Abstract
Historically the culture of mammalian cells in the laboratory has been performed on planar substrates with media cocktails that are optimized to maintain phenotype. However, it is becoming increasingly clear that much of biology discerned from 2D studies does not translate well to the 3D microenvironment. Over the last several decades, 2D and 3D microengineering approaches have been developed that better recapitulate the complex architecture and properties of in vivo tissue. Inspired by the infrastructure of the microelectronics industry, lithographic patterning approaches have taken center stage because of the ease in which cell-sized features can be engineered on surfaces and within a broad range of biocompatible materials. Patterning and templating techniques enable precise control over extracellular matrix properties including: composition, mechanics, geometry, cell-cell contact, and diffusion. In this review article we will explore how the field of engineered extracellular matrices has evolved with the development of new hydrogel chemistry and the maturation of micro- and nano- fabrication. Guided by the spatiotemporal regulation of cell state in developing tissues, we will review the maturation of micropatterning in 2D, pseudo-3D systems, and patterning within 3D hydrogels in the context of translating the information gained from 2D systems to synthetic engineered 3D tissues.
1. Introduction
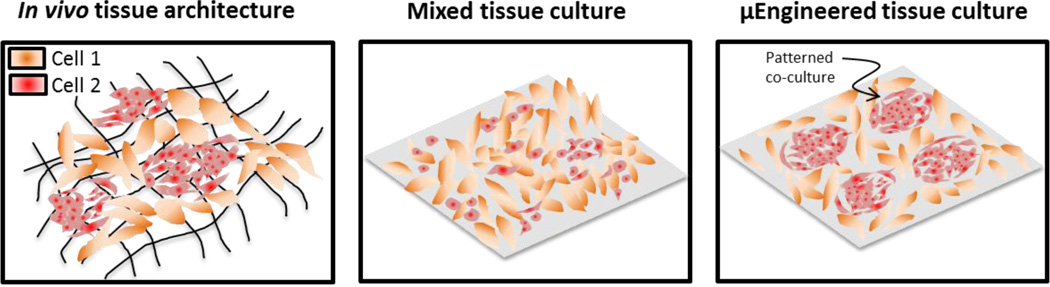
Nature has developed intricate processes in which the form and function of tissues arise in multicellular organisms. Starting from a single cell, a complex array of biophysical and biochemical cues guide the segregation of our earliest progenitors into distinct germ layers that ultimately develop into the multitude of specialized cells of the adult organism. This process is regulated by many extrinsic and intrinsic factors and central to these processes is a complex orchestration between the composition of the surrounding extracellular matrix (ECM), its viscoelastic properties, spatiotemporal gradients of soluble factors, and interactions with neighboring cells. The interplay of these parameters influence cell state, function, and coordinated assembly to precisely control tissue formation. Understanding the context in which the ECM and its cellular constituents coordinate to establish complex architectures and build functioning tissue is of great importance in developmental biology, but is also necessary in the design of materials for medicine. Here we will explore the progress and promise of engineered materials to control cellular outcomes in vitro, from new assays for cell biology to complex 3D materials that recapitulate the function of tissues (Figure 1).
Figure 1.
Approach to recapitulate structure in vitro using 2D microengineering.
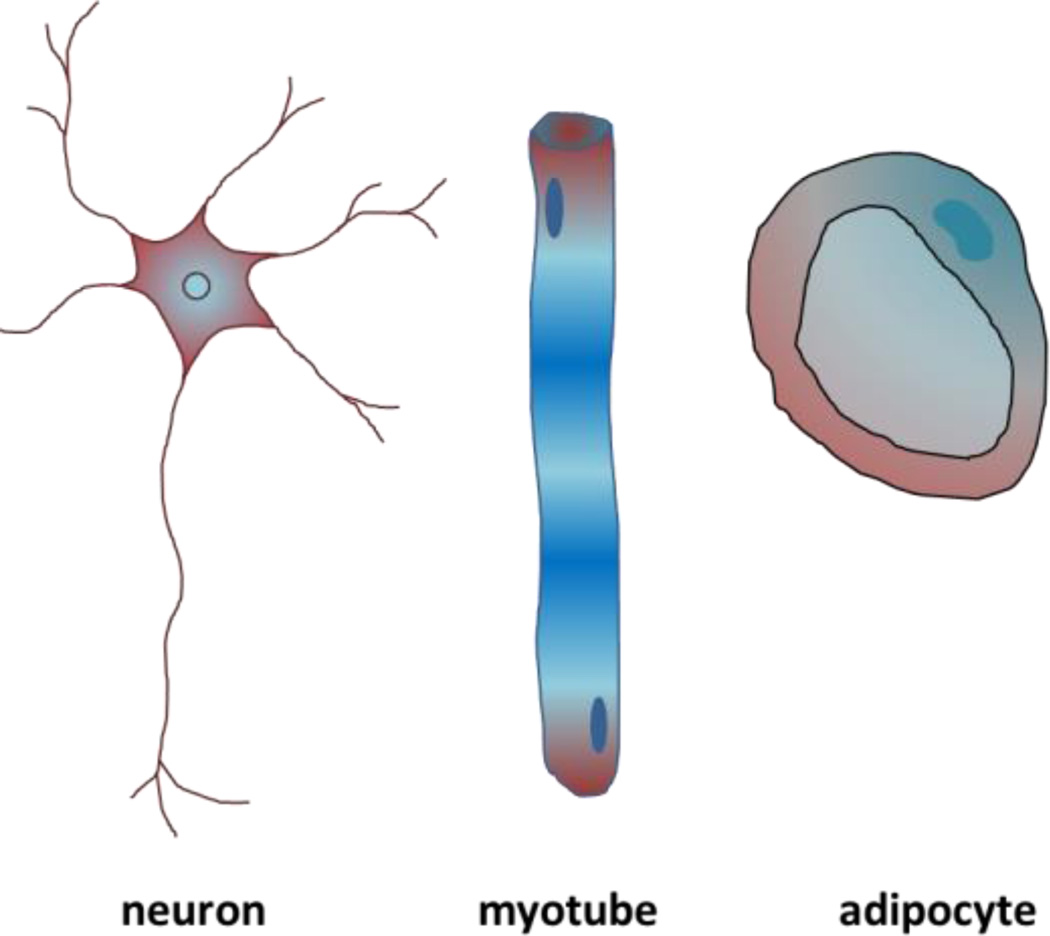
The extracellular matrix (ECM) is the non-cellular component of tissues and is comprised of a combination of polysaccharides, growth factors, and proteins including collagen, fibronectin, laminin, and elastin. The ECM guides a host of cell and tissue level functions including regulation of cellular architecture [1], directing tissue-specific stem cell specification [2], [3], guiding cell migration [4], maintaining homeostasis [5], and influencing tissue development [6]–[9], including controlling branching morphogenesis [10], [11]. One major function of the ECM is to regulate cell shape and its connectivity to surrounding cells which in turn regulates the cellular epigenetic state, gene expression, and function [6], [12]. While all the cells arise from a single fertilized egg; major morphological and functional differences can be seen in different tissue types. For instance, neurons have small bodies with long axons to transfer electric signals over long distances, myocytes are long and tubular with contracting myofibrils with a shape that is optimized for generating force along the direction of the cell, and adipocytes are round with large vacuoles which is optimum for lipid storage [13]–[15] (Figure 2).
Figure 2.
Representations of cells with different shapes found in tissue
The shape of the cell is synergistic with its function and it is acknowledged that cell shape is due in part to ECM mechanics and composition [16]–[19]. For example, during pregnancy the fibronectin distribution in the ECM changes from a fibrillar pattern during estrus to a patched pattern before disappearing on day six of pregnancy which regulates the shape of the stromal fibroblasts to change from elongated cells at estrus to a round morphology on day six [1]. This ECM remodeling serves to support the decidual cell’s morphological differentiation and creates an environment which permits the invasion and establishment of the placenta [20]. Numerous other examples exist of how ECM influences cells and tissues ranging from microscale influence on cellular morphology[17], [21], [22] and proliferation [16], [23], [24], to macroscale guidance of the stem cell niche [2], [25], [26] and tissue formation [27][18], [19], [28]. This dynamic organization and reorganization of the extracellular matrix proteins during embryogenesis and morphogenesis leads to distinct and organized tissues structures composed of a variety of cell shapes performing distinct functions. From cues that shape the early embryo to dynamic morphogenesis in the adult organism, the partitioning of cells into functional structures necessarily requires differential organization that is coordinated by the properties of the matrix.
The sensitivity of the early embryo to its surrounding microenvironment during development has been appreciated for some time [29]–[32], however only recently have we begun to decipher the complex interactions in the microenvironment that guide cellular processes. At the beginning of embryogenesis, the blastula reorganizes from the symmetric blastula into an asymmetrical gastrula during gastrulation[33]. The ECM is essential in guiding the movement of cells from the primitive streak of the blastula to form the germ layers ectoderm, mesoderm, and endoderm. The migrating cells of a chick embryo attaches onto the laminin in the ECM, the first glycoprotein to appear, and is then guided to the ventral surface of the epiblast[34]. In zebrafish gastrulation, fibronectin and laminin fibrils align in the direction of membrane protrusion formation to polarize mesoderm cells and guide migration which helps to shape the embryo [8]. When expression of fibronectin is knocked down, there is a disruption of cell convergence and extension proving that the ECM is essential to gastrulation.
Another aspect of embryogenesis guidance by the ECM is the compliance of the surrounding tissue. As an embryo develops, the stiffness of the embryo increases dramatically [35] and the stiffness of its environment can impact embryo development. When Rinaudo and colleagues cultured mouse embryos on 1kPa gels representing the uterine epithelium, they observed developmental differences between those cultured on stiff petri dishes [36]. This included increased frequency of development from zygote to the 2-cell stage and from 2-cell to blastocyst stage, increased hatching frequency, and had larger placentae once transplanted back into recipient females. Regional stiffness differences of embryos also begin to appear early on in embryogenesis including stiffening of blastula wall[37] and stiffening of the marginal zone [38] and the notochord [39] during gastrulation. This asymmetric stiffening can be guided by differences in stiffness of the ECM [37] and by its orientation, namely the orientation of fibrillary fibronectin [40]. To further understand these in vivo observations, additional in vitro studies with embryonic stem cells (ESC) has shed light on the influence of the ECM. Softer substrates promotes self-renewal and pluripotency of ESCs and create more homogeneous cell populations [41], [42] in addition to increasing cell traction at the basal surface [43]. However, stiffer substrates promotes cell growth and differentiation [44], [45].
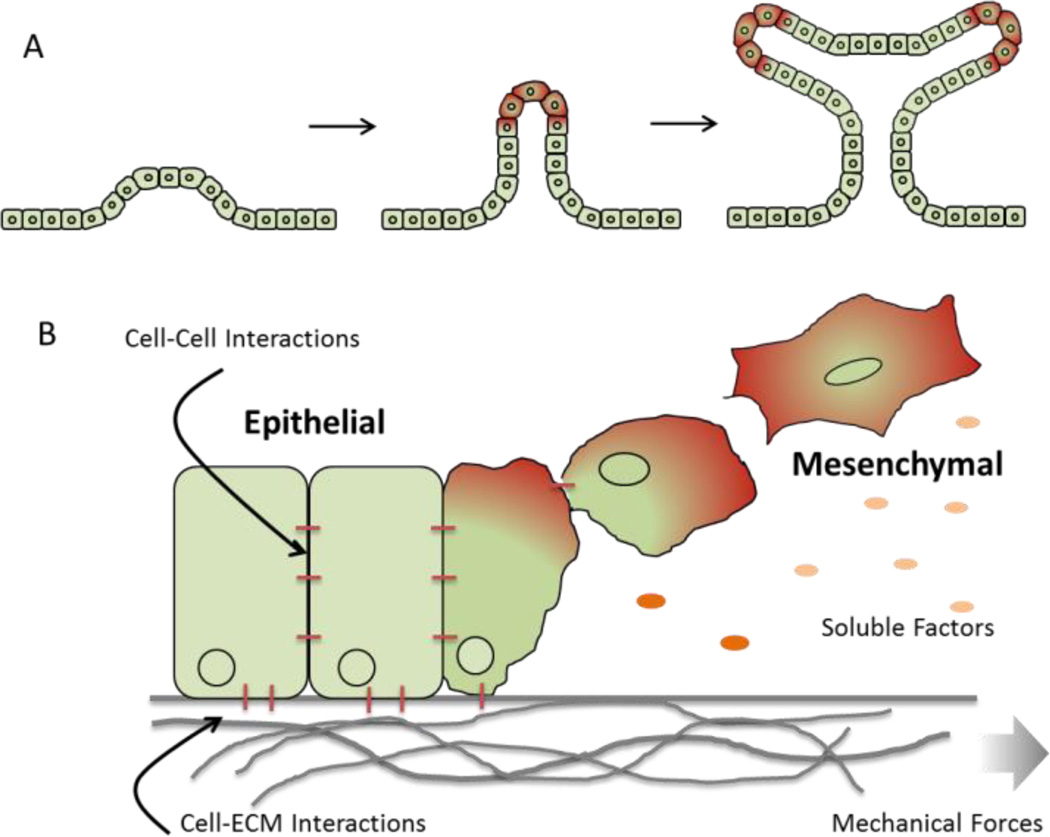
The ECM continues to play an important role in guiding cell and tissue geometry during processes like branching morphogenesis, during which the epithelial trees in the lung, kidney, mammary, and salivary glands are created [46] (Figure 3a). Branching involves repetitive epithelial cleft and bud formation [47], [48] and the ECM can provide both mechanical cues and also serve to stabilize newly formed branches. During initial salivary gland formation, focal adhesion kinase (FAK) acts as a mechanosensor and is required for the assembly of ECM fibrils within a growing cleft [49]. These clefts then lead to the assembly of fibronectin fibrils via Rho-associated kinase (ROCK)-induced actomyosin contraction [50]. Fibronectin is also critical for initiation of epithelial branching where fibrillary fibronectin accumulate in cleft forming regions and suppresses cadherin cell-cell adhesions [10]. Other ECM components like collagen play a stabilizing role and can be found in the stalks of the forming branches [51].
Figure 3.
Depiction of (A) branching morphogenesis where soluble and insoluble signals coordinate the formation of hierarchical structures in developing tissue; (B) epithelial-to-mesenchymal transition.
In addition to embryo development and initial tissue formation, important changes in tissue morphology occur during normal and pathological processes. One example is the epithelial-to-mesenchymal transition (EMT) where cuboidal, polarized epithelial cells attached to the basement membrane undergo a physiological change to adopt an elongated mesenchymal cell morphology with increased migratory capacity and increased production of ECM components [52]–[54] (Figure 3b). EMT is an important process during gastrulation [55], tissue repair [56], and cancer progression [57], [58]. The ECM composition plays a role here too where it has been shown that type I collagen gels can induce EMT of thyroidepithelial cells [59], [60]. Other in vitro studies showed that laminin can suppress EMT of mammary epithelial cells, [61] whereas fibronectin enhances EMT of human bronchial epithelial cells [62] and can direct migration of EMT induced mesothelial cells[63]. The same was seen for primary alveolar epithelial cells, where fibronectin promoted EMT whereas laminin and collagen promoted apoptosis instead of EMT [64].
The mechanical properties of the cell and tissue microenvironment can also play a role in morphogenesis where a stiffer environment can promote EMT of murine mammary gland cells and Madin–Darby canine kidney epithelial cells [65]. In the breast cancer microenvironment, the increase in and alignment of collagen fibrils increases the stiffness of the cancer microenvironment [66], [67]. This increase in stiffness then drives EMT of breast tumor cells, increasing tumor invasion potential and metastasis [68]. A recent study by Wang, Huang and colleagues showed that soft fibrin gels will promote the growth of a subpopulation of tumor initiating melanoma cells, suggesting that soft matrices may prove important for amplifying specific cell types [69]. From these studies it is clear that the interplay between mechanical properties and matrix composition will guide a range of cellular processes in a context dependent fashion.
As the influence of individual ECM components is becoming increasing clear, the synergy of these factors can sometimes produce fascinating results. When natural organs and tissues are decellularized to form scaffolds, the complex ECM proteins are left behind to guide spatiotemporal organization of multiple cell types. Gershlak et al. decellularized rat hearts of different developmental stages to study differences in ECM composition and stiffness between fetal, neonatal, and adult hearts[70]. It was found that stiffness increases ~2 fold between fetal and neonatal but not between neonatal and adult hearts. In addition, the composition of the ECM was significantly different. When these ECM components were cross-linked into polyacrylamide gels of differing stiffness, mesenchymal stem cells (MSC) responded differently depending on the ECM composition. From an increase in stiffness from 9kPa to 48kPa, MSCs showed a decrease in stress generated by gels while on adult ECM but increase in stress while on fetal ECM with a further influence on differentiation potential. In addition, cell traction force increases with substrate stiffness on fetal ECM but not neonatal or adult ECM. This work shows that both ECM composition and substrate stiffness influences tissue form and function through pathways that are regulated through multiple biophysical and biochemical cues. Establishing these structure-function relationships that govern the formation of tissue in vivo is challenging at best due to the dynamic environment and limitations associated with analyzing excised tissue [71]. Depending on the substrate stiffness, the effect of ECM composition may be different and unraveling the complexity of the natural ECM environment is a complex endeavor that will be best assessed using combinatorial strategies.
Over the past decade, the maturation of nano- and micro- engineering technologies for both ”soft” and “hard” materials have enabled researchers to precisely control cellular assemblies in vitro [72], [73]. These approaches can be used to mimic the in vivo microenvironment towards deconstructing the cues that orchestrate cellular assembly, while providing platforms that are amenable to modern microscopy techniques. More recently, dynamic hydrogel systems that can pattern material properties such as protein composition and stiffness in real time demonstrate the promise of synthetic materials that may recapitulate aspects of in vivo systems such as morphogenesis. In this review article we explore the evolution of 2D cell culture systems to engineered 3D model tissues, with a particular focus on translating information gained in 2D to inform the design of 3D materials. We highlight the importance of integrating multiple biophysical and biochemical cues to guide cellular processes in the laboratory. This review is not intended to be comprehensive but rather demonstrate the trend in translating 2D assays to 3D biomaterials; for details on recent advances in methods and materials to direct cell fate, the readers are referred to the following recent reviews [74]–[76].
2. Recreating the form and function of cells and tissues on 2D materials
2.1 Micropatterning single cells to explore geometry-function relationships
Cell shape influences a variety of cellular functions including proliferation [16], [77], [78], migration[79], and the regulation of lineage specific gene expressions[21], [80]–[84], amongst other functions [85]. In order to decouple these relationships, the microelectronics industry has provided a wealth of tools to modify the surface of materials with spatial definition in order to precisely control the shape of single cells via the presentation of ECM components including proteins and peptides.
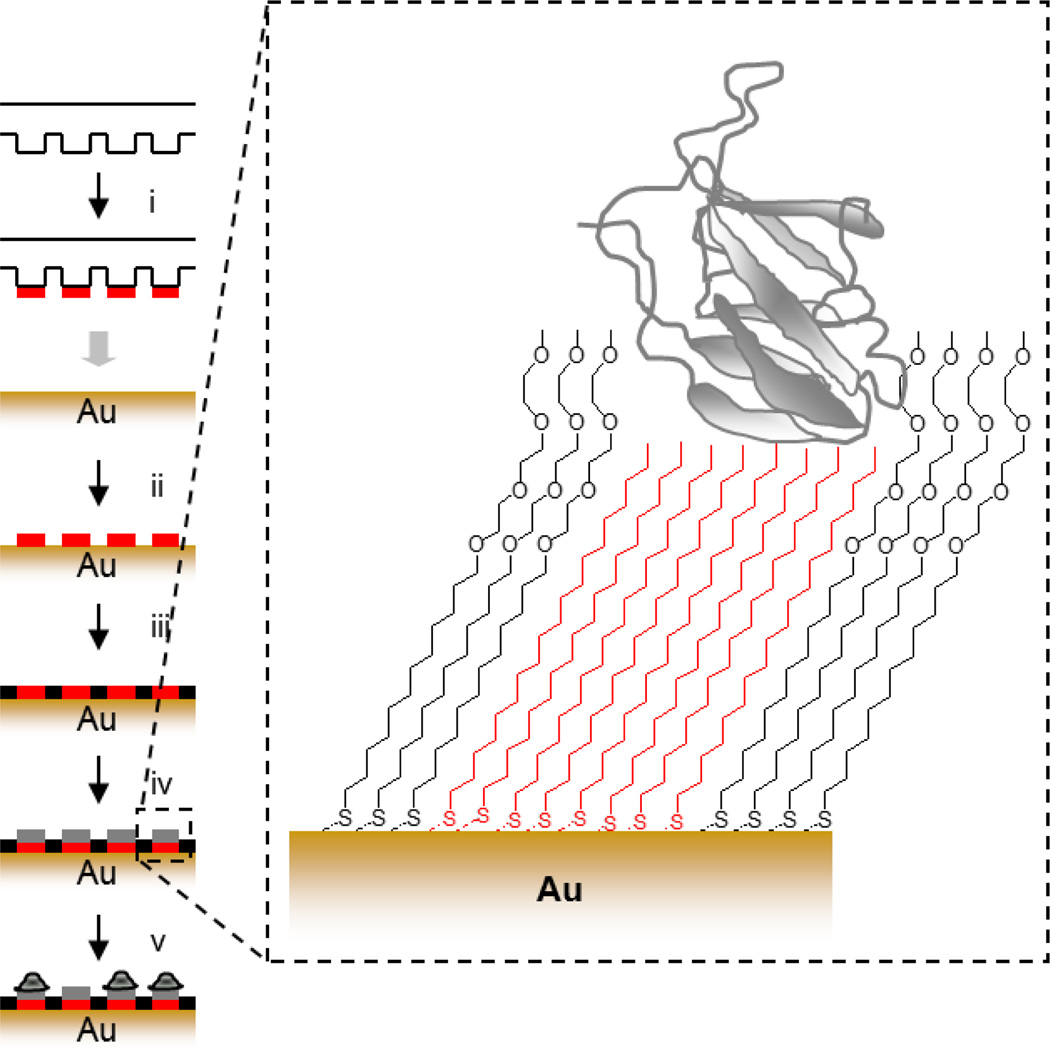
One common approach is the technique of soft-lithography (i.e. microcontact printingdeveloped by Whitesides and colleagues[86] to spatially pattern chemistry. This technique involves the use of a structured pattern mask formed via photolithography to cast an elastomer which can be used to transfer a specific chemistry to a surface (Figure 4). Microcontact printing has also been used to pattern chemistry on glass substrates via silane monomers [87] and proteins [88]. For more detail on soft lithography, we refer the reader to several reviews [89]–[91].
Figure 4.
Soft lithography strategy for patterning cells. (i) A polydimethylsiloxane (PDMS) stamp is inked with octadecanethiol, (ii) printed onto the gold surface, (iii) the intervening regions are passivated with a tri(ethylene glycol) diluent, (iv) matrix protein is physisorbed to hydrophobic regions, (v) cells are captured specifically to protein coated islands.
Other patterning techniques include localized SAM replacement which involves using a microfluidic device to remove regions of inert alkanethiol and replacing it with “active” alkanethiol [92], dip pen lithography which used an AFM tip to deposit molecules onto a surface [93], and various other related strategies (Table 1). To improve SAM stability, researchers have explored patterning under liquid medium or using inks with different properties such as low diffusion, reactive SAMs, or supramolecular interactions [94].
Table 1.
Summary of strategies for micropatterning hard and soft materials
| Substrate | Surface chemistry | Adhesive ligand | Cell Type | Reference |
|---|---|---|---|---|
| Gold | mixed alkane thiolates | Peptides (RGD) | capillary endothelial cells |
[95] |
| adsorption/agarose* | Protein (Fibronectin) | pulmonary artery endothelial cells, smooth muscle cells |
[96] | |
| gold-thiol | Chemistry (1-octadecanethiol) | [97] | ||
| gold-thiol | Protein (collagen) | [93] | ||
| Glass | silane | Protein (Collagen IV, fibronectin, laminin) |
neuroblastoma | [98] |
| adsorption/BSA* | Protein (fibronectin) | adrenal capillary endothelial |
[99] | |
| adsorption/PEG* | Protein (collagen) | Neuron, glia cells | [100] | |
| Glass/PDMS | adsorption/pluronic* | Protein (Collagen) | Hepatocytes, fibroblast | [88] |
| Graphene | reduced graphene oxide | Reduced graphene oxide | Mesenchymal stem cell | [101] |
| Hydrogel (PA) |
NHS acrylate | Protein (fibronectin) | fibroblast | [102] |
| sulfo-SANPAH | Protein (collagen) | Airway smooth muscle cell |
[103] | |
| adsorption | Protein (fibronectin) | Mammary gland epithelial cell |
[104] | |
| hydrazine hydrate | Protein (collagen) | fibroblast | [105] | |
| physical crosslinking | Protein (Collagen I, fibronectin, laminin) |
fibroblast | [106] | |
| Hydrogel (PEG) |
polymer brush | Protein (BSA) | Hippocampal neurons | [107] |
| gold-thiol/cysteamine | Peptide (cRGD) | fibroblast | [108] | |
| thiol-ene | Peptide (RGD) | fibroblast | [109] | |
| biotin-avidin | Peptide (G11GRGDS, G5CSRARKQAASIKVAVSADR) |
Bovine aortic endothelial cells, nerve cells |
[110] | |
| Polystyrene | adsorption/Poly(NIPAAm)* | Protein (fibronectin) | Rat hepatocytes | [111] |
Additive to prevent non-specific protein adhesion
Using these techniques, surface positioning and composition of ECM proteins can be precisely tuned with micrometer scale resolution and single cells can be captured in patterns to study the influence of geometric cues on cellular processes [90], [91], [112]. After initial attachment of the cells onto the substrate, the cells then acquire their new cell shape in two stages. First, the most distal contacts of the cell define the apices of the cell shape. Second, the cell borders that link and minimizes the distance between the two apices. It overcomes the non-adhesive regions by forming stress fibers and accumulating focal adhesions. These stress fibers work against the membrane tension in the cell border [113]. It was shown that irrespective of the shape of the adhesive region, compressive stress is maximum at the cell center and vanishes at the cell boundary, vanishing more rapidly at regions of high curvature or at sharp corners. Sheer stress is essentially zero for isotropic shapes. For anisotropic shapes, sheer stress also build up at the center of the shape [114]. The actinmyosin contractility generated by the patterning of fibroblasts was shown to influence rotation of the nucleus which can be controlled via apical actin fibers absent in circular geometries but present in elongated geometries [115]. In a striking example of how patterning can influence subcellular architecture, Bornens and colleagues demonstrated how the positioning of protein patterns under a cell can guide adhesion and cytoskeletal tension [113]. When the location of adhesive and non-adhesive regions are manipulated within the same given convex envelope, cells on different ECM patterns might have similar shape but different cytoskeletal networks (Figure 5a).
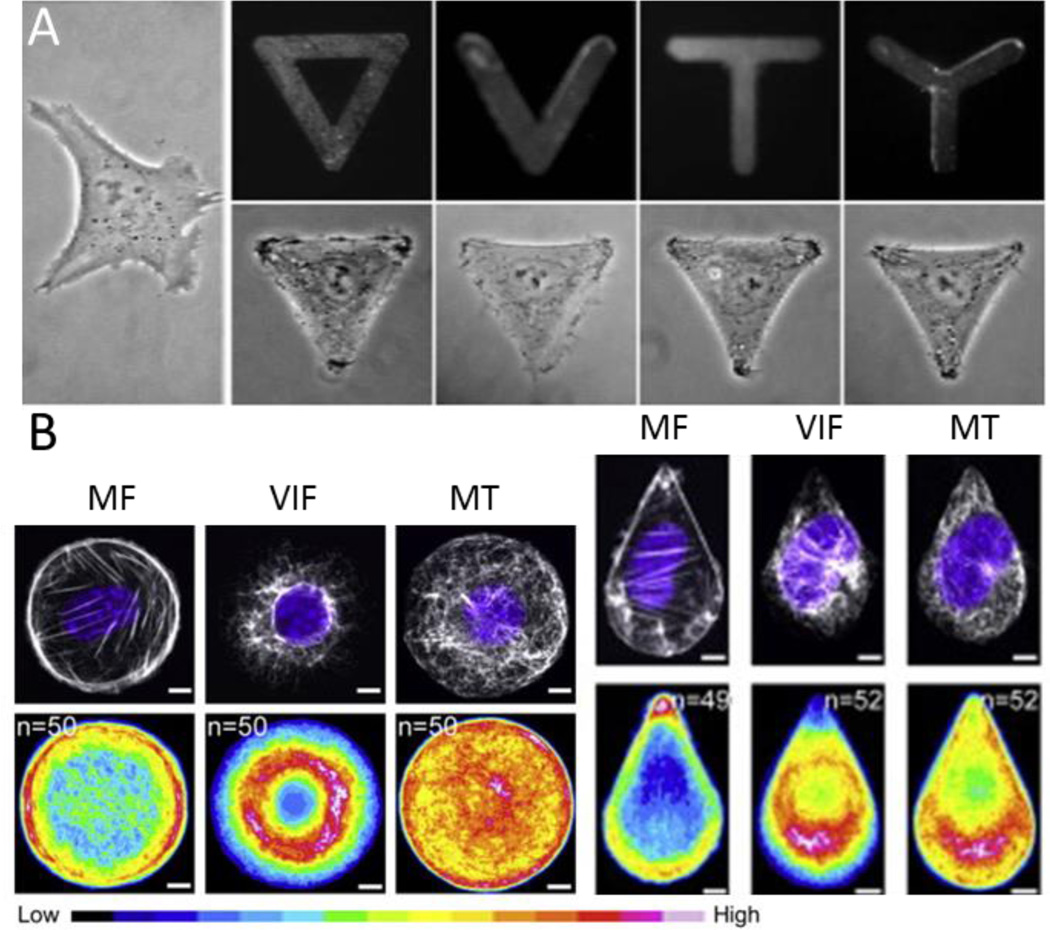
Figure 5.
(A) Adherent fibroblasts take on the adhesive geometry printed on the substrate. Reproduced with permission [113] 2006, Wiley periodicals, INC. (B) Microfilaments (MF), vimentin intermediate filaments (VIF) and microtubules (MT), differentially organize within a cell in response to geometry. Reproduced with permission[119] 2014, Elsevier.
Controlling cell shape will guide the organization of the cytoskeleton and adhesion architecture, and thereby influence cell behavior [116]–[118]. In addition to microfilaments, cell shape was recently shown to guide the formation and localization of intermediate filaments [119]. When cells are patterned in defined geometries, vimentin intermediate filaments (VIF) are primarily perinuclear in contrast to the microfilaments which localize to the circumference of the cell. Microtubules (MT) tend to be evenly distributed throughout the cell with the exception to a shape approximating a teardrop where filaments localize to the blunt edge (Figure 5b). In these cases with asymmetric shapes, VIF and MT tend to avoid concentrated areas of MF which biases the cell motility pattern. Similarly, patterning cells in asymmetric shapes will influence cell polarity and exert control over protrusion ([120]) and directional cell movement [121], [122]. When cultured in the presence of platelet-derive growth factor (PDGF), cells preferentially extend lamellipodia, filopodia, and microspikes from the corners of shapes and tractional forces were also concentrated in those regions [123]. Other cellular function such as contractile strength of vascular smooth muscle cells (VSMC) is also geometry dependent [124], [125]. Patterned VSMCs showed greater contractile range and decreased contractile strength when elongated as influenced by morphological changes in its nucleus.
In addition to subtle geometric cues, the degree in which a cell can spread will influence adhesion, cytoskeletal tension and intracellular signaling. Cells cultured on smaller area will have a higher rate of apoptosis, while spread cells show increased rate of DNA synthesis, resulting in decreased apoptosis [126]. With an increase in cell spreading, there is also an increase in nuclear volume and chromatin decondensation [77]. This is coupled with epigenetic changes including increase in histone H3 acetylation at lysine 9. An increase in actomyosin contractility, e.g. triangle versus circle, is also associated with an increase in polymerized actin and decrease in nuclear levels of histone deacetylase 3 resulting in decondensed chromatin and global histone acetylation [82]. Micropatterning has also been used to direct EMT signaling in single cells. Epithelial cells confined to small islands showed reduced metalloproteinase induced EMT but not TGFβ induced EMT [81]. When allowed to spread, epithelial cells also increased expression of myofibroblast markers in contrast to when cell spreading is restricted, epithelial-myofibroblast transition is prevented via MRTF-A signaling [127].
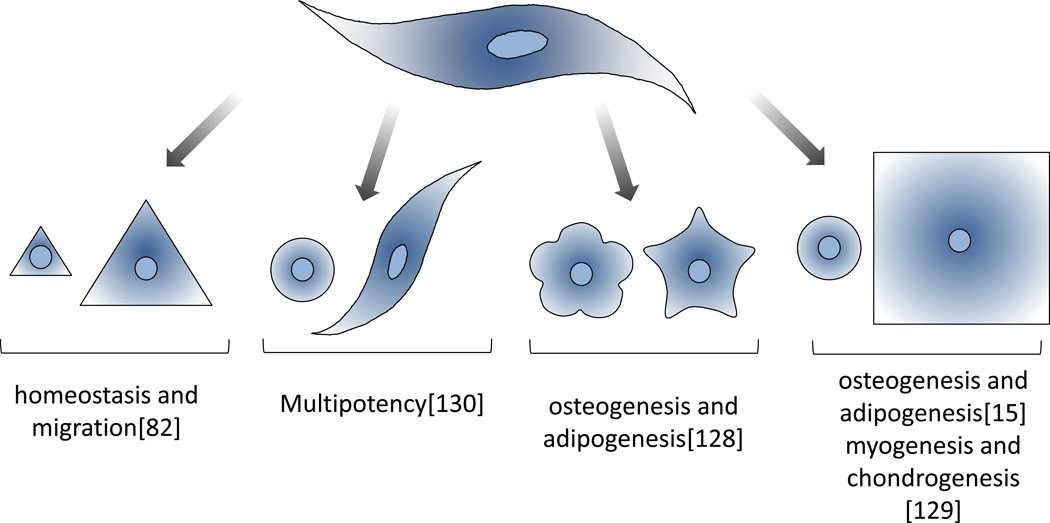
To understand how cell shape influences developmental processes, in vitro stem cell systems have proved a powerful tool in deciphering the role of geometric cues in guiding lineage outcomes[15], [128], [129]. Recently we demonstrated that restricting the spreading of single mesenchymal stem cells (MSCs) will enhance and preserve the multipotent phenotype in culture through control of cytoskeletal tension and actomyosin contractility [130]. Chen and colleagues showed that spread cells, which experience high actomyosin contractility, tend to adopt an osteogenesis outcome while rounded cells, which experience lower actomyosin contractility, prefer to undergo adipogenesis when exposed to soluble media supplements[15]. In addition to spreading, MSCs cultured in shapes of same area but increasing aspect ratio or pentagonal shapes with variable subcellular curvature at the perimeter, would differentially undergo adipogenesis or osteogenesis depending on the geometry that fostered the lowest or highest cytoskeletal tension respectively [128]. hMSCs cultured in micropatterns and exposed to TGFβ3 upregulate myogenic genes when spread and chondrogenic genes when shape is restricted [129] (Figure 6).
Figure 6.
Cells respond to geometric cues to coordinate and regulate a variety of biological activities.
2.2 Deconstructing morphogenesis using 2D micropatterning
Single cell patterning gives us great insight into the relationship between cell shape, adhesive structures and cytoskeletal tension during cellular processes. Micropatterning can also be used for multicellular systems to explore cell-cell contact, force transmission, and signaling to adjacent cells and across large populations. Cells can be patterned in individual 2-cell patterns or up to thousands of cells can be patterned into a tissue sheet. Patterning of cells in these larger geometries will help us define how cells interact with each other and behave in large tissue structures. Nelson et al. patterned endothelial cell doublets in a bowtie configuration, and showed how vascular endothelial-cadherin inhibits growth by decreasing cell spreading through changes in cell adhesion to the ECM. This cadherin induced proliferation signal can be inhibited by blocking actin-myosin generated tension [96], [131]. The two-cell bowtie configuration can be adjusted for higher cell-cell contact by the addition of triangles to the bowties shape; as the number of cell-cell contact increase, proliferations decreases [132].
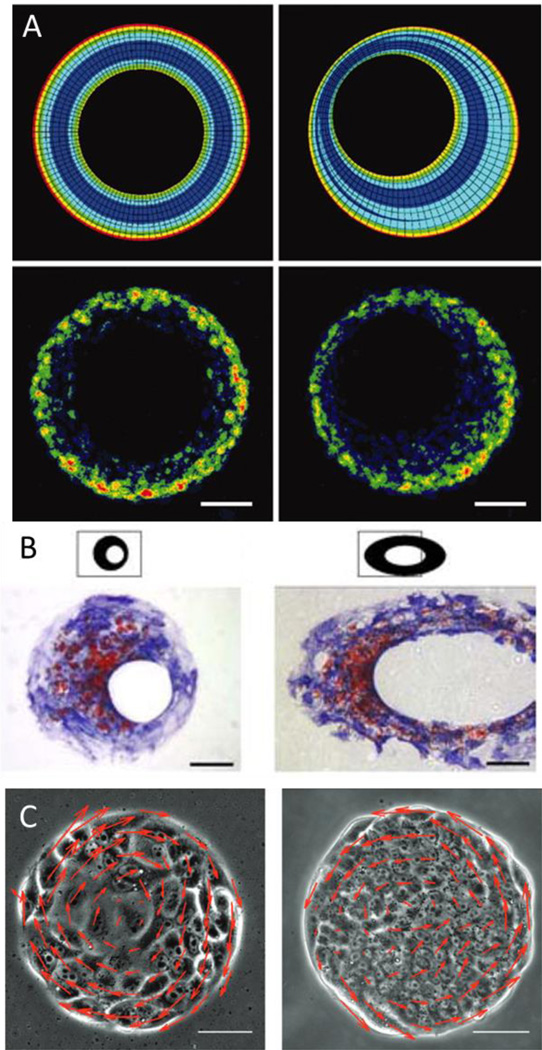
Spatial patterns of cellular growth generate mechanical stresses that help to deform tissues into their specific forms. The forces experienced by the cells can be predicted by finite-element models of multicellular mechanics which are then confirmed and measured directly by using a micromechanical force sensor array. Chen and colleagues used a combination of micropatterning large populations of cells and finite element analysis to show how a gradient of force is spatially organized from the perimeter of large patterns to the center [134] (Figure 7a). Changing the shape of perimeter features in patterned epithelial sheets was shown to promote EMT of epithelial cells in regions of high tension [135]. This is due to several factors. Cells at the edge of patterened shapes experience reduced cell-cell adhesion in addition to the increased mechanical stress from the contractile tension generated by the cellular sheet [134]. These gradients of stress are then propagated by intercellular transmission of the actomyosin cytosketeton and can influence cell function and development. At regions of higher stress at the perimenter of the patterns, myocardin-related transcription factor (MRTF)-A localized to the nucleus which combined with the decreased cell-cell contact and increased tension promotes EMT of epithelial cells in that region. Human adipose derived stem cells grown in a ring showed increased proliferation in the outer edges where cells are large and spread and increased differentiation in the inner edges where cells were small and elongated [136]. Human mesenchymal stem cells (hMSC) from bone marrow were shown to respond to perimeter geometric cues during lineage specification and commitment when exposed to mixed differentiation promoting media [137], [138]. hMSCs in regions of higher stress were shown to differentiate towards the osteoblast lineage while hMSCs preferred to undergo adipogenesis in regions that fostered a lower degree of stress (Figure 7b). Ding and colleagues further explored the role of cell-cell contact and density within multicellular sheets[139]. They seeded varying densities of MSCs on the same size adhesive islands to create populations of cells with differing amounts of cell-cell contact and cell size. When exposed to sole osteogenic or sole adipogenic media, adipogenesis increased with cell density but osteogenesis was unaffected. When exposed to both media, as density increased, adipogenesis increased and osteogenesis decreased.
Figure 7.
(A) Finite element modeling of a contractile monolayer of cells (top) demonstrates variable regions of stress which correlates with proliferation (bottom; Bromodeoxyuridine staining). Adapted with permission[134] 2005, National Academy of Sciences. (B) Diffeent regions of stress in tissue islands are shown to coordinate osteogenesis (Blue; alkaline phosphatase) and adipogenesis (red; Oil Red O). Adapted with permission[137] 2008, Wiley Periodicals Inc. (C) Patterned cells show directed migration within micropatterned islands. Reproduced with permission[133]. 2013, Royal Society of Chemistry, Integrative Biology.
Large scale patterning also allows us to study complex migration patterns which play a pivotal role in biological systems in regulating various processes such as gastrulation, morphogenesis, and tissue organization. The random motion of cells can be controlled and directed with asymmetric “ratchet” microgeometries which induces a controlled cell polarity. These ratchet shapes can be controlled to guide cells of different types into different directions which could be useful in cell sorting [79], [122]. Geometrical confinement of cells into circles induces a persistent, coordinated and synchronized rotation of cells that depends on cell density and size of the circles. The speed of such rotating large-scale movements slows down as the density increases. The rotating cells move as a solid body, with a uniform angular velocity [133] (Figure 7c). Warmflash et al showed that confinement of hESCs to a disk shaped geometry was sufficient recapitulate germ layer patterning with the addition of BMP4[140]. The disk shape is representative of the disk-shaped epithelium at gastrulation and when seeded with a density comparable to the cell number at the initial gastrula, the colony of hESCs self-organized and differentiated into the three germ layers. Studies like these allow us to separate out different biophysical cues and gain insight into their influences on tissue development.
Micropatterning through soft lithography has enabled a wide array of complex cellular processes to be explored. It allows for the precise control of individual cell shape to study the interplay between geometry, intracellular signaling and function. The techniques are reproducible and allow for the creation of complex geometries which different factors such as angle and curvature can be tuned and varied. However, limitations in the variation of viscoelasticity and matrix dynamics has led researchers to investigate other materials systems.
2.3 Micropatterned hydrogels: Integrating biophysical and biochemical cues in 2D
The majority of 2D patterning approaches employ rigid substrates that do not faithfully represent the deformable matrices observed in vivo. Hydrogels are an appealing scaffold material for cell and tissue studies due to high water content and tailored chemical and physical properties [141]–[143]. Using hydrogels to mimic in vivo microenvironments has proved useful in deconstructing the biochemical and biophysical cues that influence cellular morphology [144], [145], proliferation [146]–[149], migration [114], and differentiation [44], [150]–[153]. Recently, micropatterning techniques have been combined with hydrogel systems in order to study the interplay between matrix protein presentation, mechanics, and geometry [83], [105], [154]. Cells in vivo do not spread in the same way as cells cultured on 2D matrices, but rather adopt distinct geometries that relate to the presentation of matrix proteins and the deformability of surrounding matrix. Cells sense the stiffness of their environment and modify their shape, proliferation, and stiffness in response. In addition, as cells spread more they can increase their inherent cortical stiffness by upregulating cytoskeletal contractility. To explore the relationship between cell geometry and substrate stiffness, Tee et al cultured hMSCs on micropatterned polyacrylamide gels of varying stiffness and observed distinct behavior for cells on soft versus stiff substrates in the regulation of cell stiffness [155]. On soft substrates, cellular stiffness depends more strongly on matrix mechanics than spread area. In contrast, cells that were patterned on stiff substrates show a more pronounced role for geometry in directing cell stiffness.
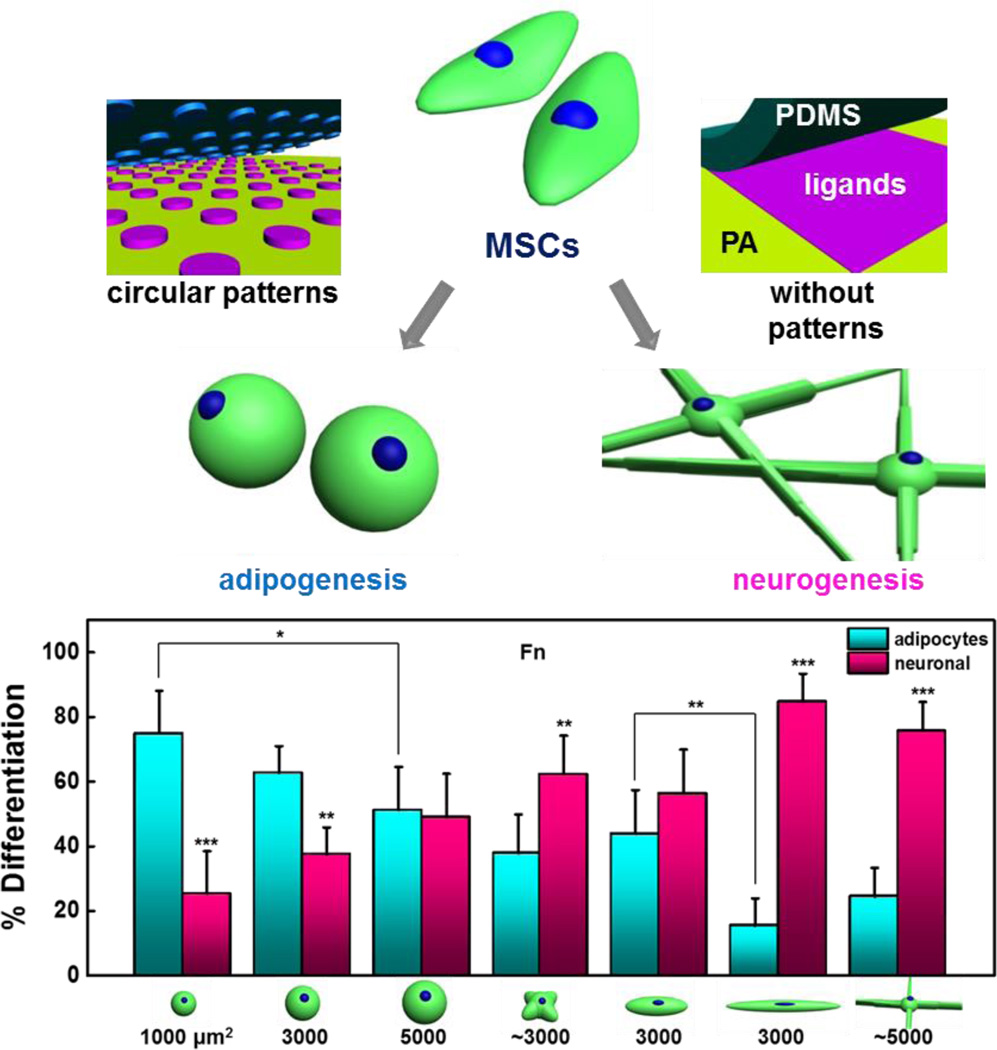
MSCs have been shown to undergo differentiation in response to substrate stiffness [156]. Since cells cultured on planar substrates show a high degree of spatial and geometric heterogeneity, which complicates studies aimed at correlating ECM properties to outcome, we used micropatterning to normalize cell shape across substrates of variable stiffness. By patterning MSCs in shapes with subcellular geometric cues that modulate actomyosin contractility across hydrogels of varying stiffness, we were able to discern the relationship between cell shape, matrix stiffness and the osteogenesis program [83]. Subcellular features that increase focal adhesion and non-muscle myosin activity were shown to promote osteogenesis. In a separate study, MSCs were micropatterned on soft hydrogels to explore neurogenesis and adipogenesis [157]. MSCs that were allowed to spread developed features consistent with the neurogenesis lineage, while cells confined to small isotropic geometries tended to specify to the adipogenesis program (Figure 8). To further explore how MSCs respond to shape and stiffness, we “switched” the matrix underlying the patterned cells—either from a stiff substrate with features that promote actomyosin contractility, or a soft substrate with features that promote neurogenesis—and measured the response of lineage specific markers [158]. Interestingly, MSCs showed a considerable amount of plasticity in the expression of early markers. Consistent with the differentiation studies, we recently demonstrated how geometric features on single cells and multicellular populations will also influence the expression of multipotency markers [159].
Figure 8.
Geometric cues patterned on soft hydrogels guide adipogenesis and neurogenesis in the absence of soluble differentiation promoting compounds. Reproduced with permission[157]. Copyright 2013, Biomaterials.
By combining micropatterning approaches with a viscoelastic polymeric system, the effect of both cell shape and matrix stiffness can be explored. These techniques combines the precise spatial control found in micropatterning along with the physical tunability of polymeric systems in order to precisely control microenvironment parameters. Nevertheless, these systems do not reflect the true dimensionality of in vivo systems, and there is a need for 3D models that can be engineered with high precision to understand how cell signaling differs from 2D to 3D.
3. Microengineering 3D biomaterials to study and direct cellular signaling and tissue organization
3.1. Beyond 2D: Nano- and Micro- templating pseudo-3D environments
While 2D biomaterial systems can give us insight into many cellular functions, the addition of a third dimension enables a closer mimic to the in vivo environment. When cells are cultured in 2D, the planar substrate induces an artificial polarity between the lower and upper surfaces of the cells. Strategies aimed at a closer approximation of tissue dimensionality involve pseudo-three dimensional (3D) or 2.5D environments, which aims to reduce the artificial polarity of 2D culture while maintaining the ease of 2D culture.
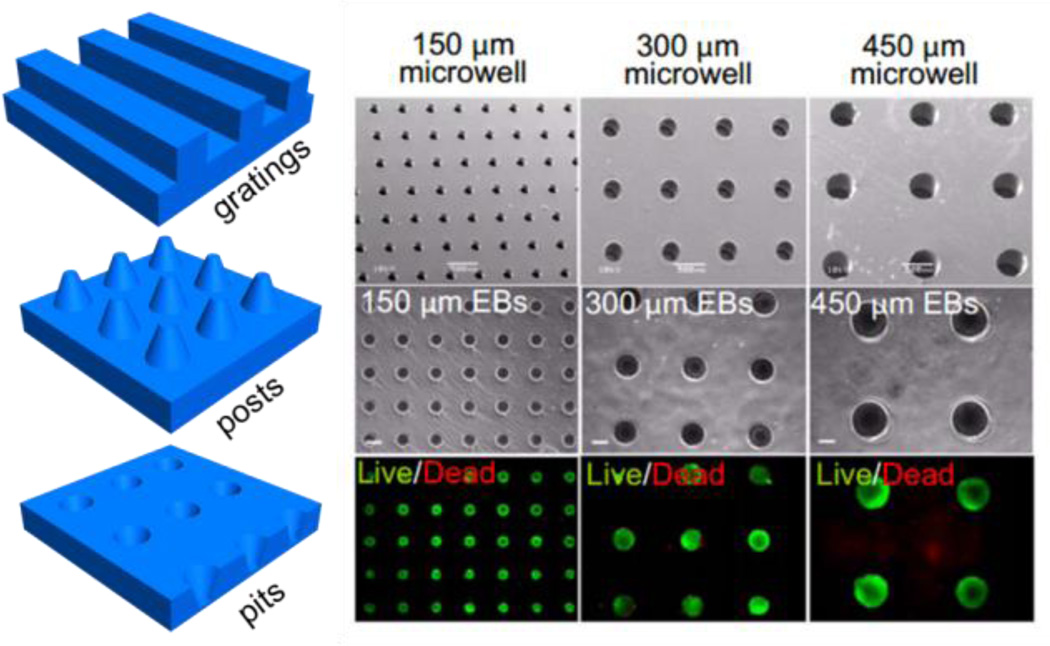
One example of a pseudo-3D environment is the creation of microwells which are topographically structured surfaces that comprise a high density of micron sized cavities of a desirable geometry. They can be created via curing a gel solution, most commonly polydimethylsiloxane (PDMS) or polyethylene glycol (PEG), onto a silicon master [160]. Microwell cultured hESC (human embryonic stem cell) demonstrated the formation of embryoid-bodies (EBs) with a defined size that maintained undifferentiated proliferation [161], [162] (Figure 9). hESCs cultured in microwells of different sizes show that cardiogenesis can be enhanced and endothelial cell differentiation decreased in larger EBs as mediated by noncanonical WNT signaling [163]. Larger concave microwells were shown to increase neuronal and cardiomyocyte differentiation [164] and were more likely to form contracting EBs [163]. EB-mediated differentiation of hESCs in microwell culture also show differences in gene expression related to development when compared to traditional 2D culture [165]. hESCs cultured in microwells show increased induction into mesoderm and endoderm lineages due to the influence of 3D culture on many signaling pathways including canonical Wnt and TGF-β signaling. The size of the embryoid body can also be controlled via controlling the size of the microwell which leads to differences in gene expression. It was seen that EBs cultured in 100µm wells expressed more ectoderm-associated genes whereas EBs cultured in 500µm microwells expressed more mesoderm related genes.
Figure 9.
Depiction of templating approaches to fabricate microwells, grooves, posts and pits. Right: Embryonic stem cells captured in microwells. Reproduced with permission[163]. Copyright 2009 Proceedings of the National Academy of Sciences of the United States of America.
Differences in cell morphology and cytoskeletal structure are observed when human adipose-derived mesenchymal stem cells (hADSCs) were cultured in microwells of varying shapes and sizes [166]. hADSCs grown on flat surfaces were flat and fibroblastic with focal adhesions located at the outer edge of the cells and connected by strong stress fibers. Comparatively, when grown in microwells, hADCs had a more 3-dimensional shape and cytoskeletal orientation. These cytosklatal networks are further mediated by the shape of the microwell. Cells cultured in square microwells formed focal adhesions at the corners and connected by strong stress fibers, whereas cells cultured in round microwells had more homogenously distributed focal adhesions and connected by weaker stress fibers.
Another form of pseudo-3D culture is topographic patterning to create a cellular and subcellular textured surfaces consisting of micron and nano sized features including grooves, pits, and pillars [167]. This approach has been harnessed to explore and direct diverse cellular functions including neural guidance [168], [169], promotion of myotubes [170], stem cell differentiation [171]–[173], and epigenetic changes[174]–[176]. In addition, topographic patterning enables approximation on a materials surface the patterned features observed within the in vivo environment. For instance, the basement membrane of tissues consist of a combination of different topography including pits, pores, protrusions, striations, particulates, and fibers [177]. To mimic these features, topographic patterning is generally performed through photopatterning, printing, and micromachining. Topographical patterning of a few micrometer wide grooves was able to induce cell alignments along the groove direction [178]. Human mesenchymal stem cells (hMSCs) cultured on 350 nm gratings have an elongated morphology with an aligned actin cytoskeleton, while on unpatterned controls, spreading cells showed a random but denser actin cytoskeleton network with altered cytoskeletal and focal adhesion protein expression [179]. Neurite growth can also be influenced by topographic cues and will follow along surface topographies [168], [169], [180] while a closely spaced array of non-adhesive PEG nanohydrogels will promote directional axon growth while limiting the attachment of astrocytes [169]. Micropatterned PDMS channels can promote neurite alignment in adult human neural stem cells; however, smaller channels force cells to deform their cytoskeleton which is unfavorable to neurogenesis [181].
In addition to grooves, pits and pillars can be fabricated in materials to reflect properties of native ECMs. 3T3 fibroblasts grown on micron sized pillars showed differences in morphology and migration [182]. Cells migrating on a surface with pillars are forced to encounter topographic stimuli which facilitate changes in behavior. Compared to flat surfaces, cells on pillar substrates have a more branched shape and have increased linear speed and decreased directional stability which is likely caused by localized stability of focal adhesions. The cells anchor to pillars via focal adhesions, followed by contraction and acceleration toward the pillar. A study by Kim et al looked at the differences between two topographic surfaces. Human epithelial cells were cultured in pillars versus pits [183] and it was observed that on pillar substrates cells migrated towards the sidewall, whereas on pit substrates cells tended to move towards the sidewalls and the bottom. These differences can be a result from the actin reorganization of the cell and the differences in focal adhesion formation at the convex and concave corners of pillar and pit substrates. For more information on topographical techniques to modulate cell signaling, we refer the readers to the following recent reviews [172], [184]–[186].
The creation of pseudo-3D systems are a novel way of combining the ease of 2D systems while overcoming its main limitation of a lack of the third dimension. However it is more restrictive in the complexity of shapes and does not completely eliminate the artificial polarity introduced by 2D systems. In addition, the generation of grooves and pits could serve to limit and organize the cell’s attachment sites which would not normally be restricted in vivo. While 2D and pseudo-3D biomaterials offer simple and reproducible techniques for isolating and studying fundamental components of the extracellular environment, these techniques contain the inevitable downside of over simplifying and not fully representing in vivo 3D architectures.
3.2 Patterning 3D biomaterials for cell and tissue engineering
One of the most common forms of 3D culture is to encapsulate cells within hydrogel scaffolds consisting of different ECM proteins or peptides [187]–[191]. This technique involves the mixing of cells and a liquid gel and allowing the solution to set into the desired shapes. Hydrogel beads have been shown to keep chondrocytes in a rounded morphology with maintenance of function [188]–[190]. Hydrogel beads incorporating small molecules can also influence encapsulated hMSCs to undergo osteogenesis and adipogenesis [192]. A peptide based hydrogelation strategy has also been developed where the application of sheer stress will result in a low viscosity gel but will maintain its rigidity allowing for the gel to be delivered via a syringe [193]. The geometry of the formed gel can also play a role in directing cell function. Fibroblasts, human umbilical endothelial cells (HUVEC), and myoblasts when encapsulated in long, rectangular gelatin methacrylate gels will elongate and self-align to the gel environment [194]. Endothelial cells cultured in channels filled with collagen gel will organize into tubes with lumen which extend up to 1 cm and exhibited cell–cell junction formation characteristic of early stage capillary vessels (Figure 10a). The tube diameter can be controlled by varying collagen concentration or channel width and branching can be guided by channel geometry [195]. An exciting recent study demonstrated the potential of guiding morphogenesis in synthetic 3D matrices, where single mouse embryonic stem cells were encapsulated within soft 90Pa fibrin gels and showed that the single ESC proliferated to formed embryoid bodies which organized into the three germ layers [196].
Figure 10.
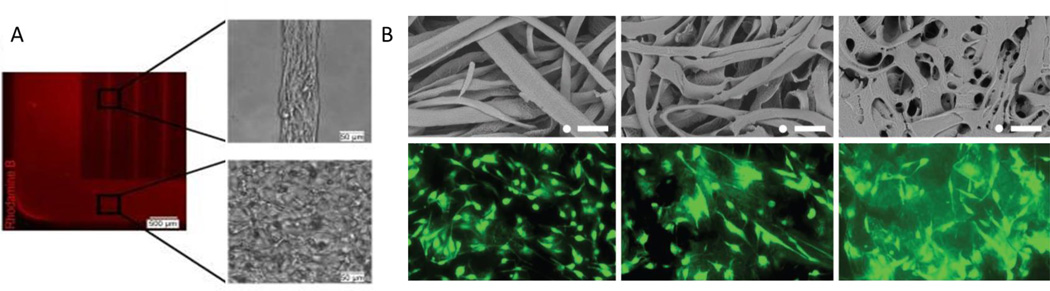
(A) Microgrooves containing collagen enable the formation of tubes with lumen. Adapted with permission [194]. 2010, Elsevier. (B) gelatin microribbons can be used to supplement structure to hydrogel matrices (left to right: 2.5%, 5%, 10% gelatin). Immunofluroescence images of cells adherent to the ribbons at after 6 d. Reproduced with permission.[197] 2013, Wiley-VCH.
Another technique that has shown utility for fabricating biomimetic 3D architectures is electrospinning, which involves the production of very thin continuous fibers that are capable of supporting cell attachment [198], [199]. These fibers are typically generated via the application of a high voltage to a polymer liquid solution sprayed from a very thin nozzle. These fibers can be created from a variety of different synthetic and natural polymers including poly(D,L-lactide-co-glycolide) (PLGA) [199], poly(epsilon-caprolactone) (PCL)[200], and collagen [201]. The fibers can have diameters as small as a few nanometers[200], can be very porous[202], and can be loaded with drugs for drug delivery [203]. Peptide-amphiphile nanofibers consisting of a peptide and an alkyl tail modification have been designed to self-assemble into nanofibers in different pH conditions without the need for additional machinery [204]. These nanofiber systems mimic the natural ECM environment which consists of interwoven protein fibers ranging in size [205]. These nanofibers have been shown to influence many cell functions [206] including guidance of neuron differentiation[207] and the differentiation of MSCs [208]. While the nanofibers are better at mimicking the ECM environment, hydrogels are better at simulating the soft nature of tissues. Yang and colleagues developed an interesting strategy for a hydrogel based scaffold made of gelatin microribbons [197]. The scaffolds are fabricated by wet-spinning a gelatin solution into microfibers and then collapsing them into ribbon like structures via drying in acetone (Figure 10b). When human adipose derived stromal cells were encapsulated in these microribbon structures, they proliferated up to 30-fold within 3 weeks.
One limitation of 3D materials as compared to 2D patterning approaches is the lack of spatial control over chemistry. One possible solution to this limitation is 3D printing which offers the promise of designing and fabricating custom scaffolds and tissues for tissue engineering. Most 3D printing techniques involves initial design of a 3D computer model which is then converted into 2D slices to be printed by a computer slice by slice. For a more in depth review of 3D printing techniques we would like to refer the reader to the following reviews [209], [210]. A newly printed device can be used for biomedical applications as scaffolds[211]–[213], be used as a mold for creation of microfluidic devices [214], or cells can be directly printed for tissue engineering applications. Scaffolds have been fabricated by printing hard extracellular components of a tissue or organ which mimics the original composition and structure, and have been demonstrated for applications in bone tissue regeneration to increase osteogenesis and vaculogenesis[211]–[213], [215]. These scaffold can be made to be biodegradable, allowing them to be replaced over time by growing bone[200] or cartilage[216].
3D printing of cells most often uses hydrogel encapsulation of cells to form a 3D structure[217]–[220]. Yoo and colleagues 3D printed multi-layered collagen hydrogels which contained layers of human skin fibroblasts and keratinocytes[218]. Each layer was first coated with a crosslinking agent. Then, the uncrossed collagen hydrogel was printed followed by the cells onto the coated surface creating a hydrogel layer. 3D printed materials have the potential to combine the fine control of 2D patterning systems with the in vivo mimicry of 3D materials to generate complex tissues with intricate microarchitecture. However, it is currently limited to a small range of materials and still cannot accomplish the micron scale tunability of 2D micropattern techniques. In addition, as the printed materials increase in size, concern regarding the vasculaturization of such materials must be addressed.
Culturing cells in 3D radically alters the interfacial interactions with the ECM compared to 2D, where cells are flatten and may lose their differentiated phenotype [221]. Cells in 3D environments also inherently have more complex cell-cell and cell-matrix interactions along with more complex dynamics for transport. Studies comparing 2D and 3D culture has shown differences in other cellular function including cell adhesion [222], [223], migration [224], gene expression [225], [226], and differentiation [227], [228]. Cells cultured in 3D environments also show differences in cytoskeletal structure and cell attachment including: focal adhesions, cytoskeletal components and associated signaling [229]. When fibroblast cells are cultured in 3D matrices, they form three times more adhesion sites when compared to 2D culture, with enhanced migration and proliferation rates.
Recent evidence has demonstrated how the composition of the matrix and the presentation of ligand can influence the way in which cells in 3D process signals. The seminal work by Discher and colleagues demonstrated that matrix elasticity directs stem cell lineage[156]. Naive MSCs cultured on collagen coated gels of varying stiffness show different morphology with increased neuron-specific markers on softer gels, increased myogenic markers on stiffer gels, and increased osteogenic markers on gels approximating the stiffness of pre-calcified bone. However, Huck and colleagues cultured hMSCs on polydimethylsiloxane (PDMS) and polyacrylamide (PAAm) hydrogels of deferring stiffness and found that cell spreading and differentiation was not effected by stiffness but rather by a decrease in collagen crosslink concentration on gels of lower stiffness with lower anchoring density[230]. The importance of ligand on MSC differentiation was also shown in 2D culture via the use of SAMs of alkane thiolates, where changing the density and affinity of ligand was shown to influence lineage specification without changes in mechanical properties [231]. Recently, Engler and colleagues cultured human adipose derived stromal cells on polyacrylamide gels of similar stiffness but different porosity and found that varying substrate porosity did not significantly affect protein tethering or differentiation potential and that matrix stiffness remains a potent lineage directing cue [232]. From these and other studies, it is clear that there is an intimate relationship between matrix stiffness and ligand presentation, and controlling these cues in engineered extracellular matrices will be crucial for guiding cell behavior to recapitulate in vivo tissue form and function.
3.3 Towards 4D control: spatiotemporal dynamics in 3D engineered extracellular matrices
Culturing cells in 3D materials more closely recapitulates the in vivo environment, when compared to conventional 2D systems; however, most of these materials only offer a static image of what is otherwise a dynamic and complex environment. Recent work on stem cells has shown the cell’s potential to self-organize in 3D culture [233], [234]. ESCs grown in suspension with retinal differentiation medium will self-organize and form patterns of optic-cup morphogenesis without external cues or forces[235]. Other examples of self-organized morphogenesis of ESCs or induced pluripotent stem cells (iPSC) includes the formation of glucose-responsive pancreatic islets [236] generation of functional thyroid [237], and functional adenohypophysis [238]. This ability for ESCs to self-pattern would be an intriguing next step for matrix engineering which could enhance the natural patterning tendencies and guide more complex cell behavior by mimicking natural ECM changes during morphogenesis in vivo. Recent advances in synthetic biomaterials has involved the design of dynamic and evolving systems which change their inherent material properties over time; thus adopting a “fourth dimension”.
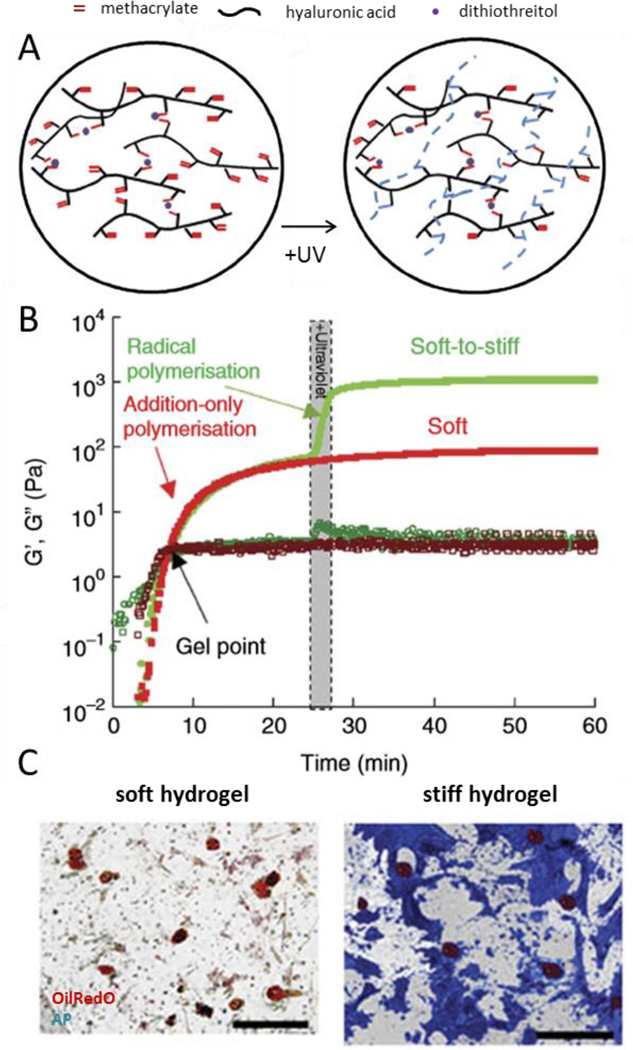
One example is a photodegradable hydrogel which consists of a PEG gel containing a nitrobenzyl ether-derived moiety [239] or a o-nitrobenzylether-based photodegradable monomer [240]. This gel system has been used to encapsulate fibrosarcoma cells in channels with precise release of the cells through illumination to study migratory effects. Burdick and colleagues developed a dynamic hydrogel which stiffens rapidly (from ~3kPa to 30kPa) in the presence of cells with the addition of light [241]. They then studied the effect of dynamic stiffening on differentiating MSCs. After the addition of media containing both adipogenic and osteogenic induction cues, the gel was stiffened at 1, 3, and 7 days. They found that adipogenic differentiation was favored the longer the stiffening event was postponed (Figure 11). Another hydrogel system involves a liposome loaded gel which contains liposomes loaded with gold nanorods and diethylenetriaminepentaacetic acid (DTPA) for softening the gel or CaCl2 for stiffening it [242]. The two differentially loaded liposomes releases their cargo at different irradiation times, thus allowing the gel to be both stiffened and softened. When fibroblasts were cultured on their system, it was seen that stiffening inhibits fibroblast spreading (Figure 11).
Figure 11.
(A) Depiction of multistage crosslinking to fabricated stiffening hydrogels. (B) Mechanical characterization during stiffening. (C) Histological staining of mesenchymal stem cells cultured on the soft gel (left) and after stiffening (right). Reproduced with permission[241]. Copyright 2012, Nature Communications.
Dynamic 4D systems can also include changes in topography over time. Yang and colleagues developed a stimuli-responsive hydrogel which formed macropores in response to different stimuli including temperature, small chelating molecules, and enzymes[243]. The gel was fabricated with a combination of stimuli-responsive porogens of gelatin, alginate, and hyaluronic acid. In the presence of stimuli such as temperature, macropores form in the scaffold as the porogen were removed. When bovine articular chondrocytes (BAC) were seeded onto the scaffold, there was increased proliferation and ECM deposition upon application of stimuli along with cell release from the scaffold. This technique could be useful both for understanding the influence of a changing topography on cells and as a tool for cell delivery. The future of 4D systems will rely on advances on materials systems with controllable architecture, dynamic signaling and reversibility that emulate the complex signaling present in living tissue.
4. Conclusions and future directions
Synthetic engineered extracellular matrices have proved useful in decoupling the environmental parameters that guide cellular processes and ultimately guide the form and function of tissue. From 2D plastic-ware to 3D dynamic hydrogels, model systems have come a long way in controlling the presentation of biochemical and biophysical cues to cells in culture towards elucidating the complex cellular orchestration underlying tissue morphogenesis. The choice of model system should reflect the questions being asked or assays being performed, as each has its own limitations.
2D patterning approaches allow precise variation in composition of the extracellular matrix, cell and tissue geometry, and substrate mechanical properties to discern the interplay between distinct factors. This level of control is difficult to achieve with the majority of 3D materials. While 2D cannot fully represent the in vivo environment, it still serves as a powerful tool for understanding fundamental relationships between cell function and microenvironment parameters. Pseudo-3D platforms are useful for replicating higher order dimensionality to study the transition from 2D to 3D, where true 3D materials are difficult if not impossible to yield coherent data on cell-cell and cell-ECM interactions. Pseudo-3D systems have proved a viable alternative, and show promise in mapping the differences between 2D and 3D towards translating the large number of 2D studies to 3D materials and in vivo systems. True synthetic 3D systems have matured considerably where the chemistry, mechanics and architecture are well controlled, and thereby allow remote interrogation of cellular interactions within material encapsulates.
Recently we have seen a new trend in engineered extracellular matrices where strategies to control both spatial and temporal aspects of the material are taking center stage. Integration of new chemistries, with temporal 3D topographic patterning, has the potential to make synthetic tissue architectures a reality. Nevertheless, we believe there will always be a role for 2D studies, where context is key, and asking the right questions with the right material is critical for defining the materials parameters that will orchestrate the complex interactions that regulate tissue in vivo and ex vivo. The future of engineered extracellular matrices involves creating more complex systems which could combine multiple factors that have been studied individually with current systems in order to understand how they interact and further enhance the influence of engineered materials on cell function. This includes combining patterning with cytokine gradients and rigidity and establishing complex multi-cellular cultures.
Biographies
Yanfen Li is a Ph.D Candidate in the Department of Bioengineering at the University of Illinois at Urbana-Champaign where she also obtained her B.A degree in Bioengineering in 2012. She is a NSF Graduate Research fellow and her research is focused on understanding the influence of biomaterials on cellular mechanics and cytoskeletal organization.

Kristopher A. Kilian is Assistant Professor of Materials Science at the University of Illinois at Urbana-Champaign and Director of the Laboratory for Bioinspired Interfacial Design, where his group develops model systems for exploring tissue form and function. He received his PhD in chemistry at the University of New South Wales in 2007, and completed postdoctoral studies at the University of Chicago in 2011. He is a 2015 recipient of the National Science Foundation CAREER award.

References
- 1.Grinnell F. Fibronectin and cell shape in vivo: studies on the endometrium during pregnancy. J. Cell Biol. 1982 Sep;94(3):597–606. doi: 10.1083/jcb.94.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadley MA. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985 Oct;101(4):1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013 Aug;14(8):467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010 Jan;339(1):83–92. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J. Cell Sci. 2010 Dec;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zagris N. Extracellular matrix in development of the early embryo. Micron. 2001 Jun;32(4):427–438. doi: 10.1016/s0968-4328(00)00011-1. [DOI] [PubMed] [Google Scholar]
- 7.Brown NH. Extracellular matrix in development: Insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 2010 Mar;29(2):89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 2010 May;341(1):126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003 Jun;423(6942):876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 11.Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J. Cell Sci. 1997 Jan;110(Pt 1):55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- 12.Bissell MJ B-HM. The influence of extracellular matrix on gene expression: is structure the message? J. Cell Sci. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- 13.Meinertzhagen IA, Takemura S, Lu Z, Huang S, Gao S, Ting C-Y, Lee C-H. From form to function: the ways to know a neuron. J. Neurogenet. 2009;23:68–77. doi: 10.1080/01677060802610604. [DOI] [PubMed] [Google Scholar]
- 14.Kuo PL, Lee H, Bray MA, Geisse NA, Huang YT, Adams WJ, Sheehy SP, Parker KK. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am. J. Pathol. 2012;181:2030–2037. doi: 10.1016/j.ajpath.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell. 2004 Apr;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 16.Gospodarowicz D, Greenburg G, Birdwell CR. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- 17.Watt FM. The extracellular matrix and cell shape. Trends Biochem. Sci. 1986 Nov;11(11):482–485. [Google Scholar]
- 18.West CM, Lanza R, Rosenbloom J, Lowe M, Holtzer H, Avdalovic N. Fibronectin alters the phenotypic properties of cultured chick embryo chondroblasts. Cell. 1979 Jul;17(3):491–501. doi: 10.1016/0092-8674(79)90257-5. [DOI] [PubMed] [Google Scholar]
- 19.West CM, Weerd H, Dowdy K, Paz A. A specificity for cellular fibronectin in its effect on cultured chondroblasts. Differentiation. 1984 Nov;27(1–3):67–73. doi: 10.1111/j.1432-0436.1984.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 20.Babiarz B, Romagnano L, Afonso S, Kurilla G. Localization and expression of fibronectin during mouse decidualization in vitro: Mechanisms of cell:matrix interactions. Dev. Dyn. 1996;206:330–342. doi: 10.1002/(SICI)1097-0177(199607)206:3<330::AID-AJA10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 22.Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: Distribution of actin, α-actinin, and myosin. Dev. Biol. 1982 Jul;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 23.Öcalan M, Goodman SL, Kühl U, Hauschka SD, von der Mark K. Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev. Biol. 1988 Jan;125(1):158–167. doi: 10.1016/0012-1606(88)90068-1. [DOI] [PubMed] [Google Scholar]
- 24.Senoo H, Hata R. Extracellular matrix regulates cell morphology, proliferation, and tissue formation. Kaibogaku Zasshi. 1994;69:719–733. [PubMed] [Google Scholar]
- 25.Hunt GC, Singh P, Schwarzbauer JE. Endogenous production of fibronectin is required for self-renewal of cultured mouse embryonic stem cells. Exp. Cell Res. 2012 Sep;318(15):1820–1831. doi: 10.1016/j.yexcr.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 2007 Dec;9(12):1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley WP, Yamada KM. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr. Opin. Genet. Dev. 2013 Aug;23(4):408–414. doi: 10.1016/j.gde.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behonick DJ, Werb Z. A bit of give and take: the relationship between the extracellular matrix and the developing chondrocyte. Mech. Dev. 2003 Nov;120(11):1327–1336. doi: 10.1016/j.mod.2003.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prather RS, First NL. A review of early mouse embryogenesis and its applications to domestic species. J. Anim. Sci. 1988 Oct;66(10):2626–2635. doi: 10.2527/jas1988.66102626x. [DOI] [PubMed] [Google Scholar]
- 30.Bolton VN, Oades PJ, Johnson MH. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J. Embryol. Exp. Morphol. 1984;79:139–163. [PubMed] [Google Scholar]
- 31.Wilmut I, Sales DI. Effect of an asynchronous environment on embryonic development in sheep. J. Reprod. Fertil. 1981;61(1):179–184. doi: 10.1530/jrf.0.0610179. [DOI] [PubMed] [Google Scholar]
- 32.CHANG MC. DEVELOPMENT AND FATE OF TRANSFERRED RABBIT OVA OR BLASTOCYST IN RELATION TO THE OVULATION TIME OF RECIPIENTS. J. Exp. Zool. 1950;114(1):197. [Google Scholar]
- 33.Snow MHL. Gastrulation in the mouse: growth and regionalization of the epiblast. 1977;42:293–303. [Google Scholar]
- 34.Zagris N, Chung AE. Distribution and functional role of laminin during induction of the embryonic axis in the chick embryo. Differentiation. 1990;43(2):81–86. doi: 10.1111/j.1432-0436.1990.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 35.Von Dassow M, Davidson LA. Natural variation in embryo mechanics: Gastrulation in Xenopus laevis is highly robust to variation in tissue stiffness. Dev. Dyn. 2009;238(1):2–18. doi: 10.1002/dvdy.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolahi KS, Donjacour A, Liu X, Lin W, Simbulan RK, Bloise E, Maltepe E, Rinaudo P. Effect of substrate stiffness on early mouse embryo development. PLoS One. 2012 Jan;7(7):e41717. doi: 10.1371/journal.pone.0041717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidson LA, Oster GF, Keller RE, Koehl MA. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev. Biol. 1999 May;209(2):221–238. doi: 10.1006/dbio.1999.9249. [DOI] [PubMed] [Google Scholar]
- 38.Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121(10):3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 39.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110(1):115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 40.Davidson LA, Keller R, DeSimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev. Dyn. 2004 Dec;231(4):888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- 41.Chowdhury F, Li Y, Poh Y-C, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010 Jan;5(12):e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lü D, Luo C, Zhang C, Li Z, Long M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials. 2014 Apr;35(13):3945–3955. doi: 10.1016/j.biomaterials.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 43.Poh Y-C, Chowdhury F, Tanaka TS, Wang N. Embryonic stem cells do not stiffen on rigid substrates. Biophys. J. 2010 Jul;99(2):L19–L21. doi: 10.1016/j.bpj.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cells Mater. 2009;18:1–13. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- 45.Eroshenko N, Ramachandran R, Yadavalli VK, Rao RR. Effect of substrate stiffness on early human embryonic stem cell differentiation. J. Biol. Eng. 2013 Jan;7(1):7. doi: 10.1186/1754-1611-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varner VD, Nelson CM. Cellular and physical mechanisms of branching morphogenesis. Development. 2014 Jul;141(14):2750–2759. doi: 10.1242/dev.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies JA. Do different branching epithelia use a conserved developmental mechanism? BioEssays. 2002;24:937–948. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- 48.Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- 49.Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev. Dyn. 2011 Sep;240(9):2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev. Biol. 2009 Dec;336(2):169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grobstein C, Cohen J. Collagenase: effect on the morphogenesis of embryonic salivary epithelium in vitro. Science. 1965;150:626–628. doi: 10.1126/science.150.3696.626. [DOI] [PubMed] [Google Scholar]
- 52.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003 Dec;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hay ED. An overview of Epithelio-Mesenchymal Transformation. Acta Anat. (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 55.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mechanisms of Development. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Parola M, Pinzani M. Hepatic wound repair. Fibrogenesis Tissue Repair. 2009;2:4. doi: 10.1186/1755-1536-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin. Cell Dev. Biol. 2008;19(3):294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Hay ED. Extracellular matrix alters epithelial differentiation. Curr. Opin. Cell Biol. 1993;5:1029–1035. doi: 10.1016/0955-0674(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 60.Greenburg G, Hay ED. Cytoskeleton and thyroglobulin expression change during transformation of thyroid epithelium to mesenchyme-like cells. Development. 1988;102:605–622. doi: 10.1242/dev.102.3.605. [DOI] [PubMed] [Google Scholar]
- 61.Chen QK, Lee K, Radisky DC, Nelson CM. Extracellular matrix proteins regulate epithelial-mesenchymal transition in mammary epithelial cells. Differentiation. 2013 Oct;86(3):126–132. doi: 10.1016/j.diff.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Câmara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair. 2010 Jan;3(1):2. doi: 10.1186/1755-1536-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi Y, Ishigaki T, Sano K, Miyamoto K, Nomura S, Horiuchi T. Three-dimensional invasion of epithelial-mesenchymal transition-positive human peritoneal mesothelial cells into collagen gel is promoted by the concentration gradient of fibronectin. Perit. Dial. Int. 2011 Jan;31(4):477–485. doi: 10.3747/pdi.2010.00166. [DOI] [PubMed] [Google Scholar]
- 64.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 2006 Aug;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-1-induced apoptosis and epithelial-mesenchymal transition. Molecular Biology of the Cell. 2012;23(5):781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003 Jan;5(5):R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011 Mar;178(3):1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015 Apr;17(5):678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, Poh Y-C, Tang K, Wang N, Huang B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat. Mater. 2012 Aug;11(8):734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gershlak JR, Resnikoff JIN, Sullivan KE, Williams C, Wang RM, Black LD. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem. Biophys. Res. Commun. 2013 Sep;439(2):161–166. doi: 10.1016/j.bbrc.2013.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maes E, Broeckx V, Mertens I, Sagaert X, Prenen H, Landuyt B, Schoofs L. Analysis of the formalin-fixed paraffin-embedded tissue proteome: pitfalls, challenges, and future prospectives. Amino Acids. 2013 Aug;45(2):205–218. doi: 10.1007/s00726-013-1494-0. [DOI] [PubMed] [Google Scholar]
- 72.Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 73.Théry M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010;123(Pt 24):4201–4213. doi: 10.1242/jcs.075150. [DOI] [PubMed] [Google Scholar]
- 74.Underhill GH, Galie P, Chen CS, Bhatia SN. Bioengineering methods for analysis of cells in vitro. Annu. Rev. Cell Dev. Biol. 2012 Jan;28:385–410. doi: 10.1146/annurev-cellbio-101011-155709. [DOI] [PubMed] [Google Scholar]
- 75.Celiz AD, Smith JGW, Langer R, Anderson DG, Winkler DA, Barrett DA, Davies MC, Young LE, Denning C, Alexander MR. Materials for stem cell factories of the future. Nat. Mater. 2014 Jun;13(6):570–579. doi: 10.1038/nmat3972. [DOI] [PubMed] [Google Scholar]
- 76.Custódio CA, Reis RL, Mano JF. Engineering biomolecular microenvironments for cell instructive biomaterials. Adv. Healthc. Mater. 2014 Jun;3(6):797–810. doi: 10.1002/adhm.201300603. [DOI] [PubMed] [Google Scholar]
- 77.Roca-Cusachs P, Alcaraz J, Sunyer R, Samitier J, Farré R, Navajas D. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in {G1} and proliferation. Biophys. J. 2008 Jun;94(12):4984–4995. doi: 10.1529/biophysj.107.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thakar RG, Cheng Q, Patel S, Chu J, Nasir M, Liepmann D, Komvopoulos K, Li S. Cell-shape regulation of smooth muscle cell proliferation. Biophys. J. 2009 Apr;96(8):3423–3432. doi: 10.1016/j.bpj.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kandere-Grzybowska K, Campbell CJ, Mahmud G, Komarova Y, Soh S, Grzybowski BA. Cell motility on micropatterned treadmills and tracks. Soft Matter. 2007;3(6):672–679. doi: 10.1039/b617308j. [DOI] [PubMed] [Google Scholar]
- 80.Le Beyec J, Xu R, Lee S-Y, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp. Cell Res. 2007;313(14):3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J. Cell. Biochem. 2008 Sep;105(1):25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain N, Iyer KV, Kumar A, V Shivashankar G. Cell geometric constraints induce modular gene-expression patterns via redistribution of {HDAC3} regulated by actomyosin contractility. Proc. Natl. Acad. Sci. U. S. A. 2013 Jul;110(28):11349–11354. doi: 10.1073/pnas.1300801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J, Abdeen AA, Huang TH, Kilian KA. Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. J. Mech. Behav. Biomed. Mater. 2014;38:209–218. doi: 10.1016/j.jmbbm.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Yao X, Peng R, Ding J. Effects of aspect ratios of stem cells on lineage commitments with and without induction media. Biomaterials. 2013;34(4):930–939. doi: 10.1016/j.biomaterials.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 85.Bissell MJ, Farson D, Tung AS. Cell shape and hexose transport in normal and virus-transformed cells in culture. J. Supramol. Struct. 1977 Jan;6(1):1–12. doi: 10.1002/jss.400060102. [DOI] [PubMed] [Google Scholar]
- 86.Kumar A, Whitesides GM. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol ‘ink’ followed by chemical etching. Appl. Phys. Lett. 1993;63:2002–2004. [Google Scholar]
- 87.Hoffmann C, Tovar GEM. Mixed self-assembled monolayers (SAMs) consisting of methoxy-tri(ethylene glycol)-terminated and alkyl-terminated dimethylchlorosilanes control the non-specific adsorption of proteins at oxidic surfaces. J. Colloid Interface Sci. 2006 Mar;295(2):427–435. doi: 10.1016/j.jcis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Liu VA, Jastromb WE, Bhatia SN. Engineering protein and cell adhesivity using PEO-terminated triblock polymers. J. Biomed. Mater. Res. 2002;60:126–134. doi: 10.1002/jbm.10005. [DOI] [PubMed] [Google Scholar]
- 89.Xia Y, Whitesides GM. SOFT LITHOGRAPHY. Annu. Rev. Mater. Sci. 1998 Aug;28(1):153–184. [Google Scholar]
- 90.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999 Dec;20(23–24):2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 91.Alom Ruiz S, Chen CS. Microcontact printing: A tool to pattern. Soft Matter. 2007;3:168. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- 92.Koepsel JT, Murphy WL. Patterning discrete stem cell culture environments via localized self-assembled monolayer replacement. Langmuir. 2009;25:12825–12834. doi: 10.1021/la901938e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson DL, Martin R, Hong S, Cronin-Golomb M, Mirkin CA, Kaplan DL. Surface organization and nanopatterning of collagen by dip-pen nanolithography. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13660–13664. doi: 10.1073/pnas.241323198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perl A, Reinhoudt DN, Huskens J. Microcontact printing: Limitations and achievements. Adv. Mater. 2009;21:2257–2268. [Google Scholar]
- 95.Roberts C, Chen CS, Mrksich M, Martichonok V, Ingber DE, Whitesides GM. Using mixed self-assembled monolayers presenting RGD and (EG)3OH groups to characterize long-term attachment of bovine capillary endothelial cells to surfaces. J. Am. Chem. Soc. 1998;120(26):6548–6555. [Google Scholar]
- 96.Nelson CM, Chen CS. Cell-cell signaling by direct contact increases cell proliferation via a PI3K–dependent signal. FEBS Lett. 2002 Mar;514(2–3):238–242. doi: 10.1016/s0014-5793(02)02370-0. [DOI] [PubMed] [Google Scholar]
- 97.Piner RD. ‘Dip-Pen’ Nanolithography. Science. 1999 Jan;283(5402):661–663. doi: 10.1126/science.283.5402.661. 80. [DOI] [PubMed] [Google Scholar]
- 98.Lom B, Healy KE, Hockberger PE. A versatile technique for patterning biomolecules onto glass coverslips. J. Neurosci. Methods. 1993;50(3):385–397. doi: 10.1016/0165-0270(93)90044-r. [DOI] [PubMed] [Google Scholar]
- 99.Ostuni E, Kane R, Chen CS, Ingber DE, Whitesides GM. Patterning mammalian cells using elastomeric membranes. Langmuir. 2000;16(20):7811–7819. [Google Scholar]
- 100.Sanjana NE, Fuller SB. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. J. Neurosci. Methods. 2004;136(2):151–163. doi: 10.1016/j.jneumeth.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Hsiao Y-S, Kuo C-W, Chen P. Multifunctional Graphene-PEDOT Microelectrodes for On Chip Manipulation of Human Mesenchymal Stem Cells. Adv. Funct. Mater. 2013;23(37):4649–4656. [Google Scholar]
- 102.Polio SR, Rothenberg KE, Stamenović D, Smith ML. A micropatterning and image processing approach to simplify measurement of cellular traction forces. Acta Biomater. 2012;8(1):82–88. doi: 10.1016/j.actbio.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang N, Ostuni E, Whitesides GM, Ingber DE. Micropatterning tractional forces in living cells. Cell Motil. Cytoskeleton. 2002 Jun;52(2):97–106. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- 104.Tseng Q, Wang I, Duchemin-Pelletier E, Azioune A, Carpi N, Gao J, Filhol O, Piel M, Théry M, Balland M. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab Chip. 2011;11(13):2231–2240. doi: 10.1039/c0lc00641f. [DOI] [PubMed] [Google Scholar]
- 105.Damljanović V, Lagerholm BC, Jacobson K. Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamide substrates for cell mechanotransduction assays. Biotechniques. 2005;39:847–851. doi: 10.2144/000112026. [DOI] [PubMed] [Google Scholar]
- 106.Tang X, Yakut Ali M, Saif MTA. A novel technique for micro-patterning proteins and cells on polyacrylamide gels. Soft Matter. 2012;8(27):7197. doi: 10.1039/C2SM25533B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Z, Yu P, Geller HM, Ober CK. Biomimetic polymer brushes containing tethered acetylcholine analogs for protein and hippocampal neuronal cell patterning. Biomacromolecules. 2013;14(2):529–537. doi: 10.1021/bm301785b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aydin D, Louban I, Perschmann N, Blümmel J, Lohmüller T, Cavalcanti-Adam EA, Haas TL, Walczak H, Kessler H, Fiammengo R, Spatz JP. Polymeric substrates with tunable elasticity and nanoscopically controlled biomolecule presentation. Langmuir. 2010;26(19):15472–15480. doi: 10.1021/la103065x. [DOI] [PubMed] [Google Scholar]
- 109.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011 Oct;3(12):925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel N, Padera R, Sanders GH, Cannizzaro SM, Davies MC, Langer R, Roberts CJ, Tendler SJ, Williams PM, Shakesheff KM. Spatially controlled cell engineering on biodegradable polymer surfaces. FASEB J. 1998 Nov;12(14):1447–1454. doi: 10.1096/fasebj.12.14.1447. [DOI] [PubMed] [Google Scholar]
- 111.Yamato M, Konno C, Koike S, Isoi Y, Shimizu T, Kikuchi A, Makino K, Okano T. Nanofabrication for micropatterned cell arrays by combining electron beam-irradiated polymer grafting and localized laser ablation. J. Biomed. Mater. Res. A. 2003 Dec;67(4):1065–1071. doi: 10.1002/jbm.a.10078. [DOI] [PubMed] [Google Scholar]
- 112.Singhvi R, Kumar A, Lopez G, Stephanopoulos G, Wang D, Whitesides G, Ingber D. Engineering cell shape and function. Science. 1994 Apr;264(5159):696–698. doi: 10.1126/science.8171320. 80. [DOI] [PubMed] [Google Scholar]
- 113.Théry M, Pépin A, Dressaire E, Chen Y, Bornens M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil. Cytoskeleton. 2006 Jun;63(6):341–355. doi: 10.1002/cm.20126. [DOI] [PubMed] [Google Scholar]
- 114.Banerjee S, Marchetti MC. Controlling cell-matrix traction forces by extracellular geometry. New J. Phys. 2013;15(3):35015. [Google Scholar]
- 115.Kumar A, Maitra A, Sumit M, Ramaswamy S, V Shivashankar G. Actomyosin contractility rotates the cell nucleus. Sci. Rep. 2014 Jan;4:3781. doi: 10.1038/srep03781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ingber D. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003 Jan;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 117.Wang N, Butler J, Ingber D. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May;260(5111):1124–1127. doi: 10.1126/science.7684161. 80. [DOI] [PubMed] [Google Scholar]
- 118.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. 1997 Feb;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shabbir SH, Cleland MM, Goldman RD, Mrksich M. Geometric control of vimentin intermediate filaments. Biomaterials. 2014 Feb;35(5):1359–1366. doi: 10.1016/j.biomaterials.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.James J, Goluch ED, Hu H, Liu C, Mrksich M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity. Cell Motil. Cytoskeleton. 2008;65:841–852. doi: 10.1002/cm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Directing cell migration with asymmetric micropatterns. Proc. Natl. Acad. Sci. U. S. A. 2005 Jan;102(4):975–978. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahmud G, Campbell CJ, Bishop KJM, Komarova YA, Chaga O, Soh S, Huda S, Kandere-Grzybowska K, Grzybowski BA. Directing cell motions on micropatterned ratchets. Nat. Phys. 2009;5(8):606–612. [Google Scholar]
- 123.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002 Aug;16(10):1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 124.Ye GJC, Aratyn-Schaus Y, Nesmith AP, Pasqualini FS, Alford PW, Parker KK. The contractile strength of vascular smooth muscle myocytes is shape dependent. Integr. Biol. (Camb) 2014;6:152–163. doi: 10.1039/c3ib40230d. [DOI] [PubMed] [Google Scholar]
- 125.Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr. Biol. (Camb) 2011;3:1063–1070. doi: 10.1039/c1ib00061f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997 May;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 127.O’Connor JW, Gomez EW. Cell adhesion and shape regulate TGF-beta1-induced epithelial-myofibroblast transition via MRTF-A signaling. PLoS One. 2013 Jan;8(12):e83188. doi: 10.1371/journal.pone.0083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107(11):4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010 Mar;28(3):564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang D, Kilian KA. The effect of mesenchymal stem cell shape on the maintenance of multipotency. Biomaterials. 2013 May;34(16):3962–3969. doi: 10.1016/j.biomaterials.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 131.Nelson CM, Chen CS. VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J. Cell Sci. 2003 Sep;116(Pt 17):3571–3581. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 132.Gray DS, Liu WF, Shen CJ, Bhadriraju K, Nelson CM, Chen CS. Engineering amount of cell-cell contact demonstrates biphasic proliferative regulation through RhoA and the actin cytoskeleton. Exp. Cell Res. 2008 Sep;314(15):2846–2854. doi: 10.1016/j.yexcr.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doxzen K, Vedula SRK, Leong MC, Hirata H, Gov NS, Kabla AJ, Ladoux B, Lim CT. Guidance of collective cell migration by substrate geometry. Integr. Biol. 2013;5(8):1026–1035. doi: 10.1039/c3ib40054a. [DOI] [PubMed] [Google Scholar]
- 134.Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. U. S. A. 2005;102(33):11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gomez EW, Chen QK, Gjorevski N, Nelson CM. Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J. Cell. Biochem. 2010;110(1):44–51. doi: 10.1002/jcb.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wan LQ, Kang SM, Eng G, Grayson WL, Lu XL, Huo B, Gimble J, Edward Guo X, Van C. M, Vunjak-Novakovic G. Geometric control of human stem cell morphology and differentiation. Integr. Biol. 2010;2(7–8):346–353. doi: 10.1039/c0ib00016g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26(11):2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Treiser MD, Yang EH, Gordonov S, Cohen DM, Androulakis IP, Kohn J, Chen CS, V Moghe P. Cytoskeleton-based forecasting of stem cell lineage fates. Proc. Natl. Acad. Sci. U. S. A. 2010 Jan;107(2):610–615. doi: 10.1073/pnas.0909597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peng R, Yao X, Cao B, Tang J, Ding J. The effect of culture conditions on the adipogenic and osteogenic inductions of mesenchymal stem cells on micropatterned surfaces. Biomaterials. 2012;33(26):6008–6019. doi: 10.1016/j.biomaterials.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 140.Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods. 2014 Aug;11(8):847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drury JL, Mooney DJ. Biomaterials. 2003;35:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 142.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem. Rev. 2001 Jul;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 143.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–5092. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 144.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 145.Smith Callahan LA, Ganios AM, Childers EP, Weiner SD, Becker ML. Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous Young’s modulus gradient. Acta BioMater. 2013;9(4):6095–6104. doi: 10.1016/j.actbio.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee C, Grodzinsky A, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001 Dec;22(23):3145–3154. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 147.Mih JD, Marinkovic A, Liu F, Sharif AS, Tschumperlin DJ. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J. Cell Sci. 2012 Dec;125(Pt 24):5974–5983. doi: 10.1242/jcs.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011 Apr;124(Pt 8):1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tan PS, Teoh SH. Effect of stiffness of polycaprolactone (PCL) membrane on cell proliferation. Mater. Sci. Eng. C. 2007 Mar;27(2):304–308. [Google Scholar]
- 150.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ren K, Crouzier T, Roy C, Picart C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cells differentiation. Adv. Funct. Mater. 2008 Jan;18(9):1378–1389. doi: 10.1002/adfm.200701297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.V Shih Y-R, Tseng K-F, Lai H-Y, Lin C-H, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011 Apr;26(4):730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 153.Wang L-S, Boulaire J, Chan PPY, Chung JE, Kurisawa M. The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials. 2010 Nov;31(33):8608–8616. doi: 10.1016/j.biomaterials.2010.07.075. [DOI] [PubMed] [Google Scholar]
- 154.Junmin Lee KAK, Abdeen Amr A, Tang Xin, Saif Taher A. Geometric guidance of integrin mediated traction force during stem cell differentiation. Biomaterials. 2015 doi: 10.1016/j.biomaterials.2015.08.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tee SYY, Fu J, Chen CS, Janmey PA. Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 2011 Mar;100(5):L25–L27. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 157.Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34:8140–8148. doi: 10.1016/j.biomaterials.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 158.Lee J, Abdeen AA, Kilian KA. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci. Rep. 2014 Jan;4:5188. doi: 10.1038/srep05188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee J, Abdeen AA, Kim AS, Kilian KA. Influence of Biophysical Parameters on Maintaining the Mesenchymal Stem Cell Phenotype. ACS Biomater. Sci. Eng. 2015;1(4):218–226. doi: 10.1021/ab500003s. [DOI] [PubMed] [Google Scholar]
- 160.Charnley M, Textor M, Khademhosseini A, Lutolf MP. Integration column: microwell arrays for mammalian cell culture. Integr. Biol. (Camb) 2009 Dec;1(11–12):625–634. doi: 10.1039/b918172p. [DOI] [PubMed] [Google Scholar]
- 161.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006 Dec;27(36):6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 162.Moeller H-C, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008 Feb;29(6):752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hwang Y-S, Chung BG, Ortmann D, Hattori N, Moeller H-C, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl. Acad. Sci. U. S. A. 2009 Oct;106(40):16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim J-H, Lee S-H. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010 May;31(15):4296–4303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]
- 165.Hsiao C, Tomai M, Glynn J, Palecek SP. Effects of 3-D microwell culture on initial fate specification in human embryonic stem cells. AIChE J. 2014 Apr;60(4):1225–1235. doi: 10.1002/aic.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu X, Wang W, Kratz K, Fang L, Li Z, Kurtz A, Ma N, Lendlein A. Controlling Major Cellular Processes of Human Mesenchymal Stem Cells using Microwell Structures. Adv. Healthc. Mater. 2014 Oct; doi: 10.1002/adhm.201400415. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 167.Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 168.Turunen S, Haaparanta A-M, Aänismaa R, Kellomäki M. Chemical and topographical patterning of hydrogels for neural cell guidance in vitro. J. Tissue Eng. Regen. Med. 2013 Apr;7(4):253–270. doi: 10.1002/term.520. [DOI] [PubMed] [Google Scholar]
- 169.Krsko P, McCann TE, Thach T-T, Laabs TL, Geller HM, Libera MR. Length-scale mediated adhesion and directed growth of neural cells by surface-patterned poly(ethylene glycol) hydrogels. Biomaterials. 2009 Feb;30(5):721–729. doi: 10.1016/j.biomaterials.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gingras J, Rioux RM, Cuvelier D, Geisse NA, Lichtman JW, Whitesides GM, Mahadevan L, Sanes JR. Controlling the orientation and synaptic differentiation of myotubes with micropatterned substrates. Biophys. J. 2009 Nov;97(10):2771–2779. doi: 10.1016/j.bpj.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Biggs MJP, Richards RG, Gadegaard N, McMurray RJ, Affrossman S, Wilkinson CDW, Oreffo ROC, Dalby MJ. Interactions with nanoscale topography: Adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage. J. Biomed. Mater. Res. - Part A. 2009;91:195–208. doi: 10.1002/jbm.a.32196. [DOI] [PubMed] [Google Scholar]
- 172.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014 Jun;13(6):558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 173.Lim JY, Dreiss AD, Zhou Z, Hansen JC, Siedlecki CA, Hengstebeck RW, Cheng J, Winograd N, Donahue HJ. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28:1787–1797. doi: 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 174.Davidson PM, Özçelik H, Hasirci V, Reiter G, Anselme K. Microstructured surfaces cause severe but non-detrimental deformation of the cell nucleus. Adv. Mater. 2009;21(35):3586–3590. [Google Scholar]
- 175.Badique F, Stamov DR, Davidson PM, Veuillet M, Reiter G, Freund JN, Franz CM, Anselme K. Directing nuclear deformation on micropillared surfaces by substrate geometry and cytoskeleton organization. Biomaterials. 2013;34(12):2991–3001. doi: 10.1016/j.biomaterials.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 176.Pan Z, Yan C, Peng R, Zhao Y, He Y, Ding J. Control of cell nucleus shapes via micropillar patterns. Biomaterials. 2012;33(6):1730–1735. doi: 10.1016/j.biomaterials.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 177.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 178.Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997 Dec;18(24):1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 179.Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li N, Folch A. Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp. Cell Res. 2005 Dec;311(2):307–316. doi: 10.1016/j.yexcr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Béduer A, Vieu C, Arnauduc F, Sol J-C, Loubinoux I, Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012 Jan;33(2):504–514. doi: 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 182.Frey MT, Tsai IY, Russell TP, Hanks SK, Wang Y-L. Cellular responses to substrate topography: role of myosin II and focal adhesion kinase. Biophys. J. 2006 May;90(10):3774–3782. doi: 10.1529/biophysj.105.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim M-H, Sawada Y, Taya M, Kino-Oka M. Influence of surface topography on the human epithelial cell response to micropatterned substrates with convex and concave architectures. J. Biol. Eng. 2014 Jan;8(1):13. doi: 10.1186/1754-1611-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lord MS, Foss M, Besenbacher F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today. 2010 Feb;5(1):66–78. [Google Scholar]
- 185.McNamara LE, McMurray RJ, Biggs MJP, Kantawong F, Oreffo ROC, Dalby MJ. Nanotopographical control of stem cell differentiation. J. Tissue Eng. 2010 Jan;2010:120623. doi: 10.4061/2010/120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dolatshahi-Pirouz A, Nikkhah M, Kolind K, Dokmeci MR, Khademhosseini A. Micro- and Nanoengineering Approaches to Control Stem Cell-Biomaterial Interactions. Journal of Functional Biomaterials. 2011;2:88–106. doi: 10.3390/jfb2030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tsuda Y, Morimoto Y, Takeuchi S. Monodisperse cell-encapsulating peptide microgel beads for 3D cell culture. Langmuir. 2010 Feb;26(4):2645–2649. doi: 10.1021/la902827y. [DOI] [PubMed] [Google Scholar]
- 188.Häuselmann HJ, Aydelotte MB, Schumacher BL, Kuettner KE, Gitelis SH, Thonar EJ. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992 Apr;12(2):116–129. doi: 10.1016/s0934-8832(11)80053-3. [DOI] [PubMed] [Google Scholar]
- 189.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc. Natl. Acad. Sci. U. S. A. 2002 Jul;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mok SS, Masuda K, Häuselmann HJ, Aydelotte MB, Thonar EJ. Aggrecan synthesized by mature bovine chondrocytes suspended in alginate. Identification of two distinct metabolic matrix pools. J. Biol. Chem. 1994 Dec;269(52):33021–33027. [PubMed] [Google Scholar]
- 191.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008 Oct;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. U. S. A. 2007 May;104(19):7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010 Sep;31(27):6941–6951. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng. Part A. 2010 Jul;16(7):2255–2263. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Poh Y-C, Chen J, Hong Y, Yi H, Zhang S, Chen J, Wu DC, Wang L, Jia Q, Singh R, Yao W, Tan Y, Tajik A, Tanaka TS, Wang N. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 2014 Jan;5:4000. doi: 10.1038/ncomms5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Han LH, Yu S, Wang T, Behn AW, Yang F. Microribbon-like elastomers for fabricating macroporous and highly flexible scaffolds that support cell proliferation in 3D. Adv. Funct. Mater. 2013;23:346–358. [Google Scholar]
- 198.Greiner A, Wendorff JH. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angewandte Chemie - International Edition. 2007;46:5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 199.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002 Jun;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 200.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003 May;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 201.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of Collagen Nanofibers. Biomacromolecules. 2002 Mar;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 202.McCann JT, Marquez M, Xia Y. Highly porous fibers by electrospinning into a cryogenic liquid. J. Am. Chem. Soc. 2006 Feb;128(5):1436–1437. doi: 10.1021/ja056810y. [DOI] [PubMed] [Google Scholar]
- 203.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009 Oct;61(12):1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 204.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. Jan;10(7–8):1160–1168. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 206.Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK. Review Paper: A Review of the Cellular Response on Electrospun Nanofibers for Tissue Engineering. J. Biomater. Appl. 2009 Jul;24(1):7–29. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 207.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005 May;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 208.Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007 Jan;28(2):316–325. doi: 10.1016/j.biomaterials.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015 Mar;9(1):4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.V Murphy S, Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 211.Tarafder S, Davies NM, Bandyopadhyay A, Bose S. 3D printed tricalcium phosphate scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013;1(12):1250–1259. doi: 10.1039/C3BM60132C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pati F, Song T-H, Rijal G, Jang J, Kim SW, Cho D-W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015 Jan;37:230–241. doi: 10.1016/j.biomaterials.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 213.Tarafder S, Dernell WS, Bandyopadhyay A, Bose S. SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. PART B-APPLIED BioMater. 2015 Apr;103(3):679–690. doi: 10.1002/jbm.b.33239. [DOI] [PubMed] [Google Scholar]
- 214.Kamei K, Mashimo Y, Koyama Y, Fockenberg C, Nakashima M, Nakajima M, Li J, Chen Y. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices. 2015 Apr;17(2):36. doi: 10.1007/s10544-015-9928-y. [DOI] [PubMed] [Google Scholar]
- 215.Seyednejad H, Gawlitta D, Kuiper RV, De Bruin A, Van Nostrum CF, Vermonden T, Dhert WJA, Hennink WE. In vivo biocompatibility and biodegradation of 3D–printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone) Biomaterials. 2012;33(17):4309–4318. doi: 10.1016/j.biomaterials.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 216.Hung KC, Tseng CS, hui Hsu S. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Advanced Healthcare Materials. 2014 doi: 10.1002/adhm.201400018. [DOI] [PubMed] [Google Scholar]
- 217.Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 218.Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009;30(8):1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 219.Khalil S, Nam J, Sun W. Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds. Rapid Prototyp. J. 2005 Feb;11(1):9–17. [Google Scholar]
- 220.Grolman JM, Zhang D, Smith AM, Moore JS, Kilian KA. Adv. Mater. 2015;27:5512. doi: 10.1002/adma.201501729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 222.Hsiong SX, Huebsch N, Fischbach C, Kong HJ, Mooney DJ. Integrin-adhesion ligand bond formation of preosteoblasts and stem cells in three-dimensional RGD presenting matrices. Biomacromolecules. 2008 Jul;9(7):1843–1851. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 2008;29:2962–2968. doi: 10.1016/j.biomaterials.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 225.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc. Natl. Acad. Sci. U. S. A. 2009 Jan;106(2):399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009 Jan;28(3):461–468. doi: 10.1038/onc.2008.394. [DOI] [PubMed] [Google Scholar]
- 227.Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int. J. Dev. Biol. 2006;50:645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 228.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001 Dec;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 230.Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WTS. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012 Jul;11(7):642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 231.Kilian KA, Mrksich M. Directing Stem Cell Fate by Controlling the Affinity and Density of {Ligand-Receptor} Interactions at the Biomaterials Interface*. Angew. Chemie Int. Ed. 2012;51(20):4891–4895. doi: 10.1002/anie.201108746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014 Aug;13(10):979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sasai Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 234.Sasai Y. Cytosystems dynamics in self-organization of tissue architecture. Nature. 2013;493(7432):318–326. doi: 10.1038/nature11859. [DOI] [PubMed] [Google Scholar]
- 235.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 236.Saito H, Takeuchi M, Chida K, Miyajima A. Generation of glucose-responsive functional islets with a three-dimensional structure from mouse fetal pancreatic cells and iPS cells in vitro. PLoS One. 2011 Jan;6(12):e28209. doi: 10.1371/journal.pone.0028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao X-H, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M, Costagliola S. Generation of functional thyroid from embryonic stem cells. Nature. 2012 doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N. Self-formation of functional adeno- hypophysis in three-dimensional culture. Nature. 2011 doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 239.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat. Protoc. 2010;5:1867–1887. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nature Communications. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 242.Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. U. S. A. 2015 Feb;112(7):1953–1958. doi: 10.1073/pnas.1421897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Han LH, Lai JH, Yu S, Yang F. Dynamic tissue engineering scaffolds with stimuli-responsive macroporosity formation. Biomaterials. 2013;34(17):4251–4258. doi: 10.1016/j.biomaterials.2013.02.051. [DOI] [PubMed] [Google Scholar]