Abstract
The blood-testis barrier (BTB) is an important ultrastructure in the testis since the onset of spermatogenesis coincides with the establishment of a functional barrier in rodents and humans. It is also noted that a delay in the assembly of a functional BTB following treatment of neonatal rats with drugs such as diethylstilbestrol or adjudin also delays the first wave of spermiation. While the BTB is one of the tightest blood-tissue barriers, it undergoes extensive remodeling, in particular at stage VIII of the epithelial cycle to facilitate the transport of preleptotene spermatocytes connected in clones across the immunological barrier. Without this timely transport of preleptotene spermatocytes derived from type B spermatogonia, meiosis will be arrested, causing aspermatogenesis. Yet the biology and regulation of the BTB remains largely unexplored since the morphological studies in the 1970s. Recent studies, however, have shed new light on the biology of the BTB. Herein, we critically evaluate some of these findings, illustrating that the Sertoli cell BTB is regulated by actin binding proteins (ABPs), likely supported by non-receptor protein kinases, to modulate the organization of actin microfilament bundles at the site. Furthermore, microtubule (MT)-based cytoskeleton is also working in concert with the actin-based cytoskeleton to confer BTB dynamics. This timely review provides an update on the unique biology and regulation of the BTB based on the latest findings in the field, focusing on the role of ABPs and non-receptor protein kinases.
Keywords: testis, Sertoli cell, seminiferous epithelial cycle, spermatogenesis, blood-testis barrier, tight junction, basal ectoplasmic specialization, actin microfilaments
1. Introduction
The concept of the blood-testis barrier (BTB) dates back to more than 100 years ago when dyes administered to rodents failed to stain the brain and the testis, illustrating the presence of the blood-brain barrier (BBB) and the blood-testis barrier (BTB) in mammals (for reviews, see (Cheng and Mruk 2012, Mruk and Cheng 2015, Setchell 2008)). Subsequent studies have shown that the BTB is very different from the BBB and other blood-tissue barriers, such as the blood-retinal barrier, since these other blood-tissue barriers are contributed almost exclusively by the tight junction (TJ) barrier between endothelial cells of the microvessels, such as those found behind the brain and the retina (Campbell and Humphries 2012, Easton 2012). Instead, the BTB is constituted by specialized junctions between adjacent Sertoli cells located near the basement membrane in the seminiferous epithelium which is composed of actin-based tight junction, basal ectoplasmic specialization (basal ES), and gap junction, as well as intermediate filament-based desmosome (for reviews, see (Cheng and Mruk 2012, Mruk and Cheng 2004, Pelletier 2011)) (Figure 1). In short, endothelial TJ barrier of the microvessels located in the interstitium between seminiferous tubules contribute virtually no barrier function. Interestingly, since the initial discovery of the BTB, relatively few functional studies were performed beyond the collection and quantification of constituents in biological fluids in the seminiferous tubule, rete testis, and interstitial fluid for comparison to blood plasma and/or serum. Findings from these studies have illustrated the significance of the BTB in the mammalian testis by restricting the flow of ions, proteins, electrolytes, sugars, and other biomolecules across the barrier (for a review, see (Setchell and Waites 1975)). Morphological studies performed in the 1970s have shown that the BTB physically divides the seminiferous epithelium into two functional compartments: the basal and the adluminal (apical) compartments. As such, the events of mitosis for self-renewal of spermatogonial stem cells and undifferentiated spermatogonia, and the differentiation of type B spermatogonia to preleptotene spermatocytes all take place outside the BTB at the basal compartment. However, meiosis I/II and the entire events of post-meiotic spermatid development (i.e., spermiogenesis) and the release of sperm at spermiation all take place behind the BTB in a specialized microenvironment known as the adluminal compartment (Figure 2). While the BTB is considered to be one of the tightest blood-tissue barriers, it continuously remodels throughout the epithelial cycle of spermatogenesis to allow the entry of selected substances/biomolecules necessary to support meiosis I/II and spermiogenesis. It also restructures itself to allow the transport of preleptotene spermatocytes across the barrier at stage VIII of the epithelial cycle so that these spermatocytes enter the adluminal compartment to be differentiated into leptotene, zygotene and pachytene spermatocytes to prepare for meiosis I/II which takes place at stage XII and XIV in the mouse and rat, respectively (for reviews, see (Cheng and Mruk 2012, Hess and de Franca 2008)). In this context, it is noted that the epithelial cycle of spermatogenesis in the mouse and rat testis is divided into stages I–XII and I–XIV, respectively (for reviews, see (Clermont 1972, Hess and de Franca 2008, Parvinen 1982, Xiao, et al. 2014b)).
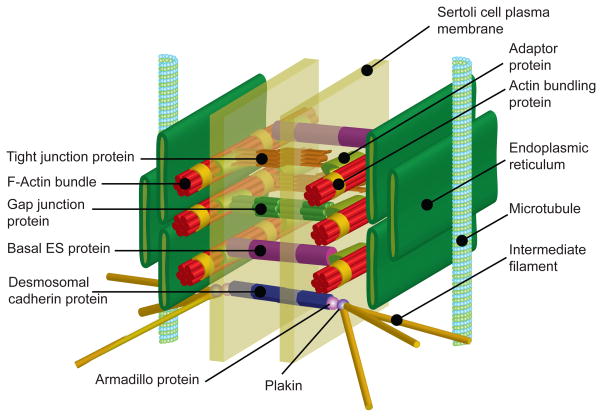
Figure 1. A schematic drawing illustrating the various junction types and their structural and spatial relationship to the actin- and microtubule (MT)-based cytoskeletons at the Sertoli cell blood-testis barrier (BTB).
In the mammalian testis, such as in rodents, the BTB is constituted by tight junction (TJ), basal ectoplasmic specialization (basal ES), and gap junction that utilize actin microfilaments for their attachment, as well as intermediate filament-based desmosome, as shown in this 3-D schematic drawing. Endothelial TJ in microvessels located in the interstitium contributes virtually no barrier function to the BTB. The corresponding protein complexes of TJ (e.g., occludin-ZO-1), basal ES (e.g., N-cadherin-β-catenin), gap junction (e.g., connexin43-plakophilin-2) and desmosome (e.g., desmoglein-2-plakoglobin-2/desmoplakin) confer cell adhesion between adjacent Sertoli cells. Actin microfilaments are polarized structures and are arranged as bundles, which together with the nearby polarized MT also provide the track for the transport of germ cells in particular spermatids and other organelles (e.g., phagosomes, endocytic vesicles) across the seminiferous epithelium besides conferring unparalleled adhesive strength to the BTB during the epithelial cycle of spermatogenesis.
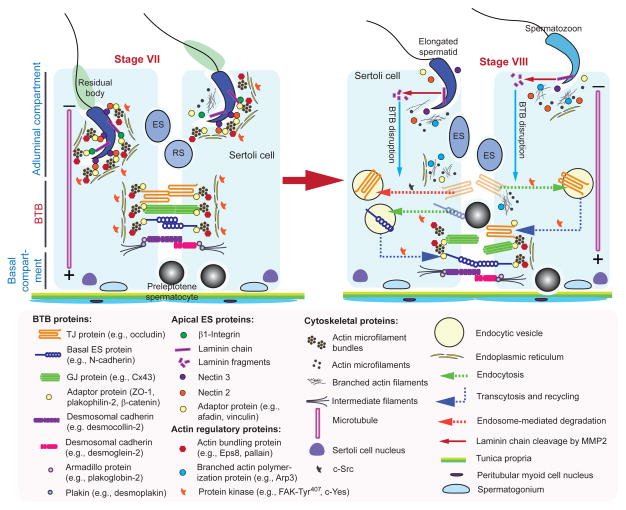
Figure 2. A schematic drawing illustrating the molecular mechanism by which preleptotene spermatocytes are transported across BTB at stage VIII of the epithelial cycle.
The cross-section of a stage VII seminiferous tubule on the left panel illustrates the intact BTB is conferred by adhesion protein complexes of the actin-based TJ, basal ES and GJ, as well as the intermediate filament-based desmosome. The apical ES is also intact which is conferred by the corresponding adhesion complexes of nectin-afadin and β1-integrin-laminins. Preleptotene spermatocytes transformed from type B spermatogonia are first detected at stage VII, and are being transported across the BTB, when apical ES also undergoes degeneration to prepare for spermiation as noted in a stage VIII tubule shown on the right panel. Biologically active laminin fragments (e.g., F5-peptide) generated by MMP-2-induced proteolytic cleavage induce BTB restructuring in which TJ (e.g., occludin), basal ES (e.g., N-cadherin), GJ (e.g., connexin 43), and desmosome (e.g., desmoglein-2) proteins undergo endocytic vesicle-mediated endocytosis, transcytosis and recycling. In this way, the “old” BTB proteins located above the preleptotene spermatocytes can be recycled to assemble a “new” BTB behind these germ cells to facilitate the transport of spermatocytes at the site, prior to the degeneration of the “old” BTB. As such, the events of spermiation and BTB remodeling which allow the release of sperm and the transport of preleptotene spermatocytes, respectively, occur at the opposite ends of the epithelium and take place simultaneously at stage VIII of the cycle.
Since the 1970s, when the ultrastructures of the BTB were reported in detail (Fawcett 1975, Fawcett, et al. 1970, Russell 1977a, 1978, Setchell and Waites 1975), few studies have examined the role of the BTB in spermatogenesis until this past decade. There are some incentives to better understand BTB functionally in recent years among investigators who seek to develop male contraceptives that exert its effects on germ cell maturation and/or meiosis behind the BTB since it poses a barrier limit the uptake of drugs into the testis (Amory, et al. 2011, Cheng, et al. 2005, Chung, et al. 2011, Grima, et al. 2001, Hild, et al. 2007, Hild, et al. 2001, Tash, et al. 2008). Studies have shown that multiple drug transporters are highly expressed by Sertoli cells which thus significantly reduce the bioavailability of many potential contraceptive drugs under investigation and development (for reviews, see (Cheng and Mruk 2012, Mruk, et al. 2011)). In short, the BTB not only poses as an important barrier to protect harmful substances from reaching the adluminal compartment to perturb spermatogenesis, but also restricts the access of potential male contraceptives to disrupt spermatogenesis transiently, rendering these contraceptives less effective. This thus potentiates the toxicity of these contraceptives because higher concentrations of these drugs are required to be administered. In this review, we highlight some of the latest developments in the field regarding the biology of the BTB since several recent reviews have addressed some other interesting aspects of the BTB biology (Cheng and Mruk 2012, Franca, et al. 2012, Kaur, et al. 2014, Mital, et al. 2011, Mruk and Cheng 2015, Pelletier 2011). In particular, we will focus on the regulation of Sertoli cell cytoskeletons by actin binding proteins (ABPs), the intrinsic activity of which may be modulated through non-receptor protein kinases to regulate the plasticity of the barrier.
2. Structure and function of the blood-testis barrier (BTB)
2.1. Structural features
Unlike other blood-tissue barrier, TJs at the BTB formed between adjacent Sertoli cells near the basement membrane of the seminiferous epithelium are surrounded by actin filament bundles, found on both sides of the Sertoli cell, that lie perpendicular to the Sertoli cell plasma membrane and sandwiched between the cisternae of endoplasmic reticulum and the plasma membrane (for reviews, see (Fawcett 1975, Setchell and Waites 1975)), making the BTB a unique blood-tissue barrier (Figure 1). These two arrays of actin microfilament bundles that enforce and support the TJ between adjacent Sertoli cells create a unique ultrastructure known as the ectoplasmic specialization (ES), and due to its restrictive presence near the basal compartment, it is called basal ES (Russell 1977a, 1978). Also, ES is found at the Sertoli-spermatid interface, similar to the basal ES ultrastructurally except that the actin microfilament bundles are only detected in the Sertoli cell, and it is restrictively present in the apical compartment, and thus named apical ES (Russell 1977b). Subsequent studies have shown that the actin microfilament bundles at the basal ES also support the function of gap junction (GJ), and as such, the BTB is comprised by both TJ and GJ and are intimately supported by actin microfilaments of the basal ES (for reviews, see (Cheng and Mruk 2010, 2012, Vogl, et al. 2008)). Thus, in order to better understand the biology of the BTB, the regulation of the Sertoli cell cytoskeletons that constitute the BTB must be elucidated. It is obvious that remodeling of the BTB to support preleptotene spermatocyte transport and the selective entry of biomolecules into the adluminal compartment requires rapid conversion of the actin microfilaments between their “bundled” to “unbundled/branched” configuration. In the sections below, recent findings regarding cytoskeletal remodeling regulated by different ABPs are evaluated.
2.2. Functions of the BTB
BTB provides a barrier that restricts the flow of small biomolecules, ions, sugars, and other substances including water between the basal and the apical compartments (transcellular barrier), and also between adjacent Sertoli cells (paracellular barrier). Studies have compared the composition of the seminiferous tubule fluid recovered from the tubule lumen or rete testis fluid in the rete testis compartment vs. the interstitial fluid in the interstitial space and blood plasma, and showed that they are considerably different (Setchell 1980, Setchell and Waites 1975, Turner, et al. 1984). It was reported that the protein content of rete testis fluid, seminiferous tubule fluid, interstitial fluid and serum of adult rats was about 0.6, 30, 40, and 60 mg/ml, respectively (Cheng, et al. 1986). These differences are contributed by the BTB as well as by the ability of the epithelial cells of different compartments (e.g., rete testis, seminiferous tubules) to secrete (or re-absorb) different proteins and fluid into (or from) the lumen. Besides these obvious functions of the BTB, it also confers polarity to the Sertoli cell in the seminiferous epithelium. One of the most typical features of Sertoli cell polarity is the conspicuous presence of the nucleus near the basement membrane. Other organelles, such as phagosomes and Golgi apparatus, are also located mostly near the basolateral region of the Sertoli cell. The BTB also physically divides the seminiferous epithelium into the apical and basal compartment in which the events of meiosis I/II and spermiogenesis all take place in a specialized microenvironment, namely the apical (adluminal) compartment. It is known that the BTB segregates post-meiotic spermatids from cells in the basal compartment (e.g., spermatogonial stem cells (SSC), spermatogonia and preleptotene spermatocytes) and cells in the interstitium (e.g., resident macrophages, Leydig cells, endothelial cells of the microvessels, fibroblasts), thereby sequestering late stage spermatocyte- and spermatid-specific antigens from the systemic circulation. Studies have shown that the BTB likely contributes relatively little to confer the immune privilege status of the testis by sequestering germ cell antigens from the systemic circulation (for reviews, see (Meinhardt and Hedger 2011, O’Bryan and Hedger 2008)) since it is known that autoantigenic germ cells (e.g., SSC, spermatogonia, preleptotene spermatocytes) reside in the basal compartment, located outside the BTB (Yule, et al. 1988). In fact, many cancer/tissue (CT) antigens (most are products of oncogenes) are abundantly expressed by germ cells in the basal compartment (e.g., pachytene spermatocytes, round/elongating/elongated spermatids and spermatozoa) as well as those in the apical compartment (for reviews, see (Cheng, et al. 2011, Simpson, et al. 2005)). It is of interest to note that the microenvironment in the apical compartment created by the BTB is essential to maintain meiosis and spermiogenesis, since irreversible disruption of the BTB is known to cause infertility, such as sterility caused by toxicants glycerol and cadmium (Hew, et al. 1993, Setchell and Waites 1970, Wiebe, et al. 2000), and also a potential male contraceptive adjudin when used at an acute high dose (Mok, et al. 2012), which are associated with a permanent BTB damage. Other studies have shown that the immune privilege status of the testis is likely contributed mostly by biological factors (e.g., cytokines) secreted by Sertoli cells (or primary cultures of Sertoli cells, but not Sertoli cell line), making Sertoli cells a prime candidate to serve as immunosuppressive cells for allogeneic and also xenogeneic transplantation (Dufour, et al. 2008, Dufour, et al. 2004, Kaur, et al. 2013, Kaur, et al. 2014, Mital, et al. 2010). In fact, recent studies have demonstrated successful long-term stability of Sertoli cell-based cell transplant therapy for several pathological conditions (Luca, et al. 2013, Luca, et al. 2015). In short, unlike other blood-tissue barriers which are conferred mostly by endothelial TJ-barrier of microvessels, BTB is constituted by coexisting TJ, basal ES and GJ, along with intermediate filament-based desmosome between adjacent Sertoli cells near the basement membrane in the epithelium of seminiferous tubules.
3. Cytoskeletons of the BTB
As briefly reviewed above, one of the most prominent features of the BTB vs. other blood-tissue barriers by electron microscopy is the prominent presence of actin microfilament bundles and microtubules (MTs) between Sertoli cells near their cortical zone that constitute the barrier (Figure 1) (Fawcett 1975, Vogl, et al. 2008). Thus, it is envisioned that these two cytoskeletons are playing a crucial role in BTB physiology during the epithelial cycle (Tables 1 and 2). In fact, the significance of actin- and MT-based cytoskeletons in maintaining the ES and tubule integrity has been reported almost three decades ago in studies using toxicants that are known to disrupt actin or MT filaments (Russell, et al. 1988, Russell, et al. 1981). Studies using environmental toxicants (e.g., 2, 5-hexanedione, carbendazim) have also demonstrated that the MT-based cytoskeleton in Sertoli cells is the primary target of these toxicants. In short, 2,5-hexanedione and/or carbendazim induce germ cell maturation arrest, disruption of fluid secretion by Sertoli cells leading to tubule atrophy, failure in spermatid transport, and an increase in cell apoptosis, thereby causing progressive germ cell exfoliation (for reviews, see (Boekelheide, et al. 2003, Johnson 2014)). Other studies have also illustrated the disruption of actin microfilaments by cadmium and glycerol leads to TJ fibrils truncation, defragmentation of actin microfilaments, and BTB disruption, coinciding with germ cell exfoliation (Hew, et al. 1993, Wiebe, et al. 2000) since many of the adhesion protein complexes at the BTB utilize actin filaments for attachment (for a review, see (Cheng and Mruk 2002)). Interestingly, many of the toxicant-mediated defects in spermatogenesis are shared by other male contraceptive drugs under development such as adjudin which are known to exert their effects behind the BTB in the adluminal compartment (for a review, see (Cheng 2014)). Studies performed in the past decade have shown that the actin-based cytoskeleton is also a target of toxicants (for a review, see (Johnson 2014)), in particular adjudin (for a review, see (Cheng 2014)). Interestingly, the precise mechanism(s) by which cytoskeletons are disrupted following exposure of male rodents and/or humans to environmental toxicants remains largely unexplored until recent years. It is noted that actin microfilaments at the basal ES confer the adhesive strength of Sertoli cell-cell junctions at the BTB which also serve as the attachment site for adhesion protein complexes of the TJ (e.g., occludin-ZO-1), basal ES (e.g., N-cadherin-β-catenin) and GJ (e.g., connexin 43) (for reviews, see (Cheng and Mruk 2002, Mruk and Cheng 2004)). Actin microfilaments, similar to MTs, are also polarized structures in mammalian cells including Sertoli cells in whch the fast-growing end called the barbed end, and the slow-growing end known as the pointed end (Pellegrin and Mellor 2007, Schnittler, et al. 2014). Studies have shown that actin dynamics are regulated by a number of ABPs (actin binding proteins), some of which, besides binding to and regulatory proteins (Table 1).
Table 1.
Actin binding proteins (ABPs) that regulate Sertoli cell actin-based cytoskeleton at the BTB*
| Actin binding protein (ABP) | Mr in the testis (kDa) | Expression pattern in the seminiferous epithelium during the epithelial cycle | Function at the BTB | Phenotype(s) following KO in mice |
|---|---|---|---|---|
| Arp3 | 45 | Expressed at the BTB in stage VII–IX tubules, highest at stage VIII) (Lie, et al. 2010a) | Induces branched actin polymerization | Arp3 KO mice died at blastocysts stage (Vauti, et al. 2007) |
| Eps8 | 97 | Highly expressed at the BTB in stage V–VI (Lie, et al. 2009a) | Actin barded end capping and bundling | Eps8 KO mice were healthy and fertile (Scita, et al. 1999) |
| Espin | 110 | Expressed in the basal compartment, consistent with its localization at the BTB (Bartles, et al. 1996) | Actin bundling | Espin KO mice were viable and fertile (Revenu, et al. 2012) |
| Ezrin | 85 | Expressed in all stages at the BTB except stage IX (Gungor-Ordueri, et al. 2014b) | Actin bundling, constituent protein of intercellular bridges | Ezrin KO mice did not survive more than 1.5-wk after birth (Saotome, et al. 2004, Tamura, et al. 2005) |
| Drebrin E | 110 | Highly expressed at the BTB in stages IV–V and considerably diminished in stages VIII–XIV (Li, et al. 2011) | An adaptor protein with high affinity to Arp3 by recruiting Arp3 to the BTB | Not known |
| Fascin1 | 54 | Expressed at the BTB in all stages but considerably diminished in stage VIII (Gungor-Ordueri, et al. 2014a) | Actin bundling | Fascin 1 KO mice were viable and fertile (Yamakita, et al. 2009) |
| Formin 1 | 180 | Expressed at the BTB at stages I–VII, highly expressed at stage VI, diminished at stage VII, undetectable at stage VIII (Li, et al. 2015b) | Induces actin polymerization from the barbed (fast-growing) end | Formin 1 KO mice were viable and fertile (Zhou, et al. 2009) |
| Palladin | 95 | Highly expressed at stages I–V, diminished at stages VII–VIII (Qian, et al. 2013b) | Actin bundling | Palladin KO mice died around E15.5 day (Luo, et al. 2005) |
| Plastin 3 | 70 | Expressed in all stages but considerably reduced at stage VIII (Li, et al. 2015c) | Actin bundling | Not known |
| Rai14 | 110 | Expressed at the BTB in all stages but considerably diminished at stages VI–VII (Qian, et al. 2013a) | Actin binding, structurally associated with palladin | Not known |
| Vinculin | 130 | Expressed at the BTB (Mulholland, et al. 2001) | Adaptor protein, it has high affinity to α-actinin, an actin cross-linking protein | Vinculin KO mice could not survive beyond E10 day (Xu, et al. 1998) |
This Table was not intended to be exhaustive, it summarized the functional properties of some actin binding proteins known to be involved in regulating Sertoli cell actin microfilaments or by recruiting proteins that can regulate actin microfilament dynamics. Abbreviations used: Arp3, actin-related protein 3; BTB, blood-testis barrier; Eps8, epidermal growth factor receptor pathway substrate 8; KO, knockout; Rai14, retinoic acid induced protein 14.
Table 2.
Microtubule associated proteins that regulate microtubule (MT) dynamics at the Sertoli cell BTB*
| MAP | Mr in the testis (kDa) | Stage-specific expression during the epithelial cycle | Function at the BTB | Phenotype(s) following KO in mice |
|---|---|---|---|---|
| EB1 | 30 | Expressed at the BTB in all stages of the epithelial cycle (Tang, et al. 2015a) | +TIP that binds to the fast (+)growing end of a MT, promotes MT assembly and links MT ends to actin; it also serves as a regulator to cross-talk with F-actin network since EB1 knockdown perturbs actin microfilament organization at the Sertoli cell BTB (Tang, et al. 2015a) | not known |
| MARK4 | 79 | Highly expressed at the BTB in stages VI–IX (Tang, et al. 2012a) | A Ser-/Thr-kinase, activates MAPs such as Tau, involved in cell polarization | MARK4 KO mice are viable and fertile (Sun, et al. 2012) |
This Table was not intended to be exhaustive, it summarized the functional properties of some MT associated proteins that regulate MT dynamics at the BTB. EB1, end binding protein 1, a plus (+)-end tracking protein, p-TIP; KO, knockout; MAP, microtubule-associated protein; MARK4, MAP/microtubule affinity-regulating kinase,
On the other hand, MTs serve as the rails (or tracks) in Sertoli cells for germ cell transport (most notably spermatids), phagosome transport, as well as for intracellular transport involved in organelle positioning, cell shape, cell polarity, cell division and endocytic vesicle-mediated trafficking (for reviews, see (Lie, et al. 2010c, O’[Donnell and O’Bryan 2014, O’Donnell 2014, Tang, et al. 2013, Tang, et al. 2015b, Vogl, et al. 2008)). MTs are also polarized structures in Sertoli cells with the fast growing plus (+) end and the slow growing minus (−) ends located near the basement membrane in the basal compartment and near the tubule lumen in the apical compartment, respectively. MT dynamics are also regulated by a number of MT binding proteins, yet few studies are found in the literature that investigate the role of these MT binding proteins on MT dynamics in the testis (Table 2) (for reviews, see (O’Donnell and O’Bryan 2014, Tang, et al. 2013, Tang, et al. 2015b)). Nonetheless, both cytoskeletal filaments undergo extensive re-organization involving polymerization (i.e., nucleation), depolymerization, stabilization, bundling, capping, branching and severing/cleavage so that their configuration and/or organization can be rapidly altered in Sertoli cells in response to changes of the epithelial cycle, similar to other mammalian and non-mammalian cells (Arous and Halban 2015, Hausott and Klimaschewski 2015, Lie, et al. 2010c, O’Donnell, et al. 2011, O’Donnell and O’Bryan 2014, O’Donnell 2014, Tang, et al. 2015b, Zheng and Iglesias 2013). Furthermore, studies have shown that the actin- and MT-based cytoskeletons are working in concert to modulate multiple mammalian cell functions including spermatogenesis in the testis (for reviews, see (Coles and Bradke 2015, O’Donnell, et al. 2011, O’Donnell 2014, Poulter and Thomas 2015, Schappi, et al. 2014, Tang, et al. 2015b)). For instance, a recent report has shown that a knockdown of EB1 [end binding protein 1, a member of the growing +TIPs (plus (+) end tracking proteins) (for reviews, see (Akhmanova and Steinmetz 2010, Jiang and Akhmanova 2011))] which is known to promote MT stabilization and to serve as an adaptor to recruit other +TIP proteins to the growing MT plus ends (Tamura and Draviam 2012), not only affects the organization of MTs but also actin microfilaments in Sertoli cells (Tang, et al. 2015a) (Table 2). In short, EB1 knockdown causes retraction of tubulin from cell cytosol including the cortical zone that support Sertoli cell-cell contacts to be closer to the cell nucleus, this in turn perturbs the Sertoli cell TJ-permeability function (Tang, et al. 2015a). Interestingly, EB1 knockdown also causes defragmentation of actin microfilaments, leading to disorganization of actin filament bundles at the ES, this thus destabilizes adhesion proteins at the Sertoli cell TJ and basal ES including CAR, ZO-1 and N-cadherin, contributing to a failure of TJ-barrier function (Tang, et al. 2015a). Collectively, these findings support the notion that the actin and MT cytoskeletons are working in concert to support spermatogenesis, and that EB1 is likely to be one of the proteins which mediates the necessary cross-talk between the two cytoskeletons.
4. Regulation of BTB actin-based cytoskeleton by ABPs and protein kinases
4.1. Introduction
As briefly reviewed above, there are few published studies that examined the regulation of MT-based cytoskeleton except for katanin (a MT cleavage protein, its mutation leads to infertility in males during to failure in spermiogenesis in mice and humans (O’Donnell, et al. 2014, O’Donnell, et al. 2012, Smith, et al. 2012)), MARK4 (microtubule affinity-regulating kinase 4, a MT stabilizing protein whose down-regulation induced by adjudin leads to spermatid exfoliation from the seminiferous epithelium in adult rats (Tang, et al. 2012b), and EB1 (a +TIP that promotes plus end polymerization of MT (Tang, et al. 2015a)). Since the involvement of MT in BTB function has recently been reviewed (for reviews, see (O’Donnell 2014, Tang, et al. 2015b)), we focus our discussion on the actin-based cytoskeleton herein since there are more recent published findings on this subject that have not been reviewed. We also provide a model herein (Figure 2) which can serve as a helpful guide to investigators in the field.
4.2. Actin binding proteins (ABPs)
As noted above, the best studied ABPs in the testis that play crucial roles in BTB regulation are actin bundling proteins (e.g., Eps8, palladin, fascins, plastins, ezrin) and branched actin nucleation proteins (e.g., Arp2/3, N-WASP) (Table 1). These two groups of proteins modulate the conversion of actin microfilaments between their bundled and unbundled/branched configuration, thereby conferring plasticity to the basal ES, which is necessary to facilitate the transport of preleptotene spermatocytes across the BTB. This rapid conversion of actin microfilaments between the bundled and unbundled/branched configuration also supports other intracellular trafficking events including the transport of organelles (e.g., transport of engulfed residual bodies by Sertoli cells near the tubule lumen at stage VIII of the cycle in the apical compartment to the basal compartment while transforming into phagosomes) and endocytic vesicle-mediated trafficking events involved in protein endocytosis, transcytosis and recycling. In fact, the basal ES generates an ultrastructure known as the basal tubulobulbar complex (basal TBC), representing giant endocytic vesicles used for protein trafficking which can be readily detected by fluorescence or electron microscopy (Russell 1979, Xiao, et al. 2014c) (for reviews, see (Vogl, et al. 2008, Vogl, et al. 2013)). As such, proteins at the degenerating “old” BTB above preleptotene spermatocytes connected in clones (Weber and Russell 1987) that are being transported across the barrier can be recycled to assemble the “new” BTB behind these spermatocytes (Smith and Braun 2012, Yan, et al. 2008a) (for reviews see (Cheng and Mruk 2010, Mruk and Cheng 2004). Thus, proteins at the BTB that serve as building blocks do not require continuous de novo synthesis throughout the epithelial cycle to avoid exhausting the limited metabolic resources and ability of the Sertoli cells. This is necessary since each Sertoli cell is required to nurture ~30–50 germ cells at different stages of their development during the epithelial cycle (Weber, et al. 1983).
4.2.a. Actin bundling proteins
As noted above, ES is constituted conspicuously by an array of actin microfilament bundles found on both sides of the adjacent Sertoli cells near the basement membrane that support TJ and GJ to confer the barrier function of the BTB (Figure 1), it is not unusual that these structures are maintained by actin bundling proteins. Indeed, Eps8 (also an actin barbed end capping protein that reduces actin microfilament branching) (Lie, et al. 2009b), palladin (Qian, et al. 2013c), ezrin (Gungor-Ordueri, et al. 2014b), plastin 3 (Li, et al. 2015a), fascin 1 (Gungor-Ordueri, et al. 2014a), and formin 1 (also an actin nucleation protein that promotes microfilament elongation besides conferring bundling of actin microfilaments) (Li, et al. 2015b) recently have been shown to be actively involved in actin microfilament bundling in Sertoli cells (Table 1) and to confer Sertoli cell TJ-permeability barrier function, since their knockdown by RNAi is known to induce reorganization of actin microfilaments including defragmentation and disorganization. These changes thereby destabilize cell adhesion protein complexes at the BTB (e.g., occludin-ZO-1, N-cadherin-β-catenin), causing their re-distribution so that they no longer localize strictly near the cell surface to support cell adhesion, and internalize to the cell cytosol. The net result of these changes perturbs the Sertoli cell TJ-barrier function. In this context, it is of interest to note that these changes involve an intriguing alteration on the spatiotemporal expression of several actin bundling and branched actin-inducing proteins. For instance, a knockdown of plastin 3 by RNAi that causes unbundling and defragmentation of actin microfilaments across the Sertoli cell cytosol is the result of a down-regulation of actin bundling/barbed-end capping protein Eps8 and also actin bundling protein palladin at the Sertoli cell-cell interface, which are coupled with an internalization of branched actin polymerization protein Arp3 (Li, et al. 2015a). These findings thus illustrate that actin bundling proteins are working in concert as a set of regulators to support actin microfilament bundles at the basal ES to confer BTB function, possibly via their spatiotemporal expression across different microdomains of the Sertoli cell, in particular at the site near the tunica propria. Studies in vivo also support the notion that these actin bundling proteins are involved in conferring actin microfilament bundles the proper configuration in the seminiferous epithelium to support basal ES/BTB function. For instance, the expression of Eps8, plastin 3, fascin 1 and formin 1 are stage-specific, being highest in stages V–VII, but rapidly and considerably diminished in late stage VII through early stage VIII (Gungor-Ordueri, et al. 2014a, Li, et al. 2015a, Li, et al. 2015b, Lie, et al. 2009b). This timely down regulation of actin bundling proteins thus support re-organization of the basal ES to facilitate BTB restructuring so that preleptotene spermatocytes can be transported across the BTB in stage VIII of the epithelial cycle (Figure 2).
4.2.b. Branched actin nucleation proteins
The best studied branched actin polymerization protein in the mammalian tissues and cells is the Arp2/3 complex which when activated by N-WASP upstream, the complex induces barbed end nucleation, effectively converting actin microfilaments from a bundled to a branched configuration (Cheng and Mruk 2011, Derivery and Gautreau 2010) (Table 1). The Arp2/3 complex, when it is working in concert with bundling proteins such as palladin, Eps8, ezrin, and plastin, confer plasticity to the actin-based cytoskeleton, necessary for cell motility, cell division, development, and also endocytic vesicle-mediated protein trafficking (Blanchoin, et al. 2014, Caceres, et al. 2015, Coticchio, et al. 2015, Dawson, et al. 2006). As depicted in Figure 2, Arp3 has been shown to work in coordination with the actin bundling proteins so that these two ABPs provide an effective mechanism to facilitate the transport of preleptotene spermatocytes. For instance, the considerable down-regulation of Eps8 (Lie, et al. 2009b) and plastin 3 (Li, et al. 2015a) at the basal ES/BTB to a level that is virtually non-detectable at stage VIII to facilitate the breakdown of actin microfilament bundles at the site is associated with a steady expression of Arp3 (Lie, et al. 2010b), the net result thus favors endocytic vesicle-mediated protein trafficking (for reviews, see (Cheng and Mruk 2010, Dawson, et al. 2006)) so that adhesion proteins from the “old” BTB near the apical region of preleptotene spermatocytes can be endocytosed, transcytosed and recycled to establish the “new” BTB behind the germ cells (Smith and Braun 2012, Yan, et al. 2008a) (Figure 2). Nonetheless, this hypothesis requires additional experiments to confirm in future studies. Furthermore, much work is needed to identify other crucial players to be involved in these cellular events.
4.3. c-Src and c-Yes
Studies have shown that Src-family kinase (SFK) signaling (including c-Src and c-Yes) are involved in actin-based cytoskeletal dynamics in mammalian cells and tissues including the Sertoli cell BTB (for reviews, see (Lavoie, et al. 2010, Xiao, et al. 2012)). For instance, treatment of Sertoli cells with an established TJ-permeability barrier using SU6656 at 20 nM, a selective inhibitor of c-Yes with an IC50 at 20 nM vs. Src, Fyn and Lyn (all are members of SFK family) at 280 nM, 170 nM and 130 nM, respectively (Blake, et al. 2000, Bowman, et al. 2001), has been shown to cause defragmentation of actin microfilaments across the Sertoli cell cytosol and also retraction of F-actin from cell cortical zone to cell cytosol, but the presence of testosterone protects Sertoli cells from the disruptive effects of the c-Yes inhibitor (Xiao, et al. 2011). These observations were subsequently confirmed by c-Yes specific knockdown by RNAi since the silencing of c-Yes by ~70% indeed perturbed F-actin organization in Sertoli cells, leading to actin microfilament defragmentation (Xiao, et al. 2013). As such, TJ protein occludin and basal ES protein N-cadherin no longer tightly associate with the BTB, leading to BTB disruption based on an in vivo study (Xiao, et al. 2013). Studies using biochemical assays and knockdown of c-Yes or c-Src using specific siRNA duplexes vs. non-targeting negative control duplexes to monitor protein endocytosis, recycling and degradation, c-Yes has been shown to promote endocytosed integral membrane proteins (e.g., JAM-A, CAR) to the pathway of transcytosis and recycling, whereas c-Src promotes intracellular degradation of endocytosed proteins at the Sertoli cell BTB (Xiao, et al. 2014c). Collectively, these findings illustrate that c-Src and c-Yes are working in concert to modulate F-actin organization at the Sertoli cell BTB, in particular their role in endocytic vesicle-mediated protein trafficking to determine the fate of endocytosed proteins differentially as depicted in a working model in Figure 2.
4.4. Focal adhesion kinase (FAK)
Studies in vitro (Lie, et al. 2012) and in vivo (Wan, et al. 2013) based on the use of phosphomimetic vs. non-phosphorylatable mutants have shown that the two phosphorylated (activated) forms of FAK, namely p-FAK-Tyr407 and p-FAK-Tyr397, are crucial regulators of ES including the basal ES/BTB and the apical ES in the rat testis (for reviews, see (Wan, et al. 2014, Xiao, et al. 2014b)). For instance, p-FAK-Tyr407 is highly expressed at the basal ES/BTB in the testis based on studies using dual-labeled immunofluorescence analysis and confocal microscopy (Lie, et al. 2012). p-FAK-Tyr407 also modulates the intrinsic branched actin nucleation activity of the Arp2/3 complex in Sertoli cells cultured in vitro with an established functional TJ-barrier (Lie, et al. 2012), illustrating p-FAK-Tyr407 is involved in actin microfilament organization at the ES. Indeed, consistent with these findings, Sertoli cell-specific deletion of N-WASP in the mouse testis, the upstream activator of the Arp2/3 complex, also leads to male infertility (Rotkopf, et al. 2011), due to a failure in spermiogenesis and a permanent BTB disruption (Xiao, et al. 2014a), possibly the result of a loss of plasticity of the F-actin network to be able to convert between bundled and unbundled/branched configuration. Also, a loss of the ability of Sertoli cells in the seminiferous epithelium to re-organize actin microfilaments following N-WASP Sertoli cell-specific KO also leads to a considerable reduction of p-FAK-Tyr438 (which corresponds to p-FAK-Tyr407 in the rat testis) since the disorganized F-actin network at the BTB fails to support proper spatiotemporal expression of p-FAK-Tyr438 to confer BTB function (Xiao, et al. 2014a). Collectively, these findings thus illustrate an intimate functional relationship between F-actin organization and p-FAK-Tyr407. In fact, Arp3 may be a putative substrate of p-FAK-Tyr407 in the rat testis, which should be carefully evaluated in future studies. Figure 2 also depicts the likely involvement of p-FAK-Tyr407 in regulating actin microfilament organization at the Sertoli cell BTB in the rat testis.
5. Regulation of the BTB via the apical ES-BTB-basement membrane axis
Earlier studies have shown that the events of spermiation and BTB remodeling that occur at the opposite ends of the seminiferous epithelium at stage VIII of the epithelial cycle are coordinated via a local functional axis known as the apical ES-BTB-basement membrane axis (Yan, et al. 2008b). In short, fragments of laminin chains at the apical ES during its degeneration to facilitate the release of sperm at spermiation are generated, possibly mediated by MMP2 cleavage (Siu and Cheng 2004). These fragments have been shown to possess potent biological activity to induce BTB restructuring by perturbing the Sertoli cell TJ-permeability barrier function (Yan, et al. 2008b). Subsequent studies have identified the biologically active domain in one of these fragments designated F5-peptide, which is capable of perturbing the Sertoli cell TJ-barrier in studies both in vitro and in vivo (Su, et al. 2012), illustrating the physiological significance of this axis in regulating BTB function during spermatogenesis. The model depicted in Figure 2 illustrates the likely role of these biologically active fragments in coordinating the events of BTB restructuring to facilitate the transport of preleptotene spermatocytes at stage VIII of the epithelial cycle. Since this topic has recently been reviewed (Cheng and Mruk 2010), we thus only briefly summarize these findings herein. One the other hand, a recent report from our laboratory has demonstrated a biologically active fragment released from collagen chains in the basement membrane, such as the NC1 (non-collagenous domain 1) domain, is also potent regulator to modulate BTB function (Wong and Cheng 2013), illustrating a functional link between the BTB and the basement membrane. Much work is needed to understand the role and the biology of biologically active laminin chains generated at the apical ES, and NC1 domain released from collagens in the basement membrane, to modulate basal ES/BTB function in the testis.
6. Future perspectives and concluding remarks
As briefly discussed herein, the BTB in the mammalian testis is regulated by cytoskeletons. There is accumulating evidence that microtubule (MT)-based cytoskeleton is also involved. For instance, the knockdown of EB1 (end binding protein 1, a MT plus (+) end targeting protein, +TIP) that is known to stabilize MTs in Sertoli cells has been shown to perturb the Sertoli cell TJ-barrier function by disorganizing MT configuration, in which MTs no longer stretch across the Sertoli cell cytosol but retract closer to the cell nuclei (Tang, et al. 2015a). Furthermore, actin microfilament defragmentation and disorganization are also noted in Sertoli cells following EB1 knockdown, which are mediated by changes in the spatiotemporal expression of Arp3 in which Arp3 is considerably expressed in cell cytosol, closer to the cell nucleus instead of at the cortical zone as in control Sertoli cell epithelium (Tang, et al. 2015a). Thus, F-actin microfilaments no longer assume a conspicuous bundled configuration that stretch across the entire Sertoli cell following EB1 knockdown (Tang, et al. 2015a), illustrating EB1 can also modulate actin microfilament organization. There is mounting evidence in the literature to suggest that some of the MT or actin microfilament regulatory proteins modulate both cytoskeletons such as in neurons (for a review, see (Coles and Bradke 2015)). For instance, formin 1, a barbed end actin nucleation protein that promotes actin polymerization to form long stretches of actin microfilaments that plays a critical role in ES dynamics including the basal ES/BTB in the rat testis (Li, et al. 2015b) is also an emerging ABP that regulates MT dynamics (for a review, see (Chesarone, et al. 2010)). The model depicted in Figure 2 illustrates the likely interactions of different classes of regulatory proteins (e.g., actin bundling proteins, branched actin polymerization proteins, and non-receptor protein kinases) to modulate cytoskeletal functions at the basal ES/BTB to regulate the transport of preleptotene spermatocytes across the barrier. It is obvious that these proteins (Tables 1 and 2) can now be investigated as a whole to better understand the precise mechanism(s) by which each class of these proteins is interacting with the other to modulate junction remodeling at the BTB. There are many questions that remain unanswered. For instance: What is the precise mechanism through which FAK, c-Src and c-Yes interact with one another to modulate ABPs and other MT binding proteins? Is this mediated by protein phosphorylation to unleash the intrinsic activities of these ABPs, such as bundling activity of palladin? Are these events regulated by cytokines and/or testosterone or other biomolecules? How do Sertoli cells across the seminiferous epithelium coordinate with one another during the epithelial cycle to modulate their cytoskeletal organization? Is this mediated by intercellular bridges? If it is, what are the biomolecules that trigger this coordination? It is likely that many of these questions will be answered in the next few years, which will provide a better picture regarding the functional regulation of the BTB to support spermatogenesis.
Acknowledgments
This work was support by grants from the National Institutes of Health (R01 HD056034 to C.Y.C. and U54 HD029990, Project 5 to C.Y.C.)
Footnotes
Conflict of interest statement: Authors have nothing to declare
References
- Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123:3415–3419. doi: 10.1242/jcs.062414. [DOI] [PubMed] [Google Scholar]
- Amory JK, Muller CH, Shimshoni JA, Isoherranen N, Paik J, Moreb JS, Amory DWS, Evanoff R, Goldstein AS, Griswold MD. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl. 2011;32:111–119. doi: 10.2164/jandrol.110.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arous C, Halban PA. The skeleton in the closet: actin cytoskeletal remodeling in β-cell function. Am J Physiol Endocrinol Metab. 2015 doi: 10.1152/ajpendo.00268.2015. in press. [DOI] [PubMed] [Google Scholar]
- Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109(Pt 6):1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, Kwon EJ, Patel SR, Rasoulpour RJ, Schoenfeld HA, Thompson S. 2,5-Hexanedione-induced testicular injury. Annu Rev Pharmacol Toxciol. 2003;43:125–147. doi: 10.1146/annurev.pharmtox.43.100901.135930. [DOI] [PubMed] [Google Scholar]
- Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDFG-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres R, Abou-Ghali M, Plastino J. Reconstituting the actin cytoskeleton at or near surfaces in vitro. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- Cheng CY. Toxicants target cell junctions in the testis - insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4:e981485. doi: 10.4161/21565562.2014.9814895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Gunsalus GL, Morris ID, Turner TT, Bardin CW. The heterogeneity of rat androgen binding protein (rABP) in the vascular compartment differs from that in the testicular tubular lumen: further evidence for bidirectional secretion of rABP. J Androl. 1986;7:175–179. doi: 10.1002/j.1939-4640.1986.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Wong EWP, Cheng CY. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis. 2011;1:209–220. doi: 10.4161/spmg.1.3.17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- Chung SS, Wang X, Roberts SS, Griffey SM, Reczek PR, Wolgemuth DJ. Oral administration of a retinoic acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology. 2011;152:2492–2502. doi: 10.1210/en.2010-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Coles CH, Bradke F. Coordinating Neuronal Actin-Microtubule Dynamics. Curr Biol. 2015;25:R677–691. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21:427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- Dufour JM, Dass B, Halley KR, Korbutt GS, Dixon DE, Rajotte RV. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant. 2008;17:525–534. doi: 10.3727/096368908785096033. [DOI] [PubMed] [Google Scholar]
- Dufour JM, Gores P, Hemendinger R, Emerich DF, Halberstadt CR. Transgenic Sertoli cells as a vehicle for gene therapy. Cell Transplant. 2004;13:1–6. doi: 10.3727/000000004772664833. [DOI] [PubMed] [Google Scholar]
- Easton AS. Regulation of permeability across the blood-brain barrier. Adv Exp Med Biol. 2012;763:1–19. doi: 10.1007/978-1-4614-4711-5_1. [DOI] [PubMed] [Google Scholar]
- Fawcett D. Ultrastructure and function of the Sertoli cell. In: Hamilton D, Greep R, editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1975. pp. 21–25. [Google Scholar]
- Fawcett DW, Leak LV, Heidger PM. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil. 1970;(Suppl 10):105–122. [PubMed] [Google Scholar]
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–1508. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- Gungor-Ordueri NE, Celik-Ozenci C, Cheng CY. Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis. Am J Physiol Endocrinol Metab. 2014a;307:E738–E753. doi: 10.1152/ajpendo.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY. Ezrin is an actin binding protein that regulates Sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology. 2014b;155:3981–3995. doi: 10.1210/en.2014-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B, Klimaschewski L. Membrane turnover and receptor trafficking in regenerating axons. Eur J Neurosci. 2015 doi: 10.1111/ejn.13025. [DOI] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- Hild SA, Marshall GR, Attardi BJ, Hess RA, Schlatt S, Simorangkir DR, Ramaswamy S, Koduri S, Reel JR, Plant TM. Development of I-CDB-4022 as a nonsteroidal male oral contraceptive: Induction and recovery from severe oligospermia in the adult male cynomolgus monkey (Macaca fascicularis) Endocrinology. 2007;148:1784–1796. doi: 10.1210/en.2006-1487. [DOI] [PubMed] [Google Scholar]
- Hild SA, Reel JR, Larner JM, Blye RP. Disruption of spermatogenesis and Sertoli cell structure and function by the indenopyridine CDB-4022 in rats. Biol Reprod. 2001;65:1771–1779. doi: 10.1095/biolreprod65.6.1771. [DOI] [PubMed] [Google Scholar]
- Jiang K, Akhmanova A. Microtubule tip-interacting proteins: a view from both ends. Curr Opin Cell Biol. 2011;23:94–101. doi: 10.1016/j.ceb.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4:e979106. doi: 10.4161/21565562.2014.979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Mital P, Dufour JM. Testis immune privilege - Assumptions versus facts. Anim Reprod. 2013;10:3–15. [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Thompson LA, Dufour JM. Sertoli cells - immunological sentinels of spermatogenesis. Sem Cell Dev Biol. 2014;30:36–44. doi: 10.1016/j.semcdb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Landry MC, Faure RL, Champagne C. Src-family kinase signaling, actin-mediated membrane trafficking and organellar dynamics in the control of cell fate: lessons to be learned from the adenovirus E4orf4 death factor. Cell Signal. 2010;22:1604–1614. doi: 10.1016/j.cellsig.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Li MW, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, Bonanomi M, Silvestrini B, Cheng CY. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Mruk DD, Wong CK, Lee WM, Han D, Cheng CY. Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis. FASEB J. 2015a;29:3788–3805. doi: 10.1096/fj.14-267997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Mruk DD, Wong CKC, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplamic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology. 2015b;156:2969–2983. doi: 10.1210/en.2015-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Mruk DD, Wong CKC, Lee WM, Han D, Cheng CY. Actin bundling protein plastin 3 is a regulator of ectoplasmic specialization (ES) dynamics during spermatogenesis in the rat testis. FASEB J. 2015c doi: 10.1096/fj.14-267997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Chan AY, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A. 2010a;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009a;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010b;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009b;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010c;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca G, Calvitti M, Mancuso F, Falabella G, Arato I, Bellucci C, List EO, Bellezza E, Angeli G, Lilli C, Bodo M, Becchetti E, Kopchick JJ, Cameron DF, Baroni T, Calafiore R. Reversal of experimental Laron Syndrome by xenotransplantation of microencapsulated porcine Sertoli cells. J Control Release. 2013;165:75–81. doi: 10.1016/j.jconrel.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Luca G, Mancuso F, Calvitti M, Arato I, Falabella G, Bufalari A, De Monte V, Tresoldi E, Nastruzzi C, Basta G, Fallarino F, Lilli C, Bellucci C, Baroni T, Aglietti MC, Giovagnoli S, Cameron DF, Bodo M, Calafiore R. Long-term stability, functional competence, and safety of microencapsulated specific pathogen-free neonatal porcine Sertoli cells: a potential product for cell transplant therapy. Xenotransplantation. 2015;22:273–283. doi: 10.1111/xen.12175. [DOI] [PubMed] [Google Scholar]
- Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, Xu G, Sun X, Kong H, Gu M, Chen S, Chen Z, Wang Z. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–515. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P, Kaur G, Dufour JM. Immunoprotective Sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. The mammalian blood-testis barrier: Its biology and regulation. Endocr Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (Ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis: chronic inflammation in an immunologically privileged tissues? Adv Exp Med Biol. 2008;636:92–114. doi: 10.1007/978-0-387-09597-4_6. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, McLachlan RI, Merriner DJ, O’Bryan MK, Jamsai D. KATNB1 in the human testis and its genetic variants in fertile and oligoasthenoteratozoospermic infertile men. Andrology. 2014;2:884–891. doi: 10.1111/andr.276. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. doi: 10.1016/j.semcdb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Rhodes D, Smith SJ, Merriner DJ, Clark BJ, Borg C, Whittle B, O’Connor AE, Smith LB, McNally FJ, de Kretser DM, Goodnow CC, Ormandy CJ, Jamsai D, O’Bryan MK. An essential role for katanin p80 and microtubule severing in male gamete production. PLoS Genet. 2012;8:e1002698. doi: 10.1371/journal.pgen.1002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibers. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Poulter NS, Thomas SG. Cytoskeletal regulation of platelet formation: Coordination of F-actin and microtubules. Int J Biochem Cell Biol. 2015;66:69–74. doi: 10.1016/j.biocel.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Qian X, Mruk DD, Cheng CY. Rai14 (retinoic acid induced protein 14) is involved in regulating f-actin dynamics at the ectoplasmic specialization in the rat testis*. PLoS One. 2013a;8:e60656. doi: 10.1371/journal.pone.0060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Mruk DD, Wong EW, Lie PP, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in adult rat testes. Endocrinology. 2013b;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013c;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C, Ubelmann F, Hurbain I, El-Marjou F, Dingli F, Loew D, Delacour D, Gilet J, Brot-Laroche E, Rivero F, Louvard D, Robine S. A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol Biol Cell. 2012;23:324–336. doi: 10.1091/mbc.E11-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotkopf S, Hamberg Y, Aigaki T, Snapper SB, Shilo BZ, Shejter ED. The WASp-based actin polymerization machinery is required in somatic support cells for spermatid maturation and release. Development. 2011;138:2729–2739. doi: 10.1242/dev.059865. [DOI] [PubMed] [Google Scholar]
- Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977a;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977b;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Russell LD. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978;190:99–112. doi: 10.1002/ar.1091900109. [DOI] [PubMed] [Google Scholar]
- Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–279. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–118. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- Russell LD, Malone JP, MacCurdy DS. Effect of the microtubule disrupting agents, colchicine and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell. 1981;13:349–367. doi: 10.1016/0040-8166(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Schappi JM, Krbanjevic A, Rasenick MM. Tubulin, actin and heterotrimeric G proteins: coordination of signaling and structure. Biochim Biophys Acta. 2014;1838:674–681. doi: 10.1016/j.bbamem.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittler H, Taha M, Schnittler MO, Taha AA, Lindemann N, Seebach J. Actin filament dynamics and endothelial cell junctions: the Ying and Yang between stabilization and motion. Cell Tissue Res. 2014;355:529–543. doi: 10.1007/s00441-014-1856-2. [DOI] [PubMed] [Google Scholar]
- Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- Setchell B. The functional significance of the blood-testis barrier. J Androl. 1980;1:3–10. [Google Scholar]
- Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Waites GMB. The blood-testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology. Section 7, Vol. V. Male Reproductive System. Washington, D.C: American Physiological Society; 1975. pp. 143–172. [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Simpson AJG, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Smith BE, Braun RE. Germ cell migration across Sertoli cell tight junctions. Science. 2012;338:798–802. doi: 10.1126/science.1219969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Milne L, Nelson N, Eddie S, Brown P, Atanassova N, O’Bryan MK, O’Donnell L, Rhodes D, Wells S, Napper D, Nolan P, Lalanne Z, Cheeseman M, Peters J. KATNAL1 regulation of Sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PLoS Genet. 2012;8:e1002697. doi: 10.1371/journal.pgen.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. doi:1110.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Tian L, Nie J, Zhang H, Han X, Shi Y. Inactivation of MARK4, an AMP-activated protein kinase (AMPK)-related kinase, leads to insulin hypersensitivity and resistance to diet-induced obesity. J Biol Chem. 2012;287:38305–38315. doi: 10.1074/jbc.M112.388934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, Suzuki Y, Noda T, Tsukita S. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–28. doi: 10.1083/jcb.200410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N, Draviam VM. Microtubule plus-ends within a mitotic cell are ‘moving platforms’ with anchoring, signalling and force-coupling roles. Open Biol. 2012;2:120132. doi: 10.1098/rsob.120132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats - an in vitro study. Endocrinology. 2015a;156:680–693. doi: 10.1210/en.2014-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217:R13–R23. doi: 10.1530/JOE-12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier. In: Ebnet K, editor. Cell Polarity. Vol. 1. Geneva: Springer International Publishing; 2015b. pp. 303–326. [DOI] [Google Scholar]
- Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, Lee WM, Mathur PP, Cheng CY. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012a;2:117–126. doi: 10.4161/spmg.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, Lee WM, Mathur PP, Cheng CY. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012b;2:117–126. doi: 10.4161/spmg.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod. 2008;78:1127–1138. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- Turner TT, Jones CC, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- Vauti F, Prochnow BR, Freese E, Ramasamy SK, Ruiz P, Arnold HH. Arp3 is required during preimplantation development of the mouse embryo. FEBS Lett. 2007;581:5691–5697. doi: 10.1016/j.febslet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol. 2013;303:319–355. doi: 10.1016/B978-0-12-407697-6.00008-8. [DOI] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, Cheng CY. p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab. 2013;305:E687–E699. doi: 10.1152/ajpendo.00254.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Tang EI, Xiao X, Cheng YH, Wong EW, Wong CK, Cheng CY. Role of non-receptor protein tyrosine kinases in spermatid transport during spermatogenesis. Semin Cell Dev Biol. 2014;30:65–74. doi: 10.1016/j.semcdb.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JE, Russell LD. A study of intercellular bridges during spermatogenesis in the rat. Am J Anat. 1987;180:1–24. doi: 10.1002/aja.1001800102. [DOI] [PubMed] [Google Scholar]
- Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Wiebe J, Kowalik A, Gallardi R, Egeler O, Clubb B. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–635. [PubMed] [Google Scholar]
- Wong EWP, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis. 2013;3:e25465. doi: 10.4161/spmg.25465. doi:23885310.23884161/spmg.23825465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–E159. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012;763:295–317. doi: 10.1007/978-1-4614-4711-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Tang EI, Massarwa R, Mok KW, Li N, Wong CK, Lee WM, Snapper SB, Shilo BZ, Schejter ED, Cheng CY. N-WASP is required for structural integrity of the blood-testis barrier. PLoS Genet. 2014a;10:e1004447. doi: 10.1371/journal.pgen.1004447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014b;29:286–298. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Wong EWP, Lee WM, Han D, Wong CKC, Cheng CY. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier: an in vitro study. Am J Physiol Endocrinol Metab. 2014c;307:E553–E562. doi: 10.1152/ajpendo.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Yamakita Y, Matsumura F, Yamashiro S. Fascin1 is dispensable for mouse development but is favorable for neonatal survival. Cell Motil Cytoskeleton. 2009;66:524–534. doi: 10.1002/cm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008a;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008b;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KSK. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. [PubMed] [Google Scholar]
- Zheng Y, Iglesias PA. Nucleating new branches from old. Cell. 2013;152:669–670. doi: 10.1016/j.cell.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Leder P, Zuniga A, Dettenhofer M. Formin1 disruption confers oligodactylism and alters Bmp signaling. Hum Mol Genet. 2009;18:2472–2482. doi: 10.1093/hmg/ddp185. [DOI] [PMC free article] [PubMed] [Google Scholar]