Abstract
Small animals are remarkably efficient climbers but comparatively poor runners, a well-established phenomenon in locomotor energetics that drives size-related differences in locomotor ecology yet remains poorly understood. Here, I derive the energy cost of legged locomotion from two complementary components of muscle metabolism, Activation–Relaxation and Cross-bridge cycling. A mathematical model incorporating these costs explains observed patterns of locomotor cost both within and between species, across a broad range of animals (insects to ungulates), for a wide range of substrate slopes including level running and vertical climbing. This ARC model unifies work- and force-based models for locomotor cost and integrates whole-organism locomotor cost with cellular muscle physiology, creating a predictive framework for investigating evolutionary and ecological pressures shaping limb design and ranging behaviour.
Keywords: biomechanics, energetics, locomotion
1. Introduction
The metabolic demands of walking, running and climbing affect the costs of foraging, predator avoidance and other essential tasks, providing a potentially powerful, integrative performance variable for investigating animal evolution. Yet, despite decades of laboratory study a unified model linking proximate mechanisms of muscle physiology to the whole-body locomotor cost of legged locomotion has remained elusive [1], limiting efforts to investigate selection pressures acting on locomotor anatomy and behaviour. Work-based models perform well for vertical climbing but fail to predict the cost of walking and running on level ground or changes in cost with speed and body size [1–5]. The ground force exerted by the limbs during each step predicts observed changes in the cost of running across species and speeds, but force-based models [6,7] ignore mechanical work and do not predict climbing costs.
Here, I develop and test a mathematical model predicting the energy cost of legged locomotion from established components of muscle physiology. Locomotor cost is primarily a function of muscle metabolism [1], and can therefore be partitioned into three metabolic components of muscle contraction: activation, cross-bridge cycling and relaxation [8–11]. Cross-bridge costs, sometimes called ‘maintenance costs’ [8–11], are associated with actin–myosin cross-bridge cycling and the development of muscle tension, and have been the focus of previous locomotor cost models [1,6,12]. Activation and relaxation costs (AR costs) are not associated with cross-bridge cycling and are instead related to other processes at the initiation and end of a contraction [8–11]. AR costs are typically ignored in locomotor cost models (but see [2,7,13]). However, the limited studies examining AR costs in cyclical contractions indicate that they account for a large portion of metabolic expenditure when muscles are stimulated repeatedly in contraction–relaxation cycles with short stimulation periods, as in legged locomotion (figure 1a).
Figure 1.
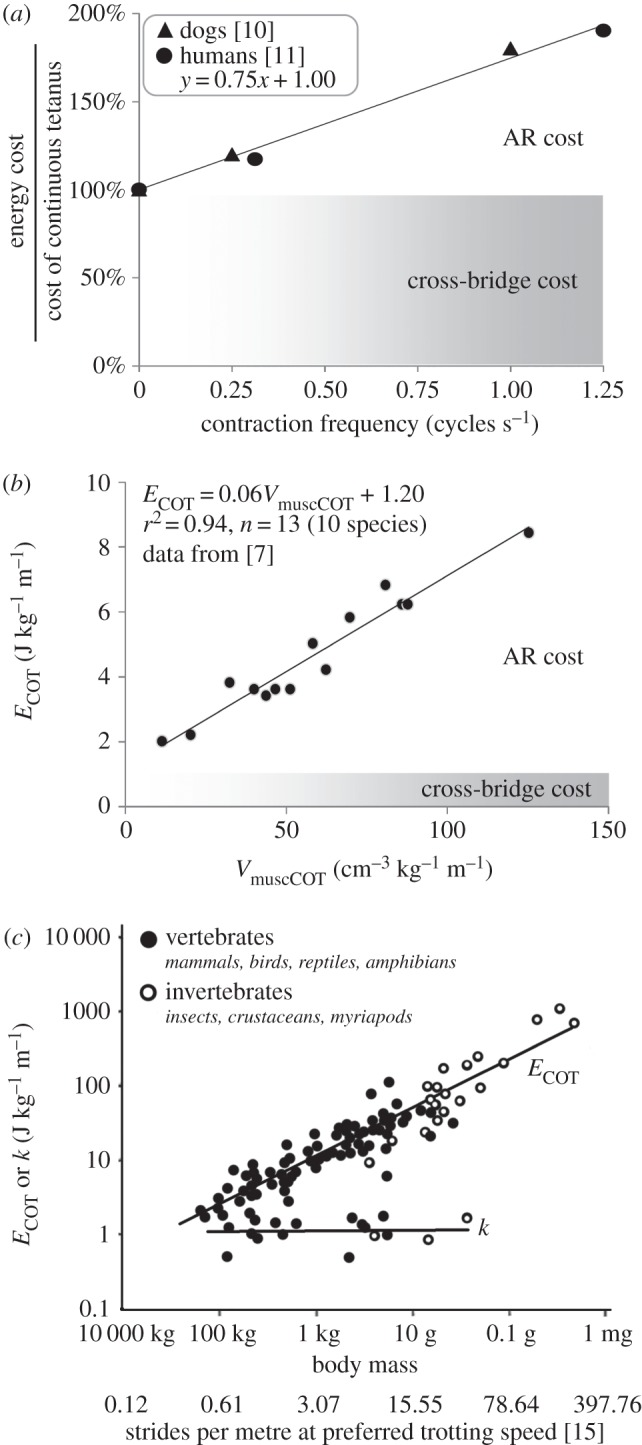
(a) AR and cross-bridge costs versus contraction cycling frequency, as a percentage of the cost of continuous contraction, from in situ studies; energy is normalized to muscle force (electronic supplementary material, text S3). (b) ECOT and VmuscCOT for running. Following equation (2.5), AR cost can be calculated from the slope of regression equation, 0.6VmuscCOT. Cross-bridge cost is given by the regression intercept, or as ECOT−AR cost. (c) Mass-specific metabolic cost, ECOT, and mechanical work, k, per metre versus body size and estimated stride cycles m−1; redrawn from [3].
2. Material and methods
The ARC model developed here combines AR and Cross-bridge cycling costs. During legged locomotion each stride is a contraction–relaxation cycle for the limb muscles involved, and AR costs are a product of the volume of muscle activated per stride, Vmusc, and stride frequency [10,11]. Cross-bridge costs are a product of the animal's total mechanical power, Pmech, and the metabolic cost rate per unit of mechanical power. Alexander [12] gives the metabolic cost of contracting muscle (ignoring AR costs) as FisovmaxΦ(v/vmax), where Fiso is the isometric contraction force of the active muscle fibres, vmax is maximum muscle shortening velocity and Φ(v/vmax) is a function relating the metabolic cost of contraction as a function of v/vmax ([12]; electronic supplementary material, figure S1). Summing AR and cross-bridge costs gives the ARC model prediction for the whole-animal rate of energy expenditure Ė,
| 2.1 |
where e is the energy consumed by AR processes per unit of Vmusc and Fiso/P is Fiso per unit of mechanical power.
(a). Activation–relaxation costs and force models
The AR cost term, eV̇musc, is determined by the rate of ground force production underlying force-based cost models [6,7] as follows. For each stride cycle, the mean vertical ground force, Fground, produced by the limbs during the period of foot–ground contact time, tc, must equal the product of body weight, Mg and stride period, T,
| 2.2 |
where j is the ground force generated per unit volume of activated limb muscle, which is independent of body size [14]. Multiplying equation (2.2) by stride frequency, T−1, gives the rate of muscle activation (i.e. the volume of muscle activated per stride multiplied by stride frequency; electronic supplementary material, text S1).
| 2.3 |
The above equation can be rewritten using the AR cost term, eV̇musc, from equation (2.1),
| 2.4 |
where c is the metabolic energy consumed per unit Fground and e = cj. The right side of equation (2.4) is identical to equation (1) in Kram and Taylor's seminal force-based model of locomotor cost [6]. However, as derived above, the term cMgtc−1 describes AR costs (i.e. the volume of muscle activated per second), not the rate of cross-bridge cycling initially proposed [6] as the mechanism linking tc to locomotor cost. Force-based models of locomotor cost [6,7] are, in effect, AR cost models.
(b). Activation–relaxation and cross-bridge model cost of transport
Dividing equation (2.1) by M and travel speed, B, gives the mass-specific energy cost per distance travelled, ECOT, often called the ‘cost of transport’. Since B is equal to step length, d, divided by tc, dividing equation (2.4) by M and B gives the AR cost of transport, eVmuscCOT = cgd−1. Pmech increases nearly linearly with MB, with only slight curvilinearity with respect to B ([2]; electronic supplementary material, text S2 and figure S2). Thus, Pmech ≈ kMB, where k is the mass-specific mechanical work per distance travelled, and dividing the cross-bridge cost term in equation (2.1) by M and B gives kFiso/PvmaxΦ(v/vmax). Hence,
| 2.5 |
As shown in figure 1b, a model of this form fits the available data for ECOT and VmuscCOT quite well. While the ARC model is consistent with previous models and data, it makes additional predictions that can be tested against observed locomotor cost within and between species.
3. Results
One test of the ARC model is whether the ratio of AR : cross-bridge cost observed for isolated muscle contracting at a given cycling frequency matches the ratio observed in an animal running at that stride frequency (electronic supplementary material, text S3). A 100 kg animal running on level ground has a predicted stride frequency of 1.84 Hz at its preferred trotting speed [15]. The limited data from in situ studies of isolated muscle suggest a cycling frequency of 1.84 Hz produces an AR : cross-bridge ratio of approximately 1.38 (figure 1a). For comparison, the predicted AR : cross-bridge ratio for a running 100 kg animal, calculated using the observed interspecific relationship between VmuscCOT and ECOT (figure 1b; electronic supplementary material, text S3), is approximately 1.34, within 5% of the estimate from in situ studies. Whole-animal measures of locomotor cost are consistent with measures of AR cost in isolated muscle.
Human walking and running provide a second test (electronic supplementary material, text S4). Inverse dynamics yields VmuscCOT values for human walking and running of 11.4 and 32.4 cm3 kg−1 m−1, respectively [7], which, using the ECOT : VmuscCOT regression in figure 1b, translates to AR costs of 0.7 and 1.9 J kg−1 m−1 (figure 1b). Subtracting these AR costs from human ECOT values yields a cross-bridge cost of 1.3 and 1.9 J kg−1 m−1 for walking and running, respectively, values consistent with the magnitude of total mechanical work during both walking (approx. 0.7 J kg−1 m−1) and running (approx. 1.5 J kg−1 m−1) and the difference in mechanical work between gaits [4]. Estimated AR costs account for approximately 33% of ECOT for human walking and approximately 50% for running, which may explain the discrepancy between mechanical work and ECOT [4].
Allometric comparisons of ECOT for running and climbing provide a final set of tests for the ARC model (electronic supplementary material, text S5). Step length, d, is proportional to effective limb length, which scales with M0.34 [16]. Since eVmuscCOT = cgd−1, AR cost is predicted to increase as zM−0.34, where z is a constant. Mass-specific mechanical work per distance, k, is independent of body mass during running ([2,3]; figure 1c), and Fiso/P and Φ(v/vmax) are expected to be independent of mass as well (electronic supplementary material, text S5). However, vmax scales with M−0.12 [17], and cross-bridge costs, kFiso/PvmaxΦ(v/vmax), are therefore predicted to decrease with body size as wM−0.12, where w is a constant. To determine z and w, we draw on the empirically determined estimates of cross-bridge cost and AR : cross-bridge cost ratio in figure 1a,b, and find these conditions are met when z = 8, w = 2 and ECOT has units J kg−1 m−1,
| 3.1 |
The ARC model (equation (3.1)) fits observed ECOT for mammals (figure 2a), and falls within the 95% confidence interval of the allometric relationship reported for a larger dataset including birds [18]. The ARC model remains in agreement with observed ECOT when varying z and w ± 20% and, consequently, varying the AR : cross-bridge cost ratio –33% to +50% (figure 2a; electronic supplementary material, text S6).
Figure 2.
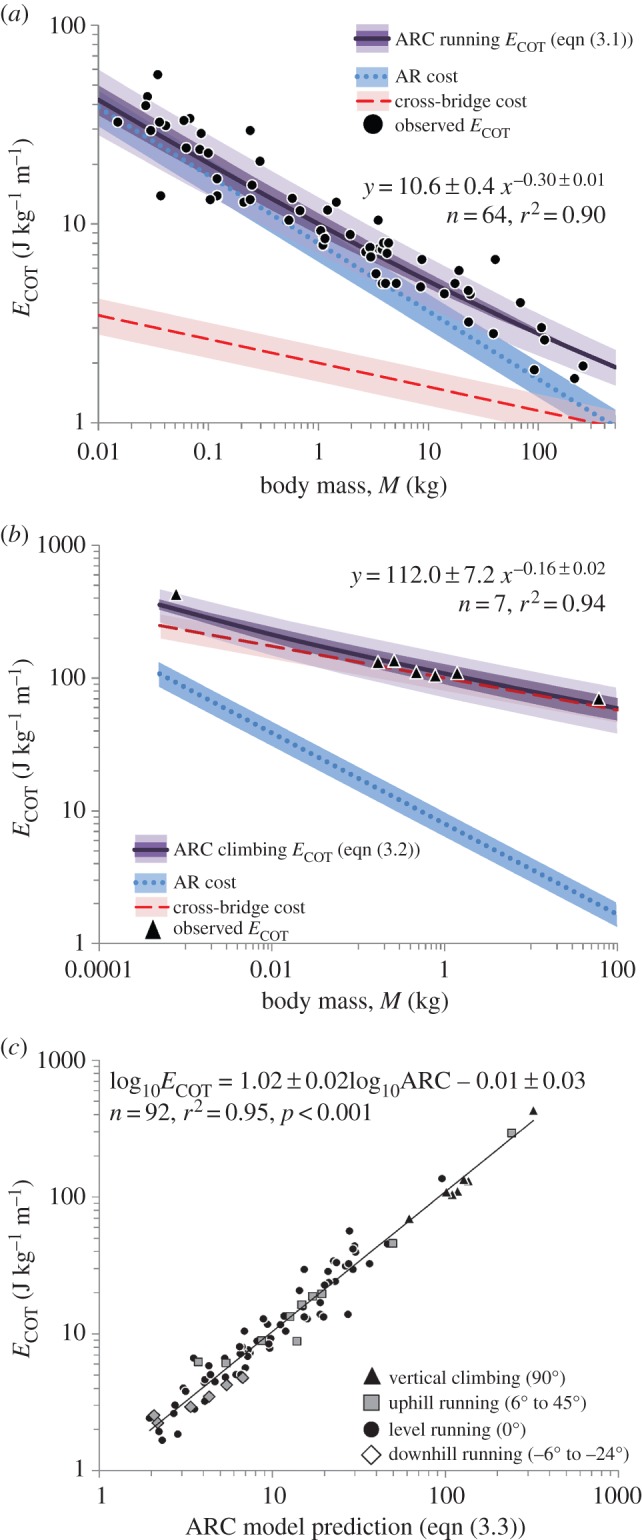
(a) Observed ECOT and ARC predictions (equation (3.1)) for level running. Shaded regions for AR and cross-bridge costs reflect varying z and w ± 20%. The darker shaded region for the ARC ECOT prediction reflects varying AR : cross-bridge ratio −33 to +50%; the light shaded region for extends ARC ECOT an additional ± 20% (electronic supplementary material, text S6). (b) Observed ECOT and ARC predictions (equation (3.2)) for vertical climbing. Shaded regions as in (a) and (c). Observed ECOT and ARC predictions (equation (3.3)) for a range of species and slopes. Data in electronic supplementary material, table S1. (Online version in colour.)
During vertical climbing, k must be at least 9.8 J kg−1 m−1, approximately 10-times greater than during level running. The cost function Φ(v/vmax) will also increase as higher ratios of v/vmax are used, such that w is estimated to be approximately 100 for vertical climbing (electronic supplementary material, text S5). AR costs are expected to remain largely unchanged, as d is similar on level and vertical substrates [19,20]. We can thus modify equation (3.1) for vertical climbing as
| 3.2 |
The trend line for observed climbing data (figure 2b) includes the ARC model (equation (3.2)) and is consistent with the weak allometry reported for non-human primates [5] and the relatively poor climbing efficiencies reported for cockroaches [19]. As with level running (equation (3.1)), the model remains in agreement with observed ECOT when varying z and w ± 20% and varying the AR : cross-bridge cost ratio between −33% and +50% (figure 2b; electronic supplementary material, text S6).
A generalized ARC model can be written that accounts for the change in cross-bridge cost at all inclines, essentially combining equations (3.1) and (3.2) (electronic supplementary material, text S5).
| 3.3 |
The above equation is a U-shaped function with respect to incline, and fits data from a broad range of species (cockroaches to zebu cattle) and slopes (−24° to +90°) with a regression slope of 1, an intercept of 0 and a high coefficient of determination (r2 = 0.95; figure 2c; electronic supplementary material, text S5).
4. Discussion
The ARC model provides a unifying framework for several well established but poorly understood [1] phenomena in terrestrial locomotion. The greater ECOT among smaller animals during level running [18] results from their shorter limbs, faster stride cycles and greater AR costs (figure 2a). Force-based cost models [6,7] capture this allometry because AR costs predominate during level running. By contrast, mechanical work predicts ECOT during vertical climbing, in which cross-bridge costs predominate. The weak scaling of cross-bridge costs results in similar climbing ECOT for most species [5] but poor climbing efficiencies at extremely small sizes [19]. Because of their high AR costs, small animals have low apparent efficiencies during running [3] but show a small marginal increase in cost during climbing, making them appear remarkably efficient climbers [5,19]. Conversely, large animals, with long legs and low AR costs, appear to be efficient runners but inefficient climbers.
Owing to the distinct allometries of AR and cross-bridge costs (figure 2a,b), mechanical work accounts for a much larger portion of running and walking ECOT for large animals. This allometry may explain why many large species, notably humans [4], have evolved a suite of work-minimizing kinematic strategies (e.g. pendular exchange of potential and kinetic energy) that appear to be absent in smaller taxa [21]. The greater contribution of mechanical work, and the weak curvilinearity in Pmech with speed, may also account for the curvilinearity in Ė with running speed reported for some large species (e.g. horses [22]; electronic supplementary material, text S2). Minimizing summed AR and cross-bridge costs may shape kinematics and kinetics in humans and other species [13].
Tests of the ARC model here are limited by the available experimental data. Additional study of in situ or isolated muscle is needed to test whether the linear relationship between AR cost and cycling frequency in figure 1a persists at the faster cycling frequencies used by smaller species (cf. figure 1a,c) as assumed here. Simultaneous measurements of Pmech and Vmusc across a range of species are also needed to test the model's allometric predictions (equation (3.1)–(3.3)). Such work may enable us to either integrate or more confidently rule out other proposed mechanisms (e.g. cross-bridge cycling frequency [6]) for explaining variation in locomotor cost.
While developed for legged terrestrial locomotion, the ARC model may prove to be a useful framework for investigating the energetics of other locomotor modes. For example, wing beat frequencies among birds and bats scale with negative allometry [23], such that smaller species have higher cycling frequencies and greater AR costs; an ARC model for flight would thus predict greater estimated efficiencies for larger flyers, consistent with empirical observation [1]. The energetics of other repetitive, muscle-driven tasks, such as tool manufacture and use or burrowing, may likewise be amenable to investigation with an ARC model. By linking whole-organism energetics to the fundamental metabolic properties of muscle, a conservative tissue [8], the ARC model may be a powerful tool for investigating the evolution of limb anatomy in a broad range of lineages, including our own.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Daniel Lieberman, David Raichlen, James Usherwood and four reviewers provided comments and discussions that improved this paper.
Data accessibility
All data for figure 2 are in the electronic supplementary material, table S1.
Competing interests
The author declares no competing interests.
Funding
This work was supported by Hunter College and the City University of New York.
References
- 1.Alexander RM. 2005. Models and the scaling of energy costs for locomotion. J. Exp. Biol. 208, 1645–1652. ( 10.1242/jeb.01484) [DOI] [PubMed] [Google Scholar]
- 2.Heglund NC, Fedak MA, Taylor CR, Cavagna GA. 1982. Energetics and mechanics of terrestrial locomotion. IV. Total mechanical energy changes as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 57–66. [DOI] [PubMed] [Google Scholar]
- 3.Full RJ, Tu MS. 1991. Mechanics of a rapid running insect: two-, four- and six-legged locomotion. J. Exp. Biol. 156, 215–231. [DOI] [PubMed] [Google Scholar]
- 4.Willems PA, Cavagna GA, Heglund NC. 1995. External, internal and total work in human locomotion. J. Exp. Biol. 198, 379–393. [DOI] [PubMed] [Google Scholar]
- 5.Hanna JB, Schmitt D, Griffin TM. 2008. The energetic cost of climbing in primates. Science 320, 898 ( 10.1126/science.1155504) [DOI] [PubMed] [Google Scholar]
- 6.Kram R, Taylor CR. 1990. Energetics of running: a new perspective. Nature 346, 265–267. ( 10.1038/346265a0) [DOI] [PubMed] [Google Scholar]
- 7.Pontzer H, Allen V, Hutchinson JR. 2009. Biomechanics of running indicates endothermy in bipedal dinosaurs. PLoS ONE 4, e7783 ( 10.1371/journal.pone.0007783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woledge RC, Curtin NA, Homsher E. 1985. Energetic aspects of muscle contraction. Monogr. Physiol. Soc. 41, 1–357. [PubMed] [Google Scholar]
- 9.Homsher E, Kean CJ. 1978. Skeletal muscle energetics and metabolism. Annu. Rev. Physiol. 40, 93–131. ( 10.1146/annurev.ph.40.030178.000521) [DOI] [PubMed] [Google Scholar]
- 10.Hogan MC, Ingham E, Kurdak SS. 1998. Contraction duration affects metabolic energy cost and fatigue in skeletal muscle. Am. J. Physiol. 274, E397–E402. [DOI] [PubMed] [Google Scholar]
- 11.Bergstrom M, Hultmann E. 1988. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J. Appl. Physiol. 65, 1500–1505. [DOI] [PubMed] [Google Scholar]
- 12.Alexander RM. 1997. Optimum muscle design for oscillatory movement. J. Theor. Biol. 184, 253–259. ( 10.1006/jtbi.1996.0271) [DOI] [PubMed] [Google Scholar]
- 13.Hubel T, Usherwood JR. 2015. Children and adults minimise activated muscle volume by selecting gait parameters that balance gross mechanical power and work demands. J. Exp. Biol. 218, 2830–2839. ( 10.1242/jeb.122135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biewener AA. 1989. Scaling body support in mammals: limb posture and muscle mechanics. Science 245, 45–48. ( 10.1126/science.2740914) [DOI] [PubMed] [Google Scholar]
- 15.Heglund NC, Taylor CR. 1988. Speed, stride frequency, and energy cost per stride: how do they change with body size and gait? J. Exp. Biol. 138, 301–318. [DOI] [PubMed] [Google Scholar]
- 16.Pontzer H. 2007. Limb length and the scaling of locomotor cost in terrestrial animals. J. Exp. Biol. 210, 1752–1761. ( 10.1242/jeb.002246) [DOI] [PubMed] [Google Scholar]
- 17.Medler S. 2002. Comparative trends in shortening velocity and force production in skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, 368–378. ( 10.1152/ajpregu.00689.2001) [DOI] [PubMed] [Google Scholar]
- 18.Taylor CR, Heglund NC, Maloiy GMO. 1982. Energetics and mechanics of terrestrial locomotion: I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 1–21. ( 10.1146/annurev.ph.44.030182.000525) [DOI] [PubMed] [Google Scholar]
- 19.Full RJ, Tullis A. 1991. Energetics of ascent: insects on inclines. J. Exp. Biol. 149, 301–317. [DOI] [PubMed] [Google Scholar]
- 20.Isler K. 2005. 3D-kinematics of vertical climbing in hominoids. Am. J. Phys. Anthropol. 126, 66–81. ( 10.1002/ajpa.10419) [DOI] [PubMed] [Google Scholar]
- 21.Reilly SM, McElroy EJ, Biknevicius AR. 2007. Posture, gait and the ecological relevance of locomotor costs and energy-saving mechanisms in tetrapods. Zoology 110, 271–289. ( 10.1016/j.zool.2007.01.003) [DOI] [PubMed] [Google Scholar]
- 22.Hoyt DF, Taylor CR. 1981. Gait and the energetics of locomotion in horses. Nature 292, 239–240. ( 10.1038/292239a0) [DOI] [Google Scholar]
- 23.Lindhe Norberg UM, Norberg RA. 2012. Scaling of wingbeat frequency with body mass in bats and limits to maximum bat size. J. Exp. Biol. 215, 711–722. ( 10.1242/jeb.059865) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for figure 2 are in the electronic supplementary material, table S1.


