Abstract
Extracellular factors may act on cells in two distinct modes: an acute increase in concentration due to regulated secretion, or a gradual increase in concentration when secreted constitutively or from a distant source. We show that cellular responses to BDNF differ dramatically depending on how BDNF is delivered. In cultured neurons, acute and gradual increases in BDNF elicited, respectively, transient and sustained activation of TrkB receptor and its downstream signaling, leading to differential expression of Homer1a and Arc. Transient TrkB activation promoted neurite elongation and spine head enlargement, whereas sustained TrkB activation facilitated neurite branch and spine neck elongation. In hippocampal slices, fast and slow increases in BDNF enhanced basal synaptic transmission and LTP, respectively. Thus, the kinetics of TrkB activation is critical for cell signaling and functions. This temporal dimension in cellular signaling may also have implications for the therapeutic drug design.
INTRODUCTION
A central question in neuronal signal transduction, and indeed in cell biology in general, is how extracellular factors elicit a complex set of signaling events to achieve specific cellular functions. Given that many intracellular signaling pathways are shared by multiple extracellular factors, how could two factors that trigger the same signaling events bring about completely different functions in the same cells? For example, while both epidermal growth factor (EGF) and nerve growth factor (NGF) activate mitogen-associated protein kinase (MAPK) in PC12 cells, EGF triggers cell division but NGF induces neurite outgrowth1, 2. In the hippocampus, both BDNF and neuregulin induce the activation of MAPK, phosphotidylinositol-3 kinase (PI3K) and phospholipase C-γ (PLC-γ) pathways3, 4. However, BDNF facilitates long-term potentiation (LTP) whereas neuregulin suppresses it5–9. An attractive idea is that two factors may activate the same signaling pathways with different kinetics. Thus, EGF induces a transient activation of MAPK in PC12 cells resulting in proliferation, while NGF induces a more sustained activation of MAPK resulting in differentiation10. Can a single extracellular factor elicit different cellular functions by activating similar signaling molecules with different kinetics?
The kinetics of signaling may be influenced not only by distinct cell surface receptors but also by how their cognate ligands are secreted or delivered. A receptor may be acutely activated upon an immediate increase in ligand concentration due to its regulated secretion. This process is mimicked by most, if not all, pharmacological studies, in which a ligand or drug is applied directly to cells grown in culture. However, in many cellular processes in vivo, cells encounter a gradual increase in the concentration of extracellular factors. A constitutively secreted factor needs to accumulate over time to reach a threshold set by the affinity of the receptor. Moreover, when a ligand is secreted from a distant source, the responding cells may experience a gradual increase in the concentration of the ligand. So far, no study has addressed the question of whether acute and gradual increases in extracellular factors would induce the same or different set of signaling events and cellular functions.
As one of the most extensively studied extracellular factors, BDNF is an ideal model to address this question. BDNF is secreted through both constitutive and regulated secretory pathways11–16. The biological function of mature BDNF is mediated by the TrkB receptor tyrosine kinase, which activates the downstream MAPK, PI3K and PLC-γ pathways4. A longstanding puzzle in the field is how BDNF regulates so many aspects of neuronal functions, ranging from cell survival and differentiation to synaptic plasticity and circuit formation17. Often very different effects have been observed in the same cells when BDNF is applied pharmacologically. For example, acute application of BDNF rapidly enhances synaptic transmission at the neuromuscular synapses in culture18, whereas long-term treatment of the same culture with BDNF promotes synapse maturation19. In hippocampal slices, Kang and his colleagues reported that bath application of BDNF induces a long-lasting enhancement of basal synaptic transmission9. In contrast, many studies demonstrated that perfusion of BDNF facilitates LTP, but not basal synaptic transmission6, 8, 20, 21, in the same CA1 synapses. One explanation for the discrepancy is that acute potentiation of synaptic efficacy requires a fast delivery of BDNF to the hippocampal slices22, 23. Can fast delivery of BDNF elicit signaling mechanisms different from that of slow delivery?
In an attempt to mimic a gradual increase of extracellular BDNF concentration ([BDNF]) in vivo, we have devised a novel method of BDNF delivery, allowing a tenfold increase in [BDNF] in neuronal cultures every 30 min. We found that a gradual increase in [BDNF] elicits a dramatically different kinetics of TrkB activation and its downstream signaling events compared to the conventional, acute increase in [BDNF]. We showed that the two modes of BDNF delivery also elicit very different morphological changes in cultured hippocampal neurons. Furthermore, fast and slow increases in [BDNF] in hippocampal slices induce differential effects on gene expression and synaptic modulation. Thus, the mode of BDNF delivery or other bio-active agents may influence their signaling and functions. These results may also have general implications for both cell biology and drug therapy.
RESULTS
Transient and sustained activation of TrkB by acute and gradual BDNF stimulation
To examine whether the kinetics of TrkB activation could be regulated by the temporal rate of BDNF stimulation, we designed two different modes of BDNF delivery to cultured hippocampal neurons (Fig. 1a, see Methods), and measured the phosphorylation of TrkB at various time points (Fig. 1b). One was an acute application of BDNF, which resulted in an immediate increase in BDNF concentration to 1 nM (“acute mode”, 25 ng/ml). The other was a gradual increase in BDNF concentration (“gradual mode”): BDNF was added to the cultures to increase its concentration tenfold every 30 minutes, from an initial 0.1 pM (10−4 nM, 2.5 pg/ml) to a final concentration of 1 nM (25 ng/ml). The acute application of BDNF triggered a robust but transient increase in TrkB phosphorylation: the level of phospho-TrkB (pTrkB) reached its peak at about 15 min but declined rapidly and returned to baseline 2 hr after BDNF application (Fig. 1b). In contrast, a gradual increase in BDNF concentration resulted in a long-lasting activation of TrkB (Fig. 1b). TrkB was activated at a relatively lower level when BDNF concentration was below 1 nM (25 ng/ml). When BDNF concentration reached 1 nM (25 ng/ml), pTrkB slowly increased and reached a maximum level within 1 hr, and the level persisted for up to 8 hr with no sign of decline. Applications of saline in a similar manner (5 times, every 30 min, “Saline” group) did not induce TrkB or MAPK activation, suggesting that the sustained TrkB activation is not due to the mechanic stress of repeated applications. A previous study reported that acute application of BDNF resulted in a rapid degradation of TrkB protein in cultured cerebellar granule neurons24. To determine whether transient and sustained activation of TrkB were due to different kinetics of TrkB synthesis and/or degradation, we measured total TrkB protein levels by Western blot. The levels of full-length TrkB remained constant at various time points after acute and gradual BDNF deliveries (Fig. 1b). We performed biotinylation assays to measure cell surface TrkB at different time points (Fig. 1c). A 15-second acute BDNF application is included as a positive control, since this treatment is known to facilitate insertion of TrkB into cell surface25. Acute BDNF application reduced surface TrkB expression, possibly due to BDNF-induced endocytosis of TrkB. In contrast, gradual BDNF application resulted in a transient and slight decrease in surface TrkB expression, which recovered after 4 hr. These results are consistent with previous findings in cultured cells25, 26, and suggest that the transient pattern of TrkB phosphorylation induced by acute BDNF application is caused possibly by reduction in TrkB receptor surface expression, rather than by down-regulation of TrkB protein.
Figure 1.
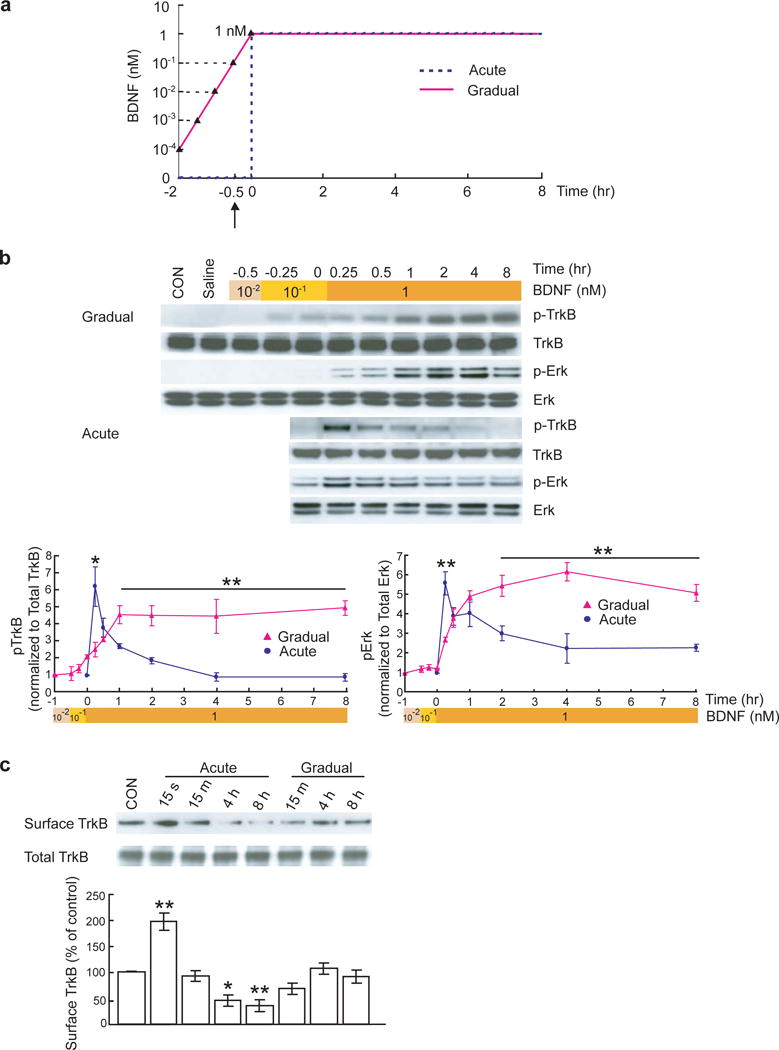
Transient or sustained TrkB activation and Erk signaling induced by acute or gradual BDNF stimulation.
(a) Protocols for application of BDNF in acute and gradual modes. Hippocampal neurons were cultured for 14 days. In the acute protocol, BDNF concentration in the culture increased immediately from 0 to 1 nM (25 ng/ml). Neuronal proteins were extracted at 0, 0.25, 0.5, 1, 2, 4, 8 hours after BDNF application. In the gradual protocol, BDNF concentration increased tenfold in every 30 min, starting from 10−4 nM (2.5 pg/ml) until it reached 1 nM (25 ng/ml; total 2 hours). Protein extraction started at 30 min after 10−2 nM (0.25 ng/ml) BDNF application (1.5 hr after the first BDNF application, indicated by arrow). When BDNF concentration reached 1 nM (25 ng/ml), protein extraction followed the same time course. (b) Transient and sustained activations of TrkB and Erk induced by acute and gradual stimulation with BDNF, respectively. Representative Western blots are shown on top of the quantitative plots. The pTrkB and pErk signals were measured by densitometry, normalized to total TrkB and Erk respectively, and data from 6 independent experiments (N = 6) were averaged and plotted. CON: vehicle control. Saline: 5 consecutive times of saline treatment with 30 min interval as a negative control. Data obtained from acute (blue line) and gradual (pink line) experiments in Fig. 1b, Fig. 2 and Fig. 6 were compared by paired t-test. In this and all other figures, data are presented as mean ± SEM, *: p < 0.05, **: p < 0.01. (c) Biotinylation assay of surface TrkB at the indicated time points after acute and gradual BDNF treatment. Data were compared to control by ANOVA.
TrkB phosphorylation is BDNF dose-dependent. Our previous study showed that TrkB was maximally phosphorylated within 5 min by 1 nM (25 ng/ml) BDNF27. To examine whether the signaling differences between the two modes reflect a concentration dependence of signaling, we performed similar experiments as in Fig. 1, but with a final BDNF concentration of 0.1 nM (2.5 ng/ml) in both acute and gradual treatments. The kinetics of signaling induced by acute and gradual BDNF applications at 0.1 nM (2.5 ng/ml) are very similar to those at 1 nM (25 ng/ml), respectively (compare Supplementary Fig. 1 to Fig. 1). Thus, it is not the final concentration, but the mode of BDNF delivery, which is important for the kinetics of TrkB signaling. To confirm that the kinetics, rather than the magnitude of the signal, accounts for the difference between the gradual and acute modes, we used BDNF at a final concentration of 1 nM (25 ng/ml) for both acute and gradual treatments.
Differential signaling kinetics induced by acute and gradual modes of BDNF delivery
We next examined whether the transient and sustained TrkB activation triggers different temporal patterns of signal transduction. In hippocampal neurons, BDNF-induced activation of MAPK, as reflected by phosphorylation of Erks (pErk), is important for gene transcription, protein synthesis and long-term synaptic plasticity28, 29. Acute and gradual BDNF application elicited very different patterns of Erk phosphorylation (Fig. 1b). Acute administration of BDNF induced a marked increase in Erk phosphorylation which peaked around 15 min, followed by a rapid decrease within the next 60 min. A stepwise increase in [BDNF], on the other hand, induced a slow and sustained increase in Erk phosphorylation that persisted for 8 hr. After BDNF concentration reached 1 nM (25 ng/ml), pErk reached a maximum level at 1 hr and then fell slowly over a long period of time. These data suggest that transient and sustained activation of TrkB, induced by acute and gradual modes of BDNF delivery, respectively, may lead to different kinetics of MAPK activation. We also examined GSK-3β and PLC-γ1 signaling, the other two important signaling pathways downstream of TrkB. An acute increase in [BDNF] elicited a transient increase in the phosphorylation levels of both GSK-3β and PLC-γ1, while gradual application of BDNF led to a sustained increase (Supplementary Fig. 2). These results suggest that the primary difference between the two modes of BDNF application is at the level of TrkB receptor, leading to differences in the kinetics of all three major signaling pathways.
Previous studies showed that the small GTPases Ras and Rap1 differentially control the kinetics of MAPK activation: Ras-dependent signaling to Erk is transient, whereas Rap1-dependent signaling to Erk is sustained30. We thus examined whether acute and gradual application of BDNF differentially activates Ras and Rap1. Ras activation was examined using GST-Raf1-RBD, and Rap1 activation was analyzed using GST-Ral-GDS31. For Ras, the kinetics of activation were similar for both acute and gradual experiments. The level of activated Ras increased and then decreased quickly, although the change induced by gradual application came slightly slower than that induced by acute application (Supplementary Fig. 3a). In contrast, the different modes of BDNF application activated Rap1 with different kinetics. In acute mode, Rap1 was activated transiently (Supplementary Fig. 3b). In gradual mode, Rap1 showed sustained activation for at least 8 hr (Supplementary Fig. 3b). These results support the notion that acute application of BDNF induces a transient Ras-MAPK signaling, whereas gradual application of BDNF triggers a sustained Rap1-MAPK signaling.
Nuclear signaling and transcription induced by gradual but not acute mode
BDNF elicits diverse biological effects in neurons. Some effects, such as acute potentiation of synaptic activity, occur rapidly and require covalent modifications of preexisting proteins18. Other effects, such as long-term regulation of synapse development and plasticity, take place slowly and depend on gene expression and protein synthesis32, 33. In the next series of experiments, we tested whether the two modes of BDNF stimulation have differential effects on gene expression. Since the transcription factor CREB is a critical mediator for BDNF-induced gene expression and is crucial for long-lasting synaptic effects of BDNF34, we first examined the kinetics of CREB activation in response to the two modes of BDNF application. To measure CREB activation, we performed Western blot using an anti-phospho-CREB (pCREB) antibody that detects phosphorylation at serine 133. As shown in Supplementary Fig. 4a, acute application of BDNF induced a rapid but transient increase in pCREB. Upon gradual BDNF stimulation, however, the increase in pCREB lasted as long as 8 hr. Immunocytochemistry revealed intense staining of pCREB in the nucleus of the hippocampal neurons. Quantification of the percentage of neurons with nuclear pCREB again indicated a transient CREB activation in response to acute BDNF application, but a long-lasting activation with gradual increase in [BDNF] (Supplementary Fig. 4b).
The MAPK-CREB signaling pathway is known to be involved in the transcription of immediate early genes (IEGs) Arc and Homer1a35, 36. Although acute application of BDNF has been shown to rapidly increase Arc and Homer1a mRNA levels, particularly in the synaptodendritic compartment36, 37, it is unclear whether distinct time courses of CREB activation induced by the two modes of BDNF stimulation dictate the expression of Arc and Homer1a proteins. Surprisingly, acute application of BDNF elicited little increase in Arc or Homer1a proteins in hippocampal cultures under our experimental conditions (Fig. 2). The protein levels of Arc and Homer1a exhibited a fairly small increase during a period of 60 min after BDNF treatment, and returned to the basal level within 2 hr. In contrast, under the gradual mode, the levels of Arc and Homer1a increased quickly as [BDNF] reached 1 nM (25 ng/ml). The elevation persisted up to 8 hr after 1 nM (25 ng/ml) BDNF stimulation (Fig. 2b). Our results suggest that sustained TrkB activation has different consequences for protein expression compared to the transient TrkB activation.
Figure 2.
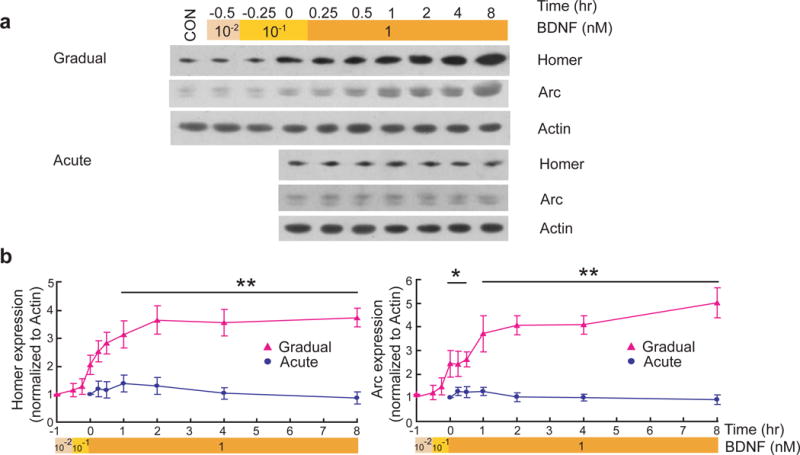
Differential expression of Homer1a and Arc by acute and gradual modes of BDNF stimulation.
Representative Western blots (a) and quantitative plots (b) of the time courses of BDNF-induced Homer1a, Arc expression in acute and gradual modes. Actin was used as loading control.
Differential effects of acute and gradual BDNF stimulation on neurite morphology
Previous studies showed that BDNF plays a central role in dendritic growth and the morphological specializations of dendrites38, 39. We therefore asked whether the two modes of BDNF stimulation could elicit differential effects on neurite growth in the developing neurons. Young hippocampal neurons (3-day old culture) were treated with BDNF either acutely or gradually, and grown for an additional 3 days. The cultures were then fixed and immunostained with anti-MAP2 antibody for morphometric analysis (Fig. 3a). Neurite growth was quantified by three parameters: total neurite length, number of first order (primary) neurites and number of branch points (Fig. 3b). Total neurite length (in μm) was 221.1 ± 12.5, 368.7 ± 17.0 and 418.1 ± 19.2 for control, gradual and acute modes, respectively. Compared to gradual stimulation, acute application of BDNF had a more pronounced effect on the number of primary dendrites: the number of first order neurites was 2.3 times higher in acute mode, but only 1.5 times higher in gradual mode, relative to control (significantly different between acute and gradual modes; p < 0.01, ANOVA). Interestingly, the number of branch points in gradual experiments averaged 4.13 ± 0.22 per cell, compared to 1.65 ± 0.14 per cell in control neurons. However, the number of branch points from acute experiments averaged 2.38 ± 0.17 per cell, only slightly more than the control (significantly different between acute and gradual experiments; p < 0.01, ANOVA). Neurons were preincubated with K252a (100 nM), and then treated with acute or gradual BDNF. K252a completely eliminated the ability of both acute and gradual BDNF to elicit neurite outgrowth (Fig. 3). Collectively, our results suggest that in early development, neurite branching seems to be sensitive to gradual BDNF stimulation, while acute BDNF stimulation promotes neurite elongation.
Figure 3.
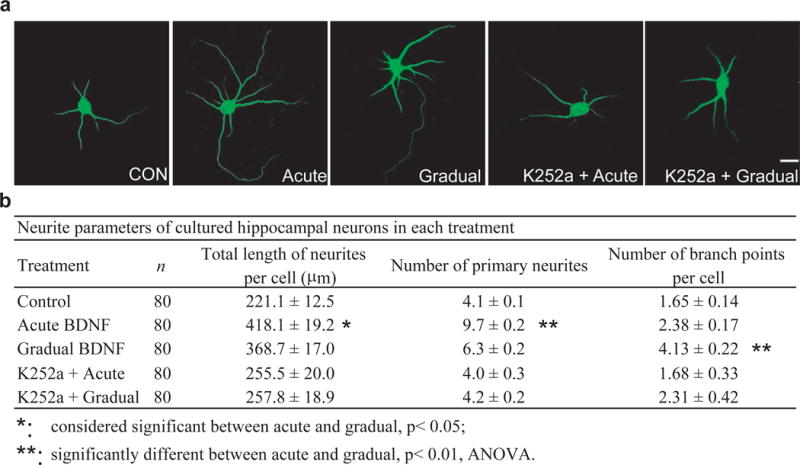
Differential effects of acute and gradual modes on neurite growth of young neurons.
(a) Representative images of MAP2-stained hippocampal neurons under different conditions. Neurons (DIV 3) were treated with BDNF in acute or gradual mode. Three days later, neurons were fixed and stained with anti-MAP2 antibody. Scale bar, 20 μm. (b) Effects of acute and gradual BDNF stimulation on neurite growth. These responses were completely inhibited when the cells were pretreated for 1 h with 100 nM K252a. Total neurite length, number of primary neurites, and number of branch points were analyzed. Data in Fig. 3 to Fig. 5 were compared by ANOVA.
Differential effects on dendritic spines
BDNF also regulates dendritic spine growth late in development38, 40. To determine whether spine growth is differentially regulated by two different modes of BDNF stimulation, we used older hippocampal neurons (21 days in culture) which exhibited many spines with well-defined heads (Fig. 4a). Twenty four hours after acute BDNF stimulation, neurons showed much more prominent dendritic spines, the majority of which were mushroom shaped, with spine heads larger than those of control neurons. Gradual BDNF stimulation for the same period (24 hr) had different effects on dendritic spine growth. More filopodia-like outgrowth was observed, and the spines were longer. Quantitative analysis indicated that upon acute BDNF stimulation, the spine density doubled (control, 2.39 ± 0.15; acute, 4.59 ± 0.18; p < 0.01, ANOVA), whereas the density of filopodia-like protrusions was only modestly increased (control, 0.58 ± 0.05; acute, 0.77 ± 0.05) (Fig. 4b). Interestingly, the most striking effect of gradual BDNF stimulation was to significantly increase the density of filopodia-like protrusion. The spine density increased only 50% (3.60 ± 0.15, p < 0.01, ANOVA) whereas filopodia density almost doubled (1.04 ± 0.07, p < 0.01, ANOVA), as compared to control.
Figure 4.
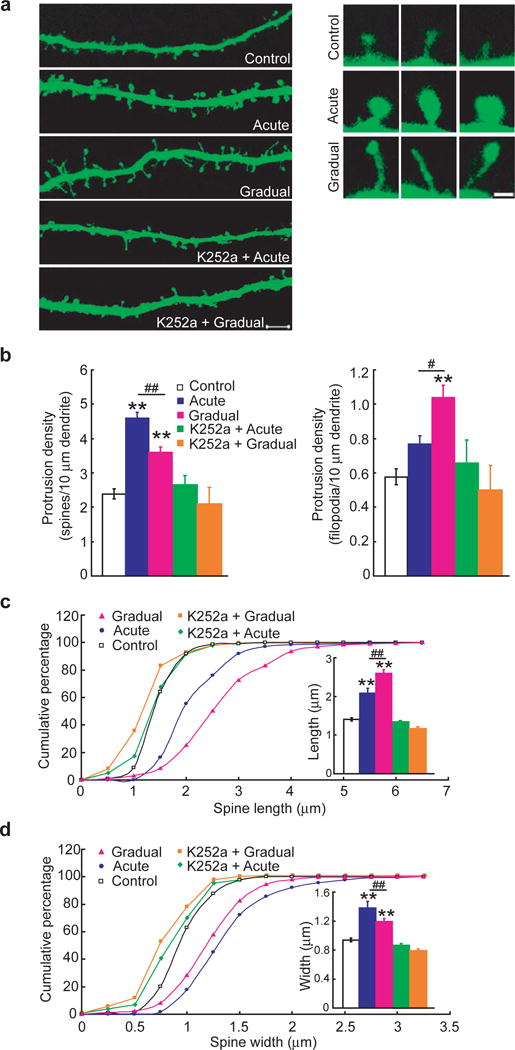
Acute and gradual modulation of dendritic spine growth of mature neurons.
(a) Examples showing dendritic spines under different conditions. Acute BDNF stimulation increased the size of spine head, whereas gradual stimulation caused spine length elongation and more filopodia-like protrusions. These responses were completely inhibited when the cells were pretreated for 1 h with 100 nM K252a. Scale bars: low magnification, 5 μm and high magnification, 1 μm. (b) Quantification of spine (left) and filopodia (right) densities (number per 10 μm dendrite length). In each experiment, 40 secondary dendrites from 40 neurons were analyzed. Five independent experiments were performed. (c, d) Quantification of the shape of dendritic spines under the indicated modes. Cumulative frequency plots showing distribution of spine length (μm) and spine head width (μm). More than 500 spines in more than 12 neurons were examined for each treatment, and 5 experiments were performed. The average spine width and length for each group are shown in the insets. **: compared to control group, p< 0.01; #: considered significant between acute and gradual, p< 0.05; ##: significantly different between acute and gradual, p< 0.01, ANOVA.
We next compared how acute and gradual modes affect the shape or morphology of spines. The length of spines and the width of spine heads from stacked z series confocal images were measured separately. The mean length of spines significantly increased in neurons treated with gradual mode of BDNF delivery (Fig. 4c). However, the mean width of spine heads significantly increased in neurons treated with acute mode of BDNF delivery (Fig. 4d). Acute BDNF treatment also caused a modest increase in spine length as compared to control, but this effect could be attributed to the increase in head diameter. In addition, pretreatment with K252a dramatically reduced BDNF-induced dendritic spine growth and morphology changes (K252a + Acute: 2.65 ± 0.29 spine density, 0.67 ± 0.14 filopodia-like protrusion density; K252a + Gradual: 2.10 ± 0.49 spine density, 0.51 ± 0.15 filopodia-like protrusion density) (Fig. 4). These results suggest that in the mature neurons, a gradual increase in [BDNF] promotes the outgrowth of filopodia-like protrusions and the length of spines, whereas an acute elevation of [BDNF] results in more spines with larger heads.
Differential effects on synaptic transmission and plasticity in hippocampal slices
As a first step towards exploring the different kinetics of BDNF signaling in vivo, we attempted to examine the effects of acute and gradual BDNF stimulation in hippocampal slices. Although it is generally accepted that certain levels of BDNF are necessary for LTP5, 6, disagreement exists as to whether BDNF itself could enhance basal synaptic transmission in the Schaffer Collateral pathway in hippocampal slices6, 8, 20, 21. It was suggested that a high perfusion rate (240 ml/hour) allows BDNF to penetrate into the slices, leading to synaptic potentiation22. However, this argument may not explain why perfusion of BDNF at a much lower rate (25 ml/hour) facilitates LTP8. To determine the kinetics of BDNF signaling in hippocampal slices, CA1 areas were dissected at different time points after BDNF (8 nM, 200 ng/ml) application in slices from 8-week old mice, and levels of TrkB and MAPK activation were examined by Western blot. Similar to acute and gradual application of BDNF to cultured neurons, fast (240 ml/hour) and slow (25 ml/hour) rate of BDNF perfusion induced a transient and sustained activation of TrkB and MAPK signaling, respectively (Fig. 5a–c). We also examined the time course expression of the IEGs Arc and Homer1a using Western blot. Consistent with our results from in vitro cultures, Arc and Homer1a levels in CA1 region were increased only in slices perfused with BDNF at slow (25 ml/hour), but not fast (240 ml/hour) rate (Fig. 5d–f).
Figure 5.
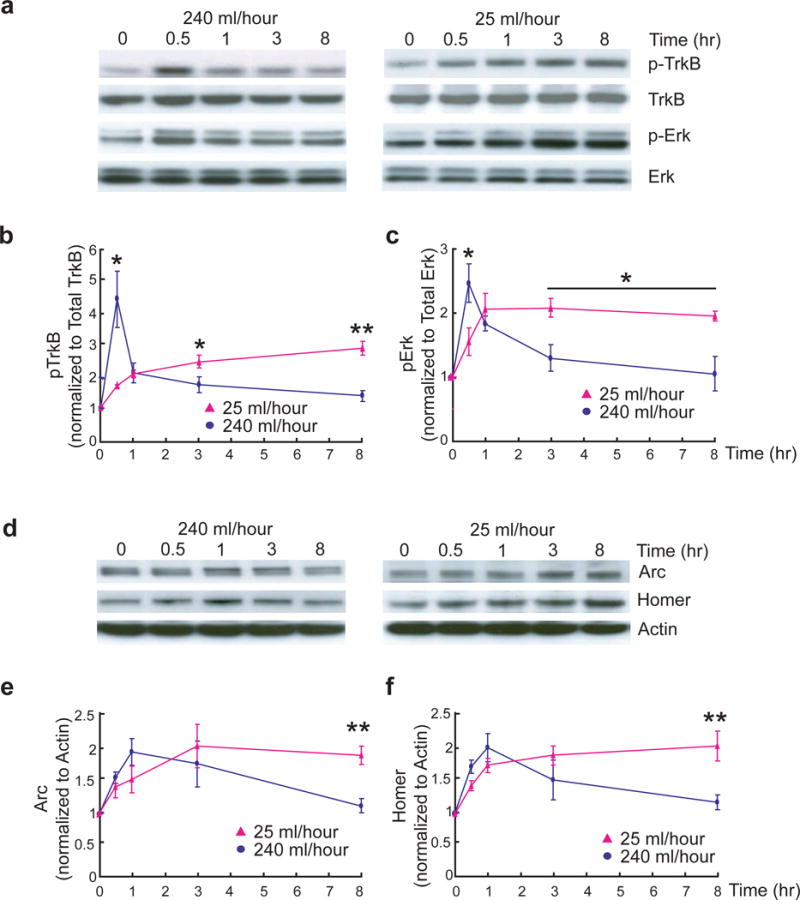
Differential signaling of fast and slow BDNF stimulation in hippocampal slices.
(a–c) Differential kinetics of TrkB and Erk activation induced by fast and slow rates of BDNF stimulation. The CA1 areas from adult hippocampal slices were quickly dissected at the indicated time points after slow (25 ml/hour) or fast (240 ml/hour) BDNF (8 nM, 200 ng/ml) perfusion. TrkB and Erks were used as loading controls. Representative Western blots (a) and quantitative plots (b–c) are presented. n = 3–5 slices/time point. N = 3 independent experiments.
(d–f) Differential expression of Homer1a and Arc in adult hippocampal slices induced by slow and fast BDNF perfusion. The experiments were performed identically as in (a). Representative Western blots (d) and quantification of data (e–f) (N = 3 repetitions of pooled slices) are shown. The same experiment was repeated using entirely independent samples, and the same results were obtained.
Thus, we reasoned that a high perfusion rate may result in a faster increase in [BDNF] mimicking the acute mode, whereas a low perfusion rate is more analogous to the gradual mode. Indeed, in slices derived from adult (8-week old) mice, basal synaptic transmission in CA1 was not affected by slow perfusion (25 ml/hour) of BDNF (Fig. 6a, 101 ± 1%). In contrast, perfusion of BDNF at a high rate (240 ml/hour) markedly enhanced synaptic efficacy (Fig. 6a, 153 ± 5%). In fact, synaptic potentiation could be induced by a brief (30 min) high-speed perfusion of BDNF, and such a potentiation could last for at least 3 hours (Fig. 6b, 149 ± 8%). Perfusion of NGF (200 ng/ml) at a high rate (240 ml/hour) had no significant effect on synaptic transmission (Supplementary Fig. 5a, 101 ± 4% at 55–60min). Pretreatment of slice with K252a (200 nM) blocked the ability of fast BDNF perfusion (240 ml/hour) to enhance synaptic transmission (Supplementary Fig. 5b, 104 ± 2% at 55–60min). These results are consistent with previous findings by Kang et al., suggesting a fast increase in [BDNF] enhances basal synaptic transmission in adult hippocampus9.
Figure 6.
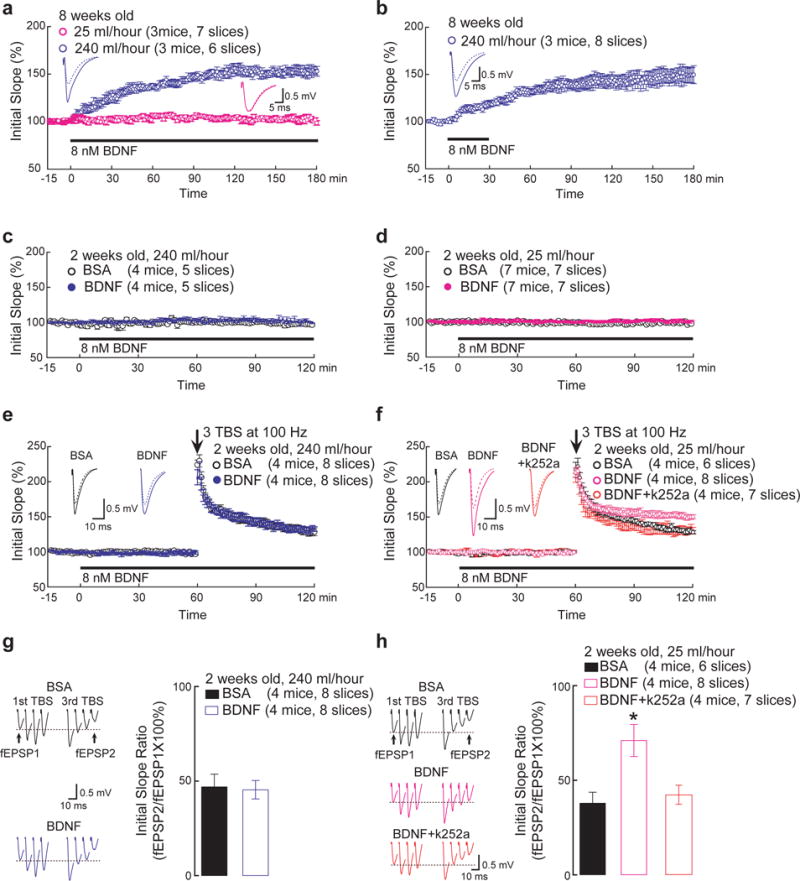
Differential physiological effects of fast and slow BDNF stimulation in hippocampal slices.
(a) Comparison of slow and fast BDNF perfusion in adult hippocampal slices. Field EPSPs were recorded at the CA1 area of adult (8-week old) hippocampal slices. After a stable baseline was established, BDNF was perfused into the slices at either fast or slow rate throughout the recording (> 3 hours). Insets are example recordings at 0 min (dotted line) and 180 min (solid line). Note that fast but not slow perfusion of BDNF potentiated synaptic efficacy (153 ± 5% vs. 101 ± 1% at 175–180 min, respectively).
(b) Effect of transient, fast BDNF perfusion on adult hippocampal slices. The experiments were done identically as in (a), except that BDNF was transiently perfused at a fast rate as indicated by the horizontal bar. Insets are example recordings at 0 min (dotted line) and 180 min (solid line). Note a significant increase in basal synaptic transmission that lasted even after BDNF was removed (149 ± 8% at 175–180min).
(c and d) Fast and slow perfusion of BDNF does not alter basal synaptic transmission in neonatal hippocampal slices.
(e, g) Effect of fast BDNF perfusion on neonatal hippocampal slices. The experiments were performed identically as in (a), except that p12–14 slices were used. (e) Fast perfusion of BDNF did not affect E-LTP induced by 3xTBS (131 ± 4% for BDNF and 128 ± 5% for control). Insets are sample recordings at 0 min (dotted line) and 120 min (solid line). (g) Synaptic fatigue during 3xTBS was indistinguishable between BDNF and control (45 ± 5% vs. 47 ± 7%, respectively; measured by the ratio of last response to the first response). Original data are shown on the left.
(f, h) Effect of slow BDNF perfusion on neonatal hippocampal slices. (f) Slow perfusion of BDNF significantly increased E-LTP (150 ± 4% at 115–120min) compared to control (130 ± 2%, p<0.001). However, this increase was blocked by co-perfusion of 200 nM K252a (127 ± 6%). Insets are example recordings at 0 min (dotted line) and 120 min (solid line). (h) Synaptic fatigue during 3xTBS was significantly attenuated by BDNF (71 ± 8%) compared to BSA control or co-perfusion of K252a (38 ± 6% and 42 ± 5%, respectively; * p<0.01, ANOVA). Original data are shown on the left.
To examine the effects of slow BDNF perfusion, we used slices derived from neonatal hippocampus (2-week old) where endogenous BDNF is low8. Neither high nor low rate of BDNF perfusion could change the basal synaptic transmission at this age (Fig. 6c and 6d). In contrast, slow perfusion of BDNF facilitated LTP induced by a relatively weak theta burst stimulation (3 bursts of four pulses at 100 Hz, or 3xTBS), which otherwise induced only small potentiation (Fig. 6f, 150 ± 4% for BDNF, 130 ± 2% for BSA). Moreover, application of 3xTBS induced a synaptic fatigue (Fig. 6h, defined as last EPSP of 3rd burst/1st EPSP of 1st burst, 38 ± 6%) at Schaffer collateral-CA1 synapses, and slow perfusion of BDNF for 2 hr significantly attenuated the synaptic fatigue (71 ± 8%). However, a high rate of BDNF perfusion had no effect on either TBS-induced LTP (Fig. 6e, 131 ± 4% for BDNF, 128 ± 5% for BSA) or synaptic fatigue (Fig. 6g, 45 ± 5% for BDNF, 47 ± 7% for BSA). Thus, in addition to eliciting differential kinetics of signaling, slow BDNF delivery facilitates LTP in developing hippocampus whereas a fast increase in [BDNF] enhances basal synaptic transmission in adult hippocampus.
Recent study has shown that BDNF causes depolarization of the postsynaptic neuron and also regulates NMDA receptors41. We therefore performed whole-cell recording experiments on hippocampal slices. BDNF regulation of synapses depends on developmental stages. In 2-week old hippocampal slices, NMDAR-mediated currents were significantly increased after fast, but not slow, perfusion of BDNF (Fig. 7a–c). At this stage, AMPAR currents are quite small and variable, and silent (NMDAR only) synapses are quite prominent. It is therefore difficult to measure the AMPAR/NMDAR current ratio accurately (data not shown). In 8-week old hippocampal slices, neither fast nor slow application of BDNF altered NMDAR-mediated EPSCs (Fig. 7d–f). In contrast, fast but not slow application of BDNF increased the AMPAR/NMDAR current ratio (Fig. 7g), possibly due to a significant increase in AMPAR-mediated synaptic currents as previously reported9.
Figure 7.
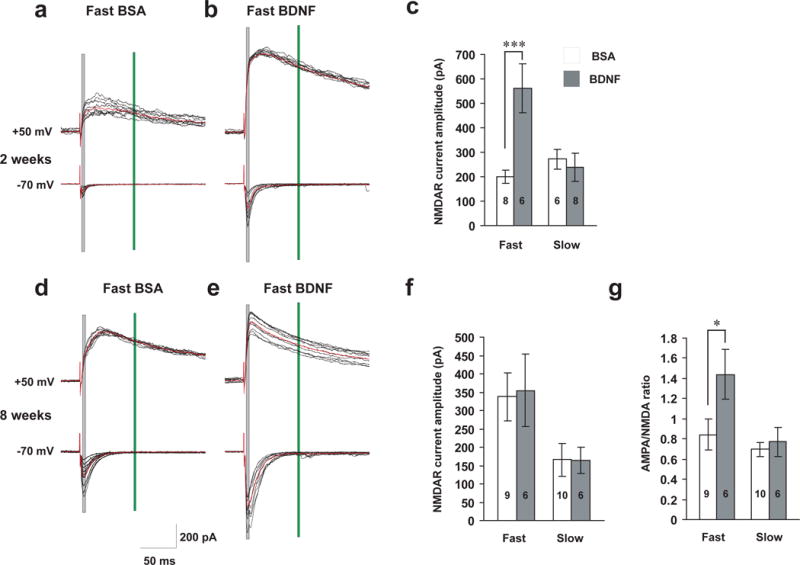
Differential regulation of NMDAR-EPSCs and AMPAR/NMDAR ratio by fast and slow BDNF application.
Whole-cell, voltage-clamp recordings were performed on CA1 pyramidal neurons of hippocampal slices. BSA or BDNF was perfused at slow (25 ml/hr) or fast (240 ml/hr) rate. (a, b, d and e) Representative traces of NMDAR (upper) and AMPAR (lower) currents evoked by extracellular stimulating presynaptic neurons at 50 μA in 2- and 8-week old animals. Black: individual current traces; Red: average waveform. Shaded grey and green rectangular areas indicate where measurements were taken to determine AMPA (at −70 mV) and NMDA (at +50 mV) current amplitudes, respectively. (c) NMDAR currents in 2-week old slices. Note that the average amplitude was significantly increased (p=0.009) after fast but not slow BDNF perfusion. (f) No differences in NMDAR currents were observed between fast and slow BDNF application in 8-week old CA1 neurons. (g) AMPA/NMDA current ratio in 8-week old CA1 neurons. The ratio was significantly increased (p<0.05) after fast but not slow BDNF perfusion.
Reversal of acute and gradual TrkB signaling by K252a
TrkB mediates many aspects of BDNF regulation of morphological plasticity and synaptic function17. Pretreatment of young hippocampal neurons with K252a (100 nM) completely eliminates the ability of both acute and gradual BDNF to elicit neurite outgrowth (Fig. 3). Neurons (DIV 21) were preincubated with K252a for 1 hr, and then treated with acute or gradual BDNF for 24 hr. K252a dramatically reduced BDNF-induced dendritic spine growth and morphology changes (Fig. 4). Furthermore, K252a (200 nM) blocks the synaptic potentiation in adult hippocampal slices induced by fast BDNF perfusion (Supplementary Fig. 5b). The effects of slow BDNF perfusion on LTP and synaptic fatigue in neonatal hippocampal slice were completely prevented by pretreatment with K252a (Figs. 6f, 6h).
Previous studies have reported that p75NTR regulates Trk signaling in several ways–by promoting ligand binding to TrkB, suppressing TrkB tyrosine autophosphorylation, and altering the endocytosis of TrkB42, 43. To determine whether the presence of p75NTR is critical for the differential responses to acute and gradual BDNF application, we repeated the experiments described in Fig. 1, using p75NTR−/− cultures. Although the magnitude of Erk signaling was slightly lower in both acute and gradual modes, BDNF application by both modes elicited the same transient and sustained kinetics of TrkB and Erk activation, respectively (Supplementary Fig. 6). These results suggest that the absence of p75NTR has little effect on the kinetics of TrkB and MAPK activation.
To further investigate whether the sustained TrkB signaling could be converted to a transient signal, we applied K252a after TrkB activity had reached its peak in the gradual mode (2 hours after stepwise BDNF application), and monitored the subsequent signaling patterns in cultured hippocampal neurons. As shown in Supplementary Fig. 7, sustained TrkB activation was largely reversed by K252a, and so were the sustained expression levels of Homer and Arc. In the gradual mode, TrkB appeared to be rapidly re-inserted into the plasma membrane after endocytosis, and became available to be reactivated by BDNF (Fig. 1c). It seems likely that K252a applied after maximal TrkB activation may have prevented reactivation of newly inserted TrkB. The difference in TrkB membrane re-insertion provides a critical explanation why gradual and acute applications of BDNF elicit very different kinetics of TrkB and MAPK activations, which in term lead to distinct physiological functions (promoting LTP vs enhancing basal synaptic transmission). We have performed similar experiments using U0126, a MEK inhibitor. As expected, U0126 applied 2 hours after gradual BDNF application reversed sustained MAPK phosphorylation and sustained expression of Homer/Arc (Supplementary Fig. 8). Since MAPK is downstream of TrkB, it is not surprising that U0126 did not affect sustained TrkB activation (Supplementary Fig. 8). Surprisingly, delayed K252a application had little effect on sustained MAPK activation (Supplementary Fig. 7). These results imply that once TrkB is activated in the sustained mode, it may trigger some special downstream signaling events, leading to long-term, irreversible biological changes. Indeed, inhibition of TrkB 40 min after TBS failed to reverse an established TBS-LTP (unpublished observation), a form of LTP dependent on the gradual signaling mode of endogenous BDNF according to the present study. Thus, while K252a could reverse the sustained TrkB activation, it may not reverse all of the biological responses induced by gradual BDNF application.
DISCUSSION
Controlling the kinetics of signal transduction may be an important mechanism to ensure functional specificity of extracellular factors. Rigorous studies have suggested that specific cellular functions of growth factor receptors can be achieved by the activation of identical signaling events with distinct kinetics1, 44. It is unclear, however, whether the same factor can elicit distinct functions on the same cells, and if so, what the underlying mechanisms may be. BDNF, as a neurotrophic factor, regulates a wide range of cellular functions in the nervous system, including dendritic and axonal growth, neuronal survival, synaptic transmission, and LTP in the hippocampus17. In the present study, we show that when delivered to the same hippocampal neurons in acute or gradual mode, BDNF can induce dramatically different cellular effects. In young neurons, an acute increase in [BDNF] promotes the elongation of neurites, whereas a gradual increase facilitates neurite branching. In more mature neurons, BDNF enlarges the heads of dendritic spines when delivered in the acute mode but elongates the spine necks in the gradual mode. In hippocampal slices, fast delivery of BDNF (acute mode) enhances basal synaptic transmission whereas slow delivery (gradual mode) facilitates LTP. These very different cellular functions of BDNF may be explained by transient and sustained TrkB activations, respectively, which in turn lead to distinct downstream signaling events. These results have revealed in a new dimension to the study of neurotrophin signaling and function, and challenged the relevance of a commonly used approach in signal transduction research. Further, our findings may have broad implications for the design of new therapeutic strategies.
Transient and sustained TrkB activations due to different modes of BDNF delivery may help to resolve a long-standing debate in the field of BDNF and synaptic plasticity: whether BDNF can elicit acute potentiation of synaptic efficacy in hippocampal slices. Analogous to the acute application of BDNF to neuronal cultures45, 46, fast perfusion of exogenous BDNF to hippocampal slices was reported to induce a rapid and long-lasting potentiation of synaptic efficacy (called BDNF-LTP) at CA1 synapses9. This result was quite provocative since most exogenous factors modulate tetanus-induced LTP without altering basal transmission. Nevertheless, subsequent attempts by a number of laboratories failed to replicate this finding6, 8, 20, 21. Notably, most of these studies have used a relatively slow rate of BDNF perfusion. Kang and his colleagues argued that a key factor in BDNF-induced synaptic potentiation is fast perfusion, which allows BDNF to penetrate into the slices22, 23. However, under the conditions that failed to observe the acute synaptic potentiation, BDNF was found to suppress GABA-mediated inhibitory synaptic currents20, 21 and facilitate synaptic responses to high-frequency stimulation8, suggesting that the molecule successfully entered the slices. Building on our new findings that acute and gradual increase in [BDNF] may lead to profoundly different biological effects in cultured hippocampal neurons, we now revisited the issue. Our data suggest that fast perfusion, even transiently, does induce synaptic potentiation (Figs. 6a and 6b), but this is not because of its ability to penetrate the slices. In fact, within 30 minutes, both fast and slow perfusion induces similar magnitude of TrkB activation (Fig. 5a). However, fast and slow BDNF perfusion results in, respectively, transient and sustained activation of both TrkB and Erk (Fig. 5a). The difference in the signaling kinetics may lead to dramatically different biological effects. Indeed, slow but not fast perfusion induces the expression of Arc and Homer1 in adult slices (Fig. 5d), reminiscent of the gradual mode of BDNF action in culture (Fig. 2). Interestingly, only a slow rate of BDNF delivery facilitates LTP in neonatal hippocampal slices (Fig. 6e and 6f). Taken together, these results provide new insights into a controversial issue that bears profound implications.
Whether endogenously secreted BDNF also elicits two distinct modes of signaling is difficult to address with current techniques. It is not possible to manipulate the kinetics of endogenous BDNF delivery at the sites of synaptic modifications, due to lack of sensitive tools that can monitor the changes, rather than the total amount, of BDNF in intact neuronal preparations. It should also be pointed out that the concentration of endogenous BDNF, measured by conventional methods such as by Western blot or ELISA, does not truly reflect that secreted at a particular synaptic site, but a balanced result of BDNF secretion and BDNF removal by internalization and/or degradation. Moreover, one would ideally want to test the functional consequences of blocking one mode (e.g. acute) but not the other (e.g. gradual). While this is currently not possible, several studies have shown that regulation of hippocampal LTP, presumably mediated by the “gradual” mode, could be blocked by removing endogenous BDNF5, 6, 8, 9. Finally, we need to take into consideration that neuronal firing in vivo might alter the signaling differences between acute and gradual delivery. Our preliminary experiments suggest that continuous chemical depolarization with high K+ sustains acute BDNF-induced TrkB and MAPK activations, and minimizes the differences between acute and stepwise BDNF administration (data not shown). It is unknown whether and to what degree the high K+ depolarization mimics neuronal firings in vivo under various physiological conditions. Unlike patterned electrical stimulation, high K+ could not produce bursts of high frequency action potentials, which are necessary for many physiological processes including LTP. Thus, a systematical investigation is necessary to address how neuronal activity, such as patterned electric stimulation, affects the signaling differences between acute and gradual delivery of endogenous BDNF.
Our findings may also be relevant to pharmacological treatments using growth factors, and chemical drugs in general. In chemotherapy, for example, continuous, slow intravenous infusion of drugs over 24 hours per day has been increasingly favored over the standard, “bullet” method (a single shot) of drug administration. The general reasoning is that the “bullet” administration results in a surge of plasma drug levels which may be toxic and intolerable by the patients, and yet the drug concentration within the body quickly reduces to non-effective levels because of drug degradation and/or body’s drug clearance capacity. Continuous infusion, on the other hand, reduces drug toxicity by eliminating the surge while maintaining a constant drug level in the system for a long period of time. We speculate that the “bullet” administration and continuous infusion of drugs are analogous to the acute and gradual modes of BDNF actions described here. The latter may lead to sustained activation of signal transduction events, which in turn may induce very different patterns of gene expression in the target cells. It is tempting to hypothesize that continuous infusion induces additional or different cellular responses, which may contribute to its superior effects over “bullet” administration. Further work is necessary to test this hypothesis.
METHODS
Cell culture and BDNF treatments
Embryonic hippocampal neurons (E18) were dissociated and plated on poly-D-lysine coated 35 mm dishes at 1,000,000 cells/well. Neurons were cultured for 14 days and then processed for biochemical experiments as described27. Dendritic morphology was studied with cultures grown for only 3 days on coverslips at 5,000 cells/coverslip. In the gradual protocol, BDNF was added to the medium from an initial concentration of 10−4 nM (2.5 pg/ml) to a final concentration of 1 nM (25 ng/ml), with a tenfold increase every 30 min (Fig. 1a). As a negative control for the “gradual experiments”, we added saline 5 consecutive times in every 30 min. For cultures with final BDNF concentrations below 1 nM (25 ng/ml), we added saline to ensure that each sample had been treated 5 consecutive times by either stepwise BDNF or saline. In the acute protocol, BDNF (1 nM, 25 ng/ml) was directly applied to the cultured neurons after 4 consecutive applications of saline treatment.
Biotinylation and immunoblotting
Cultured neurons in vitro were lysed in an ice-cold RIPA lysis buffer and subjected to Western blot, as described in Supplementary Methods. Cell surface proteins were biotinylated as described27.
Analysis of neurite complexity
Cultured hippocampal neurons at 3 days in vitro were treated with BDNF in either an acute or a gradual mode and grown for an additional 3 days. Cells were stained with mouse anti-MAP2 (1:1000, Chemicon). Images were acquired with a Nikon Neurolucida microscope (20x) by investigators blind to the experimental condition. After taking images of MAP2-positive neurons with cell body for diameter (15–20 μm), three parameters of neurite growth were analyzed: the total length of the neurite (μm), the number of primary neurites and the number of branch points per cell, using MetaMorph image analysis software neurite outgrowth application. The application settings were adjusted at the beginning of analysis and kept the same for all images in the experiment. Typically, images of 20 neurons per condition were captured for each experiment, and four independent experiments were performed.
Analysis of spine density and spine morphology
Hippocampal neurons (DIV 7) were transfected with GFP by the calcium phosphate method and examined at DIV21 using a Zeiss confocal microscope (40x, NA 1.30, 488 nm laser, LSM 510). Each image consisted of a z-stack of pictures taken at a depth interval of 0.5 μm and then projected into one image. Acquisition of microscopy images and morphometric quantifications were performed by investigators blind to the experimental condition. Morphometric measurements were performed with the aid of MetaMorph software. Each experiment was repeated at least five times with independent neuronal preparations. The densities of filopodia and spines were measured separately, as previously described27. Spine density was calculated by dividing the spine number by the measured length (in μm) of imaged secondary dendritic stretches and multiplying by 10. Quantification of dendritic spine length and width was performed as described47. The length of an individual spine was measured from the tip of the spine head to the interface with the dendritic stalk. The width of a spine was taken as a diameter of the spine head perpendicular to the length of the spine. For filopodia, the length of the spine was measured from the site of attachment to the dendritic shaft to the furthest tip of the longest spine fork. For each condition, measurements of spine densities or spine dimensions were averaged per neuron; the means from multiple neurons were then averaged to obtain the mean ± SEM for the population of neurons.
Slice electrophysiology
Transverse slices from male, C57/BL6 mice at two age groups (2-week old and 8-week old) were prepared as described in Supplementary Methods. BDNF (8 nM, 200 ng/ml) was perfused into the slices at either 240 ml/hour or 25 ml/hour. Field EPSPs were recorded (AxoClamp 2B amplifier, Axon Instruments, Foster City, CA), filtered at 1 kHz, digitized at 10 kHz (Axon Digidata 1322A), and stored for off-line analysis (Clampfit 9). LTP was induced by three sets (3xTBS) of theta burst stimulation.
Whole-cell patch recordings were made at approximately 32–34°C. Patch pipettes (3–6 MΩ) were pulled from glass capillaries (1.1 mm I.D., 1.5 mm O.D., VWR Scientific, West Chester, PA) on an electrode puller (PP-830, Narishige Co., Japan) and filled with internal solution (in mM: 130 Cs-gluconate, 11 EGTA, 2 MgCl2, 10 HEPES, 2 Mg-ATP, 0.5 Na2-GTP, 2.5 QX314, 10 Phosphocreatine, pH 7.3 adjusted with CsOH). Slices were perfused with ACSF with 10 μM Bicuculline (Sigma) to block GABAA receptor currents in all experiments. Recordings were made using an Axopatch 200B amplifier, filtered at 5 kHz, digitized at 10 kHz via an Axon Digidata 1321A, and stored for off-line analysis. Whole-cell recordings from pyramidal neurons in CA1 with a holding potential at −70 mV and +50 mV provided predominantly evoked AMPAR-mediated EPSCs and NMDAR-mediated EPSCs, respectively. The evoked AMPAR and NMDAR EPSCs recorded in voltage-clamp mode were analyzed with Clampfit 10 (Molecular Devices, CA).
Statistical analysis
Statistical analysis was performed using GraphPad InStat (GraphPad Software Inc., San Diego, CA). Statistical significance between two groups was determined with a two-tailed, paired Student’s t-test, while significance among multiple groups was determined with an analysis of variance (ANOVA), followed by the post-hoc Newman–Keuls multiple-comparison test. Differences were considered significant at p < 0.05. All values are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank the NCI, CCR Fellows Editorial Board and Dr. Guhan Nagappan for the thoughtful comments and suggestions. This work is supported by the Intramural Research Programs of NICHD and NIMH. This paper is dedicated to Dr. Wyndham H. Wilson, whose innovative research in cancer therapy helped to conceive this work.
References
- 1.Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 2.Chao MV. Growth factor signaling: where is the specificity? Cell. 1992;68:995–997. doi: 10.1016/0092-8674(92)90068-n. [DOI] [PubMed] [Google Scholar]
- 3.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- 5.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson SL, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 7.Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 8.Figurov A, Pozzo-Miller L, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 9.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 10.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canossa M, et al. Neurotrophin release by neurotrophins: implications for activity-dependent neuronal plasticity. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman LJ, et al. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:223–228. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 14.Griesbeck O, et al. Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc Res Tech. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. Embo J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 17.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Ann Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Xie KW, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Saito H, Matsuki N. Inhibition of GABAa synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang H, Jia L, Suh K, Tang L, Schuman E. Determinants of BDNF-induced hippocampal synaptic plasticity: role of the TrkB receptor and the kinectics of neurotrophin delivery. Learning and Memory. 1996;3:188–196. doi: 10.1101/lm.3.2-3.188. [DOI] [PubMed] [Google Scholar]
- 23.Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 24.Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000;275:8982–8990. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- 25.Haapasalo A, et al. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem. 2002;277:43160–43167. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- 26.Carter BD, Zirrgiebel U, Barde YA. Differential regulation of p21ras activation in neurons by nerve growth factor and brain-derived neurotrophic factor. J Biol Chem. 1995;270:21751–21757. doi: 10.1074/jbc.270.37.21751. [DOI] [PubMed] [Google Scholar]
- 27.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblum K, et al. The role of extracellular regulated kinases I/II in late-phase long-term potentiation. J Neurosci. 2002;22:5432–5441. doi: 10.1523/JNEUROSCI.22-13-05432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 30.York RD, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 31.Sasagawa S, Ozaki Y, Fujita K, Kuroda S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat Cell Biol. 2005;7:365–373. doi: 10.1038/ncb1233. [DOI] [PubMed] [Google Scholar]
- 32.Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 33.Tartaglia N, et al. Protein Synthesis-dependent and -independent Regulation of Hippocampal Synapses by Brain-derived Neurotrophic Factor. J Biol Chem. 2001;276:37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- 34.Finkbeiner S, et al. CREB: A major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 35.Ying SW, et al. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1140. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato M, Suzuki K, Nakanishi S. NMDA receptor stimulation and brain-derived neurotrophic factor upregulate homer 1a mRNA via the mitogen-activated protein kinase cascade in cultured cerebellar granule cells. J Neurosci. 2001;21:3797–3805. doi: 10.1523/JNEUROSCI.21-11-03797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci. 1998;18:8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 40.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. Epub 2003 Sep 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vesa J, Kruttgen A, Shooter EM. p75 reduces TrkB tyrosine autophosphorylation in response to brain-derived neurotrophic factor and neurotrophin 4/5. J Biol Chem. 2000;275:24414–24420. doi: 10.1074/jbc.M001641200. [DOI] [PubMed] [Google Scholar]
- 44.Kao S, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem. 2001;276:18169–18177. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- 45.Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurons. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 46.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–11769. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


