of the European Society of Cardiology on Cardiovascular Mortality and Morbidity Statistics in Europe. Eur Heart J. 1997;18:1231–48. [PubMed] [Google Scholar]
- 4.Chan PS, McNally B, Tang F, et al. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014;130:1876–82. doi: 10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–20. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–86. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 7.Morrison LJ, Neumar RW, Zimmerman JL, et al. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127:1538–63. doi: 10.1161/CIR.0b013e31828b2770. [DOI] [PubMed] [Google Scholar]
- 8.Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Curr Opin Crit Care. 2004;10:213–17. doi: 10.1097/01.ccx.0000127542.32890.fa. [DOI] [PubMed] [Google Scholar]
- 9.Hoesch RE, Koenig MA, Geocadin RG. Coma after global ischemic brain injury: pathophysiology and emerging therapies. Crit Care Clin. 2008;24:25–44. vii–viii. doi: 10.1016/j.ccc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Grabb MC, Zipfel GJ, et al. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–31. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundgreen C, Larsen FS, Herzog TM, et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–32. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 12.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–16. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 13.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 14.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 15.ECC Committee, Subcommittees and Task Forces of the American Heart Association 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(24 Suppl):IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 16.Nolan J, European Resuscitation Council European Resuscitation Council guidelines for resuscitation 2005. Section 1. Introduction. Resuscitation. 2005;67(Suppl 1):S3–6. doi: 10.1016/j.resuscitation.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen N, Wetterslev J, Friberg H, et al. Targeted temperature management after cardiac arrest. N Engl J Med. 2014;370:1360. doi: 10.1056/NEJMc1401250. [DOI] [PubMed] [Google Scholar]
- 18.Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368:1019–26. doi: 10.1056/NEJMoa1200657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stiell IG, Nesbitt LP, Nichol G, et al. Comparison of the Cerebral Performance Category score and the Health Utilities Index for survivors of cardiac arrest. Ann Emerg Med. 2009;53:241–8. doi: 10.1016/j.annemergmed.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Deye N, Cariou A, Girardie P, et al. Endovascular versus external targeted temperature management for out-of-hospital cardiac arrest patients: a randomized controlled study. Circulation. 2015;132:182–93. doi: 10.1161/CIRCULATIONAHA.114.012805. [DOI] [PubMed] [Google Scholar]
- 21.Kuboyama K, Safar P, Radovsky A, et al. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–58. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311:45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 23.Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients? insights from a large registry. Circulation. 2011;123:877–86. doi: 10.1161/CIRCULATIONAHA.110.987347. [DOI] [PubMed] [Google Scholar]
- 24.Kim YM, Yim HW, Jeong SH, et al. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms? a systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation. 2012;83:188–96. doi: 10.1016/j.resuscitation.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82:1162–7. doi: 10.1016/j.resuscitation.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Lascarrou JB, Meziani F, Le Gouge A, et al. Therapeutic hypothermia after nonshockable cardiac arrest: the HYPERION multicenter, randomized, controlled, assessor-blinded, superiority trial. Scand J Trauma Resusc Emerg Med. 2015;23:26. doi: 10.1186/s13049-015-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kory P, Fukunaga M, Mathew JP, et al. Outcomes of mild therapeutic hypothermia after in-hospital cardiac arrest. Neurocrit Care. 2012;16:406–12. doi: 10.1007/s12028-011-9664-y. [DOI] [PubMed] [Google Scholar]
- 28.Nichol G, Huszti E, Kim F, et al. Does induction of hypothermia improve outcomes after in-hospital cardiac arrest? Resuscitation. 2013;84:620–5. doi: 10.1016/j.resuscitation.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 30.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–55. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 31.Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–33. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 32.Peberdy MA, Donnino MW, Callaway CW, et al. Impact of percutaneous coronary intervention performance reporting on cardiac resuscitation centers: a scientific statement from the American Heart Association. Circulation. 2013;128:762–73. doi: 10.1161/CIR.0b013e3182a15cd2. [DOI] [PubMed] [Google Scholar]
- 33.Aurore A, Jabre P, Liot P, et al. Predictive factors for positive coronary angiography in out-of-hospital cardiac arrest patients. Eur J Emerg Med. 2011;18:73–6. doi: 10.1097/MEJ.0b013e32833d469a. [DOI] [PubMed] [Google Scholar]
- 34.Bro-Jeppesen J, Kjaergaard J, Wanscher M, et al. Emergency coronary angiography in comatose cardiac arrest patients: do real-life experiences support the guidelines? Eur Heart J Acute Cardiovasc Care. 2012;1:291–301. doi: 10.1177/2048872612465588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaway CW, Schmicker RH, Brown SP, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85:657–63. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollenbeck RD, McPherson JA, Mooney MR, et al. Early cardiac catheterization is associated with improved survival in comatose survivors of cardiac arrest without STEMI. Resuscitation. 2014;85:88–95. doi: 10.1016/j.resuscitation.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Kern KB, Lotun K, Patel N, et al. Outcomes of Comatose Cardiac Arrest Survivors With and Without STEMI: Importance of Coronary Angiography. JACC Cardiovasc Interv. 2015;8:1031–40. doi: 10.1016/j.jcin.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Mooney MR, Unger BT, Boland LL, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124:206–14. doi: 10.1161/CIRCULATIONAHA.110.986257. [DOI] [PubMed] [Google Scholar]
- 39.Nanjayya VB, Nayyar V. Immediate coronary angiogram in comatose survivors of out-of-hospital cardiac arrest—an Australian study. Resuscitation. 2012;83:699–704. doi: 10.1016/j.resuscitation.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926–34. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 41.Strote JA, Maynard C, Olsufka M, et al. Comparison of role of early (less than six hours) to later (more than six hours) or no cardiac catheterization after resuscitation from out-of-hospital cardiac arrest. Am J Cardiol. 2012;109:451–4. doi: 10.1016/j.amjcard.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomte O, Andersen GO, Jacobsen D, et al. Strong and weak aspects of an established post-resuscitation treatment protocol-A five-year observational study. Resuscitation. 2011;82:1186–93. doi: 10.1016/j.resuscitation.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Vyas A, Chan PS, Cram P, et al. Early coronary angiography and survival after out-of-hospital cardiac arrest. Circ Cardiovasc Interv. 2015 doi: 10.1161/CIRCINTERVENTIONS.114.002321. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanuttini D, Armellini I, Nucifora G, et al. Impact of emergency coronary angiography on in-hospital outcome of unconscious survivors after out-of-hospital cardiac arrest. Am J Cardiol. 2012;110:1723–8. doi: 10.1016/j.amjcard.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Noc M, Fajadet J, Lassen JF, et al. Invasive coronary treatment strategies for out-of-hospital cardiac arrest: a consensus statement from the European association for percutaneous cardiovascular interventions (EAPCI)/stent for life (SFL) groups. EuroIntervention. 2014;10:31–7. doi: 10.4244/EIJV10I1A7. [DOI] [PubMed] [Google Scholar]
- 46.Direct or Subacute Coronary Angiography for Out-of-hospital Cardiac Arrest (DISCO) ClinicalTrials.gov Identifier: NCT02309151.
- 47.Early Coronary Angiography Versus Delayed Coronary Angiography. ClinicalTrials.gov Identifier: NCT02387398.
- 48.Merchant RM, Abella BS, Khan M, et al. Cardiac catheterization is underutilized after in-hospital cardiac arrest. Resuscitation. 2008;79:398–403. doi: 10.1016/j.resuscitation.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–71. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 50.Schneider AG, Eastwood GM, Bellomo R, et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation. 2013;84:927–34. doi: 10.1016/j.resuscitation.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Rittenberger JC, Popescu A, Brenner RP, et al. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16:114–22. doi: 10.1007/s12028-011-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 53.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37:2895–903. doi: 10.1097/ccm.0b013e3181b01d8c. quiz 904. [DOI] [PubMed] [Google Scholar]
- 54.Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–10. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 55.Morrison LJ, Deakin CD, Morley PT, et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S345–421. doi: 10.1161/CIRCULATIONAHA.110.971051. [DOI] [PubMed] [Google Scholar]
- 56.Blondin NA, Greer DM. Neurologic prognosis in cardiac arrest patients treated with therapeutic hypothermia. Neurologist. 2011;17:241–8. doi: 10.1097/NRL.0b013e318224ee0e. [DOI] [PubMed] [Google Scholar]
- 57.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–11. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 58.Chan PS, Spertus JA, Krumholz HM, et al. A validated prediction tool for initial survivors of in-hospital cardiac arrest. Arch Intern Med. 2012;172:947–53. doi: 10.1001/archinternmed.2012.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herlitz J, Engdahl J, Svensson L, et al. Major differences in 1-month survival between hospitals in Sweden among initial survivors of out-of-hospital cardiac arrest. Resuscitation. 2006;70:404–9. doi: 10.1016/j.resuscitation.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Merchant RM, Berg RA, Yang L, et al. Hospital variation in survival after in-hospital cardiac arrest. J Am Heart Assoc. 2014;3:e000400. doi: 10.1161/JAHA.113.000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 2010;121:709–29. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 62.Donnino MW, Rittenberger JC, Gaieski D, et al. The development and implementation of cardiac arrest centers. Resuscitation. 2011;82:974–8. doi: 10.1016/j.resuscitation.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Stub D, Bernard S, Duffy SJ, et al. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–35. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 64.Spaite DW, Bobrow BJ, Stolz U, et al. Statewide regionalization of postarrest care for out-of-hospital cardiac arrest: association with survival and neurologic outcome. Ann Emerg Med. 2014;64:496–506.e1. doi: 10.1016/j.annemergmed.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 65.Bunch TJ, White RD, Gersh BJ, et al. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N Engl J Med. 2003;348:2626–33. doi: 10.1056/NEJMoa023053. [DOI] [PubMed] [Google Scholar]
- 66.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 67.Chan PS, Krumholz HM, Spertus JA, et al. Effectiveness of implantable cardioverter-defibrillators in survivors of inhospital cardiac arrest. Am Heart J. 2015;169:870–8.e1. doi: 10.1016/j.ahj.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]