Abstract
There is increasing evidence that the brain actively constructs action and perception using past experience. In this paper, we propose that the direction of information flow along gradients of laminar differentiation provides important insight on the role of limbic cortices in cortical processing. Cortical limbic areas, with a simple laminar structure (e.g., no or rudimentary layer IV), send “feedback” projections to lower level better-laminated areas. We hypothesize that this “feedback” functions as predictions that drive processing throughout the cerebral cortex. This hypothesis has the potential to provide a unifying framework for an increasing number of proposals that use predictive coding to explain a myriad of neural processes and disorders, and has important implications for hypotheses about consciousness.
Keywords: predictive coding, active inference, cortical processing, structural model, limbic cortices, consciousness, intrinsic networks
A general organizational framework for predictive coding in the cerebral cortex
Research and theory are converging on the idea that the brain actively constructs how we experience and act on the world. According to the principles of active inference and predictive coding, the brain functions as a hierarchical generative model of the world that follows the principles of Bayesian probability to explain sensory input based on past experience [1–3] (for an early proposal, see [4]). Signals based on this generative model, called “predictions”, are sent from higher areas in the processing hierarchy to lower areas; this corresponds to “feedback” or descending projections [5–9]. Predictions modulate the firing of sensory neurons in advance of sensory signals arriving from peripheral receptors and are compared with incoming sensory input. The difference between predictions and sensory input (called “prediction error”) is sent back up the hierarchy; this corresponds to “feedforward” or ascending projections. The reliability of the prediction error signal is also taken into account so that the impact of prediction error in updating the model is not fixed but weighted based on its reliability (or inverse of its variance, called “precision”) (see [2] for a review). Together, perceptions and actions are thought to derive from the brain's best guess about the causes of sensory events, with incoming sensory input keeping those guesses in check. In a recent paper [10], we considered the notion of systematic variation of laminar structure of the cortex and integrated a structural theory of corticocortical connections ([11,12]; see [13] for a recent review) with the principles of predictive coding to propose an interoceptive system in the brain. In this paper, we extend this logic to the entire cerebral cortex. This redefines the role of cortical limbic areas in cortical processing.
Implementing predictive coding principles within the structural model of corticocortical connections reveals that the direction of predictions and prediction errors between two cortical areas is determined by the laminar structure of those areas, such that predictions flow from less to more laminated cortices and prediction errors flow in opposite direction (as discussed in [10]). Cortical limbic areas (cingulate cortex, ventral anterior insula, posterior orbitofrontal cortex, parahippocampal gyrus and temporal pole) have the simplest laminar structure in the neocortex (Figure 1; Box 1). As a result, we hypothesize that they are at the top of the predictive hierarchy in all cortical systems, sending predictions, while the most laminated areas (e.g., primary sensory cortices) are at the lowest levels, receiving predictions. We further propose that thanks to (1) their anatomic location abutting every sensory system [13], (2) their position at the top of predictive hierarchies, and (3) their strong connectivity to each other [14–19] as well as to subcortical structures like the amygdala, the ventral striatum and the hypothalamus [20–27], limbic cortices create a highly connected, dynamic functional ensemble for information integration and accessibility in the brain. We then hypothesize that limbic cortices, by virtue of their structural and functional properties, contribute to creating a unified conscious experience. We further suggest that our hypotheses provide novel insights about the flow of information within intrinsic brain networks. Finally, we discuss how our approach may offer a unifying framework for the growing number of predictive coding models of neural processes and disorders.
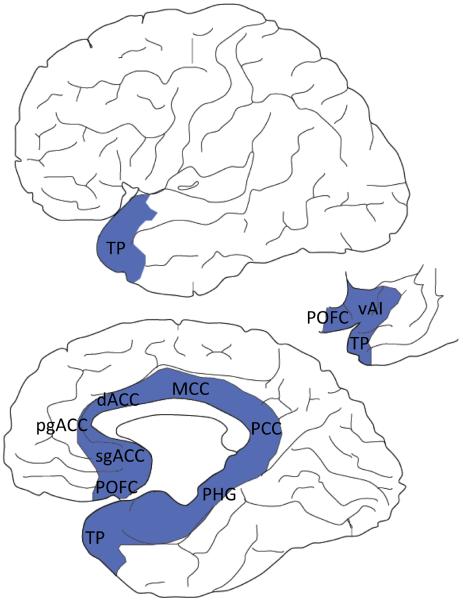
Figure 1. Limbic cortices in the human brain (modified from [117]).
Cortical limbic areas (in blue) form a ring around the corpus callosum on the medial wall of each hemisphere, continuing along the temporal cortex and the base of the brain [13]. They are neocortical areas that either lack or have a rudimentary layer IV (i.e., are agranular or dysgranular, respectively). They are located between the simpler allocortex and the better-laminated eulaminate cortex. Limbic cortices include the cingulate cortex (subgenual anterior cingulate cortex, sgACC; pregenual anterior cingulate cortex, pgACC; dorsal anterior cingulate cortex, dACC; mid cingulate cortex, MCC; posterior cingulate cortex, PCC), the ventral anterior insula (vAI), the posterior orbitofrontal cortex (POFC), the parahippocampal gyrus (PHG) and the temporal pole (TP).
Box 1. Systematic variation of laminar structure in the cerebral cortex and cortical limbic areas.
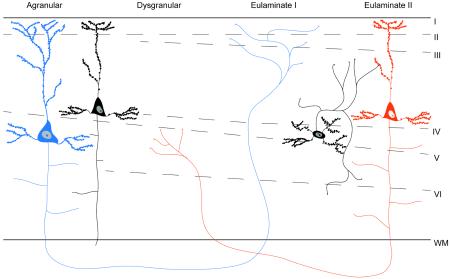
The cerebral cortex varies systematically in its degree of laminar differentiation [29,30]. Laminar differentiation increases progressively, from agranular cortices (which lack a layer IV) to dysgranular areas (with a rudimentary layer IV), then to eulaminate areas (with six layers including a well developed layer IV) and finally koniocortices (with six layers including the most developed layer IV). For the purpose of the present paper, we operationally define cortical limbic areas or limbic cortices cytoarchitecturally, rather than by location or function (following [13]). Limbic cortices are those neocortical areas that either lack a layer IV (i.e., are agranular) or have a rudimentary layer IV (i.e., are dysgranular). Limbic cortices are located between the simpler allocortex and the better-laminated eulaminate cortices [29,30]. They are also sometimes referred as periallocortex (agranular parts) and proisocortex (dysgranular parts).
Predictive coding within the laminar architecture of corticocortical connections
Predictive coding and active inference approaches to cortical processing have been implemented anatomically within the laminar architecture of the cortex. There are several models of corticocortical processing to choose from. The first papers (e.g., [6–9]) used the Felleman and Van Essen model of connections [28]. More recently, we implemented predictive coding hypotheses using the structural model of corticocortical connections [11,12] (Box 2) to propose the Embodied Predictive Interoception Coding (EPIC) model [10]. The Felleman and van Essen model identified laminar patterns for feedback and feedforward projections. The structural model went one step further to show that those patterns are predicted by the degree of laminar differentiation in the connected areas. This, together with the systematic variation in cortical structure across the cerebral cortex [29,30] has important implications for information flow. Moreover, the structural model generalizes to the entire cerebral cortex; it has successfully predicted the flow of information in frontal, temporal, parietal and occipital cortices in experiments with macaques and cats, using both experimental and computational techniques (Box 2). Other models (e.g., using the distance rule [31,32]) have proven powerful and valid for some systems (e.g., visual areas) but are known to be less suitable for predicting information flow within other systems (e.g., prefrontal areas; specifically, see Figure 6 legend in [32]).
Box 2. The structural model of corticocortical connections.
In 1997, Barbas and Rempel-Clower introduced a structural model of corticocortical connections by analyzing projection patterns within prefrontal cortices and their laminar structure in the monkey [12]. Using anterograde and retrograde tracers, they showed that there is a relationship between laminar structure in cortical columns and the distribution of projection neurons that connect those columns (for a recent review see [13]). Feedback projections originate in less differentiated cortical areas (such as agranular cortex with undifferentiated layers II and III and without a layer IV) primarily in the deep layers (layers V and VI) and terminate in superficial layers of areas with a more developed laminar structure (such as eulaminate cortices) (e.g., the blue neuron in Box 2 Figure). Feedforward projections originate in areas with higher degree of laminar differentiation (e.g. eulaminate cortices with a fully expressed layer IV) primarily in the superficial layers (II–III) and terminate in middle-deep layers (IV–VI) of areas with less-differentiated laminar architecture (for example, agranular cortex) (e.g., the red neuron in Box 2 Figure). The structural model successfully predicts the flow of information in frontal, temporal and parietal cortices in experiments with monkeys and cats (see [13] for a review) and outperforms other models of corticocortical connections [110].
A direct consequence of using the structural model to implement predictive coding is that the direction of predictions (“feedback” connections) and prediction errors (“feedforward” connections) is determined by the relative degree of laminar differentiation of the cortical areas involved [10]. Predictions originate primarily in the deep layers of cortical areas with the less laminar differentiation and terminate primarily in the superficial layers of more differentiated areas. In contrast, prediction errors originate primarily in the superficial layers of cortical areas with more laminar differentiation and terminate in the deep layers of less differentiated areas. When two areas have a comparable laminar structure, their projections originate and terminate both in superficial and deep layers (they are “lateral”). This implies that some cortical areas, such as limbic cortices (which have the least differentiated laminar structure in the entire neocortex) primarily send predictions to better-laminated cortical areas and primarily receive prediction error. By contrast, primary sensory cortices (with the most differentiated laminar structure) receive predictions from less laminated cortical areas and send prediction error. Other cortical areas (with intermediate degrees of laminar differentiation) send both predictions and prediction error depending on the relative laminar differentiation of the receiving cortices.
In the EPIC model [10], we used evidence from tract tracing studies in monkeys, as well as functional imaging evidence in humans to propose that visceromotor limbic cortices (notably the anterior and mid cingulate cortices and the ventral anterior insula) send predictions to the primary interoceptive cortex in the mid-to-posterior insula (I1), which is eulaminate in structure (extending the logic in [6–9]). Visceromotor cortical limbic areas also send predictions to subcortical structures that control the autonomic, hormonal, metabolic and immunological systems (for example, the amygdala and the hypothalamus). In this paper, we further extend our implementation of predictive coding within the structural model of corticocortical connections to hypothesize that limbic cortices are at the top of each cortical sensory system. We call this the limbic workspace model.
Limbic cortices in sensory systems
One hypothesis of our limbic workspace model is that all cortical sensory systems are structured similarly to the interoceptive system. This hypothesis builds on evidence from tract tracing studies in monkeys indicating that limbic cortices can be identified in visual (e.g., [33–36], auditory (e.g., [37–39]), and somatosensory (e.g., [36,40]) systems (also see [41,42]). The anatomical pathways in the description of the different sensory systems that follows are, as in [10], inferred in humans based on tract-tracing studies performed in monkeys, unless otherwise noted; this is similar to what has been done elsewhere [42], as inferences about the human brain are commonly made studying other species such as the macaque monkey. We acknowledge, of course, that different species have some important differences in brain structure and function.
It is well established that visual and auditory systems work via predictive coding (e.g., [5], [43–45] in humans, for a review on visual processing see [46]), and there is increasing evidence that the olfactory and gustatory sensory systems work via predictive coding as well ([47–49] in rodents, [50,51] in humans), along with a proposal that somatomotor system works similarly [6–9]. We propose that limbic cortices are at the top of each hierarchical cortical system and send predictions to better-laminated areas. Primary sensory cortices are at the bottom and send prediction error back to areas with simpler laminar structure. Evidence in support of our hypothesis can be most clearly seen in the visual, auditory and somatosensory systems (Figure 2; blue, green and red respectively), where predictions flow from cortical limbic areas (agranular and dysgranular) to multimodal association areas (e.g., lateral temporal cortex and posterior parietal cortex) (e.g., [14–17] and based on intrinsic connectivity analyses in humans [52]). These multimodal areas are eulaminate in structure (i.e. they have a well-defined layer IV) and are shared across the three systems. From there, predictions are sent to unimodal association areas (extrastriate areas for the visual system, superior temporal areas surrounding primary auditory cortex for the auditory system and the superior parietal lobule for the somatosensory system) (e.g., [36,41,53]). Unimodal association areas are eulaminate cortices with a better-developed layer IV. From these areas, predictions flow to primary sensory cortices [primary visual cortex or V1 (e.g., [33,54–56]), primary auditory cortex or A1 (e.g., [37,57]) and primary somatosensory cortex or S1 (e.g., [36,40])], which are koniocortices in structure (i.e. they contain the most well developed layer IV).
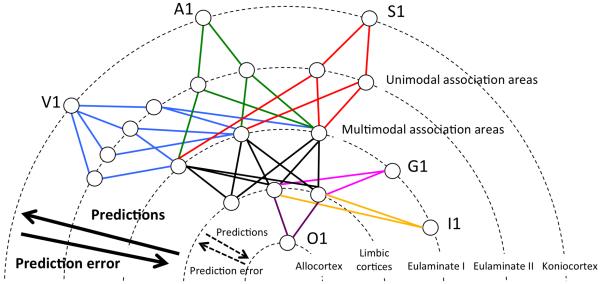
Figure 2. Schematic representation of exteroceptive and interpoceptive cortical sensory systems.
This figure is not meant to be exhaustive but representative. Each ring represents a different type of cortex, from greater (exterior circles) to less (interior circles) laminar differentiation. Primary sensory cortices (lower level of each sensory system) are indicated: A1, primary auditory cortex; G1, primary gustatory cortex; I1, primary interoceptive cortex; O1, primary olfactory cortex; S1, primary somatosensory cortex; V1, primary visual cortex. Unimodal association areas include extrastriate areas (V2, V3, V4, V5) for the visual system, superior temporal areas surrounding A1 for the auditory system and the superior parietal lobule for the somatosensory system. Multimodal association areas include the dorsolateral prefrontal cortex, lateral temporal cortex and posterior parietal cortex. Predictions flow from cortical areas with less laminar differentiation to areas with greater laminar differentiation. Prediction error flows in opposite direction. The number of cortical steps (hierarchical levels) is less in interoceptive, gustatory and olfactory systems than in exteroceptive visual, auditory and somatosensory systems.
Sensory input from the periphery (visual, auditory, and somatosensory input via the thalamus) arrives at the cortex at primary sensory cortices (V1, A1 and S1). In those areas, sensory information is represented in great detail (see e.g. the early experiments for primary visual cortex [58]) and prediction error is computed. From there, prediction error (the sensory evidence that did not match the prediction) flows through the gradients of laminar differentiation to progressively less well-laminated areas (unimodal association areas to multimodal association areas and finally to limbic cortices). Note that even though prediction and prediction errors flow hierarchically, areas within each system are not necessarily physically placed in a strictly linear fashion (for a discussion see [42]). Moreover, these systems likely influence each other at every level of the hierarchy through lateral connections.
At higher levels of the predictive hierarchy (in areas with relatively less granular differentiation), information becomes more integrated. This integration across sensory domains comes with progressive dimensionality reduction (meaning sensory detail is summarized and compressed). For example, multimodal association areas are shared across visual, auditory and somatosensory systems (e.g., [41]; see [42,59] for reviews; for evidence of a multimodal integration network in humans, see [52]).
Moreover, there are differences across systems in the amount of cortical processing. Compared to interoception (Figure 2; yellow), information from visual, auditory and somatosensory modalities is processed more extensively in the cerebral cortex. In these exteroceptive systems, predictions and prediction errors are computed across several levels of cortical processing (i.e. there are several synaptic connections between primary sensory cortices in which representations are more specialized and cortical limbic areas in which they are more integrated), whereas there are fewer steps in the interoception system. Accordingly, primary interoceptive cortices in mid and posterior insula (I1) are eulaminate in structure (i.e. they have a less developed layer IV than koniocortices of primary visual, auditory and somatosensory cortices) (see [10]). This difference in degree of laminar differentiation along which predictive signals are coded [smaller in the interoceptive system (eulaminate to limbic) vs. larger in the visual, auditory and somatosensory systems (koniocortex to limbic)] may be one reason why interoceptive perception is less differentiated and lower in dimensionality when compared to exteroceptive perception (for a description of other reasons, such as the anatomy of the ascending interoceptive circuitry, see [60]).
The gustatory system (Figure 2; pink) is structurally similar to the interoceptive system. It has few steps between limbic and primary gustatory cortex (G1) (see e.g. [14,15,17]), as G1 is eulaminate in structure (i.e. not as well laminated as koniocortices) (for a review in humans see [61]).
The olfactory system (Figure 2; purple) is structured in a way that likely reflects its ancient evolutionary origin: the primary olfactory cortex (O1) is three-layered allocortex. It abuts the anterior insula and receives olfactory input directly from the olfactory bulb without a thalamic relay (see [62] for a review in humans). Because O1 is allocortical (rather than neocortical), the neurons are not structured in columns [63,64], and therefore, strictly speaking, it is not known whether the structural model of corticocortical connections holds. Furthermore, axons leaving O1 to ipsilateral limbic cortices travel through the superficial layer I to the targeted areas [65] rather than through white matter tracts. Thus, they will reach target areas via superficial cortical layers. We can speculate, however, that predictions flow similarly from limbic cortices to O1, as odor expectations alone, even in the absence of olfactory input, are associated with activity in the main olfactory bulb ([66] in rodents; for a review of “top-down” influences on olfaction, see [49]).
Taken together, these findings are consistent with the hypothesis that predictions issued in limbic cortices involve more integrated, lower dimensional (multimodal) information, and these predictions become higher in dimensionality (as predictions issued at lower hierarchical levels within each sensory system are more specialized) until they reach primary sensory cortices, where the most specialized cortical processing occurs. As prediction error is sent from primary sensory to limbic cortices, it is compressed and summarized (for evidence consistent with this hypothesis, see [52,67–69]; for a discussion of the energy efficiency of this arrangement, see [71]). Therefore, the limbic workspace model proposes a general role of limbic cortices in cortical processing, which is compatible with more specific functions of these areas and the existence of differences across them; different cortical limbic areas may be more heavily associated with specific systems.
In a predictive coding framework, perception and action are tightly coupled, such that action can reduce prediction error (e.g., [6,7]; see also [10]). Extending this logic to the limbic workspace model, we speculate that both action and perception arise from the brain's hypotheses about the world and the body beginning as predictions in limbic cortices. Predictions are then constrained by sensory inputs, such that perceptions are largely constructions based on past experiences and their allostatic relevance, kept in check by the actual state of the world and the body, rather than the other way around.
A dynamic global workspace for conscious experience
The brain works as a generative model of the world using past experience to construct the present. We speculate that it is not an objective, accurate model, but one that is shaped by the information that the organism has encoded in its history and tailored to its allostatic needs and motivations (see also [10]). In addition to their anatomical position at the top of sensory and motor processing hierarchies, limbic cortices are strongly interconnected [14–19], and have strong bidirectional connections with subcortical structures like the amygdala, the ventral striatum and the hypothalamus [20–27]. Therefore, highly integrated neural representations in limbic cortices are easily accessible by virtually the whole brain. Interestingly, information accessibility and sharing as well as the idea of a “workspace” has been consistently described as a key feature of conscious access (e.g. [72–74]). “Global workspace” [75] theories of consciousness propose the rapid activation or “ignition” of a long-range neuronal system as the neural basis of consciousness ([72], for a review see [73]). Other theories emphasize the importance of cortico-thalamic loops (“dynamic core” theory, reviewed in [74]), or areas with dense anatomical connections known as “rich club” hubs [76] (Box 3). We contribute to these ideas by proposing that limbic cortices, thanks to their connectivity and position in hierarchical cortical information flow, are in a privileged position to contribute to the neural basis of conscious access and may provide a “workspace” for conscious experience. Representations of information in a given cortical system (e.g., visual, auditory, motor, etc.) or a combination thereof can be dynamically selected and prioritized because of their predicted relevance for the organism in a specific context [67, 70]. This implies that limbic cortices issue their predictions based primarily on the selected content. For example, as you read these lines there are many sensory details that you are not currently aware of, but you could be if those became suddenly relevant to you (e.g., the pressure of your back against the chair). As you read, these words are gaining privileged access to a workspace for consciousness, which we propose is integrated largely by cortical limbic areas. The content of specific cortical systems may be selected for its situation-specific relevance (based on priors) for the organism and sent to the workspace. From there, prioritized information can be accessed by virtually all systems in the brain, allowing a unified conscious experience. In every conscious moment, all modalities are represented, but the type of content that is prioritized may determine whether we categorize the experience as “emotion”, “perception” or “cognition”. This dynamic selection of contents in the workspace and its flexibility guarantees both differentiation and integration, which are key properties of consciousness [74], as well as overall brain function [77]: differentiation because an immense number of possible representations from each cortical system can be prioritized in the limbic workspace; integration because it provides a plausible explanation for a unified conscious experience and “stream of consciousness”.
Box 3. Functional organization of intrinsic brain networks and rich club hubs.
“Resting state functional connectivity magnetic resonance imaging” is the measurement of correlations of low frequency blood oxygen level-dependent (BOLD) signal fluctuations while a participant lays “at rest” during functional magnetic resonance imaging (i.e., is not probed with an external stimulus). Analyses reveal a number of “intrinsic” brain networks that are anatomically constrained [111–114], can be observed under light sedation [115], and account for a large proportion of the brain's metabolic budget [116]. “Rich club hubs” are the most highly connected brain areas and have been identified using diffusion tensor imaging of white matter tracts in humans [86] and reviewing tract tracing studies in monkeys [87,88]. Forty percent of the rich club hubs are contained in two of the brain's intrinsic networks [76], conventionally known as the “default mode” network [82] and the “salience” network [80]; these two networks contain most of the brain's cortical limbic circuitry, and many rich club hubs are, in fact, limbic (e.g., dorsal ACC and anterior insula). Furthermore, different intrinsic networks such as sensory networks overlap in these hubs, communicating with each other through them [76]. These findings provide a conceptual replication for the macaque tract tracing data, because they indicate that all sensory systems share cortical areas with core networks that contain limbic cortices. They suggest the intriguing hypothesis that these two networks are at the nexus of the brain's architecture for predictive coding.
Implications
Intrinsic networks and “rich club” hubs
The limbic workspace model provides insight on the relationships between different cortical areas within and across intrinsic networks (Box 3). The brain can be thought of as one large structural network showing continuous, intrinsic activity [78]. This activity has been parsed as inter-connected sub-networks that follow the white matter tracts within the brain (see [79] for a review of networks). Empirically, an intrinsic network is defined as those areas whose low frequency BOLD time courses correlate over time when a person is “at rest” (i.e. not being probed with an external stimulus). Each intrinsic network includes areas with varying degrees of laminar differentiation (including limbic cortices) such as the “salience network” [80] (which bears a strong resemblance to the “ventral attention” [81] and “multimodal” networks [52]) and the “default-mode network” [82] (sometimes called the mentalizing network [83], the construction network [84], or semantic knowledge network [85]). Within our limbic workspace model, intrinsic networks can be understood as hierarchical systems, with the flow of prediction signals within each network dictated by the structure of the cortical areas involved. In these networks, limbic cortices (e.g., the ventral anterior insula and dorsal anterior cingulate cortex for the “salience” network and the posterior cingulate cortex and sub/pregenual cingulate cortex for the “default mode” network) issue predictions to better-laminated areas in the network. This way, a single network may contain a diverse population of representations across multiple levels of cortical processing.
Similarly, our limbic workspace model provides insights into the functions of brain areas that have the strongest structural connections, known as “rich club hubs” [76,86–88], because these hubs also include areas with different degrees of laminar differentiation (Box 3). Structural and functional imaging in humans indicates that rich club hubs are “connector nodes” for intrinsic networks [76] and they have been shown to play an important role in brain communication [67,89]. Mathematical modeling indicates that when one or more rich club areas are damaged (e.g., the anterior insula or the dorsal anterior cingulate cortex, as occurs in psychopathology or chronic stress), modularity in the brain increases dramatically [90].
Integrating different functional domains and disorders
In the past several years, there has been an explosion of predictive coding approaches beyond the sensory domain, including memory [91–93], pain [94–97], emotion [10,98–100], conscious presence [101], self-recognition [102], allostasis [103], the placebo effect [104], “fear” learning [105], as well as neuropsychiatric disorders [106–108]. Each of these phenomena arises from the dynamic interaction of systems that contain cortical areas that vary in their degree of laminar differentiation. We speculate that limbic cortices, because they are at the core of the brain's architecture for prediction, serve as shared neural relevant substrate for varied phenomena whose circuitry is usually assumed to be distinct. For example, in the case of neural processing of nociception, similarly to interoception, visceromotor limbic areas (e.g. dorsal anterior cingulate) might issue predictions, while areas with higher degree of laminar differentiation like the dorsal mid to posterior insula or subcortical structures like the periaqueductal gray (PAG) will be at lower levels in the hierarchy and will send prediction error back to limbic (agranular and dysgranular) areas (for a review on connections between the PAG and limbic areas see [109]). In fact, evidence of predictions in expectance of pain in the anterior insula has been reported [94] and prediction error signals have been described in the PAG [96]. Our proposed model also suggests fruitful avenues to explore the common visceromotor predictive basis for psychiatric, metabolic, and immunologic symptom convergence in illnesses such as depression, heart disease, and cancer (see [10]).
Concluding remarks
Research and theory are converging on the idea that the brain's architecture constructs a vast repertoire of functional states as a generative model of the world. This model of the world is shaped by the organism's history and tailored to its allostatic needs and motivational goals. In this paper, we hypothesized that limbic cortices send predictions within all cortical systems, driving cortical processing across the gradients of laminar differentiation. We hypothesized that limbic cortices issue low dimensional, multimodal predictions that are specified into high-dimensional representations as they cascade to lower level cortical areas with better-laminated cytoarchitectural structure. We further speculated that cortical limbic areas, thanks to their privileged position in cortical hierarchies, their anatomical position within the brain (abutting all sensory systems), and their dense interconnectivity, are well suited to provide an integrated workspace enabling a unified experience. Ultimately, our limbic workspace model may offer a unifying anatomical and functional account to better understand the organizational principles of intrinsic networks and rich club hubs, as well as unify many healthy and pathological phenomena that have, until now, been considered as having separate circuitry (see Outstanding Questions Box).
Outstanding Questions Box.
How flexible is our generative model of the world? How easily can it be modified with new experiences?
To what degree is our generative model of the world anchored in visceromotor changes and interoception? How much do interoceptive predictions contribute to ongoing experience? Are there individual differences in this regard?
Are there structural and functional differences between cortical rich club hubs of different degree of laminar differentiation?
Are there differences in limbic predictions during the mental events that are experienced as emotions, cognitions, and perceptions?
How is a generative model of the world altered in different neuropsychiatric conditions? Are there transdisorder vulnerabilities?
Because limbic cortices function to represent integrated information across different modalities according their allostatic relevance based on past experience, this may be why scientists continue to identify limbic cortices with goals, values, or motivation. The present model of cortical processing emphasizes the importance of information integration and segregation in the brain and may help explain how the brain constructs a diverse population of representations across multiple scales of organization within a relatively constrained architecture.
Trends Box.
The brain functions as a generative model of the world that, following the principles of Bayesian probability, explains sensory input based on past experience.
The structural model of corticocortical connections allows us to hypothesize that predictions flow from less to better laminated areas and prediction errors flow in opposite direction.
Limbic cortices, with their simple laminar structure, issue predictions from the top of the hierarchy within every sensory system. The lowest levels correspond to primary sensory cortices, with a well-developed laminar structure.
Thanks to their position in cortical hierarchies and their connectivity, limbic cortices are well suited to integrate a neural “workspace” for a unified conscious experience.
This model motivates novel hypotheses about the organization of intrinsic networks and has the potential to integrate a range of neural processes and disorders.
ACKNOWLEDGEMENTS
The authors thank M. Á. García-Cabezas for helpful discussions, thoughtful feedback on earlier versions of this manuscript, and preparation of Box 2 Figure. They also thank T. Cleland for guidance on predictive coding account of olfactory and gustatory systems. This work was supported by a US National Institute on Aging grant (R01AG030311), a US National Institute of Child Health and Human Development grant (R21 HD076164), and contracts from the US Army Research Institute for the Behavioral and Social Sciences (contracts W5J9CQ12C0049 and W5J9CQ11C0046) to L.F.B., as well as a Fyssen Foundation postdoctoral fellowship to L.C. The views, opinions and findings contained in this article are those of the authors and should not be construed as an official position, policy or decision of the US National Institutes of Health or Department of the Army unless so designated by other documents.
Glossary
- Agranular cortex
Part of the neocortex that lacks a layer IV.
- Allocortex
Part of the cerebral cortex with the simplest structure (two or three layers). It comprises the primary olfactory cortex (part of the cerebral cortex which receives the projection from the olfactory bulb) and the hippocampus.
- Allostasis
Process of activating physiological systems (such as hormonal, autonomic or immune systems) with the aim of returning the body to homeostasis.
- Dysgranular cortex
Part of the neocortex with a rudimentary layer IV.
- Eulaminate cortices
Part of the neocortex with a well-developed layer IV. Eulaminate II areas have a better-developed layer IV than Eulaminate I areas. Also called granular cortex.
- Interoception
The perception and integration of autonomic, hormonal, visceral, and immunological homeostatic signals that collectively describe the physiological state of the body.
- Koniocortices
The eulaminate cortices with the most well developed layer IV.
- Limbic cortices or cortical limbic areas
Part of the neocortex with agranular or dysgranular structure. They are sometimes referred to as periallocortex (agranular) and proisocortex (dysgranular) cortex.
- Neocortex
Part of the cerebral cortex with three or more layers and columnar organization. Sometimes referred to as “isocortex”.
- Visceromotor cortices
Limbic (agranular and dysgranular) cortices that modulate the regulation of the autonomic nervous system, as well as of the hormonal and immune systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Friston K. A theory of cortical responses. Philos Trans R Soc L. B Biol Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 3.Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36:181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 4.Mumford D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol Cybern. 1992;66:241–251. doi: 10.1007/BF00198477. [DOI] [PubMed] [Google Scholar]
- 5.Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- 6.Adams RA, et al. Predictions not commands: active inference in the motor system. Brain Struct Funct. 2013;218:611–643. doi: 10.1007/s00429-012-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc L. B Biol Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastos AM, et al. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shipp S, et al. Reflections on agranular architecture: predictive coding in the motor cortex. Trends Neurosci. 2013;36:706–716. doi: 10.1016/j.tins.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015;16 doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol. 1986;252:415–422. doi: 10.1002/cne.902520310. [DOI] [PubMed] [Google Scholar]
- 12.Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- 13.Barbas H. General Cortical and Special Prefrontal Connections: Principles from Structure to Function. Annu Rev Neurosci. 2015 doi: 10.1146/annurev-neuro-071714-033936. DOI: 10.1146/annurev-neuro-071714-033936. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 15.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 16.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 17.Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- 18.Pandya DN, et al. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 19.Seltzer B, Pandya DN. Some cortical projections to the parahippocampal area in the rhesus monkey. Exp Neurol. 1976;50:146–160. doi: 10.1016/0014-4886(76)90242-9. [DOI] [PubMed] [Google Scholar]
- 20.Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- 21.Barbas H, et al. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 23.Ghashghaei HT, et al. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40:1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J. Comp. Neurol. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- 27.Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys. J. Comp. Neurol. 1993;332:175–197. doi: 10.1002/cne.903320204. [DOI] [PubMed] [Google Scholar]
- 28.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 29.Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Noback CR, Montagna W, editors. The primate brain: advances in primatology. 1970. pp. 137–208. [Google Scholar]
- 30.Zilles K, Amunts K. Architecture of the cerebral cortex. In: Mai JK, Paxinos G, editors. The human nervous system. Third Edition 2012. pp. 836–895. [Google Scholar]
- 31.Ercsey-Ravasz M, et al. A Predictive Network Model of Cerebral Cortical Connectivity Based on a Distance Rule. Neuron. 2013;80:184–197. doi: 10.1016/j.neuron.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markov NT, et al. Anatomy of hierarchy: Feedforward and feedback pathways in macaque visual cortex. J. Comp. Neurol. 2014;522:225–259. doi: 10.1002/cne.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- 34.Rockland KS, Pandya DN. Cortical connections of the occipital lobe in the rhesus monkey: interconnections between areas 17, 18, 19 and the superior temporal sulcus. Brain Res. 1981;212:249–270. doi: 10.1016/0006-8993(81)90461-3. [DOI] [PubMed] [Google Scholar]
- 35.Seltzer B, Pandya DN. Converging visual and somatic sensory cortical input to the intraparietal sulcus of the rhesus monkey. Brain Res. 1980;192:339–351. doi: 10.1016/0006-8993(80)90888-4. [DOI] [PubMed] [Google Scholar]
- 36.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 37.Seltzer B, Pandya DN. Intrinsic connections and architectonics of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;290:451–471. doi: 10.1002/cne.902900402. [DOI] [PubMed] [Google Scholar]
- 38.Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- 39.Morán MA, et al. Neural inputs into the temporopolar cortex of the rhesus monkey. J. Comp. Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- 40.Vogt BA, Pandya DN. Cortico-cortical connections of somatic sensory cortex (areas 3, 1 and 2) in the rhesus monkey. J Comp Neurol. 1978;177:179–191. doi: 10.1002/cne.901770202. [DOI] [PubMed] [Google Scholar]
- 41.Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- 42.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 43.Kok P, de Lange FP. Shape perception simultaneously up- and downregulates neural activity in the primary visual cortex. Curr Biol. 2014;24:1531–1535. doi: 10.1016/j.cub.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 44.Chennu S, et al. Expectation and attention in hierarchical auditory prediction. J Neurosci. 2013;33:11194–11205. doi: 10.1523/JNEUROSCI.0114-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wacongne C, et al. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proceedings of the National Academy of Sciences. 2011;108:20754–20759. doi: 10.1073/pnas.1117807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert CD, Li W. Top-down influences on visual processing. Nat. Rev. Neurosci. 2013;14:350–63. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner MP, Fontanini A. Encoding and tracking of outcome-specific expectancy in the gustatory cortex of alert rats. J Neurosci. 2014;34:13000–13017. doi: 10.1523/JNEUROSCI.1820-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusumoto-Yoshida I, et al. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A. 2015;112:1190–1195. doi: 10.1073/pnas.1416573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 2009;101:2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- 50.Zelano C, et al. Olfactory predictive codes and stimulus templates in piriform cortex. Neuron. 2011;72:178–187. doi: 10.1016/j.neuron.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howard JD, et al. Identity-specific coding of future rewards in the human orbitofrontal cortex. Proc Natl Acad Sci U S A. 2015;112:5195–5200. doi: 10.1073/pnas.1503550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sepulcre J, et al. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci. 2012;32:10649–10661. doi: 10.1523/JNEUROSCI.0759-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J. Comp. Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Millan L, Hollander H. Cortico-cortical projections from striate cortex of the squirrel monkey (Saimiri sciureus). A radioautographic study. Brain Res. 1975;83:405–417. doi: 10.1016/0006-8993(75)90833-1. [DOI] [PubMed] [Google Scholar]
- 55.Kaas JH, Lin CS. Cortical projections of area 18 in owl monkeys. Vis. Res. 1977;17:739–741. doi: 10.1016/s0042-6989(77)80013-8. [DOI] [PubMed] [Google Scholar]
- 56.Wong-Riley M. Reciprocal connections between striate and prestriate cortex in squirrel monkey as demonstrated by combined peroxidase histochemistry and autoradiography. Brain Res. 1978;147:159–164. doi: 10.1016/0006-8993(78)90781-3. [DOI] [PubMed] [Google Scholar]
- 57.Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 1983;221:169–184. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- 58.HUBEL DH, WIESEL TN. Receptive fields of single neurones in the cat's striate cortex. J. Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mesulam M. The evolving landscape of human cortical connectivity: facts and inferences. Neuroimage. 2012;62:2182–2189. doi: 10.1016/j.neuroimage.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013;14:143–52. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 61.Pritchard TC. Gustatory system. In: Mai JK, Paxinos G, editors. The human nervous system. 3rd ed Elsevier; 2012. pp. 1187–1218. [Google Scholar]
- 62.Van Hartevelt TJ, Kringelbach ML. The human nervous system. Elsevier; 2012. The olfactory system; pp. 1219–1238. [Google Scholar]
- 63.Johnson DM, et al. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shepherd GM. The microcircuit concept applied to cortical evolution: from three-layer to six-layer cortex. Front Neuroanat. 2011;5:30. doi: 10.3389/fnana.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmichael ST, et al. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- 66.Mandairon N, et al. Context-driven activation of odor representations in the absence of olfactory stimuli in the olfactory bulb and piriform cortex. Front Behav Neurosci. 2014;8:138. doi: 10.3389/fnbeh.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braga RM, et al. Echoes of the brain within default mode, association, and heteromodal cortices. J Neurosci. 2013;33:14031–14039. doi: 10.1523/JNEUROSCI.0570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandino L, et al. Concept representation reflects multimodal abstraction: A framework for embodied semantics. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv020. DOI: 10.1093/cercor/bhv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finlay BL, Uchiyama R. Developmental mechanisms channeling cortical evolution. Trends Neurosci. 2015;38:69–76. doi: 10.1016/j.tins.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Sepulcre J. Functional streams and cortical integration in the human brain. Neuroscientist. 2014;20:499–508. doi: 10.1177/1073858414531657. [DOI] [PubMed] [Google Scholar]
- 71.Sterling P, Laughlin S. Principles of neural design. MIT Press; 2015. [Google Scholar]
- 72.Dehaene S, et al. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci U S A. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Tononi G, Edelman GM. Consciousness and complexity. Science (80-.) 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- 75.Baars BJ. A cognitive theory of consciousness. Cambridge University Press; 1989. [Google Scholar]
- 76.Van den Heuvel MP, Sporns O. An anatomical substrate for integration among functional networks in human cortex. J Neurosci. 2013;33:14489–14500. doi: 10.1523/JNEUROSCI.2128-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tognoli E, Kelso JA. The metastable brain. Neuron. 2014;81:35–48. doi: 10.1016/j.neuron.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sporns O. Networks of the brain. Massachusetts Institute of Technology; 2011. [Google Scholar]
- 79.Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol. 2013;23:361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corbetta M, et al. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buckner RL, et al. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 83.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 84.Hassabis D, Maguire EA. The construction system of the brain. Philos Trans R Soc L. B Biol Sci. 2009;364:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Binder JR, et al. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goulas A, et al. Comparative Analysis of the Macroscale Structural Connectivity in the Macaque and Human Brain. PLoS Comput. Biol. 2014;10 doi: 10.1371/journal.pcbi.1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harriger L, et al. Rich Club Organization of Macaque Cerebral Cortex and Its Role in Network Communication. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Pasquale F, et al. A Cortical Core for Dynamic Integration of Functional Networks in the Resting Human Brain. Neuron. 2012;74:753–764. doi: 10.1016/j.neuron.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crossley NA, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. C1–8. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 93.Henson RN, Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. 2010;20:1315–1326. doi: 10.1002/hipo.20857. [DOI] [PubMed] [Google Scholar]
- 94.Ploghaus A, et al. Dissociating pain from its anticipation in the human brain. Science (80-.) 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 95.Porro CA, et al. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage. 2003;19:1738–1747. doi: 10.1016/s1053-8119(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 96.Roy M, et al. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 2014;17:1607–1612. doi: 10.1038/nn.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 98.Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Seth AK, Critchley HD. Extending predictive processing to the body: emotion as interoceptive inference. Behav Brain Sci. 2013;36:227–228. doi: 10.1017/S0140525X12002270. [DOI] [PubMed] [Google Scholar]
- 100.Ueda K, et al. Brain activity during expectancy of emotional stimuli: an fMRI study. Neuroreport. 2003;14:51–55. doi: 10.1097/00001756-200301200-00010. [DOI] [PubMed] [Google Scholar]
- 101.Seth AK, et al. An interoceptive predictive coding model of conscious presence. Front Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Apps MA, Tsakiris M. The free-energy self: a predictive coding account of self-recognition. Neurosci Biobehav Rev. 2014;41:85–97. doi: 10.1016/j.neubiorev.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sterling P. Allostasis: a model of predictive regulation. Physiol Behav. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 104.Buchel C, et al. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81:1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 105.McNally GP, et al. Placing prediction into the fear circuit. Trends in Neurosciences. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edwards MJ, et al. A Bayesian account of “hysteria.”. Brain. 2012;135:3495–3512. doi: 10.1093/brain/aws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sinha P, et al. Autism as a disorder of prediction. Proc Natl Acad Sci U S A. 2014;111:15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bar M. A cognitive neuroscience hypothesis of mood and depression. Trends Cogn. Sci. 2009;13:456–463. doi: 10.1016/j.tics.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carrive P, Morgan MM. Periaqueductal Gray. In: Mai JK, Paxinos G, editors. The human nervous system. Third Edition 2012. pp. 367–400. [Google Scholar]
- 110.Goulas A, et al. Mapping the hierarchical layout of the structural network of the macaque prefrontal cortex. Cereb Cortex. 2014;24:1178–1194. doi: 10.1093/cercor/bhs399. [DOI] [PubMed] [Google Scholar]
- 111.Deco G, et al. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 112.Hermundstad AM, et al. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc Natl Acad Sci U S A. 2013;110:6169–6174. doi: 10.1073/pnas.1219562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pernice V. How structure determines correlations in neuronal networks. PLoS Comput Biol. 2011;7:e1002059. doi: 10.1371/journal.pcbi.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van den Heuvel MP, et al. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greicius MD, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Mesulam MM. Patterns in behavioral neuroanatomy. Association areas, the limbic system, and hemispheric specialization. Princ. Behav. Neurol. 1985 [Google Scholar]