Abstract
Early HPV infection in males is difficult to detect clinically and pathologically. This study assessed histopathology in diagnosing male genital HPV. External genital lesions (n = 352) were biopsied, diagnosed by a dermatopathologist, and HPV genotyped. A subset (n = 167) was diagnosed independently by a second dermatopathologist and also re-evaluated in detail, tabulating the presence of a set of histopathologic characteristics related to HPV infection. Cases that received discrepant diagnoses or HPV-related diagnoses were evaluated by a third dermatopathologist (n = 163). Across dermatopathologists, three-way concordance was fair (k = 0.30). Pairwise concordance for condyloma was fair to good (k = 0.30–0.67) and poor to moderate for penile intraepithelial neoplasia (k = −0.05 to 0.42). Diagnoses were 44–47% sensitive and 65–72% specific for HPV 6/ 11-containing lesions, and 20–37% sensitive and 98–99% specific for HPV 16/18. Presence of HPV 6/ 11 was 75–79% sensitive and 35% specific for predicting pathologic diagnosis of condyloma. For diagnosis of penile intraepithelial neoplasia, HPV 16/18 was 95–96% specific but only 40–64% sensitive. Rounded papillomatosis, hypergranulosis, and dilated vessels were significantly (P<0.05) associated with HPV 6/11. Dysplasia was significantly (P= 0.001) associated with HPV 16/18. Dermatopathologists’ diagnoses of early male genital HPV-related lesions appear discordant with low sensitivity, while genotyping may overestimate clinically significant HPV-related disease. Rounded papillomatosis, hypergranulosis, and dilated vessels may help establish diagnosis of early condyloma.
Keywords: HPV, condyloma, penile intraepithelial neoplasia, PeIN, histopathology, biopsy
INTRODUCTION
Human papillomavirus, or HPV (family Papillomaviridiae, genus Alphapapillomavirus), is a common, highly contagious [Lacey et al., 2006] sexually transmitted infection that causes condyloma, penile intraepithelial neoplasia, and penile cancer. It has been reported that up to 60% of sexually active male college students in the United States (US) acquire a new genital HPV infection within 2 years [Partridge et al., 2007], with an estimated 20 million people infected with genital HPV at any one time.
With approximately one million new cases in the US each year [Kirnbauer and Lenz, 2012], condyloma are a frequent cause of medical office visits, (e.g., 360,000 in 2008 in the US), resulting in $6 billion in healthcare costs annually [Division of STD Prevention, 1999]. Although condyloma are not considered malignant, they are a source of pain, bleeding, and genital disfigurement [Maw et al., 1998; Giuliano et al., 2008b], which imposes a considerable psychological burden on the patient [Kirnbauer and Lenz, 2012]. The majority of condyloma are caused by low-risk (LR) types HPV 6 and 11 [Giuliano et al., 2008b; Arima et al., 2010]; however, up to half are co-infected with oncogenic high-risk (HR) HPV types 16 and 18 [Brown et al., 1999; Ball et al., 2011; Pierce Campbell et al., 2013]. Therefore, condyloma theoretically have the potential to confer risk for developing anogenital cancers [Pow-Sang et al., 2010; Blomberg et al., 2012], such as squamous cell carcinoma of the penis and anus [Blomberg et al., 2012]. Diagnosis of condyloma are also an indication to screen patients for additional sexually transmitted diseases [Centers for Disease Control 1996; Institute of Medicine Committee on Prevention and Control of Sexually Transmitted Diseases, 1997].
Early LR-HPV lesions are, therefore, important and sometimes difficult to diagnose, as they clinically resemble bowenoid papulosis, squamous dysplasia, squamous cell carcinoma, molluscum contagiosum, fibroepithelial polyp, seborrheic keratosis, and benign squamous keratosis [Wikstrom, et al., 1992; Barrasso and Gross, 1997; Von Krogh et al., 1997; Von Krogh et al., 2000]. Accurate diagnosis of subtle HPV lesions, including condyloma, early in the clinical course contributes to appropriate treatment intervention, patient education, and risk stratification for future follow-up.
The HR-HPV types, most often HPV 16 and 18, are considered to be the primary etiologic agents for cervical cancer and precancerous lesions in women (e.g., cervical, vaginal, and vulvar intraepithelial neoplasias and high-grade squamous intraepithelial lesions). In addition, HPV is responsible for a subset of squamous cell carcinomas and associated precursor lesions (penile intraepithelial neoplasia, Bowenoid papulosis, Erythroplasia of Queyrat) at other anogenital sites in men (e.g., penis and anus) [Kirnbauer and Lenz, 2012]. A biopsy is indicated to evaluate pigmented, erosive, bleeding, and/or therapy-resistant genital lesions to exclude malignancy. Although penile cancer is uncommon is the US and Europe, with an incidence of <1/100,000 men, it is more frequent in Africa, Asia, and South America and accounts for 10% of all cancers affecting men in certain areas [Van Poppel et al., 2013]. The proportion of penile intraepithelial neoplasias that progress to penile cancer remains unknown [Pierce Campbell et al., 2013].
Currently, there are no FDA-approved tests to diagnose LR- or HR-HPV infection in men, nor are there screening or diagnostic guidelines similar to the Papanicolou test, which is used in cervical cancer screening [Ivanov, 2007]. The utility and limitations of biopsy to diagnose early genital HPV lesions in men has never been investigated fully. The present study seeks to expand our knowledge concerning the relationship between clinically detectable, early external genital lesions, the presence of specific HPV types in these lesions, and the association with a diagnosis of HPV-related pathology. Additionally, this study aims to evaluate the inter-pathologist concordance in diagnosing biopsies of HPV-related male external genital lesions, compare the presence of HPV DNA with pathologic diagnosis of external genital lesions, and evaluate whether specific histopathologic features predict the presence of HPV within external genital lesion tissue.
METHODS AND STUDY DESIGN
Study Patients
Study analysis was done on 352 biopsies of external genital lesions taken from men enrolled in the HPV Infection in Men (HIM) Study, an ongoing prospective HPV natural history study among men living in the US (Tampa, FL), Brazil (São Paulo), and Mexico (Cuernavaca). The HIM Study cohort consists of >4,000 men aged 18–70 years who were recruited between 2005 and 2009 and assessed every 6 months for up to 4 years. Subjects reported no prior diagnosis of anogenital cancer or genital warts and no current symptoms of or treatment for a sexually transmitted infection, including HIV/AIDS. Additional details of the HIM Study have been published elsewhere [Giuliano et al., 2008a, 2011).
Participants who presented with an external genital lesion suspicious of condyloma or penile intraepithelial neoplasia, or of unknown etiology, and who consented to undergo shave biopsy between February 2009 and December 2011, were included in the current analysis. Participants provided written informed consent, and all procedures were approved by the human subjects committees of participating institutions.
Specimen Collection and Processing
At each study visit, participants underwent a thorough visual inspection of the skin and external genitalia (e.g., penile shaft, glans penis/coronal sulcus, scrotum, and perianal region) for the presence of suspicious external genital lesion features (e.g., wart-like architecture, erythematous or hyperpigmented papule or plaque, ulcerated surface) using light and 3× magnification. Visually distinct external genital lesions were shave or scissor snip-biopsied and subjected to pathological evaluation. If multiple lesions were present, the most representative or suspicious external genital lesion was biopsied; however, if multiple types of lesions were observed (e.g., condyloma and penile intraepithelial neoplasia), then one of each type of lesion was biopsied. Formalin-fixed paraffin-embedded (FFPE) tissue blocks from all study sites (Tampa, FL; São Paulo, Brazil; and Cuernavaca, Mexico) were processed at the University of South Florida Dermatopathology Laboratory. Four-micrometer paraffin sections were cut from each block, two for hematoxylin and eosin slides and nine for HPV genotyping, as described previously [Giuliano et al., 2008a, 2011].
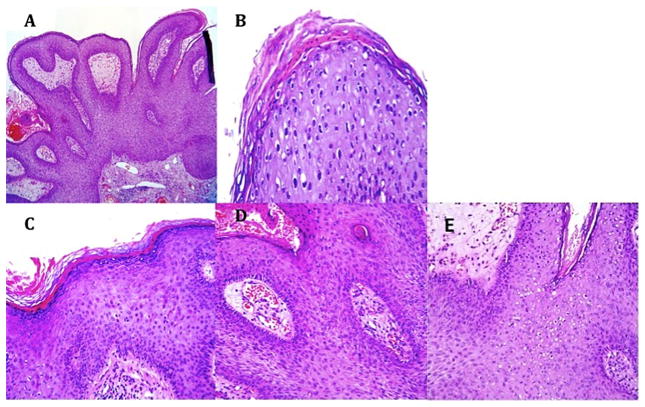
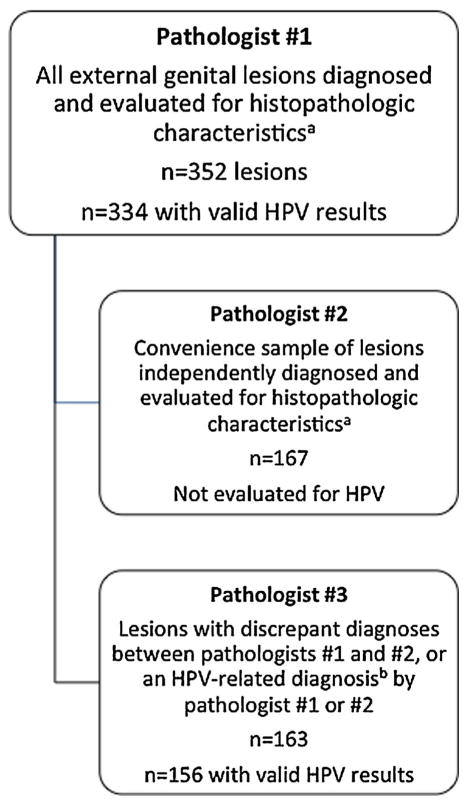
All biopsies (n = 352) were evaluated by a dermatopathologist (Pathologist #1). A second dermatopathologist (Pathologist #2) diagnosed independently the first 167 of these cases received (n = 167); time constraints prevented Pathologist #2 from evaluating all 352 slides. A convenience sample consisting of the cases evaluated by Pathologist #2 (n = 167) were evaluated further by Pathologist #1 for the presence or absence of a set of histopathologic characteristics considered to be related to HPV infection: rounded papillomatosis (Fig. 1A), parakeratosis (Fig. 1B), hypergranulosis (Fig. 1C), dilated vessels (Fig. 1D), koilocytes (Fig. 1E), or binucleation (Fig. 1E). In addition, this subset of biopsies was also evaluated for the presence or absence of horn cysts, hyper-pigmentation, and dysplasia/atypia. All tissues that received discrepant diagnoses between Pathologists #1 and 2, or HPV-related diagnoses of condyloma or penile intraepithelial neoplasia I–III by either Pathologist #1 or 2, were evaluated by a third pathologist (Pathologist #3) who had expertise in HPV (n = 163). Please see Figure 2, which illustrates how case evaluation was distributed among the three pathologists.
Figure 1.
Common histological findings in condylomas: (A) rounded papillomatosis; (B) parakeratosis; (C) hypergranulosis; (D) dilated vessels; (E) koilocytes and binucleation.
Figure 2.
Overview of specimen evaluation. aHistopathologic characteristics included rounded papillomatosis (Fig. 1A), parakeratosis (Fig. 1B), hypergranulosis (Fig. 1C), dilated vessels (Fig. 1D), koilocytes and binucleation (Fig. 1E), horn cysts, hyperpigmentation, and dysplasia/atypia. bHPV-related diagnoses included condyloma, penile intraepithelial neoplasia I, and penile intraepithelial neoplasia II/III. Note: The lesions evaluated by Pathologist #2 and 3 are not mutually exclusive. Only 155 lesions were utilized for the three-way concordance analysis.
DNA Extraction and HPV Genotyping
All external genital lesion tissue specimens underwent manual DNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, Gaithersburg, MD). Specimens were genotyped for the presence of mucosal HPV using the INNO-LiPA HPV Genotyping Extra assay (Fujirebio, Ghent, Belgium), which detects 15 LR-HPV types (6, 11, 26, 40, 43, 44, 53, 54, 66, 69, 70, 71, 73, 74, 82) and 13 high-risk HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) [Bouvard et al., 2009]. If samples tested positive for β-globin or an HPV genotype, their HPV test results were considered valid.
Statistical Analysis
Pathological diagnoses for each external genital lesion were categorized as not condyloma/HPV, suggestive but not diagnostic of HPV, condyloma, penile intraepithelial neoplasia grade I, or penile intraepithelial neoplasia grade II/III. External genital lesions suggestive but not diagnostic of HPV included entities that share common histological characteristics with condyloma but without diagnostic koilocytes, and were usually given a diagnosis of benign squamous keratosis. Some had features of seborrheic keratosis. External genital lesions characterized as not condyloma or HPV-unrelated included various benign skin conditions such as unequivocal seborrheic keratosis and fibroepithelial polyps (skin tags), basal cell carcinoma, and inflammatory conditions such as lichen planus. Three-way and pairwise pathologist concordance (n = 155) was estimated using the κ coefficient (k) and standard errors (SE).
Sensitivity and specificity were used to compare pathologists’ diagnoses to the results of the INNO-LiPA HPV genotyping assay. Separate analyses were conducted for “any HPV,” “HPV 6/11,” “HPV 16/18,” “LR-HPV,” and “HR-HPV.” Analysis for infection with “any HPV” included all pathological diagnoses thought to be HPV-related (condyloma, penile intra-epithelial neoplasia grade I, and penile intraepithelial neoplasia grade II/III) and HPV genotyping results that included the presence of at least one of the 28 assayed HPV genotypes. Evaluation of infection for “HPV 6/11” included pathological diagnoses thought to be HPV 6/11-related (condyloma) and assay positive for either HPV 6 or 11, or both. “HPV 16/18” included all pathological diagnoses thought to be HPV 16/18-related (penile intraepithelial neoplasia grade I or grade II/III) and assay positive for either HPV 16 or 18, or both. “LR-HPV” included all pathological diagnoses thought to be related to LR-HPV infections (condyloma) and HPV genotyping results that included the presence of at least one of the LR-HPV genotypes included in the assay. “HR-HPV” included all pathological diagnoses thought to be related to HR-HPV infection (penile intraepithelial neoplasia grade I and grade II/III) and HPV genotyping results that included the presence of at least one of the HR-HPV genotypes. Sensitivity and specificity were reported as percentages along with 95% confidence intervals (CIs) based on a binomial distribution.
Logistic regression was used to evaluate which histopathologic characteristics were predictive of HPV DNA detected within the external genital lesion tissue, using the results of the INNO-LiPA HPV genotyping assay. Separate analyses were conducted for “any HPV,” “LR-HPV,” and “HR-HPV” outcomes, using the same categories described above. Each characteristic was coded as a binary variable (absent vs. present). Univariate associations between each histopathologic characteristic and each HPV outcome were assessed independently. Multivariable models included those characteristics identified as statistically significant in univariate models. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Characteristics of External Genital Lesions That Underwent Pathological Evaluation
The 352 lesions included in this analysis were collected from men who ranged in age from 20 to 66 years with a median age at biopsy of 31 years (Table I). Slightly more external genital lesions were noted in men residing in Brazil (n = 137 [38.9%]), followed by the US (n = 112 [31.8%]), and Mexico (n = 103 [29.3%]). Diagnoses by Pathologist #1 included 145 condyloma, 112 suggestive but not diagnostic of HPV, 12 penile intraepithelial neoplasia, and 83 other HPV-unrelated diagnoses. All tissue specimens were assessed for the presence of HPV DNA; however, 18 lesions had an invalid HPV result (no β-globin or HPV genotype present), resulting in 334 lesions with valid HPV results. A total of 294 lesions (88.0%) tested positive for one or more HPV genotypes, and 40 (12.0%) tested negative for HPV. Of the 294 HPV-positive lesions evaluated by Pathologist #1, 132 (44.9%) were diagnosed as condyloma, 96 (32.7%) were suggestive but not diagnostic of HPV, 11 (3.7%) were penile intraepithelial neoplasia, and 55 (18.7%) were not HPV-related. Of the 163 lesions evaluated by Pathologist #3, 156 had valid HPV results.
TABLE I.
Characteristics of 352 External Genital Lesions* That Underwent Pathological Evaluation
| n (%) | |
|---|---|
| Country | |
| USA | 112 (31.8%) |
| Brazil | 137 (38.9%) |
| Mexico | 103 (29.3%) |
| Age (years) | |
| Range | 20–66 |
| Median (IQR) | 31 (25–39) |
| 18–30 | 173 (49.2%) |
| 31–44 | 141 (40.1%) |
| 45+ | 38 (10.8%) |
| Anatomical site | |
| Coronal sulcus | 37 (10.5%) |
| Glans, including meatus | 14 (4.0%) |
| Inguinal | 12 (3.4%) |
| Mons | 3 (0.9%) |
| Penile shaft | 215 (61.1%) |
| Perianal | 29 (8.2%) |
| Perineum | 1 (0.3%) |
| Scrotum | 41 (11.7%) |
| Pathological diagnosis—Pathologist #1 | |
| Not condyloma/HPV | 83 (23.6%) |
| Suggestive but not diagnostic of HPV | 112 (31.8%) |
| Condyloma | 145 (41.2%) |
| Penile intraepithelial neoplasia, grade I | 2 (0.6%) |
| Penile intraepithelial neoplasia, grades II/III | 10 (2.8%) |
| HPV genotyping (INNO-LiPA) | |
| Positive | 294 (83.5%) |
| Negative | 40 (11.4%) |
| Invalid | 18 (5.1%) |
| Rounded papillomatisis | |
| Yes | 128 (76.7%) |
| No | 39 (23.3%) |
| Parakeratosis | |
| Yes | 93 (55.7%) |
| No | 74 (44.3%) |
| Hypergranulosis | |
| Yes | 104 (62.3%) |
| No | 63 (37.7% |
| Koilocytes | |
| Yes | 37 (22.2%) |
| No | 130 (77.8%) |
| Dilated vessels | |
| Yes | 106 (63.5%) |
| No | 61 (36.5%) |
| Binucleation | |
| Yes | 10 (6.0%) |
| No | 157 (94.0%) |
| Horn cysts | |
| Yes | 8 (4.8%) |
| No | 159 (95.2%) |
| Hyperpigmentation | |
| Yes | 18 (10.8%) |
| No | 149 (89.2%) |
| Dysplasia/atypia | |
| Yes | 8 (4.9%) |
| No | 157 (95.1%) |
Multiple lesions were possible for each man.
Interpathologist Concordance
Concordance across all three pathologists was fair (k = 0.30; SE = 0.03; n = 155). Pairwise concordance tests for all diagnoses produced poor to moderate levels of agreement between pathologists (k = 0.17–0.47). For diagnoses suggesting condyloma and diagnoses of condyloma, pairwise concordance was poor to moderate (k = 0.09–0.42). For diagnoses of condyloma alone, pairwise concordance improved from fair to good range (k = 0.30–0.67). Diagnoses of penile intraepithelial neoplasia grade I–III had poor to moderate concordance (k = −0.05 to 0.42) (data not shown).
Sensitivity and Specificity of Pathologist Diagnosis and HPV Genotyping
Tables II and III provide sensitivity and specificity estimates for 334 specimens with valid HPV results reviewed by Pathologist #1 and 156 specimens with valid HPV results evaluated by Pathologist #3. In Table II, the INNO-LiPA HPV genotyping result is considered the gold standard, and in Table III, the pathological diagnosis is the gold standard.
TABLE II.
Sensitivity and Specificity Comparing Pathologic Diagnosis to HPV DNA Detected Within the External Genital Lesion
| Sensitivity (%) | 95%CI | Specificity (%) | 95%CI | |
|---|---|---|---|---|
| Pathologist #1 (n = 334) | ||||
| Any HPV-related external genital lesion vs. any HPVa | 48.6 | 42.9–54.4 | 70.0 | 55.8–84.2 |
| Condyloma vs. HPV 6/11b | 46.6 | 40.1–53.0 | 64.7 | 55.4–74.0 |
| Penile intraepithelial neoplasia I–III vs. HPV 16/18c | 36.8 | 15.2–58.5 | 98.7 | 97.5–100 |
| Penile intraepithelial neoplasia I–III vs. HR-HPVd | 18.8 | 7.7–29.8 | 99.3 | 98.3–100 |
| Condyloma vs. LR-HPVe | 46.1 | 40.3–51.9 | 73.1 | 61.0–85.1 |
| Pathologist #3 (n = 156) | ||||
| Any HPV-related external genital lesion vs. any HPVa | 44.9 | 36.6–53.2 | 77.8 | 58.6–97.0 |
| Condyloma vs. HPV 6/11b | 43.6 | 34.4–52.9 | 71.7 | 58.7–84.8 |
| Penile intraepithelial neoplasia I–III vs. HPV 16/18c | 20.0 | 00.0–44.8 | 98.0 | 95.6–100 |
| Penile intraepithelial neoplasia I–III vs. HR-HPVd | 8.7 | 0–20.2 | 97.7 | 95.2–100 |
| Condyloma vs. LR-HPVe | 42.5 | 34.2–50.9 | 81.8 | 65.7–97.9 |
All pathological diagnoses thought to be HPV-related (condyloma, penile intraepithelial neoplasia I, and penile intraepithelial neoplasia II/ III) and HPV genotyping results that included the presence of at least one of the 28 HPV genotypes included in the assay.
All pathological diagnoses thought to be HPV 6/11-related (condyloma) and HPV results that included either HPV 6 or 11, or both.
All pathological diagnoses thought to be HPV 16/18-related (penile intraepithelial neoplasia I, and penile intraepithelial neoplasia II/III) and HPV results that included either HPV 16 or 18, or both.
All pathological diagnoses thought to be related to HR-HPV infection (penile intraepithelial neoplasia I and penile intraepithelial neoplasia II/III) and HPV results that included the presence of at least one of the HR-HPV genotypes included in the assay.
All pathological diagnoses thought to be related to LR-HPV infections (condyloma) and HPV results that included the presence of at least one of the LR-HPV genotypes included in the assay.
TABLE III.
Sensitivity and Specificity Comparing HPV DNA Detected Within the External Genital Lesion to Pathologic Diagnosis
| Sensitivity (%) | 95%CI | Specificity (%) | 95%CI | |
|---|---|---|---|---|
| Pathologist #1 (n = 334) | ||||
| Any HPV vs. any HPV-related external genital lesiona | 92.3 | 88.1–96.5 | 15.6 | 10.3–21.0 |
| HPV 6/11 vs. condylomab | 75.0 | 67.9–82.1 | 34.7 | 28.0–41.5 |
| HPV 16/18 vs. Penile intraepithelial neoplasia I–IIIc | 63.6 | 35.2–92.1 | 96.3 | 94.2–98.4 |
| HR-HPV vs. Penile intraepithelial neoplasia I–IIId | 81.8 | 59.0–100 | 87.9 | 84.4–91.5 |
| LR-HPV vs. condylomae | 90.3 | 85.4–95.1 | 20.0 | 14.3–25.7 |
| Pathologist #3 (n = 156) | ||||
| Any HPV vs. any HPV-related external genital lesiona | 93.9 | 88.2–99.7 | 15.6 | 8.1–23.0 |
| HPV 6/11 vs. condylomab | 78.7 | 68.4–89.0 | 34.7 | 25.2–44.3 |
| HPV 16/18 vs. Penile intraepithelial neoplasia I–IIIc | 40.0 | 0.0–82.9 | 94.7 | 91.1–98.3 |
| HR-HPV vs. Penile intraepithelial neoplasia I–IIId | 40.0 | 0.0–82.9 | 86.1 | 80.6–91.6 |
| LR-HPV vs. condylomae | 93.4 | 87.2–99.7 | 19.0 | 11.1–26.8 |
All pathological diagnoses thought to be HPV-related (condyloma, penile intraepithelial neoplasia I, and penile intraepithelial neoplasia II/ III) and HPV genotyping results that included the presence of at least one of the 28 HPV genotypes included in the assay.
All pathological diagnoses thought to be HPV 6/11-related (condyloma) and HPV results that included either HPV 6 or 11, or both.
All pathological diagnoses thought to be HPV 16/18-related (penile intraepithelial neoplasia I, and penile intraepithelial neoplasia II/III) and HPV results that included either HPV 16 or 18, or both.
All pathological diagnoses thought to be related to HR-HPV infection (penile intraepithelial neoplasia I and penile intraepithelial neoplasia II/III) and HPV results that included the presence of at least one of the HR-HPV genotypes included in the assay.
All pathological diagnoses thought to be related to LR-HPV infections (condyloma) and HPV results that included the presence of at least one of the LR-HPV genotypes included in the assay.
Pathologic diagnoses of HPV-related lesions (condyloma, penile intraepithelial neoplasia grade I, and penile intraepithelial neoplasia grade II/III) were 45–49% sensitive and 70–78% specific in predicting the presence of any of the 28 HPV genotypes included in the assay, depending on the pathologist (Table II). The diagnosis of condyloma was 44–47% sensitive and 65–72% specific in predicting the presence of HPV 6/11. While a diagnosis of penile intraepithelial neoplasia grade I or penile intraepithelial neoplasia grade II/III had low sensitivity for detecting infection with HPV 16/18 (20–37%) or any HR-HPV type (9–19%), these diagnoses showed high specificity in predicting infection with HPV 16/18 (98–99%) or any HR-HPV type (98–99%).
An HPV-positive result (any HPV) was 92–94% sensitive but only 16% specific for predicting an HPV-related pathological diagnosis (Table III). In the prediction of pathological diagnoses of condyloma, results that included any LR-HPV type were more sensitive (90–93%) but less specific (19–20%) than results that included only HPV 6, HPV 11, or both (75–79% sensitive; 35% specific). For the prediction of penile intraepithelial neoplasia grade I–III, results that included HPV 16, HPV 18, or both were more specific (95–96%), but less sensitive (40–64%) than results that included any HR-HPV type (40–82% sensitive and 86–88% specific).
Histopathologic Characteristics Associated With HPV DNA
Table IV shows results of logistic regression analyses, used to assess how well each histological characteristic predicted the presence of HPV DNA within the external genital lesion. In univariate analyses, the presence of rounded papillomatosis (OR = 4.83, CI [1.71–13.68]; P = 0.003), parakeratosis (OR = 3.52, CI [1.18–10.52]; P = 0.024), hypergranulosis (OR = 3.63, CI [1.27–10.41]; P = 0.017), and dilated vessels (OR = 3.866, CI [1.35–11.10]; P = 0.012) were each significantly associated with an increased likelihood of detecting HPV (any HPV type) within the lesion. Potential correlation between variables was assessed prior to multivariable modeling; none of the variables showed statistically significant correlation with one another. When these four characteristics were included together in a multivariable model, none of the characteristics were independently associated with the likelihood of detecting HPV.
TABLE IV.
Histopathologic Characteristics (Presence vs. Absence) Associated With HPV DNA Detection Within External Genital Lesion Tissue
| Presence vs. absence | Univariate
|
Multivariable
|
||||
|---|---|---|---|---|---|---|
| OR | (95%CI) | P-value | OR | (95%CI) | P-value | |
| Any HPVa | ||||||
| Rounded papillomatosis | 4.8 | 1.7–13.7 | 0.003 | 2.7 | 0.8–9.8 | 0.122 |
| Parakeratosis | 3.5 | 1.2–10.5 | 0.024 | 2.8 | 0.9–9.2 | 0.084 |
| Hypergranulosis | 3.6 | 1.3–10.4 | 0.017 | 2.3 | 0.7–7.5 | 0.174 |
| Koilocytes | 2.3 | 0.5–10.4 | 0.298 | – | – | |
| Dilated vessels | 3.9 | 1.4–11.1 | 0.012 | 1.5 | 0.3–5.4 | 0.573 |
| Binucleation | 1.1 | 0.1–9.0 | 0.947 | – | – | – |
| Horn cysts | 0.7 | 0.1–6.2 | 0.749 | – | – | – |
| Hyperpigmentation | 0.5 | 0.1–1.8 | 0.276 | – | – | – |
| Dysplasia/Atypia | 1000 | 0.0–1000 | 0.980 | – | – | – |
| HPV 6/11b | ||||||
| Rounded papillomatosis | 3.8 | 1.8–8.3 | <0.001 | 2.8 | 1.1–6.8 | 0.026 |
| Parakeratosis | 1.7 | 0.9–3.4 | 0.122 | – | – | – |
| Hypergranulosis | 2.6 | 1.3–5.3 | 0.007 | 1.9 | 0.9–4.1 | 0.083 |
| Koilocytes | 1.9 | 0.8–4.7 | 0.173 | – | – | – |
| Dilated vessels | 2.2 | 1.1–4.5 | 0.025 | 1.3 | 0.6–2.9 | 0.579 |
| Binucleation | 1.0 | 0.2–3.9 | 0.964 | – | – | – |
| Horn cysts | 1.0 | 0.2–5.6 | 0.962 | – | – | – |
| Hyperpigmentation | 0.4 | 0.1–1.1 | 0.064 | – | – | – |
| Dysplasia/Atypia | 0.5 | 0.1–2.5 | 0.420 | – | – | – |
| HPV 16/18c | ||||||
| Rounded papillomatosis | 0.4 | 0.1–1.5 | 0.183 | – | – | – |
| Parakeratosis | 3.3 | 0.7–16.1 | 0.138 | – | – | – |
| Hypergranulosis | 1.4 | 0.4–5.6 | 0.643 | – | – | – |
| Koilocytes | 0.4 | 0.1–3.1 | 0.366 | – | – | – |
| Dilated vessels | 0.3 | 0.1–1.3 | 0.110 | – | – | – |
| Binucleation | 0.0 | 0.0–1000 | 0.972 | – | – | – |
| Horn cysts | 0.0 | 0.0–1000 | 0.976 | – | – | – |
| Hyperpigmentation | 2.4 | 0.5–12.6 | 0.290 | – | – | – |
| Dysplasia/Atypia | 15.4 | 2.9–82.6 | 0.001 | 15.4 | 2.9–82.6 | 0.001 |
| HR-HPVd | ||||||
| Rounded papillomatosis | 0.5 | 0.2–1.3 | 0.133 | – | – | – |
| Parakeratosis | 1.3 | 0.5–3.1 | 0.630 | – | – | – |
| Hypergranulosis | 0.6 | 0.2–1.4 | 0.243 | – | – | – |
| Koilocytes | 0.3 | 0.1–1.4 | 0.116 | – | – | – |
| Dilated vessels | 0.6 | 0.2–1.4 | 0.191 | – | – | – |
| Binucleation | 0.7 | 0.1–5.4 | 0.686 | – | – | – |
| Horn cysts | 0.0 | 0–1000 | 0.977 | – | – | – |
| Hyperpigmentation | 0.8 | 0.2–4.0 | 0.822 | – | – | – |
| Dysplasia/Atypia | 18.5 | 3.3–102.4 | <0.001 | 18.5 | 3.3–102.4 | <0.001 |
| LR-HPVe | ||||||
| Rounded papillomatosis | 6.4 | 2.4–16.9 | <0.001 | 4.9 | 1.5–15.7 | 0.008 |
| Parakeratosis | 2.3 | 0.9–6.0 | 0.078 | – | – | – |
| Hypergranulosis | 2.6 | 1.0–6.6 | 0.044 | 1.7 | 0.6–4.9 | 0.368 |
| Koilocytes | 1.8 | 0.5–6.5 | 0.372 | – | – | – |
| Dilated vessels | 4.5 | 1.7–11.9 | 0.003 | 1.8 | 0.6–5.9 | 0.309 |
| Binucleation | 1.4 | 0.2–11.5 | 0.764 | – | – | – |
| Horn cysts | 0.9 | 0.1–7.9 | 0.926 | – | – | – |
| Hyperpigmentation | 0.3 | 0.1–0.9 | 0.032 | 0.2 | 0.1–0.8 | 0.021 |
| Dysplasia/Atypia | 0.2 | 0.0–0.8 | 0.028 | – | – | – |
– Indicates that the variable was not included in the model. Bolded values denote statistical significance.
HPV genotyping results that included the presence of at least one of the 28 HPV genotypes included in the assay.
HPV results that included either HPV 6 or 11, or both.
HPV results that included either HPV 16 or 18, or both.
HPV results that included the presence of at least one of the HR-HPV genotypes included in the assay.
HPV results that included the presence of at least one of the LR-HPV genotypes included in the assay.
For HPV 6/11, the presence of rounded papillomatosis (OR = 3.83, CI [1.76–8.34]; P <0.001), hypergranulosis (OR = 2.64, CI [1.31–5.32]; P = 0.007), and dilated vessels (OR = 2.23 CI [1.11–4.48]; P = 0.025) were each significantly associated with an increased likelihood of detecting HPV 6, 11, or both within the lesion. When only those characteristics independently associated with an increased likelihood of detecting HPV 6/11 were included together in a multivariable model, rounded papillomatosis was the only feature that appeared to be independently associated with a greater likelihood of detecting HPV 6/11 (OR = 2.78, CI [1.13–6.82]; P = 0.026). Similarly, rounded papillomatosis (OR = 6.39, CI [2.42–16.85]; P <0.001), hypergranulosis (OR = 2.61, CI [1.03–6.64]; P = 0.044), and dilated vessels (OR = 4.47, CI (1.68–11.85); P = 0.003) were significantly associated with an increased likelihood of detecting a LR-HPV type within the lesion. The presence of dysplasia (OR = 0.17, CI [0.035–0.82]; P = 0.028) and hyperpigmentation (OR = 0.28, CI [0.085–0.89]; P = 0.032] were each associated with a decreased likelihood of detecting a LR-HPV type (P <0.05). After adjusting for the presence of these independently significant features in a multivariable model, only the presence of rounded papillomatosis (OR = 4.87, CI [1.51–15.65]; P = 0.008) and absence of hyperpigmentation (OR = 0.21, CI [0.06–0.79]; P = 0.021) remained significantly associated with a greater likelihood of detecting infection with a LR-HPV type.
The presence of dysplasia/atypia was the only histological characteristic independently associated with increased likelihood of detecting HPV 16/18 (OR = 15.43, CI [2.88–82.63]; P = 0.001). Similarly, dysplasia/atypia was significantly associated with the likelihood of detecting a HR-HPV type within the lesion (OR = 18.47, CI [3.33–102.35]; P < 0.001).
DISCUSSION
Currently, HPV DNA tests are only FDA-approved for use in combination with Papanicolaou (Pap) smear tests and for follow-up of women with abnormal Pap smears for cervical cancer screening [Akogbe et al., 2012]. Meanwhile, there is no accepted universal method for the accurate detection of clinically relevant HPV infection in men (i.e., condyloma, penile intraepithelial neoplasia, penile and anal squamous cell carcinoma) to facilitate appropriate follow-up surveillance and treatment intervention. The Centers for Disease Control and Prevention (CDC) in the US do not recommend screening for HPV-related disease for anal, penile, or head/neck cancers for men in the US. However, some healthcare providers offer anal Pap tests to high-risk men who may be at increased risk for anal cancer (men with HIV or men who receive anal sex) [Centers for Disease Control and Prevention, 2015].
Given that HPV DNA is detected on 65–96% of male genitalia [Giuliano et al., 2008a; Anic et al., 2013], identifying the subset of men who will develop clinically significant disease is difficult. The results of the present study found genotyping of biopsy specimens from male genitalia to be hampered by low specificity, while the histopathology underestimated the diagnosis (or had low sensitivity) when used alone. Although histopathology appeared to be a reliable method for diagnosing penile intraepithelial neoplasia in this study, the diagnosis of condyloma was more difficult.
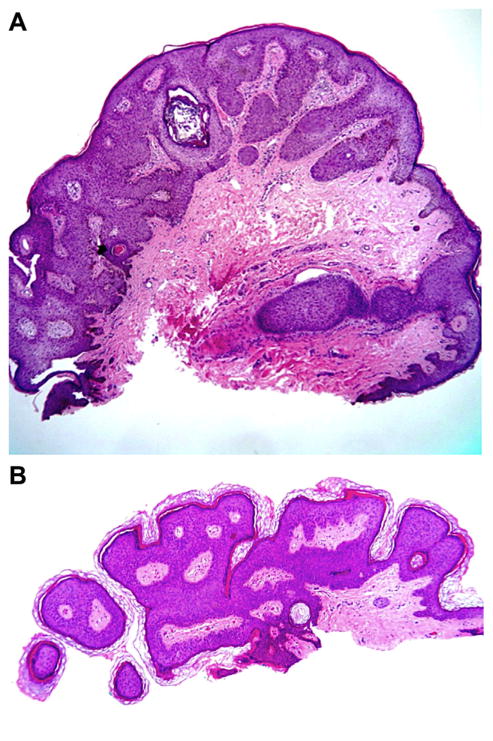
The ongoing HIM Study, which examines prospectively the natural history of genital HPV infection, has demonstrated that 112/2487 (4%) of men will develop clinically detectable condyloma within a median follow-up time of 18 months and that this risk is highest for those with incident infection with HPV types 6 and 11 [Anic et al., 2012]. While well-developed condyloma exhibit obvious features (Fig. 3), lesions biopsied early in the clinical course of HPV infection display mixed histological features and are more challenging diagnostically (Fig. 4A and B). These histologically indeterminate lesions, which do not show pathologic findings specific for HPV infection, are less likely than lesion tissue to be positive for HPV [Anic et al., 2013]. For these ambiguous cases, perhaps additional genotyping of exfoliated cells from the lesion at follow-up later in the clinical course could be considered.
Figure 3.
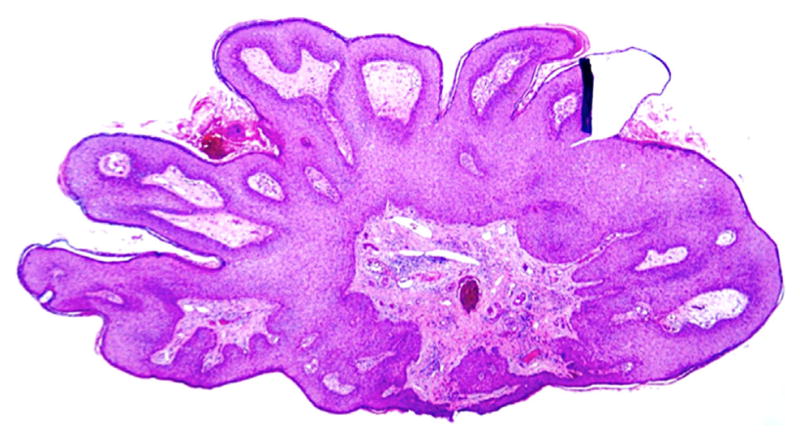
Classic condyloma with well-established features obvious on low power magnification: rounded papillomatosis, hypergranulosis, dilated vessels, koilocytes, horn cysts.
Figure 4.
(A and B) Benign squamous keratosis (BSK). These entities have some features of a condyloma: horn cysts, dilated vessels, hypergranulosis, and parakeratosis.
The present study observed significant discordance among expert pathologists for early HPV diagnosis in men, which is similar to that seen among pathologists’ readings of cervical intraepithelial lesions [Stoler and Schiffman, 2001; Parker et al., 2002; Ceballos et al., 2008]. This discordance possibly indicates a lack of accurate criteria for histological diagnosis of HPV. Although the most consistent histologic features seen in condyloma are considered to be epidermal hyperplasia, parakeratosis, koilocytosis, and papillomatosis [Kirnbauer and Lenz, 2012], our data indicate, rather, that the presence of rounded papillomatosis, hypergranulosis, dilated vessels, and parakeratosis may indicate an early condyloma. However, no combination of these features can augment specificity of the diagnosis. Surprisingly, the presence of koilocytes was not significantly predictive of the presence of HPV, likely due to their low frequency in early lesions. Alternatively, perhaps cytoplasmic vacuolization is only specific for condyloma when located within deeper portions of the epidermis such as the stratum spinosum, given that the upper portions of the epithelia of mucosal surfaces normally have some degree of cytoplasmic vacuolization already [Lever and Elder, 1997]. As the absence of viral cytopathic change does not exclude HPV, condyloma should be considered in the differential diagnosis of any squamous proliferation in a sexually active patient. The present data support findings from a previous study, in which low to moderate agreement between individual LR- and HR-HPV types and specific histology was observed [Anic et al., 2013]. While biopsy of early external genital lesions may not be useful in prediction of risk for HPV-associated neoplasia when used alone [Strand et al., 1996], these findings highlight further the low sensitivity of pathologic interpretation of clinically subtle lesions. Additional studies in the future should refine any potential screening and analysis criteria to identify men at risk for developing dysplasia and carcinoma related to HPV infection.
The present study observed that viral genotyping of HPV DNA tests appear to be more sensitive for the diagnosis of condyloma, while biopsy appears to be more specific, which is analogous to previous studies regarding cervical specimens from females [Sigurdsson et al., 1997]. The guidelines from the American College of Obstetrics and Gynecology (ACOG), which encourage long-term follow up of patients to determine the clinical significance of a positive HPV genotyping result [The American Congress of Obstetricians and Gynecologists, 2012] could also apply to men.
The present authors do not recommend generalized screening for detecting HPV in males in the US and Europe at this time, given the low incidence rate [Van Poppel et al., 2013] and prevalence of precancerous lesions and penile cancer. Additionally, it is impractical to consider routine, worldwide use of PCR assays (which are the gold standard in HPV DNA detection) given the prohibitive technical requirements and associated financial costs [Chaux et al., 2014]. However, for physicians with a high index of suspicion for HR-HPV infection who wish to evaluate a concerning lesion further, the authors recommend utilizing broad HPV DNA testing of the specimen to complement histopathology, or using skin swabs to assess beforehand whether or not skin biopsy is warranted (reserving biopsy for skin swabs that test positive for HR-HPV types). This recommendation is similar to current screening guidelines for HPV in women [The American Congress of Obstetricians and Gynecologists, 2012], although there are no official guidelines approving the use of HPV DNA testing in males. Among men at high risk of developing cancer (e.g., immunocompromised men), samples for HPV DNA testing should be harvested from exfoliated cells taken from any anatomic site where HPV is known to cause disease (penile shaft, glans penis/coronal sulcus, scrotum, perianal region, and anus) [Giuliano et al., 2007], given that high concordance has been associated previously between swab and biopsy specimens from external genital lesions for HR-HPV types [Anic et al., 2013]. A potential alternative to PCR assays to detect HR-HPV in males could be the Cobas HPV test (Roche), a recently approved first line tool to determine the need for colposcopy and Pap smear [US Food and Drug Administration, 2014]. Although ELISAs using L1 VLPs as the antigen are available and have been useful in epidemiologic studies [Kirnbauer et al., 1994], they are not suitable to diagnose individual patients due to low antibody titers and variable intervals between infection and seroconversion [Kirnbauer and Lenz, 2012].
One limitation of this study is that the HPV test used, INNO-LiPA HPV Genotyping Extra assay (Fujirebio, Ghent, Belgium), detects only Alphapapillomaviruses, so cutaneous HPV types (Betapapillomavirus) were not detected [Pierce Campbell et al., 2013; Sichero et al., 2013]. Additional limitations could possibly include inconsistencies in specimen collection across countries in addition to low viral load in the lesions, preventing detection by INNO-LiPA (despite the sensitivity of this method).
This study highlights the pitfalls in early diagnosis of HPV infection in men through biopsy alone. Routine histology is neither sensitive nor specific in predicting the presence of HPV infection, and the presence of this nearly ubiquitous virus in skin samples may not indicate a diseased state. Even expert pathologists show moderate concordance at best when analyzing skin biopsies of early lesions, further demonstrating the need for refined screening and analysis criteria in predicting men at risk of developing dysplasia and carcinoma related to HPV infection.
Acknowledgments
Grant sponsor: National Cancer Institute, National Institutes of Health (to ARG); Grant number: R01CA098803.; Grant sponsor: American Cancer Society (to CMPC); Grant number: PF-13-222-01.
ARG received research funding from Merck Sharp & Dohme Corp. MHS, LLV, and ARG are consultants of Merck Sharp & Dohme Corp. for HPV vaccines. None of the other authors have conflicts of interest to report.
We thank the HIM Study participants and teams in the USA (Moffitt Cancer Center, Tampa, FL: HY Lin, C Gage, K Eyring, K Kennedy, K Isaacs, A Bobanic, MT O’Keefe, MR Papenfuss, W Fulp), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer Estado de São Paulo, and Ludwig Institute for Cancer Research, São Paulo: ML Baggio, L Galan, L Sichero, E Gomes, E Brito, F Cernicchiaro, R Cintra, R Cunha, R Matsuo, V Souza, B Fietzek, R Hessel, V Relvas, F Silva, J Antunes, G Ribeiro, R Bocalon, R Otero, R Terreri, S Araujo, M Ishibashi, CRT-DST/ AIDS nursing team), and Mexico (Instituto Mexicano del Seguro Social and Instituto Nacional de Salud Pública, Cuernavaca: A Cruz Valdez, R Alvear Vásquez, O Rojas Juárez, R González Sosa, A Salgado Morales, A Rodríguez Galván, P Román Rodríguez, A Landa Vélez, M Zepeda Mendoza, G Díaz García, V Chávez Abarca, J Ruiz Sotelo, A Gutiérrez Luna, M Hernández Nevárez, G Sánchez Martínez, A Ortiz Rojas, C Barrera Flores).
References
- Akogbe GO, Ajidahun A, Sirak B, Anic GM, Papenfuss MR, Fulp WJ, Lin HY, Abrahamsen M, Villa LL, Lazcano-Ponce E, Quiterio M, Smith D, Schabath MB, Salmeron J, Giuliano AR. Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States. Int J Cancer. 2012;131:E282–E291. doi: 10.1002/ijc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anic GM, Lee JH, Villa LL, Lazcano-Ponce E, Gage C, Jose CSR, Baggio ML, Quiterio M, Salmeron J, Papenfuss MR, Abrahamsen M, Stockwell H, Rollison DE, Wu Y, Giuliano AR. Risk factors for incident condyloma in a multinational cohort of men: The HIM study. J Infect Dis. 2012;205:789–793. doi: 10.1093/infdis/jir851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anic GM, Messina JL, Stoler MH, Rollison DE, Stockwell H, Villa LL, Lazcano-Ponce E, Gage C, Silva RJ, Baggio ML, Salmeron J, Giuliano AR. Concordance of human papillomavirus types detected on the surface and in the tissue of genital lesions in men. J Med Virol. 2013;85:1561–1566. doi: 10.1002/jmv.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima Y, Winer RL, Feng Q, Hughes JP, Lee SK, Stern ME, O’Reilly SF, Koutsky LA. Development of genital warts after incident detection of human papillomavirus infection in young men. J Infect Dis. 2010;202:1181–1184. doi: 10.1086/656368. [DOI] [PubMed] [Google Scholar]
- Ball SL, Winder DM, Vaughan K, Hanna N, Levy J, Sterling JC, Stanley MA, Goon PK. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J Med Virol. 2011;83:1345–1350. doi: 10.1002/jmv.22111. [DOI] [PubMed] [Google Scholar]
- Barrasso R, Gross G. A clinical atlas. Berlin: Wiesbaden Ullstein-Mosby; 1997. External genitalia: Diagnosis. Human papillomavirus infection; pp. 291–361. [Google Scholar]
- Blomberg M, Friis S, Munk C, Bautz A, Kjaer SK. Genital warts and risk of cancer: A Danish study of nearly 50000 patients with genital warts. J Infect Dis. 2012;205:1544–1553. doi: 10.1093/infdis/jis228. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens-Part B: Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos KM, Chapman W, Daya D, Julian JA, Lytwyn A, McLachlin CM, Elit L. Re producibility of the histological diagnosis of cervical dysplasia among pathologists from 4 continents. Int J Gynecol Pathol. 2008;27:101–107. doi: 10.1097/pgp.0b013e31814fb1da. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Sexually transmitted disease surveillance 1995. Atlanta, GA: Centers for Disease Control and Prevention, U. S. Department of Health Services Publis Health Service; 1996. [Google Scholar]
- Centers for Disease Control and Prevention. HPV and Men—Fact Sheet. 2015 Jan 28; Retrieved March 1, 2015 from http://www.cdc.gov/std/hpv/stdfact-hpv-and-men.htm.
- Chaux A, Cubilla AL, Haffner MC, Lecksell KL, Sharma R, Burnett AL, Netto GJ. Combining routine morphology, p16(INK4a) immunohistochemistry, and in situ hybridization for the detection of human papillomavirus infection in penile carcinomas: A tissue microarray study using classifier performance analyses. Urol Oncol. 2014;32:171–177. doi: 10.1016/j.urolonc.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Division of STD Prevention. Prevention of genital HPV infection and sequelae: Report of an External Consultants’ Meeting. Atlanta, GA: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, Markowitz LE, Smith D, Harris RB. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: The HPV detection in men study. J Infect Dis. 2007;196:1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, Papenfuss MR, Abrahamsen M, Jolles E, Nielson CM, Baggio ML, Silva R, Quiterio M. The human papillomavirus infection in men study: Human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008a;17:2036–2043. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, Munoz N, Schiffman M, Bosch FX. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008b;26:K17–K28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, Smith D. Incidence and clearance of genital human papillomavirus infection in men (HIM): A cohort study. Lancet. 2011;377:932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Prevention and Control of Sexually Transmitted Diseases. The hidden epidemic: Confronting sexually transmitted diseases. Washington DC: National Academy Press; 1997. [Google Scholar]
- Ivanov S. The role of the cytological screening, HPV-test and the colposcopy for early detection of the precancerous lesions of the uterine cervix. Akush Ginekol (Sofiia) 2007;46:45–49. [PubMed] [Google Scholar]
- Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994;86:494–499. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Lenz P. Human papillomaviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3. Vol. 2. New York, NY: Saunders; pp. 1303–1317. [Google Scholar]
- Lacey CJ, Lowndes CM, Shah KV. Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Lever WF, Elder DE. Lever’s histopathology of the skin. 8. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: Perceptions regarding treatment and impact on lifestyle. Int J STD AIDS. 1998;9:571–578. doi: 10.1258/0956462981921143. [DOI] [PubMed] [Google Scholar]
- Parker MF, Zahn CM, Vogel KM, Olsen CH, Miyazawa K, O’Connor DM. Discrepancy in the interpretation of cervical histology by gynecologic pathologists. Obstet Gynecol. 2002;100:277–280. doi: 10.1016/s0029-7844(02)02058-6. [DOI] [PubMed] [Google Scholar]
- Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi LF, Stern ME, Lee SK, O’Reilly SF, Hawes SE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection in men: Incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- Pierce Campbell CM, Messina JL, Stoler MH, Jukic DM, Tommasino M, Gheit T, Rollison DE, Sichero L, Sirak BA, Ingles DJ, Abrahamsen M, Lu B, Villa LL, Lazcano-Ponce E, Giuliano AR. Cutaneous human papillomavirus types detected on the surface of male external genital lesions: A case series within the HPV Infection in Men Study. J Clin Virol. 2013;58:652–659. doi: 10.1016/j.jcv.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow-Sang MR, Ferreira U, Pow-Sang JM, Nardi AC, Destefano V. Epidemiology and natural history of penile cancer. Urology. 2010;76:S2–S6. doi: 10.1016/j.urology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Sichero L, Pierce Campbell CM, Ferreira S, Sobrinho JS, Luiza Baggio M, Galan L, Silva RC, Lazcano-Ponce E, Giuliano AR, Villa LL. Broad HPV distribution in the genital region of men from the HPV Infection in Men (HIM) study. Virology. 2013;443:214–217. doi: 10.1016/j.virol.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson K, Arnadottir T, Snorradottir M, Benediktsdottir K, Saemundsson H. Human papillomavirus (HPV) in an Icelandic population: The role of HPV DNA testing based on hybrid capture and PCR assays among women with screen-detected abnormal Pap smears. Int J Cancer. 1997;72:446–452. doi: 10.1002/(sici)1097-0215(19970729)72:3<446::aid-ijc12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Stoler MH, Schiffman M. In terobserver reproducibility of cervical cytologic and histologic interpretations: Realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–1505. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- Strand A, Rylander E, Wilander E, Zehbe I, Kraaz W. Histopathologic examination of penile epithelial lesions is of limited diagnostic value in human papillomavirus infection. Sex Transm Dis. 1996;23:293–298. doi: 10.1097/00007435-199607000-00009. [DOI] [PubMed] [Google Scholar]
- The American Congress of Obstetricians and Gynecologists. Clinical management guidelines for obstetrician-gynecologists: Screening for cervical cancer. ACOG Practice Bulletin 2014. 2012 Jul 20; [Google Scholar]
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. 2014 Apr 24; Retrieved July 20, 2014 from http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm.
- Van Poppel H, Watkin NA, Osanto S, Moonen L, Horwich A, Kataja V, Group EGW. Penile cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:115–124. doi: 10.1093/annonc/mdt286. [DOI] [PubMed] [Google Scholar]
- Von Krogh G, Gross G, Barrasso R. Human papillomavirus infections in dermatovenereology. Boca Raton, FL: CRC Press; 1997. Warts and HPV-related squamous cell tumours of the genitoanal area in adults; pp. 259–304. [Google Scholar]
- Von Krogh G, Lacey CJ, Gross G, Barrasso R, Schneider A. European course on HPV associated pathology: Guidelines for primary care physicians for the diagnosis and management of anogenital warts. Sex Transm Infect. 2000;76:162–168. doi: 10.1136/sti.76.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom A, Hedblad MA, Johansson B, Kalantari M, Syrjanen S, Lindberg M, Von Krogh G. The acetic acid test in evaluation of subclinical genital papillomavirus infection: a comparative study on penoscopy, histopathology, virology and scanning electron microscopy findings. Genitourin Med. 1992;68:90–99. doi: 10.1136/sti.68.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]