Abstract
Objectives
In injury conditions, myofibroblasts are induced to lay down matrix proteins and support the repair process. In this study, we investigated the role of myofibroblasts, particularly stellate cells, in the growth and regeneration of pancreatic β-cells.
Methods
We used both in vitro and in vivo approaches to address whether stellate cells may promote the growth of β-cells.
Results
Our experiments demonstrated that activated stellate cells support the proliferation of β-cells in vitro. In vivo, mesenchymals surrounding the pancreatic islets are activated (induced to proliferate) in the islet regeneration model of Pten null mice. These mesenchymals display markers of pancreatic stellate cells such as desmin and to a lesser extent, smooth muscle actin α. We have shown previously that targeted β-cell deletion of Pten lead to a significant increase in total islet mass. This phenotype was accompanied by an increase in peri-islet mitotic activity, particularly in islets injured by streptozotocin, a β-cell specific toxin.
Conclusion
Together with the in vitro observations, our data, here, suggests that that these mesenchymal cells may support the regeneration of the islets. Identifying how the communication occurs may provide clinically relevant mechanism for inducing β-cell regeneration.
Keywords: Regenerating islet, PTEN, ECM, stellate cells
INTRODUCTION
Regeneration of injured tissues is a complicated process that involves participation from multiple cell types. In particular, the myofibroblasts that compose the extracellular matrix (ECM) produce critical factors needed for the replication and replenishment of lost cells [1]. In the pancreas and liver, injury to the parenchymal results in activation of myofibroblasts such as the stellate cells. These myofibroblasts secrete and deposit matrix proteins to build and restructure the ECMs. Similarly, chronic inflammatory disease such as pancreatitis are also accompanied by the activation of these myofibroblasts, particularly the resident pancreatic stellate cells (PSCs) [2]. While the mechanisms for the activation of the myofibroblasts are not clear, the activated PSCs are found to secrete and express extracellular matrix (ECM) proteins including smooth muscle actin α (SMAα) and collagens. The activated myofibroblasts also produce enzymes such as metalloproteases and their inhibitors, of which the functions are to construct the ECMs. In addition, PSCs produce inflammatory cytokines and growth factors that stimulate the proliferation of various cell types to repair the injured pancreas. In line with this observation, the growth and proliferation of tumor cells in pancreatic ductal adenocarcinoma appear to be promoted by the activated PSCs [3]. While the function of the myofibroblasts has not been specifically defined, the fact that they reconstitute ECM and restructure the tissue by producing growth factors suggests a role for these cells in supporting growth and proliferation of local parenchymals.
Pancreatic β-cells are the major endocrine cell types in the islets. Diminished production of insulin by β-cells (due to loss of mass or function) to meet the metabolic demand leads to diabetes mellitus, a growing epidemic worldwide. In recent years, genetic studies have demonstrated a role for mitogenic signaling pathways in β cell regeneration [4]. However, the source of these β-cell mitogens is unknown. Activated myofibroblasts such as PSCs, being the cells that produce mitogens that may promote tumor growth, represent a candidate source for these mitogens. In this study, we investigated whether PSCs are capable of promoting the growth of β-cells. Our data indicate that co-culturing β-cells with stellate cells promotes the growth of the β-cells. Using a genetically engineered β-cell regeneration model (PtenloxP/loxP; Rip-Cre+) [5], we found that the regeneration phenotype in this model correlates with the appearance of desmin positive myofibroblasts, possibly PSCs.
MATERIALS AND METHODS
Animals
Targeted deletion of Pten in β-cells was achieved by crossing PtenloxP/loxP mice with rat insulin promoter-Cre (RIP-Cre) mice [5]. F1 generation compound heterozygous animals were backcrossed with PtenloxP/loxP mice to produce F2 generation experimental animals. These mice were then crossed with RosalacZ reporter mice to generate PtenloxP/loxP; Rosa26lacZ; RIP-Cre mice. Of the offspring generated, we used male PtenloxP/loxP; Rosa26lacZ/lacZ; RIP-Cre+ mice as our mutants and male PtenloxP/loxP; Rosa26lacZ/lacZ; RIP-Cre- or Pten+/+; Rosa26lacZ/lacZ; RIP-Cre+ mice as our controls. Animals were genotyped from tail DNA using standard genomic PCR techniques. Three months old male mice were used for the experiments. Animals were housed in a temperature-, humidity-, light-controlled room (12-h light/dark cycle), allowing free access to food and water. All experiments were conducted according to the Institutional Animal Care and Use Committee of the University of Southern California research guidelines.
Cell Culture
β-TC-6 cells were obtained from ATCC. Cells were maintained in DMEM medium with 20% FBS, penicillin and streptomycin. Mouse pancreatic stellate cell (PSCs) was a generous gift from Dr. Anna L Means in University of Vanderbilt [6]. PSCs were maintained in RPMI1640 medium with 10% FBS, 1% interferon-α, penicillin and streptomycin at 33°C. When performing experiments, PSCs were cultured under correspondent experimental conditions at 37°C. Rat hepatic stellate cells (HSCs) were isolated from the liver tissues of 500g–550g Wistar rats (Charles River, Wilmington, MA) by the USC non parenchymal cell core facility under the approval of the Institutional Animal Care and Use Committee of the University of Southern California. Primary HSC cells were maintained in glucose free DMEM medium with 10% FBS, penicillin and streptomycin.
Culturing of β-TC-6 cells with Stellate cell Conditioned Medium
Conditioned medium from PSCs and primary rat HSCs were collected from 3 day cell cultures. β-TC-6 cells (3 × 105 cells/well) were seeded in 6-well culture plates (BD Bioscience) in DMEM medium supplemented with 20% FBS, penicillin and streptomycin. The following day, the β-TC-6 cell medium was removed and washed with PBS. Then, the conditioned medium, supplemented with glucose and FBS, was added to the β-TC-6 cells. β-TC-6 cells were typsinized and counted after 3–6 days.
Indirect Co-Culture of β-TC-6 cells and Stellate Cells
β-TC-6 cells (104 cells/well) were seeded in 24-well culture plates in DMEM medium supplemented with 20% FBS, penicillin and streptomycin. PSC cells (0.5×104 cells/well) were initially grown on the bottom of transwells in RMPI1640 supplemented with 10% FBS, penicillin and streptomycin. In the second day, transwells with PSCs were removed from culture well and placed in wells within β-TC-6 cells. Co-culture of PSCs and β-TC-6 cells were in DMEM supplemented with 20% FBS, penicillin and streptomycin at 37°C degree. In the control group, blank transwells were inserted. At different time points of culturing, β-TC-6 cells from experimental and control group were trypsinized and counted to record cell number. The ratio of cell number between β-TC-6 cells and PSCs were 16 :1 when these two cells were seeded. Similar protocols were used for co-culturing of β-TC-6 with primary rat HSCs. Briefly, β-TC-6 cells (3 × 105 cells/well) were seeded in 6-well culture plates in DMEM medium supplemented with 20% FBS, penicillin and streptomycin. Rat HSCs (2 × 105 cells/culture insert) were seeded into culture inserts of 1.0 µm pore size in DMEM medium with 10% FBS, penicillin and streptomycin. The following day, the culture inserts seeded with HSCs were placed into the 6-well plates containing the β-TC-6 cells, and incubation was continued up to 3 days. At the end of the incubation period, the cells were typsinized and counted.
Tritiated Thymidine Incorporation Assay
β-TC-6 cells (3 × 105 cells/well) were seeded in 6-well culture plates (BD Bioscience) in DMEM medium supplemented with 20% FBS, penicillin and streptomycin. The following day the β-TC-6 cell medium was removed, and the HSC conditioned medium, supplemented with glucose and FBS, was added to the β-TC-6 cells. Each well received 0.5 µCi of Thymidine [Methyl-3H] (Perkin Elmer, San Jose, CA) and was incubated for 8, 24 and 30 hours at 37°C. After incubation the medium was removed and cells were washed with phosphate-buffered saline, 0.5 mL of 10% trichloroacetic acid was then added to each well for 1 hour to precipitate the DNA. Unbound thymidine was removed by a gentle wash with 4°C phosphate-buffered saline. The DNA was then dissolved in 500 µL of 0.1% Triton X100 in 10% sodium hydroxide. The final lysates were transferred to a PET flexible microplate (Perkin Elmer, San Jose, CA) containing 600 µL of scintillation fluid. Measurements were obtained using the 1450 LSC Microbeta TriLux Counter from Perkin Elmer.
Immunohistochemistry
Pancreatic tissue was fixed overnight in Zn-Formalin solution and embedded in paraffin, sectioned in 4µm slices, and stained with H&E. Immunohistochemistry was performed using the following antibodies: anti-insulin (Zymed); anti-β-galactosidase (Abcam); anti-BrdU (BD Pharmingen); anti-Glucagon (Zymed); anti-Pancreatic Polypeptide (Zymed); anti-Somatostatin (Zymed); anti-Amylase (Zymed); anti-Pan-Cytokeratin (Dako); anti-Ngn3 (generous gift from Michael German); anti-Nestin (Chemicon); anti-Pdx1 (Abcam); anti-GFAP and anti-SMAα (Dako); anti-Desmin (Thermo Scientific); anti-CD45 (BD Pharminigen).
Quantification of Desmin Staining
The quantification of desmin staining was achieved by calculating percentage of desmin positive area over peri-islet area. After staining pancreatic sections with desmin and insulin, picture for each islet was taken. For each islet, the peri-islet was determined by drawing 50µm perimeter around islet which assures the peri-islet area includes islet itself and an area which is 50µm around the edge of islet. Using software ImageJ, the desmin positive area and total peri-islet area were calculated in pixel units. The ratio of desmin positive area over peri-islet is used to represent the density of desmin staining at the peri-islet area in control and Pten null mice. Three mice were analyzed for each group. For each mice, at least three sections (240µm apart), 30 islets per mouse were analyzed in this quantification process.
In Situ X-gal Staining
Fresh pancreatic tissue was rinsed with a mild detergent used to aid in permeability of tissue to fixative and stain. Tissue was then fixed with Zn-Formalin for one hour and stained with 1mg/ml X-gal (Sigma) for 24 hours at 30°C on rocker. The following day the tissue was rinsed with PBS + 3% DMSO and paraffin embedded for slide and counterstain preparation.
Determination of Cell Proliferation
Cell proliferation was evaluated on three-month-old male mice with bromodeoxyuridine (BrdU) pulse-labeling. BrdU (1mg/ml) was administered into the drinking water. Mice were allowed free access to BrdU containing water for five continuous days. On the sixth day, the mice were sacrificed and the pancreatic tissue was processed for immunohistochemical analysis.
STZ-Induced Diabetes
Three-month-old male mice were injected i.p. with subdiabetogenic doses (50mg/kg body weight) of streptozotocin (STZ) daily for five consecutive days to produce a β-cell injury model followed by BrdU labeling. On day 10, animals were sacrificed after BrdU labeling. Pancreatic tissue was removed and processed for immunohistochemical analysis.
Statistics
Student’s t tests were performed to compare the differences between control and mutant mice on quantified data. Data are presented as Mean+SEM.
RESULTS
Stellate Cells support the growth of β-cells
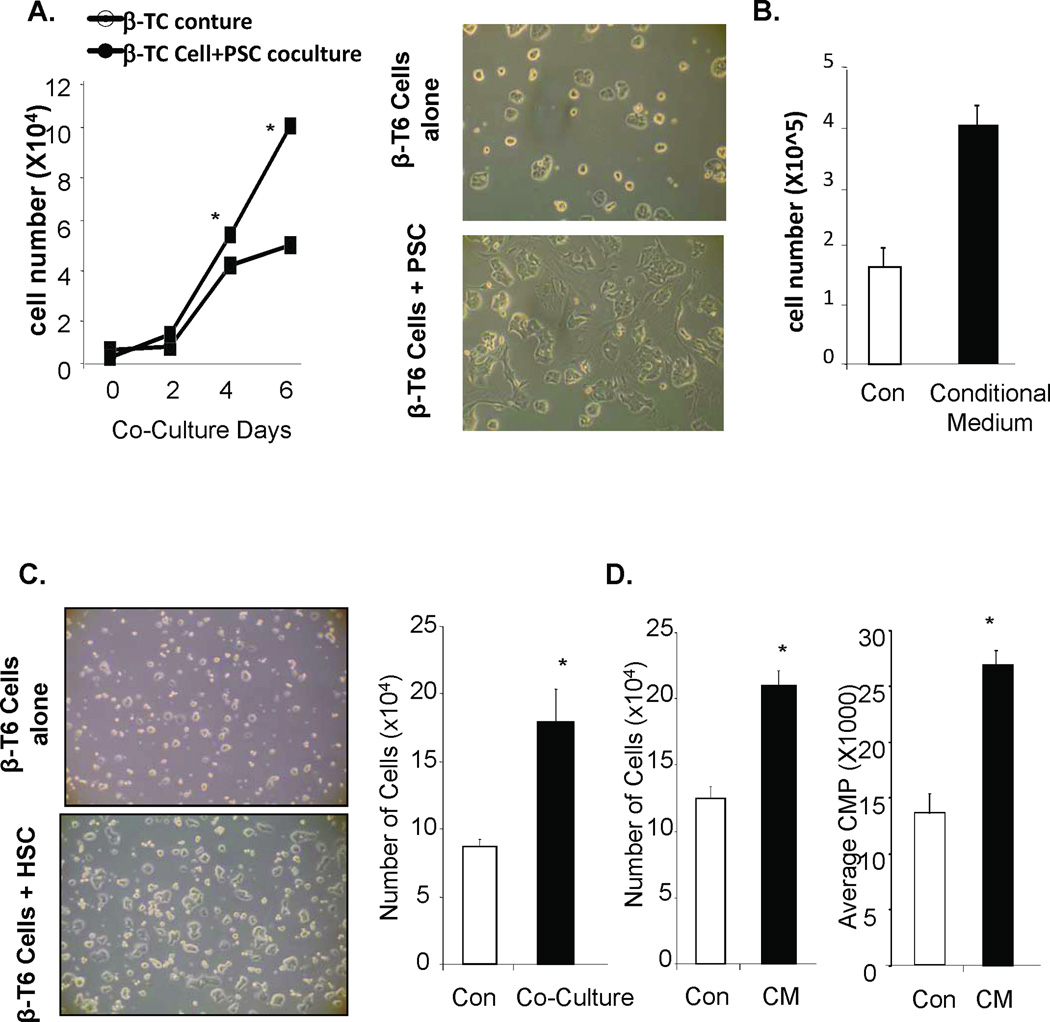
Stellate cells from ECM have been shown to support the growth of epithelial cells in malignant pancreas [3]. We investigated the hypothesis that activation of stellate cells may also support the growth of β-cells using a co-culture system of mouse pancreatic stellate cells (PSC) and β-cell (β-TC-6 cells) cell line. When co-cultured with PSC, the β-TC6 cells grow faster and display more an epithelial cell morphology by spreading out on the surface of the cell culture dish (Fig 1A). The β-TC-6 cells cultured with PSC start to grow significantly faster than those grown on mono layer single population cultures starting from day 4 of culturing (5.5×104 vs. 4.2×104) and the difference is much more dramatic at day 6 of culture (10×104 vs. 5.1×104). Similar increase of cell number (4.1×105 vs. 1.6×105) is observed when PSC conditioned medium were used to culture β-TC-6 cells (Fig 1B). Since stellate cells change their phenotypes as they are cultured in vitro, we confirmed this effect using primary hepatic stellate cells (HSCs) isolated from rat liver where stellate cells are more abundant. HSCs are 99.9% similar to PSCs [7]. We observed that β-TC-6 cells co-cultured with HSCs also grow much faster than those cultured on culture dishes. Cell count indicated a 2-fold increase (from 1.2×105 to 2.1×105) in cell number 3 days after initial co-culturing (Fig 1C, right panel). HSC conditioning medium similarly induced the proliferation of β-TC-6 cells (Fig 1D, left panel). To address whether this increase in cell number is due to increased cell proliferation, we performed 3H-thymidine incorporation study. When β-TC6 cells are cultured in conditioning medium collected from HSC culture, more than 2-fold 3H-thymidine was incorporated into the cells than when they were cultured with standard medium (Fig 1D, right panel), confirming the increased proliferation. This initial experiment, thus, suggest that the stellate cells are capable of secreting factors that signal the growth of pancreatic β-cells.
Figure 1. Stellate cells support the growth of β-cells in vitro.
(A) β-TC-6 cells cultured co-cultured with or without PSC cells using transwell. The data points represent the average number of cells (×104/mL) counted for the control and experimental cultures (n=3). The error bars represent the SEM for all samples analyzed for that group. *p≤0.05 is considered to be statistical significant. Images of β-TC-6 cells cultured with a control insert containing either no cells or PSCs and incubated for three days (right panels). (B) β-TC-6 cells cultured with a control or conditional medium collected from PSC cell line and incubated for six days. The data points represent the average number of cells (×104/mL) counted for the control and experimental cultures (n=3). The error bars represent the SEM for all samples analyzed for that group. *p≤0.05 is considered to be statistical significant. (C) Image of β-TC-6 cells cultured with a control insert containing either no cells or isolated primary HSCs and incubated for three days (left panels). Right panel, β-TC-6 cells co-cultured with HSCs and incubated for three days. The bars represent the average number of cells (×104/mL) counted for the controls and experimental conditions (n=3). The error bars represent the SEM for all samples analyzed for that group. *p≤0.05 is considered to be statistical significant. (D) Left panel, β-TC-6 cells incubated with HSC-conditioned medium. Right panel, graph is representative of 3H thymidine incorporation in proliferating β-TC-6 cells. 3H incorporation is performed at least three times with different incubation time. The bars represent the average counts per minute (CPM) for the controls and conditional medium cultures. The error bars represent the SEM for all animals analyzed for that group. n=3, *p≤0.05 is considered to be statistical significant.
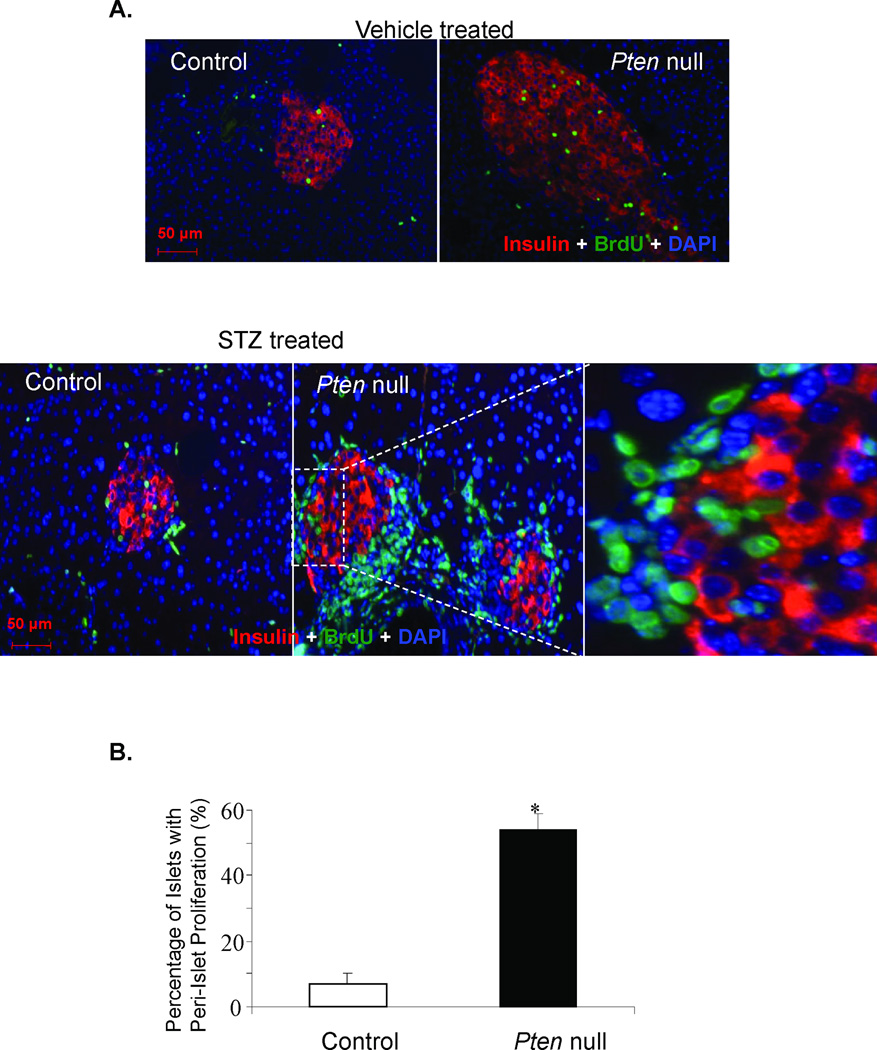
We sought to validate this in vitro observation in an in vivo setting. Like other mesenchymal cells, stellate cells can become active at site of injury. Streptozotocin, a β-cell toxin that induces injury to the pancreatic β-cells and slow recovery of β-cell mass presumably due to proliferation of the remaining β-cells [8]. A well-established effect of low-dose STZ treatment is the induction of inflammatory response and lymphocyte infiltration. We show here evidence of inflammatory cell infiltration in the STZ-treated mice (Supplemental Fig 1). CD45 positive immune cells are evident in some but not all islets. Infiltration of inflammatory cells suggests that the STZ treated pancreas sustained extensive injury and this injury may have stimulated the activation of myofibroblasts. In wild type mice, very slow turnover mitotic rate of β-cells is observed (Fig 2A) even when injury is inflicted with STZ [9]. To investigate the potential involvement of stellate cells in β-cell proliferation, we opted to a genetic model where β-cell growth is significantly augmented due to the loss of a lipid phosphatase (PTEN the phosphatase and tensin homologue deleted on chromosome 10) [5, 10–12]. PTEN is a negative regulator of the PI3K/AKT mitogenic signaling pathway and have been found to play major roles in cell proliferation. In mice lacking PTEN in β-cells (PtenloxP/loxP; Rip-Cre+, Pten null), β-cell proliferation and mass are found to be higher than that of the controls [5, 11, 12]. In these mice (Fig 2A), treatment with STZ leads to further cell proliferation and regeneration of the β-cells as the tissues attempt to repair itself [5]. In this STZ treated Pten null β-cell regeneration mouse model, we observed massive proliferation activity at the peri-islet areas (Fig 2A&B) in addition to the enhanced β-cell proliferation that was previously reported for these mice [5].
Figure 2. Peri-islet proliferating cells induced by STZ treatment do not express insulin.
(A) Control and Pten null mice were treated with either vehicle sodium citrate (top row) or STZ to induce β-cell injury (bottom row) and the pancreas was analyzed with the endocrine β-cell marker insulin (red), proliferating cell marker BrdU (green) and nuclear cell marker DAPI (blue). Top row: The left panel is the control pancreas. The right panel is the Pten null pancreas. Original magnification, 20×. Bottom row: The left panel is the control islet. The middle panel is the Pten null islets. Original magnification, 20×. The right panel is a higher magnification of the area within the dashed square. (B) Graph summarizing the ratio of the number of islets with increased per-islet proliferation compared to the number of islets with little to no peri-islet proliferation (n=4). The bars represent the average percentage of peri-islet proliferation for the controls and mutants. The error bars represent the SEM for all animals analyzed for that group. *p≤0.05 is considered to be statistical significant. Bar, 50µM.
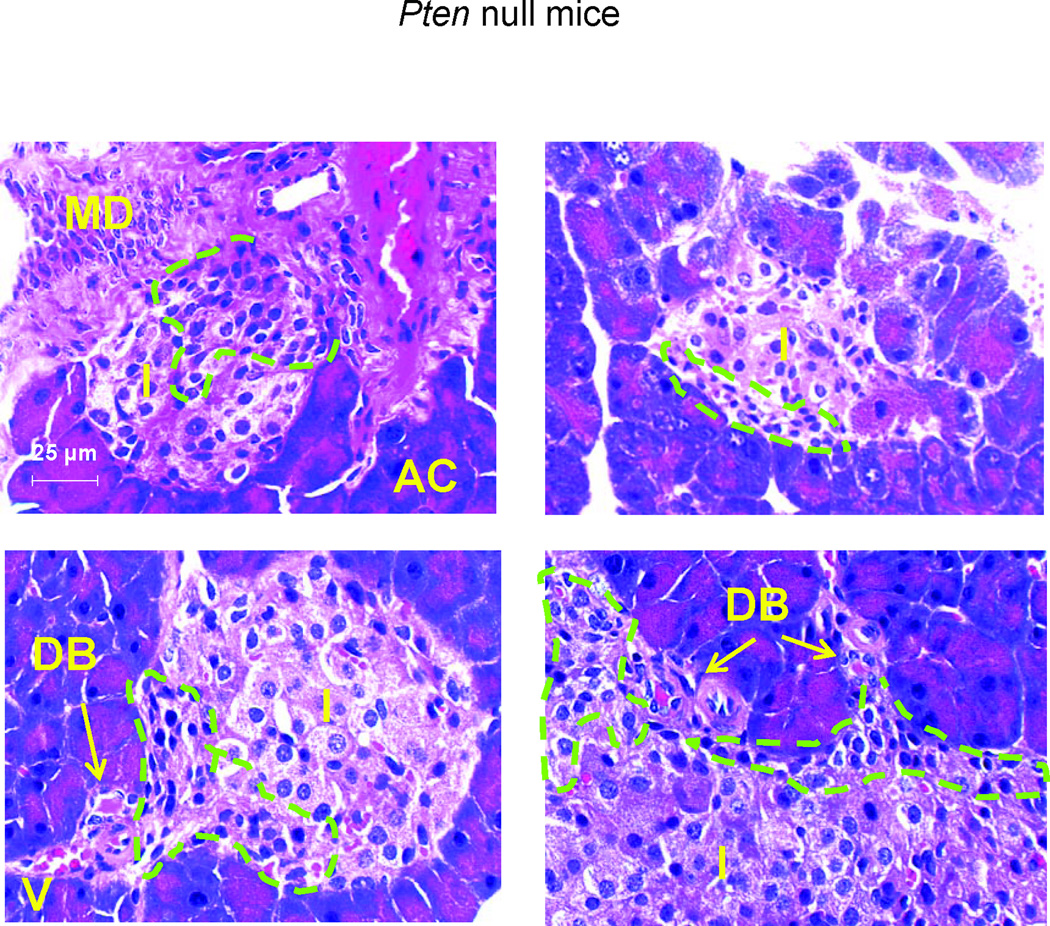
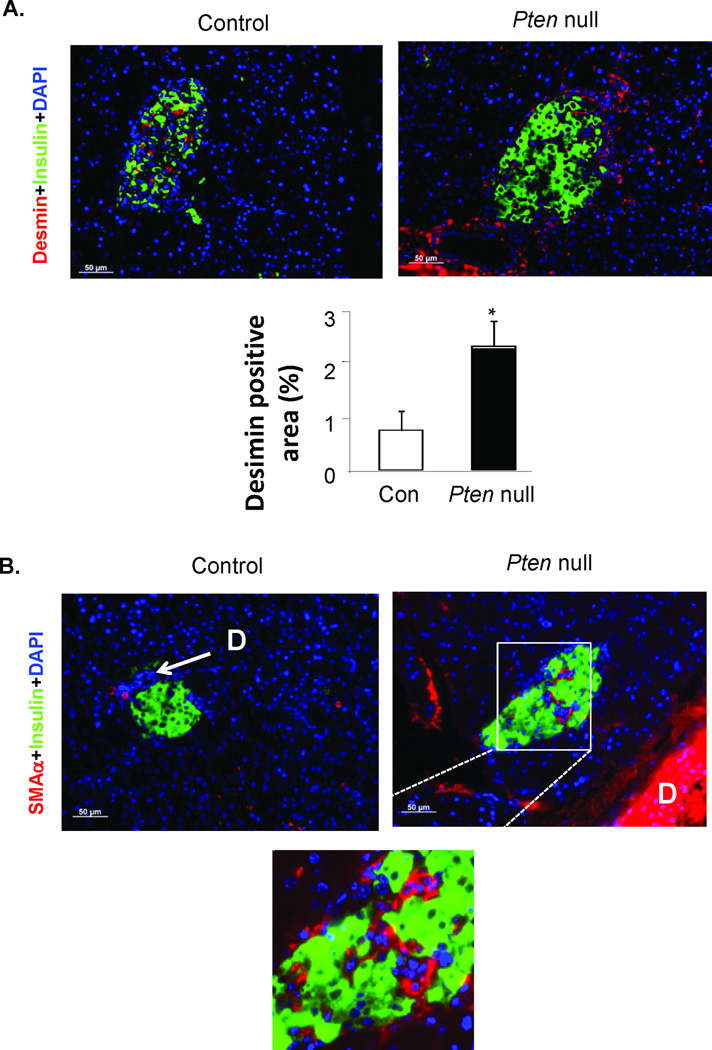
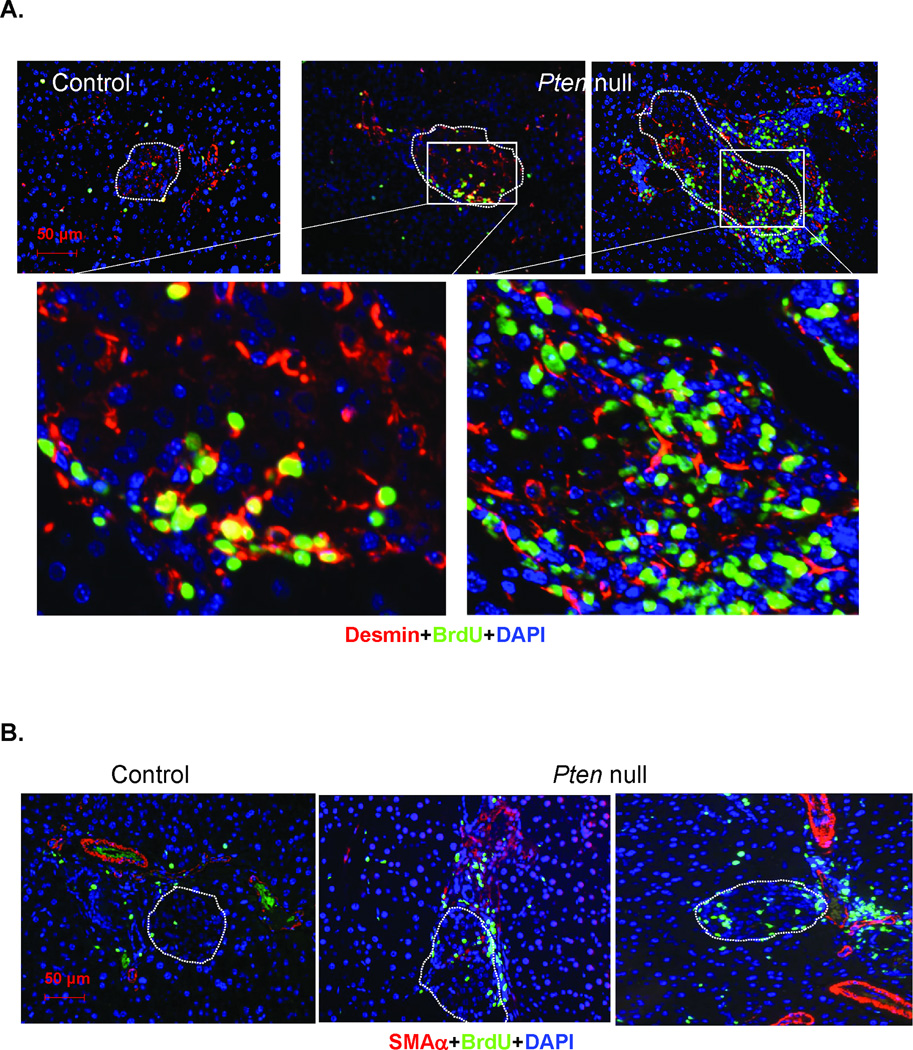
To identify these cells with high mitotic activity, we first evaluated their morphology. H&E stained sections of pancreas from the Pten null mouse model show that the “regenerating” islets are often associated with the major ductular structures or minor ductal branches/blood vessels (Fig 3). Elongated mesenchymal cells normally found near the ductular structures appear to surround or infiltrate the islets. We evaluated these pancreatic sections with immunostaining for two mesenchymal markers (red), desmin (SMAα) and smooth muscle actin α (Fig 4) together with insulin to mark the islets (green). Desmin staining is observed surrounding and throughout the islets (Fig 4A). More areas surrounding the islets were found to be desmin positive in the regenerating Pten null than the non-regenerating control model (Fig 4A, bottom panel). The major ducts in Pten null pancreas are strongly positive for SMAα staining. Minor ductular structures including those near the islets are also positive for SMAα in Pten null pancreas but less so in controls. SMAα positive cells are also found within the islets (Fig 4B, magnified inset). We also evaluated these Desmin and SMAα positive cells at the peri-islet area for cell proliferation. Indeed, many of the BrdU positive cells are also positive for desmin (Fig 5A), suggesting that these are myofibroblasts. Most of the BrdU positive myofibroblasts are minimally stained for SMAα (Fig 5B).
Figure 3. Images of H&E stained pancreas sections.
Representative images of islets from the Pten null regenerating model. MD, major duct; AC, acinar cells; I, islets; DB, ductal branches; V, blood vessels. Circled green dotted areas are examples of cells with myofibroblast morphologies. Bar, 25µm.
Figure 4. Images of desmin and smooth muscle actin α (SMAα) stained pancreas sections.
Representative images of islets from control and Pten null mice. (A) Desmin stained pancreas sections (top panel). Green, insulin; Red, Desmin. Bottom panel, quantification of the Desmin stained areas at the peri-islet area. Areas 100µm outside the islets are included in the quantification. (B) Top, SMAα stained pancreas sections. Bottom, higher magnification image of boxed area. D, duct. Bar, 50µm. Blue, DAPI.
Figure 5. PTEN loss leads to expansion of surrounding mesenchymes and pancreatic stellate cells.
(A) STZ-treated control and Pten null pancreas sections were costained with Desmin (red) and BrdU (green). Bottom row, the magnified images of the boxed areas. (B) STZ-treated control and Pten null pancreas sections were costained with smooth muscle actin α (SMAα, red) and BrdU (green). Islets are designated in dashed circles. Bar, 50µM. Blue, DAPI.
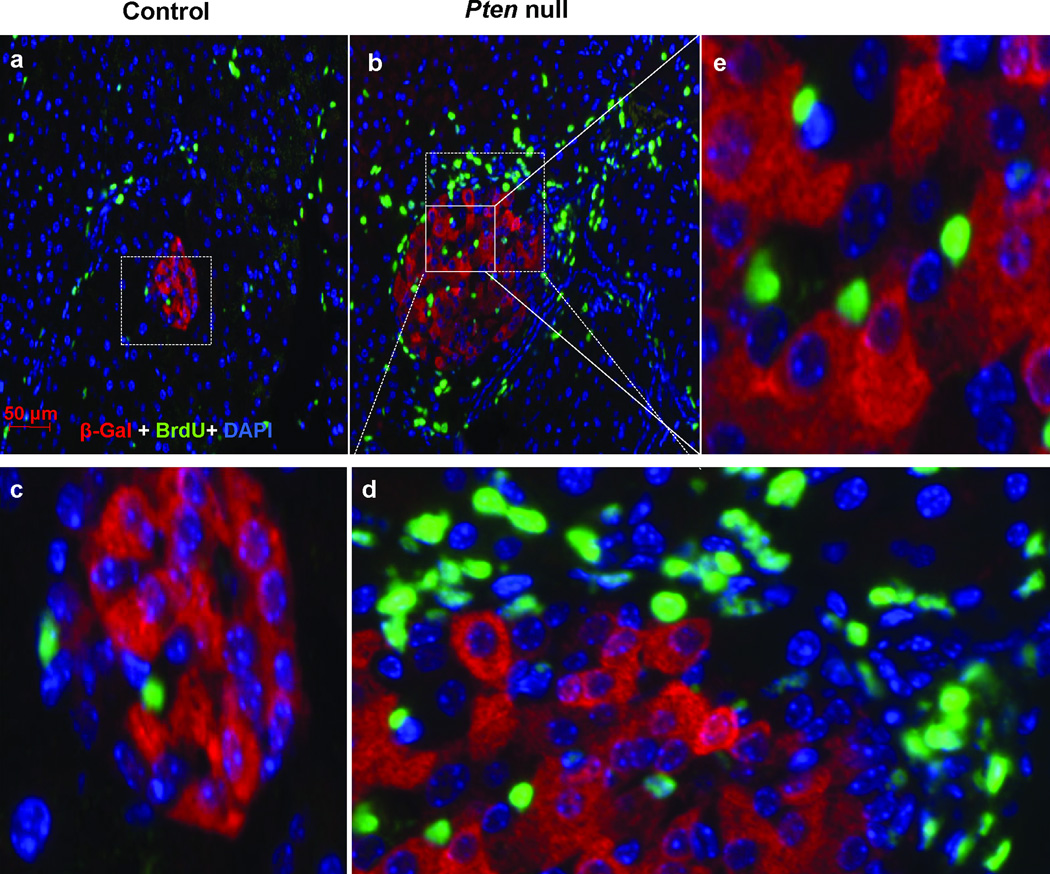
Given the recent advances in the ability to alter cell fate by manipulating transcriptional factors [13–15], we tested whether these proliferating cells were β-cells that lost their insulin expression [16]. To do this, we crossed the PtenloxP/loxP; Rip-Cre+ mice with a reporter strain Rosa26lacZ. In this model, all cells that expressed insulin at any given point during their existence are labeled with β-gal. Immunochemical analysis of pancreatic sections confirmed the expression of lacZ reporter gene in the islets of Langerhans (Supplemental Fig 2). Our results indicate that none of the surrounding cells are positive for β-gal (Fig 6 a–d). This is true for both the control (Fig 6 a,c) and the Pten null (Fig 6 b,d) pancreas. In addition, the majority of the BrdU positive cells within the islets are also negative for β-gal (Fig 6e). This result suggests that the injured β-cells produced a paracrine effect to induce the proliferation of surrounding non-β-cells under the STZ-induced injury condition.
Figure 6. Dedifferentiation of β-cells is unlikely to contribute to the peri-islet proliferating activity induced by STZ.
Control (Pten+/+; Rosa26lacZ; RIPCre+) and Pten null (PtenloxP/loxP; Rosa26lacZ; RIPCre+) mice were treated with STZ to induce β-cell injury and the pancreas was analyzed with β-gal (red), BrdU (green) and DAPI (blue). (a) Control islet. (b) Pten null islet. (c) Higher magnification of area within the dashed square of control islet. (d) Higher magnification of area within the dashed square of mutant islet. (e) Higher magnification of area within the solid square of mutant islet. Dashed circles indicate islet area. Bar, 50µm.
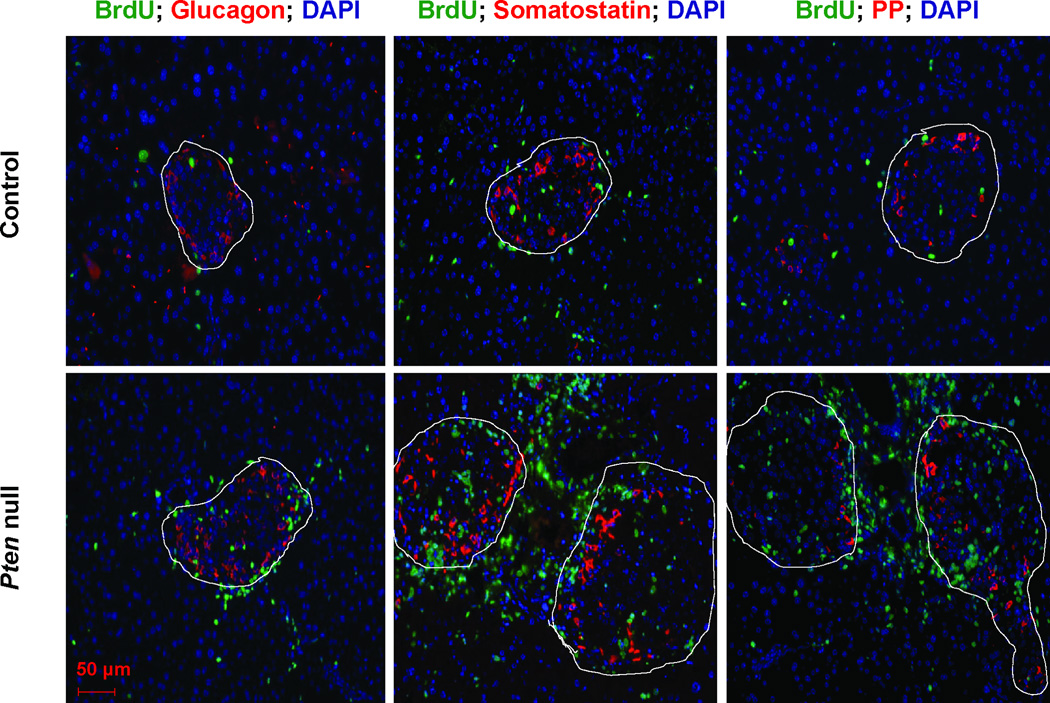
We stained the control and Pten null pancreas with glucagon (α-cells) as well as other endocrine cell markers: PP (pancreatic polypeptide cells), and somatostatin (δ-cells) together with anti-BrdU (Fig 7 & Supplemental Fig 2). Compared to β-cells, these other cell populations are present in much lower numbers in the islets and are physically located surrounding the β-cell clusters. In the untreated mice, very few or none of the peri-islet proliferating cells are positive for any of the endocrine hormones (Supplemental Fig 3). STZ treatment significantly induced proliferation in the islet area, particularly in the Pten null mice (dotted lines). Under these conditions, we still did not observe significant overlap of BrdU positive cells with the non-insulin endocrine positive cells (Fig 7). This result suggests that the non-β-cell pancreatic endocrine cell types are unlikely contributors to the mass of β-cells in the Pten null mice.
Figure 7. PTEN loss in β-cells leads to increased proliferation within and surrounding the pancreatic islets.
Control and Pten null were treated with STZ for 5 consecutive days. Pancreas were dissected and costained with endocrine cell markers: glucagon, pancreatic polypeptide, somatostatin (red), BrdU (green), and DAPI (blue). Islets are designated in dashed circles. Bar, 50µm.
Further, we stained the pancreatic sections with amylase and CK to mark the acinar cells and ductal cells, respectively. Our data shows that the proliferating BrdU positive, insulin negative cells are not these two common exocrine cell types. Very little colocalization was found for BrdU with amylase or CK (Supplemental Fig 4). We also stained the sections with several potential progenitor cell markers including Pdx-1, a pancreatic progenitor cell marker; Ngn3, a pancreatic endocrine progenitor cell marker; nestin and GFAP, both neuroendocrine markers and sometime used as quiescent stellate cell markers [17]. Our staining shows that none of the proliferating cells are these cell types (Supplemental Fig 4).
DISCUSSION
Tissue repair involves a dynamic sequence of events that leads to regeneration of the injured tissues. One of the early events after the insult is stopped is the building and deposition of extracellular matrix (ECM) to support the regeneration of lost cells. This process, while not well understood, involves the crosstalk between injured cells and other resident cells, as well as the infiltrating immune cells. In this study, we showed that stellate cells, a major source of ECM, support the growth of the injured β-cells by producing soluble factors. Using a genetically modified model for β-cell regeneration [5, 10], we showed that activation of surrounding mesenchymal cells occurs simultaneously with regeneration of the injured islet β-cells. The fast regenerating β-cells in the Pten null model are accompanied by significantly more accumulation of activated myofibroblasts surrounding the islets compared to the slower regenerating β-cells in mice with intact PTEN.
Pancreatic stellate cells (PSCs) are a type of myofibroblasts that can be activated during injury. When activated, PSCs can proliferate and migrate to the site of tissue injury [17]. They lay down extracellular matrix to promote repair of damaged tissues. These stellate cells express an array of markers including GFAP and nestin when dormant and SMAα and desmin when they are activated [17]. The peri-islet cell population with high mitotic activity that we have identified highly expresses desmin, and to a lesser extent SMAα, but not GFAP or nestin, indicating that they are a type of myofibroblast, likely activated stellate cells. The morphology of this cell population with elongated fiber-like body also resembles activated myofibroblasts found at the site of injury [18]. Our data suggests that the fast growing β-cells in Pten null islets may signal the proliferate of myofibroblasts in order to promote the regeneration of the β-cells. Research from the pancreatic cancer field suggests that there is a symbiotic relationship between cancer cell growth and activation of SMAα expressing stellate cells [17]. We showed here that such a relationship might also exist for the growth of injured islets and the surrounding myofibroblasts. In the Pten null mice, the fast regenerating β-cells may induce the activation of myofibroblasts in an effort to support their rapid growth. How loss of PTEN in the epithelial β-cell compartment led to such response in the ECM, however, is unclear. Speculations from the pancreatic cancer and fibrosis fields suggest the involvement of TGFβ in this process [17]. While TGFβ and PTEN signals do crosstalk [19, 20], whether the results seen in this model are due to an altered response in TGFβ signaling needs to be further explored.
Recently, it was shown that β-cells may reduce their insulin secretion when proliferating [16]. Although this study uses insulinoma cell lines, the implication may extend to β-cells in vivo. The primary function of β-cells is to secrete insulin. The cytoplasm of β-cells is laden with insulin granules. In order to make space in the cytoplasm for β-cells to replicate, it is not unlikely that β-cells need to shed their insulin granules. Human β-cells cultured in vitro can undergo epithelial-mesenchymal transition (EMT) to proliferate [21]. Cells produced in vitro through such EMT process were shown to produce high levels of C-peptide in SCID mice capable of rescuing hyperglycemia. Here, we demonstrate in vivo that the proliferating cells surrounding the regenerating islets in the STZ-treated Pten null mice are not β-cells that lost their insulin granules in an effort to proliferate. Our lineage analysis clearly shows that in either maintenance or STZ-stimulated stage, fast proliferating cells surrounding the islets are negative for β-gal staining, an indication of non-β-cell origin. Therefore, a potential “dedifferentiation” process does not occur in vivo in β-cell maintenance or in this STZ-treated stage in our mouse model. This conclusion is in agreement with the in vitro study using mouse islets showing that migrating cells from cultured islets are indeed fibroblasts rather than β-cells going through EMT [22]. Proliferating β-cells may reduce their insulin burden as shown in vitro with insulinoma cell lines [16]. However, they do not change their cell fate in the effort to proliferate even in the Pten deletion mice. Our data suggest that communication between β-cells and surrounding mesenchymal cells are critical for maintaining the growth of both cell types.
Regenerating pancreatic β-cells is a long-term goal for regenerative medicine and cell-based therapy given the ever-growing incidence of diabetes. Recent studies highlighted the plasticity of β-cells in their ability to grow and proliferate and also adapt and alter their identity [23–25]. Recent advances in the stem cells field also highlighted the plasticity of somatic cells. By manipulating key transcriptional factors, exocrine cells are capable of producing insulin [26]. However, they do not migrate and form clusters like the β-cells do. In chicken pancreas, intercalated islet stellate cells were found to contribute to the mass of β-cells [27]. During embryogenesis, development of the islets is coordinated with surrounding tissues. Communication between mesoderm and endoderm help set the pattern for pancreas development [28]. Similarly, regeneration of islets in adult tissues may also rely on such communications. Here, we showed that myofibroblasts, such as stellate cells, promote the growth and proliferation of β-cells in culture. In vivo, islet β-cells in the Pten null mice are surrounded by more myofibroblasts and display higher mitotic indexes in response to injury. This evidence suggests that myofibroblasts support the growth and regeneration of pancreatic β-cells. Our study indicated that communication between the stellate cell-like myofibroblast cell population and β-cells may be important for the maintenance of islets. Future studies are needed to further dissect the specific signals that promote this effect.
Supplementary Material
Acknowledgments
We thank Drs. Dor, Hamm-Alvarez, Pandol and Asahina for the helpful suggestions on this manuscript. We thank Dr. Means for providing the mouse PSC cell line.
Grants: This work was supported by NIH grants R21 DK075928-02 and R01 DK084241-01 (BLS), and a grant from the Zumberg foundation. Dr. Stiles also acknowledge support from NCI (R01CA154986-01). Dr. Bayan would like to acknowledge funding from NIDDK R36 MD005009-01. The USC non-parenchymal cell core facility is supported by a grant from NIAAA (R24 AA12885). Dr. Zeng would like to acknowledge the CBM fellowship program and the USC Provost Postdoctoral Fellowship program. Ms. Peng would like to acknowledge the CIRM fellowship.
REFERENCES
- 1.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34–41. doi: 10.1111/j.1440-1746.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 3.Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 4.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 5.Stiles BL, Kuralwalla-Martinez C, Guo W, et al. Selective deletion of Pten in pancreatic beta cells leads to increased islet mass and resistance to STZ-induced diabetes. Mol Cell Biol. 2006;26:2772–2781. doi: 10.1128/MCB.26.7.2772-2781.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaine SA, Ray KC, Branch KM, et al. Epidermal growth factor receptor regulates pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G434–G441. doi: 10.1152/ajpgi.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kordes C, Sawitza I, Haussinger D. Hepatic and pancreatic stellate cells in focus. Biol Chem. 2009;390:1003–1012. doi: 10.1515/BC.2009.121. [DOI] [PubMed] [Google Scholar]
- 8.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann K, Besch W, Zuhlke H. Spontaneous recovery of streptozotocin diabetes in mice. Exp Clin Endocrinol. 1989;93:225–230. doi: 10.1055/s-0029-1210861. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen KT, Tajmir P, Lin CH, et al. Essential role of Pten in body size determination and pancreatic beta-cell homeostasis in vivo. Mol Cell Biol. 2006;26:4511–4518. doi: 10.1128/MCB.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang KT, Bayan JA, Zeng N, et al. Adult-onset deletion of Pten increases islet mass and beta cell proliferation in mice. Diabetologia. 2014;57:352–361. doi: 10.1007/s00125-013-3085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng N, Yang KT, Bayan JA, et al. PTEN controls beta-cell regeneration in aged mice by regulating cell cycle inhibitor p16(ink4a.) Aging Cell. 2013;12:1000–1011. doi: 10.1111/acel.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine F, Itkin-Ansari P. beta-cell Regeneration: neogenesis, replication or both? J Mol Med (Berl) 2008;86:247–258. doi: 10.1007/s00109-007-0259-1. [DOI] [PubMed] [Google Scholar]
- 14.Marzioni M, Saccomanno S, Candelaresi C, et al. Pancreatic Duodenal Homeobox-1 de novo expression drives cholangiocyte neuroendocrine-like transdifferentiation. J Hepatol. 2010;53:663–670. doi: 10.1016/j.jhep.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi H. Production of pancreatic beta-cells from stem cells. Curr Diabetes Rev. 2010;6:184–190. doi: 10.2174/157339910791162934. [DOI] [PubMed] [Google Scholar]
- 16.Cozar-Castellano I, Harb G, Selk K, et al. Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes. 2008;57:3056–3068. doi: 10.2337/db08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masamune A, Kikuta K, Watanabe T, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2008 doi: 10.1136/gut.2008.154401. [DOI] [PubMed] [Google Scholar]
- 19.Assinder SJ, Dong Q, Kovacevic Z, et al. The TGF-beta, PI3K/Akt and PTEN pathways: established and proposed biochemical integration in prostate cancer. Biochem J. 2009;417:411–421. doi: 10.1042/BJ20081610. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest. 2006;116:1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershengorn MC, Hardikar AA, Wei C, et al. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 22.Atouf F, Park CH, Pechhold K, et al. No evidence for mouse pancreatic beta-cell epithelial-mesenchymal transition in vitro. Diabetes. 2007;56:699–702. doi: 10.2337/db06-1446. [DOI] [PubMed] [Google Scholar]
- 23.Dor Y, Brown J, Martinez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 24.Elghazi L, Weiss AJ, Barker DJ, et al. Regulation of Pancreas Plasticity and Malignant Transformation by Akt Signaling. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teta M, Rankin MM, Long SY, et al. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datar SP, Bhonde RR. Islet-derived stellate-like cells as a novel source for islet neogenesis in chicks. Poult Sci. 2009;88:654–660. doi: 10.3382/ps.2007-00440. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M, Jordan N, Melton D, et al. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.