Significance
There has been much debate on the Sapir–Wharf hypothesis regarding whether language affects our perceptual world. Despite much research on this topic, there remains no clear consensus on whether and how language affects categorical color perception. Here, we provide the first evidence, to our knowledge, that categorical color perception has a universal starting point prior to language acquisition. We measured the neural correlates of categorical color perception in prelinguistic infants. We found increased brain activities to colors in different categories, but not to colors in the same category. These results indicated that different color categories are differently represented in the visual cortex of prelinguistic infants, which implies that color categories may develop in the visual system before language acquisition.
Keywords: categorical color perception, cortical response, infant, visual development
Abstract
Perceptual color space is continuous; however, we tend to divide it into only a small number of categories. It is unclear whether categorical color perception is obtained solely through the development of the visual system or whether it is affected by language acquisition. To address this issue, we recruited prelinguistic infants (5- to 7-mo-olds) to measure changes in brain activity in relation to categorical color differences by using near-infrared spectroscopy (NIRS). We presented two sets of geometric figures to infants: One set altered in color between green and blue, and the other set altered between two different shades of green. We found a significant increase in hemodynamic responses during the between-category alternations, but not during the within-category alternations. These differences in hemodynamic response based on categorical relationship were observed only in the bilateral occipitotemporal regions, and not in the occipital region. We confirmed that categorical color differences yield behavioral differences in infants. We also observed comparable hemodynamic responses to categorical color differences in adults. The present study provided the first evidence, to our knowledge, that colors of different categories are represented differently in the visual cortex of prelinguistic infants, which implies that color categories may develop independently before language acquisition.
Humans can discriminate thousands of colors among continuous color space. However, we use only a handful of color terms to describe colors in our daily communication. From the analyses of data for the World Color Survey (www1.ICSI.Berkeley.EDU/wcs/), a corpus of color-naming data from 110 universal languages, many studies have revealed that particular structures of color terms used by speakers exist, and that these structures possess some common features (1, 2). Furthermore, these common features had been found even in the color perception of infants before the acquisition of the color terms (3–5). These results imply that categorical color perception may have some biological basis across cultures and languages. On the other hand, one argument for categorical color perception is that the color lexicon changes perceptual differences among colors so that colors from the same linguistic category appear much closer than colors of different categories (6, 7). A possible hypothesis is that categorical color perception has an innate perceptual foundation, and then could be modified along with the acquisition of language (8).
A recent set of studies focusing on hemispheric asymmetries in categorical color perception has added another perspective to this hypothesis. Gilbert et al. (9) found that the reaction time for detecting a colored target among differently colored distractors was faster when the target and distractors belonged to different categories than when they belonged to the same category. They named this phenomenon the color-category effect, and reported that this category effect is evident only when the target was in the right visual field (RVF) [i.e., when the information was processed through visual cortex in the left hemisphere, where language-related areas reside in adults (e.g., 10)]. Further evidence for the RVF category effect was provided in a series of behavioral, event-related potential (ERP) (11), and functional MRI (fMRI) (12) studies. One such recent study has reported that the category effect in prelinguistic infants was, unlike in adults, lateralized to the left visual field (13), but switches to the right (RVF) when the color words for the relevant categories are learned (14). However, the neurophysiological basis for this lateralization of the category effect in infants was not reported in an ERP study (15). Furthermore, recent psychophysical studies have raised questions about visual-field asymmetry and the repeatability of the category effects (16, 17).
Although evidence has suggested that prelinguistic infants hold categorical color perception, no prior study has identified how the categorical relationship of color is encoded in infants, and whether it is lateralized to a hemisphere. In the present study, we address this question by using a functional brain activity imaging technique, near-infrared spectroscopy (NIRS), to investigate the neural activity related to categorical color perception, along with behavioral experiments. A hierarchy of color-information processing has been demonstrated by several physiological studies in monkeys (18, 19) and humans (20), starting from the occipital to the temporal cortex through the ventral visual areas. Thus, we recorded responses of bilateral occipitotemporal (OT) regions in an attempt to obtain responses to categorical color differences; responses in occipital regions were also recorded to compare responses to a lower level visual feature. The results of the present study suggest the presence of some categorical color representation in the OT regions in infants, which appears to be achieved independent of or before the acquisition of language, and which is probably not driven by mere perceptual color differences.
NIRS Measurement
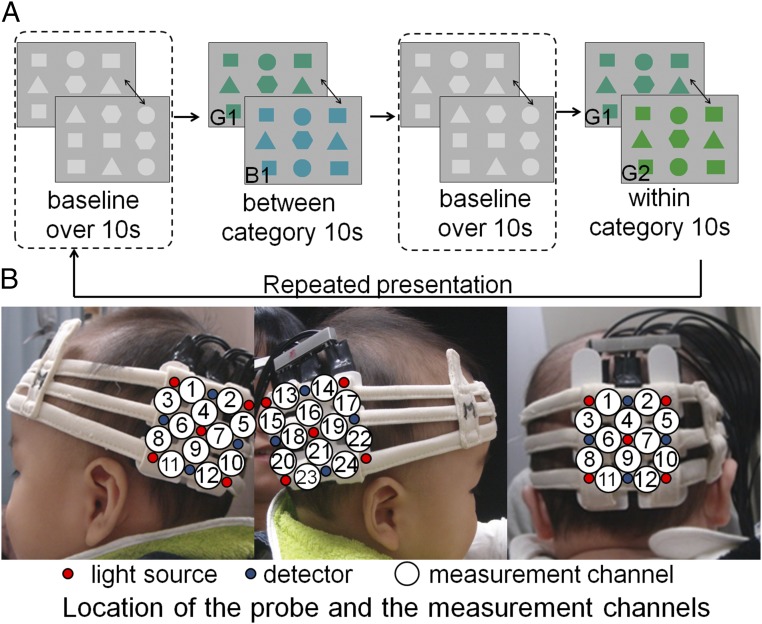
We conducted measurements of infant brain activity using a NIRS system (ETG-4000; Hitachi Medical), and then tested adults for comparison, because it is easier to measure the brain activity of infant participants using NIRS than fMRI. Additionally, previous studies by our group have revealed the interhemispheric differences in face information processing in infants by using NIRS (e.g., 21). One benefit of this method is that identical procedures and measures can be used for both infants and adults, and can be used to verify whether a similar hemodynamic response exists in both groups. In our NIRS experiment, we presented infants and adults with two sets of geometric figures (Fig. 1A). The color of the figures in one set alternated between green and blue at 1 Hz (Green 1 and Blue 1 in Table 1: between-category condition), whereas the color of the other set of figures alternated between two different shades of green at 1 Hz (Green 1 and Green 2 in Table 1: within-category condition). (Blue 1, Green 1, and Green 2 are referred to as B1, G1, and G2, hereafter.)
Fig. 1.
Experimental procedure and location of the NIRS probes. (A) In each trial, the baseline phase consisted of figures changing in shape, which had a duration of at least 10 s. The test phase consisted of color changes between categories (G1/B1) or within a category (G1/G2), which had a duration of 10 s. The presentation order of the between- and within-category phases was counterbalanced across infants. (B) Location of the NIRS probes and the measurement channels in the probe system for infants. The probe holders were placed on the left and right OT regions, slightly below T5 and T6 of the international 10–20 system in the main measurement. The probe holder was placed slightly above Oz for the measurement of the occipital region responses. The distance between the emitter and detector probes was set at 2 cm.
Table 1.
CIE xy and a*b* chromaticities of the stimuli for NIRS measurement and behavior experiment
| Stimulus | x | y | a* | b* | Color difference in CIE L*a*b* color space |
| G2 | 0.252 | 0.490 | −62.6 | 24.3 | G2-G1: ΔE* = 37.1 |
| G1 | 0.231 | 0.355 | −42.0 | −6.63 | G1-B1: ΔE* = 37.4 |
| B1 | 0.215 | 0.255 | −17.4 | −34.8 | B1-B2: ΔE* = 38.7 |
| B2 | 0.204 | 0.185 | 10.4 | −61.6 |
For easier comparisons with a previous study, the chromaticity coordinates of G1, G2, and B1 were chosen from the chromaticity coordinates specified in an article on infant color categorization (5), but were slightly modified to minimize luminance artifacts. Because our experiment alternated the color of the stimuli in a time sequence with a squared waveform, residual luminance contrast at the instance of color alternation could lead to an artifact in the infants’ cortical response. Although a previous study reported the similarity of luminous efficiency function between infants and adults except in the short wavelength range (22), it is better to minimize any cause of artifactual stimulation for recording cortical responses to color changes (23). To attempt to minimize the stimulation of luminance-sensitive mechanisms, we selected colors among those colors that selectively differ in terms of short-wavelength–sensitive cone excitations (24), while retaining an equal color difference between G1/G2 and G1/B1 in a uniform color space defined by Commission internationale de l'éclairage (International Commision on Illumination), so called CIE LAB (1976), (Table 1; details about the luminance control are provided in Supporting Information). The cone fundamentals of Smith and Pokorny (25) were used to calculate the cone responses.
The NIRS responses to the categorical perception of color under the between- and within-category conditions were contrasted against the response during the baseline period in which gray geometric patterns changed their shape at the same frequency as the color alternations. We measured the NIRS responses in the bilateral OT regions (Fig. 1B) to test lateralization in the categorical processing of color. Data were obtained from 12 infants between 5 and 7 mo of age, each of whom had more than three valid trials in both the within-category and between-category conditions (average of 3.7 trials in within-category conditions and average of 3.8 trials in the between-category conditions).
To elucidate NIRS channels that exhibited significant signal changes from the baseline during the measurement, regardless of stimulus conditions, we conducted a repeated-measure ANOVA on the time-series data for each channel, in each participant (26, 27). Channels with consistent activations would display repeatable time-series patterns between trials under the same condition. We took NIRS recording time as a main factor in ANOVA analysis by assuming no significant difference at any time point as a null hypothesis. Fig. 2A illustrates the localization of the activated channels in oxy-Hb (P < 0.01, Bonferroni-corrected) with the statistically significant main effect of time. A wide area of channels was activated under the between- and within-category conditions. However, when this ANOVA on the time-series data was carried out across all participants, we could not find a significant channel that was common among all participants (i.e., no channel exhibited n = 12 in Fig. 2A). This low signal-to-noise ratio is possibly because the absolute amplitude of NIRS signals contained nonnegligible differences among individuals. Therefore, we normalized the signal amplitude (Z-scores) using the mean and the SD of the prestimulus period (details are provided in Supporting Information) for each channel and each participant before applying further statistical analyses.
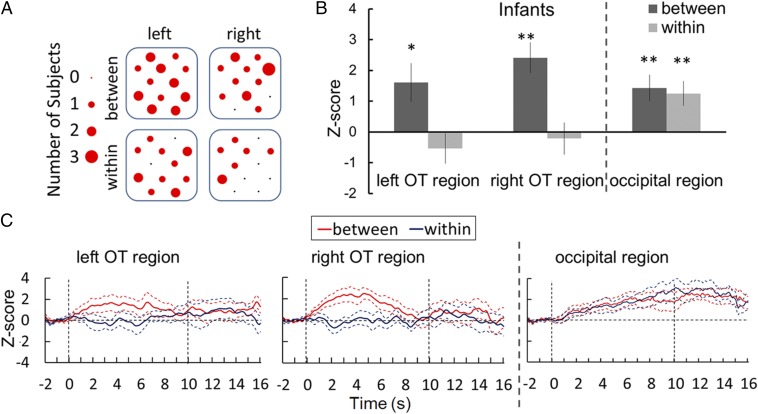
Fig. 2.
Results of the NIRS measurements in infants. (A) Channels with significant changes in oxy-Hb in the OT regions. The size of each red circle represents the number of participants who showed significant changes (P < 0.01) in the corresponding channel. The spatial arrangement of the NIRS channels is illustrated in Fig. 1B. (B) Mean Z-scores of NIRS response in 5- to 7-mo-old infants. Mean Z-scores of data in infants for each of the left temporal (Left), right temporal (Middle), and occipital (Right) regions, respectively, are shown. Each bar represents the mean Z-score of oxy-Hb averaged across 2–6 s in the stimulus onset latency. Dark and light bars represent the results for the between- and within-category conditions, respectively. The error bars represent ±1 SEM. Asterisks indicate the significance level of statistical differences: *P < 0.05; **P < 0.01. (C) Time course of changes in oxy-Hb concentrations was averaged among 5- to 7-mo-old infants during the between- and within-category conditions measured in the left and right OT regions and the occipital region. The deoxy-Hb and total-Hb changes in infants’ bilateral OT and occipital regions are illustrated in Figs. S1 and S2. Thick red and blue lines in each panel represent the mean Z-score in the between- and within-category conditions, respectively. The broken lines represent the range of ±1 SEM. The horizontal axis represents the time from the onset of the test stimulus in seconds; the vertical dashed lines at 0 and 10 s denote the onset and offset of the test stimulus presentation, respectively.
In the next analysis, we first compared the responses (Z-scores), averaged across 12 channels in each of the left and right hemispheres, against the baseline to examine the basic question of this study. The concentrations of oxy-Hb and total-Hb in both the left and right OT regions increased during the presentation of the between-category stimulus (Fig. 2C; results of deoxy-Hb and total-Hb changes are shown in Fig. S1), but not during the presentation of the within-category stimulus. To select a time window for further statistical evaluation, we binned the NIRS data by 2 s from 2 s before the stimulus onset to 12 s following the stimulus presentation and conducted an ANOVA with three factors (stimulus condition, hemisphere, and time window). The resultant ANOVA revealed that the signal during 2–6 s after the stimulus onset contains significant differences between stimulus conditions (details about the ANOVA are provided in Table S1). Therefore, we applied statistical analyses to the Z-scores after averaging them within 2–6 s after the stimulus onset (Fig. 2B). A two-tailed one-sample t test against zero response (baseline) was conducted for the left and right OT regions independently. The results revealed that the concentration of oxy-Hb and total-Hb increased significantly in both OT regions during the between-category condition [for oxy-Hb: left, t(11) = 2.44, P < 0.05; right, t(11) = 4.27, P < 0.01; and for total-Hb: left, t(11) = 2.48, P < 0.05; right, t(11) = 2.55, P < 0.05]. In contrast, no significant increase in the concentration of oxy-Hb and total-Hb was observed under the within-category condition. To test the lateralization in the processing of categorical color differences, a repeated-measure ANOVA with two factors was applied to oxy-Hb data by taking stimulus condition (between vs. within) and hemisphere (right vs. left) as the factors. This analysis revealed a significant main effect of the stimulus condition [F(1,11) = 11.973, P < 0.001], but the main effect of the hemisphere [F(1,11) = 1.036, P = 0.331; not significant (n.s.)] and interaction [F(1,11) = 1.035, P = 0.331; n.s.] were not statistically significant.
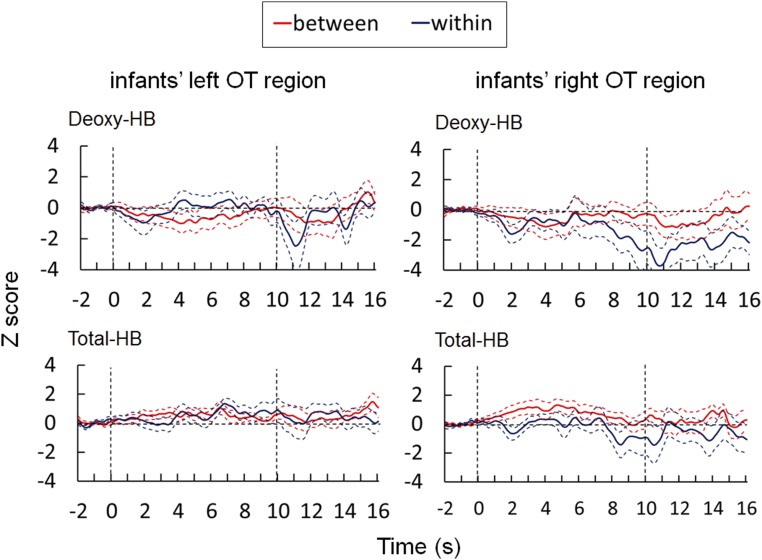
Fig. S1.
Result of the deoxy-Hb and total-Hb changes in infants’ OT regions (related to Fig. 2). Time course of the changes in deoxy-Hb, and total-Hb concentrations averaged among 5- to 7-mo-old infants during the between- and within-category conditions. Thick red and blue lines in each panel represent the mean Z-score in the between- and within-category conditions, respectively. The broken lines represent ±1 SEM. The horizontal axis represents the time from the onset of the test stimulus in seconds; the vertical dashed lines at 0 and 10 s denote the onset and offset of the test stimulus presentation, respectively. (Left) Graphs show the hemodynamic changes in the left hemisphere (1–12 channels). (Right) Graphs show the hemodynamic changes in the right hemisphere (13–24 channels).
Table S1.
Mean (and SD) of responses (Z-score) in each time bin
| Condition | Hemisphere | − 2 to 0 s | 0–2 s | 2–4 s | 4–6 s | 6–8 s | 8–10 s | 10–12 s |
| Between | Left | 0.004 (0.02) | 0.696 (1.48) | 1.411 (2.47) | 1.526 (2.21) | 1.333 (2.77) | 0.921 (1.90) | 1.839 (2.54) |
| Right | 0.000 (0.00) | 1.182 (1.23) | 2.289 (2.43) | 2.38 (2.10) | 1.335 (2.40) | 0.535 (2.20) | 1.078 (3.00) | |
| Within | Left | 0.000 (0.00) | 0.285 (1.22) | −0.246 (2.26) | −0.008 (2.62) | 0.435 (2.06) | 0.670 (2.56) | 0.666 (4.05) |
| Right | 0.000 (0.00) | −0.094 (1.12) | −0.387 (1.84) | 0.252 (2.55) | 0.162 (2.01) | 0.134 (2.22) | 0.735 (2.95) |
These analyses revealed the presence of difference in NIRS responses among categorical conditions, but a more detailed spatial distribution of the brain activity differences was necessary to examine the area of cortical regions that exhibited brain activities related to color categorization, as well as the lateralization of the responses. Despite the finding of significant differences in oxy-Hb signals averaged across 12 channels in each hemisphere (Fig. 2B), no statistically significant difference was detected in any channel when the data from the 24 channels were treated separately. This absence of statistical significance is possibly because some interparticipant differences in the relative position of the activation focus with respect to the NIRS-channel positions caused a reduction in the signal-to-noise ratio when tested across participants. To solve this problem, we reduced the spatial resolution by arbitrarily clustering 12 channels in each hemisphere to constitute four groups: anterior, posterior, dorsal, and ventral group channels (details are provided in Table 2). Multiple comparisons by t test revealed that anterior groups of channels showed a significant signal increase after Bonferroni’s correction for multiple comparisons in each hemisphere: for left [t(11) = 3.09, P < 0.01] and right [t(11) = 4.29, P < 0.01]. Other groups showed no statistical significance. To clarify the relation of NIRS responses to lingual processes, lateralization was also examined. A three-factor repeated-measure ANOVA was performed on the oxy-Hb data by taking stimulus condition, hemisphere, and channel group as the within-participant factors. This analysis revealed a significant main effect of stimulus condition [F(1,11) = 11.95, P < 0.01], but the main effects of hemisphere and channel groups, as well as interactions, were not significant. The activated areas are described in detail in General Discussion. To summarize, the grouped channels analysis indicated significant differences in oxy-Hb responses in the bilateral OT regions between stimulus conditions, which suggests that colors in different categories are differently represented in the visual cortex in infants already at the age of 5 to 7 mo; however, significant lateralization was not detected in our study.
Table 2.
Groups of channels compared by multiple t test followed by a Bonferroni’s correction
| Location | Left | Right |
| Anterior | 3ch, 6ch, 8ch | 17ch, 19ch, 22ch |
| Posterior | 5ch, 7ch, 10ch | 15ch, 18ch, 20ch |
| Dorsal | 1ch, 2ch, 4ch | 13ch, 14ch, 16ch |
| Ventral | 9ch, 11ch, 12ch | 21ch, 23ch, 24ch |
ch, channel.
Hypothetically, the different responses to the two color pairs in the bilateral OT regions could arise from lower level color attributes. For instance, previous studies reported that blue was more salient than green in infants (28–30). Furthermore, it is unknown whether the CIE LAB color space is perceptually uniform in infants. Hence, it is not possible to reject the possibility that the color difference in infants for the “between-category” pair was larger than the color difference of the “within-category” pair. If any asymmetry in lower level color attributes were present, corresponding differences would be observed in the responses of the early visual cortex at the same time. We tested this possibility by measuring another 12 infants’ NIRS responses in the occipital region (Fig. 1B). We performed statistical analyses against the mean Z-scores of a time window from 2 to 6 s after the stimulus onset (Fig. 2C; results of deoxy-Hb and total-Hb are shown in Fig. S2). There were no significant differences in the concentration of oxy-Hb between the two stimulus conditions [t(11) = 0.49, P = 0.634; n.s.]. The hemispherical difference in the occipital data was also tested, but no statistical significance was detected (details are provided in Supporting Information). In summary, the lower level color attributes do not account for the different responses in the bilateral OT regions in the present study in infants. This result indicated that colors of different categories are represented differently in the bilateral OT regions, and not in the early visual cortex.
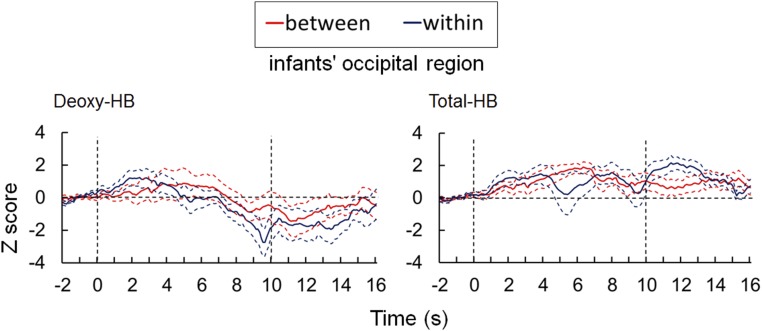
Fig. S2.
Results of the deoxy-Hb and total-Hb changes in infants’ occipital regions (related to Fig. 3). The time course of the average change in deoxy-Hb and total-Hb measured in the occipital region of infants is shown. Axes and line colors/styles are the same as in Fig. S1.
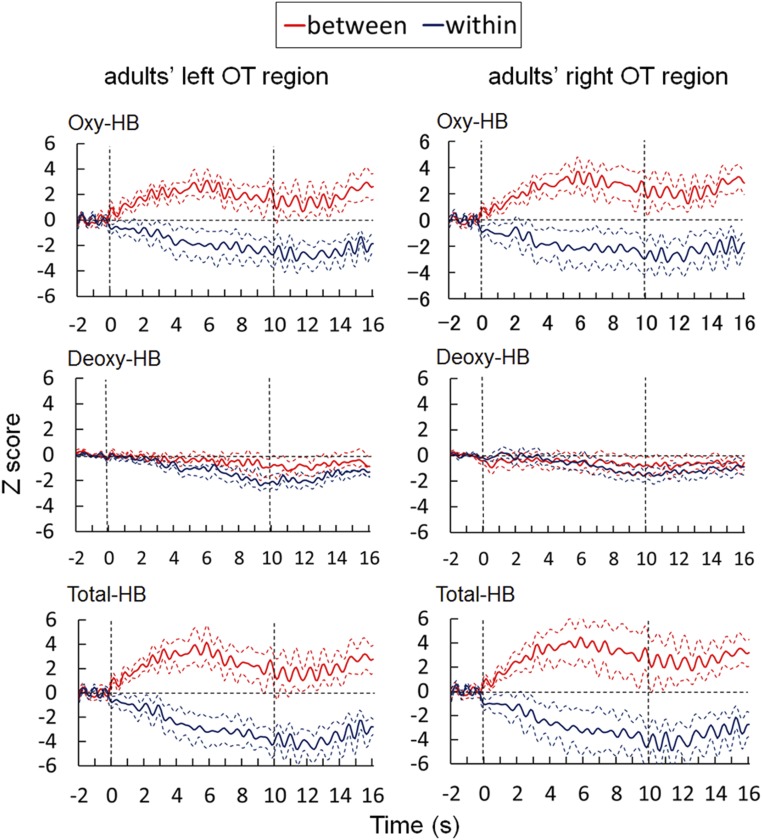
Adult participants also took part in the same NIRS measurements in the bilateral OT regions. The most important difference between the measurements in the adult and infant participants was that the border of perceptual color categories had been tested in adult participants before the NIRS recordings. We obtained NIRS data that were qualitatively similar to NIRS data in the infants. The concentrations of oxy-Hb and total-Hb in both the left and right OT regions increased during the presentation of the between-category stimulus, but not during the presentation of the within-category stimulus (more details are provided in Fig. S3). Stimulus colors were the same as for the stimulus colors used for the NIRS experiment in infants, and the differences between the two pairs of color stimuli (B1 vs. G1 and G1 vs. G2) could be primarily ascribed to a difference in color category. Therefore, it may be possible to infer that the difference in brain activities of infants under the two stimulus conditions in the NIRS measurements was primarily due to the representation of color categories.
Fig. S3.
Results of adults’ OT regions. The time course of the average change in oxy-Hb, deoxy-Hb, and total-Hb measured in the OT region of adults. Axes and line colors/styles represent the same as in Fig. S1.
Behavioral Experiment
We confirmed whether the color-category boundary in infants is similar to the color-category boundary of adults by conducting the following behavioral experiment, using a familiarization/novelty-preference procedure. Infants were familiarized with stimuli of one color and then tested for novelty preference with the original color and a novel color presented simultaneously. Infants were tested whether, for example, B1 was perceived as a color in the same group as B2 and different from the group of G1 and G2. Stimulus pairs differed in terms of between- or within-category pairs. Significant preference for only novel stimuli in a new category, but not for stimuli of the same category, would indicate the presence of categorical perception and the boundary of a color category in infants. This inference is based on the consensus that the effect of familiarization cannot be generalized across a category boundary (3, 4). We used three conditions (B1/B2, B1/G1, and G1/G2), depending on the color pair for the test of novelty preference. For instance, in the B1/G1 condition, the infants were familiarized with either B1 or G1 in the familiarization phase, and then presented with the pair of B1 and G1 in the posttest phase.
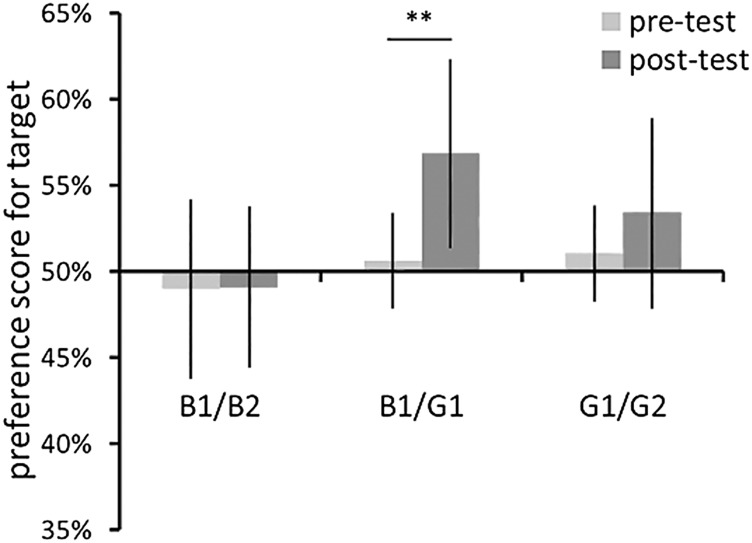
By calculating preference scores, the relative looking time for the novel stimuli divided by the total looking time for each infant, we found evidence indicating that infants discriminated stimuli according to color categories, as shown in Fig. 3. The novelty preference was evaluated by preplanned t tests to compare the scores between pre- and posttest conditions. The infants’ novelty preferences increased significantly only when the posttest stimulus was the between-category pair [B1/G1: t(11) = 3.90, P < 0.01; B1/B2: t(11) = 0.03, P = 0.975: n.s.; G1/G2: t(11) = 0.90, P = 0.386: n.s.]. This result implies that a category border was present between colors B1 and G1 in infants. To examine whether a spontaneous preference existed, a two-tailed t test was performed on the data of the pretest phase against the chance level (50%). No preference scores in the pretest phase were significantly different from chance [B1/G1 condition: t(11) = 0.32, P = 0.75; B1/B2 condition: t(11) = 0.57, P = 0.57; G1/G2 condition: t(11) = 0.23, P = 0.82; all n.s.]. Therefore, the infants appear to have distinguished the colors based on category differences, and not by simple color preference. These findings support the hypothesis that 5- to 7-mo-old infants distinguish color categories, and that the color pairs we used in the NIRS experiment (B1/G1 vs. G1/G2) differed in relation to the border of a color category. It must be noted that the absence of a novelty preference does not imply the inability of infants to discriminate colors within a category. A previous study (5) has demonstrated that infants could discriminate the same set of colors using a visual search task. Additionally, significant activation in NIRS responses for both color pairs in the occipital region (Fig. 2 B and C) may imply that both pairs of color alternations could evoke responses in the infants’ early visual cortex. Therefore, it is possible that the novelty preference measures were not sufficiently sensitive to detect the infants’ ability for discriminating two colors in the same category.
Fig. 3.
Mean preference scores for novel stimuli in the habituation experiment. Error bars indicate ±SEM. Results for the pretest phase are indicated by light gray bars, and results for the posttest phase are indicated by dark gray bars. A significant difference was found only in the between-category pair. Asterisks indicate a statistically significant difference: **P < 0.01.
General Discussion
Our findings have revealed, for the first time to our knowledge, that 5- to 7-mo-old infants have similar hemodynamic responses as adults to color alternations in relation to a color-category border. This result is consistent with the behavioral data of categorical color perception in infants (3–5, 13). These results also support the idea that categorical color perception is formed by a perceptual process that is plausibly based on an innate organization of color categories in visual processing or experience in the visual environment, and that can be independent of language.
Our results indicated that different categories of colors are represented differently in infants in the OT regions, similar to the results in adults (Fig. 2 and Fig. S3), but not in the early visual cortex (Fig. 2). Furthermore, the responses in the OT regions are likely driven by the color category, rather than by mere perceptual color difference. These results are consistent with previous studies in both macaque monkeys and humans (18–20, 31, 32), which found the presence of categorical encoding of color in the ventral pathway. Previous functional neuroimaging studies have associated area V4, located in the fusiform gyrus, as an important site for color perception in the adult human brain (33–35). A recent fMRI study reported that the categorical clustering of neural representation for color-naming was found in the ventral visual areas V4v and VO1 (20), whereas these areas seem to change their responses flexibly under different tasks (19), probably according to the top-down signal from higher order areas that define categories (31). An electrophysiological study in macaque monkeys’ inferior temporal cortex (18) showed the presence of neurons with selectivity for color categories, which was highly similar to the categories measured by psychophysics in humans (32). According to the estimation of correspondence between the channel positions in the international 10–20 EEG system and their anatomical loci (36), the significant activity shown in our grouped channel analysis was near the border of the middle temporal gyrus and the fusiform gyrus, which are close to Wernicke’s area in adults; this region of significant activity may suggest some relevance to lingual processes. However, a recent NIRS study revealed that Wernicke’s area begins to take part in the lingual process at 13–14 mo after birth, but not in infants at 6–7 mo of age (37).
Clifford et al. (15) have shown that the between-category stimuli elicited a larger peak amplitude of ERPs than the within-category stimuli, but failed to detect the lateralization (9, 13, 14). In the present study, using NIRS to measure categorical color responses, hemispheric asymmetry was not observed. In addition, lateralization of the categorical effect (9) could not be replicated by an extensive series of experiments in two recent psychophysical studies (16, 17). Three possible explanations for this absence of significant laterality are as follows: (i) the lateralization of categorical color perception is not robust or has a weak effect size, such that the statistical power was too low and was not reliably detected; (ii) it could be restricted to certain tasks; or (iii) it cannot be measured as an NIRS response. Thus, the direct relationship between color categorization and hemispheric lateralization needs to be reconsidered.
The origin and nature of color categories have been concerns of researchers from a range of disciplines for many decades. There is converging behavioral evidence for categorical responses to color in prelinguistic infants (3, 4). Furthermore, it has been reported that a chimpanzee could classify Munsell samples into several categories in a similar way to humans (38). The results of the present study imply that colors in different categories are differently represented in the visual cortex of 5- to 7-mo-old infants. Our findings support the hypothesis that categorical color perception does not necessarily originate from language.
A recent study that applied cluster analysis (39) to the data of the World Color Survey (www1.ICSI.Berkeley.EDU/wcs/), a corpus of color-naming data from 110 unwritten languages, revealed that the particular structure of color terms used by each language is drawn on a set of about three to six universal color-naming systems. Notably, the results of that study suggested that the pattern of categorization for colors between blue and green could be classified into about four types of “motifs” that are common across various mother languages, original habitations, and cultural backgrounds. On the other hand, there are several other studies in anthropology, cognitive science, and linguistics fields that argue the acquisition of color terms can modify the perceptual border of color categories, such that the same color would be categorized into different categories by different language speakers (40–42). Taken together, it seems that “some categorical color distinctions apparently exist prior to language, and then may be reinforced, modulated, or eliminated by learning a particular language,” as was suggested in a previous study (8).
Materials and Methods
Participants.
All infants were full term at birth and were healthy at the time of the experiment. Ethical approval for this study was obtained from the Ethical Committee at the Chuo University (2012-8). Written informed consent was obtained from the parents of the participants. None of the participants’ parents reported any family history of color deficiency. The participants in the infant NIRS measurement were 24 healthy infants (12 infants for the bilateral OT region measurement and 12 infants for the occipital region measurement) ranging from 5 to 7 mo of age (10 males and 14 females; mean age = 182.3 d, ranging from 154–226 d). An additional 18 infants were excluded because of an insufficient number of successful trials for analysis (fewer than three trials for either the between- or within-category condition) due to fussiness. Thirty-six infants aged 5–7 mo (16 males and 20 females; mean age = 186.9 d, ranging from 143–221 d) participated in the behavior experiment. Another 18 infants were tested but were excluded from the analysis because of fussiness or a side bias.
Apparatus.
Each infant sat on his or her parent’s lap in an experimental booth throughout the experiment. A 21-inch color cathode ray tube (CRT) display (Diamond Pro-2070SB; Mitsubishi Electric Co.) was used to present visual stimuli. The display was placed in front of the infant at a distance of about 40 cm. The infant’s looking behavior was monitored by a hidden video camera set below the CRT display, and the stimulus presentation was controlled by an experimenter. Stimuli were generated by a computer-controlled visual stimulus generator (ViSaGe; Cambridge Research Systems) with a 12-bit resolution per each primary color after a careful photometric calibration. The NIRS instrument was a Hitachi ETG-4000 system. We used a pair of sensor-probe holders, each of which contained nine optical fibers (3 × 3 arrays), and recorded changes in oxy-Hb and deoxy-Hb concentrations with 12 channels in each holder. For the measurement of bilateral OT regions, the center of each probe holder was placed slightly below T5/T6 in the international 10–20 EEG system (Fig. 1B). For the occipital region measurement, we used 12 channels (one holder set) only, and the center of the holder was placed slightly above Oz (Fig. 1B, Right). Details of the NIRS instrument are given in Supporting Information.
Stimuli and Procedure for NIRS Measurement.
Nine geometric figures, each subtended 8.5° × 8.5° in the visual angle and arranged in a 3 × 3 array, were presented as visual stimuli to the infants. A gray background (metameric to equal-energy white with a medium lightness: 25 cd/m2) was presented for the prevention of dark adaptation. The luminance of G1, G2, and B1 was equated to 20 cd/m2 on the CRT display by the photometer (details about luminance control are provided in Supporting Information). The within-category and between-category conditions were presented in alternating trials. The duration of each trial was fixed at 10 s. During the intertrial intervals (baseline period), the color of the figures was fixed to a light gray (35 cd/m2), but the figure shapes were changed periodically (once per second). The baseline-period duration was controlled to be at least 10 s (Fig. 1). The infants watched the stimuli passively while their brain activity was recorded, and they were allowed to watch the stimuli for as long as they were willing.
Data Analysis in the NIRS Measurement.
We excluded trials from analysis if the infants’ looking time for the test stimuli was less than 7 s or if they became fussy. The mean concentration of each channel within a participant was calculated by averaging data across the trials in a time series from 2 s before trial onset to 10 s after trial offset, recorded at 10 Hz. To remove significant individual differences, we normalized signals of oxy-Hb, deoxy-Hb, and total-Hb concentrations as Z-scores in each stimulus condition, channel, and participant before statistical analyses. More details are provided in Supporting Information.
Stimuli and Procedure for Behavioral Experiments.
A smiling face pattern, whose diameter subtended 10° in visual angle, was used as a stimulus on a gray background. The chromaticities of G1, G2, B1, and B2 were identical to the chromaticity of the NIRS measurement, and their luminance was equated by photometer at 20 cd/m2. The experimental session consisted of two trials in the pretest phase, six trials in the familiarization phase, and two trials in the posttest phase. The duration of each trial was 10 s in the pretest and posttest phases and 5 s in the familiarization phase. The positions of the stimuli were reversed in the two trials of the pretest and posttest phases. There were three paired conditions (B1/B2, B1/G1, and G1/G2), and each of 36 infants was assigned randomly to one of these conditions.
Data Coding and Analysis of Behavioral Experiments.
A technical staff member who did not know the stimulus identity measured the infant’s looking time, based on an offline analysis of a videotape of each infant’s face. The technical staff member recorded the infant’s looking time for the left or right presentation field when the infant was looking at the relevant field.
Luminance Control of NIRS Stimulus
Our experiment alternated the color of the stimuli in time sequence in a square waveform, and the luminance contrast between the colors would be an artifact in the infants’ brain activity recordings. According to Eisner and MacLeod (24), excitation of only long- and medium-wavelength–sensitive cones (L- and M-cones) contributes to luminance, but excitation of short-wavelength–sensitive cones (S-cones) does not. Therefore, it is optimal to design the color changes along a direction that selectively varies S-cone excitations so as to minimize stimulus differences in luminance contrast at the abrupt onset of color alternations. Responses from a luminance-based mechanism often dominate/mask responses from color mechanisms (43). Accordingly, we considered that it is better to attempt to minimize any residual luminance modulation. Therefore, we modified the stimulus colors slightly from the colors used in a previous study by Franklin et al. (5), so that the chromaticity of the test colors exclusively varies in terms of S-cone excitation, while retaining an equal color difference between G1/G2 and G1/B1 in the CIE LAB color space (the CIE xy and La*b* chromaticities of the stimuli are provided in Table 1). The cone fundamentals of Smith and Pokorny (25) were used to calculate L-, M-, and S-cone responses.
However, it is also known that the S-cone responses contribute to brightness perception (44), and it can be argued that the NIRS responses to stimuli with a lower temporal frequency (1 Hz) could be affected by the brightness. The S-cone response is considered to contribute to the brightness in a log-additive manner (44), which implies that the S-cone response ratios between colors play a major role in the perception of brightness differences. To address this concern, we compared S-cone response ratios between the two color pairs in the NIRS experiment. The ratios of S-cone responses between the NIRS stimuli were as follows: G1/G2 = 2.21 (0.345 log10 unit) and B1/G1 = 1.78 (0.251 log10 unit). This result implies that the amplitude of S-cone contribution to brightness is likely to be greater in the within-category pair (G1/G2) than in the between-category pair (B1/G1); only the latter showed significant NIRS responses. Although some difference in luminous efficiency function was reported between infants and adults at short wavelengths (22), it must be noted that the relative intensity of S-cone responses and its rank order among colors would not change. Therefore, we consider it unlikely that S-cone modulation caused any brightness artifacts in the NIRS response differences in the present study (Fig. 2 B and C).
NIRS Instrument Details
The NIRS instrument used in this study was a Hitachi ETG-4000 system (Hitachi Medical). The instrument generated two wavelengths of near-IR light (695 and 830 nm) and measured the time courses of the levels of oxy-Hb, deoxy-Hb, and total-Hb concentrations in each channel with a 0.1-s time resolution. We used newly developed NIRS sensor probes for infants (infant probe 3 × 3 mode; Hitachi Medical), which have a lower weight and softer contact on the skin than previous probes. Therefore, most of the infants appeared comfortable during experiments and were not reluctant to participate. We used a pair of probe holders, each of which contained nine optical fibers (3 × 3 arrays). Of the nine fibers, five were used for the emission of IR lights and four were used for detection. The optical fibers of each probe were kept in place with a soft silicon holder (Fig. 1B). The emitter and detector fibers were displaced by 2 cm in the infant’s holder and by 3 cm in the adult’s holder. Each pair of adjacent emitting and detecting fibers defined a single measurement channel, and this arrangement allowed the measurement of oxy-Hb and deoxy-Hb changes in 12 channels for each hemisphere (Fig. 1B). Although the spatial resolution of NIRS is not as high as the spatial resolution of fMRI, the activated region can be estimated by locating channels, each of which is composed of a certain path of IR light that connects a pair of emitter and receiver probes. Additionally, the depth of the recorded brain activity can be estimated by the separation of the emitter and receiver probes: ∼10–15 mm into the cortex in depth for 2–3 cm of between-probe separation. When the probes were positioned, the experimenter checked whether all fibers were touching the infant’s scalp correctly. The ETG-4000 system evaluates whether the contact is adequate to capture the emerging photons in each channel after the scattering and refractions of IR lights under the scalp. If adequate contact between the fibers and the infant’s scalp could not be achieved (e.g., because of interference by hair), the channels were rejected from the analysis. The ETG-4000 system automatically filters the data of oxy-Hb, deoxy-Hb, and total-Hb with a 0.02- to 1-Hz bandpass filter to remove longitudinal signal drift and noise from the instrument.
Data Analysis in the NIRS Measurement
We removed trials from the analysis if the infants’ looking time in the test stimuli was less than 7 s or if they became fussy. The mean looking time of test trials was 8.9 s (SD = 0.71). In addition, we removed the trials from analysis if the infants looked back at the face of the experimenter during the preceding baseline period or if the trials included movement artifacts, which were detected by the analysis of sharp changes in the time series of the NIRS raw data. The mean concentration of each channel for a given participant was calculated by averaging data across the trials in a time series from 2 s before trial onset to 10 s after trial offset, recorded with a time resolution of 0.1 s. On the basis of the mean concentrations in the time series, we normalized signals of oxy-Hb, deoxy-Hb, and total-Hb as Z-scores in the within-category and between-category conditions, respectively, for each channel and for each participant, because the absolute concentration values are significantly different among participants. The Z-scores (z) were calculated as the difference between the mean of the baseline (μ2) and the concentration value (μ1) divided by the SD during the baseline period (σ):
The 2 s immediately before the onset of stimulus presentation was defined as the baseline. We applied a statistical test to elucidate the channels that showed significant responses (Supporting Information, Statistical Inference of the Time Window for the NIRS Data Analysis, Results).
Statistical Inference of the Time Window for the NIRS Data Analysis
To make statistical comparisons of NIRS responses between stimulus conditions and regions (i.e., left/right, between channels/groups, etc.), we had to define the time window for the analysis. To minimize bias in the definition of this time window, the following procedure was used.
Background.
First, it must be noted that the canonical time course of NIRS responses in infants has yet to be established (27). It is also known that NIRS responses are slightly different between regions. This fact implies that the impulse response function of oxy/deoxy-Hb concentration (i.e., hemodynamic response function) has to be obtained in each participant and each brain region; however, doing so will increase participant’s burden, and it is problematic to have infants participate beyond the main experiment. Therefore, it is difficult to estimate the “expected” temporal response waveform. In addition, even though infants kept watching the stimuli, we could not confirm that infants kept paying attention during the entire stimulus presentation period (e.g., by verbal report). Therefore, it is probably inappropriate to assume that the neural activity was maintained completely during the entirety of the stimulus presentation period (i.e., 10 s in the present study). Hence, it is practically challenging to derive the typical NIRS response time course in infants by the convolution of stimulus presentation time course and canonical NIRS response in infants (given that it is established). In summary, the analysis time window had to be determined from the recorded data.
Similar to the method for evaluating the ability of a machine-learning classifier, a possible solution was to divide the dataset into two parts and to use one part to determine the time window and the other to analyze cortical responses. However, given the low number of participants and valid trials, statistical power would be dramatically reduced when only half of the data are used for statistical analyses. Therefore, we had to use all available data to select the applicable time window.
Analysis.
To elucidate which time period contains statistically significant signal changes, we applied ANOVA to the NIRS response data. The NIRS data were binned by 2 s to reduce the effect of noise during the recording. The analysis was conducted by including 2 s before stimulus onset and 12 s following the stimulus presentation, which contained the typical peak latency of the NIRS response in adults (about 6–8 s). The data were averaged across channels within either the left or right hemisphere. The within-participant design included three factors (stimulus condition, hemisphere, and time bin), and to avoid any prejudice, we sought time bins that exhibited a significant interaction between the time and stimulus conditions.
Results.
The main effect of stimulus condition [F(1,11) = 7.17, P < 0.05] and the interaction between stimulus condition and time bin [F(6,66) = 2.38, P < 0.05] were detected. According to the following analysis, the effect of stimulus condition was significant at the time bins of 2–4 s [F(1,11) = 10.3, P < 0.01] and 4–6 s [F(1,11) = 8.32, P < 0.05], whereas marginal significance was detected at 0–2 s [F(1,11) = 4.39, P = 0.06]. The findings of this analysis are presented in Table S1. The same analysis was conducted using a bin width of 1 s with similar results.
Summary.
According to the results of ANOVA, the signal 2–6 s after the stimulus onset contains meaningful information to investigate differences between stimulus conditions, and was used for the analyses in the main text. This integrating range was consistent with other NIRS studies using visual stimuli (45).
Test of Lateralization in Occipital Cortex
To examine whether significant lateralization can be found in infants, the occipital measurement data were compared between signals from channels in the right and left halves of a probe holder. The channels on the sagittal line (channels 16 and 21; Fig. 1B, Right) were excluded from the analysis. NIRS signals (oxy-Hb) from left hemisphere channels (channels 13, 15, 18, 20, and 23; Fig. 1B) and right hemisphere channels (channels 14, 17, 19, 22, and 24; Fig. 1B) were averaged within each hemisphere and for each participant during 2–6 s in the stimulus onset latency. The resulting data were tested with a two-factor ANOVA by taking the hemisphere and stimulus conditions (between-category or within-category) as within-subject factors. This result was not statistically significant in the main effect of hemisphere [F(1,11) = 0.913, P = 0.35], in stimulus conditions [F(1,11) = 0.016, P = 0.90], or in terms of interactions [F(1,11) = 1.23, P = 0.29].
These results demonstrated that no significantly lateralized brain activity could be detected in either the OT or occipital regions of NIRS data measured in the present study.
Adults' NIRS Measurement
To confirm whether categorical color responses measured in the infants’ cortices (Fig. 2 B and C) are also present in adults, we compared their hemodynamic responses with the hemodynamic responses of adults who reported a clear color-category border between B1 and G1, and not between G1 and G2. Six participants (two males and four females) with a mean age of 29.2 y (SD = 5.3) took part in the experiment. All of them had normal color vision and were unequivocally right-handed. They were asked to report the color categories for the three colors (B1, G1, and G2). The adult participants were instructed to fixate on the center of the screen and to pay attention to the visual stimuli. A total of 40 trials, which consisted of trials in the between-condition category (20 trials) and the within-condition category (20 trials), were performed by each participant.
We found a clear similarity with infants in the trend of brain activity. The concentrations of oxy-Hb and total-Hb in both the left and right OT regions increased during the presentation of between-category stimuli. Fig. S3 shows the time course of the average change in oxy-Hb, deoxy-Hb, and total-Hb in adults during the between- and within-category conditions. An explanation of axes and line colors/styles is provided in Fig. S1. To examine the presence of significant signal changes and whether there was any difference between stimulus conditions, we performed statistical analyses on the mean Z-scores from 2 to 6 s after the stimulus onset. First, a two-tailed one-sample t test was performed against the baseline. It revealed that the concentrations of oxy-Hb and total-Hb increased significantly in both OT regions under the between-category condition [oxy-Hb, left: t(5) = 2.14, P < 0.05; right: t(5) = 1.97, P < 0.05; and total-Hb, left: t(5) = 4.48, P < 0.05; right: t(5) = 3.55, P < 0.05]. In contrast, such a significant increase in the concentration of oxy-Hb and total-Hb was not observed under the within-category condition. Next, to test the significance of differences between stimulus conditions, a two-factor repeated-measure ANOVA was performed on the data of oxy-Hb by taking stimulus condition (between vs. within) and measurement area (right vs. left) as within-subject factors. This analysis revealed a significant main effect of the stimulus condition for oxy-Hb [F(1,5) = 11.97, P < 0.01], but no significant main effect of the measurement area [F(1,5) = 1.04, P = 0.83; n.s.] or interactions [F(1,5) = 5.03, P = 0.07; n.s.].
The time series of NIRS data were also tested for significant signal changes in all 24 channels by conducting the same ANOVA analysis as for the infants’ data (27). Similar to the results in infants, no channel showed a significant change in the within-category condition and no significant deoxy-Hb change was observed in either categorical condition. We also divided the 12 channels in each hemisphere into four groups, as we did with the infants. Multiple comparisons by t test revealed that the anterior [t(5) = 3.88, P < 0.05] and ventral [t(5) = 4.54, P < 0.01] groups in the left hemisphere and the anterior [t(5) = 4.12, P < 0.01] and dorsal [t(5) = 4.20, P < 0.01] groups in the right hemisphere showed significant signal increase among the groups after Bonferroni’s correction for multiple comparisons within each hemisphere; other groups showed no statistically significant changes. Compared with the infants, a larger response area has been found in adults. Limited cortical activities in the infants plausibly indicate the immature process of color categorization.
Acknowledgments
We thank Hiroko Ichikawa, Yuna Inada, Megumi Kobayashi, Yuiko Sakuta, Kazuki Sato, Wakayo Yamashita, and Yuka Yamazaki for their help in data collection. Special thanks to the infants and their parents for their kindness and cooperation. We thank Shuntaro Sasai, Angela M. Brown, and Delwin T. Lindsey for their helpful comments. We also thank the two anonymous reviewers for their thoughtful comments and suggestions. This research was supported by a Grant-in-Aid by the Japan Society for the Promotion of Science (JSPS) Fellows (232049 to J.Y.), Grants-in-Aid for Scientific Research on Innovative Areas ‘‘Shitsukan’’ from the Ministry of Education, Culture, Sports, Science and Technology, Japan (25135729 to M.K.Y. and 25135702 to I.K.), and Grants-in-Aid for Scientific Research from the JSPS (21243041 to M.K.Y. and 24330205 and 15H03460 to I.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Raw data for the measurement in the occipitotemporal regions of infants are available at figshare (figshare.com/articles/new_fileDataset_Yang_et_al_submitted_to_PNAS_for_publication_set/1558040).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512044113/-/DCSupplemental.
References
- 1.Kay P, Regier T. Resolving the question of color naming universals. Proc Natl Acad Sci USA. 2003;100(15):9085–9089. doi: 10.1073/pnas.1532837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsey DT, Brown AM. Universality of color names. Proc Natl Acad Sci USA. 2006;103(44):16608–16613. doi: 10.1073/pnas.0607708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornstein MH, Kessen W, Weiskopf S. Color vision and hue categorization in young human infants. J Exp Psychol Hum Percept Perform. 1976;2(1):115–129. doi: 10.1037//0096-1523.2.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Franklin A, Davies IRL. New evidence for infant colour categories. Br J Dev Psychol. 2004;22(3):349–377. [Google Scholar]
- 5.Franklin A, Pilling M, Davies I. The nature of infant color categorization: Evidence from eye movements on a target detection task. J Exp Child Psychol. 2005;91(3):227–248. doi: 10.1016/j.jecp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Jameson KA, Alvarado N. Differences in color naming and color salience in Vietnamese and English. Color Res Appl. 2003;28(2):113–138. [Google Scholar]
- 7.Davidoff J, Davies I, Roberson D. Colour categories in a stone-age tribe. Nature. 1999;398(6724):203–204. doi: 10.1038/18335. [DOI] [PubMed] [Google Scholar]
- 8.Kay P, Regier T. Language, thought and color: recent developments. Trends Cogn Sci. 2006;10(2):51–54. doi: 10.1016/j.tics.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert AL, Regier T, Kay P, Ivry RB. Whorf hypothesis is supported in the right visual field but not the left. Proc Natl Acad Sci USA. 2006;103(2):489–494. doi: 10.1073/pnas.0509868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wernicke C. Einpsychologische studie auf anatomischer basis. Cohn & Weigert; Breslau, Germany: 1874. Der aphasische symptomencomplex. German. [Google Scholar]
- 11.Liu Q, et al. The N2pc component in ERP and the lateralization effect of language on color perception. Neurosci Lett. 2009;454(1):58–61. doi: 10.1016/j.neulet.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 12.Ting Siok W, et al. Language regions of brain are operative in color perception. Proc Natl Acad Sci USA. 2009;106(20):8140–8145. doi: 10.1073/pnas.0903627106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin A, et al. Categorical perception of color is lateralized to the right hemisphere in infants, but to the left hemisphere in adults. Proc Natl Acad Sci USA. 2008;105(9):3221–3225. doi: 10.1073/pnas.0712286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin A, et al. Lateralization of categorical perception of color changes with color term acquisition. Proc Natl Acad Sci USA. 2008;105(47):18221–18225. doi: 10.1073/pnas.0809952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford A, Franklin A, Davies IRL, Holmes A. Electrophysiological markers of categorical perception of color in 7-month old infants. Brain Cogn. 2009;71(2):165–172. doi: 10.1016/j.bandc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Witzel C, Gegenfurtner KR. Is there a lateralized category effect for color? J Vis. 2011;11(12):16, 1–25. doi: 10.1167/11.12.16. [DOI] [PubMed] [Google Scholar]
- 17.Brown AM, Lindsey DT, Guckes KM. Color names, color categories, and color-cued visual search: Sometimes, color perception is not categorical no bias. J Vis. 2012;11(12):1–21. doi: 10.1167/11.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komatsu H, Ideura Y, Kaji S, Yamane S. Color selectivity of neurons in the inferior temporal cortex of the awake macaque monkey. J Neurosci. 1992;12(2):408–424. doi: 10.1523/JNEUROSCI.12-02-00408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koida K, Komatsu H. Effects of task demands on the responses of color-selective neurons in the inferior temporal cortex. Nat Neurosci. 2007;10(1):108–116. doi: 10.1038/nn1823. [DOI] [PubMed] [Google Scholar]
- 20.Brouwer GJ, Heeger DJ. Categorical clustering of the neural representation of color. J Neurosci. 2013;33(39):15454–15465. doi: 10.1523/JNEUROSCI.2472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsuka Y, et al. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. Neuroimage. 2007;34(1):399–406. doi: 10.1016/j.neuroimage.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Bieber ML, Volbrecht VJ, Werner JS. Spectral efficiency measured by heterochromatic flicker photometry is similar in human infants and adults. Vision Res. 1995;35(10):1385–1392. doi: 10.1016/0042-6989(95)98718-o. [DOI] [PubMed] [Google Scholar]
- 23.Kuriki I, Sun P, Ueno K, Tanaka K, Cheng K. Hue Selectivity in Human Visual Cortex Revealed by Functional Magnetic Resonance Imaging. Cereb Cortex. 2015;25(12):4869–4884. doi: 10.1093/cercor/bhv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisner A, MacLeod DIA. Flicker photometric study of chromatic adaption: selective suppression of cone inputs by colored backgrounds. J Opt Soc Am. 1981;71(6):705–717. doi: 10.1364/josa.71.000705. [DOI] [PubMed] [Google Scholar]
- 25.Smith VC, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975;15(2):161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- 26.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- 27.Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proc Natl Acad Sci USA. 2003;100(19):10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bornstein MH. Qualities of color vision in infancy. J Exp Child Psychol. 1975;19(3):401–419. doi: 10.1016/0022-0965(75)90070-3. [DOI] [PubMed] [Google Scholar]
- 29.Zemach IK, Teller DY. Infant color vision: Infants’ spontaneous color preferences are well behaved. Vision Res. 2007;47(10):1362–1367. doi: 10.1016/j.visres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Brown AM, Lindsey DT. Infant color vision and color preferences: A tribute to Davida Teller. Vis Neurosci. 2013;30(5-6):243–250. doi: 10.1017/S0952523813000114. [DOI] [PubMed] [Google Scholar]
- 31.Bird CM, Berens SC, Horner AJ, Franklin A. Categorical encoding of color in the brain. Proc Natl Acad Sci USA. 2014;111(12):4590–4595. doi: 10.1073/pnas.1315275111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchikawa K, Kuriki I, Shinoda H. Expression of color appearance in aperture and surface color modes with a category rating estimation method. Journal of the Illuminating Engineering Institute of Japan. 1994;78(2):83–93. [Google Scholar]
- 33.Conway BR, Moeller S, Tsao DY. Specialized color modules in macaque extrastriate cortex. Neuron. 2007;56(3):560–573. doi: 10.1016/j.neuron.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conway BR, Tsao DY. Color architecture in alert macaque cortex revealed by FMRI. Cereb Cortex. 2006;16(11):1604–1613. doi: 10.1093/cercor/bhj099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: New results and a review. Eur J Neurosci. 2000;12(1):172–193. doi: 10.1046/j.1460-9568.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto M, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21(1):99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Minagawa-Kawai Y, Mori K, Naoi N, Kojima S. Neural attunement processes in infants during the acquisition of a language-specific phonemic contrast. J Neurosci. 2007;27(2):315–321. doi: 10.1523/JNEUROSCI.1984-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzawa T. Color naming and classification in a chimpanzee (Pantroglodytes) J Hum Evol. 1985;14(3):283–291. [Google Scholar]
- 39.Lindsey DT, Brown AM. World Color Survey color naming reveals universal motifs and their within-language diversity. Proc Natl Acad Sci USA. 2009;106(47):19785–19790. doi: 10.1073/pnas.0910981106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberson D, Davies I, Davidoff J. Color categories are not universal: Replications and new evidence from a stone-age culture. J Exp Psychol Gen. 2000;129(3):369–398. doi: 10.1037//0096-3445.129.3.369. [DOI] [PubMed] [Google Scholar]
- 41.Winawer J, et al. Russian blues reveal effects of language on color discrimination. Proc Natl Acad Sci USA. 2007;104(19):7780–7785. doi: 10.1073/pnas.0701644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchikawa K, Boynton RM. Categorical color perception of Japanese observers: Comparison with that of Americans. Vision Res. 1987;27(10):1825–1833. doi: 10.1016/0042-6989(87)90111-8. [DOI] [PubMed] [Google Scholar]
- 43.Kuriki I, Sadamoto K, Takeda T. MEG recording from the human ventro-occipital cortex in response to isoluminant color stimulation. Vis Neurosci. 2005;22(3):283–293. doi: 10.1017/S0952523805223040. [DOI] [PubMed] [Google Scholar]
- 44.Nakano Y, Ikeda M, Kaiser PK. Contributions of the opponent mechanisms to brightness and nonlinear models. Vision Res. 1988;28(7):799–810. doi: 10.1016/0042-6989(88)90027-2. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]