Abstract
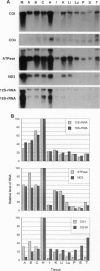
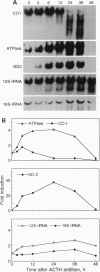
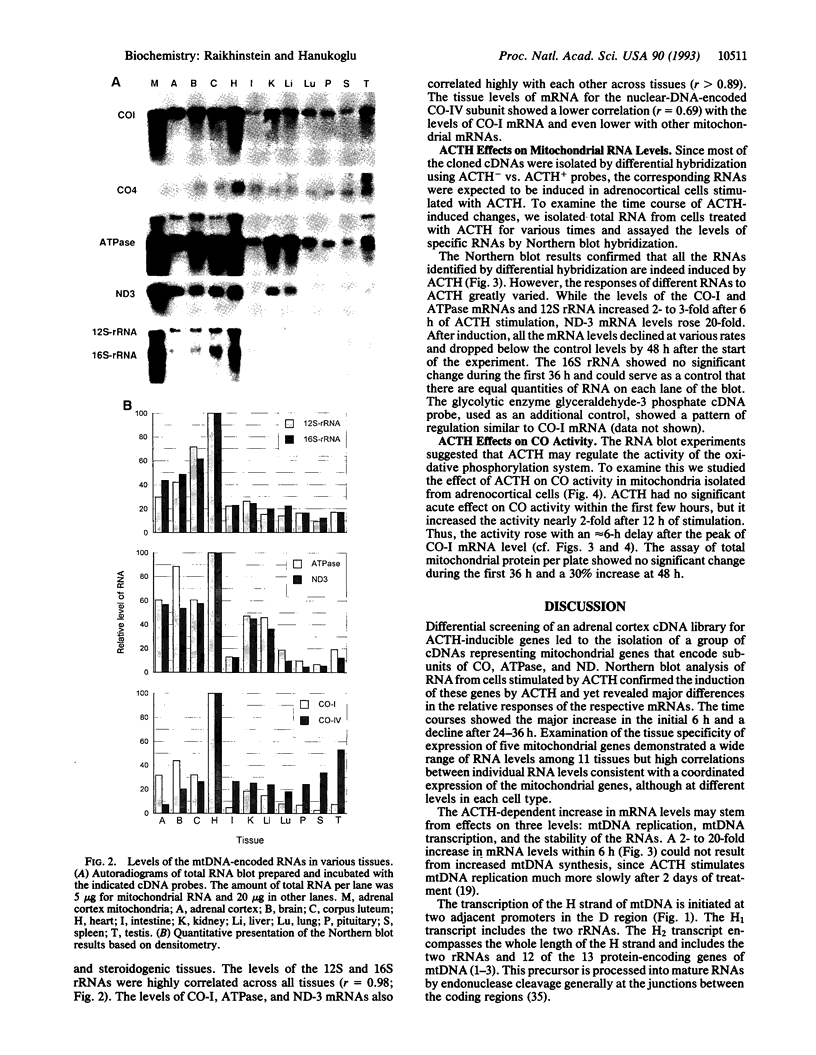
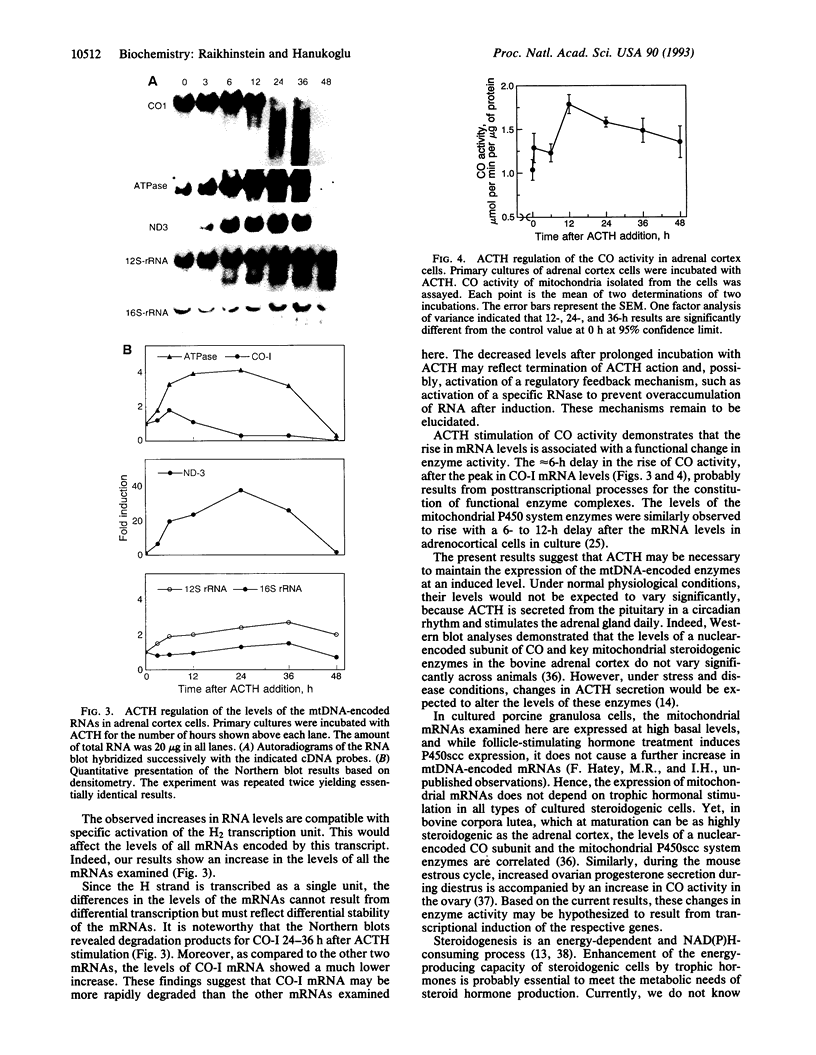
Differential screening of an adrenal cortex cDNA library for corticotropin (ACTH)-inducible genes led to the isolation of a group of cDNAs representing mitochondrial genes that encode subunits of cytochrome oxidase, ATPase, and NADH dehydrogenase. Northern blot analysis of RNA from cells stimulated by ACTH confirmed the induction of these genes by ACTH yet revealed major differences in the relative responses of the respective mRNAs. The levels of mRNAs for cytochrome oxidase subunit I and ATPase increased 2- to 4-fold and for NADH dehydrogenase subunit 3 increased 20-fold, whereas the levels of the mitochondrial 16S rRNA showed no change within 6 h of ACTH stimulation. These effects of ACTH on mitochondrial mRNA levels probably result from both activation of the H2 transcription unit that encodes mitochondrial mRNAs and alteration of mRNA stability. ACTH also increased the activity of cytochrome oxidase after 12 h of stimulation. Examination of the tissue specificity of expression of five mitochondrial genes showed a wide range of RNA levels among 11 tissues but high correlations between individual RNA levels, consistent with a coordinated expression of the mitochondrial genes, although at different levels in each cell type. Proportionately high levels of mitochondrial mRNAs were found in adrenal cortex, probably reflecting a stimulatory effect of ACTH in vivo. Overall, the results indicate that ACTH enhances the energy-producing capacity of adrenocortical cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Attardi G., Chomyn A., King M. P., Kruse B., Polosa P. L., Murdter N. N. Regulation of mitochondrial gene expression in mammalian cells. Biochem Soc Trans. 1990 Aug;18(4):509–513. doi: 10.1042/bst0180509. [DOI] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bettini E., Maggi A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. J Neurochem. 1992 May;58(5):1923–1929. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- Casanova J. L., Pannetier C., Jaulin C., Kourilsky P. Optimal conditions for directly sequencing double-stranded PCR products with sequenase. Nucleic Acids Res. 1990 Jul 11;18(13):4028–4028. doi: 10.1093/nar/18.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. C., Waterhouse T. B., Michael S. D. Changes in mitochondrial and microsomal 3 beta-hydroxysteroid dehydrogenase activity in mouse ovary over the course of the estrous cycle. Biol Reprod. 1992 Dec;47(6):992–997. doi: 10.1095/biolreprod47.6.992. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cornwall G. A., Orgebin-Crist M. C., Hann S. R. Differential expression of the mouse mitochondrial genes and the mitochondrial RNA-processing endoribonuclease RNA by androgens. Mol Endocrinol. 1992 Jul;6(7):1032–1042. doi: 10.1210/mend.6.7.1508219. [DOI] [PubMed] [Google Scholar]
- Dazord A., Gallet de Santerre D., Saez J. M. ACTH in vivo stimulation of the synthesis of a specific mitochondrial protein in the rat. Biochem Biophys Res Commun. 1981 Feb 27;98(4):885–891. doi: 10.1016/0006-291x(81)91194-3. [DOI] [PubMed] [Google Scholar]
- Dorfman D. M., Zon L. I., Orkin S. H. Rapid amplification of lambda gt11 bacteriophage library inserts from plaques using the polymerase chain reaction (PCR). Biotechniques. 1989 Jun;7(6):568–570. [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Feuchtwanger R., Hanukoglu A. Mechanism of corticotropin and cAMP induction of mitochondrial cytochrome P450 system enzymes in adrenal cortex cells. J Biol Chem. 1990 Nov 25;265(33):20602–20608. doi: 10.1016/S0021-9258(17)30545-8. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Gutfinger T., Haniu M., Shively J. E. Isolation of a cDNA for adrenodoxin reductase (ferredoxin-NADP+ reductase). Implications for mitochondrial cytochrome P-450 systems. Eur J Biochem. 1987 Dec 15;169(3):449–455. doi: 10.1111/j.1432-1033.1987.tb13632.x. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Hanukoglu Z. Stoichiometry of mitochondrial cytochromes P-450, adrenodoxin and adrenodoxin reductase in adrenal cortex and corpus luteum. Implications for membrane organization and gene regulation. Eur J Biochem. 1986 May 15;157(1):27–31. doi: 10.1111/j.1432-1033.1986.tb09633.x. [DOI] [PubMed] [Google Scholar]
- Heerdt B. G., Augenlicht L. H. Effects of fatty acids on expression of genes encoding subunits of cytochrome c oxidase and cytochrome c oxidase activity in HT29 human colonic adenocarcinoma cells. J Biol Chem. 1991 Oct 5;266(28):19120–19126. [PubMed] [Google Scholar]
- Kadowaki T., Kitagawa Y. Enhanced transcription of mitochondrial genes after growth stimulation and glucocorticoid treatment of Reuber hepatoma H-35. FEBS Lett. 1988 Jun 6;233(1):51–56. doi: 10.1016/0014-5793(88)81354-1. [DOI] [PubMed] [Google Scholar]
- Ku C. Y., Lu Q., Ussuf K. K., Weinstock G. M., Sanborn B. M. Hormonal regulation of cytochrome oxidase subunit messenger RNAs in rat Sertoli cells. Mol Endocrinol. 1991 Nov;5(11):1669–1676. doi: 10.1210/mend-5-11-1669. [DOI] [PubMed] [Google Scholar]
- Lomax M. I., Bachman N. J., Nasoff M. S., Caruthers M. H., Grossman L. I. Isolation and characterization of a cDNA clone for bovine cytochrome c oxidase subunit IV. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6295–6299. doi: 10.1073/pnas.81.20.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciakova K., Nelson B. D. Transcript levels for nuclear-encoded mammalian mitochondrial respiratory-chain components are regulated by thyroid hormone in an uncoordinated fashion. Eur J Biochem. 1992 Jul 1;207(1):247–251. doi: 10.1111/j.1432-1033.1992.tb17044.x. [DOI] [PubMed] [Google Scholar]
- Manam S., Van Tuyle G. C. Separation and characterization of 5'- and 3'-tRNA processing nucleases from rat liver mitochondria. J Biol Chem. 1987 Jul 25;262(21):10272–10279. [PubMed] [Google Scholar]
- Mazzocchi G., Nussdorfer G. G. Effects of chloramphenicol on the long term trophic action of ACTH on rat adrenocortical cells: a combined stereological and enzymological study. J Anat. 1985 Jun;140(Pt 4):607–612. [PMC free article] [PubMed] [Google Scholar]
- McNamara B. C., Jefcoate C. R. Heterogeneous pools of cholesterol side-chain cleavage activity in adrenal mitochondria from ACTH-treated rats: differential responses to different reducing precursors. Mol Cell Endocrinol. 1990 Oct 22;73(2-3):123–134. doi: 10.1016/0303-7207(90)90125-r. [DOI] [PubMed] [Google Scholar]
- Menapace L., Armato U., Whitfield J. F. The effects of corticotrophin (ACTH1-24), cyclic AMP and TPA (12-O-tetradecanoyl phorbol-13-acetate) on DNA replication and proliferation of primary rabbit adrenocortical cells in a synthetic medium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1295–1303. doi: 10.1016/s0006-291x(87)80274-7. [DOI] [PubMed] [Google Scholar]
- Mutvei A., Kuzela S., Nelson B. D. Control of mitochondrial transcription by thyroid hormone. Eur J Biochem. 1989 Mar 1;180(1):235–240. doi: 10.1111/j.1432-1033.1989.tb14638.x. [DOI] [PubMed] [Google Scholar]
- Neri G., Gambino A. M., Mazzocchi G., Nussdorfer G. G. Investigations into the effects of ACTH on the half-life of mitochondrial proteins in the rat adrenal cortex. Experientia. 1978 Jan 15;34(1):133–134. doi: 10.1007/BF01921946. [DOI] [PubMed] [Google Scholar]
- Nussdorfer G. G., Mazzocchi G., Gottardo G. Investigations of the turnover of adrenocortical mitochondria. XVI. Effects of ethidium bromide on the ACTH-induced elongation of the half-life of rat zona fasciculata mitochondria. Anat Anz. 1983;153(5):411–413. [PubMed] [Google Scholar]
- Orme-Johnson N. R. Distinctive properties of adrenal cortex mitochondria. Biochim Biophys Acta. 1990 Dec 6;1020(3):213–231. doi: 10.1016/0005-2728(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Ray D. B., Horst I. A., Kowal J. Adrenocorticotropic hormone increases specific proteins of the mitochondrial fraction that are translated inside or outside this organelle in cultured adrenal tumor cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4648–4652. doi: 10.1073/pnas.77.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riondel A. M., Rebuffat P., Mazzochi G., Nussdorfer G. G., Gaillard R. C., Bockhorn L., Nussberger J., Vallotton M. B., Muller A. F. Long-term effects of ACTH combined with angiotensin II on steroidogenesis and adrenal zona glomerulosa morphology in the rat. Acta Endocrinol (Copenh) 1987 Jan;114(1):47–54. doi: 10.1530/acta.0.1140047. [DOI] [PubMed] [Google Scholar]
- Salmenperä M., Kahri A. I. Studies on the dependence of mitochondrial 11beta- and 18-hydroxylation on the nuclear and mitochondrial DNA synthesis during ACTH-induced differentiation of cortical cells of rat adrenals in tissue culture. Exp Cell Res. 1977 Jan;104(1):223–232. doi: 10.1016/0014-4827(77)90085-4. [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Dannies P. S. Estrogen induces accumulation of the mitochondrial ribonucleic acid for subunit II of cytochrome oxidase in pituitary tumor cells. Mol Endocrinol. 1988 Apr;2(4):332–337. doi: 10.1210/mend-2-4-332. [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M. Thyroid hormone and dexamethasone increase the levels of a messenger ribonucleic acid for a mitochondrially encoded subunit but not for a nuclear-encoded subunit of cytochrome c oxidase. Endocrinology. 1990 Jul;127(1):55–62. doi: 10.1210/endo-127-1-55. [DOI] [PubMed] [Google Scholar]
- Wiesner R. J., Kurowski T. T., Zak R. Regulation by thyroid hormone of nuclear and mitochondrial genes encoding subunits of cytochrome-c oxidase in rat liver and skeletal muscle. Mol Endocrinol. 1992 Sep;6(9):1458–1467. doi: 10.1210/mend.6.9.1331777. [DOI] [PubMed] [Google Scholar]
- Wulffraat N. M., Drexhage H. A., Jeucken P., van der Gaag R. D., Wiersinga W. M. Effects of ACTH and ACTH fragments on DNA synthesis in guinea-pig adrenal segments kept in organ culture. J Endocrinol. 1987 Dec;115(3):505–510. doi: 10.1677/joe.0.1150505. [DOI] [PubMed] [Google Scholar]