Abstract
BACKGROUND
Chronic obstructive pulmonary disease (COPD) is a risk factor that increases the incidence of postoperative cardiopulmonary morbidity and mortality after lung resection. Dexmedetomidine, a selective α2-adrenoreceptor agonist, has been reported previously to attenuate intrapulmonary shunt during one-lung ventilation (OLV) and to alleviate bronchoconstriction.
OBJECTIVE
The objective is to determine whether dexmedetomidine improves oxygenation and lung mechanics in patients with moderate COPD during lung cancer surgery.
DESIGN
A randomised, double-blinded, placebo-controlled study.
SETTING
Single university hospital.
PARTICIPANTS
Fifty patients scheduled for video-assisted thoracoscopic surgery who had moderate COPD. Patients were randomly allocated to a control group or a Dex group (n = 25 each).
INTERVENTIONS
In the Dex group, dexmedetomidine was given as an initial loading dose of 1.0 μg kg−1 over 10 min followed by a maintenance dose of 0.5 μg kg−1 h−1 during OLV while the control group was administered a comparable volume of 0.9% saline. Data were measured at 30 min (DEX-30) and 60 min (DEX-60) after dexmedetomidine or saline administration during OLV.
MAIN OUTCOME MEASURES
The primary outcome was the effect of dexmedetomidine on oxygenation. The secondary outcome was the effect of dexmedetomidine administration on postoperative pulmonary complications.
RESULTS
Patients in the Dex group had a significantly higher PaO2/FiO2 ratio (27.9 ± 5.8 vs. 22.5 ± 8.4 and 28.6 ± 5.9 vs. 21.0 ± 9.9 kPa, P < 0.05), significantly lower dead space ventilation (19.2 ± 8.5 vs. 24.1 ± 8.1 and 19.6 ± 6.7 vs. 25.3 ± 7.8%, P < 0.05) and higher dynamic compliance at DEX-30 and DEX-60 (P = 0.0001 and P = 0.0184) compared with the control group. In the Dex group, the PaO2/FiO2 ratio in the postoperative period was significantly higher (P = 0.022) and the incidence of ICU admission was lower than in the control group.
CONCLUSION
Dexmedetomidine administration may provide clinically relevant benefits by improving oxygenation and lung mechanics in patients with moderate COPD undergoing lung cancer surgery.
TRIAL REGISTRATION
ClinicalTrial.gov identifier: NCT 02185430.
Introduction
Chronic obstructive pulmonary disease (COPD) is a risk factor that increases the incidence of postoperative cardiopulmonary morbidity and mortality after lung resection.1–3 COPD is a physiological condition in which patients display airflow limitations because of narrowing of peripheral bronchioles. Decreased elastic recoil and structural changes of the small airways and alveoli cause air trapping and pulmonary hyperinflation,4 and the chronic alveolar hypoxia results in medial hypertrophy of the pulmonary arteriole and structural changes, such as muscularisation.5 In COPD patients, hypoxaemia is caused by intrapulmonary right-to-left shunt and the uneven distribution of alveolar ventilation and pulmonary perfusion. The high ventilation/perfusion area interferes with effective CO2 elimination, possibly resulting in hypercapnia.6,7 One-lung ventilation (OLV) aggravates the intrapulmonary shunt and dead space, whereas gravitational effects and hypoxic pulmonary vasoconstriction (HPV) act as protective mechanisms.8,9 However, decreased pulmonary artery compliance and increased pulmonary artery stiffness are reported in early COPD without pulmonary hypertension,10,11 and the HPV responses have been reported to be less efficient in patients with COPD.12 Furthermore, the treatment strategies for hypoxaemia induced by OLV, such as positive end-expiratory pressure (PEEP) and alveolar recruitment, cannot be applied to COPD patients because of concern about high intrinsic PEEP development.
In addition to β-adrenoreceptors in the smooth muscle of the bronchus, α1 and α2-adrenoreceptors are found in the bronchial mucosa and ganglia. Bronchodilators that are currently used target the β-adrenoreceptors of the bronchial wall, as bronchodilator effects of α-adrenoreceptors have not yet been proved. Dexmedetomidine, which is a selective α2-adrenoreceptor agonist, was reported to effectively inhibit histamine-induced bronchoconstriction in an animal study13 and to decrease the intrapulmonary shunt during OLV in healthy patients.14 In addition, dexmedetomidine is known to decrease pulmonary arterial pressure directly,15 and it does not increase pulmonary arterial pressure in patients with pre-existing pulmonary hypertension.16–18 However, whether or not dexmedetomidine can exert beneficial effects on oxygenation and lung mechanics in COPD during OLV remains unclear.
The primary object of this randomised trial was to assess the effects of dexmedetomidine on oxygenation and lung mechanics during OLV in patients with moderate COPD. The secondary end point was to assess the effects of dexmedetomidine administration on postoperative pulmonary complications.
Methods
This study received approval from the institutional review board of Severance Hospital, Yonsei University Health System, Seoul, South Korea (Ref. 4-2014-0306) in July 2014 and was registered at Clinical Trials.gov (NCT 02185430). All participants provided written informed consent before participation. Patients expected to undergo video-assisted thoracoscopic surgery for lung lobectomy were included. The inclusion criteria were as follows: age more than 40 years, American Society of Anaesthesiologists’ physical status 2 or 3, a diagnosis of COPD of moderate grading, and a forced expiratory volume in 1 s (FEV1) of at least 50% and less than 80% of predicted with the presence of a post-bronchodilator FEV1/forced vital capacity ratio less than 0.70 in a preoperative pulmonary function test.19 The exclusion criteria were heart failure (New York Heart Association class > II), a history of arrhythmia or treatment with antiarrhythmic drugs, bradycardia [heart rate (HR) <45 beats min−1] or atrioventricular block, severe functional liver or kidney disease, obesity (body mass index >30 kg m−2), restrictive lung disease and/or decreased lung diffusion capacity for carbon monoxide (DLco <70%).
Enrolled patients were randomly allocated to a control (0.9% saline) group or a Dex (dexmedetomidine) group using a randomised sequence generated by a computer, and the randomisation process was centralised. A concealed envelope for random allocation was sent to the anaesthesia nurses who prepared the dexmedetomidine or saline. Therefore, the anaesthesiologist administered the drug in a blind manner. The participating anaesthetists, nurses, surgeons and patients were blinded to the treatment allocation.
Patients were monitored with standard monitoring devices upon arrival at the operating room. Anaesthesia was induced with propofol, remifentanil and rocuronium. Tracheal intubation was performed with a left-sided double-lumen tube (Broncho-Cath, Mallinckrodt, Dublin, Ireland), and the position of the double-lumen tube was confirmed with a fibre-optic bronchoscope before OLV. After induction of anaesthesia, a 20-gauge radial artery catheter was inserted, and a 7-FG central venous catheter (Arrow, Teleflex Incorporated, Wayne, PA, USA) was inserted through the right internal jugular vein. The inserted length of the central venous catheter was calculated using a height-based formula so that the tip would lie near the right atrium.20 Anaesthesia was maintained with 1.0 to 3.0% sevoflurane, remifentanil and rocuronium. The lungs were initially ventilated in autoflow pressure-controlled ventilation mode (Primus i ventilator, Dräger TM Medical, Lübeck, Germany) with a tidal volume of 8 ml kg−1 and an inspiratory : expiratory (I : E) ratio of 1 : 2.5. During OLV, the lungs were ventilated in autoflow pressure-controlled ventilation mode with a tidal volume of 6 ml kg−1 and PEEP of 3 cmH20 for protective ventilation. In all patients, PEEP of 3 cmH20 was applied but discontinued if the peak airway pressure exceeded 30 mmHg. The fraction of inspired oxygen (FiO2) was initially set at 0.6 and in cases of desaturation to SaO2 less than 95%, FiO2 was increased by 0.2 up to 1.0. The respiratory rate was adjusted to maintain an end-tidal CO2 tension (EtCO2) of 5 to 6 kPa. During the study, the depth of anaesthesia was adjusted to maintain a bispectral index of 40 to 60. If the bispectral index decreased below 40, the concentration of sevoflurane was reduced. The concentration of sevoflurane in exhaled gas was monitored. In the Dex group, dexmedetomidine (Precedex, Hospira, Lake Forest, IL, USA) was started once the patient was haemodynamically stable 30 min after the initiation of OLV; a bolus of 1.0 μg kg−1 was administered over 10 min followed by a continuous infusion at 0.5 μg kg−1 h−1 for 1 h. A comparable volume of 0.9% saline was administered to patients in the control group.
The object of this trial was to evaluate the effects of dexmedetomidine on patients with moderate COPD undergoing lung cancer surgery. The primary outcome was lung oxygenation expressed as the PaO2/FiO2 ratio. The secondary outcome was to assess the effects of dexmedetomidine administration on postoperative pulmonary complications. Arterial blood gases were analysed (GEM Premier 4000, Instrumentation Laboratory, Lexington, MA, USA) at three time points: 30 min after the initiation of OLV (OLV), 30 min after dexmedetomidine administration during OLV (DEX-30) and 60 min after dexmedetomidine administration during OLV (DEX-60). The respiratory variables included PaO2/FiO2 ratio, PaCO2, EtCO2, dead space ventilation, peak airway pressure, tidal volume and dynamic compliance. The physiological dead space ventilation (Vd/Vt) was calculated as follows according to the Hardman & Aitkenhead equation:21 Vd/Vt = 1.14 × (PaCO2–EtCO2)/PaCO2 – 0.005. Dynamic compliance and plateau airway pressure were obtained from the Primus i ventilator. Dynamic compliance was automatically calculated as tidal volume/(plateau airway pressure – PEEP). The haemodynamic measurements included HR and systolic and diastolic blood pressures. Vasoactive drugs, such as ephedrine, were administered if the mean arterial pressure decreased by more than 20% from baseline arterial pressure, and atropine was administered if HR decreased to less than 45 beats min−1. If the SaO2 measured by pulse oximetry decreased to less than 90% during OLV, two-lung ventilation was reinstuted, and the event was recorded. The double-lumen tube was removed after the end of surgery.
To evaluate the secondary end point of pulmonary complications, respiratory rate and PaO2 /FiO2 ratio were recorded 20 min after arrival at the post-anaesthetic care unit (PACU), and incidences of postoperative atelectasis, pneumonia and acute lung injury (ALI) were assessed for 72 h. In the PACU, if the patient showed signs of dyspnoea with a concurrent PaO2/FiO2 ratio less than 40 kPa, the patient required ICU admission. ALI was defined as the following: PaO2/FiO2 ratio less than 40 kPa (300 mmHg); diffuse pulmonary infiltration at 72 h; exclusion of hydrostatic pulmonary oedema because of other primary causes, including cardiac insufficiency or fluid overload.22
Statistical analysis
The primary outcome was lung oxygenation expressed by PaO2/FiO2 ratio during OLV. A previous study23 showed that the mean ± SD value of PaO2/FiO2 ratio during OLV in patients with COPD was 22.7 ± 7.0 kPa. A PaO2 /FiO2 ratio difference of 6.7 kPa between the two groups is considered to be significant. We calculated that a sample size of 23 patients was required in each group at a power of 90%, with a two-sided significance level of 0.05 by an independent t-test. To account for a 10% dropout rate, we included 25 patients in each group. Results are expressed as mean ± SD or median (IQR). Adjusted odds ratios (OR) and 95% confidence intervals (CIs) were calculated for independent variables. Intergroup comparisons at each time point were determined by unpaired t-tests, Mann–Whitney U-tests and χ2 or Fisher exact tests. Variables with repeated measures were analysed using a linear mixed model with patient indicator as a random effect and group, time, and group-by-time as fixed effects. The statistical analyses were performed with SPSS 20.0 software (IBM Corporation, Armonk, NY, USA), and P values less than 0.05 were considered statistically significant.
Results
Seventy patients were determined to be eligible for this study, but four patients were excluded at initial screening and three patients declined to participate. Of the 63 patients, 13 were excluded from the study because of incomplete data and/or changes of surgical plan. Data from 25 patients in each group were analysed (Fig. 1). There were no differences between groups regarding baseline characteristics. The frequency of ephedrine administration was significantly greater in the Dex group (Table 1).
Fig. 1.
Study flow diagram.
Table 1.
Patients’ characteristics and operative data
| Dex group (n = 25) | Control group (n = 25) | |
| Age (years) | 68.4 ± 6.4 | 69.4 ± 8.7 |
| Sex (M/F) | 12/13 | 11/14 |
| Height (cm) | 162.9 ± 6.9 | 162.3 ± 8.4 |
| Weight (kg) | 60.8 ± 8.9 | 61.3 ± 8.9 |
| BMI (kg m−2) | 22.3 ± 2.7 | 22.7 ± 2.1 |
| ASA physical status, 2/3 | 11/14 | 12/3 |
| Hypertension | 10 | 11 |
| Cardiovascular medication | ||
| RAAS inhibitor | 8 | 8 |
| Calcium channel blocker | 1 | 2 |
| β-adrenergic antagonists | 0 | 1 |
| Furosemide | 2 | 2 |
| Smoking | ||
| Ex-smoker/current smoker | 11/12 | 14/10 |
| Smoking index, pack-years | 36.7 ± 27.1 | 35.8 ± 28.9 |
| Non-smoker | 2 | 1 |
| Medication | ||
| Inhaled corticosteroid | 42.7% | 39.5% |
| Oral corticosteroid | 18.6% | 14.3% |
| Inhaled bronchodilator | 21.3% | 17.9% |
| Oral bronchodilator | 33.6% | 34.2% |
| Muscarinic antagonists | 3.7% | 3.2% |
| Preoperative SpO2, % | 93.8 ± 1.2 | 94.1 ± 1.3 |
| Preoperative spirometry | ||
| FEV1, % predicted | 60.8 ± 5.2 | 61.4 ± 4.1 |
| FVC, % predicted | 82.7 ± 8.6 | 83.1 ± 7.2 |
| FEV1/FVC, % | 55.9 ± 3.2 | 56.1 ± 5.7 |
| FEF25–75%, l s−1 | 26.4 ± 18.9 | 27.1 ± 19.3 |
| FEF50%, l s−1 | 18.9 ± 11.3 | 19.3 ± 12.7 |
| DLco, % predicted | 93.6 ± 8.2 | 92.3 ± 7.6 |
| Intraoperative data | ||
| Anaesthesia time (min) | 193.6 ± 24.6 | 189.7 ± 44.1 |
| Operation time (min) | 163.8 ± 39.4 | 158.1 ± 33.2 |
| OLV time (min) | 125.6 ± 37.1 | 124.7 ± 41.2 |
| Fluid intake (ml) | 791.4 ± 92.3 | 813.2 ± 79.5 |
| Urine output (ml) | 274.5 ± 132.6 | 354.6 ± 132.7 |
| Estimated blood loss (ml) | 122.4 ± 55.6 | 125.6 ± 84.7 |
| Number receiving ephedrine | 9* | 1 |
| Right/left lobectomy | 17/8 | 16/9 |
| Right upper lobe | 8 | 5 |
| Right middle lobe | 2 | 5 |
| Right lower lobe | 7 | 6 |
| Left upper lobe | 4 | 6 |
| Left lower lobe | 4 | 3 |
Values are mean ± SD, number or percentage of patients. ASA, American Society of Anesthesiologists; DLco, diffusion capacity of lung for carbon monoxide; FEF25–75%, forced expiratory volume between 25 and 75% of the FVC; FEF50%, forced expiratory volume 50% of the FVC; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; OLV, one-lung ventilation; RAAS, renin–angiotensin–aldosterone system; SpO2, oxygen saturation (pulse oximetry).
*P < 0.05 vs. control group.
Intraoperative data are shown in Table 2. In comparison with the control group, there were significant haemodynamic changes in patients in the Dex group. HR at DEX-30 and DEX-60 was significantly lower compared with values at the same times in the control group (P < 0.001). In addition, mean blood pressure was significantly lower in the Dex group than in the control group (P < 0.05). The end-tidal concentrations of sevoflurane were similar in the two groups.
Table 2.
Haemodynamic and respiratory changes in 50 patients with moderate chronic obstructive pulmonary disease during one-lung ventilation
| Dex group (n = 25) | Control group (n = 25) | P Group × Time | |
| Heart rate (beats min−1) | <0.001 | ||
| OLV | 76.6 ± 7.6 | 81.1 ± 11.6 | |
| DEX-30 | 63.2 ± 9.8*,** | 76.4 ± 8.3 | |
| DEX-60 | 58.8 ± 6.0*,** | 77.0 ± 8.5 | |
| MBP (mmHg) | 0.014 | ||
| OLV | 84.8 ± 13.6 | 81.1 ± 12.1 | |
| DEX-30 | 70.7 ± 10.9*,** | 82.2 ± 6.3 | |
| DEX-60 | 68.9 ± 9.5*,** | 76.5 ± 5.1 | |
| Et SEVO (vol%) | 0.078 | ||
| OLV | 2.0 ± 0.5 | 2.0 ± 0.4 | |
| DEX-30 | 1.8 ± 0.2 | 2.0 ± 0.4 | |
| DEX-60 | 1.9 ± 0.2 | 2.2 ± 0.4 | |
| BIS | 0.022 | ||
| OLV | 56.9 ± 8.9 | 54.6 ± 6.6 | |
| DEX-30 | 41.5 ± 5.5*,** | 53.6 ± 8.6 | |
| DEX-60 | 40.2 ± 7.3*,** | 52.5 ± 5.8 | |
| PaCO2 (kPa) | 0.027 | ||
| OLV | 6.2 ± 0.3 | 6.1 ± 0.3 | |
| DEX-30 | 5.6 ± 0.3*,** | 5.9 ± 0.5 | |
| DEX-60 | 5.7 ± 0.3*,** | 6.1 ± 0.6 | |
| Ppeak (cmH2O) | 0.001 | ||
| OLV | 22.3 ± 3.9 | 22.5 ± 4.9 | |
| DEX-30 | 18.2 ± 3.2*,** | 23.0 ± 4.1 | |
| DEX-60 | 18.2 ± 2.7*,** | 21.7 ± 4.3 | |
| Compliance (ml cmH2O−1) | 0.018 | ||
| OLV | 19.5 ± 4.9 | 19.2 ± 5.2 | |
| DEX-30 | 22.5 ± 3.5*,** | 17.7 ± 3.4 | |
| DEX-60 | 21.4 ± 4.2*,** | 18.1 ± 4.6 |
Values are mean ± SD. BIS, bispectral index; Compliance, dynamic compliance; Control, control group; Dex, dexmedetomidine group; DEX-30, 30 min after dexmedetomidine administration during OLV; DEX-60, 60 min after dexmedetomidine administration during OLV; Et SEVO, concentration of sevoflurane in exhaled gas; MBP, mean blood pressure; OLV, 30 min after initiation of OLV; PaCO2, arterial carbon dioxide tension; Ppeak, peak airway pressure.
*P < 0.05 versus Control group.
**P < 0.05 versus OLV within the same group.
Results of blood gas analysis and lung mechanics are shown in Fig. 2 and Table 2. The PaO2/FiO2 ratio was significantly higher at DEX-30 and DEX-60 in the Dex group compared with the control group (27.9 ± 5.8 vs. 22.5 ± 8.4 and 28.6 ± 5.9 vs. 21.0 ± 9.9 kPa, P = 0.026 and 0.009). In the Dex group, dead space ventilation at DEX-30 and DEX-60 was significantly lower compared with the control group (19.2 ± 8.5 vs. 24.1 ± 8.1 and 19.6 ± 6.7 vs. 25.3 ± 7.8%, P = 0.046 and 0.013). Dynamic compliance was higher at DEX-30 and DEX-60 in the Dex group compared with the control group. Plateau airway pressure was significantly lower in the Dex group at DEX-30 and DEX-60.
Fig. 2.
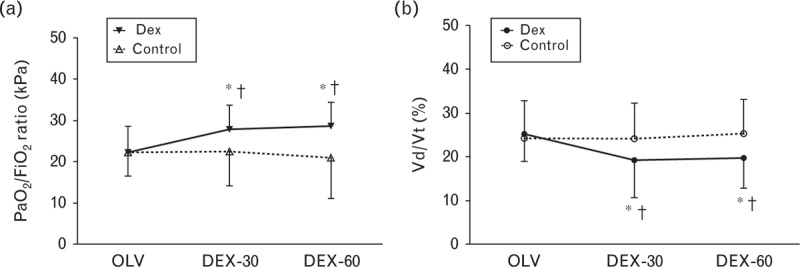
Respiratory changes in 50 patients with moderate COPD during OLV. (a) PaO2/FiO2 ratio, (b) deadspace ventilation (Vd/Vt). Data are mean with error bars showing SD. ∗P < 0.05 versus OLV, †P < 0.05 vs. control group. COPD, chronic obstructive pulmonary disease; Dex, dexmedetomidine group; Control, control group; OLV, 30 min after initiation of OLV; DEX-30, 30 min after dexmedetomidine administration during OLV; DEX-60, 60 min after dexmedetomidine administration during OLV; OLV, one-lung ventilation; Vd, dead space volume; Vt, tidal volume.
Postoperative data are shown in Table 3. In the PACU, PaO2 /FiO2 ratio was significantly higher in the Dex group than in the control group. Seven patients in the control group had episodes in which SaO2 was less than 95% and PaO2/FiO2 ratio was less than 40 kPa while breathing room air, eventually resulting in respiratory care in the ICU, with one patient ultimately diagnosed with ALI. In comparison, only one patient in the Dex group required respiratory care in the ICU after lung surgery. Patients in the Dex group had fewer incidences of atelectasis (Dex group 0%, control group 16%), focal lung infiltration (Dex group 4%, control group 8%). The duration of hospital stay did not differ between groups.
Table 3.
Postoperative outcome of 50 patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery
| Dex group (n = 25) | Control group (n = 25) | P | OR | |
| PaO2/FIO2 ratio at PACU (kPa) | 47.5 ± 7.1* | 42.2 ± 5.9 | 0.022 | |
| Need for ICU stay | 1 | 7 | 0.049 | 9.33 (1.05 to 82.78) |
| Atelectasis | 0 | 4 | 0.110 | 10.67 (0.54 to 209.65) |
| Length of hospital stay (days) | 6.5 (5 to 10) | 7.1 (6 to 11) | 0.102 | |
| Focal lung infiltration | 1 | 2 | >0.99 | 2.09 (0.18 to 24.61) |
| ALI | 0 | 1 | >0.99 | 3.12 (0.12 to 80.39) |
Values are mean ± SD, number of patients, median (IQR) OR (95% CI). ALI, acute lung injury; Dex, dexmedetomidine; OR, odds ratio; PACU, post-anaesthetic care unit.
∗P < 0.05 vs. control group.
Discussion
This randomised trial assessed the effects of dexmedetomidine on oxygenation and lung mechanics of patients with moderate COPD undergoing lung cancer surgery. Dexmedetomidine improved oxygenation by decreasing dead space ventilation and dynamic compliance in patients with moderate COPD during the surgery, with significantly higher postoperative PaO2/FiO2 ratios and fewer pulmonary complications.
High ventilation/perfusion mismatch, which is caused by luminal narrowing of pulmonary arterioles and air-trapping hyperinflation, is considered the cause of hypoxaemia and hypercapnia in COPD.6 In patients with COPD, the response to HPV does not activate effectively because of the remodelled pulmonary vascular structure and altered pulmonary vascular reactivity.24 Furthermore, even in patients with early COPD without coexisting pulmonary hypertension, decreased pulmonary vascular resistance and increased pulmonary artery stiffness can possibly inhibit the activation of HPV.11 According to the global initiative for [chronic obstructive] lung disease classification,19 COPD is assessed as moderate when FEV1 is at least 50%, and 80% or less of predicted, and severe if FEV1 is more than 30%, and 50% or less of predicted with a post-bronchodilator FEV1/ forced vital capacity ratio less than 0.70.
Previous studies of COPD have focused on hyperinflation and intrinsic PEEP because of air trapping during OLV in patients with severe COPD.25,26 Our results revealed that the PaO2/FiO2 ratio in the control group was comparable with that of previously reported PaO2/FiO2 ratios in COPD patients during OLV. 23,25,26 However, there is controversy in the literature regarding the results of some studies which have claimed significantly higher PaO2/FiO2 ratios than we found.27 Furthermore, there is a continuing dispute over intrinsic PEEP and oxygenation during OLV in COPD patients. Although Bardoczky et al.,26 assert that intrinsic PEEP and oxygenation have a negative correlation, other views have been expressed by, for example, Aschkenasy et al.,27 who stated that intrinsic PEEP may delay events of hypoxaemia in severe COPD patients when compared with healthy patients. Some authors have explained that intrinsic PEEP caused by the hyperinflation of ventilated lungs in patients with severe COPD increases the functional residual capacity and improves oxygenation during OLV.27,28 However, they did not evaluate the statistical difference.28 In light of this, Michelet et al.23 insisted that moderate to severe COPD patients had better PaO2/FiO2 ratios than healthy patients during OLV. This hypothesis was based on the idea that high intrinsic PEEP could induce more entrapped lung volume in severe COPD. In our study, intrinsic PEEP rarely occurred during OLV. In addition, Yokota et al.29 could not confirm the correlations between intrinsic PEEP and oxygenation, and preoperative forced expiratory volume and oxygenation have not been shown to correlate during OLV.30 There are larger numbers of patients with mild to moderate COPD than severe COPD.31,32 Thus, further studies are required to explore the physiological consequences of mild to moderate COPD during OLV.
In this study, dexmedetomidine administration decreased dead space ventilation and improved dynamic compliance and oxygenation. Dexmedetomidine has been reported to have a bronchodilator effect in histamine-induced bronchoconstriction when it was administered intravenously in an animal study,13 but no clinical studies have confirmed this effect. Some authors have found that dexmedetomidine improves oxygenation and respiratory dynamics during OLV in healthy patients.14,33 They suggested that a sparing effect of the inhalational agent by dexmedetomidine administration ameliorated HPV inhibition or increased perfusion in the ventilated lung by vasodilatation. However, the concentrations of sevoflurane were similar in both groups in the present study, and it seems more likely that dexmedetomidine affects lung mechanics directly in COPD to increase the threshold of hypoxaemia during OLV, rather than having an inhalation anaesthetic-sparing effect.
There is limited data about how dexmedetomidine affects the pulmonary vasculature and perfusion. Although perioperative dexmedetomidine administration is known to increase systemic blood pressure temporarily, its effect on pulmonary arterial pressure has rarely been studied. A previous study showed that dexmedetomidine administration decreased pulmonary arterial pressure when given through a peripheral vein, and it did not increase pulmonary arterial pressure even after direct injection into the pulmonary artery.15 Several studies have reported that dexmedetomidine does not elevate pulmonary arterial pressure after cardiac surgery.34,35 Clinically, administration of dexmedetomidine decreases pulmonary arterial pressure effectively in patients with existing pulmonary hypertension after cardiac surgery.18 Recently, it was reported that dexmedetomidine does not affect the pulmonary vasculature in patients with existing pulmonary hypertension.36,37 Furthermore, Nathan et al.17 reported that dexmedetomidine improved respiratory status during sedation in patients without pulmonary arterial pressure elevation. As we did not have direct evidence to verify pulmonary arterial pressure and pulmonary vascular resistance in this study, we cannot determine whether dexmedetomidine improved oxygenation by increasing pulmonary perfusion. However, considering the decrease of dead space ventilation and improvement of PaO2/FiO2 ratio after dexmedetomidine administration, dexmedetomidine could act as a pulmonary vasodilator which increases perfusion to the dead space ventilation portion of the ventilated lung in moderate COPD patients. Therefore, further study regarding the mechanism of dexmedetomidine on the pulmonary vasculature and broncho-alveolar system is needed.
Regardless of the novel findings of the present study, there are several limitations to take into account. First, although high FiO2 can possibly increase the risk of pulmonary atelectasis, lead to absent ventilation in low ventilation/perfusion areas of the lung and result in a high alveolar-arterial O2 tension difference,38 we allowed for an increase of FiO2 at an increment of 0.2 up to FiO2 1.0 in response to hypoxaemia. Because our goal was to prevent hypoxaemia during OLV, we set our focus on detection of intraoperative hypoxaemia rather than postoperative hypoxaemia. Second, this study was conducted on patients with moderate COPD. Consequently, we cannot assume the same results of dexmedetomidine administration in patients with severe COPD, which is characterised by irreversibility of airflow limitation, hyperdynamic inflation and resultant high intrinsic PEEP. Third, we could not confirm whether the enrolled patients had pulmonary hypertension before surgery. Because pulmonary artery catheters are not inserted routinely in clinical settings, we could not measure the pulmonary vascular resistance, or directly measure mixed venous oxygen tension. Fourth, the primary outcome of this trial was lung oxygenation expressed by PaO2/FiO2 ratio and respiratory mechanics during OLV. Thus, the results from this study are underpowered to confirm the secondary outcome of postoperative pulmonary complications. Fifth, it is currently recognised that inhaled anaesthetics have an effect on intrapulmonary shunt during OLV. To minimise the effects of inhaled anaesthetics and focus on the direct effects of dexmedetomidine on lung mechanics, we maintained anaesthesia with the lowest possible concentration of sevoflurane in both groups. Nonetheless, we cannot exclude possible effects of deep anaesthesia by inhaled anaesthetics on lung mechanics. Finally, this study included a relatively small number of patients and further studies will be needed to verify our findings in both moderate and severe COPD patient groups.
In conclusion, dexmedetomidine provides clinically relevant benefits in patients with moderate COPD undergoing lung cancer surgery. Dexmedetomidine administration during OLV not only improved oxygenation but also reduced dead space ventilation in patients with moderate COPD. The positive effects of dexmedetomidine extended to the postoperative period, with the potential for better outcome after lung surgery.
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: this work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2013R1A1A2059283) and funded by the Ministry of Education, Science and Technology.
Conflicts of interest: none.
Presentation: none.
Footnotes
Published online 7 January 2016
References
- 1.Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006; 81:1830–1837. [DOI] [PubMed] [Google Scholar]
- 2.Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010; 37:525–530. [DOI] [PubMed] [Google Scholar]
- 3.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002; 37:95–101. [DOI] [PubMed] [Google Scholar]
- 4.Burrows B. Differential diagnosis of chronic obstructive pulmonary disease. Chest 1990; 97:8–16. [DOI] [PubMed] [Google Scholar]
- 5.Magee F, Wright JL, Wiggs BR, et al. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 1988; 43:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner PD, Dantzker DR, Dueck R, et al. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 1977; 59:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol 2009; 106:1902–1908. [DOI] [PubMed] [Google Scholar]
- 8.Szegedi LL, D’Hollander AA, Vermassen FE, et al. Gravity is an important determinant of oxygenation during one-lung ventilation. Acta Anaesthesiol Scand 2010; 54:744–750. [DOI] [PubMed] [Google Scholar]
- 9.Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology 2015; 122:932–946. [DOI] [PubMed] [Google Scholar]
- 10.Hilde JM, Skjorten I, Grotta OJ, et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol 2013; 62:1103–1111. [DOI] [PubMed] [Google Scholar]
- 11.Ertan C, Tarakci N, Ozeke O, Demir AD. Pulmonary artery distensibility in chronic obstructive pulmonary disease. Echocardiography 2013; 30:940–944. [DOI] [PubMed] [Google Scholar]
- 12.Peinado VI, Santos S, Ramírez J, et al. Response to hypoxia of pulmonary arteries in chronic obstructive pulmonary disease: an in vitro study. Eur Respir J 2002; 20:332–338. [DOI] [PubMed] [Google Scholar]
- 13.Groeben H, Mitzner W, Brown RH. Effects of the α2-adrenoceptor agonist dexmedetomidine on bronchoconstriction in dogs. Anesthesiology 2004; 100:359–363. [DOI] [PubMed] [Google Scholar]
- 14.Xia R, Yin H, Xia ZY, et al. Effect of intravenous infusion of dexmedetomidine combined with inhalation of isoflurane on arterial oxygenation and intrapulmonary shunt during single-lung ventilation. Cell Biochem Biophys 2013; 67:1547–1550. [DOI] [PubMed] [Google Scholar]
- 15.Unal Y, Pampal HK, Arslan M, et al. The effects of dexmedetomidine on pulmonary artery pressure in experiment. Bratisl Lek Listy 2014; 115:272–274. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara H, Hirota K, Sato M, et al. Monitored anesthesia care with dexmedetomidine of a patient with severe pulmonary arterial hypertension for inguinal hernioplasty. J Anesth 2010; 24:611–613. [DOI] [PubMed] [Google Scholar]
- 17.Nathan AT, Marino BS, Hanna B, et al. Novel use of dexmedetomidine in a patient with pulmonary hypertension. Paediatr Anaesth 2008; 18:782–784. [DOI] [PubMed] [Google Scholar]
- 18.But AK, Ozgul U, Erdil F, et al. The effects of preoperative dexmedetomidine infusion on hemodynamics in patients with pulmonary hypertension undergoing mitral valve replacement surgery. Acta Anaesthesiol Scand 2006; 50:1207–1212. [DOI] [PubMed] [Google Scholar]
- 19.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365. [DOI] [PubMed] [Google Scholar]
- 20.Czepizak CA, O’Callaghan JM, Venus B. Evaluation of formulas for optimal positioning of central venous catheters. Chest 1995; 107:1662–1664. [DOI] [PubMed] [Google Scholar]
- 21.Hardman JG, Aitkenhead AR. Estimating alveolar dead space from the arterial to end-tidal CO2 gradient: a modeling analysis. Anesth Analg 2003; 97:1846–1851. [DOI] [PubMed] [Google Scholar]
- 22.Licker M, Cartier V, Robert J, et al. Risk factors of acute kidney injury according to RIFLE criteria after lung cancer surgery. Ann Thorac Surg 2011; 91:844–850. [DOI] [PubMed] [Google Scholar]
- 23.Michelet P, Blayac D, Vincent A, et al. How do COPD and healthy-lung patients tolerate the reduced volume ventilation strategy during OLV ventilation. Acta Anaesthesiol Scand 2010; 54:1128–1136. [DOI] [PubMed] [Google Scholar]
- 24.Saadjian A, Philip-Joet F, Levy S, et al. Vascular and cardiac reactivity in pulmonary hypertension due to chronic obstructive lung disease: assessment with various oxygen concentrations. Eur Respir J 1992; 5:525–530. [PubMed] [Google Scholar]
- 25.Ducros L, Moutafis M, Castelain MH, et al. Pulmonary air trapping during two-lung and one-lung ventilation. J Cardiothorac Vasc Anesth 1999; 13:35–39. [DOI] [PubMed] [Google Scholar]
- 26.Bardoczky GI, d’Hollander AA, Rocmans P, et al. Respiratory mechanics and gas exchange during one-lung ventilation for thoracic surgery: the effects of end-inspiratory pause in stable COPD patients. J Cardiothorac Vasc Anesth 1998; 12:137–141. [DOI] [PubMed] [Google Scholar]
- 27.Aschkenasy SV, Hofer CK, Zalunardo MP, et al. Patterns of changes in arterial PO2 during one-lung ventilation: a comparison between patients with severe pulmonary emphysema and patients with preserved lung function. J Cardiothorac Vasc Anesth 2005; 19:479–484. [DOI] [PubMed] [Google Scholar]
- 28.Slinger PD, Kruger M, McRae K, et al. Relation of the static compliance curve and positive end-expiratory pressure to oxygenation during one-lung ventilation. Anesthesiology 2001; 95:1096–1102. [DOI] [PubMed] [Google Scholar]
- 29.Yokota K, Toriumi T, Sari A, et al. Auto-positive end-expiratory pressure during one-lung ventilation using a double-lumen endobronchial tube. Anesth Analg 1996; 82:1007–1010. [DOI] [PubMed] [Google Scholar]
- 30.Guenoun T, Journois D, Silleran-Chassany J, et al. Prediction of arterial oxygen tension during one-lung ventilation: analysis of preoperative and intraoperative variables. J Cardiothorac Vasc Anesth 2002; 16:199–203. [DOI] [PubMed] [Google Scholar]
- 31.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370:741–750. [DOI] [PubMed] [Google Scholar]
- 32.Hoogendoorn M, Rutten-van Mölken MP, Hoogenveen RT, et al. A dynamic population model of disease progression in COPD. Eur Respir J 2005; 26:223–233. [DOI] [PubMed] [Google Scholar]
- 33.Kernan S, Rehman S, Meyer T, et al. Effects of dexmedetomidine on oxygenation during one-lung ventilation for thoracic surgery in adults. J Minim Access Surg 2011; 7:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazol JP, Lichtenstein SE, Jooste EH, et al. Effect of dexmedetomidine on pulmonary artery pressure after congenital cardiac surgery: a pilot study. Pediatr Crit Care Med 2010; 11:589–592. [DOI] [PubMed] [Google Scholar]
- 35.Gazit AZ. Dexmedetomidine and pulmonary artery pressure in the pediatric cardiac surgery patient: A new insight. Pediatr Crit Care Med 2010; 11:634–635. [DOI] [PubMed] [Google Scholar]
- 36.Friesen RH, Nichols CS, Twite MD, et al. The hemodynamic response to dexmedetomidine loading dose in children with and without pulmonary hypertension. Anesth Analg 2013; 117:953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishibe S, Imanishi H, Mieda T, Tsujita M. The effects of dexmedetomidine administration on the pulmonary artery pressure and the transpulmonary pressure gradient after the bidirectional superior cavopulmonary shunt. Pediatr Cardiol 2015; 36:151–157. [DOI] [PubMed] [Google Scholar]
- 38.Kleinsasser AT, Pircher I, Truebsbach S, et al. Pulmonary function after emergence on 100% oxygen in patients with chronic obstructive pulmonary disease: a randomized, controlled trial. Anesthesiology 2014; 120:1146–1151. [DOI] [PubMed] [Google Scholar]


