Abstract
Sjögren's Syndrome (SS) is a debilitating autoimmune disease. Patients with SS may develop xerostomia. This process is progressive, and there are no therapeutics that target disease etiology. We hypothesized BAFF receptor (BAFFR) blockade would mitigate SS disease development, and neutralization of CXCL13 and BAFF signaling would be more efficacious than BAFFR blockade alone. We treated NOD/ShiLtJ SS mice with soluble BAFF receptor (BAFFR-Fc) or anti-CXCL13/BAFFR-Fc in combination, prior to the development of clinical disease. Our results show treatment with BAFFR-Fc reduced peripheral B cells numbers and decreased sialadenitis. In addition, this treatment reduced total serum immunoglobulin as well as IgG and IgM specific anti-nuclear autoantibodies. NOD/ShiLtJ mice treated with BAFFR-Fc and anti-CXCL13 antibody were protected from salivary deficits. Results from this study suggest blockade of CXCL13 and BAFFR together may be an effective therapeutic strategy in preventing salivary hypofunction and reducing autoantibody titers and sialadenitis in patients with SS.
Keywords: Sjögren's syndrome, sialadenitis, salivary hypofunction, BAFF receptor, CXCL13, autoantibody
1. Introduction
Sjögren's Syndrome (SS) is an autoimmune disease in which the immune system targets exocrine gland tissue [1]. Both the adaptive and innate immune systems are crucial to the progression of SS [2]. Inflammatory cells are observed in salivary and lacrimal tissue, and this lymphocytic infiltration may contribute to loss of glandular function [3]. B cell dysfunction is well documented in SS, both locally and systemically. SS is characterized by the presence of numerous autoantibodies, including those directed against Ro (SSA), La (SSB), nuclear autoantigens, and rheumatoid factor (RF) [4, 5]. Since the etiology of SS is unknown, there are no therapeutics that target disease pathogenesis. Currently, treatment is palliative, and SS patients may experience significant morbidity related to xerostomia and xerophthalmia. These include loss of teeth due to dental caries, difficulty speaking and chewing, and deficits in vision. Thus, it is important to identify therapies that mitigate inflammation and loss of exocrine secretions in SS patients.
SS is characterized by lymphocytic infiltration of salivary tissue, termed focal lymphocytic sialadentitis (FLS) [3]. In SS, the percentage of the infiltrating salivary gland lymphocytes that are B cells increases with the degree of glandular inflammation [6]. B cells within salivary tissue likely contribute to SS pathogenesis, as they produce autoantibodies [7, 8], and differences in immunoglobulin (Ig) repertoires are observed between salivary and peripheral blood B cells [9]. Moreover, memory B cells are increased in the salivary tissue of SS patients [10]. Systemic B cell abnormalities are also observed in SS. For example, there is a decrease in unswitched memory B cells, altered chemokine receptor expression, and evidence for dysregulated B cell development and selection [9, 11-13].
B cells are regulated by complex cell-cell interactions and signals transduced by soluble mediators. B-cell activating factor of the TNF family (BAFF, also called BLyS, TALL-1, THANK, and zTNF4) is implicated in several autoimmune disorders, including SS [14]. BAFF is secreted mainly by macrophages, monocytes, and dendritic cells, and is also produced by nonmyeloid cells such as salivary gland epithelial cells (SGECs) [15, 16]. BAFF directs B cell maturation, development, and survival. BAFF also mediates Ig production and class switching [15]. BAFF is upregulated by interferon (IFN)-γ, interleukin (IL)-10 and CD40 ligand (CD40L) produced during inflammation and infection [17]. BAFF is the only cytokine known to activate the BAFF receptor (BAFFR), which is expressed by circulating B and T cells [18, 19]. Studies in mice demonstrate a crucial role for BAFF in B cell survival. Accordingly, mice genetically deficient in BAFF or BAFFR show reduced peripheral B cell numbers [20, 21]. Since BAFF plays a central role in maintenance of these B cells, dysregulation of this cytokine contributes to the persistence of autoreactive B cells [22]. It is important to note that BAFF transgenic mice develop SS- and lupus-like diseases. Moreover, patients with SS have elevated BAFF levels in salivary tissue, sera, and saliva [14, 23-27]. Thus, BAFF is clearly important in SS pathogenesis in both murine models and SS patients.
The chemokine CXCL13 also plays an important role in B cell physiology and is increased in SS. CXCL13 is secreted by follicular stromal cells such as follicular dendritic cells and marginal reticular cells [28]. CXCL13 binds the G protein coupled receptor CXCR5 that is expressed predominantly by peripheral B cells and T follicular helper cells [29]. CXCL13 directs B cell chemotaxis, and is increased in both murine and human SS [30-36]. Of note, blockade of CXCL13 signaling results in a modest reduction in lymphocytic infiltration of salivary tissue in SS mice [30, 37]. Thus, these data suggest CXCL13 may be integral to SS pathogenesis.
Since BAFF and CXCL13 both direct B cell function, it is not surprising that these cytokines act synergistically to regulate B cell activity. Studies in humans show BAFF increases the chemotactic response of B cells to CXCL13, and this effect is more pronounced in memory B cells than naïve. Importantly, blockade of BAFFR abrogates this migration [38]. To determine whether BAFFR neutralization alone or in combination with CXCL13 blockade mitigates SS disease development, we inhibited CXCL13 and BAFFR signaling in the NOD/ShiLtJ (NOD) model of SS. Animals were treated prior to disease development continuously until the time that they would normally develop disease. We found that salivary gland inflammation, total serum antibody and ANA specific IgG and IgM autoantibody titers were decreased in animals given BAFFR alone. Animals that received concomitant CXCL13 and BAFFR blockade also exhibited reduced salivary gland inflammation, total serum antibody and ANA specific IgG autoantibody titers. In addition, these animals also had diminished IgM titers and did not lose salivary flow. Results from this study suggest that neutralization of CXCL13 and BAFFR mediated signaling may be an effective therapeutic strategy in SS.
2. Materials and Methods
2.1. Mice
Female NOD/ShiLtJ (NOD) mice (age 3 weeks) were purchased from Jackson Labs. All animals were cared for and handled in accordance with NIH and IACUC guidelines.
2.2. Serum collection
For murine studies, sera were harvested immediately following euthanasia. Blood was collected by retro-orbital eye bleed or cardiac puncture following euthanasia in accordance with IACUC protocols.
2.3. Assessment of Saliva Production
Pilocarpine HCl (0.3 mg/100 μL) was injected intraperitoneally (Sigma-Aldrich), and saliva was collected for 10 minutes. Saliva was immediately placed on ice, centrifuged briefly, and quantified using a pipette. Saliva was stored at −80°C until use.
2.4. CXCL13 and BAFFR Neutralization
2.4.1. Reagents
Anti-CXCL13 antibody (MAb 5378) and soluble BAFFR-Fc were generously provided by Vaccinex. IgG2a isotype control and anti-CXCL13 antibodies were generated and validated as previously described [30]. To make the soluble BAFFR-Fc reagent, the murine BAFFR gene was obtained from Open Biosystems (accession # BC104127, clone ID: 40044559). PCR primers were designed to amplify the region corresponding to amino acid residues 10-71. The resultant PCR product was cloned into an expression vector encoding a signal peptide, and was placed in-frame with a 3’ amino acid linker sequence followed by the mouse IgG2a Fc domain (hinge-CH2-CH3). CHO cells were transfected with this construct using polyethylenimine max transfection reagent (Polysciences, Inc.), and the culture supernatant harvested. BAFFR-Fc was purified by affinity chromatography using POROS MabCapture protein A resin (Life Technologies). The molecular weight of BAFFR-Fc is approximately 32 kDa, and the theoretical isoelectric point is 5.76. The protein was eluted with 0.1 M glycine (pH 2.7). We performed buffer-exchange on the BAFFR-Fc protein into 20 mM Tris (pH 8.0) using PD-10 desalting columns (GE Healthcare Life Sciences). The protein was then loaded onto POROS HQ anion exchange resin (Life Technologies) and eluted fractions with successive 95 mM, 140 mM, 165 mM, and 1M NaCl step gradients. The 140 mM elution fraction was used for further processing. BAFFR-Fc was concentrated using Amicon Ultra-15 30K filters (Millipore) and buffer-exchanged into PBS (pH 7.2) using PD-10 desalting columns. The protein was passed through a Mustang E filter (Pall Corporation) to remove endotoxin. Finally, the product was sterile-filtered through a 0.2 μm syringe filter (Pall Corporation). A PyroGene Recombinant Factor C endotoxin assay (Lonza) was performed to verify endotoxin removal. Size exclusion chromatography was carried out using a TSKgel G3000SWXL column (TOSOH Bioscience) to confirm removal of aggregates and contaminants as additional quality control steps.
2.4.2. Validation of BAFFR-Fc
2.4.2.1. BAFF ELISAs
Microtiter plates were coated with murine BAFF (0.5 μg/mL, R&D systems). Serial dilutions of commercially available recombinant murine BAFFR-Fc (R&D systems) or BAFFR-Fc (Vaccinex) were performed. Biotinylated goat anti-mouse BAFFR (100 ng/ml, R&D systems) was added, followed by incubation with Streptavidin-HRP (10 μg/mL, Jackson ImmunoResearch). ELISAs were developed with TMB substrate and read at 450/570 nm.
2.4.2.2. Proliferation Assays
Splenocytes were isolated from 8-10 week old female C57BL/6 mice and cultured in 96-well microtiter plates (1 × 105 cells per well) in RPMI1640 with 10% FBS. The cells were treated with recombinant murine BAFF (0.01 μg/mL, R&D systems) and goat anti-mouse IgM (10 μg/ml Jackson ImmunoResearch) in the presence or absence of commercially available recombinant murine BAFFR-Fc (10 μg/ml, R&D systems) or BAFFR-Fc (10 μg/ml, Vaccinex) for 4 days. Cells were incubated with Alamar Blue at 37°C for 4 hours, and fluorescence read at 530/590 nm.
2.4.3. Administration protocol
NOD females were injected with 100 μg each of isotype control IgG2a antibody, BAFFR-Fc, and anti-CXCL13 antibody/BAFFR-Fc in combination. Animals were injected three times weekly for 12 weeks beginning at 4 weeks of age. Antibody was administered by intraperitoneal injection. All animals were euthanized at 16 weeks of age. Sera and saliva were collected as described above. Spleen, cervical lymph nodes (cLNs), and salivary tissue were harvested.
2.5. Histological Processing and Analysis
Salivary tissue was fixed in formalin, paraffin embedded, and processed using a Tissue Tek VIP (Sakura). H&E staining was performed using a Symphony automated platform (Ventana). Slides were scanned using Aperio software (Leica Biosystems) and lymphocytic infiltration was quantified using ImageJ. The presence of periductal FLS within submandibular salivary tissue was considered indicative of SS disease. Periductal FLS was quantified by measuring the percent of SMG tissue occupied by lymphocytes [30]. Immunohistochemical staining (IHC) was performed on salivary and splenic tissue with an IntelliPATH autostainer (BioCare Medical) using antibodies directed against B220 (2 μg/mL, clone RA3-6B2, Abcam) and CD3 (1:100 dilution, clone SP7, Abcam). Spectral phasor analysis was carried out using a custom ImageJ plugin (available from http://www.spechron.com). The phasor approach has previously been implemented to analyse lifetime [39, 40] and spectral images [41, 42]. A detailed description of this analysis is provided in the appendix.
2.6. Cell Isolation and Flow Cytometry
Lymphocytes from spleens and cLNs were isolated using mechanical disruption. Splenocytes and cLN cells were stained with B220, CD21/35 (BD Biosciences) and CD23 (BioLegend). Flow cytometry was performed using an Influx (BD) and FlowJo software was used for analysis (TreeStar Inc.).
2.7. RNA Isolation and Quantitative PCR
RNeasy kits (Qiagen) were used to isolate mRNA from salivary tissue. cDNA synthesis was performed with an iScript kit (Bio-Rad). Quantitative (q) PCR was performed with SYBR Green (Bio-Rad). Primers for Cd19 and Cd4 were published previously [30]. PCR settings were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative expression was calculated with the ΔCt method, where R = 2[Ct sample - Ct control]. All samples were analyzed in duplicate, and expression was normalized to actin.
2.8. ELISAs
Serum IgM, IgG1, and IgG3 (Bethyl Labs) were quantified by ELISA. IgG and IgM specific anti-nuclear autoantibody (ANA) levels were also determined by ELISA (Alpha Diagnostics, catalog #5210). Horseradish peroxidase conjugated to anti-IgG or IgM respectively was used to detect isotype-specific ANA (Southern Biotech). Sera were harvested as described above and appropriate serial dilutions were determined experimentally.
2.9. Hep2 Staining
Sera were diluted 1:50 and incubated with Hep-2 slides according to manufacturer instructions (MBL Bion). ANA were detected with alexa fluor 488 anti-mouse IgG (H+L) or IgM (μ chain) (Life Technologies). Slides were imaged using an Olympus BX41 fluorescence microscope with a Lumenera Infinity 3 camera.
3.0. Statistics
Statistical analyses were performed using GraphPad Prism software.
3. Results
3.1. BAFFR-Fc is specific for murine BAFF and inhibits splenocyte proliferation
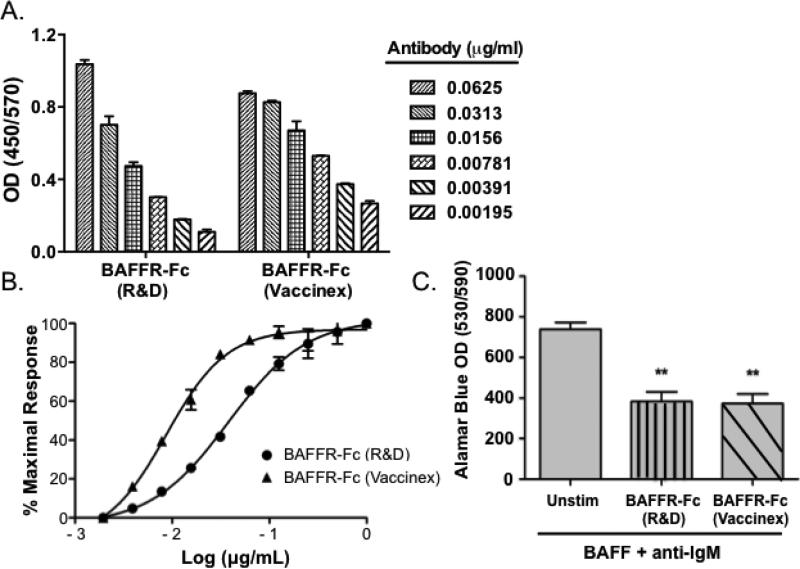
Initial studies were conducted in vitro to confirm the specificity and neutralizing capacity of BAFFR-Fc. To verify the binding of BAFFR-Fc to BAFF, we performed BAFF ELISAs where we compared the BAFFR-Fc we developed to a commercially available murine BAFFRFc. We found that both reagents bound BAFF similarly in a dose-dependent manner (Figure 1A). We calculated the half maximal effective concentration values (EC50) for the commercially available BAFFR-Fc reagent (EC50 = 0.04 μg/mL) and the reagent we developed (EC50 = 0.009 μg/mL) (Figure 1B). We then sought to determine whether the soluble BAFFR-Fc we developed could block BAFF and anti-IgM induced proliferation of murine splenocytes. To this end, we treated splenocytes with BAFF and anti-IgM, and analyzed proliferation in the presence or absence of the BAFFR-Fc reagent we generated. We used the commercially available neutralizing murine BAFFR-Fc as a positive control. We found our BAFFR-Fc reagent inhibited BAFF/anti-IgM induced proliferation with similar efficacy as compared to the positive control (p = 0.003 and 0.004, respectively) (Figure 1C). Therefore, the BAFFR-Fc we developed binds BAFF and inhibits its biological activity.
Figure 1. BAFFR-Fc binds BAFF specifically and inhibits splenocyte proliferation.
(A) BAFF ELISAs were performed with BAFFR-Fc (Vaccinex). Commercially available murine BAFFR-Fc was used as a positive control. Data represent an average of duplicate measurements and SD is shown. (B) Splenocytes were incubated with murine BAFF (0.5 μg/mL) in the presence of increasing concentrations of R&D BAFFR-Fc or Vaccinex BAFFR-Fc (0.02 - 1.0 μg/mL). The titration curve was fitted using four-parameter sigmoidal curve fit (R2 = 0.99). (C) Splenocytes were isolated from C57BL/6 females (8-10 wks). Proliferation was quantified using alamar blue incorporation. Data represent an average of triplicate measurements. Unstim = unstimulated. Significance was determined using Student's t-test. Mean and SEM are shown (**p < 0.01).
3.2. B cells are reduced in the periphery of animals treated with BAFFR-Fc
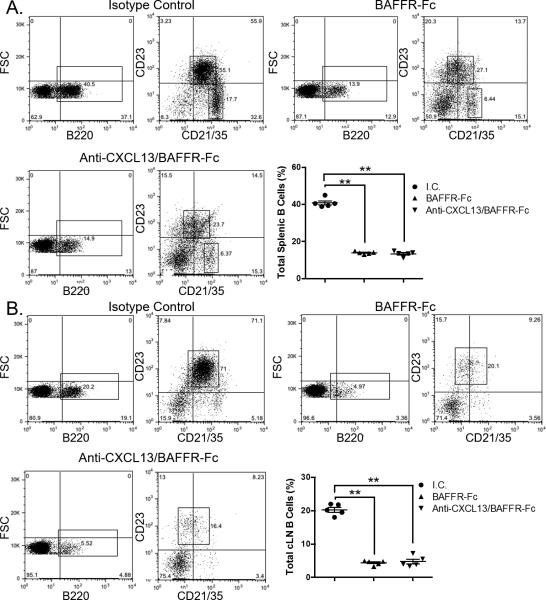
To verify the effectiveness of BAFFR-Fc and anti-CXCL13/BAFFR-Fc treatment, we isolated spleens and cLN tissue from 16-week-old female NOD mice treated with isotype control antibody (n = 10), BAFFR-Fc (n = 9), and anti-CXCL13/BAFFR-Fc (n = 9). We pooled tissue from 2 separate animals in each group for flow cytometric analysis. We saw a significant decrease in the percentage of splenic follicular and marginal zone B cells in animals treated with BAFFR-Fc alone and anti-CXCL13/BAFFR-Fc as compared to those receiving the isotype control (p = 0.008 and p = 0.008, respectively) (Figure 2A). We also observed a significant decrease in the percentage of B cells in the cLNs in these two treatment groups as compared to isotype control treated animals (p = 0.008 and p = 0.008, respectively) (Figure 2B). Thus, BAFFR-Fc administration caused a significant decrease in the percentage of B cells in secondary lymphoid organs. This decrease was also observed in animals treated with anti-CXL13/BAFFRFc, and the addition of CXCL13 neutralization did not have any additional suppressive effect on the percentage of B cells in either splenic or cLN tissue as compared to the animals treated with BAFFR-Fc alone (Figure 2).
Figure 2. B lymphocytes are reduced in spleen and cLNs of BAFFR-Fc and BAFF-Fc/anti-CXCL13 treated mice.
Spleens and cLNs were isolated from animals treated with either I.C., BAFFR-Fc, or BAFFR-Fc/anti-CXCL13. B220+ B cells from (A) spleen and (B) cLN were gated on and examined for expression of CD23 and CD21/35. I.C. = isotype control. Significance was determined using Mann-Whitney test. Mean and SEM and shown (**p < 0.01).
3.3. Lymphocytic infiltration of submandibular gland (SMG) tissue is reduced following administration of BAFFR-Fc
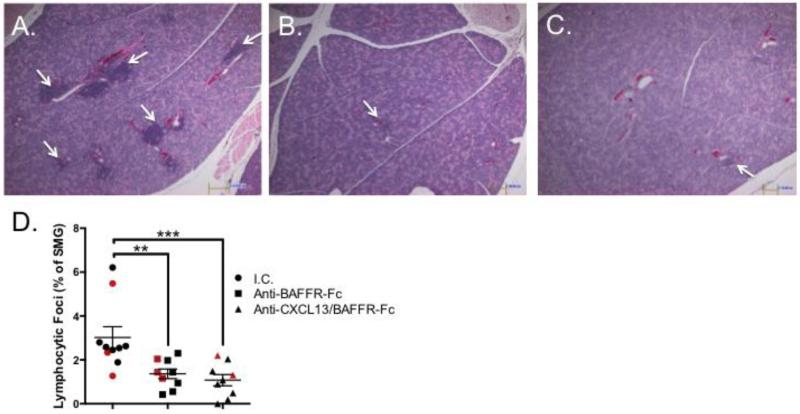
To determine whether blockade of BAFFR reduces salivary gland inflammation, we isolated SMG tissue from animals in the three treatment groups, and quantified SMG gland focus scores on H&E stained tissue. We analyzed the SMG because this gland reliably develops a robust lymphocytic infiltration in female NOD animals [43]. Previous results from our laboratory demonstrated a modest reduction in salivary gland focus scores in animals receiving anti-CXCL13 treatment alone [30]. Strikingly, we observed a dramatic reduction in SMG inflammation in animals treated with BAFFR-Fc or anti-CXCL13/BAFFR-Fc as compared to those treated with isotype control antibody (p = 0.002 and 0.0006, respectively) (Figure 3A-C). Of note, there was no difference in focus score between the BAFFR-Fc and anti-CXCL13/BAFFR-Fc treated animals (Figure 3D).
Figure 3. FLS is decreased in salivary tissue as a result of BAFF receptor blockade.
Histological analysis of H&E stained salivary tissue from NOD/ShiltJ mice treated with (A) I.C. (n = 10), (B) BAFFR-Fc (n = 9), and (C) Anti-CXCL13/BAFFR-Fc (n = 9). White arrows indicate lymphocytic foci. (D) Quantification of histological data shown in graph. Red shapes indicate animals selected for further analysis in Figure 4. I.C. = isotype control. Significance was determined using the Mann-Whitney test. Mean and SEM are shown (**p < 0.01, ***p < 0.001, N.S. non-significant). Original magnification is 40X.
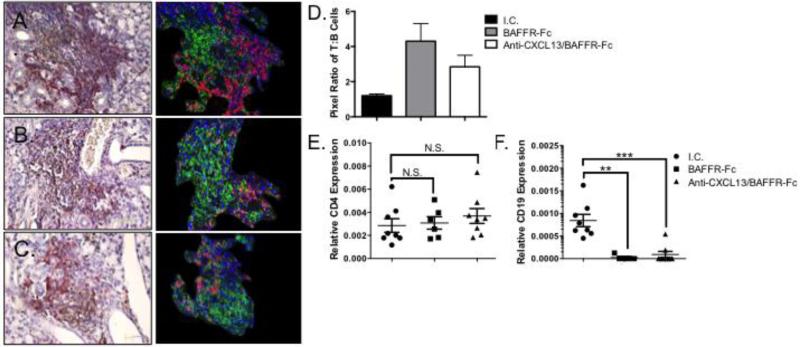
To extend these findings, we performed IHC for B and T cell markers using representative animals from each treatment group. We stained SMG tissue with antibodies directed against B220 and CD3 (Figure 4A-C, left panel). To further clarify these findings, spectral unmixing was performed (Figure 4A-C, right panel). We found both B and T cells were present within the lymphocytic foci within SMG tissue of isotype control treated animals (Figure 4A). In the SMG of the animals treated with BAFFR-Fc or anti-CXCL13/BAFFR-Fc, the remaining infiltrates consisted largely of T cells, although scant B cells were noted in some of the foci (Figure 4B and C). Using pixel density, we quantified the ratio of T to B cells in the SMG tissue. The pixel densities of T to B cells were similar in animals treated with isotype control. However, the ratio of T to B cells in the SMG was approximately 4:1 in both the BAFFR-Fc and anti-CXCL13/BAFFR-Fc treated animals (Figure 4D). To confirm these findings, we performed qPCR for T (CD4) and B cell markers (CD19) using SMG tissue. There was no difference in CD4 expression between BAFFR-Fc and anti-CXCL13/BAFFR-Fc treated animals as compared to the isotype control (p = 0.74 and 0.27, respectively) (Figure 4E). In contrast, relative CD19 transcripts were decreased significantly in the BAFFR-Fc and anti-CXCL13/BAFFR-Fc groups as compared to isotype control treated animals (p = 0.0016 and p = 0.0003, respectively) (Figure 4F). Thus, BAFFR-Fc and anti-CXCL13/BAFFR-Fc administration reduces salivary inflammation and decreases B cells specifically in the NOD model of SS.
Figure 4. Salivary B cells are dramatically reduced in BAFFR-Fc and anti-CXCL13/BAFFR-Fc treated animals.
Immunohistochemical staining was performed for CD3 (brown) and B220 (red) (Left panels). Spectral unmixing was performed to distinguish T (green) and B cells (red). Hematoxylin stained cells are shown in blue (right panels). (A) I.C. treated animals (n = 3) (B) BAFFR-Fc animals (n = 3), and (C) Anti-CXCL13/BAFFR-Fc treated animals (n = 3). A representative photomicrograph from each treatment group is shown. Original magnification is 40X. (D) The pixel ratio of T to B cells for each treatment group. (E) CD4 and (F) CD19 transcript levels were determined by qPCR using SMG tissue from I.C. (n = 8), BAFFR-Fc (n = 5) and anti-CXCL13/BAFFR-Fc (n = 8). I.C. = isotype control. Each sample was analyzed in duplicate and expression normalized to β-Actin. Significance was determined using Mann-Whitney test. Mean and SEM are shown (**p < 0.01, ***p < 0.001, N.S. non-significant).
3.4. Concomitant anti-CXCL13 and BAFFR-Fc treatment prevents loss of salivary flow
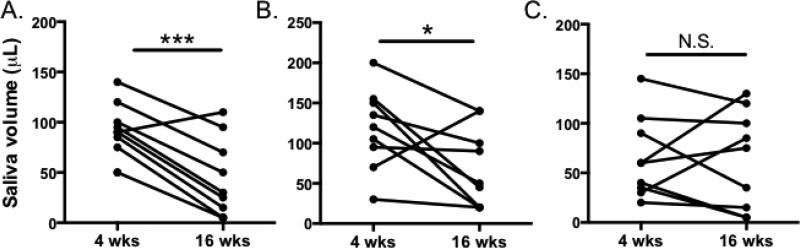
We then sought to determine whether blockade of CXCL13 and BAFFR could prevent the development of xerostomia. To this end, we measured stimulated salivary flow before treatment at 3 weeks of age, and immediately prior to euthanasia at 16 weeks of age in all treatment groups. We found that mice treated with isotype control or BAFFR-Fc had reduced salivary production as the disease progressed (p = 0.0002 and p = 0.04, respectively). However, animals that received anti-CXCL13 and BAFFR-Fc together maintained saliva production (p = 0.9) (Figure 5). These results suggest that blockade of CXCL13 and BAFFR, but not BAFFR alone, may help to prevent development of xerostomia in SS disease.
Figure 5. Blockade of CXCL13 and BAFFR-Fc protects SS mice from loss of saliva.
Saliva was collected from NOD/ShiltJ mice treated with (A) Isotype control, (B) BAFFR-Fc, or (C) anti-CXCL13/BAFFR-Fc before and after treatment. Saliva was collected for ten minutes following intraperitoneal pilocarpine injection Pre-treatment (4 wks of age) and post-treatment saliva volumes (16 wks of age) are shown. Significance was determined using paired t-tests (* p < 0.05, *** p < 0.001, N.S. = non significant).
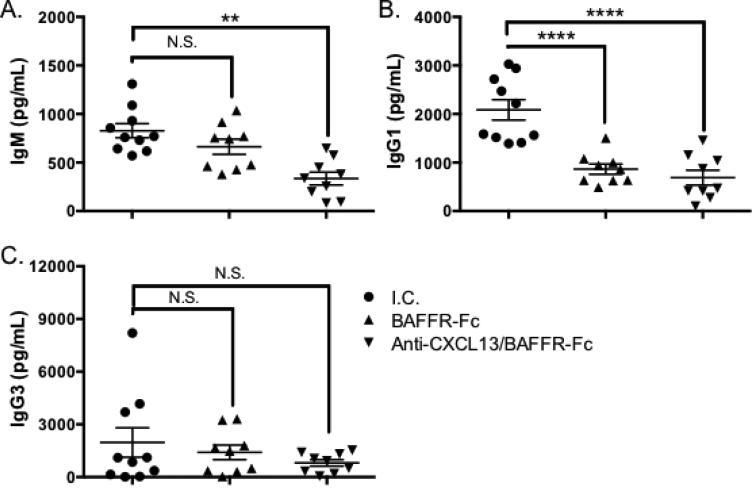
3.5. Serum antibodies are reduced in animals receiving BAFFR-Fc alone or anti-CXCL13 and BAFFR-Fc in combination
Patients and mice with SS have elevated antibody levels [44, 45]. We examined serum antibody isotypes associated with autoimmune disease in the three treatment groups. IgM levels were equivalent in the isotype control and BAFFR-Fc treatment groups. However, animals that received anti-CXCL13/BAFFR-Fc therapy had reduced serum IgM as compared to the isotype control group (p = 0.003) and those receiving BAFFR-Fc treatment (p = 0.008) (Figure 6A). IgG1 levels were decreased in mice treated with BAFFR-Fc (p < 0.0001) and anti-CXCL13/BAFFR-Fc in combination as compared to the isotype control group (p < 0.0001), although addition of anti-CXCL13 antibody did not result in any further decrease in IgG1 seen in the BAFFR-Fc treated animals. (Figure 6B). Finally, there were no differences in IgG3 levels among the three treatment groups (Figure 6C).
Figure 6. Serum Ig levels are decreased in BAFFR-Fc and anti-CXCL13/BAFFR-Fc treated animals.
Sera were harvested and (A) IgM, (B) IgG1 and (C) IgG3 levels were assessed by ELISA. Significance was determined using Mann-Whitney test. Mean and SEM are shown (**, p < 0.01, ***, p < 0.001, ****, p < 0.0001).
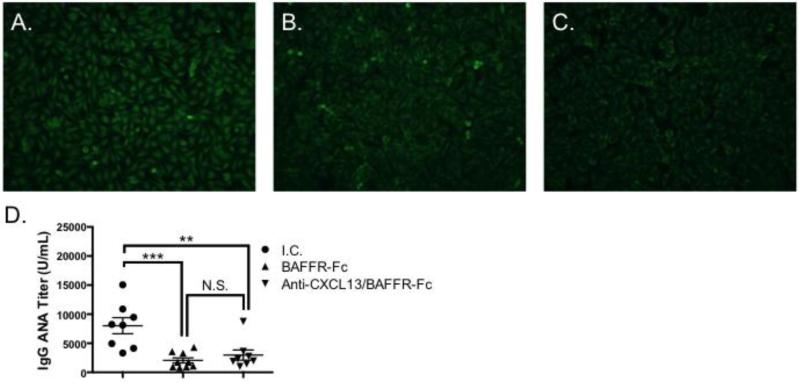
3.6. IgG autoantibodies are diminished in animals treated with BAFFR-Fc
Similar to SS patients, NOD animals develop anti-nuclear autoantibodies (ANA) with disease progression [5, 43]. We used two approaches to determine whether ANA levels were decreased in animals treated with BAFFR-Fc alone or anti-CXCL13 and BAFFR-Fc in combination. First, we performed indirect immunofluorescence on Hep-2 cells with sera from the three treatment groups. We found ANA titers were reduced in animals receiving either BAFFR-Fc or anti-CXCL13/BAFFR-Fc (Figure 7B and C) as compared to isotype control treated animals (Figure 7A). To verify these findings, we performed anti-ANA ELISAs. Mice given BAFFR-Fc or anti-CXCL13/BAFFR-Fc treatment had reduced serum IgG ANA titers as compared to those receiving the isotype control (p = 0.0006 and 0.005, respectively). Of note, there was no difference in ANA levels between animals treated with BAFFR-Fc and those given anti-CXCL13/BAFFR-Fc. (Figure 7D). Therefore, blockade of BAFFR-Fc reduces IgG autoantibodies that are associated with SS pathology.
Figure 7. Serum IgG ANA levels are decreased following treatment with BAFFR-Fc and anti-CXCL13/BAFFR-Fc.
Hep2 staining was performed using serum from (A) I.C. (n = 4) (B) BAFFR-Fc (n = 4) and (C) Anti-CXCL13/BAFFR-Fc (n = 4) treated mice. Representative staining from each treatment group is shown. Original magnification is 200X. (D) ELISAs were performed to quantify ANA titers in sera from I.C. (n = 10), BAFFR-Fc (n = 9), and anti-CXCL13/BAFF (n = 9) treated animals. I.C. = isotype control. Significance was determined using Mann-Whitney test. Mean and SEM are shown (**, p < 0.01, ***, p < 0.001).
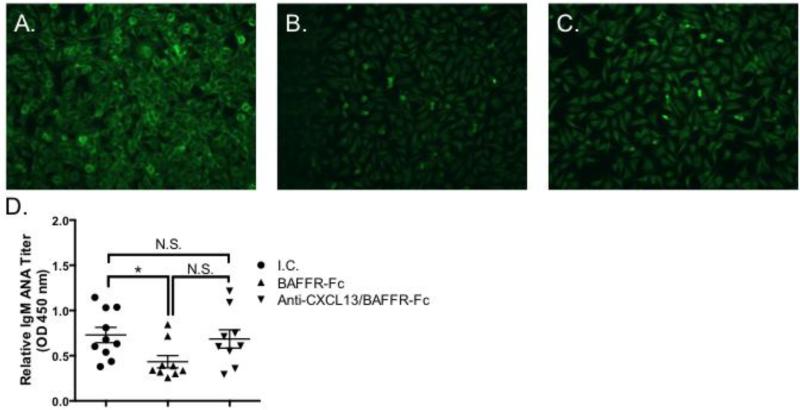
3.7 IgM autoantibody levels are similar in animals treated with isotype control and anti-CXCL13/BAFFR-Fc, but decreased in BAFFR-Fc treated mice
To determine whether IgM ANA titers differ between the three treatment groups, we first performed Hep-2 immunofluorescent staining on sera from isotype control, BAFFR-Fc, and anti-CXCL13/BAFFR-Fc treated animals. We found that BAFFF-Fc treated mice had less IgM ANA titers as compared to the isotype control and anti-CXCL13/BAFF-Fc treatment groups (Figure 8A-C). We confirmed these results with an ANA ELISA. We found animals given BAFFR-Fc had significantly lower IgM ANA levels as compared to the isotype control group (p = 0.01). However, there was no difference between isotype control treated animals and those that received anti-CXCL13/BAFFR-Fc treatment (p = 0.76) (Figure 8D). All together, these data show IgM ANA titers are reduced in BAFFR-Fc treated animals, but not in those treated with concomitant CXCL13/BAFFR blockade.
Figure 8. Serum IgM ANA levels are decreased in BAFFR-Fc treated mice, but are similar in animals treated with isotype control and anti-CXCL13/BAFFR-Fc.
Hep2 staining was performed using serum from (A) I.C. (n = 4) (B) BAFFR-Fc (n = 4) and (C) Anti-CXCL13/BAFFR-Fc (n = 4) treated mice. Representative staining from each treatment group is shown. Original magnification is 200X. (D) ELISAs were performed to quantify ANA titers in sera from I.C. (n = 10), BAFFR-Fc (n = 9), and anti-CXCL13/BAFF (n = 9) treated animals. I.C. = isotype control. Significance was determined using Mann-Whitney test. Mean and SEM are shown (*, p < 0.05, N.S = not significant).
4. Discussion
Previous work in our laboratory demonstrated CXCL13 increases with disease both locally and systemically in the NOD model, and CXCL13 is also increased in serum and saliva from SS patients. In NOD mice, neutralization of CXCL13 reduces glandular inflammation, but only to a modest extent [30]. Therefore, we sought to identify a therapeutic approach that would improve the efficacy of anti-CXCL13 treatment alone. Studies of human B cells demonstrate chemotactic synergy between CXCL13 and BAFF, particularly in the memory B cell compartment [38]. Since both CXCL13 and BAFF are elevated in SS disease, we hypothesized that blockade of these together would reduce SS disease manifestations. We found that treatment of SS mice with BAFFR-Fc diminished FLS, reduced serum IgG1 titers and ANA specific IgG and IgM titers. Concomitant treatment with anti-CXCL13 and BAFFR-Fc also reduced IgM levels and prevented loss of salivary flow. Thus, neutralization of CXCL13 and BAFFR may mitigate disease manifestations in SS patients more effectively than either treatment alone.
Murine and human studies implicate CXCL13 and BAFF in SS. Importantly, studies in humans show both BAFF and CXCL13 are increased in human salivary glands in SS [23, 26, 30-35, 46, 47]. BAFF transgenic mice develop sialadenitis and lose salivary flow [14, 25]. CXCL13 is also implicated in exocrine gland inflammation in SS. Specifically, NOD mice treated with anti-lymphotoxin beta receptor antibodies have reduced salivary and lacrimal gland inflammation [36, 37]. CXCL13 is regulated by lymphotoxin, and was reduced in both salivary and lacrimal glands in these models. Thus, both BAFF and CXCL13 are implicated in exocrine gland inflammation in SS models.
The relationship between salivary gland inflammation and hypofunction is poorly understood. Several studies in mice and humans with SS demonstrate salivary gland inflammation does not necessarily indicate loss of function. One of most comprehensive studies in SS patients to date shows that glandular inflammation only correlates weakly with salivary production [3]. Accordingly, a study examining blockade of TACI (a receptor for BAFF and the related cytokine APRIL) in NOD mice shows these animals are protected from sialadenitis but not loss of salivary function [48]. We saw similar results in the current study, as animals treated with BAFFR-Fc had reduced salivary gland inflammation but were not protected from salivary dysfunction (Figures 3 and 5). Importantly, NOD mice that received both anti-CXCL13 and BAFFR-Fc were protected from both salivary gland inflammation and loss of salivation. Therefore, addition of CXCL13 neutralization provided a protective effect beyond that afforded by treatment with BAFFR-Fc alone.
B cells are the primary cell type affected by CXCL13 and BAFFR blockade. B cells are emerging as important therapeutic targets in several types of autoimmunity, as they promote disease through interactions with T cells, secrete pro-inflammatory cytokines and autoantibodies, and migrate to diseased tissue where they may drive or exacerbate inflammation. Currently, the cause of SS is poorly understood. An intriguing etiologic agent is the role of the altered microbiome and mucosal breach. Several studies demonstrated that alterations in bacterial genera can skew Th subset development [49-52], and this likely has important consequences for B cell activation as well. A recent study showed that peptides from commensal organisms mimic the amino acid sequence and structure of an SS autoantigen (Ro60), and some of these peptides activate Ro60 specific T cells [39]. Therefore, it is possible that microbial dysbiosis could contribute to SS initiation and progression, and further studies are necessary to define the role of the microbiome in SS more precisely.
Regardless of the disease etiology, B cells are clearly dysregulated in SS disease. Both CXCR5 and BAFFR are expressed by mature B cells [29, 53, 54]. B cells expressing CXCR5 and BAFFR are detected separately in salivary tissue [12, 23]. Mature B cells rely on signals transduced by BAFFR for survival, and CXCL13 is key for organization of lymphoid follicles [55]. The added efficacy of CXCL13 and BAFFR blockade as compared to BAFFR blockade alone may result from the fact that the remaining B cells exhibit disrupted organization that mitigates their pathogenic capacity. In addition, the effects could be due to decreased migration of memory B cells [38]. However, since peripheral and salivary B cells are significantly reduced in both the BAFFR-Fc and anti-CXCL13/BAFFR-Fc treatment groups (Figures 2 - 4), the differences observed could also be attributed to T cell effects, although further work is necessary to determine this conclusively.
Since B cells are affected by blockade of CXCL13 and BAFFR, this treatment may mediate its protective effect against salivary gland dysfunction by reduction of pathogenic autoantibodies. Both IgG1 and IgG3 are elevated in SS and SLE patients, and reactivity for the SS autoantigen La is enriched in both subclasses [56]. In a separate study, anti-Ro autoantibodies derived from SS patients are largely restricted to IgG1 [57]. Studies in mice also support the importance of IgG1 antibodies in SS disease, as IgG1 antibodies are detected in salivary gland lysates of SS models [58]. Moreover, IgG1 secreting B cells from the cLNs of SS mice have higher reactivity against salivary gland antigens as compared to control cLN cells [58].
While the significance of most autoantibodies in SS is limited [9], several studies show SS patients have autoantibodies directed against the muscarinic 3 acetylcholine receptor (M3R), which are thought to reduce salivary flow by inhibiting parasympathetic innervation of the salivary tissue [59-62]. IgG anti-M3R autoantibodies are documented in SS patient sera and saliva, and serum IgG derived from SS patients inhibits salivary flow in Igμnull mice [59, 63, 64]. Finally, recent work in a SS-susceptible murine model (NZM2758) shows serum anti-Ro52 antibody levels correlate with salivary gland dysfunction, suggesting autoantibodies against Ro52 could also mediate salivary hypofunction in SS [65]. In the current study, treatment with BAFFR-Fc or anti-CXCL13/BAFFR-Fc resulted in lower titers of IgG1. However, only animals treated with anti-CXCL13/BAFFR-Fc maintained salivary function, so total IgG1 levels alone do not account for the differences observed between BAFFR-Fc and anti-CXCL13/BAFFR-Fc treated animals. Although both groups had low IgG1 titers, it is possible that IgG1 antibody specificities differ between the BAFFR-Fc and anti-CXCL13/BAFFR-Fc treatment groups.
Alternatively, differences in IgM levels between these two treatment groups may account for the differences in salivary function observed. While the contribution of IgM autoantibodies is not well studied in SS, IgM+ B cells are clearly dysregulated in this disease. Two lymphoma studies in SS patients identify clonal expansions of IgM marginal zone B cells with specificity for rheumatoid factor [66, 67]. Moreover, CD27+ memory B cells express more mutated Cμ transcripts than corresponding γ and α chains in pSS patients [68]. Thus, while previous studies have focused on the pathogenic role of IgG autoantibodies in SS [59], it remains to be determined whether IgM autoantibodies also impair salivary flow in SS. In the present study, we tested whether ANA-specific IgM was reduced in anti-CXCL13/BAFFR-Fc treated animals, since this treatment group maintained salivary flow. However, while BAFFR-Fc treated animals had lower IgM ANA titers, levels were similar between the isotype control and the anti-CXCL13/BAFFR-Fc treatment groups (Figure 8). These results are not surprising, given that studies in pSS patients have failed to show an association between ANA levels and salivary hypofunction, although high ANA titers are associated with salivary gland inflammation [44]. Therefore, while IgM autoantibodies could contribute to the loss of salivary flow observed in SS, ANA specific IgM titers do not correlate with salivary production in our treatment groups.
Importantly, we show reduction of ANA specific IgG in mice treated with BAFFR-Fc or anti-CXCL13/BAFFR-Fc, and IgM ANA is reduced in mice given BAFFR-Fc therapy. The new diagnostic criteria for SS have 3 parameters: serum autoantibodies, focal lymphocytic sialadenitis, and an ocular staining score of ≥ 3 [5]. In order for the patient to receive a diagnosis of SS, 2 of 3 of these must be positive. Patients with ANA titers of ≥ 1:320 and RF autoantibodies fulfill the serology component of the criteria. Approximately 64% (567/886) of pSS patients have elevated ANA titers (≥ 1:320) [44]. While high ANA titers are indicative of SS, the significance of these in disease progression is not well understood. Data from the SICCA study show patients with symptoms for greater than 10 years are more likely to have a positive ANA titer. In addition, higher salivary gland focus score values are associated with ANA titers ≥ 1:320 [44]. Of note, elevated ANA titers are detected in pSS patients even prior to onset of symptoms [69]. Thus, high ANA titers are associated with salivary disease manifestations, but it is unclear at present whether these autoantibodies contribute to disease pathogenesis directly, or whether they serve as more general indicators of B cell hyperactivity.
While this study showed treatment with BAFFR-Fc and CXCL13 is effective in reducing disease manifestations in a well-established SS model, it is important to note that animals were treated prior to disease development. Further studies are warranted in mice with early and advanced clinical disease to determine whether this approach is efficacious following the onset of SS. Such studies are needed to establish the therapeutic relevance of CXCL13 and BAFF blockade in SS patients.
5. Conclusion
In conclusion, neutralization of BAFFR reduces disease severity in NOD SS mice, and co-administration of anti-CXCL13 conferred additional therapeutic benefit. This study provides a rationale to perform therapeutic studies to reverse SS in the NOD mouse model, which may then become relevant for patient studies.
Supplementary Material
Highlights.
Blockade of BAFF receptor (BAFFR) signaling prevents murine Sjögren's syndrome (SS)
Treatment with anti-CXCL13 and BAFFR-Fc protects SS mice from salivary hypofunction
BAFFR-Fc alone and in combination with anti-CXCL13 may be effective in SS treatment
Acknowledgements
The authors gratefully acknowledge Ms. Natalie Bitar and Dr. Xuemei Zhong (Hematology and Oncology Section, Department of Medicine Core Facilities, Boston University Medical Center, MA) for assistance with immunohistochemical staining. We also thank Yana Moses (Dept. of Pathology and Laboratory Medicine, Long Island Jewish Medical Center) for tissue preparation and H&E staining. The authors thank Dr. Amarpreet Sabharwal for facilitating collaboration. This work was supported by a Mentored Clinical Scientist Research Career Development Award (K08 DE020882) from the NIDCR and start-up funds from the SUNY at Buffalo School of Dental Medicine awarded to JMK, and R01 AI029690 from NIAID awarded to TLR. The content is solely the responsibility of the authors and does not represent the official views of the NIDCR, NIAID, or the NIH.
Abbreviations
- ANA
Anti-nuclear autoantibodies
- BAFF
B-cell activating factor of the tumor necrosis factor family
- BAFFR
BAFF receptor
- cLN
Cervical lymph node
- FLS
Focal lymphocytic sialadenitis
- M3R
Muscarinic 3 acetylcholine receptor
- RF
Rheumatoid factor
- SMG
Submandibular gland
- SS
Sjögren's syndrome
- TACI
Transmembrane activator and CAML interactor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Low HZ, Witte T. Aspects of innate immunity in Sjogren's syndrome. Arthritis research & therapy. 2011;13:218. doi: 10.1186/ar3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavragani CP, Moutsopoulos HM. Sjogren's syndrome. Annual review of pathology. 2014;9:273–285. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 3.Daniels TE, Cox D, Shiboski CH, Schiødt M, Wu A, Lanfranchi H, Umehara H, Zhao Y, Challacombe S, Lam MY, De Souza Y, Schiødt J, Holm H, Bisio PA, Gandolfo MS, Sawaki T, Li M, Zhang W, Varghese-Jacob B, Ibsen P, Keszler A, Kurose N, Nojima T, Odell E, Criswell LA, Jordan R, Greenspan JS, S.s.I.C.C.A.R. Groups Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren's syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63:2021–2030. doi: 10.1002/art.30381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH, E.S.G.o.C.C.f.S.s. Syndrome Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiødt M, Umehara H, Vivino F, Zhao Y, Dong Y, Greenspan D, Heidenreich AM, Helin P, Kirkham B, Kitagawa K, Larkin G, Li M, Lietman T, Lindegaard J, McNamara N, Sack K, Shirlaw P, Sugai S, Vollenweider C, Whitcher J, Wu A, Zhang S, Zhang W, Greenspan J, Daniels T, S.s.I.C.C.A.S.R. Groups American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun. 2010;34:400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Tengnér P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjögren's syndrome. Arthritis Rheum. 1998;41:2238–2248. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Halse A, Harley JB, Kroneld U, Jonsson R. Ro/SS-A-reactive B lymphocytes in salivary glands and peripheral blood of patients with Sjogren's syndrome. Clin Exp Immunol. 1999;115:203–207. doi: 10.1046/j.1365-2249.1999.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen A, Lipsky PE, Dörner T. B cells in Sjögren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res Ther. 2007;9:218. doi: 10.1186/ar2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J, Burmester GR, Lipsky PE, Dorner T. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 2002;46:2160–2171. doi: 10.1002/art.10445. [DOI] [PubMed] [Google Scholar]
- 11.Roberts ME, Kaminski D, Jenks SA, Maguire C, Ching K, Burbelo PD, Iadarola MJ, Rosenberg A, Coca A, Anolik J, Sanz I. Primary Sjogren's syndrome is characterized by distinct phenotypic and transcriptional profiles of IgD+ unswitched memory B cells. Arthritis & rheumatology. 2014;66:2558–2569. doi: 10.1002/art.38734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen A, Reiter K, Ziprian T, Jacobi A, Hoffmann A, Gosemann M, Scholze J, Lipsky PE, Dorner T. Dysregulation of chemokine receptor expression and function by B cells of patients with primary Sjogren's syndrome. Arthritis Rheum. 2005;52:2109–2119. doi: 10.1002/art.21129. [DOI] [PubMed] [Google Scholar]
- 13.Youinou P, Devauchelle V, Hutin P, Le Berre R, Saraux A, Pers JO. A conspicuous role for B cells In Sjogren's syndrome. Clin Rev Allergy Immunol. 2007;32:231–237. doi: 10.1007/s12016-007-8000-y. [DOI] [PubMed] [Google Scholar]
- 14.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol. 2011;73:1–7. doi: 10.1111/j.1365-3083.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- 16.Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009;158:155–163. doi: 10.1111/j.1365-2249.2009.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto S, Nakano S, Watanabe T, Tamayama Y, Mitsuo A, Nakiri Y, Suzuki J, Nozawa K, Amano H, Tokano Y, Kobata T, Takasaki Y. Expression of B-cell activating factor of the tumour necrosis factor family (BAFF) in T cells in active systemic lupus erythematosus: the role of BAFF in T cell-dependent B cell pathogenic autoantibody production. Rheumatology (Oxford) 2007;46:1083–1086. doi: 10.1093/rheumatology/kem097. [DOI] [PubMed] [Google Scholar]
- 18.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J, Sutton IJ, Xin C, Tangye SG, Kalled SL, Mackay F, Mackay CR. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. Journal of immunology. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 19.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 20.Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 22.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Daridon C, Devauchelle V, Hutin P, Le Berre R, Martins-Carvalho C, Bendaoud B, Dueymes M, Saraux A, Youinou P, Pers JO. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 2007;56:1134–1144. doi: 10.1002/art.22458. [DOI] [PubMed] [Google Scholar]
- 24.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szodoray P, Alex P, Jonsson MV, Knowlton N, Dozmorov I, Nakken B, Delaleu N, Jonsson R, Centola M. Distinct profiles of Sjögren's syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin Immunol. 2005;117:168–176. doi: 10.1016/j.clim.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Killedar SJ, Eckenrode SE, McIndoe RA, She JX, Nguyen CQ, Peck AB, Cha S. Early pathogenic events associated with Sjogren's syndrome (SjS)-like disease of the NOD mouse using microarray analysis. Laboratory investigation; a journal of technical methods and pathology. 2006;86:1243–1260. doi: 10.1038/labinvest.3700487. [DOI] [PubMed] [Google Scholar]
- 28.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413–419. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Förster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 30.Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjogren's syndrome in mice and humans and is implicated in disease pathogenesis. Journal of leukocyte biology. 2013;94:1079–1089. doi: 10.1189/jlb.0113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, Valesini G, Pitzalis C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren's syndrome. Arthritis Rheum. 2005;52:1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 32.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 33.Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, Carsetti R, Spencer J, Valesini G, Pitzalis C. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. Journal of immunology. 2008;180:5130–5140. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 34.Amft N, Curnow SJ, Scheel-Toellner D, Devadas A, Oates J, Crocker J, Hamburger J, Ainsworth J, Mathews J, Salmon M, Bowman SJ, Buckley CD. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren's syndrome. Arthritis Rheum. 2001;44:2633–2641. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 36.Fava RA, Kennedy SM, Wood SG, Bolstad AI, Bienkowska J, Papandile A, Mavragani CP, Gatumu M, Skarstein K, Kelly JA, Browning JL. Lymphotoxin-beta Receptor blockade reduces CXCL13 in Lacrimal Glands and improves Corneal integrity in the NOD Model of Sjogren's Syndrome. Arthritis Res Ther. 2011;13:R182. doi: 10.1186/ar3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad AI. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjögren's syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther. 2009;11:R24. doi: 10.1186/ar2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badr G, Borhis G, Lefevre EA, Chaoul N, Deshayes F, Dessirier V, Lapree G, Tsapis A, Richard Y. BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood. 2008;111:2744–2754. doi: 10.1182/blood-2007-03-081232. [DOI] [PubMed] [Google Scholar]
- 39.Digman MA, Caiolfa VR, Zamai M, Gratton E. The phasor approach to fluorescence lifetime imaging analysis. Biophysical journal. 2008;94:L14–16. doi: 10.1529/biophysj.107.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fereidouni F, Esposito A, Blab GA, Gerritsen HC. A modified phasor approach for analyzing time-gated fluorescence lifetime images. Journal of microscopy. 2011;244:248–258. doi: 10.1111/j.1365-2818.2011.03533.x. [DOI] [PubMed] [Google Scholar]
- 41.Fereidouni F, Bader AN, Gerritsen HC. Spectral phasor analysis allows rapid and reliable unmixing of fluorescence microscopy spectral images. Optics express. 2012;20:12729–12741. doi: 10.1364/OE.20.012729. [DOI] [PubMed] [Google Scholar]
- 42.Fereidouni F, Bader AN, Colonna A, Gerritsen HC. Phasor analysis of multiphoton spectral images distinguishes autofluorescence components of in vivo human skin. Journal of biophotonics. 2014;7:589–596. doi: 10.1002/jbio.201200244. [DOI] [PubMed] [Google Scholar]
- 43.Cha S, Peck AB, Humphreys-Beher MG. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: an update. Crit Rev Oral Biol Med. 2002;13:5–16. doi: 10.1177/154411130201300103. [DOI] [PubMed] [Google Scholar]
- 44.Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, Banushree R, Dong Y, Helin P, Kirkham BW, Li M, Sugai S, Umehara H, Vivino FB, Vollenweider CF, Zhang W, Zhao Y, Greenspan JS, Daniels TE, Criswell LA. Primary Sjogren's syndrome as a systemic disease: a study of participants enrolled in an international Sjogren's syndrome registry. Arthritis Care Res (Hoboken) 2012;64:911–918. doi: 10.1002/acr.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi G, Hua H, Gao Y, Lin Q, Yu GY. Sialoadenitis progression in nonobese diabetic mice and its correlation with expression of apoptosis-associated proteins in salivary glands and serum IgG levels. Chin Med J (Engl) 2007;120:1426–1431. [PubMed] [Google Scholar]
- 46.Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, Sellam J, Lepajolec C, Mariette X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salomonsson S, Larsson P, Tengnér P, Mellquist E, Hjelmström P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 48.Vosters JL, Roescher N, Illei GG, Chiorini JA, Tak PP. TACI-Fc gene therapy improves autoimmune sialadenitis but not salivary gland function in non-obese diabetic mice. Oral Dis. 2012;18:365–374. doi: 10.1111/j.1601-0825.2011.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Friedrich C, Hagemann SC, Korte WH, Goharani N, Cording S, Eberl G, Sparwasser T, Lochner M. Regulatory T cells promote a protective Th17-associated immune response to intestinal bacterial infection with C. rodentium. Mucosal immunology. 2014 doi: 10.1038/mi.2014.17. [DOI] [PubMed] [Google Scholar]
- 53.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. Journal of immunology. 2007;179:7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 54.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 56.Rubin RL, Tang FL, Chan EK, Pollard KM, Tsay G, Tan EM. IgG subclasses of autoantibodies in systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. Journal of immunology. 1986;137:2528–2534. [PubMed] [Google Scholar]
- 57.Maran R, Dueymes M, Pennec YL, Casburn-Budd R, Shoenfeld Y, Youinou P. Predominance of IgG1 subclass of anti-Ro/SSA, but not anti-La/SSB antibodies in primary Sjogren's syndrome. J Autoimmun. 1993;6:379–387. doi: 10.1006/jaut.1993.1032. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen CQ, Ogunniyi AO, Karabiyik A, Love JC. Single-cell analysis reveals isotype-specific autoreactive B cell repertoires in Sjögren's syndrome. PLoS One. 2013;8:e58127. doi: 10.1371/journal.pone.0058127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, Peen E, Jonsson R, Humphreys-Beher MG. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren's syndrome. Proc Natl Acad Sci U S A. 1998;95:7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dawson LJ, Stanbury J, Venn N, Hasdimir B, Rogers SN, Smith PM. Antimuscarinic antibodies in primary Sjogren's syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum. 2006;54:1165–1173. doi: 10.1002/art.21764. [DOI] [PubMed] [Google Scholar]
- 61.Jin M, Hwang SM, Koo NY, Kim B, Kho HS, Choi SY, Song YW, Park K. Autoantibodies in Sjogren's syndrome patients acutely inhibit muscarinic receptor function. Oral Dis. 2012;18:132–139. doi: 10.1111/j.1601-0825.2011.01853.x. [DOI] [PubMed] [Google Scholar]
- 62.Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjogren's syndrome. Arthritis Rheum. 2000;43:1647–1654. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 63.He J, Qiang L, Ding Y, Wei P, Li YN, Hua H, Li ZG. The role of muscarinic acetylcholine receptor type 3 polypeptide (M3RP205-220) antibody in the saliva of patients with primary Sjogren's syndrome. Clin Exp Rheumatol. 2012;30:322–326. [PubMed] [Google Scholar]
- 64.Tsuboi H, Matsumoto I, Wakamatsu E, Nakamura Y, Iizuka M, Hayashi T, Goto D, Ito S, Sumida T. New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjogren's syndrome. Clin Exp Immunol. 2010;162:53–61. doi: 10.1111/j.1365-2249.2010.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szczerba BM, Kaplonek P, Wolska N, Podsiadlowska A, Rybakowska PD, Dey P, Rasmussen A, Grundahl K, Hefner KS, Stone DU, Young S, Lewis DM, Radfar L, Scofield RH, Sivils KL, Bagavant H, Deshmukh US. Interaction between innate immunity and Ro52-induced antibody causes Sjogren's syndrome-like disorder in mice. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du M, Diss TC, Xu C, Peng H, Isaacson PG, Pan L. Ongoing mutation in MALT lymphoma immunoglobulin gene suggests that antigen stimulation plays a role in the clonal expansion. Leukemia. 1996;10:1190–1197. [PubMed] [Google Scholar]
- 67.Martin T, Weber JC, Levallois H, Labouret N, Soley A, Koenig S, Korganow AS, Pasquali JL. Salivary gland lymphomas in patients with Sjögren's syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 2000;43:908–916. doi: 10.1002/1529-0131(200004)43:4<908::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 68.Hansen A, Gosemann M, Pruss A, Reiter K, Ruzickova S, Lipsky PE, Dörner T. Abnormalities in peripheral B cell memory of patients with primary Sjögren's syndrome. Arthritis Rheum. 2004;50:1897–1908. doi: 10.1002/art.20276. [DOI] [PubMed] [Google Scholar]
- 69.Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA. 2013;310:1854–1855. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.