Abstract
Aim:
To describe the associated ocular, neurologic, and systemic findings in a population of children with optic nerve hypoplasia (ONH) and to evaluate the relationship between ocular signs and neurologic findings.
Method:
A retrospective chart review of 53 patients with the diagnosis of ONH seen between December 1998 and September 2012 was performed. All neurodevelopmental anomalies, neuroradiologic findings, endocrinologic and systemic findings were recorded. Poor vision was defined as the visual acuity poorer than logMAR 1.0 or inadequate central steady maintained fixation.
Results:
Thirty (56.6%) of the 53 children with ONH were boys. Mean age at presentation was 56.2±46.8 months (range; 3 months to 18 years). Poor vision defined for the purpose of this study was found in 47.2% of 53 patients. Thirty-three (62.3%) children had nystagmus. Thirty-four (64.2%) children had strabismus. Thirteen (38.2%) of those with strabismus had esotropia, 20 (58.8%) had exotropia. The total number of the children with neurodevelopmental deficit was 22 (41.5%) in our study.
Conclusion:
The vision of young children with ONH should be monitored at least annually, and any refractive errors should be treated. Neuroimaging of the brain and endocrinologic evaluation is necessary in all cases with ONH.
Keywords: Endocrinologic evaluation, neurodevelopmantal anomaly, neuroimaging, optic nerve hypoplasia
INTRODUCTION
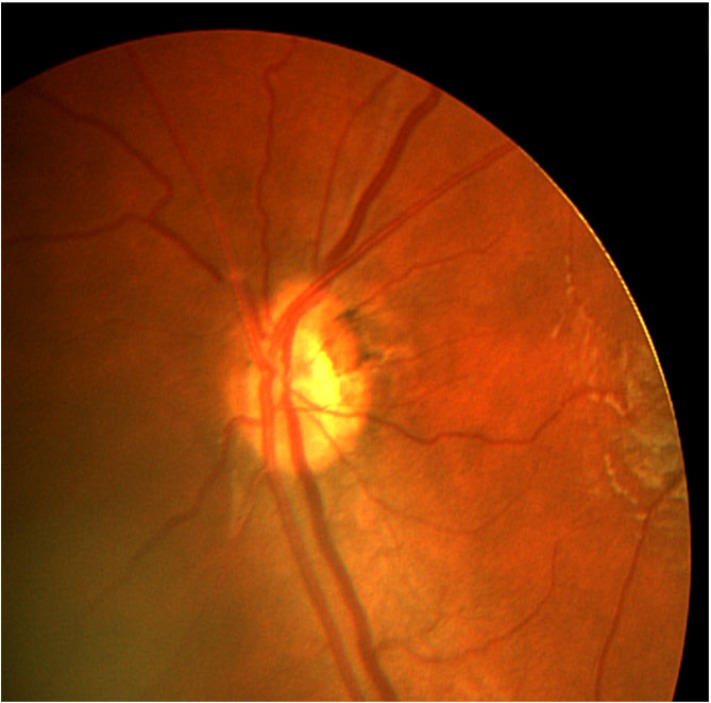
Optic nerve hypoplasia (ONH) is an ocular malformation diagnosed clinically on the basis of an abnormally small optic nerve head. Additional ophthalmoscopic features include optic nerve pallor, the “double-ring sign,” (Fig. 1) and tortuous retinal vessels. It may occur unilaterally or bilaterally, and the lost of nerve tissue can be diffuse or segmental [1-4].
Fig. (1).
An eye with optic nerve hypoplasia and double-ring sign.
It is histologically characterized by a subnormal number of optic nerve axons and is an nonprogressive, nonspecific manifestation of damage at any site of the visual pathways, sustained anytime before its full development [5-8]. It may cause a wide range of visual disabilities, and a high frequency of associated central nervous system abnormalities in children with ONH, indicating the complexity of this diagnosis [9-11]. Optic nerve hypoplasia has been associated with a broad range of congenital intracranial anomalies, including absence of the septum pellucidum, thinning of the corpus callosum, and dysplasia of the anterior third ventricle in septo-optic dysplasia (De Morsier syndrome), posterior pituitary ectopia, and cerebral hemispheric malformations [11].
Although it is very well known that ONH is associated with neurologic, endocrinologic and other systemic disorders some authors recommend that only children with bilateral ONH, nystagmus, and poor vision should undergo a thorough neuroradiographic and endocrine examination. Skarf and Hoyt [2] stated that scanning is not indicated unless the child shows clinical evidence of either hormone deficiency or a congenital anomaly. However, to perform neuroimaging and endocrinologic investigation in all cases with ONH is another approach [12,13].
The purpose of the present study was to describe the associated ocular, neurologic, and systemic findings in a population of children with ONH and to evaluate the relationship between ocular signs and neurologic findings.
METHODS
A retrospective chart review of 53 cases with the diagnosis of ONH seen between December 1998 and September 2012 was performed, with information regarding age at first examination, diagnosis (ophthalmologic and others), visual acuity, cycloplegic refraction, orthoptic condition, and fundoscopic findings being extracted from the medical files. All neurodevelopmental anomalies, neuroradiologic findings, endocrinologic and systemic findings were also recorded. As all the study cases were minors, their parents gave written informed consent prior to participation in conformity with the Declaration of Helsinki.
Snellen visual acuity was recorded whenever possible. In younger or less cooperative cases, Teller acuity cards testing or the ability to ‘‘fixate and follow’’ a target was used. Best corrected visual acuity (BCVA) values measured using Snellen chart and Teller acuity cards (corresponding visual equivalents were initially measured according to the manufacturer using the conversion scale placed on each cards) were converted into logMAR unit for statistical analysis. Poor vision was defined as the visual acuity worse than logMAR 1.0 or inadequate central steady maintained (CSM) fixation while normal vision was defined as the visual acuity equal or better than logMAR 1.0 as well as positive CSM [12].
In 56.2% of the study population, BCVA could be measured using Snellen chart and Teller acuity cards. Those with light perception or no visual-orienting behavior were arbitrarily given an acuity of 0.13 and 0.10 cycles/degree, respectively [14].
Cases whose visual acuity could be evaluated with CSM fixation did not include to parametric statistical analysis. In 9 of the 49 bilateral cases visual acuity was different in 2 eyes, the eye with poorer visual acuity was used for statistical analysis. In cases with similar visual acuity the refraction of the eye with higher spherical equivalent was used for statistical analysis. Orthoptic examination was performed with prism cover test or Hirschberg and Krimsky tests depending on the cooperation level of the case. Restriction of ocular motility as well as the presence of nystagmus and its type were noted. Cycloplegia was accomplished with one drop of 1% cyclopentolate and 1% tropicamide, repeated in five to ten minutes, with retinoscopy performed after 45 minutes. To find out the refractive errors, retinoscopy was performed in all patients and confirmations were provided via autorefractometry (Retinomax; Right Manufacturing, Virginia Beach, VA) if possible.
Spherical equivalents, found by adding the half of cylindrical values to spherical values, were used in comparisons. In 49 bilateral cases, cases were assessed as emmetropic if between 0.75 diopters of myopic and hypermetropic equivalents, hyperopic if between +1.0 and +4.75 diopters, highly hyperopic if +5.0 diopters and above, myopic if between -1.0 and -4.0 diopters, and highly myopic if -4.25 diopters and above.
SPSS, statistical software, version 11.6 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Normality of the datas was evaluated with Kolmogorov-Smirnov test. The categorical variables between the groups were analyzed by using χ2 test. Mann Whitney U test was used to compare non-categorical variables. P value of less than 0.05 was considered statistically significant.
RESULTS
Thirty (56.6%) of the 53 cases with ONH were boys. Forty-nine (92.4%) of the cases had bilateral ONH. Mean age at ophthalmologic examination was 56.2±46.8 months (range; 3 months to 18 years). Mean spherical equivalent was 0.51±3.43 dioptry (range:-8.25 to +8.5 dioptry). Thirty-three (62.3%) cases had nystagmus, none of the cases with unilateral ONH had nystagmus. Thirty-four (64.1 %) of the cases had strabismus. Characteristics of the 53 cases are shawn in Table 1.
Table 1. Characteristics of the patients [n(%)].
| All Subjects (n=53) | |
|---|---|
|
Age at examination ˂6 months 6-12 months 2-5 years ˃5 years |
3 (5.6) 8 (15.2) 21 (39.6) 21 (39.6) |
|
Type of the refraction Emmetropia Hyperopia High hyperopia Myopia High myopia |
18 (33.9) 12 (22.6) 9 (16.9) 8 (15.1) 6 (11.3) |
|
Cylindiric refraction ˂1 dioptry ≥1 dioptry |
21 (39.6) 32 (60.1) |
| Nystagmus Yes No |
33 (62.3) 20 (37.7) |
|
Type of the strabismus Ortophoric Esotropia Exotropia Hypertropia |
19 (35.9) 13 (24.6) 20 (37.7) 1 (1.8) |
Visual acuity could be assessed with the Snellen chart in 21 cases (39.6%), and with the Teller acuity cards in 8 cases (15.1%), whereas optokinetic nystagmus or CSM was taken into account according to the bilateral fixation pattern in the remaining 24 cases (45.3%). The mean best-corrected visual acuity of cases that could be measured using Snellen chart or Teller acuity cards was found as logMAR 0.52±0.40, 11 eyes had no light perception, 5 eyes had motion perception, 8 eyes had CSM fixation. Poor vision defined for the purpose of this study was found in 47.2% of 53 cases. Table 2 demonstrates the comparement of the cases with and without poor vision. Multiple regression analysis revealed that, the only important factor on BCVA was the presence of neurodevelopmental anomaly.
One case had ptosis, one case had Duane Syndrome Type 1. Two cases had albinism, iris transillumination defect and foveal hypoplasia. One case had punctual atresia and microcornea.
Total case number with neuro-developmental deficit was 22 in our study (41.5%). All of the 22 cases had bilateral ONH and none of the cases with unilateral ONH had neurodevelopmental deficit. Developmental delay was the most common neurologic associated finding and was present in 13 (24.5%) cases, there were 4 (7.5%) cases with cerebral palsy in our population. Three cases (5.6%) had seizure disorder. One case had Dandy Walker anomaly, and 1 case (1.9%) had Silver Russel Syndrome. Table 3 shows ocular findings in the cases with and without neurodevelopmental anomaly.
Twenty-five (47.8%) of the cases underwent neuroimaging, 22 of them had MRI and 3 had computed tomography. Five (20%) of these 25 cases had normal scans. Four cases had hypoplasia of corpus callosum. Eleven cases had cortical atrophy, one case had partial hydrancephaly, cerebellar atrophy, ventriculomegaly and also corpus callosum hypoplasia, one case of which has unilateral ONH had Chiary 1 Malformation (sphenoid meningosel, cerebello-toncillary ectopia) and hypophisial gland displacement, this case had also morning glory malformation in his left eye, dismorfic face, left ptosis and haemongioma at right eyelid and one case had cerebral interdigitation. Two cases had septo-optic dysplasia.
Endocrine deficits were detected in 2 (3.7%) cases. Both of them had growth hormone deficiency. One of them had hypophyseal ectopia on cranial MRI imaging.
Two cases had a history of premature birth. One case had cardiomegaly and macroglossy. One had rectal agenesia, and one patient had cleft palate. Three parents were second degree relatives.
DISCUSSION
Optic nerve hypoplasia, is the most common congenital anomaly of the optic disc. It may be an isolated finding or part of a spectrum of anatomical and functional abnormalities, which include partial or complete agenesis of the septum pellucidum, other midline brain defects, cerebral anomalies, pituitary dysfunction, and structural abnormalities of the pituitary gland [15-20].
There seems to be a slight predominance of boys in ONH, varying in proportion from 56% to 75% [15, 19, 21, 22]. This preponderance of boys was also found in this study (56.6%).
There have been varying reports in the literature regarding visual function among children with ONH, ranging from normal vision to a high frequency of children who can only perceive light or have no light perception [10, 19, 23-25]. In a study of 35 children with bilateral ONH, more than half of the eyes had visual acuity in the range of light perception or no light perception, 86% had acuity less than 20/200, and 80% were legally blind [19]. In our study 25 (47.2%) of the casess had poor vision. Multiple regression analysis revealed that the only important factor that effects the visual acuity was the presence of associated neurodevelopmental anomaly. It should be noted that 17 of 25 cases with severe visual impairment were younger than 3 years and the visual testing in this age group is unreliable. In addition, it has been shown that cases with ONH may have delayed visual maturation [26].
There are varying reports in the literature regarding refraction abnormalities among children with ONH, and a high proportion of myopia (23% over 4 D) has been reported [27]. However, in a study 40 eyes were reported on in which the refractive state has been recorded thus the spherical and cylindrical components were distributed as in the general population [28]. In the current study, fourteen eyes (26.4%) had myopic total spherical power, whereas 21 eyes (39.6%) had hyperopic total spherical power and 18 eyes (33.9%) had spherical power lower than 0.75 D. Six of the 14 myopic eyes had myopia greater than 4 D, 9 eyes of the 21 hyperopic eyes had hyperopia greater than 4.75 D. Although our study revealed a slightly higher range of hypermetropia, it is not possible to conclude that cases with ONH have hypermetropia more than myopia because age matched normal subjects that also had a tendency to have hypermetropia.
Strabismus might be the presenting symptom of ONH. In a series with 100 cases with ONH, 68 of the cases had strabismus. Of the 68 patients 32 had esotropia, 23 had exotropia and 8 had vertical strabismus. The strabismus rate was same (68%) in unilateral and bilateral ONH groups [12]. In our study, thirteen (24.1%) of the 53 cases had esotropia, whereas 20 cases had exotropia (37.8%) and only 1 case had hypertropia due to inferior oblique overaction.
An unanswered question is whether neuroradiologic investigation and endocrinologic examination is necessary in all cases with ONH. One widely accepted recommendation is that only cases with bilateral ONH, nystagmus, and poor vision should undergo a thorough neuroradiographic and endocrine examination. However, in the present study, the rate of poor vision was not statistically different in cases with isolated ONH and ONH with neurodevelopmental anomalies. Poor vision rate was 38.7% cases with isolated ONH and 59.1% in patients with neurodevelopmental anomalies. Nystagmus rate was also found to be similar in two groups. In other words in cases with neurodevelopmental anomalies 40.9% of the patients had vision better than logMAR 1.0 or had positive CSM fixation and 54.5% had no nystagmus. So, to perform neuroimaging in only patients with poor vision and/or nystagmus may cause significant misdiagnoses.
The frequency of endocrine dysfunction in cases with ONH varies considerably from one study to another (13-65%) [10, 11, 15, 19, 29-31]. Endocrinologic disorders are serious, and failure to recognize it, carries a risk of adrenal crisis, hypoglycemia, and death [32-34]. In a study, in which 55 children with ONH who had been assessed by ophthalmology and endocrine services were reviewed, 49% of the patients had endocrine dysfunction. They also found that the frequency of endocrinopathy was higher in patients with an abnormal septum pellucidum (56%) than a normal septum pellucidum (39%) [13]. Brodsky et al. [32] reported 5 children with septo-optic dysplasia who died suddenly and unexpectedly. All children had corticotropin deficiency, all had thermoregulatory disturbances, and 4 children had diabetes insipidus. They concluded that children with septo-optic dysplasia and hypocortisolism are at risk for sudden death during febrile illness. In our study there were two cases (3.8%) with growth hormone insufficiency. One of them had ectopic pituitary gland. The other had no recorded pathology on MRI examination. Another case died suddenly. There was no information about endocrinologic investigation in medical records of this patient. Age of this case was 12 months at the last examination and he died 6 months after the last examination. We feel that, the retrospective design of our study was the cause for the low rate of the endocrinopathy and it should also be emphasized that endocrinologic evaluation was not routinely performed in our clinical practice.
In conclusion, diagnosis of congenital ONH is of a great importance because of the genetic and systemic implications. Ophthalmologists are in a unique position to suspect these conditions so that appropriate investigations and therapy can be initiated. In cases with ONH, visual development may be delayed. This means that they may have been perceived as blind in the first few months of life, but that vision improves to a certain extent up to two years of age. The vision of young case with ONH should be monitored at least annually, and any refractive errors should be treated when the visual acuity reaches a functional level. Early surgical correction of strabismus is needed for children who have symmetrical functional vision in the eyes, and thus have some potential for binocularity. Based on the high incidence of structural brain malformations, which may be associated with an increased risk of endocrinopathy and developmental delay, we believe that it is advisable that neuroimaging of the brain, a detailed pediatric neurologic evaluation and endocrinologic evaluation is necessary in all patients with ONH.
Table 2. Characteristics of the patients who had vision ˂log MAR 1.0 or inadequate CSM fixation and patients who had vision ≥logMAR 1.0 or positive CSM fixation [n(%)].
| Vision ˂log MAR 1.0 or Inadequate CSM Fixation (n=25) |
Vision ≥log MAR 1.0 or Positive CSM Fixation (n=28) |
P Value | |
|---|---|---|---|
| Mean spherical equivalent (Dioptry) | 0.33±3.33 | 0.75±3.62 | 0.665 |
|
Spherical Refractive Error Emmetropia Hyperopia High hyperopia Myopia High Myopia |
9(36.0) 6(24.0) 6(24.0) 1(4.0) 3(12.0) |
9(32.1) 6(21.4) 3(10.7) 7(25.0) 3(10.7) |
0.253 |
|
Cylindiric Refractive Error ˂1 Dioptry ≥1 Dioptry |
9(36.0) 16(64.0) |
12(42.9) 16(57.1) |
0.610 |
|
Nystagmus Yes No |
11(44.0) 14(56.0) |
19(67.9) 9(32.1) |
0.080 |
|
Strabismus Yes No |
15(60.0) 10(40.0) |
10(35.7) 18(64.3) |
0.748 |
|
Neurodevelopmental anomaly Yes No |
13 (52.0) 12(48.0) |
9(32.1) 19(67.9) |
0.143 |
Table 3. Ocular findings in the patients with and without neurodevelepmental anomaly [n(%)].
| Isolated ONH (n=31) | ONH with Neurodevelopmental Anomaly (n=22) | P | |
|---|---|---|---|
| Spherical equivalent | 0.33±3.34 | 0.75±3.62 | 0.871 |
| VA Poor Not poor |
12(38.7) 19(61.3) |
13(59.1) 9(40.9) |
0.143 |
|
Spherical Refractive Error Emmetropia Hyperopia High hyperopia Myopia High Myopia |
9(29.0) 9(29.0) 4(12.9) 6(19.4) 3(9.7) |
9(40.9) 3(13.6) 5(22.7) 2(9.1) 2(13.6) |
0.834 |
| Astigmatic power | 1.26±0.97 | 1.22±1.47 | 0.886 |
|
Cylindiric Refractive Error ˂1 D ≥1 D |
14(45.2) 17(54.8) |
7(31.8) 15(68.2) |
0.328 |
|
Nystagmus Yes No |
18(58.1) 13(41.9) |
12(54.5) 10(45.5) |
0.799 |
|
Strabismus Yes No |
19(61.3) 12(38.7) |
14(63.6) 8(36.4) |
0.862 |
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Frisen L., Holmegaard L. Spectrum of ONH. Br. J. Ophthalmol. 1978;62:7–15. doi: 10.1136/bjo.62.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skarf B., Hoyt C.S. ONH in children. Association with anomalies of the endocrine and CNS. Arch. Ophthalmol. 1984;102:62–67. doi: 10.1001/archopht.1984.01040030046032. [DOI] [PubMed] [Google Scholar]
- 3.Lambert S.R., Hoyt C.S., Narahara M.H. Optic nerve hypoplasia. Surv. Ophthalmol. 1987;32(1):1–9. doi: 10.1016/0039-6257(87)90069-5. [DOI] [PubMed] [Google Scholar]
- 4.Hellstrom A., Wiklund L.M., Svensson E. The clinical and morphologic spectrum of ONH. J. AAPOS. 1999;3:212–220. doi: 10.1016/S1091-8531(99)70005-4. [DOI] [PubMed] [Google Scholar]
- 5.Whinery R.D., Blodi F.C. Hypoplasia of the optic nerve: a clinical and histopathologic correlation. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1963;67:733–738. [PubMed] [Google Scholar]
- 6.Mosier M.A., Lieberman M.F., Green W.R., Knox D.L. Hypoplasia of the optic nerve. Arch. Ophthalmol. 1978;96(8):1437–1442. doi: 10.1001/archopht.1978.03910060185017. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss M.L., Green W.R. Optic nerve aplasia and hypoplasia. J. Pediatr. Ophthalmol. Strabismus. 1979;16(4):225–240. doi: 10.3928/0191-3913-19790701-06. [DOI] [PubMed] [Google Scholar]
- 8.Novakovic P., Taylor D.S., Hoyt W.F. Localising patterns of optic nerve hypoplasia-retina to occipital lobe. Br. J. Ophthalmol. 1988;72(3):176–182. doi: 10.1136/bjo.72.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyt C.S., Good W.V. Do we really understand the difference between optic nerve hypoplasia and atrophy? Eye (Lond.) 1992;6(Pt 2):201–204. doi: 10.1038/eye.1992.39. [DOI] [PubMed] [Google Scholar]
- 10.Zeki S.M., Hollman A.S., Dutton G.N. Neuroradiological features of patients with optic nerve hypoplasia. J. Pediatr. Ophthalmol. Strabismus. 1992;29(2):107–112. doi: 10.3928/0191-3913-19920301-11. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky M.C., Glasier C.M. Optic nerve hypoplasia. Clinical significance of associated central nervous system abnormalities on magnetic resonance imaging. Arch. Ophthalmol. 1993;111(1):66–74. doi: 10.1001/archopht.1993.01090010070029. [DOI] [PubMed] [Google Scholar]
- 12.Garcia M.L., Ty E.B., Taban M., David Rothner A., Rogers D., Traboulsi E.I. Systemic and ocular findings in 100 patients with optic nerve hypoplasia. J. Child Neurol. 2006;21(11):949–956. doi: 10.1177/08830738060210111701. [DOI] [PubMed] [Google Scholar]
- 13.Birkebaek N.H., Patel L., Wright N.B., Grigg J.R., Sinha S., Hall C.M., Price D.A., Lloyd I.C., Clayton P.E. Endocrine status in patients with optic nerve hypoplasia: relationship to midline central nervous system abnormalities and appearance of the hypothalamic-pituitary axis on magnetic resonance imaging. J. Clin. Endocrinol. Metab. 2003;88(11):5281–5286. doi: 10.1210/jc.2003-030527. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A.H., Kelly J.P. Acuity, ophthalmoscopy, and visually evoked potentials in the prediction of visual outcome in infants with bilateral optic nerve hypoplasia. J. AAPOS. 2003;7(2):108–115. doi: 10.1016/S1091-8531(02)42004-6. [DOI] [PubMed] [Google Scholar]
- 15.Acers T.E. Optic nerve hypoplasia: septo-optic-pituitary dysplasia syndrome. Trans. Am. Ophthalmol. Soc. 1981;79:425–457. [PMC free article] [PubMed] [Google Scholar]
- 16.Arslanian S.A., Rothfus W.E., Foley T.P., Jr, Becker D.J. Hormonal, metabolic, and neuroradiologic abnormalities associated with septo-optic dysplasia. Acta Endocrinol. 1984;107(2):282–288. doi: 10.1530/acta.0.1070282. [DOI] [PubMed] [Google Scholar]
- 17.De Morsier G. Etudes sur les dysraphies cranio-encephaligues, III: agenesie du septum lucidum avec malformation du tractus optique. La dysplasie septo-optique. Schweiz. Arch. Neurol. Neurochir. Psychiatr. 1956;77:267–292. [PubMed] [Google Scholar]
- 18.Hoyt W.F., Kaplan S.L., Grumbach M.M., Glaser J.S. Septo-optic dysplasia and pituitary dwarfism. Lancet. 1970;1(7652):893–894. doi: 10.1016/S0140-6736(70)91717-4. [DOI] [PubMed] [Google Scholar]
- 19.Siatkowski R.M., Sanchez J.C., Andrade R., Alvarez A. The clinical, neuroradiographic, and endocrinologic profile of patients with bilateral optic nerve hypoplasia. Ophthalmology. 1997;104(3):493–496. doi: 10.1016/S0161-6420(97)30286-3. [DOI] [PubMed] [Google Scholar]
- 20.Stanhope R., Preece M.A., Brook C.G. Hypoplastic optic nerves and pituitary dysfunction. A spectrum of anatomical and endocrine abnormalities. Arch. Dis. Child. 1984;59(2):111–114. doi: 10.1136/adc.59.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards W.C., Layden W.E. Optic nerve hypoplasia. Am. J. Ophthalmol. 1970;70(6):950–959. doi: 10.1016/0002-9394(70)92474-8. [DOI] [PubMed] [Google Scholar]
- 22.Walton D.S., Robb R.M. Optic nerve hypoplasia. A report of 20 cases. Arch. Ophthalmol. 1970;84(5):572–578. doi: 10.1001/archopht.1970.00990040574003. [DOI] [PubMed] [Google Scholar]
- 23.Jan J.E., Robinson G.C., Kinnis C., MacLeod P.J. Blindness due to optic-nerve atrophy and hypoplasia in children: an epidemiological study (1944-1974). Dev. Med. Child Neurol. 1977;19(3):353–363. doi: 10.1111/j.1469-8749.1977.tb08372.x. [DOI] [PubMed] [Google Scholar]
- 24.Petersen R.A., Walton D.S. Optic nerve hypoplasia with good visual acuity and visual field defects: a study of children of diabetic mothers. Arch. Ophthalmol. 1977;95(2):254–258. doi: 10.1001/archopht.1977.04450020055011. [DOI] [PubMed] [Google Scholar]
- 25.Björk A., Laurell C.G., Laurell U. Bilateral optic nerve hypoplasia with normal visual acuity. Am. J. Ophthalmol. 1978;86(4):524–529. doi: 10.1016/0002-9394(78)90301-X. [DOI] [PubMed] [Google Scholar]
- 26.Strömland K., Hellström A. Fetal alcohol syndrome-an ophthalmological and socioeducational prospective study. Pediatrics. 1996;97(6 Pt 1):845–850. [PubMed] [Google Scholar]
- 27.Weiss A.H., Ross E.A. Axial myopia in eyes with optic nerve hypoplasia. Graefes Arch. Clin. Exp. Ophthalmol. 1992;230(4):372–377. doi: 10.1007/BF00165948. [DOI] [PubMed] [Google Scholar]
- 28.Zion V. Optic nerve hypoplasia. Ophthalmic Semin. 1976;1(2):171–196. [PubMed] [Google Scholar]
- 29.Phillips P.H., Spear C., Brodsky M.C. Magnetic resonance diagnosis of congenital hypopituitarism in children with optic nerve hypoplasia. J. AAPOS. 2001;5(5):275–280. doi: 10.1067/mpa.2001.118220. [DOI] [PubMed] [Google Scholar]
- 30.Costin G., Murphree A.L. Hypothalamic-pituitary function in children with optic nerve hypoplasia. Am. J. Dis. Child. 1985;139(3):249–254. doi: 10.1001/archpedi.1985.02140050043019. [DOI] [PubMed] [Google Scholar]
- 31.Sorkin J.A., Davis P.C., Meacham L.R., Parks J.S., Drack A.V., Lambert S.R. Optic nerve hypoplasia: absence of posterior pituitary bright signal on magnetic resonance imaging correlates with diabetes insipidus. Am. J. Ophthalmol. 1996;122(5):717–723. doi: 10.1016/S0002-9394(14)70492-1. [DOI] [PubMed] [Google Scholar]
- 32.Brodsky M.C., Conte F.A., Taylor D., Hoyt C.S., Mrak R.E. Sudden death in septo-optic dysplasia. Report of 5 cases. Arch. Ophthalmol. 1997;115(1):66–70. doi: 10.1001/archopht.1997.01100150068011. [DOI] [PubMed] [Google Scholar]
- 33.Cameron F.J., Khadilkar V.V., Stanhope R. Pituitary dysfunction, morbidity and mortality with congenital midline malformation of the cerebrum. Eur. J. Pediatr. 1999;158(2):97–102. doi: 10.1007/s004310051026. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert J.D., Scott G., Byard R.W. Septo-optic dysplasia and unexpected adult death-an autopsy approach. J. Forensic Sci. 2001;46(4):913–915. doi: 10.1520/JFS15068J. [DOI] [PubMed] [Google Scholar]