Abstract
Objectives
This study aimed to assess the factors limiting maximal exercise capacity in patients with chronic heart failure (CHF).
Background
Maximal exercise capacity, an important index of health in CHF, might be limited by central and/or peripheral factors; however, their contributions remain poorly understood.
Methods
We studied oxygen (O2) transport and metabolism at maximal cycle (centrally taxing) and knee-extensor (KE) (peripherally taxing) exercise in 12 patients with CHF and 8 healthy control subjects in normoxia and hyperoxia (100% O2).
Results
Peak oxygen uptake (VO2) while cycling was 33% lower in CHF patients than in control subjects. By experimental design, peak cardiac output was reduced during KE exercise when compared with cycling (approximately 35%); although muscle mass specific peak leg VO2 was increased equally in both groups (approximately 70%), VO2 in the CHF patients was still 28% lower. Hyperoxia increased O2 carriage in all cases but only facilitated a 7% increase in peak leg VO2 in the CHF patients during cycling, the most likely scenario to benefit from increased O2 delivery. Several relationships, peak leg VO2 (KE + cycle) to capillary-fiber-ratio and capillaries around a fiber to mitochondrial volume, were similar in both groups (r = 0.6-0.7).
Conclusions
Multiple independent observations, including a significant skeletal muscle metabolic reserve, suggest skeletal muscle per se contributes minimally to limiting maximal cycle exercise in CHF or healthy control subjects. However, the consistent attenuation of the convective and diffusive components of O2 transport (25% to 30%) in patients with CHF during both cycle and even KE exercise compared with control subjects reveals an underlying peripheral O2 transport limitation from blood to skeletal muscle in this pathology.
Keywords: blood flow, cardiac output, hyperoxia, oxygen supply, oxygen transport, oxygen use, skeletal muscle
Exercise intolerance is widely recognized as a defining symptom in patients with chronic heart failure (CHF), limiting physical activity and impairing quality of life (1). Traditionally, this attenuated exercise capacity has been attributed predominantly to the “central” limitations associated with a malfunctioning cardiac pump. However, attention has turned toward “peripheral” factors that might also contribute to the limited exercise capacity associated with CHF. A variety of alterations specific to both skeletal muscle (muscle atrophy, fiber type changes, reduced mitochondrial enzymes, decreased mitochondrial volume density) (1-4) and the vascular/skeletal muscle interface (greater sympathetic vasoconstrictor tone, decreased capillarity, and smaller capillary diameter) (5-7) have all been associated with CHF. However, the contribution of these skeletal muscle changes to limited exercise capacity in CHF is currently not well understood.
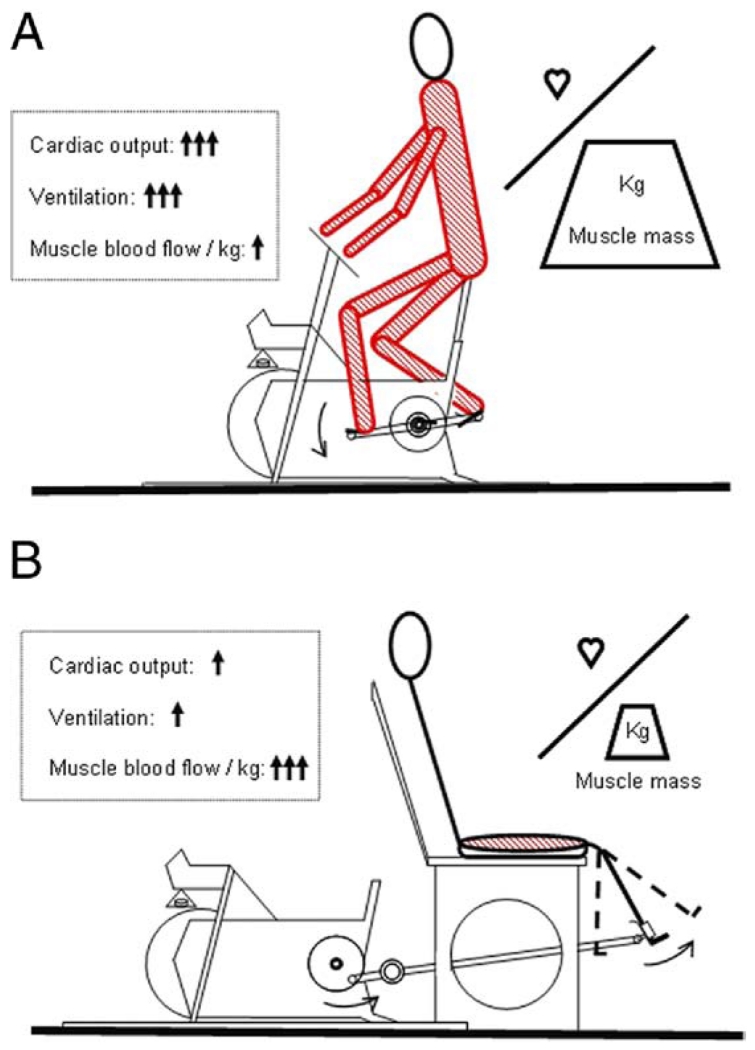
Studies of exercise limitation in CHF have predominantly used standard cycle ergometry, resulting in the recruitment of most of the muscles in the lower limbs and—to some extent— upper body muscles to accomplish the work (Fig. 1A) (8,9). With the limited maximal cardiac output associated with CHF, the involvement of such a large muscle mass is thought to be the major factor attenuating maximal exercise in this population. Although it has previously been recognized that skeletal muscle function and plasticity in CHF might be better assessed by changing exercise modality from conventional cycle exercise to a small muscle mass exercise, such as single-leg knee-extension (Fig. 1B) (9-14), identifying the role of centrally limited oxygen (O2) supply versus compromised peripheral O2 use (15,16) in both healthy matched control subjects and patients with CHF has never been attempted.
Figure 1. Exercise Modalities.
A schematic representation of the relatively small ratio of cardiac capacity to skeletal muscle recruitment during standard cycle ergometry (A), the much greater ratio during single-leg knee-extension (B), and the subsequently contrasting physiological responses to these exercise paradigms.
An additional approach to better understand exercise limitations due to O2 availability has been to raise inspired O2 concentration above that of ambient air. This method has identified exercise-trained humans as O2-supply-limited (8,17) and their sedentary counterparts as O2-demand-limited (16). However, supplemental O2 use in CHF has not been commonly employed, and the limited results have been equivocal (18-20). We contend that this technique has yet to be applied, in earnest, to the question of what limits exercise capacity in patients with CHF.
Consequently, the present study used conventional cycle ergometry and single-leg knee-extensor (KE) exercise to greatly vary muscle mass recruitment in both CHF patients and healthy matched control subjects in both normoxia and hyperoxia (100% O2) (Fig. 1). This allowed us to test the following hypotheses: 1) during conventional cycle exercise, when muscle mass is large in relation to the compromised cardiac output, CHF patients will demonstrate limited exercise capacity, predominantly explained by cardiac failure; 2) when patients with CHF are somewhat artificially freed from this cardiac limited O2 delivery at maximal exercise, by performing KE exercise or breathing hyperoxia to increase O2 availability at the exercising muscle, there will be evidence of a skeletal muscle metabolic reserve; and 3) under these more optimal conditions for muscle metabolism there will still be evidence of limited O2 transport and subsequent skeletal muscle dysfunction in patients with CHF compared with control subjects, due to inherent abnormalities in peripheral O2 transport associated with this pathology.
Methods
Subjects
Twelve male patients with CHF and 8 healthy control subjects volunteered and gave written consent to participate in the study, which had been approved by the University of California at San Diego Human Subjects Protection Program. All CHF patients were clinically stable and had symptoms compatible with New York Heart Association functional class II to III (n = 5 and n = 7, respectively). Mean left ventricular ejection fraction in the CHF patients was 25 ± 3%. Other than beta-blockers, which were withheld for 48 h before the studies, patient medications were not altered. Particular care was taken to match control subjects for age, sex, quadriceps muscle mass, and physical activity by questionnaire and interview (Table 1).
Table 1. Characteristics of Control Subjects and CHF Patients.
| Control Subjects (n = 8) |
CHF (n = 12) |
|
|---|---|---|
| Age (yrs) | 52 ± 2 | 53 ± 1 |
|
| ||
| Height (cm) | 177 ± 2 | 179 ± 2 |
|
| ||
| Body mass (kg) | 88 ± 5 | 98 ± 6 |
|
| ||
| Knee extensor (1-leg) muscle mass (kg) | 2.2 ± 0.1 | 2.2 ± 0.1 |
|
| ||
| NYHA functional class | — | II–III |
|
| ||
| Medications (no. of users) | ||
| Digoxin | 0 | 12 |
| Diuretics | 0 | 12 |
| Long-acting nitrates | 0 | 7 |
| Statins | 0 | 6 |
| Aspirin | 0 | 7 |
| Beta-blockers | 0 | 10 |
| Warfarin | 0 | 4 |
| ACE inhibitors | 0 | 9 |
| Ca++ channel blockers | 0 | 3 |
Anthropometric data, severity of disease (New York Heart Association functional class) and medications in chronic heart failure (CHF) patients and control subjects. Data are expressed as mean ± SEM; p < 0.05.
ACE = angiotensin-converting enzyme; Ca++ = calcium, doubly charged positive ion.
Catheter placement and experimental protocol
A radial arterial and common femoral venous line in addition to a thermocouple in the common femoral vein were placed upon arrival at the laboratory, as previously described (21). With catheters in place, both the patients with CHF and control subjects underwent 4 exercise tests in a balanced design, separated by at least 1.5 h for recovery (cycle and KE exercise in both normoxia and hyperoxia [100% O2]). In each trial, exercise intensity was increased progressively every 2 min to exhaustion.
Exercise modalities
Cycle exercise was performed on an electromagnetically braked cycle ergometer (Lode Excalibur Sport, Quinton Instruments, Inc., Groningon, the Netherlands). During KE exercise, the subject was seated on an adjustable chair with the ankle of 1 leg attached by a rigid bar to a cycle ergometer (Monark, Stockholm, Sweden), as previously described (10) (Fig. 1).
Measurements and calculations
Mixed expired O2 and carbon dioxide, expiratory air flow, and electrocardiography were continuously recorded and digitized (Parvo Medics, Salt Lake City, Utah). Simultaneous arterial and femoral venous blood samples were collected at rest and during the second and third minutes of each incremental work rate. At each level of work, the following variables were measured: 1) O2 partial pressure, carbon dioxide partial pressure, pH (IL model 1302, pH/blood gas analyzer; Instrumentation Laboratories, Milan, Italy), oxyhemoglobin saturation, hemoglobin concentration (IL 482 co-oximeter), and lysed whole-blood lactate concentrations (YSI 23L blood lactate analyzer; Yellow Springs Instruments, Yellow Springs, Ohio) from simultaneous arterial and common femoral venous blood samples (blood gas values were corrected to the temperature measured in the common femoral vein); and 2) common femoral venous blood flow (thermodilution) and arterial and venous vascular pressures (10). Technical aspects of these measurements and subsequent calculations have been previously provided in detail (17,22).
Cardiac output
Cardiac output was measured in duplicate at rest and during exercise with an open-circuit acetylene uptake technique, as described previously (23).
Catecholamines
Plasma epinephrine and norepinephrine (NE) were assayed in duplicate by the method of Kennedy and Zeigler (24), and the rate of NE spillover was determined as described previously (25) with the following equation:
where Cv and Ca are plasma NE concentrations in the common femoral vein and radial artery, respectively; Ee is the fractional extraction of epinephrine; and LPF is the leg plasma flow, determined from leg blood flow and hematocrit.
Muscle mass
With the use of thigh length, circumferences, and skin-fold measurements, thigh volume was calculated to allow an estimate of quadriceps muscle mass, as used previously (10,26). During cycle exercise, the amount of working muscle mass was estimated on the basis of the reported ratio of quadriceps muscle mass to other leg muscles, in a fashion similar to that for our previous estimations (8).
Muscle biopsy
On a different day, a percutaneous biopsy of vastus lateralis muscle was obtained (Bergstrom needle) from 9 of the 12 CHF patients and 6 of the 8 control subjects, as previously described (20).
Histochemistry
Transverse sections (8-mm-thick) were cut at −24°C on a cryostat (Jung-Reichert Cryocut 1800; Cambridge Instruments, Heidelberg, Germany) and kept at −20°C until histochemical processing, which was performed within a week of sectioning. After 5-min fixation in a Guth and Samaha fixative at room temperature, sections were incubated at 37°C for 1 h in lead (Pb)-adenosine triphosphatase staining medium to simultaneously stain for fiber types I and II and capillaries (27).
Tissue preparation for microscopy
The glutaraldehyde-fixed samples were completely cut into thin longitudinal strips and processed for electron microscopy as described previously (28). Electron micrographs for morphometry were taken on 70-mm films with a Zeiss 10 electron microscope (Zeiss, Peabody, Massachusetts).
Morphometry
The relative cross-sectional area and number of type I and type II fibers was estimated under a light microscope (250×) on histochemical sections by point-counting with an eyepiece square grid test A100 (29). Capillary density (i.e., capillary number/fiber cross-sectional area), capillary/fiber ratio (i.e., capillary number/fiber number), capillary number around a fiber, and fiber cross-sectional area were measured by point-counting on 1-μm-thick sections examined at a magnification of 400× with a light microscope. The volume density of mitochondria/volume of muscle fiber was estimated by point-counting at a final magnification of 49,000× on ultrathin transverse sections.
Statistical analysis
Data were analyzed with parametric statistics, after mathematical confirmation of normal distribution with Shapiro-Wilk tests. Between-group subject characteristics were assessed with independent samples t tests. Comparisons of CHF patients and control data collected during exercise were performed with both 2-way (health status [2 levels] and exercise modality [2 levels]) and 3-way (health status [2 levels], exercise modality [2 levels], and inspirate [2 levels]) analyses of variance (repeated measures). After a significant main effect and interaction, paired samples t tests were employed to make post hoc comparisons at each level of the within-subjects factor. The relationship between selected variables was identified with a Pearson Product Moment Correlation. Statistical significance was set at p < 0.05. Data are expressed as mean ± SEM.
Results
The Shapiro-Wilk tests revealed p values of > 0.05; thus the null hypothesis that the data were normally distributed was not rejected, and parametric statistics were employed. Post hoc power analyses on the statistical comparisons of major variables that revealed a difference between patients with CHF and control subjects (e.g., work load, cardiac output, blood flow, O2 delivery, oxygen uptake [VO2], diffusional oxygen conductance [DMO2], venous O2 partial pressure) all demonstrated a power of > 0.78.
There were no statistically significant differences in the physical characteristics of the patients with CHF and control subjects, and these data are presented in Table 1. The work rate and cardiovascular responses at maximal cycle exercise and KE exercise in CHF and control subjects are presented in Table 2 with specific highlights presented in the following sections.
Table 2. Cardiovascular Responses to Maximal Normoxic Cycle and KE Exercise in Control Subjects and CHF Patients.
| Cycle |
KE |
|||
|---|---|---|---|---|
| Control Subjects | CHF | Control Subjects | CHF | |
| Work load (W) | 148 ± 11 | 96 ± 8* | 35 ± 4 | 24 ± 4* |
| Pulm VO2 (l/min) | 2.03 ± 0.11 | 1.52 ± 0.15* | 1.08 ± 0.16 | 0.78 ± 0.04* |
| Pulm VO2 (ml/kg/min) | 21.2 ± 1.7 | 15.2 ± 1.1* | 11.3 ± 1.4 | 7.8 ± 0.5* |
| Pulm VCO2 (l/min) | 2.13 ± 0.16 | 1.69 ± 0.13* | 0.89 ± 0.10 | 0.73 ± 0.04* |
| RER | 1.05 ± 0.03 | 1.09 ± 0.02 | 0.86 ± 0.06 | 0.96 ± 0.06 |
| VE (l/min) | 83.2 ± 7.6 | 76.0 ± 5.0 | 36.8 ± 4.4 | 36.0 ± 3.0 |
| Cardiac output (l/min) | 15.5 ± 1.6 | 10.4 ± 1.1* | 10.3 ± 1.6 | 7.5 ± 0.7* |
| HR (beats/min) | 156 ± 11 | 148 ± 5 | 113 ± 8 | 112 ± 5 |
| Leg blood flow (l/min) | 4.93 ± 0.55 | 3.42 ± 0.27* | 3.34 ± 0.37 | 2.50 ± 0.21* |
| Leg blood flow (ml/min/100 g) | 94 ± 9.0 | 51 ± 5* | 152 ± 8 | 112 ± 10* |
| Leg O2 delivery (l/min) | 1.00 ± 0.11 | 0.65 ± 0.06* | 0.65 ± 0.07 | 0.45 ± 0.04* |
| Leg VO2 (l/min) | 0.75 ± 0.07 | 0.52 ± 0.04* | 0.46 ± 0.05 | 0.34 ± 0.02* |
| Leg VO2 (ml/min/100 g) | 11.7 ± 1.0 | 7.7 ± 0.5* | 21.1 ± 2.1 | 15.3 ± 1.3* |
| DMO2 (ml/min/mm Hg) | 18.5 ± 1.1 | 12.6 ± 0.7* | 11.1 ± 0.9 | 8.7 ± 0.7* |
| Fem arterial pressure (mm Hg) | 129 ± 18 | 115 ± 8 | 147 ± 12 | 117 ± 5* |
| Fem venous pressure (mm Hg) | 18 ± 4 | 14 ± 1 | 17 ± 5 | 17 ± 2 |
| Leg vasc res (mm Hg/ml/s) | 1.45 ± 0.29 | 1.91 ± 0.19 | 2.45 ± 0.33 | 2.54 ± 0.19 |
| CaO2 (ml/100 ml) | 20.3 ± 0.4 | 18.8 ± 0.5* | 19.5 ± 0.3 | 18.0 ± 0.6* |
| CaO2-CvO2 (ml/100 ml) | 15.4 ± 0.8 | 14.6 ± 0.5 | 13.7 ± 0.7 | 13.6 ± 0.5 |
| PaO2 (mm Hg) | 97 ± 7 | 99 ± 5 | 112 ± 4 | 102 ± 4 |
| PaCO2 (mm Hg) | 31.9 ± 1.9 | 28.5 ± 0.8* | 31.5 ± 0.9 | 30.6 ± 1.0* |
| SaO2(%) | 96 ± 1 | 96 ± 1 | 97 ± 1 | 97 ± 1 |
| [Hb] (g/dl) | 15.0 ± 0.3 | 13.9 ± 0.4* | 14.2 ± 0.2 | 13.2 ± 0.4* |
| pHa | 7.32 ± 0.02 | 7.37 ± 0.01* | 7.39 ± 0.01 | 7.41 ± 0.01* |
| [La]a (mmol/l) | 6.7 ± 1.0 | 5.4 ± 0.6 | 3.1 ± 0.4 | 2.6 ± 0.2 |
| PvO2 (mm Hg) | 21.8 ± 2.2 | 20.8 ± 1.3 | 23.4 ± 1.2 | 20.6 ± 1.0 |
| PvCO2 (mm Hg) | 68.6 ± 4.2 | 58.5 ± 1.7* | 65.7 ± 2.5 | 63.8 ± 2.3 |
| SvO2 (%) | 23 ± 4 | 22 ± 2 | 29 ± 3 | 23 ± 2 |
| PcapO2 (mm Hg) | 41.1 ± 1.7 | 39.6 ± 1.2 | 41.6 ± 0.9 | 39.2 ± 0.8 |
Cardiovascular responses to maximal normoxic cycle and knee-extensor (KE) exercise in control subjects (n = 8) and patients with chronic heart failure (CHF) (n = 12). Results are expressed as mean ± SEM.
p < 0.05 (CHF vs. control subjects).
CaO2 = arterial oxygen content; CaO2-CvO2 = arterial – venous oxygen content difference; DmO2 = muscle oxygen diffusional conductance; [Hb]a = arterial hemoglobin concentration; HR = heart rate; [La]a = arterial lactate concentration; Leg vasc res = 1-leg vascular resistance; Leg VO2 = 1-leg oxygen uptake; PaO2 and PaCO2 = oxygen and carbon dioxide partial pressures in arterial blood; PcapO2 = calculated partial pressure of oxygen in the capillaries of the exercising muscle; pHa = arterial pH; pulm VO2 = pulmonary peak oxygen uptake; pulm VCO2 = pulmonary carbon dioxide uptake; PvO2 and PvCO2 = oxygen and carbon dioxide partial pressures in femoral venous blood, respectively; RER = respiratory exchange ratio; SaO2 = arterial oxygen saturation; SvO2 = venous oxygen saturation; VE = ventilation.
Cardiac output during maximal cycle and KE exercise
Maximal cardiac output, attained by graded maximal cycle exercise, was 33% lower in the patients with CHF (p < 0.05). Despite being significantly lower than cardiac output during cycling, cardiac output in the patients with CHF at peak KE exercise was similarly attenuated by 28% (Table 2). However, the highest cardiac output attained in the CHF patients during this small muscle mass exercise modality (2.2 kg in both CHF and control subjects) (Table 1)— essential to the premise of the current experimental design—was 27% lower than that achieved during cycle exercise in this group (Table 2) (p < 0.05), documenting a functional cardiac reserve during KE exercise. The same observation was made in the control subjects who also only used 67% of their maximal cardiac output during maximal KE exercise (Table 2), although this is of less importance in terms of experimental design. Heart rate, measured independently, also supports the existence of a cardiac reserve in both groups, with a 24% and 28% lower heart rate attained during KE exercise in comparison with cycle exercise in the patients with CHF and control subjects, respectively (Table 2).
Muscle VO2 during maximal cycle and KE exercise
During cycle exercise, there was a proportional attenuation in maximal work rate (35%) and absolute peak leg VO2 (31%) in the patients with CHF compared with control subjects. Likewise during KE exercise, the patients did not achieve the same maximal work rate (31% lower) and peak leg VO2 (26% lower) as the control subjects (Table 2). However, in CHF and control subjects, both peak leg VO2 and peak muscle blood flow were significantly elevated by the transition from cycle to KE exercise when normalized by the estimated muscle mass used during the exercise (Table 2).
Oxygen transport during cycle and KE exercise
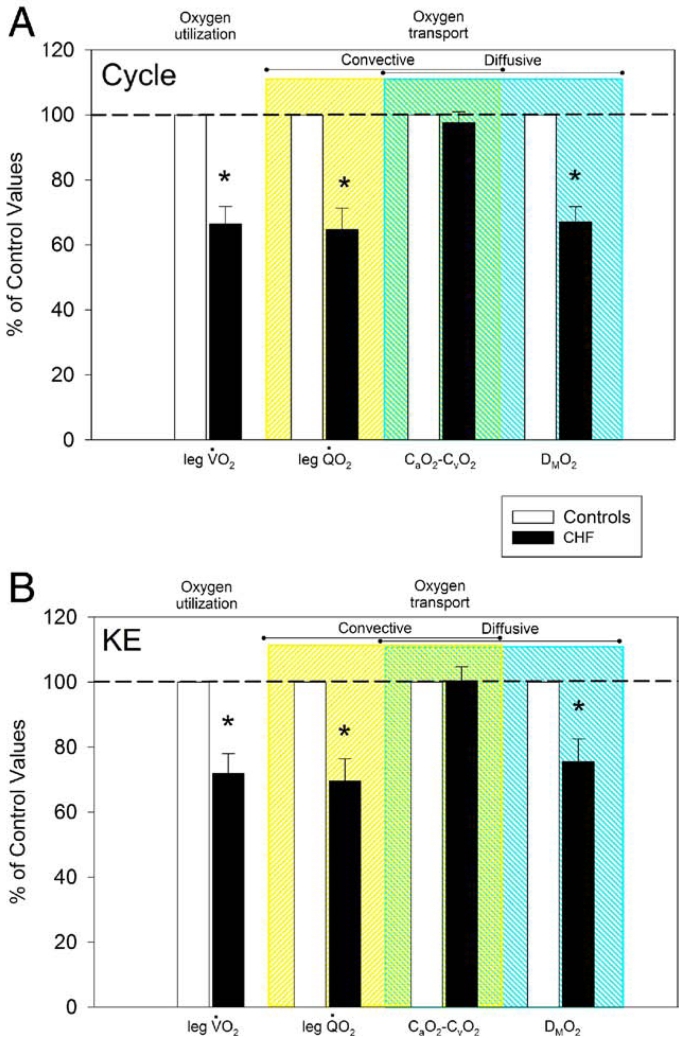
At maximal cycle and KE exercise, leg blood flow in the CHF patients was 31% and 25% lower, respectively, than in control subjects, and arterial O2 content was slightly but significantly lower in CHF than in control subjects, due to a small but significantly different hemoglobin concentration between the 2 groups (Table 2). As a consequence, leg O2 delivery was 35% and 26% lower during cycling and KE exercise, respectively, in CHF than in control subjects (convective O2 transport) (Table 2, Fig. 2). At maximal cycle and KE exercise, DMO2 (diffusive O2 transport) was 32% and 22% lower in the CHF patients, respectively, than in control subjects (Table 2, Fig. 2). The difference between arterial and venous oxygen content (CaO2 – CvO2), a variable that can be affected by both convective and diffusive O2 transport, was not different between patients and control subjects during both cycle and KE exercise (Fig. 2).
Figure 2. Oxygen Transport and Use.
A comparison of oxygen transport and use parameters assessed at maximal cycle (A) and knee-extensor (KE) (B) exercise in patients with chronic heart failure (CHF) (n = 12) and control subjects (n = 8). Control data have been normalized to 100% as a point of reference for the data collected in the patients with CHF. CaO2-CvO2 = arterial-venous oxygen content difference; DMO2 = diffusional oxygen conductance; leg VO2 = 1-leg oxygen uptake; QO2 = 1-leg oxygen delivery.
Catecholamines
Measurements of arterial and venous epinephrine, NE, and blood flow to facilitate the calculation of NE spillover from the muscle during maximal cycle and KE exercise revealed a greater arterial NE concentration during KE exercise in the patients and greater NE spillover during both exercise modalities in the patients with CHF (Table 3).
Table 3. Arterial and Venous Epinephrine, NE, and Calculated NE Spillover From the Muscle During Maximal Cycle and KE Exercise in Control Subjects and CHF Patients.
| Cycle |
KE |
|||
|---|---|---|---|---|
| Control Subjects | CHF | Control Subjects | CHF | |
| [e]a (nmol/l) | 0.86 ± 0.2 | 0.65 ± 0.1 | 0.64 ± 0.1 | 0.89 ± 0.3 |
|
| ||||
| [e]v (nmol/l) | 0.76 ± 0.2 | 0.43 ± 0.1 | 0.38 ± 0.1 | 0.39 ± 0.1 |
|
| ||||
| [NE]a (nmol/l) | 8.6 ± 2.0 | 13.1 ± 3.6 | 4.0 ± 0.9 | 9.0 ± 2.1* |
|
| ||||
| [NE]v (nmol/l) | 8.4 ± 1.3 | 13.9 ± 5.8 | 3.7 ± 0.9 | 6.7 ± 2.4 |
|
| ||||
| NE spillover (nmol/l/min/kg) | 2.47 ± 0.53 | 7.01 ± 2.2* | 1.82 ± 0.43 | 3.31 ± 0.65* |
Arterial and venous epinephrine, norepinephrine (NE), and calculated NE spillover from the muscle during maximal cycle and knee-extensor (KE) exercise in control subjects (n = 8) and patients with chronic heart failure (CHF) (n = 12). Results are expressed as mean ± SEM.
p < 0.05 (CHF vs. control subjects).
[e]a = arterial epinephrine concentration; [e]v = femoral venous epinephrine concentration; [NE]a = arterial norepinephrine concentration; NE spillover = norepinephrine spillover in 1 leg; [NE]v = femoral venous norepinephrine concentration.
The effect of 100% O2 breathing
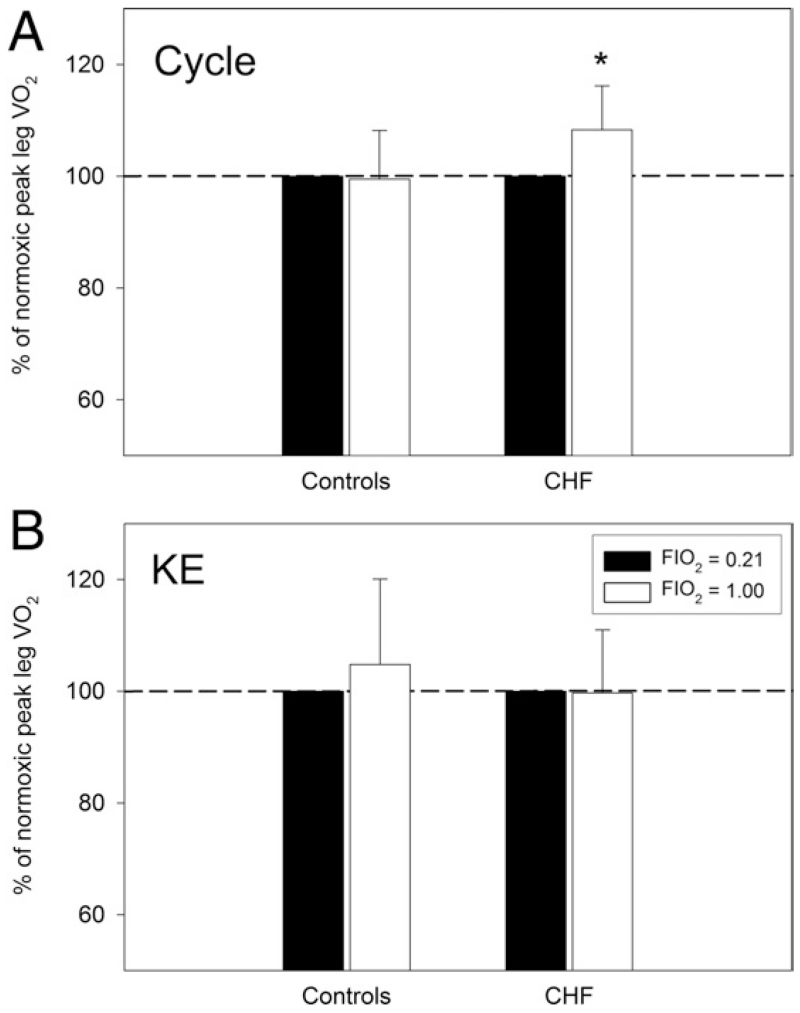
At peak cycle exercise, hyperoxic breathing in CHF increased CaO2 by 10% (p < 0.05), whereas it had no effect on leg blood flow. Therefore, leg O2 delivery increased similarly by 8% (p < 0.05), and leg VO2 also increased by 7% (p < 0.05) (Fig. 3). In control subjects, O2 breathing increased CaO2 by 10% and also had no effect on leg blood flow. Leg O2 delivery thereby increased by 8% (p < 0.05), but leg VO2 was unchanged (Fig. 3). At peak KE exercise, hyperoxic breathing increased CaO2 in patients with CHF by 7% and in control subjects by 8% (p < 0.05) and, without reducing blood flow, increased O2 delivery (p < 0.05) but had no effect on leg VO2 in either group (Fig. 3).
Figure 3. Effect of Hyperoxic Breathing.
The effect of hyperoxia (100% oxygen) on peak leg VO2 assessed at maximal cycle (A) and KE exercise (B) in patients with CHF (n = 12) and control subjects (n = 8). Control data have been normalized to 100% as a point of reference for the data collected in the patients with CHF. Abbreviations as in Figure 2.
Structural and functional/structural data
The muscle characteristics for both patients with CHF and control subjects, determined from needle biopsy samples, revealed that mitochondrial volume density in the CHF group was 25% lower (p < 0.05), compared with control subjects; and although not significant, there was a tendency for the patients with CHF to exhibit smaller muscle fibers, which resulted in a trend toward increased capillary density (Table 4). Additionally, there were several significant correlations between both structural and functional/structural variables when data from both patients and control subjects were included (Figs. 4 and 5).
Table 4. Vastus Lateralis Muscle Characteristics.
| Control Subjects (n = 6) |
CHF (n = 9) |
|
|---|---|---|
| Fiber cross-sectional area (μm2) | 3,853 ± 606 | 3,092 ± 237 |
| % area of type I fibers | 41 ± 4 | 37 ± 4 |
| % area of type II fibers | 59 ± 4 | 63 ± 4 |
| Capillary density (capillaries/mm2) | 415 ± 46 | 442 ± 21 |
| Capillary/fiber ratio | 1.52 ± 0.10 | 1.36 ± 0.12 |
| Number of capillaries around a fiber | 3.8 ± 0.1 | 3.3 ± 0.3 |
| Mitochondrial volume density (%) | 4.4 ± 0.4 | 3.3 ± 0.3* |
| Lipid droplets volume density (%) | 0.30 ± 0.06 | 0.36 ± 0.07 |
Number of subjects for cross-sectional area: chronic heart failure (CHF): n = 7; control subjects: n = 5.
p < 0.05 (CHF vs. control subjects).
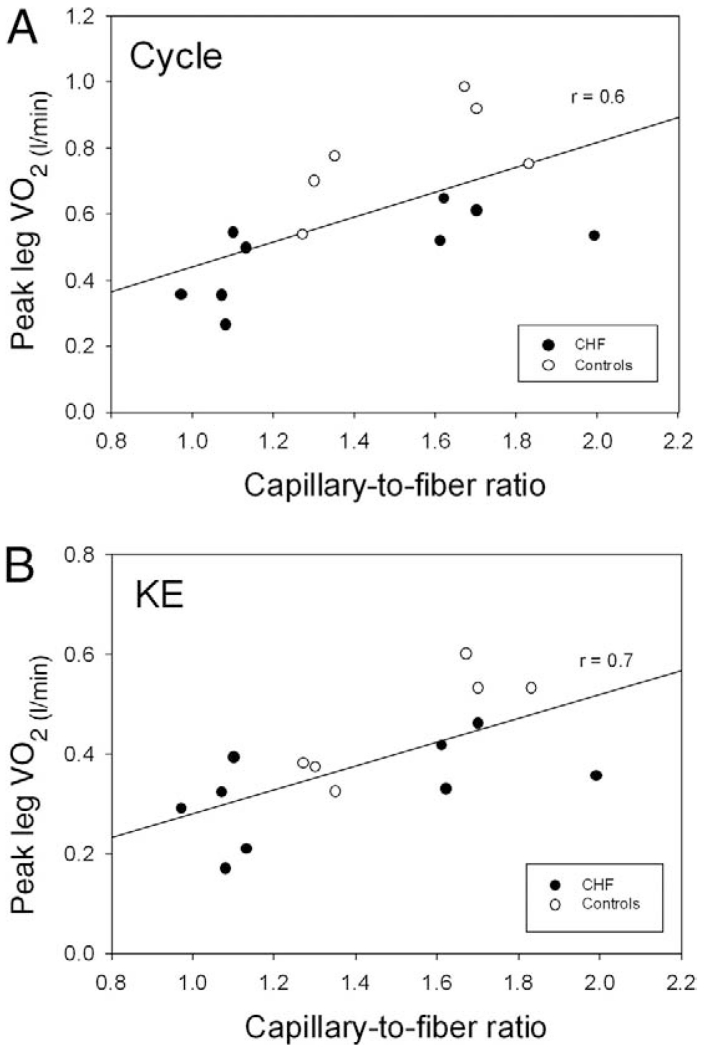
Figure 4. Capillarity and Peak Oxygen Uptake.
The relationship between capillary/fiber ratio and peak VO2 assessed at maximal cycle (A) and KE exercise (B) in patients with CHF (n = 9) and control subjects (n = 6). The decision to combine the correlation for both patients and control subjects was based upon the limited number of the data points and observation that the addition of the patients improved the original relationship displayed by the control subjects. Abbreviations as in Figure 2.
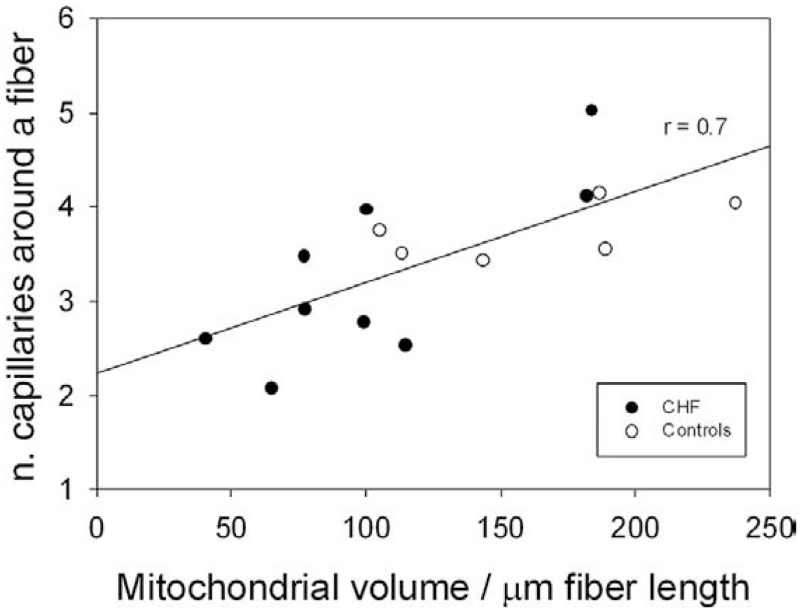
Figure 5. Mitochondrial Volume and Capillarity.
The relationship between mitochondrial volume and number of capillaries around a muscle fiber in patients with chronic heart failure (CHF) (n = 9) and control subjects (n = 6). The decision to combine the correlation for both patients and control subjects was based upon the limited number of the data points and observation that the addition of the patients improved the original relationship displayed by the control subjects.
Discussion
A large cardiac reserve during exercise was made accessible, by experimental design, to CHF patients and control subjects by adopting 1-leg KE exercise, contrasted with conventional 2-leg cycling, thereby reducing the recruited muscle mass (Fig. 1). With this approach the peak leg VO2, normalized for the active muscle mass, was significantly greater during KE exercise (with cardiac reserve) than cycle exercise (without cardiac reserve). Likewise, O2-enriched breathing increased O2 availability during both cycle and KE exercise, but this only increased peak leg VO2 during cycling in the patients with CHF. Together, these observations imply that, during whole body exercise such as cycling, cardiac output and O2 delivery contribute significantly to the exercise limitation experienced by patients with CHF.
However, this multi-faceted research also provides evidence that, despite improvements in muscle peak leg VO2 afforded by increased O2 availability, the convective and diffusive components of O2 transport from blood to skeletal muscle in patients with CHF are still compromised during KE exercise when compared with well-matched control subjects. Correlative assessment of the current morphometric and functional data reveal that CHF does not alter several important relationships that are highly predictive of skeletal muscle function and health. In the following text, we provide a more detailed interpretation and attempt to reconcile these apparently conflicting findings.
Evidence of a skeletal muscle metabolic reserve
In the current study the assessment of cardiac output during both cycle and KE exercise illustrates that, by switching from a large to a small muscle mass exercise modality, a cardiac reserve was consistently available to both patients with CHF and control subjects (Table 2). With this important proof of concept in place, the measurement of peak 1-leg VO2 and the subsequent normalization to muscle mass involved in the exercise (8), a clear muscle blood flow and metabolic reserve was revealed in patients with CHF and control subjects that was accessible during KE exercise (Table 2). This finding in patients with CHF was identical to that of control subjects. Presumably, this was a consequence of the documented cardiac reserve associated with this exercise modality. Many prior studies have suggested that CHF-induced skeletal muscle exhibits a shift in fiber type distribution, reduced oxidative capacity, reduced mitochondrial-based enzymes, and decreased mitochondrial volume density, all as a consequence of muscle atrophy or myopathy or both (1-4). Therefore, in this population, it is a significant observation that there seems to still be a metabolic reserve at maximal exercise, as revealed by freeing the skeletal muscle from the restraints imposed by the failing heart.
In the present study, we found that patients with CHF exhibited a skeletal muscle metabolic reserve, by significantly increasing peak VO2, while breathing 100% O2 during maximal cycle exercise but not during KE exercise (Fig. 3). That is, when breathing hyperoxia—which resulted in an elevated arterial O2 content—these patients were able to achieve a 5% greater cycle work rate and a 7% increase in peak VO2. This was in contrast to the healthy control subjects who failed to increase peak VO2 above that attained in normoxia in both modalities. This suggests that during cycle exercise the skeletal muscle of patients with CHF was limited by O2 supply, and this was not the case during KE exercise in which O2 delivery/unit of muscle mass was already significantly elevated in comparison with cycle exercise. The control subject responses were consistent with the published data in terms of healthy but sedentary human muscle failing to improve when provided with greater O2 availability (16,30), suggesting ambient O2 levels are either perfectly matched or in excess of metabolic capacity in these healthy but sedentary subjects.
Contribution of convective and diffusive O2 transport in limiting peak VO2
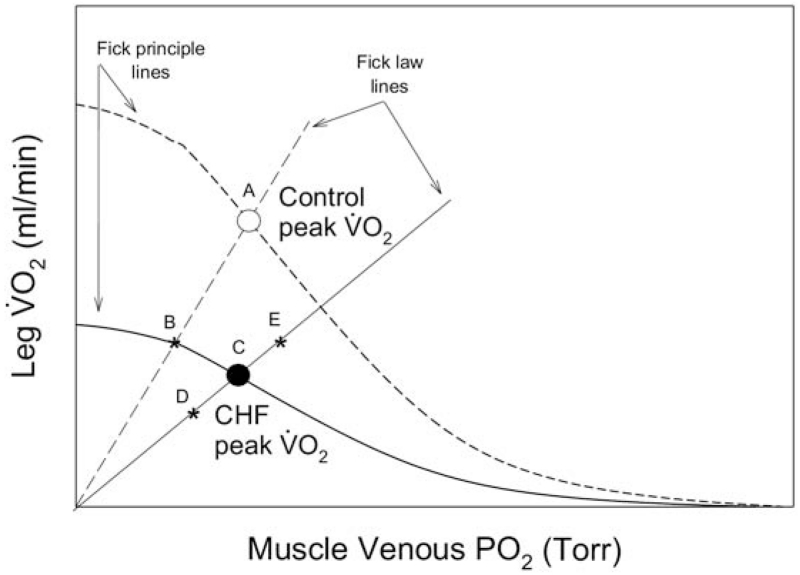
The current data afford the opportunity to assess the contributions of both the convective (bulk delivery of O2) and diffusive (movement of O2 from hemoglobin to mitochondria) elements in determining peak VO2 in CHF patients. A reasonable method by which to understand these interactions is by the representation of both the diffusive component of O2 transport, which is defined by Fick’s law of diffusion (VO2 = DMO2 × PvO2), and the convective component, which is defined by the Fick Principle (VO2 = blood flow [CaO2 – CvO2]), in a model that links peak VO2 and effluent muscle O2 partial pressure (PvO2) to explain limitations to maximal exercise capacity (Fig. 6) (15). If we first consider the scenario of cycle exercise, here it can be clearly seen that if the only difference between the control subjects and the CHF patients were the reduced convective component of O2 transport (reduced cardiac output) (Table 2), then peak VO2 would have fallen from A to B as a result of this pathology (6). However, the CHF patients also revealed a significantly attenuated DMO2 (diffusive component) (Fig. 2) in addition to the lower convective component of O2 transport, which actually resulted in a greater fall in peak maximal VO2 from A to C (Fig. 6). It is important to recognize that, on the basis of the current findings, the same schematic can be used to illustrate the results of the KE exercise studies. This is because, although cycle exercise was undoubtedly completely taxing cardiac output capacity and KE exercise had a cardiac reserve (Table 2), both the convective and diffusive components of O2 transport of the patients with CHF were diminished in both exercise models (Figs. 2 and 6).
Figure 6. Convective and Diffusive Components of O2 Transport.
A schematic illustration of the convective and diffusive components that interact to determine peak oxygen uptake (VO2) in both CHF and control subjects during both cycle (large muscle mass) and KE exercise (small muscle mass). In both scenarios patients with CHF exhibit an attenuated diffusional conductance (slope of the Fick law line). Additionally, it should be noted that the Fick principle lines are not straight, because they are directly reflective of the hemoglobin dissociation curve. Therefore, a left-shifted hemoglobin dissociation curve (greater hemoglobin oxygen affinity), resulting in a lower venous oxygen partial pressure (PO2), will bisect the Fick law line earlier and reduce maximal VO2 and vice versa. This would be the equivalent of anchoring the existing CHF Fick principle line at its origin on the x axis and altering its shape (a left-shifted O2 dissociation curve) to pass through point D (reduced peak VO2) or a right-shifted O2 dissociation curve that would pass through point E (elevated peak VO2). Explanations for point A, B, and C are given in the text. Abbreviations as in Figure 2.
The explanation for these apparently similar limitations to maximal exercise in what seem to be very different scenarios might well uncover the peripheral difference between control subjects and patients with CHF. Specifically, according to their documented cardiac output capacity during cycle exercise, the patients with CHF could be expected to match the KE exercise blood flow of the control subjects (14); however, these patients did not (Table 2). It is speculated that this might have been the result of the continued relatively elevated NE spillover in the KE exercise model that was also apparent during cycle exercise (Table 3). This increase in NE spillover is a plausible explanation for the attenuated blood flow and thus lower convective O2 transport during KE exercise (Figs. 2 and 6). However, with the current approach, the potential role of heart failure medications (only beta-blockers were discontinued) in this attenuated peripheral muscle blood flow response cannot be ruled out.
To better understand the diffusive O2 transport differences between the patients and control subjects, it is worth highlighting the findings related to the arterial–venous O2 difference that was similar between groups and the DMO2 that was significantly attenuated in the patients with CHF. This can be explained by the fact that the lower blood flow exhibited by the patients with CHF allows for a longer capillary transit time and therefore a greater time for O2 extraction and the maintenance of arterial–venous O2 difference. However, DMO2—which is expressed per unit of time—takes this longer period for gas exchange into account and reveals that, despite the adoption of a small muscle mass exercise model, DMO2 remained significantly attenuated in the patients with CHF compared with control subjects (Figs. 2 and 6). This distinction between arterial–venous O2 extraction—which is a consequence of both convective O2 delivery and diffusive O2 transport—and DMO2—which is solely a consequence of diffusive movement—provides important mechanistic insight into the limitations to O2 transport specific to patients with CHF. Indeed, these data reveal that, during both cycle and KE exercise, the patients with CHF exhibit an attenuated ability to move O2 from blood to cell, and this contributes to the reduction in exercise capacity in both scenarios.
Finally, to gain a complete understanding of this model and the determinants of peak VO2, it is interesting to note that previously, with animal models, our group has experimentally supported the concept that manipulations in O2 driving force from blood to cell (changing hemoglobin O2 affinity, see Fig. 6 legend) do indeed yield the predicted changes in peak VO2 (9,31). Additionally and specifically germane to the topic of improving the diffusional O2 transport to muscle in CHF, it was recently documented that decreasing O2 affinity (right shifting the O2 dissociation curve) improved exercise capacity in mice with CHF (32), suggesting that this might indeed be one mechanism by which exercise capacity can be improved in this pathology.
Skeletal muscle structure and functional relationships in CHF
A significant strength of the current study is the potential to not only examine the skeletal muscle structure but also to combine these data with the functional assessment of peak VO2 during both cycle and KE exercise (Fig. 4). Indeed, other such studies of skeletal muscle structure and function within and between species have revealed design features that are uniform throughout muscles of widely varying metabolic demand. One of these features is that the size of the capillary-to-fiber interface rather than diffusion distance relates most closely to the structural capacity for O2 flux into muscle fibers (33). Here again, a degree of normality is apparent in the patients with CHF who demonstrate essentially the same relationship as control subjects for capillary-to-fiber ratio and peak VO2 during both cycling and KE exercise (Fig. 4). Additionally, recent studies have revealed that the size of the capillary-to-fiber interface is matched to mitochondrial volume/fiber length in response to stimuli such as chronic hypoxia (34), electrical stimulation (35), and physical activity (36). These observations suggest that another regulated design feature in skeletal muscle is matching the structural capacity for O2 flux to fiber metabolic demand (36). Therefore, the current data, in which mitochondrial volume seems to be well-related to the number of capillaries around a muscle fiber, reveal a positive and physiologically expected relationship that was similar for control subjects and CHF patients alike. Thus, although on average the patients had a significantly reduced mitochondrial volume and not a significantly reduced number of capillaries around a muscle fiber, the relationship between these 2 variables was, as expected, reasonably strong (Fig. 5).
With the backdrop to this study being the goal to include control subjects who were well-matched with the CHF patients (relatively inactive), the combined structure and functional relationships reported here suggest that the patients exhibited relatively normal findings but still have the tendency to exhibit the characteristics of inactivity compared with control subjects. Thus, the finding of an attenuated DMO2 in these patients cannot be simply attributed to structural abnormalities.
Conclusions
This study has documented through a manipulation of muscle mass recruitment during exercise that, even under conditions where cardiac function is not limiting, both skeletal muscle blood flow—and therefore convective O2 transport—and diffusion of O2 from blood to muscle are compromised. The explanation for these apparently similar limitations to maximal exercise in patients with CHF, in what seem to be very different scenarios, supports a significant peripheral difference between control subjects and patients. The peripheral factors that might contribute to this limitation are numerous. Current data did not specifically implicate structural properties of the muscle but suggest that relatively elevated NE spillover might play a role by affecting local muscle blood flow. Such conclusions must be tempered by recognition of the role of numerous other vasoconstrictors and the possible impact of heart failure medications on regional blood flow distribution.
Acknowledgments
The authors wish to thank all the patients and the subjects for their committed participation in this involved study. Special thanks goes to Nancy Gardetto for her help and support in taking care of the patients. The technical support of Harrieth Wagner, Nicholas Busan, and Jeff Struthers was also essential.
This research was funded in part by National Institutes of Health Grants from the National Heart, Lung, and Blood Institute P01 HL-17731 and PO1 HL 09183-01A1, the Sam and Rose Stein Institute for Research on Aging, and Tobacco-Related Disease Research Program Grant 15RT-0100. At the time of the study, Dr. Esposito was on leave from University of Brescia, Italy. Dr. Esposito was partly supported by “Centro per lo studio del trattamento dello scompenso cardiaco,” University of Brescia, Italy. Lynne Warner Stevenson, MD, served as Guest Editor for this paper.
Abbreviations and Acronyms
- CaO2
arterial oxygen concentration
- CHF
chronic heart failure
- CvO2
venous oxygen content
- DMO2
diffusional oxygen conductance
- KE
knee-extensor
- NE
norepinephrine
- O2
oxygen
- QO2
oxygen delivery
- VO2
oxygen uptake
REFERENCES
- 1.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 2.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27:140–5. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 5.Duscha BD, Kraus WE, Keteyian SJ, et al. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–63. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–81. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 7.LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation. 1986;74:245–51. doi: 10.1161/01.cir.74.2.245. [DOI] [PubMed] [Google Scholar]
- 8.Richardson RS, Grassi B, Gavin TP, et al. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–53. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- 9.Richardson RS, Frank LR, Haseler LJ. Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. Int J Sports Med. 1998;19:182–7. doi: 10.1055/s-2007-971901. [DOI] [PubMed] [Google Scholar]
- 10.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–53. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 11.Tyni-Lenne R, Gordon A, Jensen-Urstad M, Dencker K, Jansson E, Sylven C. Aerobic training involving a minor muscle mass shows greater efficiency than training involving a major muscle mass in chronic heart failure patients. J Card Fal. 1999;5:300–7. doi: 10.1016/s1071-9164(99)91334-9. [DOI] [PubMed] [Google Scholar]
- 12.Tyni-Lenne R, Dencker K, Gordon A, Jansson E, Sylven C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail. 2001;3:47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson G, Kaijser L, Rong H, Isberg B, Sylven C, Saltin B. Exercise capacity in heart failure patients: relative importance of heart and skeletal muscle. Clin Physiol. 1996;16:183–95. doi: 10.1111/j.1475-097x.1996.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 14.Magnusson G, Kaijser L, Sylven C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- 15.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev. 2000;28:10–4. [PubMed] [Google Scholar]
- 16.Cardus J, Marrades R, Roca J, et al. Effects of FiO2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc. 1998;30:697–703. doi: 10.1097/00005768-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Hyperoxia increases leg maximal oxygen uptake. J Appl Physiol. 1993;75:2586–94. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- 18.Moore DP, Weston AR, Hughes JM, Oakley CM, Cleland JG. Effects of increased inspired oxygen concentrations on exercise performance in chronic heart failure. Lancet. 1992;339:850–3. doi: 10.1016/0140-6736(92)90288-e. [DOI] [PubMed] [Google Scholar]
- 19.Paul B, Joseph M, De Pasquale CG. Domiciliary oxygen therapy improves sub-maximal exercise capacity and quality of life in chronic heart failure. Heart Lung Circ. 2008;17:220–3. doi: 10.1016/j.hlc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–16. [PubMed] [Google Scholar]
- 21.Richardson RS, Knight DR, Poole DC, et al. Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am J Physiol. 1995;268:H1453–61. doi: 10.1152/ajpheart.1995.268.4.H1453. [DOI] [PubMed] [Google Scholar]
- 22.Agusti AGN, Roca J, Barbera JA, Casademont J, Rodriguezroisin R, Wagner PD. Effect of Sampling Site On Femoral Venous Blood Gas Values. J Appl Physiol. 1994;77:2018–22. doi: 10.1152/jappl.1994.77.4.2018. [DOI] [PubMed] [Google Scholar]
- 23.Barker RC, Hopkins SR, Kellogg N, et al. Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol. 1999;87:1506–12. doi: 10.1152/jappl.1999.87.4.1506. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy B, Zeigler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–54. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 25.Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise: role of muscle mass. Am J Physiol. 1989;257:H1812–8. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- 26.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204:63P–6P. [PubMed] [Google Scholar]
- 27.Rosenblatt JD, Kuzon WM, Jr., Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987;62:85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res. 1987;33:98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- 29.Weibel E. Practical methods for biological morphometry. Academic Press; London: 1979. [Google Scholar]
- 30.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97:1077–81. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- 31.Hogan MC, Bebout DE, Wagner PD. Effect of increased HbO2 affinity on VO2max at a constant O2 delivery in dog muscle in situ. J Appl Physiol. 1991;70:2656–62. doi: 10.1152/jappl.1991.70.6.2656. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Takeda T, Omiya S, et al. Reduction in hemoglobin-oxygen affinity results in the improvement of exercise capacity in mice with chronic heart failure. J Am Coll Cardiol. 2008;52:779–86. doi: 10.1016/j.jacc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Mathieu-Costello O, Saurez RK, Howchachka PW. Capillary-to-fiber geometry and mitochondrial density in hummingbird flight muscle. Respir Physiol. 1992;89:113–32. doi: 10.1016/0034-5687(92)90075-8. [DOI] [PubMed] [Google Scholar]
- 34.Mathieu-Costello O, Agey PJ, Wu L, Szewczak JM, MacMillen RE. Increased fiber capillarization in flight muscle of finch at altitude. Respir Physiol. 1998;111:189–99. doi: 10.1016/s0034-5687(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 35.Mathieu-Costello O, Agey PJ, Wu L, Hang J, Adair TH. Capillary-to-fiber surface ratio in rat fast-twitch hindlimb muscles after chronic electrical stimulation. J Appl Physiol. 1996;80:904–9. doi: 10.1152/jappl.1996.80.3.904. [DOI] [PubMed] [Google Scholar]
- 36.Poole DC, Mathieu-Costello O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirc. 1996;3:175–86. doi: 10.3109/10739689609148286. [DOI] [PubMed] [Google Scholar]