Abstract
Personal protection (PP) techniques, such as insecticide-treated nets, repellents and medications, include some of the most important and commonest ways used today to protect individuals from vector-borne infectious diseases. In this study, we explore the possibility that a PP intervention with partial coverage may have the counterintuitive effect of increasing disease burden at the population level, by increasing the biting intensity on the unprotected portion of the population. To this end, we have developed a dynamic model which incorporates parameters that describe the potential effects of PP on vector searching and biting behaviour and calculated its basic reproductive rate, R0. R0 is a well-established threshold of disease risk; the higher R0 is above unity, the stronger the disease onset intensity. When R0 is below unity, the disease is typically unable to persist. The model analysis revealed that partial coverage with popular PP techniques can realistically lead to a substantial increase in the reproductive number. An increase in R0 implies an increase in disease burden and difficulties in eradication efforts within certain parameter regimes. Our findings therefore stress the importance of studying vector behavioural patterns in response to PP interventions for future mitigation of vector-borne diseases.
Keywords: vector-borne infectious diseases, vector ecology, disease transmission, bednets, insecticide-treated nets, insect repellents
1. Introduction
The collective human mortality rate due to vector-borne diseases is estimated to be more than 1.5 million per annum [1]. Important examples include malaria, dengue, leishmaniasis, yellow fever, Lyme disease and the West Nile virus [2–5].
One of the most important measures of the risk of infectious disease outbreak is the basic reproductive rate, R0. R0 measures the average number of secondary infections caused by a single infection in a naive host population. R0 thus establishes a threshold criteria for disease invasion; a disease has the potential to invade a population if R0 > 1, and is unable to persist when R0 < 1 [6–9]. In general, the higher R0 is, the more difficult it will be to eradicate the disease [6]. In this study, we explore how personal protection (PP) interventions that protect a portion of individuals in the community affect R0.
We use PP to refer broadly to interventions that operate at the individual or household level (rather than the community level), whether or not they kill the vectors in addition to their individualistic protection. Examples include the use of medication, personal use of insect repellent and bednets, including insecticide-treated nets (ITN) [10–14]. Residual spraying of households can also be considered as PP by our definition, if application decisions are made at the household level.
In most applied interventions, only a portion of the population receives or uses a suggested treatment. Although PP provides direct benefits to protected individuals, its population-level effects can be complex and may not always be beneficial in cases where its coverage is not complete. For example, the use of insect repellents by only a part of the population (the treated group, TG) can increase the number of bites on untreated individuals (untreated group, UTG), as repelled vectors seek alternative hosts [15]. When bites are concentrated in a fraction of the host population, the disease risk of the entire population, as represented by R0, may increase [11,16–22]. The fact that certain treatments which benefit the TG may indirectly harm the UTG is of interest, since it raises ethical concerns that may limit their usage. An even more troubling scenario is when a PP harms the UTG to such a degree that the overall net effect is an increase in disease risk for the entire population. The possible outcomes of a partial treatment coverage given to a population are summarized in figure 1.
Figure 1.
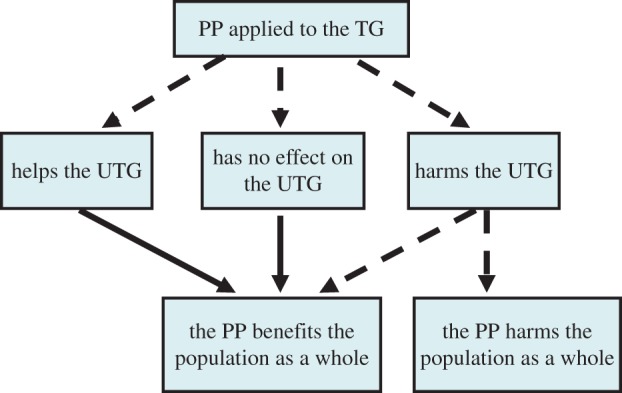
The different possible effects of PP given to the TG on the disease risk of the UTG and the entire host population. Solid and dashed arrows denote whether the outcome is obligatory or not, respectively. (Online version in colour.)
In this study, we apply a model that was originally designed to analyse a system with two host species and one vector species [20,23,24] to a single host population divided into two different groups, the TG and the UTG, and develop it to account for possible effects of PP on vector biting patterns. The goal is to analyse how partial PP coverage affects R0 under different assumptions about the vector behavioural patterns, in particular, its response to ITN, bednets and insect repellents. The analysis of R0 identified the conditions under which partial PP coverage will either increase or decrease it.
2. Model outlines
We explore how the basic reproductive number, R0, depends on individual-level treatment with variable coverage rates of the host population. To calculate R0, we modified a previously developed modelling framework [20,24] originally designed for multiple host species, to consider different host types—specifically hosts receiving (TG) and not receiving (UTG) PP treatments. We chose this model because it is a relatively simple framework that includes the details we need to explore the effects of PP on R0. Our model considers only one host species: in particular, we do not account here for the possibility that vectors divert from biting human hosts to bite domestic animals or other non-human targets.
The model quantifies the dynamics of the susceptible, infected and removed compartments of the TG and the UTG (denoted by the subscripts T and U, respectively). By using the next-generation-operator technique, we calculate the expression of R0 from the model equations [7] (see the electronic supplementary material for the model description and equations):
| 2.1 |
In equation (2.1), gi = piqi/δi is the transmission ability of host group i (i = T or U), defined as the product of probability of transmission to a vector pi, probability of transmission from a vector qi and the infectiousness duration (1/δi, see table 1 for parameters definitions) [20]. V and d are the density and the death rate of the vector, respectively, ki is the vector biting rate (number of bites per vector per unit time) on hosts belonging to group i, and Ni is host group i density. In equation (2.1), we assumed frequency-dependent biting (i.e. the biting rate of an individual vector is independent of the host total population density, N = NT + NU, see the electronic supplementary material). In general, the assumption of frequency-dependent biting leads to a decrease in R0 when host population size increases while vector density remains constant, since bites are then distributed on more host individuals, thus reducing the frequency at which individual hosts are bitten [25,26]. Similar expressions of R0 have been obtained in the past for both, metapopulation [16] and multi-host models [20,26]. The current model builds on these by modelling the factors affecting the biting rates ki, as elaborated below.
Table 1.
Definition of the model parameters. i = T or U.
| parameter | meaning |
|---|---|
| ki | biting rate, i.e. the number of bites per unit time on hosts from group i an individual vector has |
| Ni | host group i population size |
| V | vector population size |
| r | vector fixed birth rate |
| N | the total host population size, i.e. N = Nu + NT |
| pi | the efficiency that an infected vector would infect a susceptible individual of host group i during one feeding event |
| qi | the efficiency that an infected individual of host group i would infect a susceptible vector during one feeding event |
| δi | recovery rate of host in group i, i.e. 1/δi is the infectiousness duration |
| d | vector death rate for coverage rate x (i.e. d is a function of x) |
| d0 | basal vector death rate, i.e. death rate without treatment or without killing effect |
| k | the total biting rate of the vector, i.e. the number of bites per unit time of individual vector on the entire host population, i.e. k = kU + kT |
| gi | the transmission ability of hosts in group i. gi = piqi/δi |
| L | vector latent period |
| x | the treated population proportion, i.e. NT = xN and NU = (1 − x)N |
| hi1 | post-biting handling time. The time the vector needs to handle a host from group i after a successful biting attempt |
| hi2 | pre-biting handling time. The time the vector spends when occupying with host individual of group i before biting it |
| hi | the total handling time of host on group i. The amount of time the vector needs to spend in handling hosts of group i in order to achieve a single bite |
| bi | the protection time. The average time units the vector needs for a successful biting attempt on host group i individual |
| Ai | the general searching efficiency, the number of bites per unit time on host group i incurred by a vector that forages in host i population with unit density (Ni = 1) and zero handling times |
| ai | host i searching efficiency. The attractiveness of hosts belong to group i |
| βi | the probability that the vector would successfully bite an individual from host group i |
| η | a constant that determines the ability of a treatment to increase the vector death rate, or alternatively, decrease its life expectancy |
We adapt a ‘classical’ saturated (Holling type II) functional response to calculate vector biting rates on each type of host [27–29]. The type II functional response is a well-known model that has been successfully applied to various consumer–resource systems [30], and which provide a natural basis to model the way vector bites are distributed as a function of searching efficiencies, host densities and handling times with respect to each group. Under these assumptions (see the electronic supplementary material), it can be shown that the vector biting rate on host group i, ki [27,28,30] is given by:
| 2.2a |
| 2.2b |
| 2.2c |
where
| 2.3 |
In equation (2.2a), hi1 (i = T or U), the post-biting handling time, is the time the vector spends in handling host i after it has been successfully bitten. Biologically, hi1 may include the time the vector needs for (a) resting and digesting the blood meal, (b) egg production, (c) searching for a proper incubation site and (d) laying eggs. hi2 is the pre-biting handling time, that is, the time the vector is occupied by an individual host when flying around and trying to locate a biting site. hi in equation (2.2a) represents the total handling time, that is, the average time the vector needs to spend in handling a single bite from hosts in group i; hi is a weighted sum of the time the vector spends in handling a successful bite (hi1 + hi2 = the pre- and the post-handling times) and associated in unsuccessful attempts (hi2 = the pre-biting handling time, equation (2.2a)). βi (equation (2.3)) is the probability that a biting attempt on a host from group i will be successful. We assumed that the pre-biting time hi2 is exponentially distributed with mean, bi, the protection time, which measures the mean time it takes the vector to achieve a successful bite on group i. Ai in equation (2.2b) represents the general searching efficiency, that is the average biting rate the vector has when foraging in a population of unit density (Ni = 1) neglecting all handling times (i.e. when hi1 = hi2 = 0), thus, ai can be interpreted as the attractiveness of group i to the vectors.
To model treatments that may kill the vectors, we assume that the killing acts to decrease the vector longevity, or alternatively, increase its death rate, d (provided that its life expectancy is exponentially distributed) [31]. As a simple approximation for simulation purposes, we assume that d depends bilinearly on the time the vector is exposed to the pesticide, hT2, and the TG coverage rate x:
| 2.4 |
where, d0 is the basal vector death rate without the killing treatment, and η is a constant representing the treatment killing efficiency; it is zero when the treatment does not kill the vector and positive when it does. From the above, NT = xN, Nu = (1 − x)N, where N is the total host population density (N = NT + NU). With the aid of equations (2.1)–(2.4), R0 can be written as:
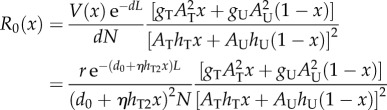 |
2.5 |
where V(x) is the vector population size when a proportion x of the population is treated. In cases where the treatment does not kill the vectors, V(x) is expected to be constant (assuming that the vector population is at equilibrium), when η > 0, V(x) may decrease with x (it can be, of course, that a treatment, such as ITN, does not substantially affect the vector population size) [31]. From the model equations (electronic supplementary material, equation S1), the vector equilibrial population is r/d, where r is the vector's fixed birth rate that is assumed to be limited by other factors in the habitat (e.g. incubation sites, etc.). The expression r/d enables us to calculate the decrease in the vector population size due to the killing effect of ITN via d in equation (2.4). We add the factor exp(−dL) to equation (2.1) and obtain equation (2.5) to account for the decrease in R0 due to a possible vector latency period, L. This factor represents the probability that an infected vector will survive the fixed latent period, L, and becomes infectious [6,31]. Definitions of all model parameters can be found in table 1.
Every PP can be characterized by different sets of the model parameters (e.g. AT, AU, hT, hU, etc.; see also table 1 and equation (2.5)). In this research, we will concentrate mainly on the effects of bednets, ITN, and insect repellents used by individuals, on R0. The main goal of this study is to explore the conditions under which these PP techniques will reduce R0 below its value in an otherwise untreated population, i.e. solving the inequality:
| 2.6 |
where R0(x) is R0 when a proportion x of the population is treated (equation (2.5)) and R0(0) = R0(x = 0) = rexp(−d0L) gu/(d02NhU2) (equation (2.5)).
3. Results
3.1. General consideration
In general, the response of R0 to PP coverage takes two main forms depending on model parameters (figure 2): (i) the net effect of the PP always decreases R0, or (ii) PP increases R0 if the TG proportion is below a certain threshold xt, and decreases R0 thereafter (figure 2). A detailed analysis of equation (2.5), including an expression of the threshold xt for η = 0 can be found in the electronic supplementary material. When R0 is near 1, an increase in it will generally result in an increased population morbidity.
Figure 2.
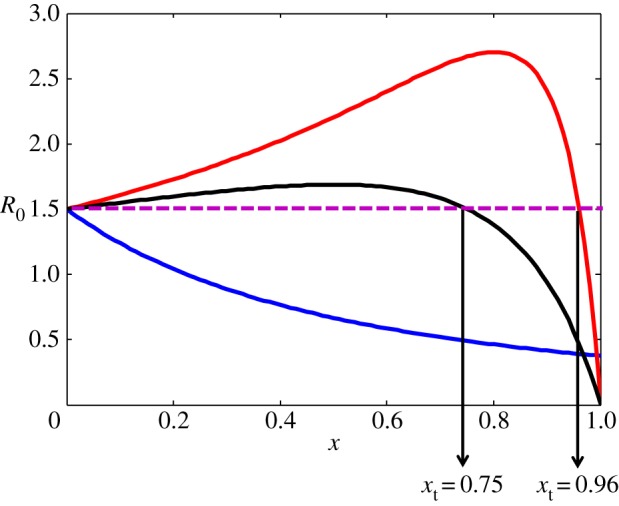
The dependence of R0 on coverage rate. Purple horizontal line: R0 for a naive (untreated) population (i.e. R0(x = 0)). Blue line: hT = 0.5, hU = 0.25, AT = AU = 0.1 and xt = −2 (see the electronic supplementary material). R0 decreases with every TG proportion thus any PP coverage is beneficial to the population. Red and black lines: AT = 0.1, AU = 5, hT = 0.5, hU = 0.06 and xt = 0.96 and AT = 0.1, AU = 5, hT = 0.5, hU = 0.03, and xt = 0.75, respectively. R0 decreases with every TG proportion x > xt and increases with x < xt. In all graphs gT = gU, η = 0 and R0(x = 0) = rgUexp(−d0L)/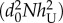 = rgTexp(−d0L)/
= rgTexp(−d0L)/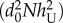 = 1.5. When R0 < 1, the disease is extinguished. (Online version in colour.)
= 1.5. When R0 < 1, the disease is extinguished. (Online version in colour.)
Figure 2 demonstrates that for the chosen parameters, R0 can reach 2.70 for coverage of 80%, exceeding the value for the untreated population by more than 80% (R0(x = 0) = 1.5). As such an increase can lead to dramatic rise in disease burden, we will explore how common PP techniques affect the model parameters, and consequently R0(x), thus enabling us to link between different PP and the behaviour of R0(x) exemplified in figure 2.
3.2. The dependency of R0 on the use of bednets, insecticide-treated nets and insect repellents
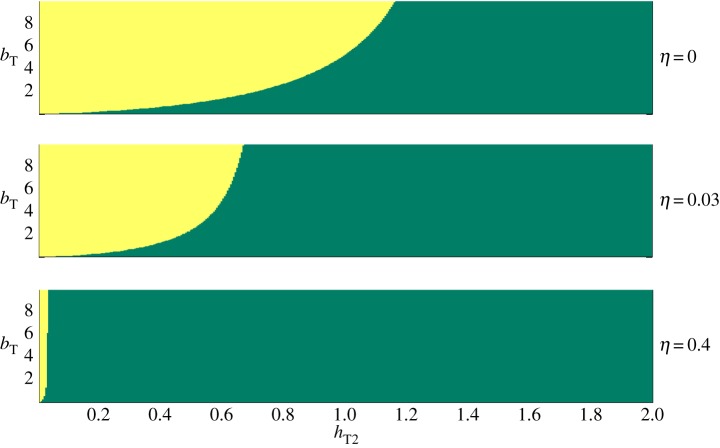
Insect repellents may affect the time the vector spends in trying to bite a host from the treated group, once located, hT2 (the pre-biting time); the vector may spend less time in trying to bite a host with repellent, or alternatively, it may spend more time around a host with repellent in trying to locate an untreated skin area. The repellent also increases the protection time, bT, and it may also make the host less attractive from a distance, thus decreasing aT (equation (2.2b)). Likewise, bednets may affect hT2; they can increase or decrease it, depending on whether nets cause the vectors to give up a protected host quickly, or alternatively, cause them to spend more time in trying to find holes or proximal body parts. Nets also increase bT, the protection time. If bednets are also impregnated with insecticide (ITN), they also increase η, and consequently the killing rate, d (equation (2.4)). Figure 3 illustrates how the form of R0(x) (the dependency of R0 on coverage rate, x, as exemplified in figure 2) varies over the parameter space of hT2, bT and η. To the best of our knowledge, there are insufficient data available to estimate these parameters with accuracy. We therefore use upper bounds of several days for hT2 and bT—the life expectancy and egg production time of many vectors species [32]. We have also set d0 = 0.088 d−1 and L = 0.1 d−1, the death rate and the latency period of typical Anopheles spp. (malaria vectors) [6,31]. We have chosen a range of η between 0 (bed nets without killing effect) and 0.3. When η = 0.3, the vector equilibrial population, r/d (equation (2.5)) decreases by 23%, an upper bound for a population decrease due to ITN according to a field study on Anopheles albimanus [33]. Figure 3 is thus intended to point out on general principles, not to provide quantitative information.
Figure 3.
The effect of PP with various vector pre-biting, hT2, and protection, bT, times, respectively, and killing ability, η (all times are in days) on the qualitative behaviour of R0 as function of the proportion of the treated group within the population. In green: the treatment always decreases R0. In yellow, the treatment increases R0 below a threshold coverage rate, and decreases R0 above it. In all simulations, gT = gU, hT1 = 3, hU = 3, aT = aU, L = 0.1 d−1, d0 = 0.088 d−1, i.e. the treatment does not affect the host transmission ability and the attractiveness to vectors. (Online version in colour.)
Figure 3 shows parameter ranges where partial PP coverage can increase R0 (yellow area). The border between the green and the yellow areas shows critical values of the pre-biting and protection times (hT2 and bT, respectively). For example, when η = 0.03 and the pre-biting time, hT2 = 0.39 days (figure 3b), there exists a critical value of the TG protection time, bTc = 1.7 days, for which PP reduces R0 at any coverage level if the protection time is less than bTc (green area), but increases R0 below a threshold coverage level when the protection time is longer than bTc (yellow area). Thus, if it is harder for the vectors to successfully bite treated hosts (i.e. bT increases), the disease risk for the entire population may increase when R0 is near the threshold value of 1. This is because when bT is long, vectors are more likely to be diverted from treated to untreated hosts, concentrating more bites per capita on the UTG.
Other parameters behave similarly. When pre-biting time hT2 is large, R0 decreases for every coverage rate (green zone), and if it is large enough (e.g. hT2 > hT2c = 1.17d, figure 3a), this decrease is independent of the value of bT. The killing parameter, η, has a critical value as well; the higher it is, the wider the parameter range of bT and hT2 under which R0 decreases for every PP coverage. If a PP intervention has very strong killing ability (i.e. η is high), then it can decrease R0 irrespective of the coverage rate over the whole range of hT and bT we studied (in the simulations of figure 3, this occurs, for example, when η > 11, data not shown).
4. Discussion
In this study, we have investigated the effects of partial PP coverage and found that under some circumstances, it is plausible that partial coverage with popular PP techniques used today, such as ITN, bednets and repellents, can lead to substantial increases in the reproductive number, R0. This result is similar to the diversity amplification effect which occurs due to vector preference towards specific host species in a two-species community [20]. In both cases, an increase in R0 occurs when vectors divert from one host group (TG or less preferred host species) to the other (the UTG or the preferred host species).
Previous models have pointed out the potential negative effects that partial bednet coverage can have due to diversion of vectors from the TG to the UTG, when combined with low killing efficiency [11,21,34]. The effects estimated, however, were very low, particularly at the level of the entire population [11,31,32]. It has also been speculated that total population morbidity could increase with increased bednet coverage in a case where bednets were combined with vaccines that could affect the population immunity of specific vulnerable host groups [35]. Field studies, however, praise the use of ITN for their success in reducing malaria incidence, or in decreasing other important metrics of disease risk (e.g. entomological inoculation rate, human biting rate and vector population) [36–41].
This study is the first to systematically explore the effect of partial PP coverage on R0 over a wide range of plausible parameters, and the first to find a potential for substantial increase in population-level risk; when R0 is near its threshold (i.e. 1), any increase in it is expected to lead an increased population-level morbidity. It is important to note, however, that when morbidity and force of infection are high, an increase in R0 caused by protecting part of the population will not always be expected to increase population-level morbidity, since the effects of increasing R0 would be outweighed by the direct effect of protecting part of the population. Yet, the increase in R0 in these cases may still lead to an increased risk in a portion of the population.
Our model makes the subtle yet important distinction between protection and diversion [11,21,31]. In previous studies, both diversion and protection have been related to the probability that a vector will give up a protected host and turn to look for another victim [11,21,31]. In the present framework, however, diversion is equivalent to the time duration the vector spends in trying to bite a protected host, hT2, that is, its pre-biting time. The shorter the pre-biting time, hT2, the stronger the diversion effect of the respective PP. The protection, bT, in our model, is equivalent to the mean time the vector needs for a successful biting attempt of a protected host, irrespective of the time the vector actually spends in that attempt (hT2). In our study therefore, protection and diversion are two independent properties, and hence may be affected differently by different PP interventions. Figure 3 demonstrates the counterintuitive dependency of R0 on protection due to this differentiation; a more protective PP can increase R0 when the vector pre-biting handling time, hT2, is short, and the coverage rate is below a certain threshold. For longer hT2, however, every PP coverage reduces R0 (figure 3). Consequently, an increase in R0 may therefore occur due to a change in the vector foraging behaviour. If vectors switch quickly on encountering nets (or on encountering ITNs), the pre-biting time, hT2, will decrease, and consequently R0 will increase for some levels of coverage (assuming high enough protection times, figure 3). Such a change of the vector behaviour is realistic. Avoiding landing on ITNs and spending less time in trying to bite protected individuals are traits that can increase vector longevity and fitness, and thus may spread within the population relatively fast. Changes in vector behaviour correlated with ITN usage have already been observed: mosquitoes change their activity time, host species (from human to livestock) and feeding site (indoor or outdoor) within several years in high coverage areas [39,42–45]. Unfortunately, to the best of our knowledge, there are no field or laboratory measurements regarding the time allocation used by vectors while foraging for potential hosts.
This study expands on previous work by supplementing simulation-based exploration of the various effects of PP on disease burden with analytical results on R0, thus increasing generality and providing a better mechanistic understanding [46]. Our results are most applicable to cases where R0 is near its threshold (i.e. 1). Under such circumstances, an increase in morbidity may occur for PP partial coverage rates if certain vector behavioural patterns exist, especially a decrease in its pre-biting handling time. This study, therefore, stresses the importance of field research on the vector's time allocation to foraging for potential hosts and its relation to the PP techniques widely used today for future elimination and mitigation of vector-borne infectious diseases.
Supplementary Material
Authors' contributions
E.M. and A.H. conceived the study. E.M. wrote the first draft of the manuscript. All authors revised the manuscript, contributed to later drafts, developed the model and analysed the results. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was supported by the Bill and Melinda Gates Foundation Global Health Program http://www.gatesfoundation.org/ (grant no. OPPGH5336). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hill CA. 2005. Arthropode borne diseases: vector control in genomics era. Nat. Rev. Microbiol. 3, 262–268. ( 10.1038/nrmicro1101) [DOI] [PubMed] [Google Scholar]
- 2.Woolhouse MEJ, Taylor LH, Haydon DT. 2001. Population biology of multihost pathogens. Science 292, 1109–1112. ( 10.1126/science.1059026) [DOI] [PubMed] [Google Scholar]
- 3.Dobson A, Cattadori I, Holt RD, Ostfeld RS, Keesing F, Krichbaum K, Rohr JR, Perkins SE, Hudson PJ. 2006. Sacred cows and sympathetic squirrels: the importance of biological diversity to human health. PLoS Med. 3, 714–718. ( 10.1371/journal.pmed.0030231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT. 2009. Epidemic dynamics at the human–animal interface. Science 326, 1362–1367. ( 10.1126/science.1177345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bern C, Courtenay O, Alvar J. 2010. Of cattle, sand flies and men: a systematic review of risk factor analyses for south Asian visceral leishmaniasis and implications for elimination. PLoS. Neglect. Trop. D 4, e599 ( 10.1371/journal.pntd.0000599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RM, May RM. 1991. Infectious diseases in humans. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.van den Driessche P, Watmough J. 2002. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48. ( 10.1016/S0025-5564(02)00108-6) [DOI] [PubMed] [Google Scholar]
- 8.Mandal S, Sarkar RR, Sinha S. 2011. Mathematical models of malaria—a review. Malar. J. 10, 202 ( 10.1186/1475-2875-10-202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts MG. 2007. The pluses and minuses of R0. J. R. Soc. Interface 4, 949–961. ( 10.1098/rsif.2007.1031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killeen GF, Smith TA, Ferguson HM, Mshinda H, Abdulla S, Lengeler C, Kachur SP. 2007. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 4, 1246–1258. ( 10.1371/journal.pmed.0040229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Killeen GF, Smith TA. 2007. Exploring the contributions of bed nets, cattle, insecticides, and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behavior and mortality. Trans. R. Soc. Trop. Med. Hyg. 101, 867–880. ( 10.1016/j.trstmh.2007.04.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatton ML, Cheng Q. 2010. Interrupting malaria transmission: quantifying the impact of interventions in regions of low to moderate transmission. PLoS ONE 5, e15149 ( 10.1371/journal.pone.0015149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin JT, et al. 2010. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 7, e1000324 ( 10.1371/journal.pmed.1000324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC. 2011. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS ONE 6, e20179 ( 10.1371/journal.pone.0020179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore SJ, Davies CR, Hill N, Cameron MM. 2007. Are mosquitoes diverted from repellent-using individuals to non-users? Results of a field study in Bolivia. Trop. Med. Int. Health 12, 532–539. ( 10.1111/j.1365-3156.2006.01811.x) [DOI] [PubMed] [Google Scholar]
- 16.Dye C, Hasibeder G. 1986. Population-dynamics of mosquito-borne disease-effect of flies which bite some people more frequently than others. Trans. R. Soc. Trop. Med. Hyg. 80, 69–77. ( 10.1016/0035-9203(86)90199-9) [DOI] [PubMed] [Google Scholar]
- 17.Dye C, Wolpert DM. 1988. Earthquakes, influenza and cycles of Indian Kala-Azar. Trans. R. Soc. Trop. Med. Hyg. 82, 843–850. ( 10.1016/0035-9203(88)90013-2) [DOI] [PubMed] [Google Scholar]
- 18.Smith DL, Dushoff J, McKenzie FE. 2004. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2, 1957–1964. ( 10.1371/journal.pbio.0020368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolhouse MEJ, et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342. ( 10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller E, Huppert A. 2013. The effects of host diversity on vector-borne disease: the conditions under which diversity will amplify or dilute the disease risk. PLoS ONE 8, e80279 ( 10.1371/journal.pone.0080279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu WD, Novak RJ. 2009. Predicting the impact of insecticide-treated bed nets on malaria transmission: the devil is in the detail. Malar. J. 8, 256 ( 10.1186/1475-2875-8-256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killeen GF, Moore SJ. 2012. Target product profiles for protecting against outdoor malaria transmission. Malar. J. 11, 17 ( 10.1186/1475-2875-11-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA. 2012. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B 279, 925–933. ( 10.1098/rspb.2011.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yakob L, Bonsall MB, Yan G. 2010. Modeling knowlesi malaria transmission in humans: vector preference and host competence. Malar. J. 9, 329 ( 10.1186/1475-2875-9-329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross R. 1910. The prevention of malaria, p. 669 London, UK: John Murray. [Google Scholar]
- 26.Rogers DJ. 1988. The dynamics of vector-transmitted diseases in human communities. Phil.Trans. R. Soc. Lond. B 321, 513–539. ( 10.1098/rstb.1988.0106) [DOI] [PubMed] [Google Scholar]
- 27.Holling CS. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398. ( 10.4039/Ent91385-7) [DOI] [Google Scholar]
- 28.Holling CS. 1966. The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 48, 1–86. ( 10.4039/entm9848fv) [DOI] [Google Scholar]
- 29.Antonovics J, Iwasa Y, Hassell MP. 1995. A generalized-model of parasitoid, venereal, and vector based transmission processes. Am. Nat. 145, 661–675. ( 10.1086/285761) [DOI] [Google Scholar]
- 30.Hassel PM. 1978. The dynamics of arthropod predator–prey systems, 1st edn Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 31.Le Menach A, Takala S, McKenzie FE, Perisse A, Harris A, Flahault A, Smith DL. 2007. An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malar. J 6, 10 ( 10.1186/1475-2875-6-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa M. 1978. Insects anti man, 2nd edn, 286 p. Tel Aviv, Israel: Hakibbutz Hameuchad. [Google Scholar]
- 33.Arredondo-Jimenez JI, Rodrigues MH, Loyola EG, Bown D. 1997. Behavior of Anopheles albimanus in relation to pyrethroid-treated bednets. Med. Vet. Entomol 11, 87–94. ( 10.1111/j.1365-2915.1997.tb00294.x) [DOI] [PubMed] [Google Scholar]
- 34.Killeen GF, Chitnis N, Moore SJ, Okumu FO. 2011. Target product profile choices for intra-domiciliary malaria vector control pesticide products: repel or kill? Malar. J. 10, 207 ( 10.1186/1475-2875-10-207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Artzy-Randrup Y, Dobson AP, Pascual M.. 2014. Synergistic and antagonistic interactions between bednets and vaccines in the control of malaria. Proc. Natl Acad. Sci. USA 112, 3014–3019. ( 10.1073/pnas.1409467112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killeen GF, et al. 2007. Cost-sharing strategies combining targeted public subsidies with private-sector delivery achieve high bednet coverage and reduced malaria transmission in Kilombero Valley, southern Tanzania. BMC Infect. Dis. 7, 121 ( 10.1186/1471-2334-7-121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell TL, et al. 2010. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar. J. 9, 187 ( 10.1186/1475-2875-9-187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teklehaimanot A, Sachs JD, Curtis C.. 2007. Malaria control needs mass distribution of insecticidal bednets. Lancet 369, 2143–2146. ( 10.1016/s01406736(07)60951-9) [DOI] [PubMed] [Google Scholar]
- 39.Bogh C, Pedersen EM, Mukoko DA, Ouma JH. 1998. Permethrin-impregnated bednet effects on resting and feeding behavior of lymphatic filariasis vector mosquitoes in Kenya. Med. Vet. Entomol. 12, 52–59. ( 10.1046/j.1365-2915.1998.00091.x) [DOI] [PubMed] [Google Scholar]
- 40.Lindblade KA, Gimnig JE, Kamau L, Hawley WA, Odhiambo F, Olang G, Ter Kuile FO, Vulule JM, Slutsker L. 2006. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. J. Med. Entomol. 43, 428–432. ( 10.1603/0022-2585(2006)043%5B0428:iosuoi%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 41.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, Walker ED, Kitron U. 2011. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar. J. 10, 356 ( 10.1186/1475-2875-10-356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djenontin A, Chandre F, Djegbe I, Guis H, Corbel V.. 2012. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 206, 1622–1629. ( 10.1093/infdis/jis565) [DOI] [PubMed] [Google Scholar]
- 43.Padonou GG, et al. 2012. Decreased proportions of indoor feeding and endophily in Anopheles gambiae s.l. populations following the indoor residual spraying and insecticide-treated net interventions in Benin (West Africa). Parasite. Vector. 5, 262 ( 10.1186/1756-3305-5-262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. 2011. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10, 80 ( 10.1186/1475-2875-10-80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takken W. 2002. Do insecticide-treated bednets have an effect on malaria vectors? Trop. Med. Int. Health 7, 1022–1030. ( 10.1046/j.1365-3156.2002.00983.x) [DOI] [PubMed] [Google Scholar]
- 46.May RM. 2004. Uses and abuses of mathematics in biology. Science 303, 790–793. ( 10.1126/science.1094442) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.