Abstract
Much of the evidence for the idea that individuals differ in their propensity to innovate and solve new problems has come from studies on captive primates. Increasingly, behavioural ecologists are studying innovativeness in wild populations, and uncovering links with functional behaviour and fitness-related traits. The relative importance of genetic and environmental factors in driving this variation, however, remains unknown. Here, we present the results of the first large-scale study to examine a range of causal factors underlying innovative problem-solving performance (PSP) among 831 great tits (Parus major) temporarily taken into captivity. Analyses show that PSP in this population: (i) was linked to a variety of individual factors, including age, personality and natal origin (immigrant or local-born); (ii) was influenced by natal environment, because individuals had a lower PSP when born in poor-quality habitat, or where local population density was high, leading to cohort effects. Links with many of the individual and environmental factors were present only in some years. In addition, PSP (iii) had little or no measurable heritability, as estimated by a Bayesian animal model; and (iv) was not influenced by maternal effects. Despite previous reports of links between PSP and a range of functional traits in this population, the analyses here suggest that innovativeness had weak if any evolutionary potential. Instead most individual variation was caused by phenotypic plasticity driven by links with other behavioural traits and by environmentally mediated developmental stress. Heritability estimates are population, time and context specific, however, and more studies are needed to determine the generality of these effects. Our results shed light on the causes of innovativeness within populations, and add to the debate on the relative importance of genetic and environmental factors in driving phenotypic variation within populations.
Keywords: innovation, necessity hypothesis, developmental stress hypothesis, personality, heritability, problem solving
1. Introduction
A central aim in evolutionary ecology is to understand the relative importance of plasticity and evolutionary processes in driving phenotypic variation within populations [1,2]. Plasticity and microevolution are not necessarily independent processes, however, because the extent to which traits change over a gradient might also be heritable [3,4]. In general, the majority of evolutionary ecology studies in wild populations have primarily focused on morphological and life-history traits. In contrast, behavioural traits have been relatively poorly studied [5,6]. Here, we examine the extent to which innovative problem-solving performance (PSP), a trait that is thought to be of substantial functional significance [7], is likely to be influenced by environmental and genetic factors in a natural population, shedding light on the microevolutionary potential of this trait.
Innovation can be defined as solving a novel problem or finding a new solution to an old problem [8] and reflects behaviour not previously witnessed in that individual or population [7]. Innovativeness, or more precisely, the propensity to innovate, is viewed by many as an important component of animal cognition [9–12] as defined in the very broadest sense [13]. While associative learning seems to be a likely cognitive process contributing to individual variation in innovative problem-solving success [14–18], identifying all of the cognitive components underlying innovation and its proxies remains a major challenge [17,19–21]. At the same time, considerable debate surrounds whether and how individual differences in various cognitive abilities might ever be measured in evolutionary ecological studies, given the enormous difficulty associated with controlling for factors such as motivation, and given that some genes have pleiotropic effects on cognitive and purportedly non-cognitive traits [22–25]. A major challenge is to understand the adaptive significance of innovativeness and indeed of its underlying components [7]. At the individual level, does innovativeness confer any real benefit for the innovator, and might innovativeness be under selection? Do costs explain variation among and within species? Comparative analyses provide compelling evidence for the idea that innovativeness can impact evolutionary processes and delivers real benefits. Innovative species are more likely to survive, for example, and successfully colonize new habitats when introduced outside their ranges [26], while rates of speciation are higher than expected along lineages containing innovative species [27]. Evolutionary conclusions, however, are tempered by statistical and practical limitations of the comparative approach [28]. Individual-level studies within or across natural populations coupled with experiments to test causality are needed to understand better the evolutionary processes and ecological drivers underlying innovation.
Intuitively the expectation is that innovators should gain a selective benefit, as innovations can provide access to new resources [29,30] and potential mates [8,10]. Some studies suggest that innovations correlate positively to traits closely aligned with fitness ([18,31,32] but see [33]). Less clear is whether the benefits accrued in the long term are any better than strategies adopted by non-innovators, not least because there is no evidence of direct links with robust measures of fitness, such as survival and the total number of offspring recruited by an individual into the breeding population over its lifetime [34]. One study estimated selection on innovative PSP in an iteroparous bird but found no link with the number of offspring recruited to the breeding population (so-called recruitment selection), despite a strong link with fecundity [18]. An antagonistic association between PSP and sensitivity to predators during reproduction was the likely explanation. Specifically in comparison to non-solvers, problem solvers were more likely to desert their nests in response to being trapped for identification purposes when chicks were 8 days old, even though they and their offspring were equally viable. More generally, engaging in innovative behaviours is likely to incur a range of costs [7], including reduced vigilance [35], increased risks of exploitation [36], poisoning [37] and parasitism [38]. It may also trade-off with other behaviours, e.g. competitive ability [39]. Selection for innovativeness may therefore occur only under some circumstances [40].
Irrespective of whether costs and benefits of innovation lead to any effect on fitness, the evolutionary potential of any trait depends on the relative importance of additive genetic (the heritable component) and other components of variation, such as environmental or maternal effects [2,41]. That the heritability of innovation as such has never been estimated perhaps is not surprising given that the literature is strongly biased towards non-behavioural traits [42]. Nevertheless, heritability estimates are available for a range of traits thought to be strongly linked to innovativeness [20], including personality traits such as exploration behaviour (EB) [43–45], and cognitive traits such as associative learning (reviewed by Dukas and co-workers [46–48]). Many of these estimates come from laboratory populations [49]; indeed, heritability has never been estimated for any cognitive trait in a natural animal population [48]. In contrast, there is a rich body of literature on heritability of various cognitive traits in humans [50–52].
Environmental factors are known to influence cognition and personality [53]. The ‘developmental stress’ hypothesis predicts that young songbirds experiencing stressful or poor-quality rearing environments exhibit impaired development of neural structures and song learning performance [54]. This hypothesis has been extended to other cognitive traits generally and has received empirical support from food-deprivation experiments resulting in long-term impairments of learning, memory and spatial cognition, in several bird and mammal species [55–57]. This hypothesis thus predicts a positive correlation between rearing environment quality and cognitive performance later in life, an idea that has yet to be tested for innovativeness. Alternatively, innovative phenotypes may develop in subordinate, low-quality individuals that have experienced stressful rearing environments; indeed, innovation has been described as an alternative strategy that is expressed when other behavioural options have failed or are unavailable [7,58]. This ‘necessity drives innovation’ hypothesis has received mixed support from studies in the wild and in the laboratory (e.g. [39,58–63]). Many of these studies report differences between groups that vary in dominance, for example between the ages and sexes, but some studies have explicitly shown a negative correlation between innovativeness and competitive ability over and above these dominance groups [39]. Although these effects tend to be interpreted as environmentally mediated, a gene × environment interaction cannot be easily ruled out since additive genetic differences may only be apparent at a specific age or in a specific environment [64,65]. Clearly, in addition to the possibility that innovativeness has a heritable component of variation, a range of environmental factors are also likely to explain the tendency to innovate but the importance of these in natural populations has not been tested.
(a). The study system
The great tit (Parus major) is a small iteroparous, socially monogamous and sexually dimorphic songbird, and for decades has been an influential model organism in many different areas of research including life-history variation [66], optimal foraging [67], phenotypic plasticity [3,68], evolutionary dynamics [69] and personality [44,70]. Strong selection on timing reproduction to coincide with peak caterpillar abundance has also made the great tit an important model species for the study of the phenology of trophic interactions [71]. High breeding density, short dispersal distance and short generation time all mean that the causes of phenotypic variation in the species have been studied intensively and, for example, heritability has been estimated for more traits in the great tit than for any other wild animal species [72]. While traditionally most of these studies have used parent–offspring regression or brood manipulation to separate environmental and genetic causes of variation, these approaches are increasingly being replaced by the animal model [41], a statistical tool that uses pedigree data to estimate the different components of variation underlying a given trait in a population more effectively and without interfering with natural processes [72]. In addition to additive genetic effects, maternal effects, and both natal and breeding environmental effects (particularly proxies for habitat quality such as local oak tree density and local population density) explain substantial variation in many traits [44,73–76]. Similarly whether birds are born locally or have immigrated to the population can also have an effect on individual variation in a range of phenotypes [74,77]. In contrast to life-history and morphological traits, the animal model has rarely been used to examine components of variation underlying behavioural traits, and in great tits the animal model approach has been applied only to EB [44], which revealed equally important additive genetic and permanent environmental effects. The paucity of animal models for behavioural traits in natural populations is surprising given that animal models do not necessarily need long-term pedigrees to prove effective [74].
The great tit is also becoming an important species in the study of innovativeness. Not only does it occupy a wide variety of different habitats, a hallmark trait of innovative species [30], the great tit is also an innovative forager [29,30,78,79], and innovations can spread through populations via social networks [80]. In our study population at Wytham Wood, success in a novel, food-motivated lever-pulling task (i.e. PSP) is positively correlated with success in another foraging test, string pulling, and is consistent over time, supporting the idea that it is a measure of innovativeness [81]. It also correlates positively with associative learning speed [82], despite this test being conducted in a very different context (laboratory versus wild) and calling upon different perceptual and motor responses. Personality can also influence innovativeness [83]; indeed, there is growing interest in the relationship between personality and cognition [21,84], which some argue should be studied in combination [24,40,57]. EB in a novel environment (henceforth EB) captures variation in what is emerging as an important personality axis: the reactive–proactive axis [85]. Previously we found no link between EB and PSP in our population, albeit with a smaller sample size [81], though EB has been linked to cognitive traits in some tit populations [86,87].
Whatever the underlying causal mechanisms, PSP has also been linked to functional behaviour and life-history variation in our study population, including clutch size, sensitivity to disturbance leading to nest desertion, competitive ability during winter and foraging behaviour during provisioning [18,39,88]. Despite these links, there is no net effect on fitness and thus the evolutionary potential of innovativeness remains unclear. Our aim here was to explore the environmental and genetic determinants of variation in our population, controlling for a range of fixed effects, some of which themselves were likely to have un-quantified quantitative genetic components of variation. Specifically, we conducted an animal model to estimate the heritability of innovativeness, and in separate general linear models (GLM) and restricted maximum-likelihood (REML) analyses with a larger dataset, we explored links between innovativeness and a range of individual factors (age, sex and personality), and natal environment (nest, local habitat and population density) in driving variation in the population (see table 1 for a full list of variables used in the analyses).
Table 1.
Names, types and characteristics of effects explored in the REML and Bayesian animal model analyses. ‘Imm.’, immigrant; ‘env’, environment.
| variable name | type | data available for | type of effect |
|---|---|---|---|
| innovative PSP (1/0) | response | Imm. and Wytham-born | individual |
| age (immature/adult) | fixed | Imm. and Wytham-born | individual |
| sex (male/female) | fixed | Imm. and Wytham-born | individual |
| individual identity (ring number) | random | Imm. and Wytham-born | individual |
| EB | fixed | Imm. and Wytham-born | individual |
| natal origin (immigrant/resident) | fixed | Imm. and Wytham-born | large-scale env |
| time of season (days since 1 Sept.) | fixed | Imm. and Wytham-born | temporal |
| winter (winter assayed) | fixed | Imm. and Wytham-born | temporal env |
| natal year (year) | fixed | Imm. and Wytham-born | temporal env |
| nesting attempt identity (unique) | random | Wytham-born | local natal env |
| individual natal mass (g) | fixed | Wytham-born | individual natal |
| tesselated territory size (ha; local population density) | fixed | Wytham-born | local natal env |
| large oaks (within 50 m of nest) | fixed | Wytham-born | local natal env |
| natal clutch size (integer) | fixed | Wytham-born | natal nest |
| natal brood mass (g) | fixed | Wytham-born | natal nest |
| natal lay date (first egg in the natal nest) | fixed | Wytham-born | temporal natal environment |
2. Material and methods
(a). Assaying innovative problem-solving performance
Great tits in Wytham Woods (51°46′ N, 1°20′ W), Oxfordshire, UK, are easily trapped and ringed, at the nest as adults or as nestlings, and during the winter using mist-nets around feeders. Standardized assays of innovative PSP were conducted on individually marked birds temporarily taken into captivity during the winter (November to March) between 2007 and 2010. As spontaneous innovations are rare, by definition, a commonly employed method to study innovativeness is to subject individuals to a novel problem that they have never encountered before ([19], reviewed in [20]). A total of 831 individuals underwent 942 PSP assays during the winters of 2007–2010, though only the first assays (when first solutions took place) were used throughout this paper, and in some analyses only the first sibling within a brood was included (see below) when multiple siblings were assayed in winter. Birds were housed singly in wire cages and presented with a problem-solving task never encountered previously, consisting of a vertical transparent Perspex tube containing a platform baited with four waxworms (Galleria mellonella) that were supported by a horizontal lever. To obtain the reward, birds had to remove the lever. To encourage birds to approach the task, one waxworm was freely available in a feeding dish just below the device, which was eaten by 99% of birds. Individuals were given 3 h to solve this task and were classified as ‘solvers' or ‘non-solvers' based on their performance. After being assayed, all individuals were released back into the wild at the site of capture.
(b). Demographic data, personality and natal conditions
In Wytham Wood, great tits breed almost exclusively in nest-boxes. Some fledglings leave the wood, while others, and birds that have bred in Wytham, tend to stay in Wytham during the winter. All nest-box nestlings are ringed and thus non-ringed juveniles caught in the non-breeding season are assumed to be immigrants. This is considered a reasonable assumption, given that the proportion of nestlings born in natural cavities in Wytham is estimated to be very low [89,90]. Age, sex [91] and natal origin (immigrant or Wytham-born) can thus be easily determined whenever individuals are trapped. Reproductive life-history traits and success data are collected every year for the entire population during weekly visits to nest-boxes in spring (April to June). The date the first egg was laid (days since 31 March), clutch size, fledged brood size (not all eggs hatch and some chicks die) and fledging mass (at 14 days old just before fledging) were recorded for all nesting attempts. Parents were trapped and identified, or ringed, when nestlings were approximately 7–12 days old. Thus routine monitoring of breeding success and trapping of adults generates information on natal conditions and pedigree of birds subsequently assayed for innovativeness in the winter.
EB assays were conducted during the non-breeding season (winter, November to March), the morning after being captured in the wild and the problem-solving assay completed, and were initiated by opening the trapdoor connecting the individual cage with the novel environment room. The novel environment assay room was based on the design used in other studies [92]: the room was split into five equally sized three-dimensional zones, each of which contained an artificial ‘tree’ (1.5 m high) with four artificial branches. Principal components analysis was used to reduce 12 different behavioural variables to a single component and was used as the combined measure of activity, novel object and area exploration, which we refer to as EB [44].
Nest-boxes varied greatly in terms of their local density and the quality of surrounding habitat. In the winter of 2011–2012, the locations of every nest-box at Wytham (N = 1020) were digitally mapped to ±3 m. An index of local population density at the individual level was estimated using a Dirichlet tessellation technique, which formed Thiessen polygons around occupied great tit nest-boxes in each year [73,74], which we refer to as tessellated territory size (territory size). In Wytham, territory size is significantly related to individual variation in several life-history traits including clutch size, fledgling mass and recruitment rate [73]. Newly emerged oak tree Quercus spp. leaves support the highest densities of the winter moth Operophtera brumata caterpillars, the main food of the great tit, and in general oak trees support more insect species than any other tree [93]. Broods near oak trees receive a higher proportion of caterpillars than broods far from oak trees [75] and birds breeding in areas of high oak tree density lay earlier, lay larger clutches and produce heavier fledglings [74,94]. Thus we used the number of large oak trees (with breast height diameter more than or equal to 50 cm) within a 75 m radius of each nest-box as a component of natal habitat quality [74]. The number of oaks within 75 m of the nest was used rather than the number of oaks within a Thiessen polygon, as this is the scale at which oak tree abundance has been shown to best predict reproductive performance in the Wytham tits [74,94]. Oak tree abundance and local population density estimates were generated at each nest for each individual nesting attempt.
(c). Mixed model and quantitative genetic analysis
Generalized linear (GLM) and generalized linear mixed models (GLMM) were used to explore links between a range of environmental factors and PSP. We ran one set of GLMs with all individuals; in this case, for Wytham-born birds we selected only one individual from each natal nest (N = 734 individuals) in order to avoid adding a random term that would otherwise have been needed to account for the nest, as this led to an unstable model owing to aliasing with winter season; all immigrants were included because natal nest was unknown. Natal data were unavailable for immigrants and thus we ran another set of models including only the Wytham-born birds (GLMM with nest attempt as the random effect to account for multiple individuals from the same nest). In the dataset including all individuals, two different analyses were conducted. First, we examined the link between PSP and the main fixed factors across all winters, including temporal effects (winter assayed, time of season), EB, age, sex and natal origin (immigrant/local born), but not information on the natal environment, which was unavailable for immigrants. Second, we tested whether main effects were dependent on annual environmental conditions by testing for all two-way interactions with winter; the expectation that effects may only be visible in some winters was justified on the basis that the expression of genotypes and phenotypes commonly varies annually.
For the analysis of natal environment effects using the dataset restricted to Wytham-born birds, to simplify the model and to avoid having to use both natal year and winter assayed in the same model, we included only juveniles who formed the vast majority of Wytham-born birds (total N = 311). Thus, for juveniles, winter assayed is the same as natal year. First, we tested for main effects of natal environmental conditions, including natal year, natal reproductive conditions (clutch size, laying date, individual fledging mass) and local environmental conditions (local population density and oak tree abundance). In all of these models, we retained individual factors used in the full dataset analysis. Second, we tested whether effects of different environmental conditions were dependent on the natal year; as in the case for the analysis on the full dataset, the expectation that effects may only be visible in some natal years was justified on the basis that the expression of genotypes and phenotypes commonly varies annually.
The quantitative genetic analysis was conducted using an animal model approach in a Bayesian framework. Bayesian animal models are computationally demanding and thus in the first instance we explored the phenotypic data using a series of frequentist linear models with Genstat v. 17, with the binary trait PSP (1/0) and a probit link function, as described above. The R package MCMCglmm [95] was used to fit animal models in order to estimate additive genetic and environmental components underpinning individual variation in PSP. The animal model is a particular form of linear mixed-effects model where phenotypic data are paired with a pedigree to obtain an estimate of the additive genetic VA and other variance components [96,97]. The pedigree gives an expectation for how the additive genetic ‘merit’ of each individual (or ‘breeding value’, treated as a random effect) should covary among related individuals (e.g. full siblings, half-siblings, cousins, etc.). The full pedigree for the Wytham Woods study area contains records from over 93 000 ringed individuals going back to 1959. Not all of these individuals were informative in the current context, however, in that most were unrelated to the individuals scored for problem solving. The full pedigree was therefore pruned using the function prunePed in the pedantics package in R [98]. The resulting pruned pedigree consisted of a list of 3404 individuals and their parents. The phenotypic data used in the animal model analysis consisted of measures of PSP for 820 individuals across four different breeding seasons (2006–2009); 744 of these had a known position in the pedigree but the other birds were included to improve the overall estimate of total phenotypic variation. For 102 out of 420 of these that were born in Wytham, the mother was also scored for PSP (either in the same winter or in a previous winter), and five of these also had PSP scores for their grandmother. Ninety-seven of the 420 Wytham-born birds also had PSP scores for their fathers, with none of them having PSP scores for their grandfather. Mothers recorded breeding more than once typically, but not always, bred with the same male. There were 294 unique mothers associated with the 820 individuals scored for PSP. Of these, 87% were recorded as a mother in only a single breeding season, 11% in two seasons and 2% in three seasons. Thus the power was low to distinguish true maternal effects from common/early environmental effects. Further details are given in electronic supplementary material, table S1.
PSP was scored as a binary trait (1 = problem solved; 0 = problem not solved) and modelled as a threshold character [99]. The assumption here is that phenotypic variation measured on the binary scale is caused by underlying variation in an unobservable, normally distributed character called the ‘liability’ relative to a threshold: individuals with liability scores above the threshold solve the problem and those below do not. As for any quantitative trait, continuous variation on the liability scale is assumed to result from genetic and environmental differences between individuals. Accurately separating additive genetic from non-additive genetic effects (e.g. dominance or epistasis variance) was not possible in this case given the data and pedigree structure, so we focused only on additive genetic effects. The only fixed effect in the model was EB, identified in the initial exploratory mixed models as being important (see §3a).
The simplest animal model followed the following equation:
| 2.1 |
where li is a normally distributed liability, μ is the population mean, EB refers to the fixed effect, ai is an additive genetic effect (breeding value), ei is a residual ‘environmental’ effect and the i subscript refers to individuals. Binary data do not provide enough information to simultaneously estimate VA (the variance in ai) and the residual ‘environmental’ variance (the variance in ei); the latter was therefore fixed at 1 and the probability of problem-solving was linked to the liability through a probit link function, which renders VA and other variance components estimable [100]:
| 2.2 |
We also tested for a maternal effect by fitting maternal identity as an additional random effect:
| 2.3 |
where mi represents the maternal effect.
The heritability h2 of innovative PSP was then calculated on the liability scale [101] as:
| 2.4 |
where VM was the maternal effect variance (included only if supported by the data, see below), the first 1 in the denominator represented the residual variance and the second 1 represented the ‘probit link’ variance [100,101].
Following Villemereuil et al. [100], we used parameter-expanded priors for the variance components based on χ2 distributions with 1 d.f. (in MCMCglmm: V = 1, ν = 1000, α.µ = 0, α.V = 1). The MCMC chain was run for 1 million iterations with a thinning interval of 1000 after a burn-in of 10 000 in order to obtain effective sample sizes of approximately 1000. The absolute autocorrelations between subsequent MCMC samples was always less than 0.10. Model selection was based on deviance information criterion (DIC), which accounts for both model fit, based on an expected deviance parameter, and model complexity based on the effective number of parameters estimated in the model [102]. The simplest models contained only VR (fixed at 1) plus either VA or VM. These models were then compared against one where VR, VA and VM were included (i.e. the model described in equation (2.3)). The model with the lowest DIC estimate was considered the best. If models were within two DIC values of each other, the simpler one was chosen as the model of best fit [102]. Parameter estimates were calculated as the means of the posterior distributions estimated from the model of best fit. The 95% highest posterior density was also estimated for each parameter.
3. Results
(a). Natal origin, individual and temporal variation
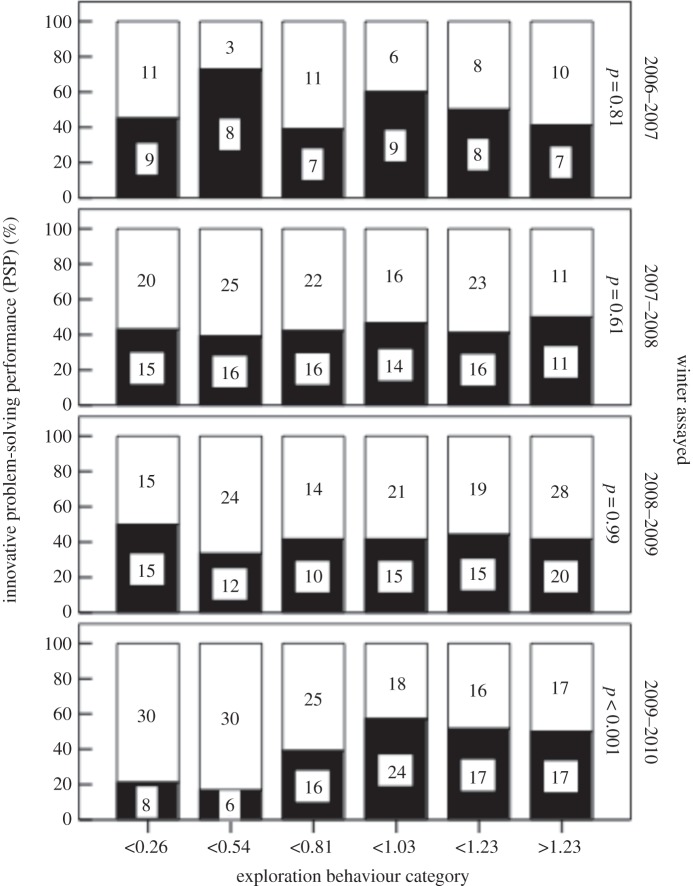
Forty-two per cent of birds solved the task when first presented with the device (total N = 734). The entire dataset model suggested no variation in PSP among winters, but PSP did increase over time within a winter (table 2a; β ± s.e. = 0.006 ± 0.002). There was also a weak positive relationship between PSP and EB (table 2a; β ± s.e. = 0.26 ± 0.11; figure 1) but no other individual factor was significant.
Table 2.
GLM of innovative PSP from the 2006–2007 to the 2009–2010 winters, including immigrant and Wytham-born great tits. (a) Model 1 shows the significance of main effects when each term is removed individually from the full model. (b) Model 2 shows the effects from a model including all main effects and their interactions with winter; each interaction was tested by removal from the full model.
| fixed term | Wald statistic | d.f. | p |
|---|---|---|---|
| (a) model 1 | |||
| winter | 2.70 | 3 | 0.440 |
| time of season | 9.35 | 1 | 0.002 |
| age | 0.17 | 1 | 0.681 |
| sex | 0.03 | 1 | 0.875 |
| EB | 5.66 | 1 | 0.017 |
| natal origin | 1.38 | 1 | 0.241 |
| (b) model 2 | |||
| winter × time of season | 9.70 | 3 | 0.021 |
| winter × age | 18.23 | 3 | <0.001 |
| winter × sex | 1.04 | 3 | 0.792 |
| winter × EB | 10.71 | 3 | 0.013 |
| winter × natal origin | 7.25 | 3 | 0.064 |
Figure 1.
Innovative PSP was positively associated with EB (an assay of the reactive–proactive personality axis) in one out of the four winters that assays were conducted. Sample sizes shown in bars. Dark bars, number of birds that solved the foraging task; white, numbers that did not solve.
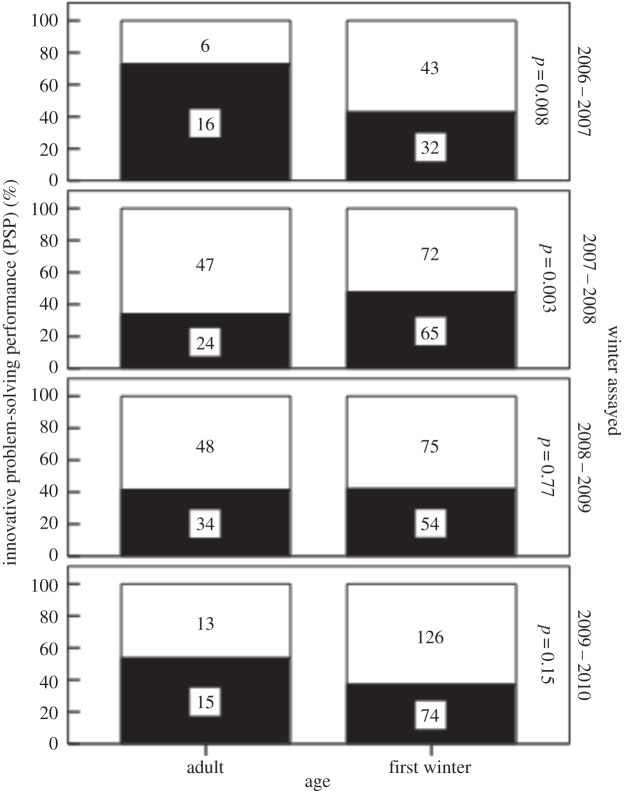
Model 2 suggested there were differences among winters for three of the five main effects (table 2b). First, the age interaction repeats a result published previously [81]; in 2006, adults had a higher performance but in 2007, juvenile birds had a higher PSP (figure 2). Second, the interaction between winter and EB was driven by a significant relationship between PSP and EB in the last season alone, explaining why no similar effect was reported in an earlier analysis [81]. Third, post hoc exploratory analyses (not shown) suggested that the apparent winter time of season × interaction (table 2) was an artefact caused primarily by low success among a limited sample size early in two winters (2006–2007 and 2007–2008). There was also a tendency for natal origin to influence PSP, but only in 2008–2009 when Wytham-born birds were more likely to solve than immigrants (49% versus 35%, see electronic supplementary material, figure S1)
Figure 2.
Variation in innovative PSP with respect to age (whether birds were in their first winter or were adults), and the winter assays were conducted. p-values were taken from post hoc tests on data for individual winters, with all main effects in the model (time of season, age, sex, EB and natal origin). Numbers in bars refer to sample sizes. Dark bars, number of birds that solved the foraging task; white, numbers that did not solve.
(b). Natal environment effects
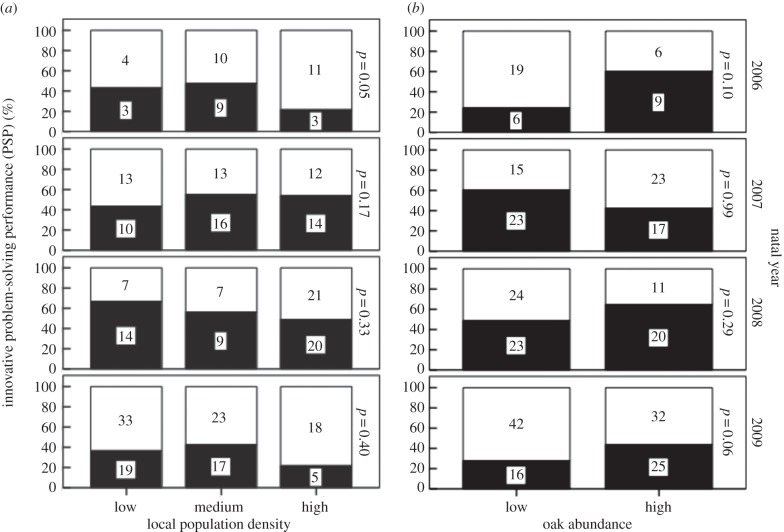
The first model tested for average effects across all natal years (table 3, model 1). PSP among juveniles varied depending on natal year (mean PSP for 2006–2009 were 0.37, 0.51, 0.54 and 0.36, respectively). None of the natal local environment variables, nor the natal nest variables appeared to influence PSP across all years combined. However, in some years, local population density and habitat quality did influence PSP (table 3, model 2; figure 3). Specifically, in three of the four years, birds born in oak-rich territories had higher PSP success, though significantly so only in 2009. Similarly, PSP was especially low where local population density was highest in 2006. None of the natal nest variables had an interacting effect with year.
Table 3.
GLMM of innovative PSP (assayed during winter) in relation to individual factors, natal breeding parameters and local environmental effects during the natal (breeding) season. Only Wytham-born great tit juveniles are included since breeding parameters were unavailable for immigrants. (a) Model 1 shows the significance of main effects when each term is removed individually from the full model. (b) Model 2 shows the effects from a model including all main effects and their interactions with natal year; each interaction was tested by removal from the full model. Nest attempt was the random effect in both models to control for multiple individuals originating from the same nest. TTS is tesselated territory size, a measure of local population density.
| fixed terms | W | d.f. | p |
|---|---|---|---|
| (a) model 1 | |||
| EB | 0.21 | 1 | 0.648 |
| sex | 0.84 | 1 | 0.361 |
| days | 1.09 | 1 | 0.298 |
| natal year | 8.64 | 3 | 0.036 |
| local population density (TTS) | 0.14 | 1 | 0.709 |
| oak abundance | 0.42 | 1 | 0.517 |
| lay date | 1.15 | 1 | 0.284 |
| clutch size | 0.63 | 1 | 0.427 |
| natal mass | 1.21 | 1 | 0.272 |
| (b) model 2 | |||
| EB | 0.17 | 1 | 0.679 |
| sex | 0.72 | 1 | 0.397 |
| days | 0.80 | 1 | 0.372 |
| natal year × TTS | 9.92 | 3 | 0.021 |
| natal year × oak abundance | 8.00 | 3 | 0.048 |
| natal year × lay date | 5.91 | 3 | 0.119 |
| natal year × clutch size | 5.04 | 3 | 0.172 |
| natal year × natal mass | 4.27 | 3 | 0.236 |
Figure 3.
Innovative PSP during winter in relation to two environmental factors in the preceding natal year, and how these varied with respect to natal year: (a) tesselated territory size, inversely related to local population density, and (b) the number of large oak trees within 50 m of each nest. In some winters, innovativeness was lower among juveniles who had been born in high local density areas, or where there were fewer oak trees. Graph based on raw data. Sample sizes shown in bars. Dark bars, number of task-solvers; white, numbers of non-solvers.
(c). Quantitative genetic analysis
The quantitative genetic model with the lowest DIC was the one in which VA and VM were estimated simultaneously (table 4, model 3). However, the model with the next lowest DIC included only VA (table 4, model 1), with a DIC within 2 of that of model 3. The model that included VM but not VA (table 4, model 2) had the highest DIC but was within 2 DIC units from the model that included VA but not VM. Collectively, these model comparisons indicate that statistical support for additive genetic and maternal variance components was weak. In line with this, the means of the posterior distributions for VA and VM were small (relative to VR, which was fixed at 1) and the lower credible intervals for both parameters were effectively 0 (and the posterior modes were also close to zero). The mean of the posterior distribution of h2, calculated according to equation (2.5), was 0.04 (lower credible interval ≤ 0.01, upper credible interval = 0.15). The estimated h2 was very similar when VM was excluded from the denominator (table 4) or when no fixed effects were included in the model (results not shown)
Table 4.
Results of the animal models. VA = additive genetic variance. VM = maternal effect variance. VR = residual variance (always fixed at 1). h2 = heritability. DIC = deviance information criterion. Values for variance components are posterior means, with lower and upper 95% credible intervals in parentheses.
| model | variance components | DIC | VA | VM | h2 |
|---|---|---|---|---|---|
| 1 | VR + VA | 1061.53 | 0.09 (0.00, 0.38) | 0.04 (0.00, 0.16) | |
| 2 | VR + VM | 1063.23 | 0.08 (0.00, 0.31) | ||
| 3 | VR + VA + VM | 1059.62 | 0.09 (0.00, 0.36) | 0.08 (0.00, 0.32) | 0.04 (0.00, 0.15) |
4. Discussion
Our results found little if any support for the idea that innovativeness was influenced by additive genetic effects, by maternal effects, or by conditions in the natal nest. Instead innovativeness varied temporally, with local environment conditions, and with a range of individual factors including age, whether birds were immigrants, personality, and where and when birds were born, though most of these effects were present only in some seasons. The necessity drives innovation and developmental stress hypotheses provide the most likely explanation for these effects.
(a). Quantitative genetics
Despite previously demonstrated links with several functional traits in our population [18,39,88], our measure of innovativeness appears to have little or no additive genetic variation. The posterior distributions of h2 generated by the Bayesian animal models peaked close to zero and were narrow, suggesting that h2 was likely closer to zero than to the upper 95% confidence interval at 0.16. These findings contrast with a range of quantitative genetic studies on traits closely allied to innovativeness—personality and cognitive—that have sometimes found substantial additive genetic variation and heritability. Many of these studies are on captive populations, however, and do not necessarily reflect patterns found in the wild ([43], reviewed by Croston et al. [48] and Drent et al. [103]). Others involve relatively small sample sizes and are unlikely to be able to separate additive genetic from confounding environmental components of variation effectively (e.g. [104,105]), and thus likely over-estimated heritabilities [96]. Much larger sample sizes than were available in our study would be necessary to demonstrate conclusively whether our estimate of h2 differed from zero [42], especially because for a binary trait the error variance is difficult to separate from other components. The fact that the upper credible interval for h2 was relatively low (0.16) gives some confidence that the true h2 was indeed very low and that we did not suffer unduly from a lack of statistical power (if power were an issue, then the posterior distribution for h2 would have been much wider, given that low sample size increases the uncertainty around parameter estimates). Given the influence of a range of ‘environmental’ factors on PSP in our population, for example age and local ecological conditions, it is feasible that G × E effects are important but remain unaccounted for in the residual variance component and that additive genetic variation is likely to be stronger in some subsets of data (e.g. [106]), but we did not have the power to test this.
Even if h2 for innovativeness in our population was marginally different from zero, clearly the trait has very little microevolutionary potential. This is generally thought to be true for certain kinds of traits, including physiological and fitness-related traits [107–109] because additive genetic variation tends to be swamped by high environmental variation. Even so, despite their h2 being lower, such polygenic traits can actually harbour more additive genetic variation than traits less closely related to fitness, perhaps because they have a higher mutational target [110]. In our case, though, it seems that h2 was low because VA was low. Despite the emphasis that is often put on the evolutionary potential of behavioural traits, studies show that the heritability of behavioural traits is not significantly different from life-history traits and their potential to respond to selection may be no stronger than that for life-history or physiological traits [111–115]. Dochtermann et al. [116] make the point that although mean h2 across studies was 0.17, VA tended to be half of the repeatability or ‘personality’ component of variation, and interpreted this as meaning that the evolutionary potential of the repeatable differences between individuals is generally high. This may be technically true but the relevance from a functional and population processes perspective seems limited because selection acts on phenotypic variation generally, not phenotypic variation corrected for temporary environmental effects.
One explanation suggested for traits with low h2 is that in contrast to relatively simple traits controlled by few genes, complex traits are linked to numerous other traits, all of which themselves are influenced by environmental variation, swamping the additive genetic variation [117,118], although VA may still be high in absolute terms given that many loci affect such complex traits [119]. This may be especially true for innovativeness, which is likely driven by a range of cognitive and non-cognitive traits. Alternatively, additive genetic variation in traits closely linked to fitness may be lost through consistent selection [118,120]. Notwithstanding the possibility that we could conceivably have missed important G × E effects, our estimate of VA specifically was also close to zero, suggesting that past selection may have eroded any genetic variation and that innovativeness has reached evolutionary equilibrium in the population. Although it remains possible that in our population the trait has never had an additive genetic basis, and therefore never underwent microevolution, comparative analyses suggest that this is unlikely to be true generally since innovativeness has a strong phylogenetic signal [26,27]. Finally, it is worth remembering that interpreting heritability is non-trivial and depends on a range of statistical issues, for example the method used [115], the nature of the trait, and whether the measured trait captures variation in the intended phenotypes effectively.
(b). Environmental and other effects
(i). Temporal variation
Supporting the results of the animal model, the mixed model analyses at the phenotypic level suggest that innovativeness in Wytham was influenced largely by environmental effects, in the broadest sense (i.e. non-genetic effects). Illustrating the limitations of drawing conclusions from short-term studies, the season individuals were born had a substantial influence on individual innovativeness, and annual PSP varied from 0.36 to 0.54, pointing to large-scale environmental factors. Post hoc inspection of variation across the four years (table 5) shows that PSP among juveniles was highest in the two winters after parents had synchronized their breeding poorly with food availability (correlation coefficient r = −0.93). Foraging conditions post-fledging are likely to be poor when adults synchronize breeding with food availability poorly [121], leading to higher innovation among juveniles, thus supporting the necessity drives innovation hypothesis. Similarly PSP was higher among juvenile birds in the two winters when over-winter fledgling-survival was low (r = −0.981), which were also the two coldest winters (table 5), again pointing to necessity. Whether competition during winter or summer drove the annual patterns of innovation is unclear, not just because N = 4 years, but also because synchronicity with food during breeding, over-winter survival, and winter temperature patterns were all inter-correlated across years (table 5). In addition to these cohort effects, the influence of other factors on innovativeness (see below) invariably depended on the winter when the birds were assayed (table 2), or natal year (table 3). Temporal environmental variation was therefore probably the most important factor driving variation in innovativeness.
Table 5.
Annual summary data for proportion of juveniles solving the task. The timing of breeding is the number of days between the mean lay date and the ‘half fall’ winter moth larvae date for those years; higher values are better timed [121]. Over-winter survival of fledglings is the average number of nestlings per nest that recruited back into the breeding population the following breeding season. Daily mean temperature data were downloaded from the Met Office (www.metoffice.gov.uk/climatechange/science/monitoring/ukcp09/, 50 km grid square: 400 000 E, 200 000 N).
| season | PSP | timing of breeding relative to food (high values = better timed) | following winter temperature (mean daily temperature Sept–Nov, °C) | following over-winter survival of fledglings (recruitment) |
|---|---|---|---|---|
| 2006–2007 | 0.37 | 32.8 | 12.9 | 0.66 |
| 2007–2008 | 0.51 | 26.5 | 10.7 | 0.29 |
| 2008–2009 | 0.54 | 28.3 | 10.0 | 0.22 |
| 2009–2010 | 0.36 | 32.7 | 11.7 | 0.58 |
(ii). Individual factors
In contrast with these results on cohort effects, the analysis on differences in solving rates among different individual classes did not provide support for the necessity drives innovation hypothesis. First, although juveniles were more likely to innovate than adults in one winter, the opposite was the case in at least one other, suggesting that experience was just as significant, or that cohorts may differ consistently over their lifetime. Similarly, we expected immigrants to be at a disadvantage in our population [74,77], and to be more likely to innovate, but their PSP did not differ from local-born birds. And because slow explorers tend to be poor competitors during winter [39], we expected also a negative correlation with EB but in fact found a positive correlation (see below). Finally, there was no influence of sex on PSP even though females tend to be subordinate. Although this limited evidence at the individual level for necessity driving innovation is not surprising given conflicting evidence from the literature, it contrasts with another study from our population [62], which found that juveniles were more likely to solve an innovative task than adults. However, the tasks used by Morand-Ferron et al. [62] were presented in the wild where juveniles were more willing than adults to participate in the trials, but not more efficient when doing so. And while it was based on a single year's data, the study also showed that those individuals who solved in captivity were no more likely to solve in the wild, which points to considerable plasticity in our measure of innovativeness. Although the temporal effects we discuss in the preceding section suggest that necessity may be important acting through annual variation in environmental conditions, and possibly competition, in contrast these data provide little evidence for necessity owing to individual differences in competitive ability.
Previously we reported no link between the reactive–proactive personality axis, assayed by EB, and PSP with 3 years of data [81]. Here, we show an overall weak positive correlation. This contradicts the ‘necessity drives innovation’ hypothesis, which predicts a negative correlation, since EB tends to correlate positively with competitive ability as discussed above. It does support an alternative hypothesis that bolder ‘proactive’ individuals are more likely to exhibit feeding innovations because they rapidly explore new environments and approach novel objects, as reported elsewhere [19,59,83,122]. However, the effect was present primarily in the last year and was relatively weak. Given the pervasive environmental effects throughout the analyses, it is possible that both traits are linked to a third, probably environmental factor, rather than PSP and EB being intrinsically linked. Such context specificity is widely, if not typically, reported in the personality literature, pointing to considerable plasticity. The weak effects here do not necessarily preclude personality from being an important contributing factor to innovativeness in great tits because correlations between traits are likely population- and time-specific ([123], but see [124]), and because other personality traits such as persistence may also be involved [125–127].
(iii). Nestling environment
Although none of the natal local environment variables, or the natal nest variables appeared to influence PSP across all years, in some years PSP was lower among individuals born where local population density was high and where habitat quality was lower, as indicated by the availability of the great tit's preferred habitat, oak trees. These effects support the developmental stress hypothesis. Developmental stress is known to influence some forms of cognition [55–57] but to our knowledge this is the first evidence for developmental stress influencing innovativeness. It is surprising that lay date did not influence PSP because it is generally considered to be one of the most important predictors of food availability. Furthermore, if anything the post hoc analyses on mean effects across years suggested the opposite effect, i.e. years in which birds timed their breeding well led to lower PSP, suggesting that necessity may have driven greater innovation post-fledging when parents timed their breeding poorly. Fledging mass was also not linked to PSP, suggesting that food availability during the nestling stage as such was not the driving factor. Arnold et al. [128] reported a link between the availability of an important amino acid found in spiders and cognitive development in the blue tit (Cyanistes caeruleus). Given that invertebrate density in general is higher in oak-rich habitats and is also likely to be higher where competition is less pronounced in low population density, our results on individual differences in PSP could instead be best explained by dietary composition during early nestling development. Thus while the post hoc analysis above suggests that relatively high PSP may have been caused out of necessity arising from limited food availability in two of the years, developmental effects linked to better habitat quality at a smaller spatial scale may have led to increased PSP during the nestling stage.
5. Conclusion
Although it is possible that significant additive genetic variation could remain undetected, owing to G × E effects for example, our analysis suggests that environmental factors played the major role in generating individual differences in the propensity to innovate in Wytham great tits. Cohorts facing conditions thought to be associated with overall reduced food availability, higher competition levels and/or harsher metabolic demands, included more problem solvers (post hoc analyses), as did first winter birds in one of the four years (figure 2), suggesting that necessity can indeed trigger innovation. At the same time, in some years individuals born in good quality territories were more, not less likely to innovate than those born in lower quality territories (figure 3), again leading to cohort effects, and in this case providing support for the developmental stress hypothesis. Thus the developmental stress and necessity hypotheses found support in these results. Together with links we found previously with a range of functional behaviours and fitness-related traits in the same population, these analyses suggest that individual differences in innovativeness reflect non-genetic variation driven by stochastic temporal and spatial environmental variation. This agrees with the idea that innovation is an emergent property of a combination of traits (e.g. personality, motor abilities, learning ability) with no underlying additive genetic variation of its own [129], while reflecting individual differences caused by behavioural plasticity [130].
Undoubtedly the generality of our findings are limited for many reasons, two of which we mention here. First, all of the effects reported are correlative and have yet to be tested experimentally. Second, our measure was based on one food-motivated, novel problem-solving task which, although correlated to success on a different problem-solving task and to associative learning speed [81,82], is limiting given the large number and kinds of innovations recorded previously for this species [30]. Together with the binary nature of our measure, this means that we could not easily explore innovation plasticity within individuals using a reaction norm approach [124,131–134]. Such an approach might allow for G × E and non-constant additive genetic variation to be detected and characterized [3].
Our results add to our understanding of individual innovativeness and behavioural variation and to the ongoing debate as to the relative importance of plasticity and genetic variation within populations generally [135]. We hope our paper stimulates other researchers to take a similar approach in their study systems, perhaps incorporating a range of different innovation tasks. Finally, it is worth noting that detailed study of the different mechanisms underlying innovativeness—personality and cognitive, for example—may ultimately be needed to determine the causes and population-level consequences of variation in the tendency to innovate among individuals [22,40].
Supplementary Material
Acknowledgements
Thanks to B. Sheldon for support and all the Wytham fieldworkers for collecting the breeding data, and to S. Bouwhuis, D. Cram and S. Patrick for help with some of the assays. The Wytham study population is managed by B. Sheldon. S. Reader provided valuable editorial input.
Ethics
The experimental protocols were all subject to ethical review by the Department of Zoology (Oxford) ethical committee and were conducted with full approval from the UK's Home Office. Birds were caught under ringing licences from the British Trust for Ornithology and were taken into captivity under Natural England licence. All individual researchers had the required licences.
Data accessibility
The data are part of a larger study but is freely available upon request from the author or by contacting the Edward Grey Institute.
Authors' contributions
J.L.Q. conceived the idea for the project, did the non-quantitative genetic analyses and wrote the paper with substantial input from all authors. E.F.C. did most of the assaying, with input from J.M.-F. and J.L.Q. T.E.R. did the quantitative genetic analysis. All authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data.
Competing interests
We have no competing interests.
Funding
This work was funded by a Royal Society research grant (rg072245) to J.L.Q., a BBSRC studentship (BB/D526702/1) to E.F.C. and an NSERC postdoctoral fellowship (90464080) to J.M.-F.
References
- 1.Futuyma DJ. 1998. Evolutionary biology, 3rd edn Sunderland, MA: Sinauer. [Google Scholar]
- 2.Roff DA. 1997. Evolutionary quantitative genetics. New York, NY: Chapman and Hall. [Google Scholar]
- 3.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. ( 10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 4.Hutchings JA. 2011. Old wine in new bottles: reaction norms in salmonid fishes. Heredity 106, 421–437. ( 10.1038/hdy.2010.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 6.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. 2012. Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol. Ecol. 26, 1101–1118. ( 10.1007/s10682-012-9563-5) [DOI] [Google Scholar]
- 7.Reader SM, Laland KN (eds). 2003. Animal innovation. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Kummer H, Goodall J. 1985. Conditions of innovative behaviour in primates. Phil. Trans. R. Soc. Lond. B 308, 203–214. ( 10.1098/rstb.1985.0020) [DOI] [Google Scholar]
- 9.Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246. ( 10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 10.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim. Behav. 81, 1209–1216. ( 10.1016/j.anbehav.2011.03.004) [DOI] [Google Scholar]
- 12.Roth G, Dicke U. 2005. Evolution of the brain and intelligence. Trends Cogn. Sci. 9, 250–257. ( 10.1016/j.tics.2005.03.005) [DOI] [PubMed] [Google Scholar]
- 13.Shettleworth SJ. 2010. Cognition, evolution, and behavior. New York, NY: Oxford University Press. [Google Scholar]
- 14.Overington SE, Cauchard L, Côté K-A, Lefebvre L. 2011. Innovative foraging behaviour in birds: what characterizes an innovator? Behav. Process. 87, 274–285. ( 10.1016/j.beproc.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 15.Tebbich S, Griffin AS, Peschl MF, Sterelny K. 2016. From mechanisms to function: an integrated framework of animal innovation. Phil. Trans. R. Soc. B 371, 20150195 ( 10.1098/rstb.2015.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin AS. 2016. Innovativeness as an emergent property: a new alignment of comparative and experimental research on animal innovation. Phil. Trans. R. Soc. B 371, 20150544 ( 10.1098/rstb.2015.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin AS, Guez D. 2014. Innovation and problem solving: a review of common mechanisms. Behav. Process. 109, 121–134. ( 10.1016/j.beproc.2014.08.027) [DOI] [PubMed] [Google Scholar]
- 18.Cole EF, Morand-Ferron J, Hinks A, Quinn JL. 2012. Cognitive ability influences life history variation in the wild. Curr. Biol. 22, 1808–1812. ( 10.1016/j.cub.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 19.Webster SJ, Lefebvre L. 2001. Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Anim. Behav. 62, 23–32. ( 10.1006/anbe.2000.1725) [DOI] [Google Scholar]
- 20.Griffin AS, Diquelou M, Perea M. 2014. Innovative problem solving in birds: a key role of motor diversity. Anim. Behav. 92, 221–227. ( 10.1016/j.anbehav.2014.04.009) [DOI] [Google Scholar]
- 21.Griffin AS, Guillette LM, Healy SD. 2015. Cognition and personality: an analysis of an emerging field. Trends Ecol. Evol. 30, 207–214. ( 10.1016/j.tree.2015.01.012) [DOI] [PubMed] [Google Scholar]
- 22.Morand-Ferron J, Cole EF, Quinn JL. 2016 Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. ( 10.1111/brv.12174) [DOI] [PubMed] [Google Scholar]
- 23.Rowe C, Healy SD. 2014. Measuring variation in cognition. Behav. Ecol. 25, 1287–1292. ( 10.1093/beheco/aru090) [DOI] [Google Scholar]
- 24.Quinn JL, Cole EF, Morand-Ferron J. 2014. Studying microevolutionary processes in cognitive traits: a comment on Rowe and Healy. Behav. Ecol. 25, 1297–1298. ( 10.1093/beheco/aru141) [DOI] [Google Scholar]
- 25.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sol D, Stirling D, Lefebvre L. 2005. Behavioral drive or behavioral inhibition in evolution: subspecific diversification in holarctic passerines. Evolution 59, 2669–2677. ( 10.1111/j.0014-3820.2005.tb00978.x) [DOI] [PubMed] [Google Scholar]
- 28.Martins EP. 2000. Adaptation and the comparative method. Trends Ecol. Evol. 15, 296–299. ( 10.1016/s0169-5347(00)01880-2) [DOI] [PubMed] [Google Scholar]
- 29.Estok P, Zsebok S, Siemers BM. 2010. Great tits search for, capture, kill and eat hibernating bats. Biol. Lett. 6, 59–62. ( 10.1098/rsbl.2009.0611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim. Behav. 78, 1001–1010. ( 10.1016/j.anbehav.2009.06.033) [DOI] [Google Scholar]
- 31.Cauchard L, Boogert NJ, Lefebvre L, Dubois F, Doligez B. 2013. Problem-solving performance is correlated with reproductive success in a wild bird population. Anim. Behav. 85, 19–26. ( 10.1016/j.anbehav.2012.10.005) [DOI] [Google Scholar]
- 32.Keagy J, Savard JF, Borgia G. 2011. Complex relationship between multiple measures of cognitive ability and male mating success in satin bowerbirds, Ptilonorhynchus violaceus. Anim. Behav. 81, 1063–1070. ( 10.1016/j.anbehav.2011.02.018) [DOI] [Google Scholar]
- 33.Isden J, Panayi C, Dingle C, Madden J. 2013. Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim. Behav. 86, 829–838. ( 10.1016/j.anbehav.2013.07.024) [DOI] [Google Scholar]
- 34.Clutton-Brock T. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 35.Morand-Ferron J, Quinn JL. 2011. Larger groups of passerines are more efficient problem solvers in the wild. Proc. Natl Acad. Sci. USA 108, 15 898–15 903. ( 10.1073/pnas.1111560108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morand-Ferron J, Veillette M, Lefebvre L. 2006. Stealing of dunked food in Carib grackles (Quiscalus lugubris). Behav. Process. 73, 342–347. ( 10.1016/j.beproc.2006.08.006) [DOI] [PubMed] [Google Scholar]
- 37.Bostic DL, Banks RC. 1966. A record of stingray predation by brown pelican. Condor 68, 515 ( 10.2307/1365329) [DOI] [Google Scholar]
- 38.Vas Z, Lefebvre L, Johnson KP, Reiczigel J, Rózsa L. 2011. Clever birds are lousy: co-variation between avian innovation and the taxonomic richness of their amblyceran lice. Int. J. Parasitol. 41, 1295–1300. ( 10.1016/j.ijpara.2011.07.011) [DOI] [PubMed] [Google Scholar]
- 39.Cole EF, Quinn JL. 2012. Personality and problem-solving performance explain competitive ability in the wild. Proc. R. Soc. B 279, 1168–1175. ( 10.1098/rspb.2011.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morand-Ferron J, Quinn JL. 2015. The evolution of cognition in natural populations. Trends Cogn. Sci. 19, 235–237. ( 10.1016/j.tics.2015.03.005) [DOI] [PubMed] [Google Scholar]
- 41.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer. [Google Scholar]
- 42.Merilä J, Sheldon BC. 2001. Avian quantitative genetics. Curr. Ornithol. 16, 179–255. [Google Scholar]
- 43.van Oers K, Drent PJ, de Goede P, van Noordwijk AJ. 2004. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. Lond. B 271, 65–73. ( 10.1098/rspb.2003.2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn JL, Patrick SC, Bouwhuis S, Wilkin TA, Sheldon BC. 2009. Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215. ( 10.1111/j.1365-2656.2009.01585.x) [DOI] [PubMed] [Google Scholar]
- 45.Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P. 2012. Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv. Int. 22, 1–34. ( 10.1017/s0959270912000020) [DOI] [Google Scholar]
- 46.Dukas R. 2004. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 35, 347–374. ( 10.1146/annurev.ecolsys.35.112202.130152) [DOI] [Google Scholar]
- 47.Dukas R, Ratcliffe JM. 2009. Cognitive ecology II. Chicago, IL: University of Chicago Press. [Google Scholar]
- 48.Croston R, Branch CL, Kozlovsky DY, Dukas R, Pravosudova VV. 2015. Heritability and the evolution of cognitive traits. Behav. Ecol. 26, 1447–1459. ( 10.1093/beheco/arv088) [DOI] [Google Scholar]
- 49.Kawecki TJ. 2010. Evolutionary ecology of learning: insights from fruit flies. Popul. Ecol. 52, 15–25. ( 10.1007/s10144-009-0174-0) [DOI] [Google Scholar]
- 50.Greenwood PM, Parasuraman R. 2003. Normal genetic variation, cognition, and aging. Behav. Cogn. Neurosci. Rev. 2, 278–306. ( 10.1177/1534582303260641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henrich J, Heine SJ, Norenzayan A. 2010. The weirdest people in the world? Behav. Brain Sci. 33, 61–83. ( 10.1017/s0140525x0999152x) [DOI] [PubMed] [Google Scholar]
- 52.Plomin R, Spinath FM. 2004. Intelligence: genetics, genes, and genomics. J. Pers. Soc. Psychol. 86, 112–129. ( 10.1037/0022-3514.86.1.112) [DOI] [PubMed] [Google Scholar]
- 53.Naguib M, Florcke C, van Oers K. 2011. Effects of social conditions during early development on stress response and personality traits in great tits (Parus major). Dev. Psychobiol. 53, 592–600. ( 10.1002/dev.20533) [DOI] [PubMed] [Google Scholar]
- 54.Nowicki S, Peters S. 1998. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 38 179–190. ( 10.1093/icb/38.1.179) [DOI] [Google Scholar]
- 55.Boogert NJ, Fawcett TW, Lefebvre L. 2011. Mate choice for cognitive traits: a review of the evidence in nonhuman vertebrates. Behav. Ecol. 22, 447–459. ( 10.1093/beheco/arq173) [DOI] [Google Scholar]
- 56.Buchanan KL, Grindstaff J, Pravosudov VV. 2013. Condition-dependence, developmental plasticity and cognition: implications for ecology and evolution. Trends Ecol. Evol. 28, 290–296. ( 10.1016/j.tree.2013.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farrell T, Kriengwatana B, MacDougall-Shackleton S. 2015. Developmental stress and correlated cognitive traits in songbirds. Comp. Cogn. Behav. Rev. 10, 1–23. ( 10.3819/ccbr.2015.100001) [DOI] [Google Scholar]
- 58.Laland KN, Reader SM. 1999. Foraging innovation is inversely related to competitive ability in male but not in female guppies. Behav. Ecol. 10, 270–274. ( 10.1093/beheco/10.3.270) [DOI] [Google Scholar]
- 59.Bouchard J, Goodyer W, Lefebvre L. 2007. Social learning and innovation are positively correlated in pigeons (Columba livia). Anim. Cogn. 10, 259–266. ( 10.1007/s10071-006-0064-1) [DOI] [PubMed] [Google Scholar]
- 60.Gajdon GK, Fijn N, Huber L. 2006. Limited spread of innovation in a wild parrot, the kea (Nestor notabilis). Anim. Cogn. 9, 173–181. ( 10.1007/s10071-006-0018-7) [DOI] [PubMed] [Google Scholar]
- 61.Keagy J, Savard J-F, Borgia G. 2009. Male satin bowerbird problem-solving ability predicts mating success. Anim. Behav. 78, 809–817. ( 10.1016/j.anbehav.2009.07.011) [DOI] [Google Scholar]
- 62.Morand-Ferron J, Cole EF, Rawles JEC, Quinn JL. 2011. Who are the innovators? A field experiment with 2 passerine species. Behav. Ecol. 22, 1241–1248. ( 10.1093/beheco/arr120) [DOI] [Google Scholar]
- 63.van Schaik CP, Burkart J, Damerius L, Forss SIF, Koops K, van Noordwijk MA, Schuppli C. 2016. The reluctant innovator: orangutans and the phylogeny of creativity. Phil. Trans. R. Soc. B 371, 20150183 ( 10.1098/rstb.2015.0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson AJ, Pemberton JM, Pilkington JG, Coltman DW, Mifsud DV, Clutton-Brock TH, Kruuk LEB. 2006. Environmental coupling of selection and heritability limits evolution. PLoS Biol. 4, 1270–1275. ( 10.1371/journal.pbio.0040216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harden KP, Turkheimer E, Loehlin JC. 2007. Genotype by environment interaction in adolescents’ cognitive aptitude. Behav. Genet. 37, 273–283. ( 10.1007/s10519-006-91134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyce MS, Perrins CM. 1987. Optimizing great tit clutch size in a fluctuating environment. Ecology 68, 142–153. ( 10.2307/1938814) [DOI] [Google Scholar]
- 67.Cowie RJ. 1977. Optimal foraging in great tits (Parus major). Nature 268, 137–139. ( 10.1038/268137a0) [DOI] [Google Scholar]
- 68.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 69.Garant D, Kruuk LEB. 2005. How to use molecular marker data to measure evolutionary parameters in wild populations. Mol. Ecol. 14, 1843–1859. ( 10.1111/j.1365-294X.2005.02561.x) [DOI] [PubMed] [Google Scholar]
- 70.Both C, Dingemanse NJ, Drent PJ, Tinbergen JM. 2005. Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 74, 667–674. ( 10.1111/j.1365-2656.2005.00962.x) [DOI] [Google Scholar]
- 71.Perrins CM. 1970. The timing of birds’ breeding season. Ibis 112, 242–255. ( 10.1111/j.1474-919X.1970.tb00096.x) [DOI] [Google Scholar]
- 72.Kruuk LEB, Charmantier A, Garant D. 2014. The study of quantitative genetics in wild populations, pp. 1–15. Oxford, UK: Oxford University Press. [Google Scholar]
- 73.Wilkin TA, Garant D, Gosler AG, Sheldon BC. 2006. Density effects on life-history traits in a wild population of the great tit Parus major: analyses of long-term data with GIS techniques. J. Anim. Ecol. 75, 604–615. ( 10.1111/j.1365-2656.2006.01078.x) [DOI] [PubMed] [Google Scholar]
- 74.Wilkin TA, Perrins CM, Sheldon BC. 2007. The use of GIS in estimating spatial variation in habitat quality: a case study of lay-date in the great tit Parus major. Ibis 149, 110–118. ( 10.1111/j.1474-919X.2007.00757.x) [DOI] [Google Scholar]
- 75.Wilkin TA, King LE, Sheldon BC. 2009. Habitat quality, nestling diet, and provisioning behaviour in great tits Parus major. J. Avian Biol. 40, 135–145. ( 10.1111/j.1600-048X.2009.04362.x) [DOI] [Google Scholar]
- 76.Basso A, Coslovsky M, Richner H. 2014. Parasite- and predator-induced maternal effects in the great tit (Parus major). Behav. Ecol. 25, 1105–1114. ( 10.1093/beheco/aru088) [DOI] [Google Scholar]
- 77.Quinn JL, Cole EF, Patrick SC, Sheldon BC. 2011. Scale and state dependence of the relationship between personality and dispersal in a great tit population. J. Anim. Ecol. 80, 918–928. ( 10.1111/j.1365-2656.2011.01835.x) [DOI] [PubMed] [Google Scholar]
- 78.Duyck I, Duyck J. 1984. Great tit using instrument to pick up food. Wielewaal 50, 416. [Google Scholar]
- 79.Fisher J, Hinde RA. 1949. The opening of milk bottles in birds. Br. Birds 42, 347–357. [Google Scholar]
- 80.Aplin LM, Farine DR, Morand-Ferron J, Cockburn A, Thornton A, Sheldon BC. 2015. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541. ( 10.1038/nature13998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cole EF, Cram DL, Quinn JL. 2011. Individual variation in spontaneous problem-solving performance among wild great tits. Anim. Behav. 81, 491–498. ( 10.1016/j.anbehav.2010.11.025) [DOI] [Google Scholar]
- 82.Morand-Ferron J, Hamblin S, Cole EF, Aplin LM, Quinn JL. 2015. Taking the operant paradigm to the field: associative learning in wild songbirds. PLoS ONE. 10, e0133821 ( 10.1371/journal.pone.0133821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenberg R. 2003. The role of neophobia and neophilia in the development of innovative behaviour of birds. In Animal innovation (eds Reader SM, Laland KN), pp. 175–196, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 84.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coppens CM, de Boer SF, Koolhaas JM. 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028. ( 10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Titulaer M, van Oers K, Naguib M. 2012. Personality affects learning performance in difficult tasks in a sex-dependent way. Anim. Behav. 83, 723–730. ( 10.1016/j.anbehav.2011.12.020) [DOI] [Google Scholar]
- 87.Amy M, van Oers K, Naguib M. 2012. Worms under cover: relationships between performance in learning tasks and personality in great tits (Parus major). Anim. Cogn. 15, 763–770. ( 10.1007/s10071-012-0500-3) [DOI] [PubMed] [Google Scholar]
- 88.Cole EF, Quinn JL. 2014. Shy birds play it safe: personality in captivity predicts risk responsiveness during reproduction in the wild. Biol. Lett. 10, 20140178 ( 10.1098/rsbl.2014.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenwood PJ, Harvey PH, Perrins CM. 1979. The role of dispersal in the great tit (Parus major): the causes, consequences and heritability of natal dispersal. J. Anim. Ecol. 48, 123–142. ( 10.2307/4105) [DOI] [Google Scholar]
- 90.Kidd LR, Sheldon BC, Simmonds EG, Cole EF. 2015. Who escapes detection? Quantifying the causes and consequences of sampling biases in a long-term field study. J. Anim. Ecol. 84, 1520–1529. ( 10.1111/1365-2656.12411) [DOI] [PubMed] [Google Scholar]
- 91.Svensson L. 1984. Identification guide to European Passerines. Tring, UK: British Trust for Ornithology. [Google Scholar]
- 92.Verbeek MEM, Drent PJ, Wiepkema PR. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121. ( 10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 93.Southwood TRE. 1961. The number of species of insect associated with various trees. J. Anim. Ecol. 30, 1–8. ( 10.2307/2109) [DOI] [Google Scholar]
- 94.Wilkin TA, Sheldon BC. 2009. Sex differences in the persistence of natal environmental effects on life histories. Curr. Biol. 19, 1998–2002. ( 10.1016/j.cub.2009.09.065) [DOI] [PubMed] [Google Scholar]
- 95.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 96.Kruuk LEB. 2004. Estimating genetic parameters in wild populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890. ( 10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson AJ, Reale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 98.Morrissey MB, Wilson AJ. 2010. Pedantics: an R package for pedigree-based genetic simulation and pedigree manipulation, characterization and viewing. Mol. Ecol. Resour. 10, 711–719. ( 10.1111/j.1755-0998.2009.02817.x) [DOI] [PubMed] [Google Scholar]
- 99.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. Essex, UK: Longman. [Google Scholar]
- 100.Villemereuil P, Gimenez O, Doligez B. 2013. Comparing parent–offspring regression with frequentist and Bayesian animal models to estimate heritability in wild populations: a simulation study for Gaussian and binary traits. Methods Ecol. Evol. 4, 260–275. ( 10.1111/2041-210x.12011) [DOI] [Google Scholar]
- 101.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 102.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. 2002. Bayesian measures of model complexity and fit. J. R. Stat. Soc. B 64, 583–616. ( 10.1111/1467-9868.00353) [DOI] [Google Scholar]
- 103.Drent PJ, van Oers K, van Noordwijk AJ. 2003. Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51. ( 10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dingemanse NJ, Both C, Drent PJ, van Oers K, van Noordwijk AJ. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938. ( 10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- 105.Hopkins WD, Russe JL, Schaeffer J. 2014. Chimpanzee intelligence is heritable. Curr. Biol. 24, 1649–1652. ( 10.1016/j.cub.2014.05.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dingemanse NJ, Barber I, Wright J, Brommer JE. 2012. Quantitative genetics of behavioural reaction norms: genetic correlations between personality and behavioural plasticity vary across stickleback populations. J. Evol. Biol. 25, 485–496. ( 10.1111/j.1420-9101.2011.02439.x) [DOI] [PubMed] [Google Scholar]
- 107.Kruuk LEB, Clutton-Brock TH, Pemberton JM, Brotherstone S, Guinness FE. 2000. Heritability of fitness in a wild mammal population. Proc. Natl Acad. Sci. USA 97, 698–703. ( 10.1073/pnas.97.2.698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merilä J, Sheldon BC. 1999. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity 83, 103–109. ( 10.1046/j.1365-2540.1999.00585.x) [DOI] [PubMed] [Google Scholar]
- 109.Roff DA, Mousseau TA. 1987. Quantitative genetics and fitness: lessons from Drosophila. Heredity 58, 103–118. ( 10.1038/hdy.1987.15) [DOI] [PubMed] [Google Scholar]
- 110.Merila J, Sheldon BC. 2000. Lifetime reproductive success and heritability in nature. Am. Nat. 155, 301–310. ( 10.1086/303330) [DOI] [PubMed] [Google Scholar]
- 111.Dingemanse NJ, Réale D. 2005. Natural selection and animal personality. Behaviour 142, 1165–1190. ( 10.1163/156853905774539445) [DOI] [Google Scholar]
- 112.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 113.van Oers K, Mueller JC. 2010. Evolutionary genomics of animal personality. Phil. Trans. R. Soc. B 365, 3991–4000. ( 10.1098/rstb.2010.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wolf M, Weissing FJ. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968. ( 10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stirling DG, Réale D, Roff DA. 2002. Selection, structure and the heritabilty of behaviour. J. Evol. Biol. 15, 277–289. ( 10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- 116.Dochtermann NA, Schwab T, Sih A. 2014. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282, 20142201 ( 10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Price T, Schluter D. 1991. On the low heritability of life-history traits. Evolution 45, 853–861. ( 10.2307/2409693) [DOI] [PubMed] [Google Scholar]
- 118.Houle D. 1992. Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ellegren H, Sheldon BC. 2008. Genetic basis of fitness differences in natural populations. Nature 452, 169–175. ( 10.1038/nature06737) [DOI] [PubMed] [Google Scholar]
- 120.Fisher RA. 1930. The genetical theory of natural selection. London, UK: Oxford University. [Google Scholar]
- 121.Hinks AE, Cole EF, Daniels KJ, Wilkin TA, Nakagawa S, Sheldon BC. 2015. Scale-dependent phenological synchrony between songbirds and their caterpillar food source. Am. Nat. 186, 84–97. ( 10.1086/681572) [DOI] [PubMed] [Google Scholar]
- 122.Seferta A, Guay PJ, Marzinotto E. 2001. Learning differences between feral pigeons and zenaida doves: the role of neophobia and human proximity. Ethology 107, 281–293. ( 10.1046/j.1439-0310.2001.00658.x) [DOI] [Google Scholar]
- 123.Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. ( 10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- 124.Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL. 2011. Variation in personality and behavioural plasticity among and within four populations of the great tit Parus major. J. Anim. Ecol. 81, 116–126. ( 10.1111/j.1365-2656.2011.01877.x) [DOI] [PubMed] [Google Scholar]
- 125.Overington SE, Griffin AS, Sol D, Lefebvre L. 2011. Are innovative species ecological generalists? A test in North American birds. Behav. Ecol. 22, 1286–1293. ( 10.1093/beheco/arr130) [DOI] [Google Scholar]
- 126.Benson-Amram S, Holekamp KE. 2012. Innovative problem solving by wild spotted hyenas. Proc. R. Soc. B 279, 4087–4095. ( 10.1098/rspb.2012.1450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thornton A, Samson J. 2012. Innovative problem solving in wild meerkats. Anim. Behav. 83, 1459–1468. ( 10.1016/j.anbehav.2012.03.018) [DOI] [Google Scholar]
- 128.Arnold KE, Ramsay SL, Donaldson C, Adam A. 2007. Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring. Proc. R. Soc. B 274, 2563–2569. ( 10.1098/rspb.2007.0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sol D. 2015. The evolution of innovativeness: exaptation or specialized adaptation? In Animal creativity and innovation: research and theory (eds Kaufman AB, Kaufman JC), pp. 163–182. New York, NY: Academic Press. [Google Scholar]
- 130.Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 131.Nussey DH, Wilson AJ, Brommer JE. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844. ( 10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 132.Kirkpatrick M, Heckman N. 1989. A quantitative genetic model for growth, shape, reaction norms, and other infinite-dimensional characters. J. Math. Biol. 27, 429–450. ( 10.1007/bf00290638) [DOI] [PubMed] [Google Scholar]
- 133.Stinchcombe JR, Kirkpatrick M, Function-valued Traits Working Group 2012. Genetics and evolution of function-valued traits: understanding environmentally responsive phenotypes. Trends Ecol. Evol. 27, 637–647. ( 10.1016/j.tree.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 134.Dingemanse NJ, Kazem AJN, Reale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 135.Merilä J, Hendry A. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are part of a larger study but is freely available upon request from the author or by contacting the Edward Grey Institute.