Abstract
The development of language comprehension abilities in childhood is closely related to the maturation of the brain, especially the ability to process syntactically complex sentences. Recent studies proposed that the fronto-temporal connection within left perisylvian regions, supporting the processing of syntactically complex sentences, is still immature at preschool age. In the current study, resting state functional magnetic resonance imaging data were acquired from typically developing 5-year-old children and adults to shed further light on the brain functional development. Children additionally performed a behavioral syntactic comprehension test outside the scanner. The amplitude of low-frequency fluctuations was analyzed in order to identify the functional correlation networks of language-relevant brain regions. Results showed an intrahemispheric correlation between left inferior frontal gyrus (IFG) and left posterior superior temporal sulcus (pSTS) in adults, whereas an interhemispheric correlation between left IFG and its right-hemispheric homolog was predominant in children. Correlation analysis between resting-state functional connectivity and sentence processing performance in 5-year-olds revealed that local connectivity within the left IFG is associated with competence of processing syntactically simple canonical sentences, while long-range connectivity between IFG and pSTS in left hemisphere is associated with competence of processing syntactically relatively more complex non-canonical sentences. The present developmental data suggest that a selective left fronto-temporal connectivity network for processing complex syntax is already in functional connection at the age of 5 years when measured in a non-task situation. The correlational findings provide new insight into the relationship between intrinsic functional connectivity and syntactic language abilities in preschool children.
Keywords: Language development, Resting-state fMRI, Amplitude of low-frequency fluctuation, Functional connectivity, Syntactic complexity
Highlights
-
•
resting state ALFF correlated in adults between left IFG and left pSTS.
-
•
resting state ALFF correlated in children between left IFG and right homolog area.
-
•
intrahemispheric connectivity co-varies with syntactic processing skills in children.
1. Introduction
The maturation of the brain during childhood is vital for normal development of language abilities. So far, however, functional magnetic resonance imaging (fMRI) data are still rare to describe the developing language–brain relationship during early development (for review see Friederici, 2006). Therefore, the available data do not allow us to relate certain stages of language development to particular aspects of brain maturation.
In adults, the neural basis of language comprehension has been investigated both using electrophysiological measures (Deutsch and Bentin, 2001, Friederici, 2002, Hagoort, 2003) as well as hemodynamic measures (for a review see Price, 2010; Vigneau et al., 2006). Language comprehension has been associated with activation in the inferior frontal cortex (Broca’s area) and the superior temporal cortex (Wernicke’s area). Hemodynamic studies indicate that the left inferior frontal gyrus (IFG) and the posterior part of the left superior temporal gyrus and sulcus (pSTG/pSTS) subserve the processing of complex syntactic sentence structures in particular (for reviews see Friederici, 2011, Friederici, 2012). These two areas showed stronger selective activation for sentences with syntactically more complex non-canonical word order than for sentences with syntactically less complex canonical word order (e.g., Friederici et al., 2006; Kinno et al., 2008; Obleser et al., 2011).
In recent years, efficient connectivity analysis such as dynamic causal modeling (DCM) was employed to further identify the connections within language-relevant brain regions during language processing. By using activation peaks associated with object-cleft sentences over syntactically less complex subject-cleft sentence processing, including IFG, pSTS, and other perisylvian cortical areas, the IFG was identified as the input where syntactic complexity modulated the flow of information from IFG to pSTS (den Ouden et al., 2012). A recent study by Makuuchi and Friederici (2013) showed converging results. In this study, four regional clusters were identified from the activation of syntactically more complex object-first sentences created by dislocating object-noun phrases from their original position of the basic subject-first sentences to a new position: IFG and the inferior frontal sulcus (IFS), the inferior parietal cortex (IPC) and the posterior part of the temporal cortex including pSTG/pSTS; the prevailing model indicated information flow from IFG via IFS and inferior parietal cortex to the pSTS. These findings provided evidence for the importance of this connection for the parsing of complex syntactic sentences. A study with adults and 7-year-old children combined information about structural nerve fiber connections from diffusion-weighted imaging (DWI) data and fMRI data from a language task, and found that adults make use of a more confined fronto-temporal language network than children because of a still ongoing maturation of the structural fronto-temporal connection in children (Brauer et al., 2011).
Since the seminal findings of Biswal et al. (1995) describing spontaneous low frequency (<0.1 Hz) fluctuations (LFFs) in the resting human brain, a line of research has been opened into characterizing functional connectivity and, more specifically, resting-state networks. Lohmann et al. (2010) have shown that default network information can be extracted from task-dependent fMRI data by removing specific experimental stimulation using low-frequency filtering. This method provides insight in domain-selective default networks such as the so-called default language network. As shown in this study, the default language network indicated low-frequency fronto-temporal correlations in fMRI data obtained from language studies independent of task and experimental specifics (Lohmann et al., 2010). Furthermore, the development of this default language network was shown to be characterized by a trend from interhemispheric connectivity in 7-year-old children to more confined intrahemispheric connectivity in adults (Friederici et al., 2011).
In the present study, we first employed a seed-independent, voxel-wise functional resting-state MRI measure (amplitude of low-frequency fluctuation, ALFF) to calculate the intensity of regional spontaneous brain activity in order to examine the correlations among the amplitude of low-frequency blood oxygen level-dependent (BOLD) fluctuations. ALFF is a regional measure for detecting the frequency of BOLD oscillations, and it provides information of regional spontaneous activity. Such an approach was proposed by Zang et al. (2007) and successfully applied as a measure of functional connectivity (Tadayonnejad et al., 2015). For ALFF, the square root of power spectrum is integrated in a low-frequency range for detecting the regional intensity of spontaneous fluctuations in BOLD signal. In the present study, we chose four regions as regions of interest (ROIs), including IFG and pSTS in both hemispheres. Activation peaks of task-evoked fMRI have previously been employed on resting-state fMRI data in order to explore related functional networks in various domains such as attention control (e.g., Dosenbach et al., 2007), emotional processing (e.g., Alaerts et al., 2015), voice-selective processing (e.g., Abrams et al., 2013), among others. The coordinates of ROIs selected for the current analysis were based on seeds from language networks that previously had been successfully applied to describe LFF language networks (Friederici et al., 2011), and both left regions have been reported relevant for processing syntactic information in numerous studies (e.g., Bahlmann et al., 2007; Ben-Shachar et al., 2003; Bornkessel et al., 2005; Friederici et al., 2006; Makuuchi et al., 2009; Moro et al., 2001; Musso et al., 2003; Newman et al., 2010; Röder et al., 2002). Since previous research showed right hemispheric regions to be additionally involved in young children when processing sentence-level information (Brauer and Friederici, 2007, Brauer et al., 2008, Holland et al., 2001), the right homolog areas were included in the analysis allowing to investigate potential interregional within- as well as cross-hemispheric correlations and also developmental differences in the interhemispheric connectivity between 5-year-olds and adults. The current study reports correlations in ALFF and relates them to findings on the language network in LFF as reported previously (Friederici et al., 2011).
We expected to find developmental differences in interregional connectivity within language-relevant regions from 5-year-olds to adults. Next, focusing on 5-year-olds data, we explored to what extent behavioral performance for processing syntactic complexity tested outside the scanner is related to resting-state functional connectivity (RSFC). Seed-based functional connectivity is an approach by which correlations are obtained between the time course of a given seed and the time course of all the other regions within the mask, thereby providing a detailed map of specific connectivity for a brain area of interest. RSFC-behavior correlations across subjects have been widely employed to investigate the neural basis of individual differences in performance (e.g., Hampson et al., 2006; Kelly et al., 2008; Koyama et al., 2011; Seeley et al., 2007; Wang et al., 2010; Zou et al., 2013). The left IFG was chosen as seed in functional connectivity (FC) analysis and the relationship between FC maps and performance in distinct syntactically complex sentences was analyzed. We hypothesized a selective left frontal-to-temporal connectivity for adults which should not yet be present for children at this age, while for children we expected stronger interhemispheric correlations. Furthermore, the left-hemispheric long-range connectivity from left IFG to left pSTS was expected to be associated with the ability to parse syntactically complex information in children.
2. Materials and methods
2.1. Participants
Forty-six typically developing preschool children aged 5 years (23 males; mean age 5.5 years, range 5.0–5.9 years) and thirty-three adults (17 males; mean age 25.06 years, range 20–32 years) participated in the study. Prior to participation, the children’s parents and adult participants gave written, informed consent, and children gave verbal assent for attendance. All children were assessed for their nonverbal intelligence quotient (IQ) using the German version of the Kaufman Assessment Battery for Children (K-ABC) (Melchers and Preuss, 2003). Raw scores were converted into age-dependent standardized scores (sample mean ± SD: 107.66±9.26, range 88–126). All participants were right-handed, monolingual German speakers with no history of neurological, medical, or psychological disorders. The study was approved by the ethical review board of the local university.
2.2. Behavioral testing
All children completed a picture-sentence matching test outside the scanner as used in previous studies (Knoll et al., 2012, Schipke et al., 2012) which comprised of two syntactic conditions: simple canonical subject-initial sentences (SO) and syntactically more complex non-canonical object-initial sentences (OS). Noun phrases in sentences were case marked by nominative case (NOM, subject) or accusative case (ACC, object). There were 75 sentences in each syntactic condition. Stimulus examples are as follows:
-
(1)
Subject-initial sentence (SO):
[der Tiger](NOM) zieht [den Fuchs](ACC)
[the tiger](SBJ) pulls [the fox](OBJ)
The tiger pulls the fox.
-
(2)
Object-initial sentence (OS):
[den Fuchs](ACC) zieht [der Tiger](NOM)
[the fox](OBJ) pulls [the tiger](SBJ)
The tiger pulls the fox.
Sentences contained animate (animals) as well as inanimate nouns (things) to increase semantic variation. Although behavioral performance was taken for each subcondition separately, sentences were pooled into the two syntactic conditions of SO and OS sentences. This was done for two reasons: firstly, animacy of nouns was not the main focus of the present research question on syntactic sentence processing of subject-initial and object-initial sentence structures, and secondly, a 2×3 repeated-measures ANOVA including syntax (SYN) conditions (subject-initial, object-initial) and semantics (SEM) sub-conditions (subject animate: SA, object animate: OA, both animate: BA) revealed only a significant main effect of SYN (F(1,40)=33.91, p<.001), whereas the main effect of SEM did not reach significance (F(2,39)=3.02, p=.06) nor was there a significant SYN×SEM interaction (F(2,39) <1). Behavioral performance in each of the sub-conditions in percent was: OS–BA: 78.05, SO–BA: 93.60, OS–SA: 82.93, SO–SA: 96.04, OS–OA: 82.01, SO–OA: 96.04.
All items were spoken by a trained female native speaker in a well-pronounced, child-directed manner. All sentences were recorded and digitized at 44.1 kHz, 16-bit mono. They had an average length of approximately 3.3 s. Children were asked to point to one of two presented pictures, one of which corresponded to the auditorily presented sentence. The corresponding picture appeared in 50% of the trials on the right/left side of the screen, and actions were performed in 50% of the trials from left-to-right/right-to-left. The number of hit was recorded for each condition. Two children failed to complete the test.
2.3. MRI scanning
All data were acquired using a 3T magnetic resonance scanner (Siemens Tim Trio) with a 12-channel head coil. During resting-state acquisition, children were instructed to lie as still as possible with eyes open while watching a calm screensaver showing a lava lamp in order to reduce potential mind-wandering. Resting-state fMRI whole-brain volumes were acquired by a T2*-weighted gradient-echo echo-planar imaging (EPI) sequence, TR 2000 ms, TE 30 ms, flip angle 90°, slice thickness 3 mm, gap 1 mm, FOV 19.2 cm, matrix 64×64, 28 slices, 100 volumes, duration 3.3 min. High-resolution 3D structural images were acquired with a T1-weighted, magnetization prepared rapid gradient echo (MPRAGE) sequence, TR 1480 ms, TE 3.46 ms, flip angle 10°; slice thickness 1.5 mm, gap 0 mm; matrix 250×250; spatial resolution 1×1×1.5 mm3, duration of 6 min.
2.4. Preprocessing
Data preprocessing was carried out using the Data Processing Assistant for Resting-State fMRI (DPARSF) (Chao-Gan and Yu-Feng, 2010, http://www.restfmri.net) based on Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm) and Resting-State fMRI Data Analysis Toolkit (REST) (Song et al., 2011, http://www.restfmri.net). Preprocessing included: (1) discarding the first 3 EPI volumes from each resting-state scan to allow for signal equilibration; (2) slice timing by shifting the signal measured in each slice relative to the acquisition of the slice at the mid-point of each TR; (3) 3D motion correction using a least squares approach and a 6 parameter (rigid body) spatial transformation; (4) reorienting functional images, and then co-registering MPRAGE image to the mean functional image of each participant; (5) segmenting MPRAGE images into gray matter, white matter (WM) and cerebrospinal fluid (CSF), and creating a study-specific template via diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) (Ashburner, 2007). In order to account for head size differences between children and adults, the template was generated using all participants to reduce warping amount differences between groups. DARTEL is a high-dimensional image registration technique, allowing for optimal mapping between subjects; it registers all subjects into a common space, where the degree of applied deformation is the same for each individual (Ashburner, 2007). The study-specific template was firstly normalized to Montreal Neurological Institute (MNI) space with 6 mm full-width-at-half-maximum (FWHM) Gaussian kernel, and all functional images were normalized to this template and resampled to voxel size 3×3×3 mm3.
Nuisance regression was implemented by using a component based noise correction method (CompCor) (Behzadi et al., 2007). Specifically, regressors included principal components (PC) extracted from subject-specific WM and CSF mask (5 PC parameters) as well as Friston 24-parameter model (6 head motion parameters, 6 head motion parameters one time point before, and 12 corresponding squared items) (Friston et al., 1996). CompCor procedure included detrending, variance normalization, and PC analysis (PCA) according to Behzadi et al. (2007). Compared to mean signal regression, where average signals are extracted from WM and CSF mask, signals captured by principal components derived from these noise ROIs can better account for voxel-specific phase differences in physiological noise due to the potential of PCA to identify temporal pattern of physiological noise (Thomas et al., 2002). The Friston 24-motion-parameter model was used as head motion regressor because it has been found to likely become a standard for the field given its statistical superiority over smaller sets of motion parameters (Power et al., 2014, Satterthwaite et al., 2013, Yan et al., 2013a).
Given recent concerns regarding the confounding influence of micromovements in intrinsic functional connectivity analyses (Power et al., 2012, Satterthwaite et al., 2012, Van Dijk et al., 2012), time series of framewise displacement (FD) (Jenkinson et al., 2002) were computed as it is preferable for its consideration of voxel-wise differences in its derivation (Yan et al., 2013a). Three children were excluded because of head motion (mean FD Jenkinson) greater than mean+2*SD (threshold 0.483 mm) (after Yan et al., 2013b). For the remaining data, the mean volumes below the threshold of FD=0.5 mm was 96.15 (SD 5.42) for children 99.76 (SD 0.79) for adults with no significant within-group variance. The threshold of FD=0.5 mm is slightly higher than suggested for adult data (Power et al., 2014), but appropriate for child data and hence used for both groups. The average of mean FD in children was 0.15 mm (SD=0.11 mm, range=0.05–0.45 mm), and in adults 0.08 mm (SD=0.03 mm, range=0.03–0.15 mm), which reached significance between groups (t(72)=−3.69, p<.001). Therefore, FD was used as a covariate in subsequent analyses.
Neither scrubbing (Power et al., 2012) nor interpolation (Carp, 2013) was implemented here to ensure the reliability of results (Yan et al., 2013a, Zuo et al., 2013) and to avoid alteration of data frames. ALFF calculation is based on Fast Fourier transform (FFT) which cannot be applied to scrubbed data due to alteration of its temporal structure by removal of frames (Yan et al., 2013a). Except for ALFF calculation, temporal filtering was performed with a band-pass of 0.01–0.1 Hz as recommended by previous studies (Lohmann et al., 2010, Yan et al., 2013a, Yan et al., 2013b).
2.5. ALFF and correlation calculation
ALFF analysis was done using REST (Song et al., 2011, http://www.restfmri.net). The procedure was the same as used in previous studies (Liu et al., 2014, Yang et al., 2007, Zang et al., 2007, Zhang et al., 2010). The time series for each voxel were first transformed to the frequency domain using FFT. The square root was calculated at each frequency of the power spectrum and the averaged square root was calculated across 0.01–0.1 Hz at each voxel, obtaining the ALFF (Zang et al., 2007). Finally, z transformation was implemented by subtracting global mean and dividing by standard deviation of all ALFF in the given brain gray mask.
For correlation analysis, two core regions of the language network (left IFG, left pSTS) as well as their right-hemispheric counterparts were selected as ROIs as identified in a previous study (Friederici et al., 2011), ‘tal2mni’ routine (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) was applied and results anatomically verified. This resulted in the following ROIs: left IFG at -58, 17, 20; rigth IFG at 58, 17, 20; left pSTS at −57, −44, 12; right pSTS at 57, −44, 12 (all coordinates in MNI space). For each participant, mean ALFF values within each ROI (voxel level) were computed and entered into Pearson’s correlation analyses between these ROIs.
2.6. RSFC–behavior correlation analysis
RSFC analysis was performed for children using REST software and focusing on left IFG ROI connectivity as it is a major cortical hub relevant for processing syntactic complexity. For RSFC calculation, the mean time series of left IFG was first computed for each participant by averaging the time series of all the voxels in the left IFG (radius 6 mm), and then individual level RSFC correlation maps (r-map) were produced for the whole brain.
Next, r-maps were converted into z-maps with application of Fisher’s r-to-z transformation to obtain normal distribution. Subsequently, RSFC–behavior correlation analysis was conducted using ‘REST Correlation Analysis’ command in the REST software. Pearson’s correlation coefficients between Fisher-z-transformed RSFC strength and performance in the two task conditions (SO, OS) as well as their direct comparison were calculated within a volume of interest (VOI) of the perisylvian language regions, including the inferior frontal as well as middle and superior temporal cortices within both hemispheres (Fig. 1) according to Friederici et al. (2011). Additionally, left V1 (BA 17) served as a language-unrelated control region, which was abstracted from Brodmann template in the REST toolbox. Analysis procedure was the same as outlined above. All group level statistical analyses were controlled for age, gender, mean FD, and IQ. Finally, all statistical r-maps were transformed into z maps by implementing ‘rest_TFRtoZ’ function in the REST toolbox (Song et al., 2011, http://www.restfmri.net) and further corrected for multiple comparisons using Gaussian Random Field (GRF) theory (Z>2.3, cluster-wise p<.05, GRF corrected) with minimal a cluster size of 40 voxels.
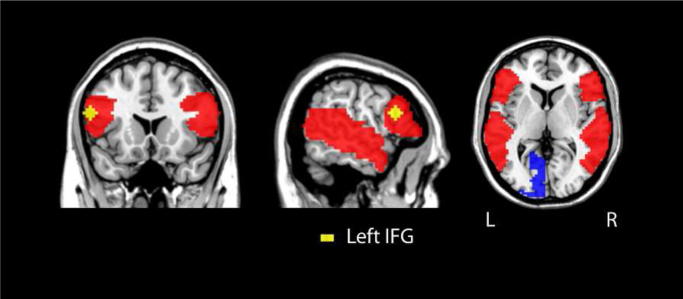
Fig. 1.
Volume of interest (VOI) mask (red) used for correlation analysis. The VOI comprises a total of 6532 voxels (176,364 mm3) covering right and left perisylvian language regions. Left IFG (yellow) served as a seed region of interest for resting-state functional connectivity (RSFC). Left BA 17 (blue) served as a language-unrelated control region for RSFC. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
3. Results
3.1. Behavioral results
Children’s performance in the picture-sentence matching test resulted in a mean accuracy for SO=95.22% (SD 7.04) and for OS=81.00% (SD 16.31), suggesting a significant advantage for SO sentences (t(40)=5.82, p<.001). Both SO and OS showed significant performance above chance (SO: t(40)=40.61, p<.001, OS: t(40)=12.02, p<.001). Accuracy for both SO and OS was not significantly correlated with age, sex, or IQ.
3.2. ALFF-based functional connectivity
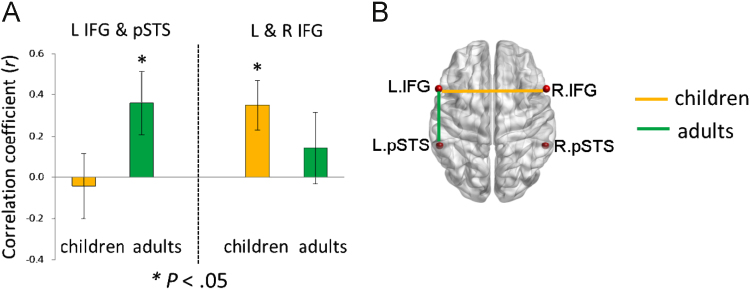
The results showed significant correlation between left IFG and left pSTS for adults, but not for 5-year-old children (Fig. 2A). At the same time, there was a significant correlation between left and right IFG for children, but not for adults (Fig. 2A). No other correlations were significant. The correlations for the two groups are depicted in Fig. 2B with BrainNet Viewer (Xia et al., 2013, http://www.nitrc.org/projects/bnv).
Fig. 2.
Correlations of amplitudes of low frequency fluctuations between language areas for children and adults. (A) Shows a significant correlation in ALFF between left IFG and left pSTS for adults, whereas significant correlation between left and right IFG was observed for children. Error bars represent standard error of the mean. The map in (B) illustrates the regions of interests and their correlational relationships. Significant correlations are indicated with lines (orange: children, green: adults). No other correlations were significant. L: left hemisphere; R: right hemisphere; IFG: inferior frontal gyrus; pSTS: posterior superior temporal sulcus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Interhemispheric connectivity as illustrated in Fig. 2 shows the quasi-comparison between adults' and children's network connectivities with strong interhemispheric connectivity for children compared to long range intrahemispheric connectivity for adults.
3.3. RSFC–behavior correlation analysis
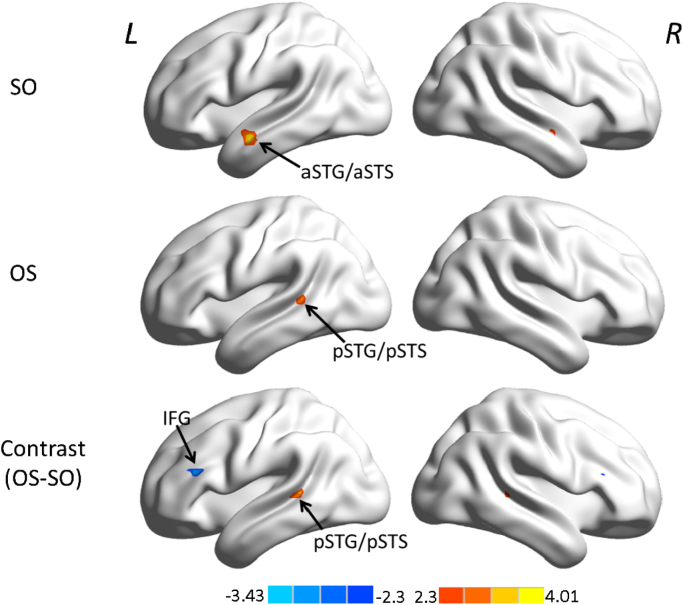
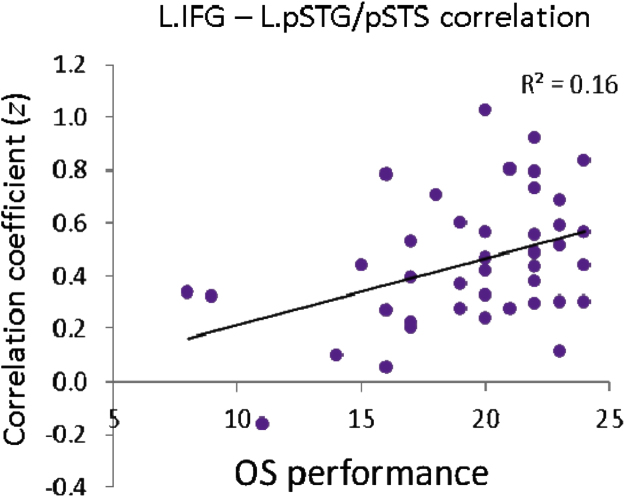
In a next step, functional connectivity was associated with children’s performance in sentence processing. By correlating RSFC maps of left IFG with scores from behavioral conditions (SO and OS), we found divergent correlation patterns for SO and OS performances. As shown in Fig. 3, correlation between the left IFG seed and SO performance was observed in bilateral but strongly left-lateralized anterior STG/STS (aSTG/aSTS). Correlation between the left IFG seed and OS performance was found in the left pSTG/pSTS. The individual variation in the contribution to correlation between left IFG and left pSTG/pSTS with OS performance is shown in Fig. 4. The direct contrast between two correlation maps revealed stronger correlation in bilateral pSTG/pSTS for OS performance, and stronger correlation within bilateral IFG for SO performance, both dominant within the left hemisphere. Peak coordinates of both correlations as well as the contrast are reported in Table 1. When seeded in the control region BA 17, no correlation within the VOI was observed.
Fig. 3.
Resting-state functional connectivity in its relation to sentence comprehension performance in children. Statistical maps of the correlation between functional connectivity of the left IFG (−58, 17, 20) and performance in simple syntax (SO) (first row), complex syntax (OS) (second row), as well as the direct contrast (third row, blue for SO, yellow-red for OS), Z>2.3, cluster-wise p<.05, GRF corrected. SO performance is associated with connectivity to aSTG/aSTS as well as local connectivity within the IFG as seen in the direct contrast, while OS performance is associated with stronger long-range connectivity to the pSTG/pSTS, mainly in the left hemisphere. L, left hemisphere; R, right hemisphere; aSTG/aSTS, anterior superior temporal gyrus and sulcus; pSTG/pSTS, posterior superior temporal gyrus and sulcus; IFG, inferior frontal gyrus. Results are illustrated with BrainNet Viewer (Xia et al., 2013, http://www.nitrc.org/projects/bnv). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Fig. 4.
Individual variation in the contribution to correlation between left IFG and left pSTG/pSTS with OS performance in children as shown in Fig. 3.
Table 1.
MNI peak coordinates of RSFC–behavior correlation.
| RSFC–behavior correlation | Cluster location | Cluster size (voxels) | Peak (MNI) | Peak Z | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| SO | L.aSTG/aSTS | 142 | −51 | −6 | −15 | 4.01 |
| R.aSTG/aSTS | 43 | 48 | −9 | −12 | 3.75 | |
| OS | L.pSTG/pSTS | 52 | −51 | −42 | 6 | 3.43 |
| Contrast (OS–SO) | L.pSTG/pSTS | 67 | −51 | −39 | 6 | 3.27 |
| R.pSTG/pSTS | 42 | 51 | −39 | 6 | 2.74 | |
| L.IFG | 59 | −39 | 24 | 15 | −3.43 | |
| R.IFG | 41 | 36 | 18 | 18 | −2.86 | |
Notes: L: left hemisphere; R: right hemisphere; aSTG/aSTS: anterior superior temporal gyrus and sulcus; pSTG/pSTS: posterior superior temporal gyrus and sulcus; IFG: inferior frontal gyrus.
4. Discussion
The present study set out to investigate the functional connectivity of language-relevant brain regions during resting state and its relation to syntactic language performance. Correlations among intrinsic BOLD oscillations in predefined language-relevant ROIs revealed long-range functional association between IFG and pSTS within the left hemisphere for adults, whereas interhemispheric association between bilateral IFG was observed for 5-year-old children, suggesting immaturity of the left fronto-temporal functional network within the perisylvian region in children at 5 years of age. Furthermore, an association with language performance in children was found. Long-range intrinsic connectivity between left IFG and left pSTG/pSTS was correlated with the performance in syntactically relatively more complex non-canonical sentences (OS), whereas no such fronto-temporal connectivity was associated with performance in processing syntactically less complex canonical sentences (SO). In previous studies, a selective network connecting the language-relevant frontal and temporal regions, particularly the inferior frontal and superior temporal areas, has been described for adults in numerous fMRI experiments on syntactic processing (for reviews see Friederici, 2011, Friederici, 2012; Price, 2010). The current findings imply that the network of these areas is already associated with the processing of complex syntax at the age of 5 years, even though the entire functional network is not yet fully mature, as indicated by ALFF-based functional connectivity between language-related regions in children and adults (Friederici et al., 2011).
Behavioral results of the present study suggest that children at 5 years of age are able to process syntactic information even in non-canonical OS sentences already above chance. Still, they performed significantly better on simple SO compared to syntactically more demanding OS sentences. The results show that young children at age 5 years are already developing the ability to comprehend syntactically complex utterances. The very good performance of 5-year-old children on SO sentences may be due to the fact that they may apply an agent-first strategy rather than performing a full syntactic analysis (Kamide et al., 2003). Better performance for subject-initial over object-initial sentence structures is in line with previous behavioral results from children at age 5 and 6 years (Knoll et al., 2012) or 7 years (Dittmar et al., 2008). It is, moreover, consistent with other recent findings of significant above chance accuracy for object-relative sentences in children at age 6 years (Skeide et al., 2015). But these results stand in contrast to other studies (e.g., Lindner, 2003; Schipke et al., 2011), in which children at about this age were found to perform not significantly above chance level for object-initial sentences. A number of factors might contribute to this inconsistency such as word familiarity or alternatively available linguistic cues for sentence interpretation. According to the competition model of language processing, sentence interpretation is supported by linguistic cues, among them case marking, word order, and animacy, with a language-specific weighting of these various factors (Bates and MacWhinney, 1982, MacWhinney, 2013). In the current study, in addition to unambiguous case marking, the varying animacy information of nouns across sentences might have drawn attention to the availability of such additional cues for sentence interpretation. Previous studies have often used material with only animate nouns thereby providing no additional semantic cues (Dittmar et al., 2008, Schipke et al., 2012). The availability of animacy cues in the current study might have contributed to the relatively good performance of children in comprehending sentences, although animacy did not significantly interact with the syntax.
The current analyses revealed significant correlations of low-frequency BOLD oscillation power between left IFG and left pSTS in adults, but not so in children who rather showed strong correlations between left IFG and its right-hemispheric homolog. In other words, these correlation patterns reveal a selective fronto-temporal functional association between the left hemispheric language-relevant regions in adults. For children, on the other hand, this left-hemispheric long-range network association is not yet established as in adults. For children rather interhemispheric association is observed which is in line with previous task-based fMRI findings (Brauer and Friederici, 2007, Szaflarski et al., 2006). The right hemisphere had been shown to have an important role in prosodic aspects of sentence processing, and a stronger role of prosody in early language processing had been proposed (Wartenburger et al., 2007). The stronger involvement of the right hemisphere had been discussed as a resource that supports language processing when left-hemispheric fronto-temporal connectivity is not yet mature as in early childhood (Holland et al., 2007) or decreasing as in elder age (Antonenko et al., 2013, Holland et al., 2007, Wartenburger et al., 2007). Models of structural brain development propose a stronger bilaterality in the maturing brain and relate this phenomenon to language development (Broce et al., 2015). The increasing correlation strength from left IFG to left pSTS with age is in line with the interpretation that a selective fronto-temporal functional connectivity characterizes a developmental trend within the default language network from childhood to adulthood (Friederici et al., 2011). Moreover, the interhemispheric connectivity in 5-year-olds is consistent with LFFs results of fMRI data (Friederici et al., 2011, Perani et al., 2011) and also resting-state functional connectivity (Fox et al., 2009, Fransson et al., 2011), and contrasting the prominent left-intrahemispheric functional connectivity in adults (Lohmann et al., 2010, Perani et al., 2011).
Seed-based functional connectivity was conducted with a seed in the left IFG (BA 44) which has been identified a main hub for processing complex syntax in numerous studies (for review see Friederici, 2011). For example, the brain activation in left IFG increases systematically as syntactic complexity increases (Friederici et al., 2006). The left IFG is involved in sentence embedding (nested structures) in German (Makuuchi et al., 2009) and activated in embedding and syntactic movement (Santi and Grodzinsky, 2010). Moreover, an enhanced activation in the left pSTG/pSTS has been reported for the processing of syntactic information in syntactically complex sentences (Friederici et al., 2006, Kinno et al., 2008, Röder et al., 2002). The present results revealed stronger coupling of RSFC between left IFG and left pSTG/pSTS for OS performance, but not for SO performance in 5-year-old children, indicating that competence of processing syntactically complex sentences is positively associated with selective RSFC strength between these regions. Notably, the present results are consistent with findings from task-dependent fMRI experiments (Kinno et al., 2008, Knoll et al., 2012, Thompson et al., 2010), which consistently reported enhanced selective activation in both left IFG and left pSTG/pSTS when processing syntactically complex sentences. The coherent overlapping correlation between left IFG and left pSTG/pSTS in the present LFF data are taken to reflect the inherent relationship between intrinsic brain activity and syntactic processing competence. On the basis of the present results we infer that correlations between frontal and temporal language-relevant regions in the perisylvian cortex are selectively modulated by the ability to process syntactically complex utterances.
In contrast, we found that SO performance was positively correlated with RSFC between IFG and aSTG. The aSTG has been referred to as a brain region for local, less complex syntactic and combinatorial processes. Previous research has associated increased activation in the aSTG during task fMRI employing simple dependencies in artificial grammar sequences (Friederici et al., 2006), syntactic violation tasks (Friederici et al., 2003), as well as natural language listening paradigms (Brennan et al., 2012). The IFG is structurally connected to the aSTG via a ventral fiber pathway, while the connection to the pSTG/pSTS is implemented via a dorsal pathway, which have been shown to be associated to the ability to process either simple (ventral pathway) or more complex (dorsal pathway) syntactic dependencies (Friederici et al., 2006). From the perspective of brain maturation, the ventral pathway matures earlier than the dorsal pathway (Brauer et al., 2013). We also observed stronger local correlation within the IFG for SO performance compared to OS performance. This local connectivity included the involvement of pars triangularis (BA 45) and IFS. As a subregion of IFG, BA 45 plays a role in language processing, such as syntactic movement (Grodzinsky, 2000, Santi and Grodzinsky, 2010) and semantic processes (Friederici, 2002, Hagoort, 2005). The left IFS had been argued to serve a function for supporting syntactic working memory (Makuuchi et al., 2009). Thus, both BA 45 and IFS are involved in processing sentences. The stronger local and ventral connectivity for SO performance suggests attributions to the processing of syntactically less complex sentences, while the long-range connectivity is regarded crucial for the processing of more complex syntactic structures (Skeide et al., 2015).
As observed in a previous study, the left dorsal fronto-temporal structural connection between the language-relevant areas in IFG and pSTG/pSTS develops as the brain matures and is still structurally underdeveloped at the age of seven years (Brauer et al., 2011). This is in line with our present ALFF-based functional connectivity findings of an immature left fronto-temporal functional association at age five years. Importantly, competence of parsing syntactically complex OS sentences was related to left fronto-temporal functional connectivity. Together with the structural findings from 7-year-olds, it can be interpreted that although the structural connection between left IFG and pSTG/pSTS is still immature, yet resting-state functional connectivity is at least already partly in place already at age five when it is required for the processing of syntactically complex utterances.
We should, however, note a few limitations when interpreting the results described in this paper. First, considering the difficulties of data acquisition from young children, only 100 volumes of resting-state data were collected for the current study with a total duration of 3.3 min. Van Dijk et al. (2010) observed stable correlation strengths at acquisition times of about 5 min. Moreover, recent studies found good inter-session reliability for functional homogeneity analyses with scan durations as brief as 3 min (Zuo et al., 2013) as well as high reliability of resting-fMRI measures available for scan durations of 3 min (Yan et al., 2013a). Second, given strong apriori hypotheses for selective perisylvian networks, a ROI-based rather than a whole-brain approach was chosen to examine correlations of low-frequency BOLD oscillation. This is, however, because of strong hypotheses based empirical support from numerous research on the role of these regions for syntactic processes from previous fMRI studies in adults (Friederici et al., 2011, Kinno et al., 2008, Makuuchi et al., 2009, Thompson et al., 2010, Tomasi and Volkow, 2012) and in children (Brauer and Friederici, 2007, Brauer et al., 2008, Knoll et al., 2012, Nuñez et al., 2011, Skeide et al., 2014, Yeatman et al., 2010). A ROI-based approach appeared most appropriate to answer the hypotheses. As a final remark, it is important to keep in mind that the results presented here were based on resting state fMRI data, and did not stem from task fMRI experiments. Hence, the findings should be interpreted in resting-state fMRI context with the assumption that they reflect intrinsic neural activity and in combination with behavioral data reveal relationships between functional connectivity and behavioral ability in syntactic processing. Third, we used a less stringent primary threshold (p<.01) with a corresponding cluster-level p<.05, which is more liberal than the primary threshold of p<.001, suggested for avoiding spanning of clusters across anatomical regions and loosing spatial specificity (Woo et al., 2014). However, that was not the case for the present data. Moreover, other studies have successfully used Gaussian random field theory with the same thresholds as applied in the current study, Z>2.3, cluster-level p<.05 (e.g., Alaerts et al., 2015; Pirnia et al., 2015; Salomons et al., 2015).
5. Conclusion
This study revealed the development of the fronto-temporal resting-state connectivity from 5-year-olds to adults by examining the correlation of intrinsic low-frequency BOLD oscillations in language-related regions. Notably, the findings of an interhemispheric coupling of left and right IFG in 5-year-olds and long-range connectivity from IFG to pSTS within left hemisphere in adults are consistent with previous LFFs analyses of fMRI data (Friederici et al., 2011). A stronger long-range connectivity in adults corresponds to a developmental trajectory of a proper selective left-hemispheric language network (Brauer et al., 2011, Friederici et al., 2011). The RSFC–behavior relationships showed stronger long-range IFG connectivity with left pSTG/pSTS for OS performance, but stronger local and ventral correlation within the IFG and to the aSTG for SO performance. In contrast to processing relatively simple syntactic sentences (SO), processing syntactically complex sentences (OS) is associated with stronger long-range coupling between left IFG and left pSTG/pSTS. The present results support the notion that fronto-temporal functional connectivity within the language network in the left hemisphere is crucial for the processing of syntactically complex sentences (den Ouden et al., 2012, Friederici et al., 2011). They indicate that this connectivity is specific for the language network, no connectivity differences were observed for the control seed in BA 17. Although the adult-like left fronto-temporal connection is still not fully structurally developed in 5-year-olds, these two regions are already able to cooperate and correlate with syntactic processes at this age. The findings provide novel insight into the relationship between intrinsic functional connectivity and syntactic language abilities in preschool children.
Acknowledgments
We thank Jeanine Auerswald, Kodjo Vissiennon, and Riccardo Cafiero for their contributions to data acquisition and to Johannes Stelzer for comments on a previous version of the manuscript. This research was supported by a Grant of the European Research Council to A.F. (ERC-2010-AdG 20100407, Neurosyntax).
References
- Abrams D.A., Lynch C.J., Cheng K.M., Phillips J., Supekar K., Ryali S., Uddin L.Q., Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc. Natl. Acad. Sci. USA. 2013;110:12060–12065. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts K., Nayar K., Kelly C., Raithel J., Milham M.P., Di Martino A. Age-related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2015 doi: 10.1093/scan/nsv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D., Brauer J., Meinzer M., Fengler A., Kerti L., Friederici A.D., Floel A. Functional and structural syntax networks in aging. Neuroimage. 2013;83:513–523. doi: 10.1016/j.neuroimage.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bahlmann J., Rodriguez‐Fornells A., Rotte M., Münte T.F. An fMRI study of canonical and noncanonical word order in German. Hum. Brain Mapp. 2007;28:940–949. doi: 10.1002/hbm.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E., MacWhinney B. Functionalist approaches to grammar. In: Wanner E., Gleitman L., editors. Language Acquisition: The State of the Art. Cambridge University Press; New York: 1982. [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M., Hendler T., Kahn I., Ben-Bashat D., Grodzinsky Y. The neural reality of syntactic transformations evidence from functional magnetic resonance imaging. Psychol. Sci. 2003;14:433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bornkessel I., Zysset S., Friederici A.D., von Cramon D.Y., Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Perani D., Friederici A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013;127:289–295. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brauer J., Neumann J., Friederici A.D. Temporal dynamics of perisylvian activation during language processing in children and adults. Neuroimage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J., Nir Y., Hasson U., Malach R., Heeger D.J., Pylkkänen L. Syntactic structure building in the anterior temporal lobe during natural story listening. Brain Lang. 2012;120:163–173. doi: 10.1016/j.bandl.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broce I., Bernal B., Altman N., Tremblay P., Dick A.S. Fiber tracking of the frontal aslant tract and subcomponents of the arcuate fasciculus in 5–8-year-olds: relation to speech and language function. Brain Lang. 2015;149:66–76. doi: 10.1016/j.bandl.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: comment on power et al. Neuroimage. 2013;76:436–438. doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden D.-B., Saur D., Mader W., Schelter B., Lukic S., Wali E., Timmer J., Thompson C.K. Network modulation during complex syntactic processing. Neuroimage. 2012;59:815–823. doi: 10.1016/j.neuroimage.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch A., Bentin S. Syntactic and semantic factors in processing gender agreement in Hebrew: evidence from ERPs and eye movements. J. Mem. Lang. 2001;45:200–224. [Google Scholar]
- Dittmar M., Abbot‐Smith K., Lieven E., Tomasello M. German children’s comprehension of word order and case marking in causative sentences. Child Dev. 2008;79:1152–1167. doi: 10.1111/j.1467-8624.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Friederici Towards a neural basis of auditory sentence processing. Trends Cogn. Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The neural basis of language development and its impairment. Neuron. 2006;52:941–952. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn. Sci. 2012;16:262–268. doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Bahlmann J., Heim S., Schubotz R.I., Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc. Natl. Acad. Sci. USA. 2006;103:2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D., Brauer J., Lohmann G. Maturation of the language network: from inter-to intrahemispheric connectivities. PLoS One. 2011;6:e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D., Rueschemeyer S.-A., Hahne A., Fiebach C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y. The neurology of syntax: Language use without Broca's area. Behav. Brain Sci. 2000;23:1–21. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. Neuroimage. 2003;20:S18–S29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn. Sci. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hampson M., Driesen N.R., Skudlarski P., Gore J.C., Constable R.T. Brain connectivity related to working memory performance. J. Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holland S.K., Vannest J., Mecoli M., Jacola L.M., Tillema J.-M., Karunanayaka P.R., Schmithorst V.J., Yuan W., Plante E., Byars A.W. Functional MRI of language lateralization during development in children. Int. J. Audiol. 2007;46:533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kamide Y., Scheepers C., Altmann G.T. Integration of syntactic and semantic information in predictive processing: cross-linguistic evidence from German and English. J. Psycholinguist. Res. 2003;32:37–55. doi: 10.1023/a:1021933015362. [DOI] [PubMed] [Google Scholar]
- Kelly A.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kinno R., Kawamura M., Shioda S., Sakai K.L. Neural correlates of noncanonical syntactic processing revealed by a picture-sentence matching task. Hum. Brain Mapp. 2008;29:1015–1027. doi: 10.1002/hbm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L.J., Obleser J., Schipke C.S., Friederici A.D., Brauer J. Left prefrontal cortex activation during sentence comprehension covaries with grammatical knowledge in children. Neuroimage. 2012;62:207–216. doi: 10.1016/j.neuroimage.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Zuo X.-N., Kelly C., Mennes M., Jutagir D.R., Castellanos F.X., Milham M.P. Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner K. The development of sentence-interpretation strategies in monolingual German-learning children with and without specific language impairment. Linguistics. 2003;41:213–254. [Google Scholar]
- Liu J., Ren L., Womer F.Y., Wang J., Fan G., Jiang W., Blumberg H.P., Tang Y., Xu K., Wang F. Alterations in amplitude of low frequency fluctuation in treatment‐naïve major depressive disorder measured with resting‐state fMRI. Hum. Brain Mapp. 2014;35:4979–4988. doi: 10.1002/hbm.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G., Hoehl S., Brauer J., Danielmeier C., Bornkessel-Schlesewsky I., Bahlmann J., Turner R., Friederici A. Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb. Cortex. 2010;20:1286–1292. doi: 10.1093/cercor/bhp190. [DOI] [PubMed] [Google Scholar]
- MacWhinney, B., 2013. The logic of the Unified Model. In: The Routledge handbook of second language acquisition (pp. 211–227). London: Routledge.
- Makuuchi M., Bahlmann J., Anwander A., Friederici A.D. Segregating the core computational faculty of human language from working memory. Proc. Natl. Acad. Sci. USA. 2009;106:8362–8367. doi: 10.1073/pnas.0810928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M., Friederici A.D. Hierarchical functional connectivity between the core language system and the working memory system. Cortex. 2013;49:2416–2423. doi: 10.1016/j.cortex.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Melchers P., Preuss U. Swets & Zeitlinger; Frankfurt/Main: 2003. Kaufman-Assessment Battery of Children (K-ABC) [Google Scholar]
- Moro A., Tettamanti M., Perani D., Donati C., Cappa S.F., Fazio F. Syntax and the brain: disentangling grammar by selective anomalies. Neuroimage. 2001;13:110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Musso M., Moro A., Glauche V., Rijntjes M., Reichenbach J., Büchel C., Weiller C. Broca's area and the language instinct. Nat. Neurosci. 2003;6:774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Newman S.D., Ikuta T., Burns T., Jr The effect of semantic relatedness on syntactic analysis: an fMRI study. Brain Lang. 2010;113:51–58. doi: 10.1016/j.bandl.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez S.C., Dapretto M., Katzir T., Starr A., Bramen J., Kan E., Bookheimer S., Sowell E.R. fMRI of syntactic processing in typically developing children: structural correlates in the inferior frontal gyrus. Dev. Cogn. Neurosci. 2011;1:313–323. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Meyer L., Friederici A.D. Dynamic assignment of neural resources in auditory comprehension of complex sentences. Neuroimage. 2011;56:2310–2320. doi: 10.1016/j.neuroimage.2011.03.035. [DOI] [PubMed] [Google Scholar]
- Perani D., Saccuman M.C., Scifo P., Anwander A., Spada D., Baldoli C., Poloniato A., Lohmann G., Friederici A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. USA. 2011;108:16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirnia T., Woods R.P., Hamilton L.S., Lyden H., Joshi S.H., Asarnow R.F., Nuechterlein K.H., Narr K.L. Hippocampal dysfunction during declarative memory encoding in schizophrenia and effects of genetic liability. Schizophr. Res. 2015;161:357–366. doi: 10.1016/j.schres.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Röder B., Stock O., Neville H., Bien S., Rösler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage. 2002;15:1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Salomons T.V., Nusslock R., Detloff A., Johnstone T., Davidson R.J. Neural emotion regulation circuitry underlying anxiolytic effects of perceived control over pain. J. Cogn. Neurosci. 2015;27:221–233. doi: 10.1162/jocn_a_00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A., Grodzinsky Y. fMRI adaptation dissociates syntactic complexity dimensions. Neuroimage. 2010;51:1285–1293. doi: 10.1016/j.neuroimage.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E., Eickhoff S.B., Hakonarson H., Gur R.C., Gur R.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke C.S., Friederici A.D., Oberecker R. Brain responses to case-marking violations in German preschool children. Neuroreport. 2011;22:850–854. doi: 10.1097/WNR.0b013e32834c1578. [DOI] [PubMed] [Google Scholar]
- Schipke C.S., Knoll L.J., Friederici A.D., Oberecker R. Preschool children’s interpretation of object‐initial sentences: neural correlates of their behavioral performance. Dev. Sci. 2012;15:762–774. doi: 10.1111/j.1467-7687.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Syntax gradually segregates from semantics in the developing brain. Neuroimage. 2014;100:106–111. doi: 10.1016/j.neuroimage.2014.05.080. [DOI] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Brain functional and structural predictors of language performance. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv042. [DOI] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Schmithorst V.J., Byars A.W. fMRI study of language lateralization in children and adults. Hum. Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R., Yang S., Kumar A., Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. J. Affect. Disord. 2015;172:241–250. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.G., Harshman R.A., Menon R.S. Noise reduction in BOLD-based fMRI using component analysis. Neuroimage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Thompson C.K., den Ouden D.-B., Bonakdarpour B., Garibaldi K., Parrish T.B. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia. 2010;48:3211–3227. doi: 10.1016/j.neuropsychologia.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Resting functional connectivity of language networks: characterization and reproducibility. Mol. Psychiatry. 2012;17:841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Herve P.-Y., Duffau H., Crivello F., Houde O., Mazoyer B., Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wang L., Negreira A., LaViolette P., Bakkour A., Sperling R.A., Dickerson B.C. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20:345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenburger I., Steinbrink J., Telkemeyer S., Friedrich M., Friederici A.D., Obrig H. The processing of prosody: evidence of interhemispheric specialization at the age of four. Neuroimage. 2007;34:416–425. doi: 10.1016/j.neuroimage.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Craddock R.C., Zuo X.N., Zang Y.F., Milham M.P. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage. 2013;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Long X.Y., Yang Y., Yan H., Zhu C.Z., Zhou X.P., Zang Y.F., Gong Q.Y. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Ben-Shachar M., Glover G.H., Feldman H.M. Individual differences in auditory sentence comprehension in children: An exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114:72–79. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y.F., He Y., Zhu C.Z., Cao Q.J., Sui M.Q., Liang M., Tian L.X., Jiang T.Z., Wang Y.F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lu G., Zhong Y., Tan Q., Chen H., Liao W., Tian L., Li Z., Shi J., Liu Y. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum. Brain Mapp. 2010;31:1851–1861. doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q., Ross T.J., Gu H., Geng X., Zuo X.N., Hong L.E., Gao J.H., Stein E.A., Zang Y.F., Yang Y. Intrinsic resting‐state activity predicts working memory brain activation and behavioral performance. Hum. Brain Mapp. 2013;34:3204–3215. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Xu T., Jiang L., Yang Z., Cao X.Y., He Y., Zang Y.F., Castellanos F.X., Milham M.P. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65:374–386. doi: 10.1016/j.neuroimage.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]