Abstract
Objective
To compare time to pregnancy and live birth among couples with varying intervals of pregnancy loss date to subsequent trying to conceive date.
Methods
In this secondary analysis of the Effects of Aspirin in Gestation and Reproduction trial, 1,083 women, aged 18–40 years with 1–2 prior early losses and whose last pregnancy outcome was a non-ectopic or non-molar loss, were included. Participants were actively followed for up to six menstrual cycles, and for women achieving pregnancy, until pregnancy outcome. We calculated intervals as start of trying to conceive date minus pregnancy loss date. Time to pregnancy was defined as start of trying to conceive until subsequent conception. Discrete Cox models, accounting for left truncation and right censoring, estimated fecundability odds ratios (OR) adjusting for age, race, BMI, education, and subfertility. While intervals were assessed prior to randomization and thus reasoned to have no relation with treatment assignment, additional adjustment for treatment was evaluated given that low-dose aspirin was previously shown to be predictive of time to pregnancy.
Results
Couples with a 0–3 month (n=765 [76.7%]) versus >3 month (n=233 [23.4%]) interval were more likely to achieve a live birth (53.2% versus 36.1%) with a significantly shorter time to pregnancy leading to live birth (median (IQR) 5 cycles (3, 8), adjusted fecundability OR: 1.71 [95% CI: 1.30, 2.25]). Additionally adjusting for low-dose aspirin treatment did not appreciably alter estimates.
Conclusion
Our study supports the hypothesis that there is no physiological evidence for delaying pregnancy attempt after an early loss.
INTRODUCTION
After an early pregnancy loss (1, 2) couples often seek counseling on how long to wait before attempting conception again. Many clinicians recommend waiting at least 3 months (3, 4) with the World Health Organization recommending a minimum of 6 months (5, 6). However, there are no data to support these recommendations, and previous studies have shown that the uterus may be more receptive to a pregnancy directly following an early loss (7).
Most studies addressing pregnancy spacing concentrate on the interval between live births and subsequent pregnancies (interpregnancy interval [IPI]), with the majority of findings indicating that an IPI of less than 18 months is associated with increased risk for poor maternal and perinatal outcomes (7-10). What has not been well studied is the optimal timing following a non-ectopic, non-molar, <20-week gestational age pregnancy loss. Studies to date have been limited in enrolling already pregnant women and then determining how their IPI affects pregnancy outcomes (6, 11-14). While these studies answer the question of “When should couples achieve a pregnancy after a loss?” the more relevant public health question is “When should couples start trying to achieve pregnancy after a loss?” We set out to assess the relationship between the related but distinct construct of intertrying interval, time from last pregnancy loss to conception attempt, and fecundability. Our a priori hypothesis is that there would be no difference in reproductive success among women who started trying to conceive within versus greater than 3 months of their pregnancy loss.
MATERIALS AND METHODS
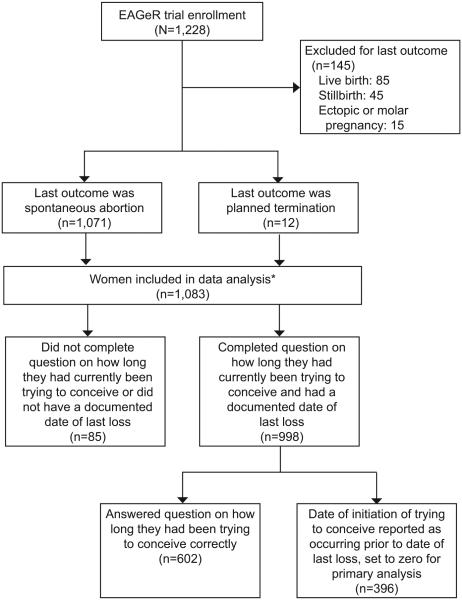
The Effects of Aspirin in Gestation and Reproduction (EAGeR) trial (2007–2011), a multicenter, block-randomized, double-blinded, placebo-controlled trial to evaluate the effect of preconception-initiated daily low dose aspirin on reproductive outcomes in women with a history of pregnancy loss, enrolled 1228 women, aged 18–40, with one to two prior losses. Trial results of primary outcomes indicate that preconception low-dose aspirin treatment increases the probability of becoming pregnant, but does not prevent pregnancy loss, among women with one pregnancy loss in the previous year (15). Details of the study design and protocol have been published previously (16). Briefly, women were included if they had regular menstrual cycles of 21–42 days in length, no known history of infertility, and were trying or stated intention to start trying to conceive. Women whose last outcome was either a spontaneous abortion (n=1071, 98.9%) or a planned termination (n=12, 1.1%) were included in this analysis while women whose last outcome was a live birth (n=85; 7.0%), stillbirth (n=45, 3.7%), or ectopic or molar pregnancy (known to require longer follow-up care) (n=15, 1.2%) were excluded resulting in a study sample of 1,083 women for this analysis (99.8% of whom had a last loss of ≤19 weeks gestation, with 54.1% having had a last loss of ≤8 weeks).
Women were followed for up to six menstrual cycles while trying to conceive and through delivery if they became pregnant. The study was approved by the Institutional Review Board (IRB) at each site, with each site serving as the IRB designated by the National Institutes of Health under a reliance agreement. All participants gave written informed consent prior to randomization. A Data Coordinating Center was responsible for developing a computerized remote data capture system, training study site personnel in data entry, and data management throughout the trial (16).
Inter-trying interval, defined as time from last pregnancy loss to time attempting a subsequent conception (Figure 1), was our primary exposure. Date of loss and gestational age of last loss were obtained from the participant’s previous physician who provided details regarding the prior loss via a standardized form. Additionally, each participant completed an extensive health and reproductive history questionnaire at baseline. The majority of women (n=1041, 96.1%) had a medically documented date of last loss. For women without a medically documented date of last loss, we relied on their self-report, resulting in 1074 (99.2%) women having a date of last loss. Date of starting to try to conceive was obtained from the baseline health and reproductive history questionnaire. Specifically, each couple was asked the question “How long have you currently been trying to become pregnant?” with answers completed in number of months (1006 [92.9%] completed the question). When the reported date of initiation of trying to conceive was reported as occurring prior to the date of last loss, the intertrying interval was defined as zero months, i,e, assuming no interruption in attempting conception. From the 1074 women with a documented loss date and the 1006 women who responded to the specific intertrying interval question, we were able to successfully calculate the intertrying interval for 998 women (92.2%). As outlined below, multiple imputation was used to impute intertrying intervals for the remaining 85 women (17) (Figure 2).
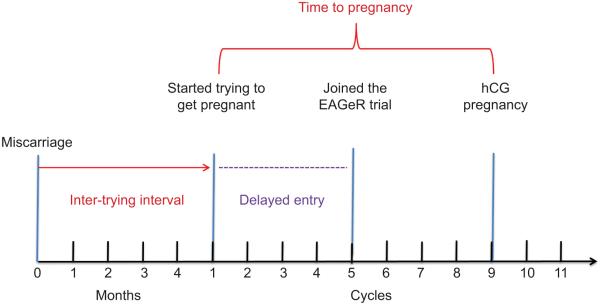
Figure 1.
An illustration of the relationship between the variables included in the survival model, where inter-trying interval is the exposure of interest, time to pregnancy is the outcome of interest, and dotted line represents the delayed entry time. EAGeR, Effects of Aspirin in Gestation and Reproduction; hCG, human chorionic gonadotropin.
Figure 2.
Flow diagram outlining participants included and excluded in this analysis from the original Effects of Aspirin in Gestation and Reproduction (EAGeR) trial study population. *Multiple imputation used for 85 women to correct for bias due to missing information.
Primary outcomes of this study were hCG-detected pregnancy and live birth. Pregnancy during the trial was ascertained by a urine pregnancy test (clinic and or home with the majority [89%] having both) and confirmed by a 6–7 week ultrasound. Live birth was defined as live delivered infant as indicated from medical records. Secondary outcomes included pregnancy loss, types of pregnancy loss, and obstetric complications (preeclampsia, gestational diabetes, and preterm birth < 37 weeks) as previously described (18) (19).
For the primary statistical analyses, the intertrying interval was categorized dichotomously (0 to 3 months, > 3 months), based on prior recommendations on intertrying interval and pregnancy loss (3, 12). We additionally assessed intertrying interval based on 3-month intervals (0–3, >3–6, >6–9, >9–12, and >12 months). Participant demographic, lifestyle, and reproductive history characteristics between intertrying intervals (0 to 3 months, > 3 months) were compared using chi-squared or where appropriate Fisher’s exact test for categorical variables, and Student’s t test for continuous variables.
Among women who achieved pregnancy, time to pregnancy was defined as conception cycle (via positive pregnancy test) minus number of menstrual cycles reported for trying to become pregnant. Given that time to pregnancy is inherently discrete (20), we used cycles as our unit of time for assessing time to pregnancy but kept our exposure in months since this is the unit used for relevant recommendations (5). Women who did not achieve pregnancy were censored at end of follow-up or withdrawal date. Discrete Cox proportional hazards regression models were used to estimate the fecundability odds ratio (FOR) and 95% confidence intervals (CI) corresponding to the cycle-specific probability of conception. In order to account for left truncation (21), time trying to achieve pregnancy as indicated by number of menstrual cycles prior to enrollment was incorporated into the model as the delayed entry time. For time to pregnancy leading to a live birth, a competing risks approach was applied to estimate cause-specific fecundability odds ratios, where women achieving pregnancy that ended in a loss were censored at the time of positive pregnancy test (22).
Based on a review of the prior literature, we considered the potential confounders of age (continuous), partner’s age (continuous), BMI (continuous), race (white vs. non-white), education (> versus ≤ high school), income (≤$19K, $20–39K, $40–74K, $75–99K, $≥100K), smoking (never, sometimes, daily), alcohol (never, sometimes, daily), physical activity (low, moderate, high), marital status (married vs. other), subfertility (yes vs. no with yes being a report of ever trying for more than 12 months to achieve a pregnancy), parity (0, 1, ≥2), prior number of losses (1 or 2), gestational age of prior loss (continuous), and whether a dilation and curettage (D & C) was performed for last loss (yes vs. no). While we did not consider treatment as a confounder given that our exposure (intertrying interval) was assessed prior to randomization and thus was reasoned to have no relation with treatment assignment, we did evaluate whether additionally adjusting for treatment appreciably altered estimates given that low-dose aspirin was previously shown to be predictive of time to pregnancy (23, 24). The choice of covariates to include in fully adjusted models was determined by directed acyclic graphs and statistical testing for confounding identification. Final models adjusted for age, race, BMI, education, and subfertility. Multiple imputation was performed to impute missing exposure and covariate data (17), thus all 1083 women were included in all analyses performed. Analyses were conducted using SAS software (version 9.4; SAS Institute, Inc) and R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Several sensitivity analyses were conducted to assess the robustness of our results. In our primary analyses, we corrected time at risk for those couples who had included time prior to their loss when reporting how long they had been trying to conceive by calculating the minimum number of months among the reported time trying and the number of months since the most recent loss. While this is an improvement compared to dropping these women from the analyses altogether, this strategy may still result in misclassification of intertrying intervals, given our assumption that all couples reporting an implausible value started trying to conceive immediately after their loss. To determine the robustness of the FOR estimates to this assumption, we performed two types of sensitivity analyses, based on multiple imputation and Monte Carlo simulations. Specifically, standard multiple imputation techniques to impute plausible values for delayed entry times were applied based on potential predictors of this value. Additionally, as an alternate strategy, we applied Monte Carlo sampling techniques to randomly assign a feasible time at risk for those couples reporting implausible intertrying interval values. This procedure was performed 500 times, and average FORs and 95% CIs were calculated using Rubin’s combining rules (25).
RESULTS
The majority of women (76.6%) had an intertrying interval of 0–3 months while 23.4% had an intertrying interval of >3 months (9.0% >3–6 months, 2.3% >6–9 months, 1.7% >9–12 months, and 10.3% >12 months). Women with a 0–3 month versus >3 month intertrying interval were slightly younger (mean 28.6 years versus 29.4 years), had a partner slightly younger (mean 29.8 years versus 31.0 years), lower BMI (mean 26.0 kg/m2 versus 27.2 kg/m2), more likely to be white (96.9% versus 91.9%), have above a high school education (89.4% versus 80.7%), never smokers (96.5% versus 91.9%), and more likely to be married (93.1% versus 87.9%) (Table 1). In terms of reproductive history, women with a 0–3 month versus >3 month intertrying interval had less frequently reported subfertility (6.6% versus 10.3%), a slightly younger age of menarche (12.5 years versus 12.8 years), younger gestational age of last loss, and an older age of first intercourse (mean age 19.8 years versus 18.6 years).
Table 1.
Demographic, lifestyle, and reproductive history of Effects of Aspirin in Gestation and Reproduction study population by inter-trying interval (ITI)*
| Inter-trying interval | ||||
|---|---|---|---|---|
| Characteristics | Total n=998 |
0–3mo n=765 (76.7) |
>3mo n=233 (23.4) |
P-value1 |
| Age, years (mean ± SD) | 28.8 ± 4.8 | 28.6 ± 4.8 | 29.4 ± 4.8 | 0.02 |
| Partner age, years (mean ± SD) | 30.1 ± 5.4 | 29.8 ± 5.3 | 31.0 ± 5.7 | 0.01 |
| BMI, kg/m2 (mean ± SD) | 26.2 ± 6.5 | 26.0 ± 6.4 | 27.2 ± 7.0 | 0.01 |
| Race | 0.001 | |||
| White | 955 (95.7) | 741 (96.9) | 214 (91.9) | |
| Non-White | 43 (4.3) | 24 (3.1) | 19 (8.2) | |
| Education | <0.001 | |||
| > High School | 871 (87.2) | 683 (89.2) | 188 (80.7) | |
| ≤ High School | 126 (12.6) | 81 (10.6) | 45 (19.3) | |
| Low-dose aspirin Treatment | 499 (50.0) | 388 (50.7) | 111 (47.6) | 0.42 |
| Smoking in past year | 0.003 | |||
| No | 952 (95.4) | 738 (96.5) | 214 (91.9) | |
| Yes | 46 (4.6) | 27 (3.5) | 19 (8.2) | |
| Alcohol consumption in past year | 0.05 | |||
| Never | 656 (65.7) | 516 (67.4) | 140 (60.1) | |
| Sometimes | 313 (31.3) | 237 (31.0) | 86 (36.9) | |
| Often | 21 (2.1) | 14 (1.8) | 7 (3.0) | |
| Coffee consumer | 272 (27.3) | 201 (26.3) | 71 (30.5) | 0.20 |
| Physical Activity | 0.91 | |||
| Low | 251 (25.2) | 190 (24.8) | 61 (26.2) | |
| Moderate | 419 (42.0) | 322 (42.1) | 97 (41.6) | |
| High | 328 (32.9) | 253 (33.1) | 75 (32.2) | |
| Income | 0.77 | |||
| ≥$100 000 | 393 (39.4) | 293 (38.3) | 100 (42.9) | |
| $75 000-99 999 | 123 (12.3) | 96 (12.6) | 27 (11.6) | |
| $40 000-74 999 | 152 (15.2) | 117 (15.3) | 35 (15.0) | |
| $20 000-39 999 | 255 (25.6) | 211 (26.3) | 54 (23.2) | |
| ≤$19 999 | 75 (7.5) | 58 (7.6) | 17 (7.3) | |
| Marital Status | 0.03 | |||
| Married | 917 (91.9) | 712 (93.1) | 205 (87.9) | |
| Living with a partner | 57 (5.7) | 39 (5.1) | 18 (7.7) | |
| Other | 24 (2.4) | 14 (1.8) | 10 (4.3) | |
| Previous subfertility | 74 (7.4) | 50 (6.5) | 24 (10.3) | 0.05 |
| Age of Menarche (years) | 12.7 ± 1.5 | 12.5 ± 1.5 | 12.8 ± 1.5 | 0.01 |
| Ever Hormonal Prescriptions | 796 (79.8) | 602 (78.7) | 194 (83.4) | 0.12 |
| Previous Number of Live Births | 0.59 | |||
| 0 | 498 (49.9) | 376 (49.2) | 122 (52.4) | |
| 1 | 345 (34.6) | 266 (34.8) | 79 (33.9) | |
| 2 | 155 (15.5) | 123 (16.1) | 32 (13.7) | |
| Previous Number of Losses | 0.10 | |||
| 1 | 669 (67.0) | 523 (68.4) | 146 (62.7) | |
| 2 | 329 (33.0) | 242 (31.6) | 87 (37.3) | |
| D & C performed on prior loss | 324 (32.5) | 250 (32.7) | 74 (31.8) | 0.79 |
| Gestational age of prior loss (weeks) | 0.01 | |||
| ≤7.99 | 439 (44.0) | 336 (43.9) | 101 (43.3) | |
| 8-13.99 | 503 (50.4) | 397 (51.9) | 106 (45.5) | |
| 14-19.99 | 52 (5.2) | 30 (3.9) | 22 (9.4) | |
| 20-31.99 | 3 (0.003) | 2 (0.3) | 1 (0.004) | |
| Age of first intercourse (years) | 19.5 ± 4.2 | 19.8 ± 4.3 | 18.6 ± 3.8 | <0.001 |
| Past month’s intercourse frequency | 0.88 | |||
| ≥3-6 per week | 315 (31.5) | 242 (31.6) | 72 (30.9) | |
| 1-2 per week to 2-3 per month | 579 (58.0) | 439 (57.4) | 140 (60.1) | |
| <1 per month | 54 (5.4) | 42 (5.5) | 12 (5.2) | |
Analyses performed via chi-square or Fisher’s exact test as appropriate for categorical variables and Student’s t test for continuous variables. Values reported are n (%) unless otherwise noted. All variables are complete except for missing n=85 for ITI (i.e., did not complete question on how long they had currently been trying to conceive or did not have a documented date of last loss), n=1 for partner age, n=1 for education, n=8 for past year’s alcohol consumption, n=4 for previous subfertility report, n=11 for age of menarche, n=1 for gestational age of prior loss, n=2 for age of first intercourse, and
Women with a 0–3 month versus >3 month intertrying interval were more likely to achieve a pregnancy (68.6% versus 51.1%) and achieve a pregnancy leading to a live birth (53.2% versus 36.1%) (Table 2). Median (IQR) for time to pregnancy among women with 0–3 month versus >3 month was 5 cycles (3, 8) versus 6 cycles (3, 9) and time to pregnancy leading to live birth, 5 cycles (3, 8) versus 6 cycles (4, 9). After adjusting for age, race, BMI, education, and subfertility, women with a 0–3 month versus >3 month intertrying interval had a shorter time to pregnancy (FOR: 1.58 [95% CI: 1.25, 2.00]) and shorter time to pregnancy leading to a live birth (FOR: 1.71 [95% CI: 1.30, 2.25]) (Table 3). There was no significant increased risk for any pregnancy complication (including pregnancy loss, preterm birth, preeclampsia, and gestational diabetes) among women with an intertrying interval 0–3 months versus >3 months. Additional adjustment for other demographic and reproductive history potential confounders including partner’s age, smoking, alcohol intake, parity, previous number of losses, recency of loss, gestational age of last loss, age of first intercourse, age of menarche, and D & C performed for last loss did not alter FOR (1.52 [95% CI: 1.20, 1.92]) or FOR leading to a live birth (1.65 [95% CI: 1.26, 2.16]), nor did further adjustment for low-dose aspirin (Table 4).
Table 2.
Pregnancy outcome of Effects of Aspirin in Gestation and Reproduction study population by intertrying interval
| Intertrying Interval | ||||
|---|---|---|---|---|
|
| ||||
| Characteristics | Total n=998 |
0-3mo n=765 (76.7%) |
>3mo n=233 (23.4%) |
P- value* |
| Pregnancy (n [%]) | 644 (64.5) | 525 (68.9) | 119 (51.1) | <0.001 |
| Live birth (n [%]) | 491 (49.2) | 407 (53.2) | 84 (36.1) | <0.001 |
| Preterm birth† | 22 (8.8) | 19 (9.2) | 3 (6.8) | 0.62 |
| Peri-implantation Loss (n [%]) | 49 (4.9) | 38 (5.0) | 11 (4.7) | 0.88 |
| Clinical Loss (n [%]) | 113 (11.2) | 88 (11.5) | 25 (10.7) | 0.74 |
| Gestational Age of Loss‡ | 9.6 ± 5.2 | 9.7 ± 4.3 | 9.9 ± 3.5 | 0.77 |
| Pre-eclampsia§ | 52 (8.2) | 42 (8.5) | 10 (7.2) | 0.63 |
| Gestational Diabetes§ | 20 (3.3) | 19 (3.6) | 1 (0.9) | 0.11 |
Analyses performed via chi-square or Fisher’s exact test as appropriate for categorical variables and Student’s t test for continuous variables.
Among the live births (n=491)
Among those with a clinical loss (n=113)
Among women achieving pregnancy (n=644)
Table 3.
Fecundability odds ratio for pregnancy and pregnancy leading to a live birth (95% CI) by intertrying interval (0–3 months versus >
| Time to pregnancy | FOR (95% CI) | |||||
|
| ||||||
|
Intertrying
interval |
Pregnancy
n (%) |
Unadjusted |
Adjusted Model
1* |
Adjusted Model
2† |
Sensitivity
Analysis 1‡ |
Sensitivity
Analysis 2§ |
|
| ||||||
| 0–3 months | 525 (68.9) | 1.69 (1.35, 2.13) | 1.58 (1.25, 2.00) | 1.52 (1.20, 1.92) | 1.31 (1.03, 1.67) | 1.35 (1.07, 1.73) |
| >3 months | 119 (51.1) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
|
| ||||||
| Time to pregnancy leading to live birth | FOR (95% CI) | |||||
|
| ||||||
| 0-3 months | 407 (53.2) | 1.85 (1.42, 2.41) | 1.71 (1.30, 2.25) | 1.65 (1.26, 2.16) | 1.49 (1.13, 1.99) | 1.56 (1.18, 2.06) |
| >3 months | 84 (36.1) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
|
| ||||||
| 3 months) | ||||||
Adjusted for age, race, BMI, education, and subfertility
Adjusted for Model 1 covariates plus partner’s age, smoking, alcohol intake, parity, previous number of losses, recency of loss, gestational age of last loss, age of first intercourse, age of menarche, and D & C performed for last loss
Sensitivity analysis using multiple imputation to impute the plausible values for delayed entry times. Model 1 adjustments.
Sensitivity analysis using Monte Carlo simulations to randomly assign time at risk for those couples with implausible values for time trying to conceive. Average FOR [95% CI] reported for 500 simulations. Model 1 adjustment.
Table 4.
Fecundability odds ratio (FOR) for pregnancy and pregnancy leading to a live birth (95% CI) by inter-trying interval (0–3 months versus > 3 months) stratified by LDA treatment
| LDA Treatment | ||||
|
| ||||
| Time to Pregnancy | FOR (95% CI) | |||
|
| ||||
| Inter-trying interval |
Pregnancy
n (%) |
Unadjusted |
Adjusted
Model 1* |
Adjusted
Model 2† |
|
| ||||
| 0–3 months | 278 (71.7) | 1.65 (1.21, 2.26) | 1.50 (1.09, 2.06) | 1.46 (1.06, 2.02) |
| >3 months | 61 (55.0) | 1.0 | 1.0 | 1.0 |
|
| ||||
| Time to Pregnancy Leading to a Live Birth | FOR (95% CI) | |||
|
| ||||
| Inter-trying interval |
Pregnancy
n (%) |
Unadjusted |
Adjusted
Model 1* |
Adjusted
Model 2† |
|
| ||||
| 0–3 months | 216 (55.7) | 1.69 (1.18, 2.41) | 1.52 (1.06, 2.18) | 1.50 (1.04, 2.16) |
| >3 months | 46 (41.4) | 1.0 | 1.0 | 1.0 |
|
| ||||
| Placebo | ||||
|
| ||||
| Time to Pregnancy | FOR (95% CI) | |||
|
| ||||
| Inter-trying interval | Pregnancy n (%) | Unadjusted |
Adjusted
Model 1* |
Adjusted
Model 2† |
|
| ||||
| 0–3 months | 247 (65.5) | 1.70 (1.22, 2.35) | 1.60 (1.14, 2.24) | 1.54 (1.10, 2.16) |
| >3 months | 58 (47.5) | 1.0 | 1.0 | 1.0 |
|
| ||||
| Time to Pregnancy Leading to a Live Birth | FOR (95% CI) | |||
|
| ||||
| Inter-trying interval |
Pregnancy
n (%) |
Unadjusted |
Adjusted
Model 1* |
Adjusted
Model 2† |
|
| ||||
| 0–3 months | 191 (50.7) | 1.98 (1.34, 2.93) | 1.86 (1.24, 2.78) | 1.83 (1.21, 2.77) |
| >3 months | 38 (31.2) | 1.0 | 1.0 | 1.0 |
Adjused for age, race, BMI, education, and subfertility.
Adjusted for Model 1 covariates plus partner’s age, smoking, alcohol intake, parity, previous number of losses, recency of loss, gestational age of last loss, age of first intercourse, age of menarche, and D & C performed for last loss.
In regards to alternative cut points for intertrying intervals, compared to an intertrying interval of >3–6 months, women with an intertrying interval 0–3 months had shorter time to pregnancy with a FOR of 1.24 (0.90, 1.72), while women with longer intertrying intervals had longer time to pregnancies: intertrying interval >6–9 months (FOR: 0.90, 95% CI: 0.44, 1.83); intertrying interval >9–12 months (FOR: 0.83, 95% CI: 0.38, 1.81); >12 months (FOR 0.60, 95% CI: 0.38, 0.95) after adjusting for age, race, BMI, education, and subfertility. Similar decreased success in achieving pregnancy leading to live birth was seen with increasing intertrying intervals (data not shown).
In the sensitivity analysis using multiply imputed values for the misspecified intertrying intervals, women with a 0–3 month versus a >3 month intertrying interval had an attenuated but still significantly shorter time to pregnancy (FOR: 1.31 [95% CI: 1.03, 1.67]) and time to pregnancy leading to live birth (FOR: 1.49 [95% CI: 1.13, 1.99]). Similar shorter time to pregnancy was observed after applying Monte Carlo simulation techniques to randomly assign time at risk for those couples who had included time prior to their loss when reporting how long they had been trying to conceive, average FOR for pregnancy, 1.35 (95% CI: 1.07, 1.73) and pregnancy leading to a live birth, 1.56 (95% CI: 1.18, 2.06).
DISCUSSION
In a preconception cohort of women with a history of 1–2 spontaneous pregnancy losses, women who waited 3 months or less, versus longer, from their most recent pregnancy loss to start trying again had higher live birth rates. Notably, women with the longest intertrying interval of >12 months had reduced fecundability compared to women with an intertrying interval of 0–3 or >3–6 months. Our findings also demonstrated no increased risk for pregnancy complications, including peri-implantation losses, among women with a short interval. Our results indicate that there is no physiologic basis for delaying pregnancy attempt after a non-ectopic, non-molar, <20-week gestational age pregnancy loss. Recommendations to delay pregnancy attempts for at least 3–6 months among couples who are psychologically ready to begin trying (4, 26, 27) may be unwarranted and should be revisited.
While several professional women’s health organizations concur on the recommended interval of at least 24 months after a live birth before attempting another pregnancy (27), there are no consistent guidelines on how long a woman should wait after experiencing a pregnancy loss. The “depletion hypothesis” may partially explain potential detrimental effects for a short interval between a live birth, but not a pregnancy loss, and a subsequent pregnancy (11, 28). This hypothesis proposes that decreasing levels of folate in the mother from the fifth month of gestation, continuing into the postpartum period during breastfeeding, lead to poorer birth outcomes including neural tube defects, intrauterine growth restriction, and preterm birth among women with short inter-pregnancy intervals. As most pregnancy losses occur prior to 20 weeks of gestation, as in our study where >99% occurred prior to 20 weeks, women conceiving after an early pregnancy loss are not at risk for depletion of vital nutrients and consequently not likely at risk for adverse outcomes. Hypothesized advantages to attempting pregnancy immediately after a pregnancy loss include enhanced growth-supporting capacities and increased uterine blood volume and flow (7).
While our study supports the hypothesis that there is no physiological reason for delaying pregnancy attempt after a loss, whether a couple needs time to heal emotionally following a loss may be dependent on many factors. While emotional versus physical readiness may require individual couple assessment, previous research has found that a speedy new pregnancy and birth of a living child lessens grief among couples who are suffering from a pregnancy loss (29).
Our study has many strengths and is an improvement over previous studies given that we enrolled women pre-conceptionally, obtained detailed demographic, lifestyle, and reproductive history information prior to conception, and closely followed participants through delivery with details of pregnancy outcomes carefully and objectively determined. While these differences in demographic and reproductive history characteristics were statistically different, they are unlikely to be clinically meaningful. Nevertheless, our study is not without limitations. While information on prior loss was obtained via medical records, our assessment of starting to try to conceive after the last loss was obtained via self-report and thus subject to recall error. However, there is no other source of this data than self-report. Additionally, there may be differences between women with equivalent intertrying intervals in regards to time at risk of pregnancy due to such factors as fertility tracking or intercourse frequency. Future studies that enroll women pre-conceptionally immediately after a loss, and follow them prospectively through pregnancy outcome are needed to corroborate our findings. Finally, while low-dose aspirin was shown to neither confound nor modify the relationship between intertrying intervals and pregnancy outcomes, it is currently not part of routine care among women with an early pregnancy loss and thus additional studies are warranted to corroborate our findings.
In summary, we previously reported that women in the EAGeR trial who achieved pregnancy within 3 versus > 3 months of their last loss had no significant differences in live birth rates or adverse pregnancy outcomes (18). In the present study we demonstrate that women who begin trying to achieve pregnancy within 3 months have just as fast, if not faster, time to pregnancy leading to a live birth, with no risk of pregnancy complications, as women who wait until after 3 months to start trying. Additionally, we found that women with a long intertrying interval, >12 months versus 0–3 or >3–6 months, had significantly lower fecundability after taking into account many confounding factors including a history of subfertility. Taken together, our findings suggest that the traditional recommendation to wait at least 3 months after a pregnancy loss before attempting to conceive may be unwarranted.
Acknowledgments
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426).
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented in part at the annual meeting of the Society for Epidemiologic Research, Denver CO, June 17, 2015.
REFERENCES
- 1.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;12(368):601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;28(319):189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 3.Katz VL. Spontaneous and recurrent abortion: etiology, diagnosis, treatment. Comprehensive gynecology. In: Katz VL, Lentz GM, Lobo RA, Gershenson DM, editors. Comprehensive Gynecology. 5th Mosby Elsevier; Philadelphia (PA): 2007. p. 381. [Google Scholar]
- 4.After a Miscarriage: Getting Pregnant Again Pregnancy Loss. Available at: http://americanpregnancy.org/pregnancy-loss/after-miscarriage-getting-pregnant-again/. Retrieved June 13, 2015.
- 5.World Health Organization Report of a WHO technical consultation on birth spacing, Geneva Switzerland 13-15 June 2005. Available at: http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf. Accessed June 13, 2015.
- 6.Conde-Agudelo A, Belizan JM, Breman R, Brockman SC, Rosas-Bermudez A. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(Suppl 1):S34–40. doi: 10.1016/j.ijgo.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340:589–94. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]
- 8.Fuentes-Afflick E, Hessol NA. Interpregnancy interval and the risk of premature infants. Obstet Gynecol. 2000;95:383–90. doi: 10.1016/s0029-7844(99)00583-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhu BP, Haines KM, Le T, McGrath-Miller K, Boulton ML. Effect of the interval between pregnancies on perinatal outcomes among white and black women. Am J Obstet Gynecol. 2001;185:1403–10. doi: 10.1067/mob.2001.118307. [DOI] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–23. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- 11.Love ER, Bhattacharya S, Smith NC, Bhattacharya S. Effect of interpregnancy interval on outcomes of pregnancy after miscarriage: retrospective analysis of hospital episode statistics in Scotland. BMJ. 2010;341:c3967. doi: 10.1136/bmj.c3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentolila Y, Ratzon R, Shoham-Vardi I, Serjienko R, Mazor M, Bashiri A. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459–64. doi: 10.3109/14767058.2013.784264. [DOI] [PubMed] [Google Scholar]
- 13.Davanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591. doi: 10.1136/bmjopen-2012-001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Behery MM, Siam S, Seksaka MA, Ibrahim ZM. Reproductive performance in the next pregnancy for nulliparous women with history of first trimester spontaneous abortion. Arch Gynecol Obstet. 2013;288:939–44. doi: 10.1007/s00404-013-2809-9. [DOI] [PubMed] [Google Scholar]
- 15.Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM. Preconception lowdose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet. 2014;384:29–36. doi: 10.1016/S0140-6736(14)60157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A Randomised Trial to Evaluate the Effects of Low-dose Aspirin in Gestation and Reproduction: Design and Baseline Characteristics. Paediatr Perinat Epidemiol. 2013;27:598–609. doi: 10.1111/ppe.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat Med. 2010;29:2920–31. doi: 10.1002/sim.3944. [DOI] [PubMed] [Google Scholar]
- 18.Wong LF, Schliep KC, Silver RM, Mumford SL, Perkins NJ, Ye A, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1–11. doi: 10.1016/j.ajog.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA. Nomenclature for pregnancy outcomes: time for a change. Obstet Gynecol. 2011;118:1402–8. doi: 10.1097/AOG.0b013e3182392977. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg C, Wilcox A, Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd Lippencott Williams & Wilkins; Philadelphia (PA): 2008. Methodologic Issues in Reproductive Epidemiology; pp. 585–608. [Google Scholar]
- 21.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27:491–502. doi: 10.1111/ppe.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schisterman EF, Mumford SL, Schliep KC, Sjaarda LA, Stanford JB, Lesher LL, et al. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab. 2015;100:1785–91. doi: 10.1210/jc.2014-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauck WW, Anderson S, Marcus SM. Should we adjust for covariates in nonlinear regression analyses of randomized trials? Control Clin Trials. 1998;19:249–56. doi: 10.1016/s0197-2456(97)00147-5. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Nonresponse in Surveys. J. Wiley & Sons; New York: 1987. [Google Scholar]
- 26.Daugirdaite V, van den Akker O, Purewal S. Posttraumatic stress and posttraumatic stress disorder after termination of pregnancy and reproductive loss: a systematic review. J Pregnancy. 2015:646345. doi: 10.1155/2015/646345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein RR, Croughan MS, Robertson PA. Neonatal outcomes in immediate versus delayed conceptions after spontaneous abortion: a retrospective case series. Am J Obstet Gynecol. 2002;186:1230–34. doi: 10.1067/mob.2002.123741. [DOI] [PubMed] [Google Scholar]
- 28.Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet. 2001;358:2074–77. doi: 10.1016/S0140-6736(01)07105-7. [DOI] [PubMed] [Google Scholar]
- 29.Cuisinier M, Janssen H, de Graauw C, Bakker S, Hoogduin C. Pregnancy following miscarriage: course of grief and some determining factors. J Psychosom Obstet Gynaecol. 1996;17:168–74. doi: 10.3109/01674829609025678. [DOI] [PubMed] [Google Scholar]