Abstract
When individuals mate outside the pair bond, males should employ behaviours such as aggression or vocal displays (e.g. duetting) that help assure paternity of the offspring they care for. We tested whether male paternity was associated with aggression or duetting in the red-backed fairy-wren, a species exhibiting high rates of extra-pair paternity. During simulated territorial intrusions, aggression and duetting were variable among and repeatable within males, suggesting behavioural consistency of individuals. Males with quicker and stronger duet responses were cuckolded less often than males with slower and weaker responses. In contrast, physical aggression was not correlated with male paternity. These results suggest that either acoustic mate guarding or male–female vocal negotiations via duetting lead to increased paternity assurance, whereas physical aggression does not.
Keywords: duetting, aggression, extra-pair mating, fairy-wren
1. Introduction
The majority of socially monogamous bird species exhibit extra-pair mating [1]. In species with paternal care, males should evolve behaviours that help assure paternity of offspring produced by their social mates [2]. In territorial species, aggression in response to intruders may serve to repel rivals [3] and decrease the likelihood that the male is cuckolded [4]. Duetting, the synchronous combination of male and female vocalizations [5], may also decrease extra-pair mating by signalling commitment between pair members or by directly guarding against extra-pair copulations [6–8]. However, the relative influence of aggression and vocal displays on paternity has never been experimentally tested and is key to understanding the evolution of promiscuity.
We tested whether behavioural responses to simulated territorial intrusions were correlated with genetic paternity in a population of red-backed fairy-wrens (Malurus melanocephalus), an Australian passerine. Males foray onto neighbouring territories pursuing extra-pair females [9,10], eliciting a response from the resident male including physical aggression and song. We used artificial mounts and song playback to simulate intrusions and recorded the aggressive and duetting responses of territory holders. We predicted a negative association between the proportion of extra-pair young (EPY) in a male's nest and either aggression, duetting or both, depending on which behaviours affect paternity allocation.
2. Material and methods
(a). Simulated territorial intrusions
We conducted these experiments in Samsonvale, Queensland (GPS = 27°16′ S, 152°51′ E). We presented an artificial mount and song playback on territories and video recorded the behaviour of the territorial pair (for details, see [11] and the electronic supplementary material). We reviewed the videos and quantified attacks, songs and latency to/duration of approach to various distances (table 1). Because there are two subspecies that differ in plumage (red versus orange) and song [11–14], we presented six different treatments representing combinations of local, foreign and heterospecific plumage and song. We used principal components analysis (PCA) of two separate subsets of behaviours to create one composite score representing physical aggression towards the mount (‘Aggression PC1’) and one representing duetting behaviour (‘Duet PC1’, table 1). Red-backed fairy-wrens are facultative cooperative breeders, but we only tested older males (i.e. after-second-year) exhibiting orange–black nuptial plumage, with no auxiliaries.
Table 1.
Two PCAs used to quantify aggression and duetting. A dash indicates that the behaviour was not included in that PCA.
| Aggression PC1 | Duet PC1 | |
|---|---|---|
| eigenvalue | 6.72 | 2.45 |
| per cent variation | 67.2 | 81.5 |
| time spent within 10 m | 0.35 | — |
| time spent within 5 m | 0.37 | — |
| time spent in mount bush | 0.36 | — |
| time spent within 0.5 m | 0.33 | — |
| time spent attacking mount | 0.19 | — |
| latency to 10 m | −0.26 | — |
| latency to 5 m | −0.31 | — |
| latency to mount bush | −0.35 | — |
| latency to 0.5 m | −0.34 | — |
| latency to attack mount | −0.27 | — |
| total duets | — | 0.56 |
| ratio duets : solos | — | 0.59 |
| latency to duet | — | −0.58 |
(b). Repeatability of aggression and duetting
We used three subsets of the data to analyse repeatability across multiple trials to confirm that behaviours were representative of a natural response and to determine temporal consistency. Because the PCA data were non-Gaussian, we calculated repeatability using an overdispersed binomial generalized linear mixed model in the R v. 3.1.1 [15] package rptR [16], followed by randomization tests. First, we chose the subset of males that received all six treatments (N = 44 males) and calculated repeatability in both aggression and duetting. Then, because previous analyses revealed that response was strongest to the local song type but equivalent between red and orange mount colour [11], we calculated repeatability in aggression again on the subset of males that received local song with red or orange mount (N = 51 males). Finally, we estimated repeatability in duetting again, using this same restricted subset, plus the female present (N = 18 males). In summary, we estimated repeatability of aggression and duetting twice: once with a large dataset including responses to all six trials, and once with a narrower subset including only the two trials eliciting the strongest responses most representative of a typical territorial intrusion.
(c). Aggression, duetting and cuckoldry
We assessed paternity using seven microsatellite loci ([17], electronic supplementary material). To explore relationships between aggression, duetting, and cuckoldry, we ran two binomial generalized linear models with logit-link functions using the R v. 3.1.1 [15] package lme4. For aggression, proportion of EPY in a male's nest was the response variable, and average Aggression PC1 and trial date were fixed effects. This model used the two-trial dataset for males with at least one nest (N = 35). For duetting, proportion of EPY in a male's nest was the response variable, and average Duet PC1 and trial date were fixed effects. This model used the two-trial, female-present dataset for males with at least one nest (N = 13).
3. Results
We assigned genetic paternity to 97% (181/186) of offspring, and the five unassigned offspring were confirmed EPY due to low assignment probability with their social father. Forty-seven per cent of offspring (88/186) were EPY, and 60% (44/73) of nests contained at least one EPY.
Both male aggression and duetting behaviour were significantly repeatable across the six- and two-trial datasets (table 2). Both behaviours were marginally, but non-significantly, more repeatable when analysed across the two trials expected to elicit strong, comparable responses.
Table 2.
Repeatability of aggression and duetting in both the six-trial (all combinations of mount and song) and two-trial (red or orange mount and local song) datasets.
| behaviour | dataset | N (males) | R | 95% CI | p |
|---|---|---|---|---|---|
| Aggression PC1 | six-trial | 44 | 0.29 | 0.09–0.44 | 0.001 |
| Aggression PC1 | two-trial | 51 | 0.32 | 0.00–0.61 | 0.001 |
| Duet PC1 | six-trial | 44 | 0.24 | 0.00–0.47 | 0.001 |
| Duet PC1 | two-trial | 18 | 0.29 | 0.00–0.72 | 0.03 |
There was a significant negative relationship between Duet PC1 and proportion of EPY in a male's nest (estimate = −4.44, z = −2.37, p = 0.018, figure 1a). In contrast, there was no significant association between Aggression PC1 and proportion of EPY in a male's nest (estimate = −0.08, z = −0.11, p = 0.913, figure 1b). Trial date had no significant effect on either model (duet model: estimate = 0.04, z = 0.83, p = 0.406; aggression model: estimate = −0.01, z = −0.60, p = 0.547).
Figure 1.
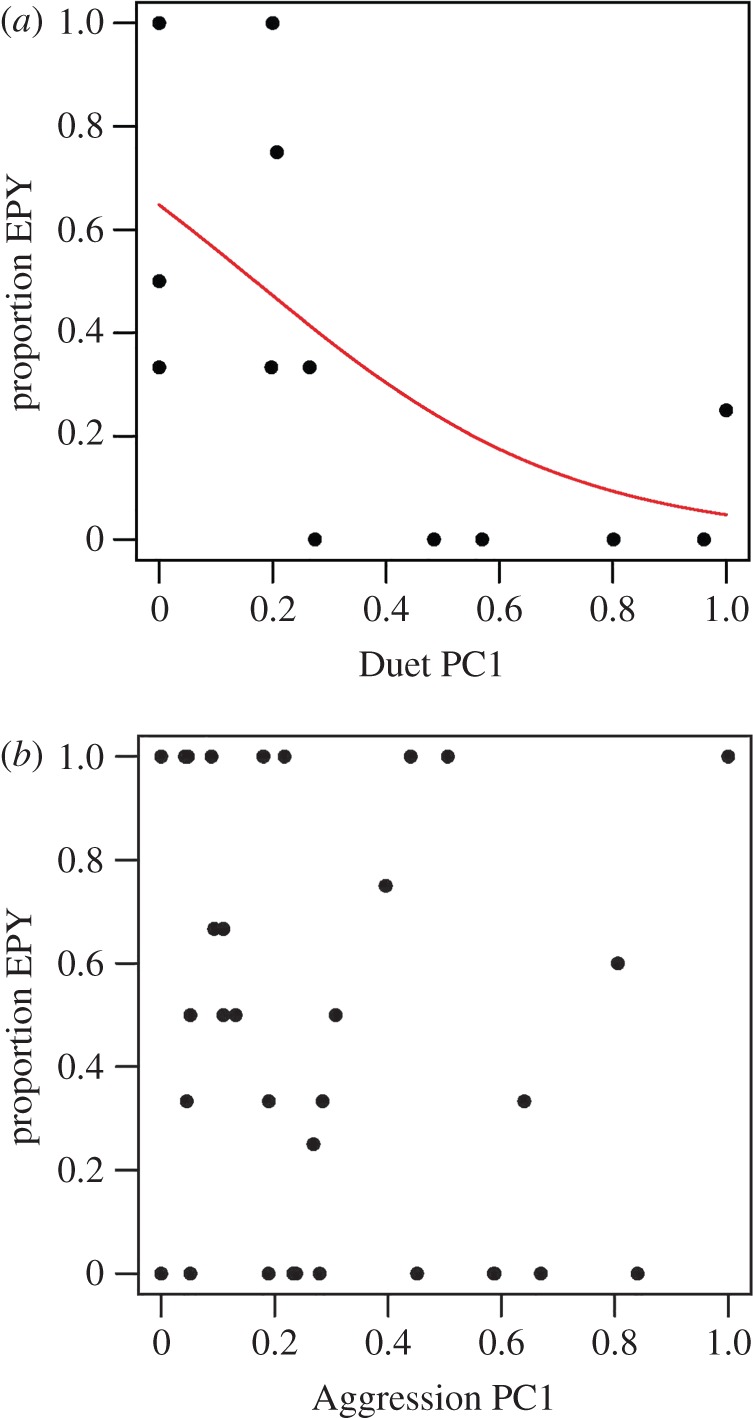
Relationships between duetting (a), aggression (b) and proportion of EPY in a male's nest. x-values were scaled 0–1 for visualization only; scaling did not transform the data. (Online version in colour.)
4. Discussion
When presented with simulated territorial intrusions, male red-backed fairy-wrens responded with physical aggression and duetting, both of which were repeatable across variable contexts, including heterospecific mounts and songs. This pattern suggests that although males modify their aggressive and vocal responses to some extent, there are consistent among-individual differences in both of these behaviours [18]. Here, we examined the hypothesis that they are a behavioural strategy to increase paternity assurance.
Aggression was not significantly associated with cuckoldry (figure 1b), suggesting that aggressive males were not particularly successful at deterring rivals from copulating with their mates. This pattern could arise if extra-pair copulations are initiated by females off-territory or occur pre-dawn [19,20]. Aggression may be more effective at defending resources on the territory rather than mates or may maintain territory boundaries.
In contrast, males with a fast and strong duet response were cuckolded significantly less often by their social mates (figure 1a). There are several reasons a strong duet response could relate to high paternity assurance. First, if males acoustically mate guard, those with stronger duet responses may more effectively mask their female's acoustic signal that would otherwise attract rivals [6–8]. Because we did not have data on duet initiation, we could not determine if females initiated duets that were joined by males, as would be predicted by this hypothesis. Nonetheless, previous work on another population of red-backed fairy-wrens failed to demonstrate acoustic mate guarding [21,22]. Another possibility is that duetting is a general ‘keep out’ signal produced by the pair to prevent usurpation of a partner or pair bond position [23]. However, previous work suggested that duetting functions mostly to establish and maintain the territory rather than deter intruding males per se [21]. Finally, duetting may strengthen the pair bond, especially if the female joins the male's song to signal her commitment [24–26]. These hypotheses are not mutually exclusive, and duetting may serve multiple functions. A forthcoming study of male versus female duet initiation/joining and paternity will help distinguish among these hypotheses. In red-backed fairy-wrens, there is support for territory establishment and defence [21,22], but the results presented here suggest that strong duetting response is also associated with increased paternity assurance. This pattern has been inferred previously from behavioural evidence or life-history trends [27–29], but this study is, we believe, the first to demonstrate a clear link based on within-population variance in duetting and genetic paternity. Finally, this result contrasts with a recent study on crimson-breasted shrikes that found a positive relationship between duetting and cuckoldry [30], further supporting multiple functions of duetting across species.
Supplementary Material
Supplementary Material
Acknowledgements
Jenélle Dowling, Derrick Thrasher and Searcy/Uy laboratory members improved the manuscript. Amanda Corwin and Mateusz Dziekan transcribed videos. Queensland University of Technology and Seqwater provided field support.
Ethics
We secured approval from animal ethics and permitting agencies (Cornell IACUC no. 0105, James Cook University Ethics no. A1340, Queensland SPP no. WISP07773610).
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
All authors designed the study, revised and approved the manuscript, and are accountable for this work. D.T.B. and E.I.G. collected the data. D.T.B. analysed the data and wrote the manuscript.
Competing interests
We have no competing interests.
Funding
Funding and equipment were provided by Macaulay Library, Cornell Lab of Ornithology and NSF grant no. 0964826 to M.S.W.
References
- 1.Griffith SC, Owens IPF, Thuman KA. 2008. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 2.Kokko H, Morrell LJ. 2005. Mate guarding, male attractiveness, and paternity under social monogamy. Behav. Ecol. 16, 724–731. ( 10.1093/beheco/ari050) [DOI] [Google Scholar]
- 3.Mays HL Jr, Hopper KR. 2004. Differential responses of yellow-breasted chats, Icteria virens, to male and female conspecific model presentations. Anim. Behav. 67, 21–26. ( 10.1016/j.anbehav.2003.01.003) [DOI] [Google Scholar]
- 4.Raouf SA, Parker PG, Ketterson ED, Nolan V, Ziegenfus C. 1997. Testosterone affects reproductive success by influencing extra–pair fertilizations in male dark–eyed juncos (Aves: Junco hyemalis). Proc. R. Soc. Lond. B 264, 1599–1603. ( 10.1098/rspb.1997.0223) [DOI] [Google Scholar]
- 5.Farabaugh SM. 1982. The ecological and social significance of duetting. In Acoustic communication in birds, vol. 2 (eds Kroodsma DE, Miller DH), pp. 85–124. New York, NY: Academic Press. [Google Scholar]
- 6.Hall ML. 2004. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430. ( 10.2307/25063377) [DOI] [Google Scholar]
- 7.Hall ML. 2009. A review of vocal duetting in birds. In Advances in the study of behavior (eds Naguib M, Janik VM), pp. 67–121. Burlington, MA: Academic Press. [Google Scholar]
- 8.Dahlin CR, Benedict L. 2014. Angry birds need not apply: a perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 120, 1–10. ( 10.1111/eth.12182) [DOI] [Google Scholar]
- 9.Karubian J. 2002. Costs and benefits of variable breeding plumage in the red-backed fairy-wren. Evolution 56, 1673–1682. ( 10.1554/0014-3820(2002)056[1673:CABOVB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 10.Potticary AL, Dowling JL, Barron DG, Baldassarre DT, Webster MS. 2016 Subtle benefits of cooperation to breeding males of Malurus melanocephalus. Auk 133. [Google Scholar]
- 11.Greig EI, Baldassarre DT, Webster MS. 2015. Differential rates of phenotypic introgression are associated with male behavioral responses to multiple signals. Evolution 69, 2602–2612. ( 10.1111/evo.12756) [DOI] [PubMed] [Google Scholar]
- 12.Baldassarre DT, Thomassen HA, Karubian J, Webster MS. 2013. The role of ecological variation in driving divergence of sexual and non-sexual traits in the red-backed fairy-wren (Malurus melanocephalus). BMC Evol. Biol. 13, 75 ( 10.1186/1471-2148-13-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greig EI, Webster MS. 2013. Spatial decoupling of song and plumage generates novel phenotypes between 2 avian subspecies. Behav. Ecol. 24, 1004–1013. ( 10.1093/beheco/art005) [DOI] [Google Scholar]
- 14.Baldassarre DT, White TA, Karubian J, Webster MS. 2014. Genomic and morphological analysis of a semipermeable avian hybrid zone suggests asymmetrical introgression of a sexual signal. Evolution 68, 2644–2657. ( 10.1111/evo.12457) [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 16.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 17.Baldassarre DT, Webster MS. 2013. Experimental evidence that extra-pair mating drives asymmetrical introgression of a sexual trait. Proc. R. Soc. B 280, 20132175 ( 10.1098/rspb.2013.2175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 19.Double M, Cockburn A. 2000. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. Lond. B 267, 465–470. ( 10.1098/rspb.2000.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockburn A, Dalziell AH, Blackmore CJ, Double MC, Kokko H, Osmond HL, Beck NR, Head ML, Wells K. 2009. Superb fairy-wren males aggregate into hidden leks to solicit extragroup fertilizations before dawn. Behav. Ecol. 20, 501–510. ( 10.1093/beheco/arp024) [DOI] [Google Scholar]
- 21.Dowling JL, Webster MS. 2013. The form and function of duets and choruses in red-backed fairy-wrens. EMU 113, 282–293. ( 10.1071/MU12082) [DOI] [Google Scholar]
- 22.Dowling J, Webster MS. 2015. An experimental test of duet function in a fairy-wren (Malurus) with moderate cuckoldry rates. Behav. Ecol. 27, 228–236. ( 10.1093/beheco/arv144) [DOI] [Google Scholar]
- 23.Sonnenschein E, Reyer H-U. 1983. Mate-guarding and other functions of antiphonal duets in the slate-coloured boubou (Laniarius funebris). Ethology 63, 112–140. ( 10.1111/j.1439-0310.1983.tb00083.x) [DOI] [Google Scholar]
- 24.Hall ML. 2006. Convergent vocal strategies of males and females are consistent with a cooperative function of duetting in Australian magpie-larks. Behaviour 143, 425–449. ( 10.2307/4536354) [DOI] [Google Scholar]
- 25.Hall ML, Magrath RD. 2007. Temporal coordination signals coalition quality. Curr. Biol. 17, R406–R407. ( 10.1016/j.cub.2007.04.022) [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Cáceres KD. 2015. Plain wrens Cantorchilus modestus zeledoni adjust their singing tempo based on self and partner's cues to perform precisely coordinated duets. J. Avian Biol. 46, 361–368. ( 10.1111/jav.00575) [DOI] [Google Scholar]
- 27.Benedict L. 2008. Occurrence and life history correlates of vocal duetting in North American passerines. J. Avian Biol. 39, 57–65. ( 10.1111/j.0908-8857.2008.04103.x) [DOI] [Google Scholar]
- 28.Topp SM, Mennill DJ. 2008. Seasonal variation in the duetting behaviour of rufous-and-white wrens (Thryothorus rufalbus). Behav. Ecol. Sociobiol. 62, 1107–1117. ( 10.1007/s00265-007-0538-4) [DOI] [Google Scholar]
- 29.Douglas SB, Heath DD, Mennill DJ. 2012. Low levels of extra-pair paternity in a neotropical duetting songbird, the rufous-and-white wren (Thryothorus rufalbus). Condor 114, 393–400. ( 10.1525/cond.2012.110028) [DOI] [Google Scholar]
- 30.van den Heuvel IM, Cherry MI, Klump GM. 2014. Crimson-breasted Shrike females with extra pair offspring contributed more to duets. Behav. Ecol. Sociobiol. 68, 1245–1252. ( 10.1007/s00265-014-1735-6) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.


