Abstract
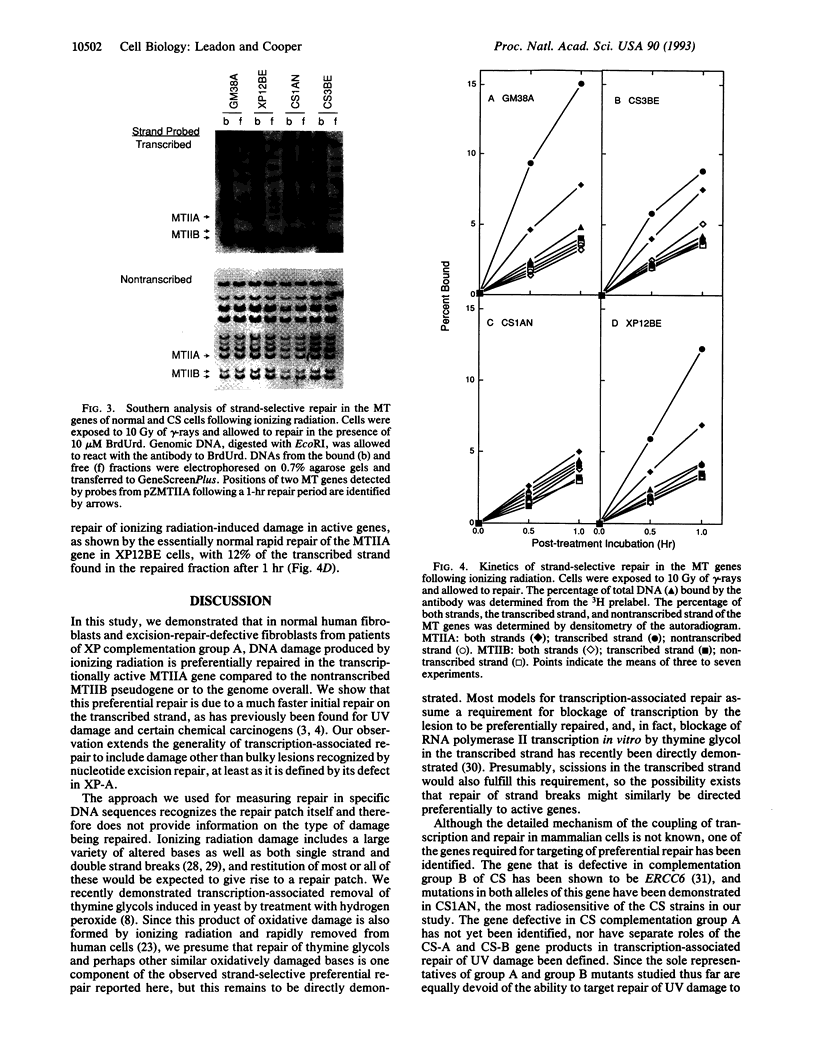
Cells from patients with Cockayne syndrome (CS), which are sensitive to killing by UV although overall damage removal appears normal, are specifically defective in repair of UV damage in actively transcribed genes. Because several CS strains display cross-sensitivity to killing by ionizing radiation, we examined whether ionizing radiation-induced damage in active genes is preferentially repaired by normal cells and whether the radiosensitivity of CS cells can be explained by a defect in this process. We found that ionizing radiation-induced damage was repaired more rapidly in the transcriptionally active metallothionein IIA (MTIIA) gene than in the inactive MTIIB gene or in the genome overall in normal cells as a result of faster repair on the transcribed strand of MTIIA. Cells of the radiosensitive CS strain CS1AN are completely defective in this strand-selective repair of ionizing radiation-induced damage, although their overall repair rate appears normal. CS3BE cells, which are intermediate in radiosensitivity, do exhibit more rapid repair of the transcribed strand but at a reduced rate compared to normal cells. Xeroderma pigmentosum complementation group A cells, which are hypersensitive to UV light because of a defect in the nucleotide excision repair pathway but do not show increased sensitivity to ionizing radiation, preferentially repair ionizing radiation-induced damage on the transcribed strand of MTIIA. Thus, the ability to rapidly repair ionizing radiation-induced damage in actively transcribing genes correlates with cell survival. Our results extend the generality of preferential repair in active genes to include damage other than bulky lesions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carothers A. M., Zhen W., Mucha J., Zhang Y. J., Santella R. M., Grunberger D., Bohr V. A. DNA strand-specific repair of (+-)-3 alpha,4 beta-dihydroxy-1 alpha,2 alpha-epoxy-1,2,3,4-tetrahydrobenzo[c]phenanthrene adducts in the hamster dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11925–11929. doi: 10.1073/pnas.89.24.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. L., Little J. B. Cross-sensitivity of certain xeroderma pigmentosum and Cockayne syndrome fibroblast strains to both ionizing radiation and ultraviolet light. Mol Gen Genet. 1981;181(4):562–563. doi: 10.1007/BF00428755. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., Brouwer J., van de Putte P., McCormick J. J. Preferential repair and strand-specific repair of benzo[a]pyrene diol epoxide adducts in the HPRT gene of diploid human fibroblasts. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5413–5417. doi: 10.1073/pnas.89.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschavanne P. J., Chavaudra N., Fertil B., Malaise E. P. Abnormal sensitivity of some Cockayne's syndrome cell strains to UV- and gamma-rays. Association with a reduced ability to repair potentially lethal damage. Mutat Res. 1984 Feb;131(2):61–70. doi: 10.1016/0167-8817(84)90012-9. [DOI] [PubMed] [Google Scholar]
- Deschavanne P. J., Diatloff-Zito C., Macieria-Coelho A., Malaise E. P. Unusual sensitivity of two cockayne's syndrome cell strains to both UV and gamma irradiation. Mutat Res. 1981 Jul-Sep;91(4-5):403–406. doi: 10.1016/0165-7992(81)90022-1. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Xeroderma pigmentosum, Cockayne's syndrome, helicases, and DNA repair: what's the relationship? Cell. 1992 Dec 11;71(6):887–889. doi: 10.1016/0092-8674(92)90384-o. [DOI] [PubMed] [Google Scholar]
- Htun H., Johnston B. H. Mapping adducts of DNA structural probes using transcription and primer extension approaches. Methods Enzymol. 1992;212:272–294. doi: 10.1016/0076-6879(92)12017-k. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Coon H. G., Petinga R. A., Barrett S. F., Rahe A. E., Robbins J. H. National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20014, USA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunala S., Brash D. E. Excision repair at individual bases of the Escherichia coli lacI gene: relation to mutation hot spots and transcription coupling activity. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11031–11035. doi: 10.1073/pnas.89.22.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Lawrence D. A. Preferential repair of DNA damage on the transcribed strand of the human metallothionein genes requires RNA polymerase II. Mutat Res. 1991 Jul;255(1):67–78. doi: 10.1016/0921-8777(91)90019-l. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Lawrence D. A. Strand-selective repair of DNA damage in the yeast GAL7 gene requires RNA polymerase II. J Biol Chem. 1992 Nov 15;267(32):23175–23182. [PubMed] [Google Scholar]
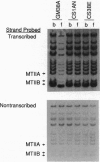
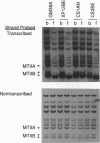
- Leadon S. A., Snowden M. M. Differential repair of DNA damage in the human metallothionein gene family. Mol Cell Biol. 1988 Dec;8(12):5331–5338. doi: 10.1128/mcb.8.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Three complementation groups in Cockayne syndrome. Mutat Res. 1982 Dec;106(2):347–356. doi: 10.1016/0027-5107(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Sancar A. A new mechanism for repairing oxidative damage to DNA: (A)BC excinuclease removes AP sites and thymine glycols from DNA. Biochemistry. 1989 Oct 3;28(20):7979–7984. doi: 10.1021/bi00446a002. [DOI] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H., McBride O. W. Chromosomal location of human metallothionein genes: implications for Menkes' disease. Science. 1984 Jun 8;224(4653):1104–1106. doi: 10.1126/science.6719135. [DOI] [PubMed] [Google Scholar]
- Scicchitano D. A., Hanawalt P. C. Repair of N-methylpurines in specific DNA sequences in Chinese hamster ovary cells: absence of strand specificity in the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1989 May;86(9):3050–3054. doi: 10.1073/pnas.86.9.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Witkin E. M., Sancar A. Escherichia coli mfd mutant deficient in "mutation frequency decline" lacks strand-specific repair: in vitro complementation with purified coupling factor. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11574–11578. doi: 10.1073/pnas.88.24.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Thoma F. Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell. 1990 May 18;61(4):675–684. doi: 10.1016/0092-8674(90)90479-x. [DOI] [PubMed] [Google Scholar]
- Sweder K. S., Hanawalt P. C. Preferential repair of cyclobutane pyrimidine dimers in the transcribed strand of a gene in yeast chromosomes and plasmids is dependent on transcription. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10696–10700. doi: 10.1073/pnas.89.22.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleth C., van de Putte P., Brouwer J. New insights in DNA repair: preferential repair of transcriptionally active DNA. Mutagenesis. 1991 Mar;6(2):103–111. doi: 10.1093/mutage/6.2.103. [DOI] [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]