Abstract
Background
Health care spending is known to be highly skewed, with a small subset of the population consuming a disproportionate amount of health care resources. Patients with cancer are high-cost users because of high incremental health care costs for treatment and the growing prevalence of cancer. The objectives of the present study included characterizing cancer-patient trajectories by cost, and identifying the patient and health system characteristics associated with high health system costs after cancer treatment.
Methods
This retrospective cohort study identified Ontario adults newly diagnosed with cancer between 1 April 2009 and 30 September 2010. Costs of health care use before, during, and after cancer episodes were used to develop trajectories of care. Descriptive analyses examined differences between the trajectories in terms of clinical and health system characteristics, and a logistic regression approach identified predictors of being a high-cost user after a cancer episode.
Results
Ten trajectories were developed based on whether patients were high- or low-cost users before and after their cancer episode. The most common trajectory represented patients who were low-cost in the year before cancer, survived treatment, and continued to be low-cost in the year after cancer (31.4%); stage ii cancer of the male genital system was the most common diagnosis within that trajectory. Regression analyses identified increases in age and in multimorbidity and low continuity of care as the strongest predictors of high-cost status after cancer.
Conclusions
Findings highlight an opportunity to proactively identify patients who might transition to high-cost status after cancer treatment and to remediate that transition.
Keywords: Multimorbidity, resource intensity, costs
INTRODUCTION
Health care spending is known to be highly skewed, with a small subset of the general population consuming a disproportionate amount of health care resources1–5. In recent years, policymakers have increasingly sought additional knowledge about the needs of patients who are found to be high-cost users of the health care system, with the aim of identifying opportunities to better manage the care for those individuals. A growing emphasis has therefore been placed on characterizing subgroups of high-cost patients so as to better understand the complexity that drives their resource use and to facilitate improvements in the organization, delivery, and quality of care for those patients3,6,7.
The methods used to identify the sources of high expenditures have taken broad approaches in which the focus tends to be on all users of the health care system, with costs categorized by provider type or setting8,9. Canadian data along these lines have shown that cancer is one of five diagnostic conditions with the largest hospital care expenditures10. The broad approaches do not assess patient-level costs and belie the notion that costs are highly concentrated within certain groups of patients. Patients with cancer have high costs across the health care system both because of high incremental costs for treatment11–15 and because of increasing prevalence16. Ontario-based research identified cancer treatment as one of the most common causes for hospitalization among the highest-cost health care usersa,17.
The existing literature on cancer-related costs has provided considerable insight into the amount of spending during cancer treatment and has been useful in considering the effects of alternative treatment modalities. Additionally, the literature highlights opportunities for better management of cancer treatment12,13,15,18. However, treatment for comorbid conditions that existed before and continue to exist beyond a cancer episode might also affect a patient’s interaction with the health care system and thus contribute to the cost of care, aside from costs related to cancer treatment.
Understanding health care utilization by patients and patterns of cost before and after cancer could offer important insights into how patient complexity affects cancer treatment costs and outcomes. Exploring the effects of comorbid conditions and the interactions of patients with non-cancer-care providers would yield opportunities for all providers in the circle of care to consider the implications of a cancer diagnosis and treatment on overall patient health after treatment.
The aims of the present study were therefore to characterize cancer patient trajectories using cost as a measure of heath care utilization before cancer, during cancer treatment (considering both survival and death), and after cancer treatment; and to determine which patient and health system characteristics are associated with high health system costs after cancer treatment. Overall, our purpose was to identify and characterize cancer patients who become or remain high-cost users after their cancer treatment and also to identify cancer and non-cancer care-system factors that might affect post-cancer costs.
METHODS
This retrospective cohort study used administrative data to identify, from the Ontario Cancer Registry, adults 18 years of age and older who were newly diagnosed with cancer between 1 April 2009 and 30 September 201019. At the Institute for Clinical Evaluative Sciences (ices), patient records from population-based health administrative databases were linked using encoded identifiers for all residents of Ontario. Databases held at ices were used to access clinical and demographic data for the patients, as well as the costs of their health system utilization. Approval to complete this study was granted by the Sunnybrook Health Sciences Centre Research Ethics Board.
In the study, costs were used to measure patient resource intensity and served as a proxy for patient complexity20. Costs of health care resource use were quantified for patients before, during, and after their cancer episode by algorithms developed for patient-level costing using health administrative data and implemented at ices21. Table i describes the databases used to assess health care costs.
TABLE I.
Databases and cost components used in the calculation of health system costs before, during, and after cancer treatment
| Database | Description | Cost component |
|---|---|---|
| Ontario Health Insurance Plan (OHIP) | Contains claims paid by OHIP for services provided by all eligible health care providers, including physicians, groups, and laboratories. |
|
| National Ambulatory Care Reporting System | Contains data from hospital- and community-based ambulatory care services, including day surgery, outpatient clinics, and emergency departments. |
|
| Discharge Abstract Database | Contains information on patient separations, notably:
|
|
| Client Agency Program Enrolment | Registry of patients enrolled in a primary care model. Data elements include program type (family health team, family health organization, family health network, etc.) and patient enrolment status. |
|
| Ontario Drug Benefit (ODB) | Contains claims for prescription drugs covered under the ODB program. Primarily includes drug claims for individuals 65 years of age and older, but also coverage under special ODB programs. |
|
| National Rehabilitation Reporting System | Contains client data from adult inpatient rehabilitation facilities, such as
|
|
| Continuing Care Reporting System | Contains information about residents receiving facility-based continuing care services. Range of services includes complex continuing care, extended or chronic care, and residential care providing nursing services (that is, long-term care). |
|
| Home Care Database | Captures information on all services provided or coordinated by Ontario Community Care Access Centres, including client data, intake and assessment information, admission and discharge, diagnosis and procedures, and care delivery. |
|
| Ontario Mental Health Reporting System | Contains data on patients in adult designated inpatient mental health beds in acute and psychiatric facilities. Data elements include admission and discharge information, diagnoses, service utilization or intervention and procedures. |
|
| Assistive Devices Program | Contains data on Ontario residents with long-term disability receiving personalized assisted devices to support basic needs, such as insulin pumps and supplies, home oxygen, and respiratory and ventilator equipment. |
|
Costs for each encounter that generated an encounter-specific payment [for example, prescriptions, fee-for-service (ffs) physician visits] were measured as the fee paid for the service. Costs for hospital encounters were determined using the appropriate resource intensity weight for that particular care setting and the weighted cost derived based on Ontario spending. Costs for long-term care were measured as a fixed per diem based on prevailing government payment rates. Emergency department and oncologist physicians receive substantial alternative payments that are not visit-related, and the algorithms also ascribed those payments, generally on an average per-patient approach. Capitation payments were calculated based on the payment rate and the particular model of primary care for each patient’s physician in each month of the study period. Team-based payments for family health teams and physician pay-for-performance bonuses were not ascribed to individual patients and thus were not included in the analysis.
Patient-Level Factors
Patient and health system measures were both defined based on earlier research in cancer costing11,12,14,22,23, were extracted from the administrative data to predict health resource costs, and were applied to describe the characteristics of patients with varying trajectories across the health care system. Cancer site and stage were extracted from the Ontario Cancer Registry. Patient age at the date of diagnosis, sex, postal code, and date of death were identified using the Registered Persons Database, which includes records for all Ontarians eligible for public health insurance. Patient postal codes were linked to the 2006 Canadian census to identify the neighborhood income quintile.
To evaluate the role of non-cancer conditions, we identified whether patients had been diagnosed with any of 15 conditions previously used to study multimorbidity in Ontario, including congestive heart failure, chronic obstructive pulmonary disease, asthma, osteoarthritis or other arthritis, rheumatoid arthritis, osteoporosis, chronic coronary syndrome, acute myocardial infarction, hypertension, cardiac arrhythmia, diabetes, dementia, depression, stroke, or renal failure24. The number of co-morbid conditions for each patient was categorized as 0, 1, 2, 3, or 4 or more.
System-Level Factors
We measured a number of health system factors so as to ascertain the relationship between health care delivery and patient cost trajectories. We focused on physician visits to capture involvement in the treatment of cancer and non-cancer conditions in both the institutional and the community care setting. All encounters with physicians regardless of the site of care were included. All physician billings were ascertained from Ontario Health Insurance Plan claims and were specified as cancer-related or non-cancer-related using International Classification of Diseases (9th revision) diagnostic codes 140–239. All visits to medical or radiation oncologists were classified as cancer-related. Physician status as primary care or specialist was determined using the ices Physician Database.
In Ontario, physicians are free to choose to remain in a traditional ffs payment plan or to enrol in a primary care practice model. Since 2006, Ontario has expanded the types of models available, some of which include additional team support and resources such as social workers, chronic disease specialists, and after-hours care25,26. We included the practice model of each patient’s primary care physician, categorized as Traditional FFS, Enrolment FFS, Capitation Group, Capitation Team, and Other.
The Continuity of Care Index (coci) was used to measure the concentration of visits to multiple providers27. The coci ranges from 0 to 1.0, with values close to 0 indicating complete dispersion among a large number of providers, and values close to 1.0 indicating that a single provider handles most of the patient’s care. The coci seeks to identify whether visits are concentrated with a single provider or a small subset of providers, or whether visits are distributed more evenly across multiple providers, thereby indicating lower continuity of care27,28.
We also measured rurality, which was defined using the Rurality Index for Ontario, in which a score greater than 40 was considered rural29. Patients were categorized into health regions based on Ontario’s Local Health Integration Network boundaries. These two measures were included to account for regional differences in accessibility of cancer-related and non-cancer-related care.
Analysis
Episodes of cancer care were created using the date of diagnosis as the index date. Patients diagnosed with cancer before 1 October 2010 were followed from their diagnosis until a maximum follow-up date of 31 March 2012. Episodes of cancer care ended with the date of the last cancer visit preceding a period of 3 months without a cancer-related visit. Deaths during the study period and patients receiving ongoing treatment at 31 March 2012 were identified. If a patient had a new record in the Ontario Cancer Registry within 3 months of their last cancer-related visit, they were grouped with patients who were considered to be receiving ongoing cancer treatment. The identification of incident cancer patients was restricted to 18 months before the maximum follow-up date so that 1 full year of patient costs after cancer treatment could be captured, given the assumption that cancer treatment was expected to last 6 months on average for most patients.
Patients were classified as high-cost if their 1-year costs were in the top 10% of spending for all Ontario residents in the relevant year of comparison. Patients whose costs are in the top 10% account for approximately 80% of all health care spending in Ontario18. The top 10% thresholds used to classify patients as high-cost in each fiscal year of the study were $3,041 (2007–2008), $3,620 (2008–2009), $3,764 (2009–2010), and $3,668 (2010–2011). Notably, all patients had costs well above those levels while receiving treatment for cancer, meaning that they were considered high-cost users during their cancer treatment. Trajectories of care were then created by classifying patients as high- or low-cost in the year before the cancer episode, as survived or died during cancer treatment or receiving ongoing treatment, and as either high- or low-cost or died in the year after cancer treatment. The result was 10 mutually exclusive trajectories. A descriptive analysis of patient and health system measures was then performed for the 10 trajectories (reported as percentages and means with standard deviation).
Given that the primary goal of the study was to determine which patients are most likely to be high-cost users after their cancer treatment, we aimed to identify patient and health system characteristics that are associated with high health system costs after cancer treatment. Thus, for the regression analyses, the study population was limited to patients who had completed their cancer treatment and survived to the end of the study period. Logistic regressions were conducted to predict high-cost status after cancer treatment separately for patients who had a high-cost or low-cost status before cancer treatment.
Measures of interest included patient age, sex, cancer type and stage, multimorbidity, rurality for the patient, and socioeconomic status for the patient’s neighborhood of residence. We also assessed the relationship between modifiable health system measures and high-cost outcomes to determine how interventions and care management changes might affect the likelihood of patients being high-cost users after cancer treatment. Those health system factors included the reimbursement and practice model of the primary care physician, continuity of care, and the intensity of primary and specialist care for both cancer-related and non-cancer-related visits during cancer treatment. Patient geography was also included to determine if post-cancer costs varied with geographic location.
Bivariate analyses for all measures included in the regression were performed to ensure that spurious findings were not reported because of high correlations between independent variables (data not shown). Statistically significant differences between the patients with high- or low-cost status after cancer treatment (stratified by pre-cancer cost categories) were ascertained using t-tests (means), Kruskal–Wallis tests (medians), or chi-square tests (categorical variables) (data not shown). Analyses were performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Cohort Characteristics
The patient cohort included 88,749 adults newly diagnosed with cancer between 1 April 2009 and 30 September 2010. A large proportion of patients in the cohort were between the ages of 45 and 64 years (37.6%) and resided in urban settings (86%). The average duration of cancer treatment was 6.9 months, with the most common disease site being the digestive system (20.6%), followed by the male genital system (14.6%), and the respiratory system (13.9%). Nearly a quarter of all cancers were stage ii at the time of diagnosis (23.4%).
The most common trajectory, which represented almost one third of the patients (31.4%), was low-cost in the year before cancer, survived treatment, and then continued to be low-cost in the year after cancer. The next most common trajectory, which accounted for nearly 15% of the cohort, included patients who were low-cost before cancer, but high-cost in the year after cancer treatment ended. About one third of cancer patients (35.6%) were high-cost users before their cancer, with 18% dying during cancer treatment and 10% dying in the year after treatment. At the end of the study observation period, 7% of cancer patients were receiving ongoing treatment. Slightly more than 20% of patients had a changing trajectory, in which they moved either from low-cost to high-cost or from high-cost to low-cost after cancer treatment.
Table ii shows cancer characteristics across trajectories. Stage ii cancer of the male genital system and breast cancer (stage i or ii) were, respectively, the most commonly diagnosed cancers in the top two trajectories (both low-cost before cancer). Stage iv cancer of the respiratory system was the most common diagnosis and stage for all trajectories that ended with death (high–died, low–died, high–survived–died, low–survived–died). Little variation was observed in the average treatment duration, except for the trajectories that involved ongoing treatment (low–ongoing, high–ongoing).
TABLE II.
Cancer characteristics for the top 10 trajectories
| Care trajectorya | Mean duration of cancer treatment (months) | Five most common cancer sites [% (stage)] | ||||
|---|---|---|---|---|---|---|
| Low–Survived–Low | 196.4±146.9 | 10.2 Male genital system (II) | 9.0 Breast (I) | 7.3 Breast (II) | 5.1 Female genital system (I) | 3.5 Digestive system (II) |
| Low–Survived–High | 198.7±153.5 | 16.9 Male genital system (II) | 7.4 Breast (I) | 7.1 Breast (II) | 5.2 Digestive system (II) | 5.1 Digestive system (III) |
| Low–Ongoing | 541.3±143.8 | 11.0 Breast (II) | 8.9 Digestive system (IV) | 7.8 Digestive system (III) | 7.0 Breast (III) | 5.3 Breast (I) |
| Low–Died | 183.3±172.8 | 23.0 Respiratory system (IV) | 14.0 Digestive system (IV) | 6.9 Respiratory system (III) | 4.0 Digestive system (III) | 2.5 Digestive system (II) |
| Low–Survived–Died | 189.5±164.0 | 15.9 Respiratory system (IV) | 11.1 Digestive system (IV) | 6.3 Respiratory system (III) | 5.2 Digestive system (III) | 3.2 Digestive system (II) |
| High–Survived–Low | 174.8±143.6 | 12.3 Male genital system (II) | 4.9 Digestive system (I) | 4.7 Digestive system (II) | 4.7 Respiratory system (I) | 4.6 Breast (I) |
| High–Survived–High | 164.0±139.4 | 11.7 Male genital system (II) | 6.1 Digestive system (II) | 5.4 Digestive system (I) | 5.2 Breast (II) | 5.1 Respiratory system (I) |
| High–Ongoing | 551.7±144.4 | 6.2 Digestive system (III) | 6.0 Digestive system (IV) | 5.6 Respiratory system (III) | 5.0 Respiratory system (IV) | 4.8 Digestive system (II) |
| High–Died | 138.1±151.0 | 17.9 Respiratory system (IV) | 8.5 Digestive system (IV) | 8.1 Respiratory system (III) | 3.5 Digestive system (III) | 2.9 Digestive system (II) |
| High–Survived–Died | 142.0±145.5 | 12.1 Respiratory system (IV) | 6.7 Respiratory system (III) | 6.1 Digestive system (IV) | 4.1 Digestive system (III) | 3.7 Digestive system (II) |
Pre-cancer cost, outcome during cancer episode, post-cancer cost.
Table iii shows patient and health system factors for all 10 trajectories. Comparisons of the 10 trajectories indicated that patients in the low–survived–low group were younger (58.5 ± 13.2 years) than those who became high-cost users. Mean age for the high–survived–high group was 71.9 years. Low-cost patients were more likely to live in higher-income neighborhoods. Multimorbidity varied considerably; the highest proportion of patients with 4 or more chronic conditions fell into high-cost trajectories: high–high (39.6%), high–died (38.1%), and high–survived–died (43.5%). Almost no variation was observed in terms of rurality. An examination of regional variation across Local Health Integration Networks found the same distribution across all trajectories (data not shown).
TABLE III.
Demographic characteristics for the top 10 trajectories
| Care trajectorya | Pts [n(%)] | Mean age (years) | Age group [ n (%)] | Sex [n(%)] | Neighbourhood income quintile [n(%)] | Rurality [ n(%)] | Multimorbidity at cancer diagnosis [n(%)] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||||||||
| 18–44 Years | 45–64 Years | 65–74 Years | 75–84 Years | 85+ Years | Women | Men | 1 (lowest) | 2 | 3 | 4 | 5 (highest) | Urban | Rural | 0 | 1 | 2 | 3 | 4+ | |||
| Low–Survived–Low | 27,896 (31.4) | 58.5±13.2 | 3,810 (13.7) | 15,092 (54.1) | 6,003 (21.5) | 2,498 (9.0) | 493 (1.8) | 14,773 (53.0) | 13,123 (47.0) | 4,212 (15.1) | 5,231 (18.8) | 5,496 (19.7) | 6,172 (22.1) | 6,685 (24.0) | 24,220 (86.8) | 3,654 (13.1) | 9,015 (32.3) | 8,973 (32.2) | 5,920 (21.2) | 2,770 (9.9) | 1218 (4.4) |
| Low–Survived–High | 13,004 (14.7) | 64.4±13.1 | 992 (7.6) | 5,087 (39.1) | 3,927 (30.2) | 2,457 (18.9) | 541 (4.2) | 6,120 (47.1) | 6,884 (52.9) | 2,289 (17.6) | 2,591 (19.9) | 2,529 (19.4) | 2,697 (20.7) | 2,862 (22.0) | 11,135 (85.6) | 1,863 (14.3) | 2,735 (21.0) | 3,662 (28.2) | 3,347 (25.7) | 1,971 (15.2) | 1289 (9.9) |
| Low–Ongoing | 4,518 (5.1) | 58.2±13.0 | 636 (14.1) | 2,444 (54.1) | 978 (21.6) | 399 (8.8) | 61 (1.4) | 2,659 (58.9) | 1,859 (41.1) | 743 (16.4) | 846 (18.7) | 921 (20.4) | 1,004 (22.2) | 988 (21.9) | 3,917 (86.7) | 600 (13.3) | 1,487 (32.9) | 1,402 (31.0) | 931 (20.6) | 454 (10.0) | 244 (5.4) |
| Low–Died | 8,054 (9.1) | 66.9±12.9 | 320 (4.0) | 3,146 (39.1) | 2,131 (26.5) | 1,796 (22.3) | 661 (8.2) | 3,654 (45.4) | 4,400 (54.6) | 1,672 (20.8) | 1,748 (21.7) | 1,534 (19.0) | 1,578 (19.6) | 1,485 (18.4) | 6,794 (84.4) | 1,253 (15.6) | 2,088 (25.9) | 2,212 (27.5) | 1,918 (23.8) | 1,129 (14.0) | 707 (8.8) |
| Low–Survived–Died | 3,779 (4.3) | 69.0±12.9 | 118 (3.1) | 1,268 (33.6) | 974 (25.8) | 987 (26.1) | 432 (11.4) | 1,698 (44.9) | 2,081 (55.1) | 834 (22.1) | 822 (21.8) | 731 (19.3) | 697 (18.4) | 682 (18.0) | 3,220 (85.2) | 556 (14.7) | 951 (25.2) | 955 (25.3) | 913 (24.2) | 551 (14.6) | 409 (10.8) |
| High–Survived–Low | 5,258 (5.9) | 64.7±14.3 | 535 (10.2) | 1,759 (33.5) | 1,572 (29.9) | 1,120 (21.3) | 272 (5.2) | 2,541 (48.3) | 2,717 (51.7) | 961 (18.3) | 1,028 (19.6) | 1,015 (19.3) | 1,112 (21.1) | 1,122 (21.3) | 4,499 (85.6) | 757 (14.4) | 778 (14.8) | 1,289 (24.5) | 1,351 (25.7) | 977 (18.6) | 863 (16.4) |
| High–Survived–High | 11,322 (12.8) | 71.9±11.8 | 308 (2.7) | 2,051 (18.1) | 3,763 (33.2) | 3,851 (34.0) | 1,349 (11.9) | 5,405 (47.7) | 5,917 (52.3) | 2,434 (21.5) | 2,399 (21.2) | 2,202 (19.4) | 2,138 (18.9) | 2,108 (18.6) | 9,740 (86.0) | 1,577 (13.9) | 563 (5.0) | 1,297 (11.5) | 2,352 (20.8) | 2,627 (23.2) | 4483 (39.6) |
| High–Ongoing | 1,869 (2.1) | 66.9±13.1 | 109 (5.8) | 580 (31.0) | 613 (32.8) | 447 (23.9) | 120 (6.4) | 907 (48.5) | 962 (51.5) | 401 (21.5) | 373 (20.0) | 343 (18.4) | 374 (20.0) | 370 (19.8) | 1,599 (85.6) | 270 (14.4) | 182 (9.7) | 371 (19.9) | 436 (23.3) | 388 (20.8) | 492 (26.3) |
| High–Died | 8,212 (9.3) | 74.4±11.2 | 126 (1.5) | 1,281 (15.6) | 2,272 (27.7) | 3,081 (37.5) | 1,452 (17.7) | 3,894 (47.4) | 4,318 (52.6) | 1,840 (22.4) | 1,751 (21.3) | 1,557 (19.0) | 1,562 (19.0) | 1,476 (18.0) | 7,036 (85.7) | 1,173 (14.3) | 543 (6.6) | 1,073 (13.1) | 1,672 (20.4) | 1,794 (21.8) | 3130 (38.1) |
| High–Survived–Died | 4,837 (5.5) | 76.0±11.0 | 39 (0.8) | 668 (13.8) | 1,152 (23.8) | 1,862 (38.5) | 1,116 (23.1) | 2,343 (48.4) | 2,494 (51.6) | 1,178 (24.4) | 1,033 (21.4) | 900 (18.6) | 906 (18.7) | 806 (16.7) | 4,148 (85.8) | 688 (14.2) | 238 (4.9) | 535 (11.1) | 928 (19.2) | 1,033 (21.4) | 2103 (43.5) |
Pre-cancer cost, outcome during cancer episode, post-cancer cost.
Table iv summarizes health system factors across trajectories. Patients who died during or after cancer treatment were more likely have primary care physicians who were part of team-based capitation models (“family health teams”) and less likely to have primary care physicians who were capitated but not part of a team-based model. Other differences were less remarkable and somewhat subject to smaller numbers of patients within specific trajectories. The mean number of physician visits was higher across all categories of visits—cancer-related or non-cancer-related, family physician or specialist—in trajectories that ended in death, but particularly in trajectories in which death occurred during the cancer episode (7.9 and 9.7 non-cancer specialist visits per month for the low–died and high–died groups respectively). Trajectories with high costs after a cancer episode had only slightly higher mean numbers of physician visits (primary care, specialist, non-cancer-related, cancer-related). The coci varied little across the trajectories.
TABLE IV.
Health services use during treatment in the top 10 trajectories
| Care trajectorya | Primary care model [ n(%)]a | Mean visits per month with ... | Mean continuity of care index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Enrolment fee-for-serviceb | Enrolment capitationc | Team capitationd | Othere | Traditional fee-for-service | Family physician | Oncology | Other specialist | ||||
|
|
|
||||||||||
| Cancer-related | Non-cancer-related | Non-cancer-related | Cancer-related | ||||||||
| Low–Survived–Low | 10,566 (37.9) | 6,058 (21.7) | 7,509 (26.9) | 349 (1.3) | 3,414 (12.2) | 0.24 ± 0.73 | 0.82±1.36 | 0.56±0.84 | 2.73±2.70 | 1.19±1.59 | 0.17±0.14 |
| Low–Survived–High | 4,559 (35.1) | 2,756 (21.1) | 3,973 (30.6) | 163 (1.3) | 1,553 (11.9) | 0.32 ± 0.99 | 1.15±2.17 | 0.65±1.05 | 3.37±3.44 | 1.20±1.65 | 0.17±0.14 |
| Low–Ongoing | 1,361 (30.2) | 721 (15.9) | 1,925 (42.6) | 22 (0.5) | 489 (10.8) | 0.49 ± 0.89 | 0.99±1.07 | 1.43±1.12 | 2.48±1.52 | 1.03±1.03 | 0.12±0.08 |
| Low–Died | 2,018 (25.1) | 1,198 (14.9) | 3,803 (47.2) | 92 (1.1) | 943 (11.7) | 3.67 ± 5.02 | 5.50±6.38 | 2.11±2.89 | 7.88±7.04 | 3.09±4.57 | 0.13±0.10 |
| Low–Survived–Died | 972 (25.7) | 694 (18.4) | 1,569 (41.5) | 39 (1.0) | 505 (13.4) | 1.81 ± 3.38 | 3.43±4.99 | 1.48±2.10 | 5.86±5.65 | 2.11±3.40 | 0.14±0.11 |
| High–Survived–Low | 1,980 (37.7) | 1,119 (21.3) | 1,473 (28.0) | 85 (1.6) | 601 (11.4) | 0.37 ± 1.38 | 1.23±2.23 | 0.49±0.91 | 3.72±3.96 | 1.42±2.14 | 0.17±0.12 |
| High–Survived–High | 4,118 (36.4) | 2,355 (20.8) | 3,428 (30.3) | 181 (1.6) | 1,240 (11.0) | 0.38 ± 1.27 | 1.79±3.15 | 0.47±0.95 | 4.77±4.86 | 1.37±2.19 | 0.17±0.13 |
| High–Ongoing | 559 (29.9) | 284 (15.2) | 876 (46.9) | 11 (0.6) | 139 (7.4) | 0.68 ± 1.17 | 1.46±1.42 | 1.19±1.17 | 3.00±1.75 | 1.0704±1.08 | 0.12±0.09 |
| High–Died | 2,373 (28.9) | 1,418 (17.3) | 3,539 (43.1) | 96 (1.1) | 786 (9.6) | 4.25 ± 5.73 | 6.84±7.30 | 1.61±2.88 | 9.65±8.06 | 3.44±5.33 | 0.15±0.12 |
| High–Survived–Died | 1,368 (28.3) | 951 (19.7) | 1,956 (40.4) | 56 (1.1) | 506 (10.5) | 1.77 ± 3.64 | 4.52±6.21 | 1.09±2.02 | 7.46±6.91 | 2.13±3.85 | 0.16±0.12 |
Pre-cancer cost, outcome during cancer episode, post-cancer cost.
Comprehensive care model, family health groups.
Family health network, family health organization.
Family health team.
Community health group, community-sponsored agreement, group health centre, health services organization, primary care group, primary care network, Rural and Northern Group, South Eastern Area Medical Organization, St. Joseph’s Health Centre.
Table v outlines total health system costs—before, during, and after a cancer episode—across all trajectories. Patients who died incurred the highest total cost throughout their entire trajectory. In particular, patients whose care was highly resource-intensive but who died during the cancer episode had the highest total health system costs, largely comprising costs incurred during treatment (mean cost: $264,152).
TABLE V.
Total health system costs across trajectories
| Care trajectorya | Mean costs (CA$) | ||
|---|---|---|---|
|
| |||
| Pre-cancer | During cancer | After cancer | |
| Low–Survived–Low | 1,292±881 | 54,385±123,005 | 1,441±937 |
| Low–Survived–High | 1,699±989 | 70,010±136,936 | 15,352±21,372 |
| High–Survived–High | 14,916±21,114 | 107,664±188,141 | 21,401±30,392 |
| High–Died | 16,521±22,514 | 264,152±294,699 | |
| Low–Died | 1,733±1,010 | 211,945±247,883 | |
| High–Survived–Low | 9,632±10,771 | 80,321±160,309 | 2,011±1,000 |
| High–Survived–Died | 20,496±27,830 | 185,203±229,628 | 163,763±286,863 |
| Low–Ongoing | 1,388±943 | 44,238±33,946 | |
| Low–Survived–Died | 1,754±1,015 | 138,423±207,870 | 151,596±331,536 |
| High–Ongoing | 11,489±13,949 | 48,728±37,938 | |
Pre-cancer cost, outcome during cancer episode, post-cancer cost.
Subgroup Predictive Models
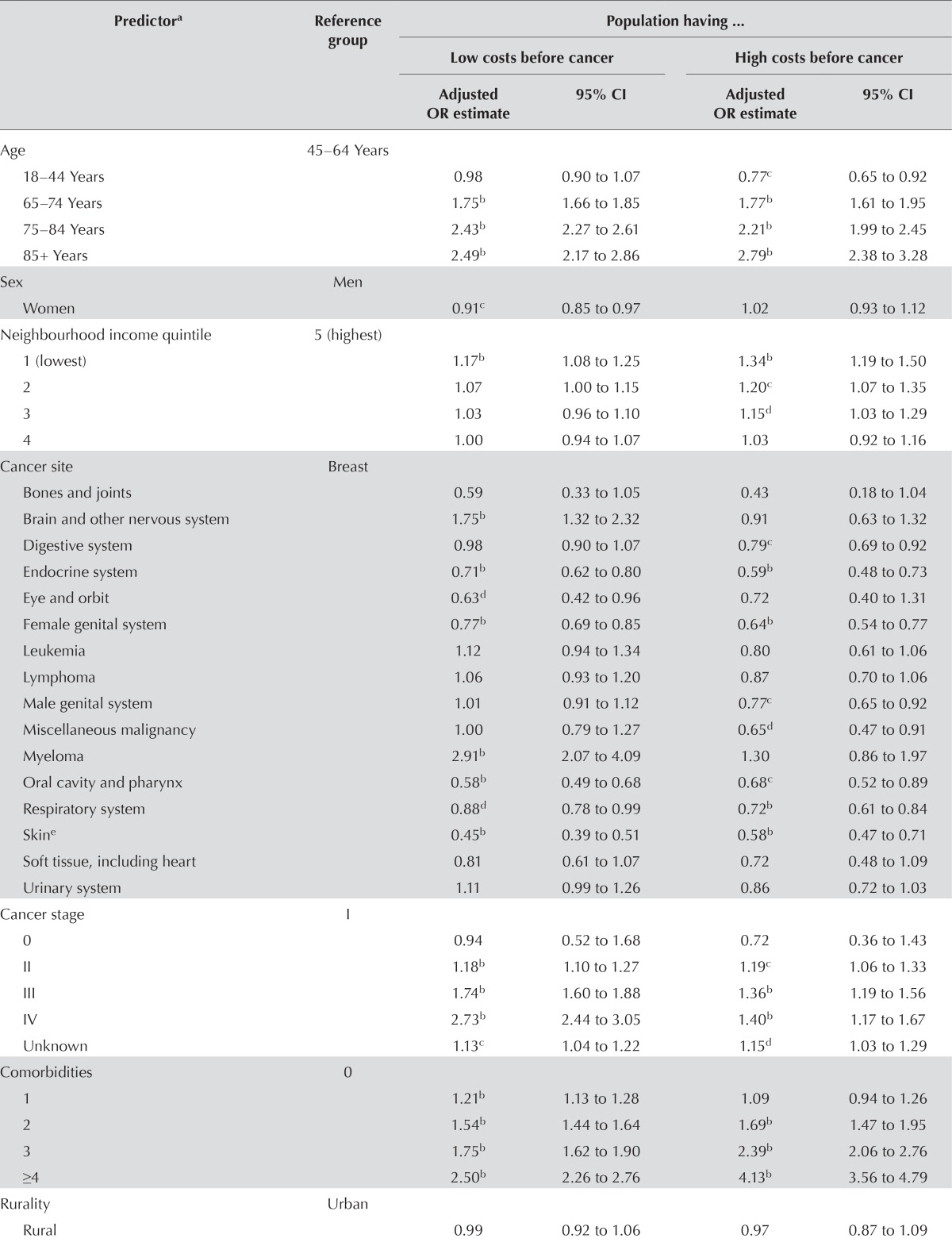
Table vi shows findings from the stratified logistic regressions predicting high-cost status after cancer for patients who survive. For brevity, only the results of the multivariate analyses are shown; however, the bivariate analyses of all variables that were significant in the multivariate analyses showed the same direction and significance (5% level).
TABLE VI.
Logistic regression model predicting high costs after cancer treatment
| Predictora | Reference group | Population having ... | |||
|---|---|---|---|---|---|
|
| |||||
| Low costs before cancer | High costs before cancer | ||||
|
|
|
||||
| Adjusted OR estimate | 95% CI | Adjusted OR estimate | 95% CI | ||
| Age | 45–64 Years | ||||
| 18–44 Years | 0.98 | 0.90 to 1.07 | 0.77c | 0.65 to 0.92 | |
| 65–74 Years | 1.75b | 1.66 to 1.85 | 1.77b | 1.61 to 1.95 | |
| 75–84 Years | 2.43b | 2.27 to 2.61 | 2.21b | 1.99 to 2.45 | |
| 85+ Years | 2.49b | 2.17 to 2.86 | 2.79b | 2.38 to 3.28 | |
| Sex | Men | ||||
| Women | 0.91c | 0.85 to 0.97 | 1.02 | 0.93 to 1.12 | |
| Neighbourhood income quintile | 5 (highest) | ||||
| 1 (lowest) | 1.17b | 1.08 to 1.25 | 1.34b | 1.19 to 1.50 | |
| 2 | 1.07 | 1.00 to 1.15 | 1.20c | 1.07 to 1.35 | |
| 3 | 1.03 | 0.96 to 1.10 | 1.15d | 1.03 to 1.29 | |
| 4 | 1.00 | 0.94 to 1.07 | 1.03 | 0.92 to 1.16 | |
| Cancer site | Breast | ||||
| Bones and joints | 0.59 | 0.33 to 1.05 | 0.43 | 0.18 to 1.04 | |
| Brain and other nervous system | 1.75b | 1.32 to 2.32 | 0.91 | 0.63 to 1.32 | |
| Digestive system | 0.98 | 0.90 to 1.07 | 0.79c | 0.69 to 0.92 | |
| Endocrine system | 0.71b | 0.62 to 0.80 | 0.59b | 0.48 to 0.73 | |
| Eye and orbit | 0.63d | 0.42 to 0.96 | 0.72 | 0.40 to 1.31 | |
| Female genital system | 0.77b | 0.69 to 0.85 | 0.64b | 0.54 to 0.77 | |
| Leukemia | 1.12 | 0.94 to 1.34 | 0.80 | 0.61 to 1.06 | |
| Lymphoma | 1.06 | 0.93 to 1.20 | 0.87 | 0.70 to 1.06 | |
| Male genital system | 1.01 | 0.91 to 1.12 | 0.77c | 0.65 to 0.92 | |
| Miscellaneous malignancy | 1.00 | 0.79 to 1.27 | 0.65d | 0.47 to 0.91 | |
| Myeloma | 2.91b | 2.07 to 4.09 | 1.30 | 0.86 to 1.97 | |
| Oral cavity and pharynx | 0.58b | 0.49 to 0.68 | 0.68c | 0.52 to 0.89 | |
| Respiratory system | 0.88d | 0.78 to 0.99 | 0.72b | 0.61 to 0.84 | |
| Skine | 0.45b | 0.39 to 0.51 | 0.58b | 0.47 to 0.71 | |
| Soft tissue, including heart | 0.81 | 0.61 to 1.07 | 0.72 | 0.48 to 1.09 | |
| Urinary system | 1.11 | 0.99 to 1.26 | 0.86 | 0.72 to 1.03 | |
| Cancer stage | I | ||||
| 0 | 0.94 | 0.52 to 1.68 | 0.72 | 0.36 to 1.43 | |
| II | 1.18b | 1.10 to 1.27 | 1.19c | 1.06 to 1.33 | |
| III | 1.74b | 1.60 to 1.88 | 1.36b | 1.19 to 1.56 | |
| IV | 2.73b | 2.44 to 3.05 | 1.40b | 1.17 to 1.67 | |
| Unknown | 1.13c | 1.04 to 1.22 | 1.15d | 1.03 to 1.29 | |
| Comorbidities | 0 | ||||
| 1 | 1.21b | 1.13 to 1.28 | 1.09 | 0.94 to 1.26 | |
| 2 | 1.54b | 1.44 to 1.64 | 1.69b | 1.47 to 1.95 | |
| 3 | 1.75b | 1.62 to 1.90 | 2.39b | 2.06 to 2.76 | |
| ≥4 | 2.50b | 2.26 to 2.76 | 4.13b | 3.56 to 4.79 | |
| Rurality | Urban | ||||
| Rural | 0.99 | 0.92 to 1.06 | 0.97 | 0.87 to 1.09 | |
| Continuity of care index | Low | ||||
| High | 0.91b | 0.86 to 0.95 | 0.89c | 0.82 to 0.96 | |
| Primary care model | Traditional FFS | ||||
| Enrolment FFS | 0.93 | 0.87 to 1.01 | 0.94 | 0.83 to 1.06 | |
| Enrolment capitation | 0.97 | 0.89 to 1.05 | 1.02 | 0.89 to 1.17 | |
| Team capitation | 1.07 | 0.99 to 1.16 | 1.07 | 0.94 to 1.22 | |
| Other | 1.04 | 0.84 to 1.30 | 1.12 | 0.82 to 1.54 | |
| Length of cancer episode | 1.01b | 1.01 to 1.02 | 1.01d | 1.00 to 1.02 | |
| Visits per month to ... | |||||
| Specialist, non-cancer-related | 1.09b | 1.08 to 1.10 | 1.08b | 1.06 to 1.09 | |
| Family physician, non-cancer related | 1.06b | 1.04 to 1.08 | 1.08b | 1.06 to 1.11 | |
| Family physician, cancer related | 1.02 | 0.99 to 1.05 | 0.95b | 0.92 to 0.98 | |
| Specialist, cancer-related, and Oncology | 1.03b | 1.02 to 1.04 | 1.01 | 0.99 to 1.03 | |
The predictive models were adjusted for Local Health Integration Network; however, any effects were nonsignificant (data not shown).
p < 0.001.
p < 0.01.
p < 0.05.
Excluding basal and squamous cell.
OR = odds ratio; CI = confidence interval; FFS = fee-for-service.
An examination of the predictors of high-cost status after cancer yielded similar results from both logistic models. When modelling the odds of high-cost status after treatment for patients who were low-cost users before cancer, the presence of a greater number of pre-cancer chronic conditions was significantly associated with a transition to high-cost status after cancer treatment. Among individuals who were low-cost users before cancer, 1 additional disease increased the odds of high-cost status after cancer treatment by 20% [odds ratio (or): 1.21; 95% confidence interval (ci): 1.13 to 1.28], and compared with the presence of no comorbid conditions, the presence of 4 or more conditions was associated with an odds of high-cost status after cancer that was higher by a factor of 2.5 (95% ci: 2.26 to 2.76). Although the presence of 1 condition (compared with none of the conditions considered in the study) did not affect the odds of high-cost status after cancer for individuals who incurred high costs before a cancer diagnosis, 4 or more conditions increased the odds of remaining high-cost by a factor of more than 4 (or: 4.13; 95% ci: 3.56 to 4.79).
Although the primary care model had no significant association with high-cost status after cancer, high continuity of care was associated with a likelihood of being high-cost after cancer that was nearly 10% lower for both groups (or for low pre-cancer costs: 0.91; 95% ci: 0.86 to 0.95; or for high pre-cancer costs: 0.89; 95% ci: 0.82 to 0.96). Although the number of cancer-related visits was associated with small and mixed effects on the likelihood of being high-cost after cancer, the number of non-cancer-related primary care visits was associated with a slightly increased likelihood of high-cost status after cancer for both baseline cost groups (or for low pre-cancer costs: 1.06; 95% ci: 1.04 to 1.08; or for high pre-cancer costs: 1.08; 95% ci: 1.06 to 1.11).
Other notable relationships included a higher odds of high-cost status after cancer treatment for individuals in the lowest income quintile (compared with the highest quintile), for men, for older individuals, and for patients diagnosed with a higher stage of cancer. Among patients who had low health care costs before cancer, the likelihood of becoming a high-cost user after cancer was higher for those with brain and myeloma cancer sites than for those with breast cancer. For both high- and low-cost patients before cancer, those with endocrine, female genital, respiratory, and skin cancers were all less likely to be high-cost after cancer than were those with breast cancer. Cancer stage was associated with high post-cancer costs (or for stage ii: 1.18; or for stage iii: 1.74; or for stage iv: 2.73). Rurality was nonsignificant in the models, and we observed few differences in the likelihood of high-cost status after cancer treatment by Local Health Integration Network (data not shown).
DISCUSSION
Findings from the present study, particularly the descriptive analysis of the 10 trajectories, highlight the marked difference between patients who died during or after their treatment, patients who exited their cancer episode as high-cost users of the system, and patients who returned to being low-cost users after cancer. The predictive models confirmed the observations in the descriptive analysis, whereby advanced age, multiple comorbidities, advanced cancer stage, and low continuity of care were all strong predictors of high-cost status after cancer treatment, regardless of whether the patient was a high- or low-cost user before cancer. Of particular note, the ors associated with multimorbidity were among the strongest predictors of post-cancer costs, and the highest levels of comorbidity were at least equivalent to comparing an 85-year-old with a 45-year-old cancer patient. Cancer site seems to have a larger effect in predicting the transition to high resource intensity for patients who were low-cost users before cancer, illustrating the ability of cancer to complicate the health of an individual, creating a need for increased physician visits and health care resource use even after treatment completion and survival. And although cancer stage was associated with high post-cancer costs, the association was smaller than it was for multimorbidity.
Our study is the first to examine costs for cancer patients based on stages in the cancer continuum of care (from before to after cancer) so as to assess the importance of patient complexity from conditions other than cancer. Partitioning the cohort into trajectories of care based on costs before, during, and after their cancer episode allowed for a broad consideration of resource intensity. That analysis provides a novel approach that enhances our understanding of care trajectories beyond the traditional method of evaluating patterns of hospital visits to define models of care (for example, visits to the emergency department)30.
Previous studies have shown the benefit of high continuity of care in reducing the number of emergency department visits by cancer patients at the end of life32. Our results indicate that continuity of care has broader implications in predicting resource intensity after an episode of cancer; continuity of care was associated with lower total health system costs after cancer. To the extent that high costs reflect complex patient management practices, it seems quite logical that a higher degree of clinical management concentrated among fewer physicians would be associated with lower system costs. From the patient’s perspective, such an approach could imply less time spent in waiting rooms and fluctuating between various providers.
The results highlight a recurring trend in the literature: an aging population with multiple comorbidities constitutes a patient population that is becoming increasingly costly to the health care system32. Not only is cancer treatment complex for these individuals, but many are also exiting the cancer system as complex patients; they would likely benefit from a more integrated approach to care during the survivorship stage of their cancer trajectory.
The patients who were low-cost upon entry into the cancer system but who exited as high-cost users constitute a group that warrants further research. An examination of models that could identify this group in advance could potentially inform interventions. Confirmed by the observation that increased continuity of care is likely to mitigate the risk of high-cost status after treatment, primary care involvement in the circle of care during cancer treatment has also been cited to be likely to lessen many of the challenges that arise when coordinating care for a patient with cancer33,34. Although the involvement of family physicians for cancer-related visits was not a significant predictor of cost after cancer for patients with low pre-cancer costs, such involvement was shown to be protective against high costs after cancer for patients with high pre-cancer costs.
The delivery of cancer care spans a longitudinally diverse range of providers and settings depending on the patient’s journey, prognosis, and personal preferences, and care is often fragmented and poorly coordinated33,35. Given the complex and evolving nature of the needs of cancer patients36–38, the coordination of treatment between cancer-care and non-cancer-care providers is important not only during cancer treatment, but especially as patients transition out of the cancer system34,36. Moreover, the presence of comorbid conditions and their co-management during cancer treatment and in the survivorship stage also present important challenges for patients and providers alike37.
The study population and analyses reported here have substantive limitations. We created episodes of cancer care based on visits to cancer physicians, visits in which cancer diagnoses were recorded, and cancer treatments. We defined the end of an episode to be the last such visit, when followed by a gap of more than 3 months before the next cancer-related visit. It might be that some patients experience a gap of more than 3 months even though their cancer is not resolved; they would therefore be misclassified as to trajectory. Nonetheless, our approach to understanding an episode of cancer treatment could be highly useful as a stopping rule for ascribing costs for cancer care in analyses of health administrative data. We were unable to identify the costs of some health services use as cancer-related or non-cancer-related because of limitations in detecting the purpose of services in the drug, home care, rehabilitation, continuing care, and long-term care data. Additionally, all patient characteristics, including multimorbidity and cancer diagnosis and staging, were measured at the date of diagnosis; analyses did not take into account changes in health conditions during the course of treatment or after treatment.
CONCLUSIONS
The present study is the first to look at trajectories of cancer care by cost and to use a more global approach in predicting high resource intensity by way of total cost across all cancer sites, stages, and a variety of patient- and system-level characteristics. We found that multimorbidity, age, and continuity of care affected the transition to high-cost user status after cancer treatment. Those findings highlight an opportunity to identify, during a cancer episode, patients who might transition into high-cost users and potentially to intervene to remediate that transition.
ACKNOWLEDGMENTS
The authors acknowledge Longdi Fu for statistical support and YuQing Bai for preparation of the multimorbidity data. This study was conducted at ices and was supported by Cancer Care Ontario (cco) and the Health System Performance Research Network (no. 06034), which receive funding from the Ontario Ministry of Health and Long-Term Care (mohltc). Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (cihi). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of cihi. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by cco, ices, or the mohltc is intended or should be inferred.
A brief summary of the cohort characteristics are available at the cco Web site: http://www.cancercare.on.ca/complexcancer patients. Results presented in this paper were obtained from additional analyses conducted after that Web site was made public.
Footnotes
Guilcher S, Wodchis WP, Bronskill SE, Gandhi S. Who are the high-cost users? A method for person-centred attribution of health care spending. Presented at the Canadian Association for Health Services Research Annual Conference; Toronto, ON; 12–14 May 2014.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on conflicts of interest disclosure, and we declare that we have none.
REFERENCES
- 1.Reid R, Evans R, Barer M, et al. Conspicuous consumption: characterizing high users of physician services in one Canadian province. J Health Serv Res Policy. 2003;8:215–24. doi: 10.1258/135581903322403281. [DOI] [PubMed] [Google Scholar]
- 2.Stanton M. The high concentration of US health care expenditures. In: Rutherford M, editor. Research in Action. Rockville, MD: United States, Agency for Healthcare Research and Quality; 2006. [Available online at: http://archive.ahrq.gov/research/findings/factsheets/costs/expriach/expendria.pdf; cited 24 September 2015] [Google Scholar]
- 3.Ontario Association of Community Care Access Centres. Ontario Federation of Community Mental Health and Addiction Programs, and Ontario Hospital Association Ideas and Opportunities for Bending the Health Care Cost Curve: Advice for the Government of Ontario. s.l.: s.n.; 2010. [Available online at: https://www.oha.com/KnowledgeCentre/Library/Documents/Bending%20the%20Health%20Care%20Cost%20Curve%20(Final%20Report%20-%20April%2013%202010).pdf; cited 14 September 2015]
- 4.Jiang HJ, Barrett ML, Sheng M. Characteristics of Hospital Stays for Nonelderly Medicaid Super-Utilizers, 2012. Statistical brief 184 Rockville, MD: United States, Agency for Healthcare Research and Quality; 2014. [Available online at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb184-Hospital-Stays-Medicaid-Super-Utilizers-2012.pdf ; cited 19 September 2015] [PubMed] [Google Scholar]
- 5.Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5:e007771. doi: 10.1136/bmjopen-2015-007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deber RB, Lam KCK. Handling the high spenders: implications of the distribution of health expenditures for financing health care. Proceedings of the 2009 American Political Science Association Annual Meeting; Toronto, ON. 3–6 September 2009; [Available online at: http://www.researchgate.net/publication/228293945_Handling_the_High_Spenders_Implications_of_the_Distribution_of_Health_Expenditures_for_Financing_Health_Care; cited 18 September 2015] [Google Scholar]
- 7.Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies; 2013. [Available online at: http://www.rwjf.org/en/library/research/2013/10/super-utilizer-summit.html; cited 14 September 2015] [Google Scholar]
- 8.Lynn J, Straube BM, Bell KM, Jencks SF, Kambic RT. Using population segmentation to provide better health care for all: the “Bridges to Health” model. Milbank Q. 2007;85:185–208. doi: 10.1111/j.1468-0009.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway P, Goodrich K, Machlin S, Sasse B, Cohen J. Patient-centered care categorization of U.S. health care expenditures. Health Serv Res. 2011;46:479–90. doi: 10.1111/j.1475-6773.2010.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada (phac) Economic Burden of Illness in Canada. Ottawa, ON: PHAC; 2014. [Available online at: http://www.phac-aspc.gc.ca/ebic-femc/index-eng.php; cited 9 September 2015] [Google Scholar]
- 11.Mittmann N, Isogai PK, Saskin R, et al. Population-based home care services in breast cancer: utilization and costs. Curr Oncol. 2012;19:e383–91. doi: 10.3747/co.19.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira C, Bremner KE, Pataky R, et al. Understanding the costs of cancer care before and after diagnosis for the 21 most common cancers in Ontario: a population-based descriptive study. CMAJ Open. 2013;1:E1–8. doi: 10.9778/cmajo.20120013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Oliveira C, Bremner KE, Pataky R, et al. Trends in use and cost of initial cancer treatment in Ontario: a population-based descriptive study. CMAJ Open. 2013;1:E151–8. doi: 10.9778/cmajo.20130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittmann N, Liu N, Porter J, et al. Utilization and costs of home care for patients with colorectal cancer: a population-based study. CMAJ Open. 2014;2:E11–17. doi: 10.9778/cmajo.20130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21:281–93. doi: 10.3747/co.21.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison LF, Wilkins K. Canadian Trends in Cancer Prevalence. Ottawa, ON: Statistics Canada; 2012. [Available online at: http://www.statcan.gc.ca/pub/82-003-x/2012001/article/11616-eng.pdf; cited 1 October 2015] [PubMed] [Google Scholar]
- 17.Wodchis W, Austin P, Henry D. A three year study of high cost users. CMAJ. 2016 doi: 10.1503/cmaj.150064. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbera L, Paszat L, Qiu F. End-of-life care in lung cancer patients in Ontario: aggressiveness of care in the population and a description of hospital admissions. J Pain Symptom Manage. 2008;35:267–74. doi: 10.1016/j.jpainsymman.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Clarke EA, Marrett LD, Kreiger N. Cancer registration in Ontario: a computer approach. IARC Sci Publ. 1991;95:246–57. [PubMed] [Google Scholar]
- 20.MaCurdy T, Bhattacharya J. Challenges in Controlling Medicare Spending: Treating Highly Complex Patients. Burlingame, CA: Acumen; 2014. [Available online at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/HighCostComplexPatients.pdf; cited 3 September 2015] [Google Scholar]
- 21.Wodchis WP, Bushmeneva K, Nikitovic M, McKillop I. Guidelines on Person-Level Costing Using Administrative Databases in Ontario. Toronto, ON: Health System Performance Research Network; 2013. [Available online at: http://www.hsprn.ca/uploads/files/Guidelines_on_PersonLevel_Costing_May_2013.pdf; cited 4 September 2015] [Google Scholar]
- 22.Cheung MC, Earle CC, Rangrej J, et al. Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer. 2015;121:3307–15. doi: 10.1002/cncr.29485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587–91. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glazier RH, Klein-Geltink J, Kopp A, Sibley LM. Capitation and enhanced fee-for-service models for primary care reform: a population-based evaluation. CMAJ. 2009;180:E72–81. doi: 10.1503/cmaj.081316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glazier R, Zagorski B, Rayner J. Comparison of Primary Care Models in Ontario by Demographics, Case Mix and Emergency Department Use, 2008/09 to 2009/10. Toronto, ON: Institute for Clinical Evaluative Sciences; 2012. [Available online at: http://www.ices.on.ca/Publications/Atlases-and-Reports/2012/Comparison-of-Primary-Care-Models; cited 30 September 2015] [Google Scholar]
- 27.Bice T, Boxerman S. A quantitative measure of continuity of care. Med Care. 1977;15:347–9. doi: 10.1097/00005650-197704000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006;63:158–88. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- 29.Kralj B. Measuring Rurality—RIO2008_BASIC: Methodology and Results. Toronto, ON: Ontario Medical Association; 2009. [Available online at: https://www.oma.org/Resources/Documents/2008RIO-FullTechnicalPaper.pdf; cited 1 September 2015] [Google Scholar]
- 30.Walker H, Anderson M, Farahati F, et al. Resource use and costs of end-of-Life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27:79–88. [PubMed] [Google Scholar]
- 31.Burge F, Lawson B, Johnston G. Family physician continuity of care and emergency department use in end-of-life cancer care. Med Care. 2003;41:992–1001. doi: 10.1097/00005650-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Canadian Institute for Health Information (cihi). Health Care in Canada 2011: A Focus on Seniors and Aging. Ottawa, ON: CIHI; 2011. [Available online at: https://secure.cihi.ca/estore/productFamily.htm?locale=en&pf=PFC1677; cited 21 September 2015] [Google Scholar]
- 33.Brazil K, Sussman J, Bainbridge D, Whelan T. Who is responsible? The role of family physicians in the provision of supportive cancer care. J Oncol Pract. 2010;6:19–24. doi: 10.1200/JOP.091060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sussman J, Baldwin LM. The interface of primary and oncology specialty care: from diagnosis through primary treatment. J Natl Cancer Inst Monogr. 2010;2010:18–24. doi: 10.1093/jncimonographs/lgq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brazil K, Whelan T, O’Brien MA, Sussman J, Pyette N, Bainbridge D. Towards improving the co-ordination of supportive cancer care services in the community. Health Policy. 2004;70:125–31. doi: 10.1016/j.healthpol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt ME, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 37.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712–19. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 38.Adler NE, Page AEK, on behalf of the U.S. Institute of Medicine Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting, editors. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]