Abstract
This study evaluates contemporary trends in the use and outcomes of adult patients undergoing extracorporeal membrane oxygenation (ECMO) in U.S. hospitals. All adult discharges in the Nationwide Inpatient Sample database during the years 2002-2012 that included ECMO were used to estimate the total number of U.S. ECMO hospitalizations (n = 12,407). Diagnostic codes were used to group patients by indication for ECMO use into postcardiotomy, heart transplant, lung transplant, cardiogenic shock, respiratory failure, and cardiopulmonary failure. A Mann-Kendall test was used to examine trends over time using standard statistical techniques for survey data. We found that ECMO use increased significantly from 2002-2012 (P = 0.003), whereas in-hospital mortality rate fluctuated without a significant difference in trend over time. No significant trend was observed in overall ECMO use from 2002-2007, but the use did demonstrate a statistically significant increase from 2007-2012 (P = 0.0028). The highest in-hospital mortality rates were found in the postcardiotomy (57.2%) and respiratory failure (59.2%) groups. Lung and heart transplant groups had the lowest in-hospital mortality rates (44.10% and 45.31%, respectively). The proportion of ECMO use for postcardiotomy decreased from 56.9% in 2002 to 37.9% in 2012 (P = 0.026) and increased for cardiopulmonary failure from 3.9% to 11.1% (P = 0.026). We concluded that ECMO use in the United States increased between 2002 and 2012, driven primarily by increase in national ECMO use beginning in 2007. Mortality rates remained high but stable during this time period. Though there were shifts in relative ECMO use among patient groups, absolute ECMO use increased for all indications over the study period.
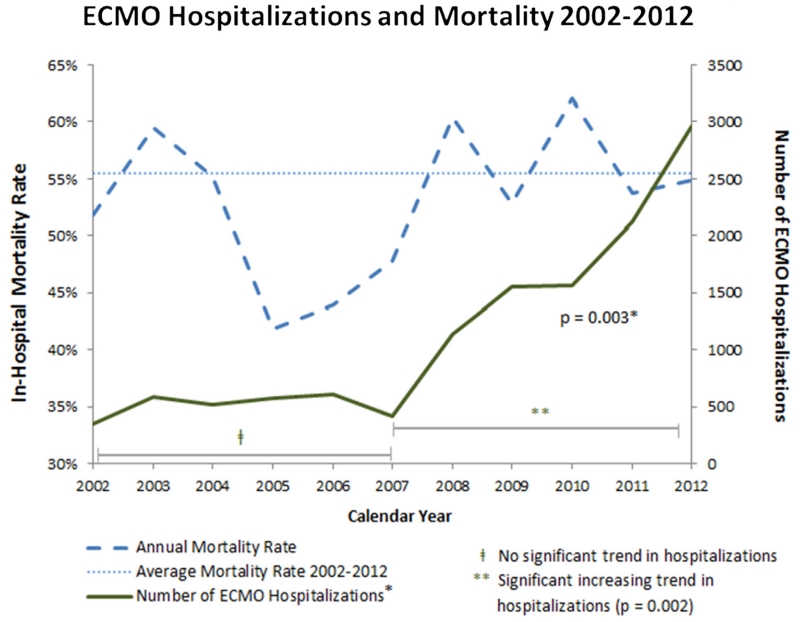
ECMO Use and Mortality for 2002-2012.
INTRODUCTION
The first successful use of extracorporeal membrane oxygenation (ECMO) support in an adult was reported by Hill and colleagues in 1972 in a patient with acute respiratory distress syndrome.1 The first multicenter National Institutes of Health (NIH)-sponsored ECMO clinical trial occurred in 1975 and randomized adults to 1 week of venoarterial ECMO or mechanical ventilator support. Equally high mortality rates were reported in both arms, but ECMO had greater than 90% mortality and no clinical benefit despite higher cost and greater resource use.2 Though early results in adults were discouraging, ECMO in neonatal and pediatric populations had better results and was more readily adopted.3 Single-institution retrospective studies and multi-institutional registries have reported survival rates ranging from 40%-80% in neonatal (0-30 days) and pediatric populations with respiratory indications generally having better outcomes.4,5
ECMO use in adults as a rescue therapy in the setting of cardiac, pulmonary, or cardiopulmonary failure in adults experienced a resurgence following the relative success of ECMO in the pediatric population.4 More recently, a multicenter randomized trial of venovenous ECMO support for acute respiratory distress syndrome published in 2009,6 the use of ECMO in the H1N1 global flu pandemics,7 and improvement in ECMO equipment and patient management have affected ECMO use and potential outcomes. Among current ECMO providers and experts there exists the perception that ECMO use in adults has increased significantly in recent years. Studies of ECMO use have demonstrated increased resource use and costs associated with ECMO in the United States but not increased use.8 Whether ECMO use has increased across the country instead of just in ECMO referral centers in recent years remains relatively unknown as also the clinical indications potentially driving any changes in ECMO use.
MATERIALS AND METHODS
The study was approved by the Intuitional Review Board at the University of Pennsylvania. A total of 87 million records from the National Inpatient Sample (NIS) for 2002-2012 were obtained from the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. The NIS is the largest publicly available all-payer database and includes patients from Medicare, Medicaid, private insurance, and the uninsured. Records are collected in a rigorous fashion to reflect a 20% sample of all U.S. hospital admissions with the exception of admissions to rehabilitation hospitals and federal hospitals such as Veterans Affairs hospitals. The most recently available sample NIS from 2012 comprised data from more than 4500 hospitals in 46 states. Each year of NIS data includes approximately 7-8 million records, each with a corresponding discharge weight. Representative sampling and tabulation of appropriate discharge weights are achieved by characterizing participating institutions across 5 strata: ownership or control, bed size, teaching status, urban or rural location, and US region. Weighted, each year of NIS data estimates approximately 36 million hospital stays.9
The International Classification of Diseases, 9th Revision, was used to identify discharge records with the ECMO procedural codes of 39.65 (ECMO) and 39.66 (percutaneous ECMO). Other inclusion criteria included age ≥ 18 years. ECMO patients were also classified by clinical indication using a modified version of a previously standardized method.8 In all, 8 mutually exclusive clinical indications were used: (1) postcardiotomy, (2) cardiogenic shock, (3) respiratory failure and severe lung disease, (4) cardiopulmonary failure, (5) pre– and post–heart transplantation, (6) pre– and post–lung transplantation, (7) trauma or drowning, and (8) miscellaneous. Owing to limited numbers and lack of more specific clinical information, the trauma or drowning and miscellaneous patients were excluded from the study. For the specific codes and methods of these mutually exclusive clinical indications, see Appendix 1.
The primary outcome of the study was discharge survival with ECMO patients stratified by year and clinical indication. Secondary outcomes included length of stay and time from ECMO placement until hospital discharge. SAS statistical software (SAS Institute Inc, Cary, NC) and survey techniques were used to estimate means, frequencies, and variance. Mean, standard errors, and 95% CI are presented for continuous variables. ANOVA was used for multigroup comparisons along with post hoc pairwise comparisons. Categorical variables were analyzed using Pearson’s chi-squared test, and Mann-Kendall tests were used to examine trends over time. P-values less than 0.05 were considered statistically significant. All analysis was performed on SAS version 9.4 or Microsoft Excel.
RESULTS
Baseline patient characteristics are listed in Table 1 for the entire study period (n = 12,157) as well as comparing ECMO patients in 2002-2006 (n = 2639) and 2007-2012 (n = 9519). Overall, the average age was 51.9 years (95% CI: 51.0-52.8). Patients in 2007-2012 were more likely to be younger (53.5 vs 51.4, P = 0.05). ECMO discharges in the 2007-2012 era were also less likely to be initially classified as elective (24% vs 40%, P < 0.01), more likely to be from urban, teaching hospitals (91% vs 85%), and showed longer average lengths of stay (21.5 days vs 17.5 days, P = 0.04).
Table 1.
Baseline Patientand Institutional Characteristics
| Characteristics | All (n = 12,157) |
2002-2006 (n = 2639) |
2007-2012 (n = 9519) |
P |
|---|---|---|---|---|
| Male (%) | 62 | 57 | 62 | 0.03 |
| White (%) | 68 | 76 | 66 | 0.01 |
| Age (mean, 95% CI) | 51.9 (51.0-52.8) | 53.5 (51.3-55.6) | 51.4 (50.5-52.4) | 0.05 |
| Elective admission (%) | 28 | 40 | 24 | <0.01 |
| Hospital type(%) | ||||
| Rural | 1 | 2 | 1 | 0.14 |
| Urban, nonteaching | 9 | 13 | 8 | 0.07 |
| Urban, teaching | 90 | 85 | 91 | 0.03 |
| Died in hospital | 56 | 52 | 58 | 0.12 |
| Length of stay (days) | 20.6 (18.8-22.4) | 17.5 (14.6-20.4) | 21.5 (14.3-18.2) | 0.04 |
| ECMO insertion to discharge (days) | 15.7 (13.9-17.4) | 13.4 (10.9-15.8) | 16.2 (14.3-18.2) | 0.17 |
ECMO use increased significantly over the entire study period, 2002-2012 (Mann-Kendall P = 0.003). The number of unweighted ECMO discharges captured in the NIS database increased from 352 in 2002 to 2715 in 2012. Figure 1 shows the trend in annual ECMO hospitalizations and mortality. There is an inflection point in the graph of ECMO use starting in 2007, and the trend toward increased ECMO use was driven primarily by significantly increased use across the 2007-2012 period (P = 0.002). ECMO use from 2002-2006 remained relatively constant without a significant trend in use.
Figure 1.
ECMO Use and Mortality for 2002-2012. (Color version of figure is available online at http://www.semthorcardiovascsurg.com.)
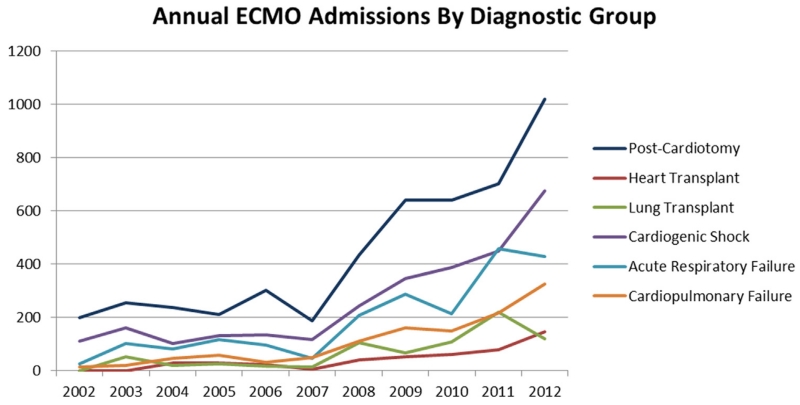
The proportion of total ECMO use by clinical indication and the change in overall use by clinical indication over time from 2002-2012 are shown in Figure 2. Use for all indications increased significantly over the entire study period. There were also significant changes in the relative proportion of certain clinical indications: postcardiotomy-associated ECMO decreased from 56.9% of all ECMO admissions in 2002 to 37.9% of ECMO admissions in 2012 (P = 0.03), whereas cardiopulmonary failure associated ECMO increased from 3.9% in 2002 to 11.1% in 2012 (P = 0.03).
Figure 2.
ECMO Clinical Indications for 2002-2012. (Color version of figure is available online at http://www.semthorcardiovascsurg.com.)
Overall mortality for the entire study period was 56% (95% CI: 52%-60%) and ranged from 43% in 2006 to 63% in 2010 (Fig. 1). There was no significant trend over time or difference when stratified by era: 2002-2006 (52%, 95% CI: 44%-58%) vs 2007-2012 (58%, 95% CI: 53%-61%, P = 0.12). From 2002-2012, postcardiotomy ECMO demonstrated a significantly decreasing trend in mortality (P = 0.02) and cardiopulmonary failure showed significantly increasing mortality (P = 0.02). There were no significant mortality trends within the other clinical indications. Evaluating the mortality in the later period of ECMO use (2007-2012), there was a significant difference in the mortality between clinical indications (Table 2). Lung and heart transplantations had the lowest mortalities at 44% and 45%, respectively, and cardiogenic shock and postcardiotomy had the highest mortalities at 63% and 60%, respectively.
Table 2.
Mortality by Clinical Indication for 2007-2012
| Indication | 2007-2012 |
Pairwise Comparisons |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mortality (95% CI) |
Cardiogenic Shock |
Postcardiotomy | Cardiopulmonary Failure |
Acute Respiratory Failure |
Heart Transplant |
Lung Transplant |
|
| Cardiogenic Shock |
458 | 0.63 (0.57-0.69) |
0.49 | 0.22 | 0.03 | <0.01 | 0.01 | |
| Postcardiotomy | 751 | 0.60 (0.55-0.65) |
0.47 | 0.09 | 0.0114 | 0.03 | ||
| Cardiopulmonary failure |
210 | 0.56 (0.48-0.65) |
0.62 | 0.1 | 0.15 | |||
| Acute respiratory failure |
341 | 0.54 (0.48-0.60) |
0.18 | 0.22 | ||||
| Heart transplant | 80 | 0.45 (0.34-0.55) |
0.97 | |||||
| Lung transplant | 133 | 0.44 (0.31-0.58) |
||||||
| P | P = 0.018 | – | – | – | – | – | – | |
DISCUSSION
There are 3 primary findings in this study. Most significantly, ECMO use across the United States increased from 2002-2012, driven primarily by increased use starting in 2007. Secondly, ECMO mortality has remained relatively stable and did not demonstrate a significant trend over the entire study period nor in the more recent era from 2007-2012. Lastly, the use of ECMO increased for every indication across the study period, but this increase was accompanied by changes in the relative case mix of ECMO discharges and the emergence of trends in mortality over time within some, but not all, clinical indications.
The statistically significant trend in increasing use of ECMO in adults across the United States is a novel finding. Maxwell et al8 evaluating cost and use of ECMO between 1998 and 2009 found a significant increasing trend in costs and length of stay but did not find a significant increase in use over the study period. Paden et al5 published an update of the Extracorporeal Life Support Organization Registry with complete reporting through 2011, which showed progressive increases in all adult ECMO use starting around 2005 and in adult respiratory ECMO use starting in 2009; however, this database is international and the report did not include statistics to facilitate interpretation of trends. The consistent finding across all studies, however, is that each subsequent year shows increasing ECMO use with the greatest increases occurring most recently.
Despite a significant increase in the use of ECMO nationwide, the primary outcome of discharge mortality in this study did not show a significant trend. The finding of preserved clinical outcomes in the setting of expanded cardiopulmonary and resuscitation use and technology diffusion is consistent with expanded use in some cardiovascular and resuscitative technologies10,11 but differs from others.12-14 Whether the preservation of outcomes in the setting of increased use is due to previous centers expanding their annual ECMO volume, new centers achieving comparable outcomes, improved ECMO technology, or some combination of these and other factors is not directly captured in NIS data. Given the clinical severity of the ECMO patient population as well as the unique complications of ECMO compared with other standard cardiopulmonary resuscitation techniques, these questions are probably best answered in large, multicenter studies or clinical trials of adult ECMO in the United States, which are yet to be undertaken or published.15
The high and varying mortality rates exhibited in the current study fall within the range of mortality rates reported by other large, multicenter ECMO studies.5,8,16 Other single-center studies of ECMO use in adults and in pediatric patients have reported lower mortality rates but the limited ability for risk adjustment of patients from the NIS or account for the selection bias of single-center studies prevents a direct comparison of mortality rates.3,17 The results of this study demonstrate that there still remains the clinical challenge of both an appropriate expansion of ECMO use as well as decreasing its associated morbidity and mortality.
For specific clinical indications, there were differences both in terms of proportional increases and associated mortality. Postcardiotomy was the predominant indication for ECMO use in every year of this study, and it was the only clinical indication that demonstrated decreasing mortality over the study period. Although the absolute number of postcardiotomy cases increased every year, postcardiotomy was also the only clinical indication that demonstrated a significant decrease in its proportion of the annual overall ECMO use from 2002 until 2012. ECMO use for cardiopulmonary failure was the only clinical indication that demonstrated a significantly increased proportion of ECMO use from 2002-2012, but heart and lung transplantation–associated ECMO, which had no discharges in 2002, grew to represent 4% and 6%, respectively, of the overall ECMO use by 2012. The proportion of ECMO use associated with respiratory failure doubled from 8% in 2002 to 16% in 2012, possibly because of positive findings from trials conducted during the study period and ECMO use to treat patients in respiratory failure during the global influenza pandemics.6,7,18
The trends of ECMO use, clinical indication, and mortality are independently evaluated in this study, but they are also related in ways that help describe overall changes in ECMO use in the United States. Although the most significant finding is increased use starting after 2007, ECMO use in this later era was also associated with changing patient demographics, particularly with more younger patients; decreased proportion of elective admission; increased use in urban teaching centers; and longer lengths of hospital stays. Along with the trends of proportionally decreased postcardiotomy use and increased cardiopulmonary failure and previous studies showing increased associated ECMO costs,8 these finding suggest that an important area of likely increased ECMO use is in salvage situations and tertiary referral centers. The lack of a trend or significant improvement in mortality may also be related to the steady use of ECMO use for cardiogenic shock, which has the highest mortality in this study, and increased use for cardiopulmonary failure. High mortality rates in these patients populations most likely reflect the extremely high-risk nature of this patient population and may still represent a number of clinical successes in what otherwise may be a clinically unsalvageable situation.
STUDY LIMITATIONS
There are significant limitations to this study starting with the use of administrative NIS data. The sampling frame for the NIS changed annually between 2002 and 2011, so the same hospitals are not consistently represented in the database and thus the evaluation of longitudinal outcomes and center-clustered analysis are not possible. The sampling methodology for the NIS also changed in 2012 in an effort to improve the accuracy with which a representative clinical sample is collected. New, back-dated discharge weights were distributed to permit consistent analysis over time before 2012, but the change underscores the variability of weights in making national estimates. The NIS database also lacks relevant, detailed clinical information including whether patients were placed on venoarterial or venoveno ECMO.
Central Message
ECMO use has increased significantly, starting primarily in 2007, with changing clinical indications but stable mortality rates.
Perspective
This study demonstrates that recent ECMO use has increased significantly and with increasingly varied clinical indications. Mortality has remained stable throughout the period of increased ECMO use. These results support further ECMO technology diffusion across the United States and mounting clinical interest to expand ECMO as a salvage platform in increasingly heterogeneous clinical environments.
APPENDIX 1. ICD-9 CODES FOR ECMO CLINICAL INDICATIONS
Diagnosis and Procedure Code-Based Criteria to Define Extracorporeal Membrane Oxygenation Groups
| Group | Code Type | Code | Description | Related Criteria |
|---|---|---|---|---|
| Postcardiotomy | ICD-9-CM Vol 3 (Procedure) | 35.× | Operations on valves and septa of heart |
Absence of heart or lung transplant procedure codes |
| ICD-9-CM Vol 3 (Procedure) | 36.× | Operations on vessels of heart |
||
| ICD-9-CM Vol 3 (Procedure) | 37.1 | Cardiotomy and pericardiotomy |
||
| ICD-9-CM Vol 3 (Procedure) | 37.3× | Pericardiectomy and excision of lesion of heart |
||
| ICD-9-CM Vol 3 (Procedure) | 441.× | Major aortic dissection or aneurysm repair |
||
| Heart transplant | ICD-9-CM Vol 3 (Procedure) | 37.51 | Heart transplantation | Absence of lung transplant procedure codes |
| Lung transplant | ICD-9-CM Vol 3 (Procedure) | 33.5 | Lung transplantation | Absence of heart transplant procedure code |
| ICD-9-CM Vol 3 (Procedure) | 33.6 | Heart-lung transplantation |
||
| Cardiogenic shock | CCS diagnoses | 107 | Cardiac arrest and ventricular fibrillation |
Absence of cardiac surgical, heart, or lung transplant procedure codes |
| CCS diagnoses | 108 | Congestive heart failure, nonhypertensive |
and | |
| CCS diagnoses | 100 | Acute myocardial infarction |
Absence of respiratory failure CCS diagnosis codes |
|
| CCS diagnoses | 97 | Pericarditis, endocarditis, and myocarditis; cardiomyopathy |
or | |
| CCS diagnoses | 106 | Cardiac dysrhythmias | Presence of respiratory failure CCS diagnosis code(s) but primary CCS diagnosis code for hospitalization to qualify for cardiogenic shock group |
|
| CCS diagnoses | 101 | Coronary atherosclerosis and other heart disease |
||
| CCS diagnoses | 103 | Pulmonary heart disease | ||
| Respiratory failure | CCS diagnoses | 131 | Respiratory failure, insufficiency, or arrest (adult) |
Absence of cardiac surgical, heart, or lung transplant procedure codes |
| CCS diagnoses | 122 | Pneumonia | and | |
| CCS diagnoses | 123 | Influenza | Absence of cardiogenic shock CCS diagnosis codes |
|
| CCS diagnoses | 130 | Pneumothorax or pulmonary collapse |
or | |
| CCS diagnoses | 132 | Lung disease due to external agents |
Presence of cardiogenic shock CCS diagnosis code(s) but primary CCS diagnosis code for hospitalization to qualify for respiratory failure group |
|
| CCS diagnoses | 126 | Other upper respiratory tract infections |
||
| CCS diagnoses | 3 | Bacterial infection | ||
| Cardiopulmonary failure |
CCS diagnoses | (see aforementioned cardiogenic shock and respiratory failure codes) |
Absence of cardiac surgical, heart, or lung transplant procedure codes |
|
| At least 1 CCS diagnosis code from cardiogenic shock and at least 1 CCS diagnosis code from respiratory failure |
||||
| Primary CCS diagnosis code for hospitalization is not among cardiogenic shock or respiratory failure group codes |
||||
| Trauma/drowning | CCS diagnoses | 2601- 2615 |
ICD-9-CM E-codes for trauma and drowning |
Absence of cardiac surgical, heart, or lung transplant procedure codes |
| Absence of diagnosis codes for respiratory failure or cardiogenic shock |
||||
| Miscellaneous | Absence of codes to qualify for any of the 6 aforementioned groups |
|||
CCS, clinical classifications software; ICD-9-CM, International Classification of Diseases, 9th Revision-clinical modification.
Footnotes
Presented at the 2015 AATS, Seattle, WA, April 29, 2015.
References
- 1.Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care. 2000;4:156–168. doi: 10.1186/cc689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zapol WM, Snider MT, Hill J, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: A randomized prospective study. J Am Med Assoc. 1979;242(20):2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 3.Karamlou T, Vafaeezadeh M, Parrish AM, et al. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. 2013;145(2):470–475. doi: 10.1016/j.jtcvs.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Shanley CJ, Hirschl RB, Schumacher RE, et al. Extracorporeal life support for neonatal respiratory failure. A 20-year experience. Ann Surg. 1994;220:269–282. doi: 10.1097/00000658-199409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paden ML, Rycus PT, Thiagarajan RR, et al. Update and outcomes in extracorporeal life support. Semin Perinatol. 2014;38(2):65–70. doi: 10.1053/j.semperi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 7.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, et al. Extracorporeal membrane oxygenation for 2009 influenza a(h1n1) acute respiratory distress syndrome. J Am Med Assoc. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell BG, Powers AJ, Sheikh AY, et al. Resource use trends in extracorporeal membrane oxygenation in adults: An analysis of the nationwide inpatient sample 1998-2009. J Thorac Cardiovasc Surg. 2014;148(2):416–421. e1. doi: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 9.HCUP Nationwide Inpatient Sample (NIS): Healthcare Cost and Utilization Project (HCUP). 2002-20012. Agency for Healthcare Research and Quality; Rockville, MD: 2012. [Google Scholar]
- 10.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the united states. J Am Med Assoc. 2013;310(19):2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 11.Arrich J, Holzer M, Herkner H, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;4:CD004128. doi: 10.1002/14651858.CD004128.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Malas M, Arhuidese I, Qazi U, et al. Perioperative mortality following repair of abdominal aortic aneurysms: Application of a randomized clinical trial to real-world practice using a validated nationwide data set. JAMA Surg. 2014;149:1260–1265. doi: 10.1001/jamasurg.2014.275. [DOI] [PubMed] [Google Scholar]
- 13.Fischer M, Fischer NJ, Schüttler J. One-year survival after out-of-hospital cardiac arrest in Bonn city: Outcome report according to the ‘Utstein style’. Resuscitation. 1997;33(3):233–243. doi: 10.1016/s0300-9572(96)01022-2. [DOI] [PubMed] [Google Scholar]
- 14.Absalom AR, Bradley P, Soar J. Out-of-hospital cardiac arrests in an urban/rural area during 1991 and 1996: Have emergency medical service changes improved outcome? Resuscitation. 1999;40(1):3–9. doi: 10.1016/s0300-9572(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 15.Crow S, Fischer AC, Schears RM. Extracorporeal life support: Utilization, cost, controversy, and ethics of trying to save lives. Semin Cardiothorac Vasc Anesth. 2009;13(3):183–191. doi: 10.1177/1089253209347385. [DOI] [PubMed] [Google Scholar]
- 16.Diddle JW, Almodovar MC, Rajagopal SK, et al. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med. 2015;43(5):1016–1025. doi: 10.1097/CCM.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 17.Chrysostomou C, Morell VO, Kuch BA, et al. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg. 2013;146(2):317–325. doi: 10.1016/j.jtcvs.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240(4):595–607. doi: 10.1097/01.sla.0000141159.90676.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]