Abstract
The evolutionary origin of innovativeness remains puzzling because innovating means responding to novel or unusual problems and hence is unlikely to be selected by itself. A plausible alternative is considering innovativeness as a co-opted product of traits that have evolved for other functions yet together predispose individuals to solve problems by adopting novel behaviours. However, this raises the question of why these adaptations should evolve together in an animal. Here, we develop the argument that the adaptations enabling animals to innovate evolve together because they are jointly part of a life-history strategy for coping with environmental changes. In support of this claim, we present comparative evidence showing that in birds, (i) innovative propensity is linked to life histories that prioritize future over current reproduction, (ii) the link is in part explained by differences in brain size, and (iii) innovative propensity and life-history traits may evolve together in generalist species that frequently expose themselves to novel or unusual conditions. Combined with previous evidence, these findings suggest that innovativeness is not a specialized adaptation but more likely part of a broader general adaptive system to cope with changes in the environment.
Keywords: creativity, behavioural plasticity, brain evolution, pace-of-life, developmental constrains
1. Introduction
Innovativeness designates the possibility of constructing plastic behavioural responses to ecological challenges [1–3], thereby potentially enhancing the fitness of animals when exposed to unusual or novel situations [4]. In Barbados, for example, bullfinches (Loxigilla barbadensis) have developed a novel technique to open sugar packets from restaurant terraces [5,6], which enables them to exploit an important resource opportunity inaccessible to most other animals. By changing the relationship with the environment, innovative behaviours provide opportunities for animals to modify their niche and even invade new adaptive zones that can subsequently favour evolutionary divergence [7,8]. While the implications of innovativeness are increasingly appreciated among evolutionary ecologists, the way selection has shaped the ability to innovate is less clear.
From an evolutionary perspective, the ability to innovate is considered by some to be an adaptive specialization related to modular cognitive processes [9]. However, innovating means responding to problems the animal has rarely experienced before, and hence it is unlikely to be selected by itself. This distinguishes innovativeness from more specialized cognitive abilities like food hoarding or song learning, which are easier to understand as a result of exposure to consistent selection pressures. Indeed, experimental work has failed to identify any cognitive specialization associated with enhanced innovative abilities [10,11]. Instead, some animals seem to possess from the start the machinery needed to invent sophisticated behaviours even when these are rarely used in the wild [12]. These findings are in line with growing evidence that innovative propensity is part of a domain-general cognitive mechanism [13] regulated by large areas of the brain [1,14,15] and affected by physiology and morphology [16,17].
The alternative to the adaptive specialization perspective is that innovative propensity is not the direct target of selection, at least in the early stages of evolution, but is a by-product of a combination of traits that have evolved for other functions yet predispose individuals to solve problems by adopting novel behaviours [17–19]. Although theoretically more plausible, the challenge is to understand how and why these adaptations may evolve together in an animal. Building on previous work [20–22], we develop here the argument that the adaptations enabling animals to innovate evolve together because they are jointly part of a life-history strategy for coping with environmental changes.
Life history subsumes a variety of adaptive mechanisms affecting how individuals allocate time and energy in reproduction, development and survival [23,24]. Because life history largely influences population dynamics in changing environments [25] and is closely associated with lifestyle [26], it is possible that the adaptations required to innovate have evolved together with certain life-history strategies through correlated selection or shared genetic/physiological mechanisms in lineages exposed to environmental changes. For example, the fast–slow continuum of life-history variation might create a link between the traits underlying innovations by generating differences in the need to collect, assess, retain and use environmental information [17,27]. In addition, the possibility of constructing plastic behavioural responses to ecological challenges might directly affect the evolution of life histories by enhancing adult survival, a theory known as the cognitive buffer hypothesis [28,29].
While the benefits of innovating are obvious, the fact that some animals appear to be less innovative than others suggests that innovativeness might have substantial costs or that it might be highly constrained by other aspects of life history [22]. Costs that may outweigh benefits and select for reduced innovativeness may be direct or indirect. Direct costs include the time and energy needed to innovate and the risks individuals take during the process by exposing themselves to pathogens and predators. Indirect costs are primarily related to the need to invest in neural structures, which are energetically expensive to produce and maintain and require long developmental periods. The idea that the time and energy available are crucial for innovating not only constitutes a life-history approach by itself [22], but also creates a number of additional potential links between innovativeness and life history (see below).
Despite the existence of sound theoretical arguments, direct evidence for a link between innovativeness and life history is currently absent. In the only study to date that has comprehensively tested it, Ricklefs [22] found no evidence that incubation period and lifespan are associated with the propensity to innovate. The only current evidence comes from the existence of an association between slow life histories and enlarged brains, one of the main predictors of innovativeness in birds and primates [1,13–15]. Yet, disentangling whether the link arises from either benefits or costs/constraints is difficult in the absence of direct measures of innovativeness [30]. To clarify the relationship between innovativeness and life history, we summarize previous evidence and present new analyses aimed at asking (i) whether and how feeding innovative propensity is linked to life history in birds, (ii) whether the link is caused by brain size or developmental constraints, and (iii) what selective scenarios might have enabled innovativeness and life-history traits to evolve together.
2. Framework to investigate the innovativeness–life-history association
Because the life history of animals tends to be evolutionarily conserved [31], the existence of an association between innovativeness and life history is unlikely to be easily inferred by comparing individuals or populations. A more promising approach is to compare species differing in life-history strategy. Characterizing innovativeness for a large number of species with contrasting lifestyles is not easy, however. To tackle this difficulty, Lefebvre et al. [1] proposed to quantify its product (i.e. innovation frequency), assuming that species that exhibit enhanced innovative skills should be more often observed innovating in the wild than those that are less innovative [32,33].
In the past 20 years, Lefebvre and collaborators have assembled a global database of novel feeding behaviours of birds based on exhaustive reviews of ornithology journals from North America, Europe, Australia, New Zealand, the Indian subcontinent, southern Africa and South America. To be considered an innovation, the reported behaviour needed to contain words such as ‘novel’, ‘first description’, ‘not noted before’ and ‘unusual’. Each innovation was then classified as a (i) consumer innovation, if it involves a novel food item, or (ii) technical innovation, if the searching and handling techniques are themselves novel regardless of whether the food type is novel or not. This distinction is important because while consumer innovations primarily reflect opportunistic feeding, technical innovations are thought to reflect more cognitively demanding technical intelligence [15]. The current database of feeding innovations includes 2608 reports for 1018 species.
The frequency with which a species is observed using novel foods or new feeding techniques in the wild depends not only on its innovative ability, but also on the extent to which the environment elicits innovative behaviours and the probability that these new behaviours are observed and reported [32]. We tackled these potential biases in two ways. On one hand, we followed Overington et al. [15] and based our analyses exclusively on species that had been observed innovating at least once. This is a conservative way to exclude animals that have not been reported innovating because they are rare, secretive or live in inaccessible regions. On the other hand, we modelled feeding innovation frequency as a function of life-history strategies by means of a Gaussian linear mixed model, using a Bayesian approximation in the R-package ‘MCMCglmm’ [34]. By including species and continents as random effects, this approach made possible the integration of global information originating at the continental scale. Although this already controls for much of the variation in detectability and reporting bias, we further controlled such confounds by including geographical range size, migratory behaviour, body size and research effort as fixed factors in the models. In addition, MCMCglmm allowed us to investigate the association between innovative propensity and life history while controlling for phylogenetic dependencies.
With the above Bayesian framework, we tested all major hypotheses linking innovativeness and life history, as detailed in the next sections. Once all the important links were well established, we used phylogenetic path analyses to identify the most likely causal scenarios. Following von Hardenberg & Gonzalez-Voyer [35,36], we applied the concept of d-separation to predict the minimal set of conditional probabilistic independence constraints that were expected to be true for the causal model to be correct. To test whether the defined conditional independencies were fulfilled in the observational data, we combined all the values of probabilities that the non-adjacent variables are statistically independent conditional on their parent variables using Fisher's C statistic. Probabilities and standardized path coefficients were estimated by means of MCMCglmm, but the analyses were conducted at the species level rather than species × continent level. For additional details on the data and procedures, we refer the reader to the electronic supplementary material.
3. Innovativeness and life history under the cognitive buffer hypothesis
If animals can resolve many of their problems by building plastic behavioural responses and this in turn ensures that survival is high in most years, then selection should favour the combination of adaptations that facilitate such behavioural changes [20,21,28,29]. According to the demographic theory of life-history evolution [23], this would in turn contribute to prolonging lifespan. The link between innovativeness and life history should be further reinforced if a long life increases exposure to environmental changes [37], which would enhance the value of gathering environmental information and constructing appropriate behavioural responses. The cognitive buffer hypothesis thus predicts that feeding innovation frequency should be linked with long-lived strategies, although the correlation should not be strong because other mechanisms besides enhanced innovativeness can also increase longevity [21,38]. In addition, the hypothesis also predicts that the brain should be one of the underlying causes of this link [39]. These two predictions were evaluated with three different metrics describing the extent to which the species prioritize future over current reproduction: the fast–slow continuum, brood value and maximum recorded lifespan (figure 1; electronic supplementary material, figure S1).
Figure 1.
Covariation of the fast–slow (FS) continuum and brood value (BV) with each other and with body size, fecundity and productivity. Colours indicate the types of development as follows: altricial, red; precocial, green; semi-altricial, blue; semi-precocial,white. (Online version in colour.)
(a). Fast–slow continuum
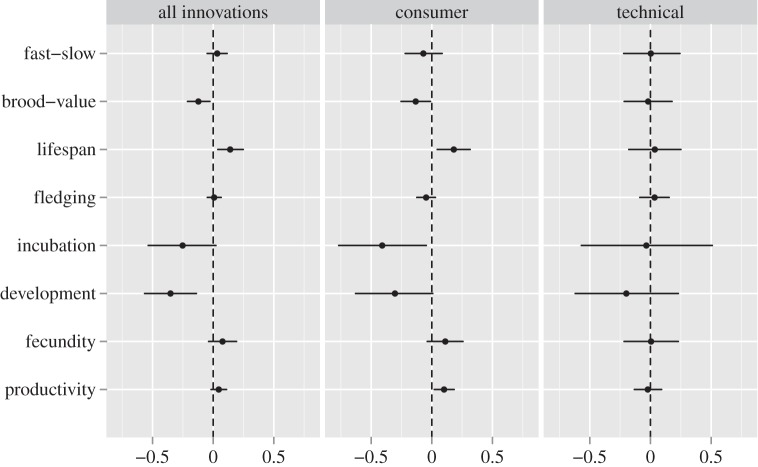
The fast–slow continuum defines a trade-off of life-history variation that ranges from species that have great reproductive effort, develop fast and die young (fast-lived) at one end to species that have low fecundity, slow development and long lives at the other end [40]. We described this continuum by means of a factor analysis based on the correlation matrix of fecundity (clutch size × number of broods per year), maximum lifespan and the lengths of the hatchling and fledging periods, all log-transformed (electronic supplementary material, figure S1). Contrary to expectations, the fast–slow continuum was not associated with innovative propensity, whether measured as total frequency of innovations (figure 2; electronic supplementary material, table S1) or separated into technical and consumer innovations (electronic supplementary material, table S2).
Figure 2.
Effect size of the regression coefficients of life-history traits on feeding innovation frequency estimated with Bayesian phylogenetic mixed models (see details in electronic supplementary material, tables S1–S18). Each trait or attribute has been tested independently of the others. The effect is considered significant when its credibility interval (CI) does not overlap zero. All parameters are estimated with the same sample size (n = 510 feeding innovations reports belonging to 385 species) and represent the average of models based on 10 randomly chosen phylogenies. Error bars are the 95% credible interval.
(b). Brood value and lifespan
The fast–slow continuum as defined above does not capture the variety of mechanisms by which life history can affect population dynamics. One reason for this is that a species may have a high annual productivity either by laying large clutches or by reproducing several times within a breeding season, and these two strategies have distinct consequences for populations exposed to environmental changes [41]. A more general metric to characterize species that prioritize future over current reproduction is provided by the brood value concept, which is expressed as log10{1/[(broods per year) × (reproductive lifespan)]} [42]. A high brood value means that all reproductive effort is allocated into a few reproductive events and, hence, that each brood has high fitness value, whereas a low brood value means that the effort is distributed among many attempts, whether in the same season or in different ones. Being less dependent on the length of the developmental period, this metric is also largely independent of body size (electronic supplementary material, figure S1).
Using the brood value metric, we found evidence that species that prioritize future reproduction tend to be more innovative (figure 2; electronic supplementary material, table S3), a finding that held for consumer but not technical innovations (electronic supplementary material, table S4). This latter discrepancy can reflect the fact that the innovation record is more complete for consumer (n = 1548) than for technical innovations (n = 1060). Alternatively, the differences may reflect different ways of being innovative. Adopting new types of food may not require very sophisticated cognitive abilities compared with inventing new feeding techniques. Nevertheless, it still requires a combination of traits like neophilia, discrimination and associative learning, which are also essential for more sophisticated innovations [17].
In our dataset, there were relatively few species that attain high brood values by having short lifespan and multiple broods in the same season, and hence brood value largely reflected maximum lifespan (electronic supplementary material, figure S1). Indeed, our results indicate that lifespan also predicted variation in innovation frequency (electronic supplementary material, tables S5 and S6). This contrasts with Ricklefs [22], who found no evidence for such an association. The discrepancies between our results and those of Ricklefs [22] can result from our enlarged dataset (2608 innovations in seven regions of the world in our case versus 427 in two regions in Ricklefs [22]) and the availability of higher resolution analytical methods that allow studying innovations at the species level while controlling for phylogenetic and regional effects.
Beyond brood value and lifespan, none of the other life-history traits were consistently associated with innovation frequency except developmental mode (figure 2; electronic supplementary material, tables S7–S18), an issue that we will discuss later on. Because the results did not qualitatively change whether using maximum lifespan or brood value, in the next sections we will focus on maximum lifespan to make the results easier to interpret. Results with brood value are presented in the electronic supplementary material.
(c). Brain size as nexus linking innovativeness and life history
Previous work has identified brain size (corrected for body size) as a major predictor of innovative propensity [1,14,15], a result that is confirmed in our analyses (electronic supplementary material, tables S19 and S20). Because brain size has also been suggested to enhance lifespan by improving physiological regulation [21,28,38], it remains unclear whether the association between innovative propensity and brood value is direct or indirectly caused through their common association with brain size. As a way to distinguish between these alternatives, we explored direct, indirect and common causal relationships among these traits by means of a phylogenetic path analysis [35,36]. To control for the allometric effect of body size on brain size (by which larger species tend to have larger brains), we previously estimated the residuals of a log–log phylogenetic least-square regression of brain mass against body mass (hereafter, relative brain size; see the electronic supplementary material for details) [4]. Although the use of residuals is open to criticisms [43], this was justified in our case for two reasons: (i) there was a strong, linear relationship between brain size and body mass (R2 = 0.88), which would have created problems of colinearity if both absolute brain mass and body mass were included as predictors in a same model; and (ii) we were interested in removing allometric effects from brain mass (as innovation propensity is related to relative brain size but not absolute brain size [15]) but not from innovation propensity. However, the conclusions did not change whether using one method or another.
The estimated path coefficients suggest that the link between lifespan and innovativeness arose in part owing to the common causal effect of brain size (figure 3a). However, there was also evidence for a direct effect of innovation frequency on lifespan (figure 3a; electronic supplementary material, tables S19 and S20). Although a brain can be disproportionally large relative to body size because of selection for a large brain but also as a result of selection for a smaller body, body size was not associated with innovation propensity (electronic supplementary material, tables S21 and S22). Thus, our results do not merely reflect differences in body size.
Figure 3.
(a) Phylogenetic path model deconstructing the direct, indirect and common causal relationships explaining covariation between innovation propensity and maximum lifespan as a function of relative brain size for 473 avian species. Path coefficients have been estimated with a MCMCglmm on standardized values, including research effort as fixed effect and phylogeny as random factor. (b) Best-supported phylogenetic path models deconstructing direct, indirect and common causal effects accounting for the covariation between innovation propensity and lifespan for 471 avian species. IP, innovation propensity; ML, maximum lifespan; HB, habitat breadth; BS, relative brain size; DM, developmental mode (altricial versus all other categories); C, Fisher's C statistic, which can be approximated to a χ2 distribution with 2 k d.f. Continuous and dashed lines illustrate respectively significant and non-significant paths.
4. Life history and innovativeness under alternative hypotheses
Although our findings fit well with the cognitive buffer hypothesis, a number of alternatives also need to be considered.
(a). Brain maturation hypothesis
Because a large brain takes longer time to grow, an association between innovativeness and life history is predicted even when brain size has been selected for reasons other than enhancing survival via the cognitive buffer [30,38]. We tested this hypothesis by examining whether innovation frequency covaries with the time to hatching and developmental mode (precocial, semi-precocial, semi-altricial and altricial). Like Ricklefs [22], we found no consistent evidence that incubation period is related to innovation frequency (figure 2; electronic supplementary material, tables S11, S12, S23 and S24). However, altricial species exhibited a tendency to be more innovative than precocial species (electronic supplementary material, tables S13, S14, S25 and S28).
(b). Delayed benefits hypothesis
According to the delayed benefits hypothesis, a long developmental period is necessary to acquire the behavioural skills needed to survive as adults [38]. New Caledonian crows, a species with an unusual ability for innovative tool use, have one of the longest known periods of extended parental provisioning in birds, with some parents regularly feeding juveniles for up to 10 months post-fledging [44]. The delayed benefits hypothesis is compatible with the cognitive buffer because it suggests some benefit of enhanced innovativeness during adulthood. However, an extended development adds costs in terms of delayed reproduction and substantial parental care not explicitly considered by the cognitive buffer hypothesis. We tested the hypothesis by modeling innovation frequency as a function of the age at first reproduction and the duration of the period from hatching to fledging (fledging period). Neither the age at first reproduction nor the length of the fledging period were related to innovative propensity (figure 2; electronic supplementary material, tables S7, S10, S29 and S30). Thus, the hypothesis cannot account for the observation that innovative propensity is associated with lifespan.
In a refinement of the delayed benefits hypothesis, Allman & Hasenstaub [45] suggested that big-brained animals should spend a disproportionate amount of time during their development outside, where environmental stimulation is enhanced, rather than within the egg or the mother. We consequently also tested whether species with enhanced innovative propensity are characterized by fledging periods that are disproportionally longer given their incubation period. We did so by including incubation as covariate in the model, thereby adjusting the length of the fledging period by the length of the incubation period. Again, we did not find any evidence that fledging is related to innovation propensity (electronic supplementary material, tables S31 and S32). Although we did not have information on the period of post-fledging care, during which species presumably learn many important skills for proficient feeding, current evidence does not provide general support for the delayed benefits hypothesis.
5. Contexts that select for innovativeness and life history
In previous sections, we reported evidence that part of the covariation between innovativeness and life history comes from their common association with brain size. However, there was also evidence for links not mediated by brain size. These links can be causal or can arise by correlated evolution if both enhanced innovativeness and long lifespan provide benefits under similar selective pressures. We discuss below the possibility that ecological generalism generates correlated selection for innovativeness and lifespan.
In the foraging context, innovations are particularly useful when resources are scarce or novel. By being able to exploit alternative foods, individuals can alleviate adverse conditions [46]. In black-capped chickadees (Poecile atricapillus), a common garden experiment revealed that individuals from harsh environments significantly outperformed conspecifics from the milder southern population in a novel problem-solving task and also had larger telencephalons [47]. Harsh/novel environments may also select for life-history strategies that increase the value of adults over the value of offspring. For example, analyses of species introduced outside their native range has revealed that survival is not only more likely if the species is big-brained and innovative [4], but also if it prioritizes future over current reproduction by spreading the reproductive effort over many events [41,48]. This provides advantages in terms of bet-hedging and storage effect, thus reducing the costs of losing a breeding attempt under uncertain conditions and adaptive mismatches.
Although features of the external environment may be important in generating correlated evolution, almost all environmental conditions can also be exploited by specialized adaptations; in cold environments, an alternative strategy to deal with low temperatures and food scarcity is hibernation. This suggests that often it is not the environment itself but the way the animal interacts with it, along with constraints, that really matters to understand how the ability to innovate has evolved.
Lefebvre et al. [1] have suggested that opportunist–generalist lifestyles where individuals interact frequently with new food opportunities might favour the evolution of innovation components. In spatially and temporally heterogeneous environments, for instance, many animals rely on a variety of food sources that are too scarce or unstable over time to allow specialization. Food resources might not only differ in quality, but some might even contain toxins. Such a scenario should select for individuals that are more persistent in sampling and discriminating between different options and that are less impulsive in taking decisions [27], features that are essential for innovating [17]. Given that morphology can limit innovation by affecting the type and diversity of motor patterns exhibited by the animal [16,17], the unspecialized morphology typical of generalists should also facilitate innovative lifestyles.
The evolution of innovative lifestyles in ecological generalists can nonetheless be contingent on life-history strategy. In general, selection for the traits underlying innovations is expected to be more intense in long-lived species. Like with innovativeness, selection favouring generalism might be more intense in long-lived species that, despite exhibiting more risk-averse lifestyles, have more time to explore and adopt new ecological opportunities and are not so constrained to have small neural structures [17,28,37]. Nevertheless, not all theories link generalism to future returns strategies. Under the ‘time-limited disperser model’ [49], an animal's time budget will determine the costs of rejecting suboptimal resources. Because species that prioritize current reproduction have less time available for searching, the costs of rejecting suboptimal resource patches are increased, favouring a generalist lifestyle. Conversely, species that prioritize future over current reproduction (and thus have a low brood value) should be less time-limited, potentially leading to an increased specialization.
Although the association between ecological generalism and life history has been poorly investigated empirically, there is instead growing evidence that ecological generalism is associated with innovativeness [50,51]. In birds, in particular, habitat generalists have higher rates of consumer innovations (but not technical innovations), and diet generalists (which are not necessarily habitat generalists) have higher rates of both consumer and technical innovations [50]. To address whether ecological generalism also explains the link between innovativeness and lifespan, we estimated three key components of the foraging niche (i.e. habitat breadth, diet breadth and diversity of foraging techniques) using a new approach based on information theory [52].
In agreement with Ducatez et al. [50], species with broader habitat tolerances and broader diets had a consistently higher propensity to innovate, an effect that was not simply attributed to differences in geographical range size or other confounds (electronic supplementary material, tables S33 and S34). In contrast, the diversity of foraging techniques did not correlate with innovation frequency, despite evidence suggesting that innovation is greatly affected by motor diversity [16,17]. Interestingly, species with longer lifespan were also more likely to be habitat generalists (electronic supplementary material, table S35). Taken together, these results are consistent with the interpretation that ecological generalism can simultaneously affect life history and innovativeness by correlated evolution rather than exclusively by shared mechanisms.
6. Integrating findings
Our analyses identified relative brain size, developmental mode and ecological generalism as attributes potentially explaining why long-lived species tend to be more innovative. Conclusions are similar when using brood value instead of lifespan to quantify the extent to which species prioritize future over current reproduction (electronic supplementary material, tables S36–S52). To further understand the nature of such associations, we tested a number of causal scenarios with the phylogenetic path analysis proposed by von Hardenberg & Gonzalez-Voyer [35,36] (figure 3b; electronic supplementary material, figure S2). From all the scenarios investigated, only three appropriately fit the data according to d-separation criteria. In these scenarios, lifespan is directly affected by innovation propensity (although the direction could be the other way around) and both are in turn associated by the indirect and common effects of relative brain size and ecological generalism. In contrast, development does not act as a common cause linking innovativeness and lifespan. Although developmental mode affects lifespan, the generally assumed view that altricial species have larger brains than precocial species [9,53] is not supported when phylogenetic effects are taken into account. Developmental mode may explain a significant proportion of the variation in whole brain size in birds [53], but this likely reflects a phylogenetically conserved constraint rather than being a major agent of brain size selection.
7. Concluding remarks
Our analyses, coupled with previous evidence, suggest that in birds, (i) innovative propensity is linked to life histories that prioritize future over current reproduction, (ii) the link is in part caused by differences in brain size, and (iii) innovative propensity and life-history traits may evolve together in generalist species that frequently expose themselves to novel or unusual conditions.
The covariation between innovative propensity and life history could reflect the fact that both attributes have evolved together (either through correlated evolution or shared mechanisms) or that one is the cause of the other. Although our results do not allow us to really establish causalities, our path analyses suggest that all three scenarios are plausible. First, correlated selection may have favoured enhanced behavioural plasticity and low brood values (derived from long lives) in species constantly challenged by new problems such as ecological generalists. Second, large brains needed to innovate require longer developmental periods, and this may have constrained the evolution of innovativeness in species with a fast pace-of-life. Finally, a future returns strategy may have further selected in large-brained lineages when exposed to environmental changes that can be dealt with behaviourally. This last finding contributes to the current debate over why the brain is associated with a slow pace-of-life. In primates, Barton & Capellini [30] concluded that the general pattern of slower life histories in large-brained species is a direct consequence of developmental costs. While our results do not deny the existence of developmental costs, they nonetheless suggest that the benefits of innovation, as an expression of general behavioural plasticity that buffer individuals against extrinsic mortality, can also help us understand why brain size variation correlates with life histories.
Previous work has emphasized that the combination of traits that enhance innovativeness can be selected in environments that generate uncertainties and/or that frequently confront individuals with novel problems (reviewed in [17]). However, the possibility that under such environmental circumstances, innovativeness is more strongly favoured in animals that actively expose themselves to new challenges is less often recognized. Although we do not deny the importance of other selective scenarios, it seems plausible that behavioural and ecological plasticity might have reinforced each other in facilitating the evolution of innovative lifestyles in long-lived species. Indeed, most innovative animals, like primates, parrots and crows, tend to be ecological generalists. The importance of ecological generalism in explaining variation in innovativeness adds to growing evidence that innovativeness is not a specialized adaptation but more likely part of a more general adaptive system to cope with changes in the environment. It also highlights that understanding the evolution of innovativeness requires an eco-evo perspective in which the animals are not considered as passive agents of selection but, by constructing their own niches, as agents that largely contribute to shape the selective forces that act on them.
Selection rarely acts on single traits, but rather on suites of traits that respond to selection in a correlated way [54]. These combinations lead to emergent properties that cannot be anticipated by considering each trait separately. We propose that innovativeness is part of a slow pace-of-life syndrome to cope with environmental changes. Considering innovativeness as a complex of traits is not only theoretically plausible, but it is currently backed by important empirical evidence. Although the evolutionary origin of innovativeness is far from being resolved, recent theoretical and empirical progress provides the necessary advances to guide future research.
Supplementary Material
Acknowledgements
We thank Simon Reader, Kevin Laland and Emma Flynn for inviting us to the nice workshop that prompted this paper, Jarrod Hadfield and Alejandro González-Voyer for statistical advice, Alejandro González-Voyer and two anonymous reviewers for insightful comments on a previous version of the manuscript, and Simon Reader, Andrea Griffin, Julie Morand-Ferron, Denis Réale and Luc-Alain Giraldeau for discussions over the past years.
Data accessibility
The datasets supporting this article are available in the electronic supplementary material.
Authors' contributions
D.S., F.S., S.D. and L.L. conceived, designed and gathered data, D.S., F.S. and S.D. ran the analyses, D.S. wrote the paper, and F.S., S.D. and L.L. critically reviewed and approved it.
Competing interests
We have no competing interests.
Funding
This paper is part of the project CGL2013-47448-P from the Spanish Government to D.S.
References
- 1.Lefebvre L, Whittle P, Lascaris E, Finkelstein A. 1997. Feeding innovations and forebrain size in birds. Anim. Behav. 53, 549–560. ( 10.1006/anbe.1996.0330) [DOI] [Google Scholar]
- 2.Reader SM, Laland KN. 2003. Animal Innovation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Ramsey G, Bastian ML, van Schaik C. 2007. Animal innovation defined and operationalized. Behav. Brain Sci. 30, 393–407. ( 10.1017/S0140525X07002373) [DOI] [PubMed] [Google Scholar]
- 4.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducatez S, Audet JN, Lefebvre L. 2013. Independent appearance of an innovative feeding behaviour in Antillean bullfinches. Anim. Cogn. 16, 525–529. ( 10.1007/s10071-013-0612-4) [DOI] [PubMed] [Google Scholar]
- 6.Reader SM, Nover D, Lefebvre L. 2002. Locale-specific sugar packet opening by Lesser Antillean Bullfinches in Barbados. J. F. Ornithol. 73, 82–85. ( 10.1648/0273-8570(2002)073%5B0082:LSSPOB%5D2.0.CO;2) [DOI] [Google Scholar]
- 7.Lapiedra O, Sol D, Carranza S, Beaulieu JM. 2013. Behavioural changes and the adaptive diversification of pigeons and doves. Proc. R. Soc. B 280, 20122893 ( 10.1098/rspb.2012.2893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morand-Ferron J, Sol D, Lefebvre L. 2007. Food-stealing in birds: brain or brawn. Anim. Behav. 74, 1725–1734. ( 10.1016/j.anbehav.2007.04.031) [DOI] [Google Scholar]
- 9.Healy SD, Rowe C. 2007. A critique of comparative studies of brain size. Proc. R. Soc. B 274, 453–464. ( 10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teschke I, Cartmill EA, Stankewitz S, Tebbich S. 2011. Sometimes tool use is not the key: no evidence for cognitive adaptive specializations in tool-using woodpecker finches. Anim. Behav. 82, 945–956. ( 10.1016/j.anbehav.2011.07.032) [DOI] [Google Scholar]
- 11.Teschke I, Wascher CAF, Scriba MF, von Bayern AMP, Huml V, Siemers B, Tebbich S. 2013. Did tool-use evolve with enhanced physical cognitive abilities? Phil. Trans. R. Soc. B 368, 20120418 ( 10.1098/rstb.2012.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bird CD, Emery NJ. 2009. Insightful problem solving and creative tool modification by captive nontool-using rooks. Proc. Natl Acad. Sci. USA 106, 10 370–10 375. ( 10.1073/pnas.0901008106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reader SM, Laland KN. 2002. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA 99, 4436–4441. ( 10.1073/pnas.062041299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim. Behav. 78, 1001–1010. ( 10.1016/j.anbehav.2009.06.033) [DOI] [Google Scholar]
- 16.Griffin AS, Guez D. 2014. Innovation and problem solving: a review of common mechanisms. Behav. Process. 109, 121–134. ( 10.1016/j.beproc.2014.08.027) [DOI] [PubMed] [Google Scholar]
- 17.Sol D. 2015. The evolution of innovativeness: exaptation or specialized adaptation? In Animal creativity and innovation: research and theory (eds Kaufman A, Kaufman J), pp. 163–182. San Diego, CA: Academic Press. [Google Scholar]
- 18.Reader SM. 2007. Environmentally invoked innovations and cognition. Behav. Brain Sci. 30, 420–421. ( 10.1017/S0140525X07002518) [DOI] [Google Scholar]
- 19.Chiappe D, Gardner R. 2011. The modularity debate in evolutionary psychology. Theory Psychol. 22, 669–682. ( 10.1177/0959354311398703) [DOI] [Google Scholar]
- 20.Allman J, McLaughlin T, Hakeem A. 1993. Brain weight and life-span in primate species. Proc. Natl Acad. Sci. USA 90, 118–122. ( 10.1073/pnas.90.1.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Schaik CP, Deaner RO. 2003. Life history and cognitive evolution in primates. In Animal social complexity (eds de Waal FBM, Tyack PL), pp. 5–25. Cambridge, MA: Harvard University Press. [Google Scholar]
- 22.Ricklefs RE. 2004. The cognitive face of avian life histories. Wilson Bull. 116, 119–196. ( 10.1676/04-054) [DOI] [Google Scholar]
- 23.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Roff D. 2002. Life history evolution. Sunderland, MA: Sinauer Associates, INC. [Google Scholar]
- 25.Lewontin RC. 1965. Selection for colonizing ability. In The genetics of colonizing species (eds Baker H, Stebbins G), pp. 77–94. London, UK: Academic Press. [Google Scholar]
- 26.Sibly RM, Witt CC, Wright NA, Venditti C, Jetz W, Brown JH. 2012. Energetics, lifestyle, and reproduction in birds. Proc. Natl Acad. Sci. USA 109, 10 937–10 941. ( 10.1073/pnas.1206512109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sol D. 2009. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 5, 130–133. ( 10.1098/rsbl.2008.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sol D. 2009. The cognitive-buffer hypothesis for the evolution of large brains. In Cognitive ecology (eds Dukas R, Ratcliffe RM), pp. 111–134. Chicago, IL: Chicago University Press. [Google Scholar]
- 30.Barton RA, Capellini I. 2011. Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl Acad. Sci. USA 108, 6169–6174. ( 10.1073/pnas.1019140108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett PM, Owens IPF. 2002. Evolutionary ecology of birds: life histories, mating systems and extinction. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246. ( 10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre L. 2011. Taxonomic counts of cognition in the wild. Biol. Lett. 7, 631–633. ( 10.1098/rsbl.2010.0556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadfield J, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508. ( 10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Voyer A, von Hardenberg A. 2014. An introduction to phylogenetic path analysis. In Modern phylogenetic comparative methods and their application in evolutionary biology (ed. LZ Garamszegi), pp. 201–229 Berlin, Germany: Springer. [Google Scholar]
- 36.von Hardenberg A, Gonzalez-Voyer A. 2013. Disentangling evolutionary cause–effect relationships with phylogenetic confirmatory path analysis. Evolution 67, 378–387. ( 10.1111/j.1558-5646.2012.01790.x) [DOI] [PubMed] [Google Scholar]
- 37.Dukas R. 1998. Evolutionary ecology of learning. In Cognitive ecology: the evolutionary ecology of information processing and decision making (ed. R Dukas), pp. 129–174. Chicago, IL: University of Chicago Press. [Google Scholar]
- 38.Deaner RO, Barton RA, van Schaik CP. 2003. Primate brains and life histories: renewing the connection. In Primate life histories and socioecology (eds PM Kappeler, ME Pereira), pp. 233–265. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 39.Allman J. 2000. Evolving brains. New York, NY: Scientific American Library. [Google Scholar]
- 40.Saether B-E. 1988. Pattern of covariation between life-history traits of European birds. Nature 331, 616–617. ( 10.1038/331616a0) [DOI] [PubMed] [Google Scholar]
- 41.Sol D, Maspons J, Vall-llosera M, Bartomeus I, Garcia-Pena GE, Pinol J, Freckleton RP. 2012. Unraveling the life history of successful invaders. Science 337, 580–583. ( 10.1126/science.1221523) [DOI] [PubMed] [Google Scholar]
- 42.Bókony V, Lendvai ÃZ, Liker A, Angelier F, Wingfield JC, Chastel O, Lendvai AZ.. 2009. Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598. ( 10.1086/597610) [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Berthou E. 2001. On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J. Anim. Ecol. 70, 708–711. ( 10.1046/j.1365-2656.2001.00524.x) [DOI] [Google Scholar]
- 44.Hunt GR, Gray RD. 2007. Genetic assimilation of behaviour does not eliminate learning and innovation. Behav. Brain Sci. 30, 412–413. ( 10.1017/S0140525X07002439) [DOI] [Google Scholar]
- 45.Allman J, Hasenstaub A. 1999. Brains, maturation times, and parenting. Neurobiol. Aging 20, 447–454. ( 10.1016/S0197-4580(99)00076-7) [DOI] [PubMed] [Google Scholar]
- 46.Sol D. 2003. Behavioural flexibility: a neglected issue in the ecological and evolutionary literature? In Animal innovation (eds Reader SM, Laland KN), pp. 63–82. Oxford, UK: Oxford University Press. [Google Scholar]
- 47.Roth TC, LaDage LD, Pravosudov VV. 2010. Learning capabilities enhanced in harsh environments: a common garden approach. Proc. R. Soc. B 277, 3187–3193. ( 10.1098/rspb.2010.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sol D, González-Lagos C, Moreira D, Maspons J, Lapiedra O. 2014. Urbanisation tolerance and the loss of avian diversity. Ecol. Lett. 17, 942–995. ( 10.1111/ele.12297) [DOI] [PubMed] [Google Scholar]
- 49.Ward A. 1986. Optimal habitat selection in time-limited dispersers. Am. Nat. 129, 568–579. ( 10.1086/284658) [DOI] [Google Scholar]
- 50.Ducatez S, Clavel J, Lefebvre L. 2014. Ecological generalism and behavioural innovation in birds: technical intelligence or the simple incorporation of new foods? J. Anim. Ecol. 84, 79–89. ( 10.1111/1365-2656.12255) [DOI] [PubMed] [Google Scholar]
- 51.Overington SE, Griffin AS, Sol D, Lefebvre L. 2011. Are innovative species ecological generalists? A test in North American birds. Behav. Ecol. 22, 1286–1293. ( 10.1093/beheco/arr130) [DOI] [Google Scholar]
- 52.De Cáceres M, Sol D, Lapiedra O, Legendre P. 2011. A framework for estimating niche metrics using the resemblance between qualitative resources. Oikos 120, 1341–1350. ( 10.1111/J.1600-0706.2011.19679.x) [DOI] [Google Scholar]
- 53.Bennett PM, Harvey PH. 1985. Relative brain size and ecology in birds. J. Zool. 207, 151–169. ( 10.1111/j.1469-7998.1985.tb04920.x) [DOI] [Google Scholar]
- 54.Badyaev A, Ghalambor C. 2001. Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82, 2948–2960. ( 10.1890/0012-9658(2001)082%5B2948:EOLHAE%5D2.0.CO;2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available in the electronic supplementary material.