Significance
The ability to detect dominance relationships is essential for survival because it helps individuals weigh the potential costs and benefits of engaging in a physical competition. Here we show that infants as young as 6 mo of age are capable of detecting dominance relations when provided with an ecologically relevant cue such as social group size. Furthermore, infants can infer the social dominance relationship between two competing individuals based on the size of the group to which they belong, and expect individuals from a numerically larger group to get their way. These findings reveal that infants may have an evolutionarily ancient cognitive capacity to represent social dominance relations that is shared with other species within the animal kingdom.
Keywords: social dominance, infancy, conflict, group size
Abstract
Detecting dominance relationships, within and across species, provides a clear fitness advantage because this ability helps individuals assess their potential risk of injury before engaging in a competition. Previous research has demonstrated that 10- to 13-mo-old infants can represent the dominance relationship between two agents in terms of their physical size (larger agent = more dominant), whereas younger infants fail to do so. It is unclear whether infants younger than 10 mo fail to represent dominance relationships in general, or whether they lack sensitivity to physical size as a cue to dominance. Two studies explored whether infants, like many species across the animal kingdom, use numerical group size to assess dominance relationships and whether this capacity emerges before their sensitivity to physical size. A third study ruled out an alternative explanation for our findings. Across these studies, we report that infants 6–12 mo of age use numerical group size to infer dominance relationships. Specifically, preverbal infants expect an agent from a numerically larger group to win in a right-of-way competition against an agent from a numerically smaller group. In addition, this is, to our knowledge, the first study to demonstrate that infants 6–9 mo of age are capable of understanding social dominance relations. These results demonstrate that infants’ understanding of social dominance relations may be based on evolutionarily relevant cues and reveal infants’ early sensitivity to an important adaptive function of social groups.
Competition for valuable resources such as mates, food, and territory (1) is commonplace across the animal kingdom. To minimize the cost of fighting (e.g., energy spent and personal injury or death), natural selection appears to have favored the emergence of cognitive adaptations that help individuals predict whether they stand a chance against an opponent (2–5). For example, many species, including ants, bees, birds, chimpanzees, and humans, appear to represent dominance relationships among conspecifics and use this information to decide whether to engage in or avoid a physical conflict (6–10). One such cue often associated with dominance ranking is physical size, with larger individuals often benefiting from greater strength and power over smaller individuals. Natural selection has also favored adaptations that exploit this inference, such that under threat, certain species adopt postures that make them appear bigger (11, 12) in order to intimidate an opponent.
Underscoring the possibility that representations of social dominance may be part of humans’ evolved psychology, recent evidence has demonstrated that preverbal human infants infer social dominance relationships by comparing the physical size of two competing agents (13). In this earlier study, infants were introduced to two agents (one twice as large as the other), each with the goal of crossing to the opposite side of a platform. When both agents tried to cross the platform at the same time, their paths conflicted. Infants were shown two scenarios: one in which the larger agent yielded to the smaller agent, and one in which the smaller agent yielded to the larger agent. Although 10–13 mo olds expected a smaller agent to yield to a larger agent, younger infants (8–9 mo) failed to show any systematic belief about which agent should prevail. Therefore, only older infants were able to use the relative physical size of two competing agents to infer which one would get the right of way.
Because younger infants did not reliably use physical size as a cue to social dominance, it remains unclear whether the younger infants were incapable of representing dominance relationships in general, or if they lacked sensitivity to this particular cue. To address this issue, the present study examined whether infants’ understanding of social dominance extends to cues beyond physical size—namely, to numerical group size, and if so, whether such a sensitivity emerges earlier in development.
For many group-living animals, including social insects (7), wolves (14), hyenas (15), lions (16), primates (6), and human children and adults (5, 10), the ability to infer social dominance by assessing the numerical size of one’s own group relative to another is particularly important for survival (15, 17). The importance of this capacity to evaluate one’s own group size relative to another is illustrated by groups of chimpanzees patrolling their territory borders. To advertise the numerical strength of their group to others (18, 19) and deter opposing groups from approaching (20, 21), both males and females will engage noisy pant-hoot calling. In general, both chimpanzees and lions are more likely to approach if they outnumber intruders, but will stay silent and refrain from engaging in intergroup conflict if they do not (6, 16, 22, 23). Consequently, a group’s decision to engage in competition is more likely to occur if there are more individuals in one’s own group than in the opposing group (22, 24). Further, the relationship between numerical group size and inferences about social dominance has also been recently observed among children ages 6–8 y (5). School-aged children predicted that alliance strength would determine the likelihood of success in a conflict, such that two individuals aligned together were expected to win against a single individual. Coupled with the evidence reviewed from behavioral ecology, numerical group size may serve as an evolutionarily relevant cue to social dominance that humans are sensitive to within the first few years of life.
Indeed, if young human infants have core knowledge of social relationships, as some have argued (13, 25), along with the capability to track the numerical size of small groups (26), it is possible that infants may be able to draw on both capacities to support inferences about the social dominance relationship between groups that differ in numerical size. If infants infer that individuals from larger groups are more dominant than individuals from smaller groups, this would demonstrate that infants’ understanding of social dominance can extend beyond the direct relationship between two competing individuals. Specifically, such a finding may shed light on whether infants already have an understanding of how social alliances operate—namely, that group members may help their own during a conflict, which confers a benefit to having more alliance members in close proximity during a conflict (10).
Here, we explored whether infants can infer the dominance relationship between two agents from groups that differ in numerical size by modifying the methodology designed by Thomsen et al. (13). In our study, infants were first introduced to two groups that differed in numerical size (but equated for total surface area) and color. Next, infants were familiarized to an agent from each group independently achieving their goal of crossing a platform. When both of these agents attempted to cross the platform simultaneously, they bumped into one another. Therefore, the only way an agent could continue along their goal path was if one agent yielded to the other by moving out of the way.
In study 1, we investigated whether 9- to 12-mo-old infants use numerical group size as a cue to social dominance. In study 2, we examined whether 6- to 9-mo-old infants (who have not yet been shown to represent social dominance relationships between individuals) would also be sensitive to the cue of numerical group size. Infants in studies 1 and 2 viewed the same sequence of events.
Study 1
We presented 48 infants between the ages of 9 and 12 mo (mean age = 10.68 mo, SD = 25 d, 25 females) with short animations that depicted the actions and goals of two novel agents, each belonging to a group that differed in numerical size and color (Fig. 1). Crucially, whereas the two groups differed in numerical size, the total surface area of the groups was matched. Therefore, only numerical size (and not continuous extent) could be used to determine the dominance relationship among individuals from these groups. Infants were first familiarized to one agent from each group crossing a platform alone (Movies S1 and S2). Only one agent from each group crossed the platform and both of these agents were identical in physical size. Then, infants saw both agents attempt to cross the platform at the same time, resulting in the two agents bumping into each other (Fig. 2 and Movie S3). Last, infants viewed two outcome trials, one where the agent from the numerically larger group prevailed (expected outcome; Movie S4) and one where the agent from the numerically smaller group prevailed (unexpected outcome; Movie S5). Infants’ looking time to each trial was recorded. We reasoned that if infants use numerical group size to infer which agent is more dominant, then infants should be more surprised (and therefore look longer) when the agent from the numerically smaller group prevails (unexpected outcome).
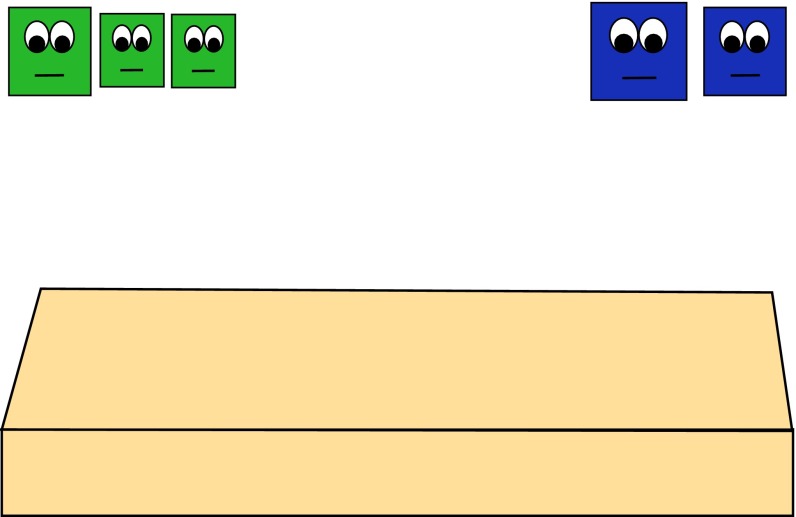
Fig. 1.
Example of the numerically larger group (n = 3) and numerically smaller group (n = 2) introduced at the start of the study.
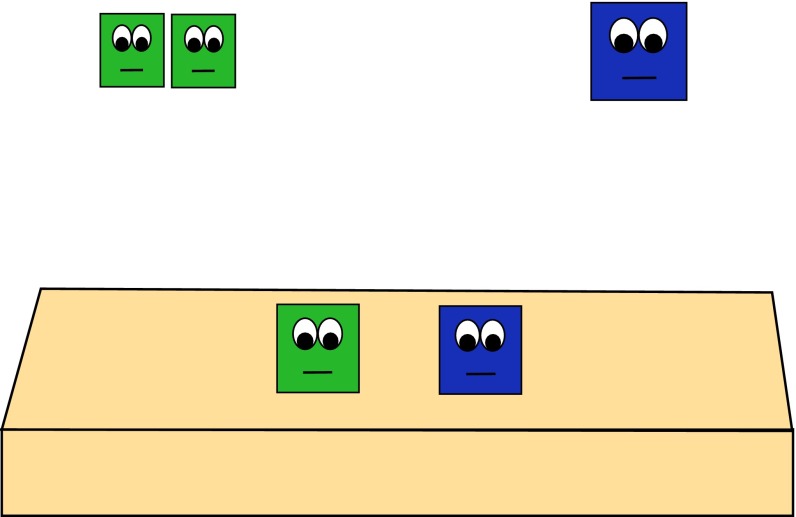
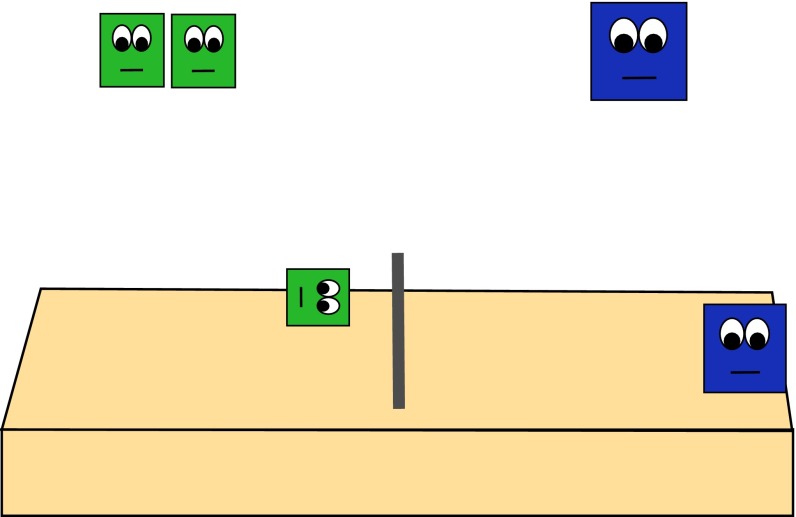
Fig. 2.
Example of one agent from both the numerically larger group and numerically smaller group blocking each other’s goal path.
We ran an ANOVA with a difference score (calculated from infants’ looking times to the unexpected and expected outcomes) entered as the dependent variable, and entered two between-subjects factors: trial order (expected outcome trial first vs. unexpected outcome trial first) and gender. No main effect of trial order was found (F1, 47 = 1.04, P = 0.31). In addition, no main effect of gender (F1, 47 = 0.004, P = 0.95) or interaction between trial order and gender (F1, 47 = 0.32, P = 0.58) was observed. To rule out the possibility of age differences, we ran the same analysis and entered age as a covariate. We found no significant differences due to age (F1,47 = 0.52, P = 0.47).
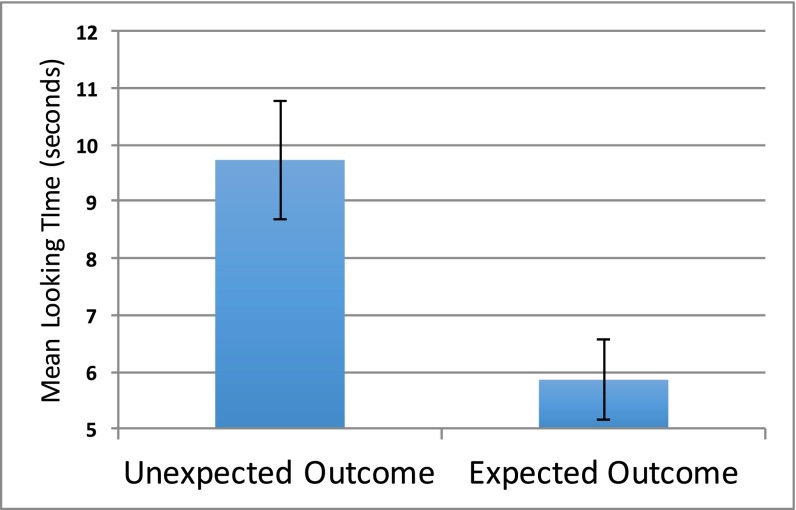
As predicted, infants looked longer to the unexpected outcome trial, in which an agent from the numerically larger group yielded to an agent from the numerically smaller group (mean = 9.72 s) compared with the expected outcome trial: (mean = 5.86 s), 95% CI [1.43, 6.30], t(47) = 3.19, P = 0.003, d = 0.62 (Fig. 3). Our main finding was further supported when the data were examined nonparametrically. Of 48 participants, 36 (75% of the sample) looked longer to the unexpected outcome trial in comparison with the expected outcome trial: χ2 (1, 47) = 12.00, P = 0.001.
Fig. 3.
Mean looking time to unexpected outcome trial compared with expected outcome trial for 9- to 12-mo-old infants. Error bars denote SE of the mean.
To our knowledge, this study is the first to demonstrate that infants use the numerical size of a group as a cue to social dominance, and expect an agent from a numerically larger group to be dominant. Although physical size (13) and numerical group size are both sufficient cues to dominance, our study shows that physical size is not a necessary cue, because the two competing agents in our study were matched along this dimension. Importantly, infants are not only capable of differentiating between the numerical quantity of groups and determining whether one group is larger or smaller (26), but they use this information to infer the dominance relationship between competing individuals from those groups. Furthermore, it is important to note that the noncompeting group members from both groups did not assist in any way during the conflict. Thus, infants must have inferred which competing agent would be dominant through their alliance with a numerically larger group; this may suggest that infants understand that the presence of alliance members confer a competitive advantage, even if they are not directly involved in an observed conflict.
Study 2
It is possible that the reported failure of infants’ ability to reason about social dominance relationships before 9 mo of age (25) reflects a genuine lack of infants’ capacity to establish such representations. Alternatively, younger infants may be capable of understanding social dominance relations, but simply do not use physical size to infer dominance. To determine whether infants’ ability to infer social dominance from the numerical size of a group emerges before their sensitivity to relative physical size, we conducted the same experiment as in study 1 with a sample of 48 infants between the ages of 6 and 9 mo. Importantly, this sample included the age ranges that reportedly fail to use physical size to reason about social dominance (13). These infants (mean age = 7.40 mo, SD = 30 d, 20 females) viewed the same sequence of events as the 9- to 12-mo-old infants in study 1.
We ran an ANOVA with a difference score (calculated from infants’ looking times to the unexpected and expected outcomes) entered as the dependent variable, and entered two between-subjects factors: trial order (expected outcome trial first vs. unexpected outcome trial first) and gender. A main effect of trial order was found (F1, 47 = 9.37, P = 0.004, ηp2 = 0.18). This effect was mainly driven by infants viewing the unexpected outcome trial first, such that infants looked longer on average when viewing the unexpected outcome trial first, as opposed to viewing the unexpected outcome trial second (Table S1). No main effect of gender (F1,47 = 2.14, P = 0.15) or interaction between trial order and gender (F1,47 = 0.00, P = 0.98) was observed. To rule out the possibility of age differences, we ran the same analysis and entered age as a covariate. We found no significant differences due to age (F1,47 = 1.45, P = 0.24).
Table S1.
Study 2 results
| Trial no. | Trial type | Mean looking time, s |
| 1 | Unexpected outcome | 11.11 |
| Expected outcome | 5.79 | |
| 2 | Unexpected outcome | 8.34 |
| Expected outcome | 5.92 |
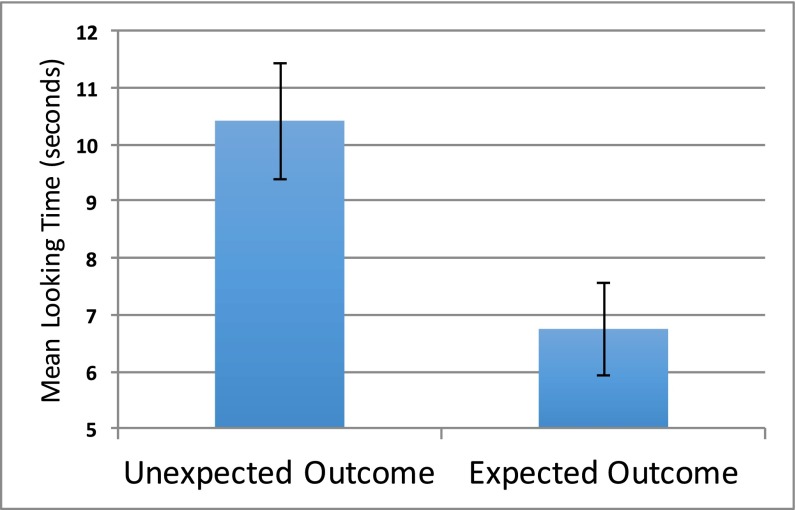
A paired-samples t test comparing the mean looking time (s) that infants ages 6–9 mo spent looking at the screen following the unexpected outcome trial (mean = 10.41 s) and the expected outcome trial (mean = 6.75 s) was significant, 95% CI [1.37, 5.95], t(47) = 3.22, P = 0.002, d = 0.57 (Fig. 4), replicating our finding observed with the older sample from study 1. Thus, even younger infants are more surprised when an agent from a numerically smaller group prevails at the expense of an agent from a numerically larger group. As with study 1, this finding was further supported when the data were examined nonparametrically. Of 48 participants, 35 (73% of the sample) looked longer to the unexpected outcome trial in comparison with the expected outcome trial, χ2 (1, 48) = 10.08, P = 0.001.
Fig. 4.
Mean looking time to unexpected outcome trial compared with expected outcome trial for 6- to 9-mo-old infants. Error bars denote SE of the mean.
Results from study 2 demonstrate once again that infants can use the relative numerical size of two groups to infer the social dominance relationship between competing individuals from those groups. Like older infants, younger infants expect an agent from a numerically larger group to be socially dominant. This result is especially interesting in light of previous experiments where infants younger than 10 mo of age failed to represent social dominance relationships among two competing individuals that differed in their physical size (13).
Study 3
In studies 1 and 2, we presented infants with two groups varying in numerical size, but matched in total surface area. Infants looked longer when an agent from the numerically larger group yielded to a same sized agent from the numerically smaller group. Though we interpret this result as evidence that infants use numerical size of a group to reason about the dominance relationship between individuals from those groups, there is another possible interpretation to consider. With the limited cues to depth inherent in our computer display, the relative size vs. distance of each individual on the screen might have been difficult to discern after viewing one agent yield to the other agent on the platform. It is possible that infants noticed this change in state and remained fixated on the screen to compare the relative size and distance of the agent that yielded to the remaining individual(s) from the agents’ own group. Accordingly, this account would predict that infants looked longer to our unexpected outcome trial, because there were more size and depth comparisons to assess between the individuals in the larger group (three individuals) relative to the smaller group (two individuals).
To address this alternative hypothesis, we conducted a study with 48 infants between the ages of 6 and 12 mo (mean age = 8.40 mo, SD = 1.44 mo, 22 females). In this study, we modified the stimuli presented in studies 1 and 2 by eliminating the conflict between an agent from each group. To begin, participants viewed the group introduction sequence (same as studies 1 and 2). Then, participants observed one agent from each group move simultaneously to the platform below. In one trial, infants viewed the agent from the numerically larger group attempt to cross the platform. However, a barrier already present in the middle of the platform obstructed the agent’s path (Fig. 5). Once the agent reached the middle of the platform, the agent bumped into the barrier three times. Then, the agent bowed down and retreated toward the back of the platform (in a manner identical to the retreating motion observed in studies 1 and 2; Movie S6). In the other trial, infants viewed the same sequence of events when the agent from the numerically smaller group attempted to cross the platform (Movie S7). Because this new stimuli eliminated conflicting goals between agents, infants should not automatically reason about social dominance relationships. Thus, if infants’ attention to each trial is driven by an effort to compare the relative size and distance of the retreating agent to the remaining individual(s) from the agents’ own group, then infants should look longer to the trial in which the agent from the numerically larger group retreats toward the back of the platform.
Fig. 5.
Example of one agent from the numerically larger group (n = 3) being obstructed by a barrier.
We ran an ANOVA with a difference score (calculated from infants’ looking times to the trial in which an agent from the larger group retreats and the trial in which an agent from the smaller group retreats) entered as the dependent variable, and entered two between-subjects factors: trial order (agent from larger group retreats first vs. agent from smaller group retreats first) and gender. A main effect of trial order was found (F1,47 = 6.27, P = 0.016, ηp2 = 0.13). This effect was mainly driven by infants looking longer, in general, to the second trial presented (Table S2). No main effect of gender (F1,47 = 0.12, P = 0.73) or interaction between trial order and gender (F1,47 = 0.37, P = 0.55) was observed. To rule out the possibility of age differences, we ran the same analysis and entered age as a covariate. We found no significant differences due to age (F1,47 = 0.23, P = 0.63).
Table S2.
Study 3 results
| Trial no. | Trial type | Mean looking time, s |
| 1 | Agent from larger group retreats | 9.20 |
| Agent from smaller group retreats | 8.51 | |
| 2 | Agent from larger group retreats | 11.86 |
| Agent from smaller group retreats | 11.89 |
A paired-samples t test comparing the mean looking time (s) to each trial type revealed that infants did not look significantly longer to the trial in which the agent from the larger group retreated (mean = 10.53 s) compared with the trial in which the agent from the numerically smaller group retreated (mean = 10.20 s), 95% CI [−2.20, 2.86], t(47) = 0.26, P = 0.79, d = 0.04. Furthermore, when the data were examined nonparametrically, half of the infants (n = 24) looked longer to the trial in which the agent from the larger group retreated, and half looked longer to the trial in which the agent from the smaller group retreated, χ2 (1, 47) = 0.00, P = 1.00. Therefore, infants’ mean looking times were not driven by one particular trial type. Although a null result, this finding suggests that it is unlikely infants in studies 1 and 2 looked longer to an agent from a numerically larger group yielding to an agent from a numerically smaller group because participants were trying to use changes in apparent physical size to make sense of the size and depth of the 2D characters on the screen.
Discussion
To our knowledge, this is the first study to demonstrate that infants as young as 6 mo of age can represent the dominance relationship between two competing agents in terms of the numerical size of their respective social groups. Whereas previous research has demonstrated that 10- to 13-mo-olds are capable of using physical size to predict whether an individual should be dominant or subordinate to another (13), younger infants were unable to do so. Thus, our data suggest that the reported failure among younger infants to represent social dominance in this earlier study may have been due to the specific cue tested—specifically, understanding the relationship between physical size and social dominance may require more time for infants to learn.
Why might numerical group size be an earlier emerging cue to social dominance than physical size? Interestingly, perceptual cues such as physical size may not always serve as a reliable indicator of social dominance, especially among species that form cooperative social relationships with conspecifics. For example, in nonhuman primates, male chimpanzees striving to achieve a higher status position cannot attain this on their own, and must rely on the support of other males (27, 28). Supporting a male that achieves a higher status position confers benefits to the subordinate males as well. Specifically, higher-status males can provide greater mating opportunities to coalition partners as well as support and protection during a conflict against other coalitions or neighboring groups (6, 27, 29). Therefore, higher-status positions are not necessarily reserved for the largest individuals, but rather can be achieved by smaller (and/or younger) individuals that can successfully cultivate social alliances (30, 31). In comparison with physical size, numerical group size may be a more reliable or salient indicator of social dominance. Often the consequences of being outnumbered are greater than the consequences of being outsized. The mob behavior of several avian species on larger birds of prey being just one example where group size trumps physical size (32). Another possibility is that infants respond to group size earlier than physical size not because of conceptual changes in their representations of social dominance, but because infants find number more salient than physical size (which corresponds with surface area). For instance, Brannon et al. (33) showed that 6-mo-old infants could discriminate a twofold increase in number but not a twofold increase in surface area.
The early sensitivity to the relationship between numerical size and social status may in part be due to both assessments sharing a common representational system. Specifically, in adults, social status comparisons (e.g., military rank) are processed in the same brain region (inferior parietal cortex) in which numerical ratio discrimination is computed (34, 35). Further, judgments of numerical quantity and social status exhibit a similar constraint; this is known as the numerical distance effect and semantic distance effect, respectively, where individuals take longer to compare two points closer on a scale (e.g., 34 vs. 35; associate professor vs. assistant professor) than points further on the same scale (e.g., 30 vs. 50; associate professor vs. janitor) (34–36).
Although infants appear to attribute greater dominance to an individual from a numerically larger group, it is unclear what kinds of inferences infants make about group members that do not directly participate in the conflict. For instance, even though the two competing agents in studies 1 and 2 were physically the same size, the relative size of these individuals with respect to their own group members differed. More specifically, the competing agent from the numerically larger group was always physically larger than the other members of her group, and the competing agent from the numerically smaller group was always physically smaller than the other member of her group. Given the differences in relative physical size within groups, it is possible that infants used relative physical size to evaluate within-group dominance rankings first, before using these rankings to predict the outcome of a between-groups competition. Consequently, infants may expect an agent with a higher within-group dominance ranking (i.e., the largest individual within the group) to also have a between-groups advantage, even when facing an opponent that is identical in size.
Although future research will need to explore this possibility, this account cannot fully explain our findings based on the methodology we used. First, Thomsen et al. (13) showed that infants younger than 10 mo of age were unable to use the physical size of agents to represent dominance relationships. Thus, if infants relied on physical size to evaluate within-group dominance relationships before assessing between-group dominance relationships, only infants older than 10 mo of age would have expected an individual from a numerically larger group to be dominant over an individual from a numerically smaller group in our study. However, we found that 6- to 9-mo-old infants can use the relative numerical size of two groups to infer the social dominance relationship between competing individuals from those groups. Second, Mascaro and Csibra (25) demonstrated that 12- and 15-mo-old infants have to witness one agent prevail over another agent when encountering competing goals to make inferences about the agents’ dominance relationship. Because none of the infants in our study observed a direct competition between agents within the same group, our infants would not have enough information to assess dominance relationships within each group.
Future research may also want to examine infants’ expectations of the behavior of group members during a conflict. It is possible that infants may expect individuals from the same group to help an own group member during a perceived conflict. Consistent with this hypothesis, in a recent study with 6- to 8-y-olds, alliance strength was found to be an important predictor of a group’s success, such that allies were expected to win against a single individual with no allies (5). Because infants as young as 3 mo of age understand that an agent’s goal (such as trying to climb up a hill) can be helped or hindered by another individual (37, 38) and by 7 mo also expect social group members to act alike (39), it is possible that infants may expect additional members of a group to help one another during a conflict. This hypothesis could be directly tested by examining infants’ surprise when an individual from one group refuses to help someone from their own group or helps someone from an opposing group.
Furthermore, how infants weigh the benefits of increased group size with the potential cost of loss or injury of group members can speak to theories of intergroup conflict that posit distinct functional roles for different members of a group (10). For example, Lanchester (10) has proposed that the Linear and Square Law can help assess the benefits of increased group size with the cost of losing individuals in battle. More specifically, the Linear Law is based on the assumption that there is little-to-no fitness advantage for individuals from the numerically larger group to concentrate attacks on individuals from the numerically smaller group until they are needed to replace others that have been removed from the battle (owing to injuries or fatalities). According to the Linear Law individual fighting abilities and the number of individuals in a group will both influence the group’s resource holding potential (the capacity for one’s group to impose costs on the other group), such that the resource holding potential of the larger group will increase as a linear function of their numerical size advantage. Therefore, a larger group may strategize to send out their “best” fighters first, to eliminate more members of the smaller group.
Conversely, the Square Law assumes that all individuals from the numerically larger group will simultaneously attack the individuals from the numerically smaller group (10). According to the Square Law, numerical group size has the greatest influence on the group’s resource holding potential, and is less dependent on individual fighting ability. Consequently, the resource holding potential of the larger group will increase as a square function of their numerical size advantage. Therefore, working together as a unit provides the greatest advantage when fighting a smaller group of individuals.
Studies with infants’ and young toddlers’ expectations of the behavior of group members during an intergroup conflict may help reveal the extent to which they hold beliefs about the timing of when a group member will intervene (before or after a group member is defeated), how many group members will intervene on behalf of a compatriot (one or many), and the particular order with which group members may intervene (larger individuals first or random). Identifying the constraints on infants’ reasoning about the role of group members in intergroup conflict may help to clarify which (or whether) such laws are part of an early emerging system for reasoning about intergroup cognition.
Materials and Methods
All research was conducted in accordance with the Behavioral Research Ethics Board guidelines (approval no. H10-00147). The University of British Columbia approved all experiments. A legal guardian provided consent on behalf of each participant.
Studies 1 and 2.
Participants.
For studies 1 and 2, 48 infants for each age group were recruited from a local science center and tested in a soundproof testing room located on-site. In study 1, we analyzed the data from infants between the ages of 9 and 12 mo (mean age = 10.68 mo, range = 9.36–12.00 mo, SD = 25 d, 25 females). According to parental report, 47% of infants included in the final sample were classified as Caucasian, 32% as East Asian, and 21% as other ethnicities. An additional 28 participants were excluded from the sample because they did not watch the screen during the critical sequence in which one of the agents bowed down and moved out of the other agent’s path of motion (n = 12), fussed out (n = 10), or because of sibling or parental interference (n = 6). These exclusion rates are typical for studies with infants in community-based testing centers where infants are removed from otherwise highly stimulating environments before their participation in the study, and are comparable to the exclusion rate for the study conducted by (13), which also investigated infants’ understanding of social dominance in a museum setting.
In study 2, we analyzed the data from infants between the ages of 6 and 9 mo (mean age = 7.40 mo, range = 6.00–9.12 mo, SD = 30 d, 20 females). According to parental report, 66% of infants included in the final sample were classified as Caucasian, 18% as East Asian, and 16% as other ethnicities. An additional 21 participants were excluded from the sample because they did not watch the screen during the critical sequence in which one of the agents bowed down and moved out of the other agent’s path of motion (n = 11), fussed out (n = 7), or because of sibling or parental interference (n = 3).
Procedure.
For all studies, the procedure was identical. Each participant was seated on the lap of their caregiver in a sound proof testing room for the duration of the study, ∼140 cm from the center of a television screen measuring 48” in diameter. To ensure that caregivers would not influence their child’s behavior, they were instructed to either keep their eyes closed or were asked to wear a pair of opaque glasses for the duration of the study. Caregivers were also asked to remain silent and to not otherwise direct the child’s’ attention during the course of the study. The experimenter sat adjacent to the infant and caregiver, separated by a distance of ∼4 feet and hidden behind a black curtain. The experimenter remained behind the curtain and out of the infants’ line of sight for the duration of the study.
Stimuli.
For studies 1 and 2, the stimuli were identical.
Group introduction sequence.
All participants were first familiarized to two groups of novel animated characters – one group of blue characters and one group of green characters similar to the animations designed by Thomsen et al. (13) (Fig. 1). To introduce these groups, the green characters appeared on the opposite side of the screen from the blue characters, and infants observed each set of characters bounce in synchrony with other members of their group for a duration of 3 s. For example, members of the green group bounced together, followed by members of the blue group (order was counterbalanced across participants). One group was always numerically larger than the other, and this was counterbalanced between participants. We chose numerical groups consisting of two agents and three agents, because previous research has demonstrated that infants as young as 6 mo are able to reliably distinguish between the ratio of 2:3 individual objects in a variety of contexts and methodologies (26). To ensure that infants’ attention was exclusively drawn to numerical magnitude and not overall continuous extent (40), we equated the amount of surface area occupied by each group on the screen.
Goal familiarization trials.
Following the group introduction sequence, participants observed one agent from one of the groups move to a platform on the bottom of the screen and then move to the opposite side of the screen. To ensure that infants understood that the agent had the goal of crossing the platform, this sequence repeated for a minimum of two trials and a maximum of four trials. The total number of trials depended on whether the infant looked away from the screen (for at least 1 s), as others have previously done (13). Next, infants observed one agent from the other group perform the same actions, albeit moving across the screen in the opposite direction as the first agent, once again for a maximum of four repetitions of the same sequence or until the infant looked away for at least 1 s. The order in which infants viewed the agent from the numerically larger (or numerically smaller) group cross the platform first in the first set of familiarization trials was counterbalanced across participants. Importantly, both agents who crossed the platform were identical in physical size (Movies S1 and S2).
Intertrial.
The intertrial began with infants viewing the group introduction sequence, in which both groups bounced one at a time. After the group introduction sequence, one agent from each group (the same agents viewed in the goal familiarization trials) moved to the platform below simultaneously and proceeded to move to the opposite side from which they came. Moving across the platform, albeit in opposing directions, the two agents bumped into each other in the middle of the platform, slightly backed up, and then bumped into each other again. For a third and final time, the agents slightly backed up and then bumped into each other again. (Fig. 2 and Movie S3). This intertrial firmly established that when each agent pursued their goal of crossing the platform at the same time, a conflict ensued.
Test trials.
Following the intertrial event, infants viewed two test trials in which one agent on the platform yields to the other agent. Thus, after the two agents bumped into each other a third time during the intertrial event, one of the agents bowed down and moved out of the way so the other agent could cross to the other side of the platform. Based on our hypothesis that infants may use the numerical size of two groups to infer dominance relationships among individuals from those groups, in the unexpected outcome trial, the agent from the numerically larger group moved out of the way so that the agent from the numerically smaller group could cross the platform (Movie S5). In the expected outcome trial, the agent from the numerically smaller group moved out of the way to allow the agent from the numerically larger group could cross the platform (Movie S4). The order of these two trials was counterbalanced. After the agent crossed the platform, the animation froze and total looking duration for that trial was recorded until the infant looked away for more than two consecutive seconds, or until 30 s had elapsed.
The methods described in the goal familiarization trial, intertrial, and test trials described each agent moving in an identical manner to Thomsen et al. (13). The only modifications made to the stimuli were done to address our particular research question, such that the two competing agents were of the same physical size and were first shown bouncing exclusively with the color-matched group members from which they belonged (either in the upper left- and right-hand corners of the screen).
Study 3.
Participants.
For study 3, 48 infants were recruited from a local science center and tested in a soundproof testing room located on-site. We analyzed the data from 48 infants between the ages of 6 and 12 mo (mean age = 8.40 mo, range = 6.12–11.52 mo, SD = 1.44 mo, 22 females). According to parental report, 41% of infants included in the final sample were classified as Caucasian, 24% as East Asian, and 35% as other ethnicities. An additional 13 participants were excluded from the sample due to fussing out (n = 8) or sibling/parental interference (n = 5).
Procedure.
Identical to studies 1 and 2.
Stimuli.
In study 3, infants saw two test trials. Each trial began with infants viewing the group introduction sequence (as above). After the group introduction sequence, one agent from each group (the same agents viewed in the goal familiarization trials in studies 1 and 2) moved simultaneously to the platform below. One agent remained stationary, and the other proceeded to move to the opposite side from which they came. In one trial, infants viewed the agent from the numerically larger group attempt to cross the platform. However, a barrier placed in the middle of the platform obstructed the agent’s path. Once the agent reached the middle of the platform, the agent bumped into the obstacle for a total of three times. Then, the agent bowed down and moved along the platform (in a direction perpendicular from which they were moving previously; Movie S6). Once the agent bowed and moved to the back of the platform, the animation froze and total looking duration for that trial was recorded until the infant looked away for more than two consecutive seconds, or until 30 s had elapsed. In the other trial, infants viewed the agent from the numerically smaller group attempt to cross the platform, who was also obstructed by the barrier (Movie S7). The order of these two trials was counterbalanced across participants.
Supplementary Material
Acknowledgments
We would like to thank the Living Lab at Science World at TELUS World of Science in Vancouver, the Early Development Research Group at the University of British Columbia, and the participating parents and children in these studies. This research was funded by a Social Sciences and Humanities Research Council Insight Development Grant (435-2013-0286) (to A.S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514879113/-/DCSupplemental.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life. John Murray; London: 1859. [PMC free article] [PubMed] [Google Scholar]
- 2.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211(4489):1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 3.Geist V. Life Strategies, Human Evolution, Environmental Design: Toward a Biological Theory of Health. Springer; New York: 1978. [Google Scholar]
- 4.Smith JM. The theory of games and the evolution of animal conflicts. J Theor Biol. 1974;47(1):209–221. doi: 10.1016/0022-5193(74)90110-6. [DOI] [PubMed] [Google Scholar]
- 5.Pietraszewski D, Shaw A. Not by strength alone: Children’s conflict expectations follow the logic of the asymmetric war of attrition. Hum Nat. 2015;26(1):44–72. doi: 10.1007/s12110-015-9220-0. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ML, Wrangham RW. Intergroup relations in chimpanzees. Annu Rev Anthropol. 2003;32:363–392. [Google Scholar]
- 7.Batchelor TP, Briffa M. Fight tactics in wood ants: Individuals in smaller groups fight harder but die faster. Proc Biol Sci. 2011;278(1722):3243–3250. doi: 10.1098/rspb.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgar MA. Predator vigilance and group size in mammals and birds: A critical review of the empirical evidence. Biol Rev Camb Philos Soc. 1989;64(1):13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 9.Bowles S, Choi J-K, Hopfensitz A. The co-evolution of individual behaviors and social institutions. J Theor Biol. 2003;223(2):135–147. doi: 10.1016/s0022-5193(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 10.Lanchester FW. Mathematics in warfare. In: Newman JR, editor. The World of Mathematics. Vol 4. Simon and Schuster; New York: 1956. pp. 2138–2157. [Google Scholar]
- 11.Brown JH, Maurer BA. Body size, ecological dominance and Cope’s rule. Nature. 1986;324(6094):248–250. [Google Scholar]
- 12.Buston P. Social hierarchies: Size and growth modification in clownfish. Nature. 2003;424(6945):145–146. doi: 10.1038/424145a. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen L, Frankenhuis WE, Ingold-Smith M, Carey S. Big and mighty: Preverbal infants mentally represent social dominance. Science. 2011;331(6016):477–480. doi: 10.1126/science.1199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mech L, Adams L, Meier T, Burch J, Dale B. The Wolves of Denali. Univ of Minnesota Press; Minneapolis: 1998. [Google Scholar]
- 15.Boydston EE, Morelli TL, Holekamp KE. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta) Ethology. 2001;107(5):369–385. [Google Scholar]
- 16.McComb K, Packer C, Pusey A. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim Behav. 1994;47(2):379–387. [Google Scholar]
- 17.Tomasello M. The ultra-social animal. Eur J Soc Psychol. 2014;44(3):187–194. doi: 10.1002/ejsp.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark AP. Rank differences in the production of vocalizations by wild chimpanzees as a function of social context. Am J Primatol. 1993;31(3):159–179. doi: 10.1002/ajp.1350310302. [DOI] [PubMed] [Google Scholar]
- 19.Ghiglieri MP. The Chimpanzees of Kibale Forest: A Field Study of Ecology and Social Structure. Columbia Univ Press; New York: 1984. [Google Scholar]
- 20.Marler P. Social Organization, Communication and Graded Signals: The Chimpanzee and the Gorilla. Cambridge Univ Press; Cambridge, UK: 1976. [Google Scholar]
- 21.Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford Univ Press; Oxford: 2000. [Google Scholar]
- 22.Wilson ML, Hauser MD, Wrangham RW. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim Behav. 2001;61(6):1203–1216. [Google Scholar]
- 23.Heinsohn R. Group territoriality in two populations of African lions. Anim Behav. 1997;53(6):1143–1147. doi: 10.1006/anbe.1996.0316. [DOI] [PubMed] [Google Scholar]
- 24.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Belknap Press of Harvard Univ Press; Cambridge, MA: 1986. [Google Scholar]
- 25.Mascaro O, Csibra G. Representation of stable social dominance relations by human infants. Proc Natl Acad Sci USA. 2012;109(18):6862–6867. doi: 10.1073/pnas.1113194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feigenson L, Dehaene S, Spelke E. Core systems of number. Trends Cogn Sci. 2004;8(7):307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Nishida T, Hosaka K. Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In: McGrew WC, Marchant LF, Nishida T, editors. Great Ape Societies. Cambridge Univ Press; Cambridge, UK: 1996. pp. 114–134. [Google Scholar]
- 28.de Waal FB. Sex differences in the formation of coalitions among chimpanzees. Ethol Sociobiol. 1984;5(4):239–255. [Google Scholar]
- 29.Duffy KG, Wrangham RW, Silk JB. Male chimpanzees exchange political support for mating opportunities. Curr Biol. 2007;17(15):R586–R587. doi: 10.1016/j.cub.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Univ of Chicago Press; Chicago: 1992. [Google Scholar]
- 31.Hand JL. Resolution of social conflicts: Dominance, egalitarianism, spheres of dominance, and game theory. Q Rev Biol. 1986;61:201–220. [Google Scholar]
- 32.Lorenz K. On Aggression. Harcourt, Brace & World; New York: 1966. [Google Scholar]
- 33.Brannon EM, Abbott S, Lutz DJ. Number bias for the discrimination of large visual sets in infancy. Cognition. 2004;93(2):B59–B68. doi: 10.1016/j.cognition.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Chiao JY, et al. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47(2):354–363. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Chiao JY. Neural basis of social status hierarchy across species. Curr Opin Neurobiol. 2010;20(6):803–809. doi: 10.1016/j.conb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Chiao JY, Bordeaux AR, Ambady N. Mental representations of social status. Cognition. 2004;93(2):B49–B57. doi: 10.1016/j.cognition.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Hamlin JK, Wynn K, Bloom P. Three-month-olds show a negativity bias in their social evaluations. Dev Sci. 2010;13(6):923–929. doi: 10.1111/j.1467-7687.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhlmeier V, Wynn K, Bloom P. Attribution of dispositional states by 12-month-olds. Psychol Sci. 2003;14(5):402–408. doi: 10.1111/1467-9280.01454. [DOI] [PubMed] [Google Scholar]
- 39.Powell LJ, Spelke ES. Preverbal infants expect members of social groups to act alike. Proc Natl Acad Sci USA. 2013;110(41):E3965–E3972. doi: 10.1073/pnas.1304326110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feigenson L, Carey S, Spelke E. Infants’ discrimination of number vs. continuous extent. Cognit Psychol. 2002;44(1):33–66. doi: 10.1006/cogp.2001.0760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.