Significance
Xeroderma pigmentosum (XP) is a genetic disorder caused by defective repair of DNA damage. Affected patients are mutated in one of eight genes and develop skin pigmentation changes, skin cancers, ocular surface abnormalities, and, in some cases, acute sunburn and neurodegeneration. The XP proteins are involved in different steps in the repair of DNA damage. Examination of 89 patients, the largest reported cohort under long-term follow-up, by the same multidisciplinary team of clinicians and scientists has revealed unexpected clinical heterogeneity dependent on the affected gene and the exact mutation. Our findings provide new insights into the mechanisms of carcinogenesis, ocular surface disease, and neurodegeneration, as well as providing improved clinical management and more definitive prognostic predictions.
Keywords: UV radiation, nucleotide excision repair, skin cancer, ocular disease, neurodegeneration
Abstract
Xeroderma pigmentosum (XP) is a rare DNA repair disorder characterized by increased susceptibility to UV radiation (UVR)-induced skin pigmentation, skin cancers, ocular surface disease, and, in some patients, sunburn and neurological degeneration. Genetically, it is assigned to eight complementation groups (XP-A to -G and variant). For the last 5 y, the UK national multidisciplinary XP service has provided follow-up for 89 XP patients, representing most of the XP patients in the United Kingdom. Causative mutations, DNA repair levels, and more than 60 clinical variables relating to dermatology, ophthalmology, and neurology have been measured, using scoring systems to categorize disease severity. This deep phenotyping has revealed unanticipated heterogeneity of clinical features, between and within complementation groups. Skin cancer is most common in XP-C, XP-E, and XP-V patients, previously considered to be the milder groups based on cellular analyses. These patients have normal sunburn reactions and are therefore diagnosed later and are less likely to adhere to UVR protection. XP-C patients are specifically hypersensitive to ocular damage, and XP-F and XP-G patients appear to be much less susceptible to skin cancer than other XP groups. Within XP groups, different mutations confer susceptibility or resistance to neurological damage. Our findings on this large cohort of XP patients under long-term follow-up reveal that XP is more heterogeneous than has previously been appreciated. Our data now enable provision of personalized prognostic information and management advice for each XP patient, as well as providing new insights into the functions of the XP proteins.
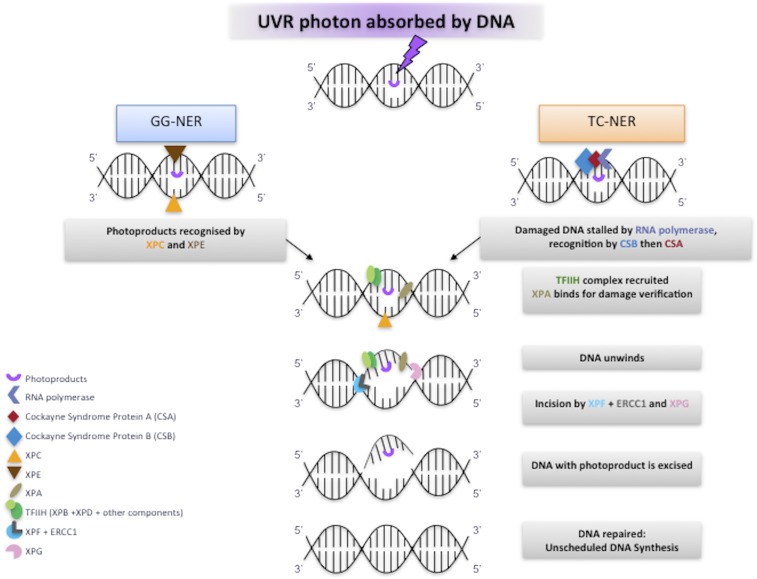
Xeroderma pigmentosum (XP) is an autosomal recessive genodermatosis, with clinical features predominantly recognized in the dermatological, ocular, and neurological systems. XP patients may present with severe sunburn on minimal sun exposure or with pigmentary changes at exposed sites and multiple early age skin cancers. The incidence has been estimated at 2.3 per million live births in Western Europe (1), but is higher in Japan (2) and North Africa (3). XP patients have a 2,000- and 10,000-fold increased incidence of melanoma and nonmelanoma skin cancers, respectively (4). About 50% of XP patients show severe sunburn reactions on minimal sun exposure (5), and approximately one third of patients have progressive neurological degeneration associated with neuronal loss (4, 5). XP has been intensively studied at the molecular and cellular levels, as affected individuals are defective in the repair of UV radiation (UVR)-induced DNA damage. The majority of patients have mutations in one of seven genes (XPA through G), whose products are involved in nucleotide excision repair (NER) of UVR and other types of DNA damage (6) (Fig. S1).
Fig. S1.
Role of XP proteins in NER. The figure represents a scheme for NER, showing only the roles of the XP proteins. For simplicity, other proteins involved have not been included. In GG-NER, XPE and XPC are involved in damage recognition, TFIIH-containing XPB and XPD are recruited and open out the DNA structure in the vicinity of the damage. XPA verifies the damaged structure before dual incisions by XPF-ERCC1 and XPG. After the damaged section has been removed, DNA polymerases and ligases fill in the gap. In TC-NER, blockage of RNA polymerase by the damage is the recognition signal. Proteins CSB and CSA, defective in Cockayne syndrome, are recruited, and these, in turn recruit TFIIH to enable the rest of the NER process to proceed.
There are two subbranches of NER distinguished by the initial recognition step (Fig. S1). Transcription-coupled NER (TC-NER) is a process whereby damage formed on the transcribed strand of actively transcribed regions of DNA is repaired rapidly. The rest of the genome is repaired by a slower global genome NER (GG-NER). Importantly the damage recognition proteins XPC and XPE are not required for TC-NER, whereas the other XP proteins are involved in both subpathways.
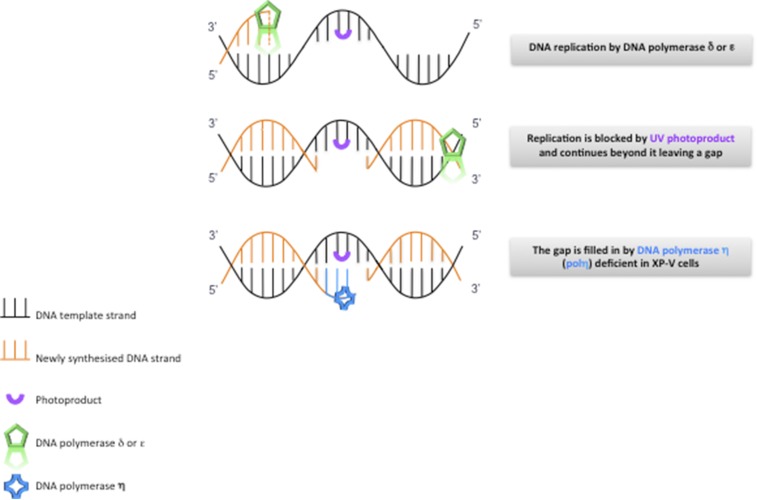
About 20% of XP patients, the so-called XP variants, have normal NER but are defective in translesion synthesis past UVR-induced DNA damage (Fig. S2) (7), as a consequence of mutations in the POLH gene encoding DNA polymerase ƞ, an enzyme vital for replicating past unrepaired UVR photo-products (8, 9). This deficiency confers sensitivity to fibroblasts that are exposed to UVR followed by incubation with caffeine (10). This UVR+caffeine sensitivity is used as a diagnostic test (11).
Fig. S2.
Translesion synthesis. DNA replication, carried out by DNA polymerases δ or ε, is blocked by a UVR lesion, and in many cases reinitiates beyond the lesion. The resulting gap is filled in by DNA polymerase ƞ, which is deficient in XP variants.
XP patients in which TC-NER remains intact (groups C, E, and V) do not, in general, suffer from neurological problems (4) and have normal sunburn reactions (5), whereas those in groups in which both subbranches of NER are affected often develop neurological problems and have an abnormal sunburn reactions.
In 2010, funding was obtained from the National Commissioning Group of the UK National Health Service to establish an XP specialized clinic, where all XP patients in the United Kingdom are offered specialist dermatological, ophthalmological, neurological, psychological, and clinical genetic examination, as well as DNA repair testing and mutation analysis. Our findings on 89 XP patients, the largest reported cohort under long-term follow-up, are presented here. We estimate that this represents 90% of the XP patients in the United Kingdom. Our study differs from previous analyses in that this large cohort has been examined by the same clinicians using 60 different phenotypical end points, and in all cases, this has been correlated with the pathological mutation. These end points have enabled us to identify previously unrecognized phenotypes in some XP groups, as well as several unexpected genotype-phenotype relationships in patients with unusual phenotypes. Our findings also provide new insights into the functions of the XP proteins.
Results
A few general points have emerged from our analyses, which will be discussed in more detail below [aspects of points 1–3 have been discussed previously (5)]:
-
i)
Most patients in XP-A, -B, -D, -F, and -G groups have severe sunburn reactions from an early age. In contrast the TC-NER–competent groups XP-C, -E, and -V have normal sunburn reactions for skin type. We recently provided a detailed analysis of this phenomenon (5), which has also been recently noted in the US XP population (4).
-
ii)
XP patients with extreme sunburn reactions are likely to be diagnosed very early and photo-protected from a young age. As a consequence, in such cases pigmentary changes are relatively mild and skin cancers are relatively uncommon.
-
iii)
In contrast, the first symptoms in XP-C patients are photo-distributed lentigines around the age of 2 y. They have a later age of XP diagnosis and therefore accumulate more photo-damage, leading to an earlier age of first skin cancer (4, 5).
-
iv)
XP-E and XP-V patients tend to be diagnosed much later; they may have two decades or more without any symptoms. They therefore accumulate more UVR-induced mutations and can develop hundreds of skin tumors in later life.
-
v)
Paradoxically, therefore, the XP-C, -E, and -V groups, which have generally been described as milder on the basis of their cellular sensitivity to UVR and lack of neurological abnormalities (12), tend to develop more skin cancers than the groups classified as more severe using the same criteria.
-
vi)
XP-C patients are particularly susceptible to ocular problems relative to their skin changes compared with the other groups.
-
vii)
Within the XP population, XP-F and XP-G patients seem to be remarkably resistant to the development of skin cancers.
We developed a scoring system to indicate the severity of the ocular and neurological features of each patient (SI Methods). By necessity, this is somewhat simplistic. Note that all scores are likely to increase with age of the patients, especially those with neurological abnormalities. More detailed breakdown of dermatological, ocular disease, and neurological features by complementation group is provided in Tables S1–S3, respectively.
Table S1.
General and dermatological features
| Clinical features | Total | Mild XP-A* | % | XP-A | % | XP-B | % | XP-C | % | XP-D | % | XP-E | % | XP-F | % | XP-G | % | XP-V | % |
| Total cases reported | 89 | 10 | 11 | 8 | 9 | 2 | 2 | 28 | 31 | 14 | 16 | 4 | 4 | 3 | 3 | 8 | 9 | 12 | 13 |
| Men | 38 | 6 | 60 | 4 | 50 | 0 | 14 | 50 | 7 | 50 | 0 | 2 | 67 | 4 | 50 | 7 | 58 | ||
| Women | 41 | 4 | 40 | 4 | 50 | 2 | 100 | 14 | 50 | 7 | 50 | 4 | 100 | 1 | 33 | 4 | 50 | 5 | 42 |
| Median age as of December 2014 (y) | 35 | 17 | 45 | 14 | 24 | 56 | 23 | 19 | 55 | ||||||||||
| Median age at diagnosis (y) | 32 | 8 | 36 | 5 | 4 | 47 | 9 | 8 | 44 | ||||||||||
| Age at onset of cutaneous symptoms (y) (average) | 4 | 0.4 | 1 | 3 | 0.6 | 17 | 1 | 0.6 | 27 | ||||||||||
| Sunburn severity score (median) | 1 | 3 | 3 | 0 | 3 | 1 | 3 | 3 | 0 | ||||||||||
| Mean age at onset of lentigines stated (y) | 3 | 7 | 3 | 3 | 8 | 15 | 15 | 6 | 15 | ||||||||||
| Age photoprotection started (y) (average) | 24 | 3 | 2 | 10 | 10 | 17 | 15 | 10 | 39 | ||||||||||
| Presence of lentigines at exposed sites | 10 | 100 | 7 | 88 | 2 | 100 | 28 | 100 | 10 | 7 | 4 | 100 | 1 | 33 | 6 | 75 | 12 | 100 | |
| Hypopigmented macules | 7 | 70 | 3 | 38 | 2 | 100 | 24 | 86 | 10 | 71 | 4 | 100 | 0 | 5 | 63 | 7 | 58 | ||
| Cutaneous atrophy/actinic damage present | 1 | 10 | 0 | 0 | 2 | 100 | 7 | 25 | 9 | 64 | 3 | 75 | 0 | 0 | 11 | 92 | |||
| Malignant skin neoplasms | |||||||||||||||||||
| Present | 2 | 20 | 1 | 0 | 1 | 50 | 15 | 54 | 9 | 64 | 3 | 75 | 0 | 0 | 10 | 83 | |||
| Absent | |||||||||||||||||||
| Age first skin cancer (y) (median) | 38 | 8 | 22 | 11 | 14 | 15 | NA | NA | 36 | ||||||||||
| Type of first cutaneous neoplasm | |||||||||||||||||||
| BCC | 1 | 11 | 8 | 2 | NA | NA | 3 | ||||||||||||
| SCC | 1 | 2 | 1 | NA | NA | 2 | |||||||||||||
| Melanoma/MIS | 1 | 1 | 2 | 1 | NA | NA | 5 |
This is the group of XP-A with the c.555+8A > G mutation. NA, Not applicable.
Table S3.
Neurological disease features
| Clinical features | Total | Mild XP-A | % | XP-A | % | XP-B | % | XP-C | % | XP-D | % | XP-E | % | XP-F | % | XP-G | % | XP-V | % |
| Total cases reported | 89 | 10 | 11 | 8 | 9 | 2 | 2 | 28 | 31 | 14 | 16 | 4 | 4 | 3 | 3 | 8 | 9 | 12 | 13 |
| Median age as of December 2014 (y) | 35 | 17 | 45 | 14 | 24 | 56 | 23 | 19 | 55 | ||||||||||
| Total cases reported with neurologic abnormalities | 0 | 0 | 7 | 88 | 2 | 100 | 1 | 4 | 11 | 79 | 0 | 0 | 1 | 33 | 7 | 88 | 1 | 8 | |
| Age at onset of symptoms (y) (median) | 4 | 26 | 24 | 12 | 38 | 15 | 51 | ||||||||||||
| Average Neuro score (maximum 8) | 0 | 4 | 3 | 0 | 4 | 0 | 2 | 4 | 0 | ||||||||||
| Maximum Neuro score in group | 0 | 8 | 4 | 1 | 8 | 0 | 6 | 6 | 1 | ||||||||||
| Peripheral neuropathy | 0 | 0 | 6 | 75 | 1 | 0 | 0 | 0 | 11 | 79 | 0 | 0 | 1 | 33 | 2 | 25 | 0 | 0 | |
| Cerebellar signs | 0 | 0 | 8 | 114 | 1 | 50 | 0 | 0 | 9 | 64 | 0 | 0 | 1 | 33 | 6 | 75 | 0 | 0 | |
| Sensorineural hearing loss | 0 | 0 | 3 | 43 | 2 | 100 | 1 | 4 | 10 | 71 | 0 | 0 | 1 | 33 | 6 | 75 | 1 | 8 | |
| Cognitive impairment | 0 | 0 | 8 | 114 | 1 | 50 | 1 | 4 | 10 | 71 | 0 | 0 | 1 | 33 | 7 | 88 | 0 | 0 | |
| Bulbar signs (dysphagia, PEG) | 0 | 0 | 1 | 14 | 0 | 0 | 0 | 0 | 2 | 14 | 0 | 0 | 0 | 0 | 2 | 25 | 0 | 0 | |
| Neuro-ophthalmological signs | 0 | 0 | 7 | 100 | 1 | 50 | 0 | 0 | 8 | 57 | 0 | 0 | 1 | 33 | 6 | 75 | 0 | 0 | |
| Functional score | |||||||||||||||||||
| Normal gait | 10 | 100 | 1 | 13 | 2 | 100 | 28 | 100 | 4 | 29 | 4 | 100 | 2 | 67 | 3 | 38 | 12 | 100 | |
| Assisted gait | 5 | 63 | 8 | 57 | 1 | 33 | 2 | 25 | |||||||||||
| Wheelchair bound | 1 | 13 | 2 | 14 |
XP-A (MIM278700).
Eighteen XP-A patients from 14 families have attended the clinic (Table 1). With one exception (XP15BR), they are of South Asian or Somali origin. All are homozygous for the causative mutations. We have divided them into two clearly distinct subgroups.
Table 1.
XP-A, XP-B, and XP-C patients
| XP ID no | Group | Age (y) | Age XP diagnosis (y) | Ethnicity | UDS* | Allele 1 | Protein change | Allele 2† | Protein change | SSS | Age first cancer (y) | Type first cancer | No. MM | No. NMSC | Ocular severity score (maxiumum 12) | Neuro severity score (maximum 8) |
| XP111BR | A | 8 | 5 | Bangladeshi | 3 | c.253C > T | p.Gln85X | 3 | — | 0 | 0 | 2 | 3/5‡ | |||
| XP54PV | A | 8 | 7 | Pakistani | ND | c.648_649delGA | p.Lys217fs | 3 | 8 | SCC | 0 | 2 | 3 | 4/5‡ | ||
| XP57BR | A | 15 | 4 | BangladeshI | 1 | c.640dupA | p.Met214fs | 1 | — | 0 | 0 | 2 | 4 | |||
| XP80BR | A | 15 | 8 | Somalian | 2 | c.314G > A | p.Cys105Tyr | 3 | — | 0 | 0 | 3 | 4 | |||
| XP81BR | A | 19 | 12 | Somalian | 5 | c.314G > A | p.Cys105Tyr | 1 | — | 0 | 0 | 3 | 5 | |||
| XP15BR | A | 23 | <1 | Caucasian | 0 | c.266_267dupAA | p.Val90fs | 3 | — | 0 | 0 | 1 | 5 | |||
| XP114BR | A | 24 | 22 | Pakistani | 8 | c.682C > T | p.Arg228X | 3 | — | 0 | 0 | 6 | 5 | |||
| XP20BR | A | 33 | 13 | Pakistani | 8 | c.682C > T | p.Arg228X | 3 | — | 0 | 0 | 6 | 8 | |||
| XP9BI | A | 7 | 6 | Pakistani | ND | c.555+8A > G | 0 | — | 0 | 0 | 1 | 0 | ||||
| XP103BR | A | 8 | 4 | Pakistani | 20 | c.555+8A > G | 0 | — | 0 | 0 | 1 | 0 | ||||
| XP53BR | A | 19 | 7 | Pakistani | 3 | c.555+8A > G | 1 | — | 0 | 0 | 2 | 0 | ||||
| XP116BR | A | 31 | 31 | Indian Asian | 12 | c.555+8A > G | 1 | 30 | BCC | 0 | 7 | 4 | 0 | |||
| XP2PR | A | 34 | 33 | Pakistani | ND | c.555+8A > G | 1 | — | 0 | 0 | 1 | 0 | ||||
| XP89BR-S | A | 35 | 34 | Pakistani | ND | c.555+8A > G | 2 | — | 0 | 0 | 2 | 0 | ||||
| XP1PR | A | 36 | 35 | Pakistani | ND | c.555+8A > G | 2 | — | 0 | 0 | 3 | 0 | ||||
| XP88BR | A | 37 | 31 | Pakistani | 15 | c.555+8A > G | 3 | — | 0 | 0 | 2 | 0 | ||||
| XP89BR | A | 44 | 39 | Pakistani | 5 | c.555+8A > G | 3 | — | 0 | 0 | 2 | 0 | ||||
| XP1CB | A | 80 | 67 | Indian Asian | 13 | c.555+8A > G | 0 | 46 | MIS | 10 | 35 | 3 | 0 | |||
| XP84BR | B | 35 | 28 | Caucasian | 14 | c.296T > C | p.Phe99Ser | 3 | — | 0 | 0 | 3 | 1 | |||
| XP1SA | B | Died55 | 45 | Mixed | 21 | c.296T > C | p.Phe99Ser | c.1273C > T | p.Arg425X | 2 | 22 | MM | 1 | 7 | 3 | 4 |
| XP117BR | C | 5 | 5 | Caucasian | 8 | c.299+2delT | Splice | c.2429_2441del13 | p.Gly810fs | 0 | 4 | BCC | 0 | 0 | 1 | 0 |
| XP93BR | C | 6 | 1 | Pakistani | 1 | c.1243C > T | p.Arg415X | 0 | — | 0 | 2 | 0 | 0 | |||
| XP66TU | C | 6 | 3 | Caucasian | 20 | c.924_938del15 | p.Leu309_313del | c.1021G > T | p.Ala341Ser | 0 | 3 | SCC | 0 | 4 | 3 | 0 |
| XP112BR-S | C | 7 | 3 | Bangladeshi | ND | c.1808G > A | p.Trp603X | c.2420+2T > C | Splice | 0 | — | 0 | 0 | 2 | 0 | |
| XP102BR | C | 7 | 4 | Caucasian | 14 | c.1111delA | p.Thr371fs | deletion exon 1 | 0 | — | 0 | 0 | 3 | 0 | ||
| XP112BR | C | 9 | 7 | Bangladeshi | 9 | c.1808G > A | p.Trp603X | c.2420+2T > C | Splice | 0 | — | 0 | 0 | 2 | 0 | |
| XP4LE | C | 9 | 5 | Pakistani | 5 | c.2176_2192del17 | p.Glu726fs | 0 | 6 | BCC | 0 | 2 | 5 | 0 | ||
| XP73BR | C | 10 | 2 | Pakistani | 7 | c.1243C > T | p.Arg415X | 0 | — | 0 | 0 | 6 | 1 | |||
| XP94BR | C | 10 | 6 | Sri Lankan | 11 | c.877C > T | p.Arg293X | 0 | — | 0 | 0 | 7 | 0 | |||
| XP82BR | C | 10 | 2 | Caucasian | 15 | c.395_398delATTG | p.Asp132fs | deletion XPC exon 14–15 | 0 | 6 | SCC | 0 | 3 | 2 | 0 | |
| XP76BR | C | 11 | 3 | Pakistani | 10 | c.1243C > T | p.Arg415X | 0 | — | 0 | 0 | 4 | 0 | |||
| XP78BR | C | 12 | 5 | Pakistani | 28 | c.1243C > T | p.Arg415X | 0 | — | 0 | 0 | 5 | 0 | |||
| XP3LE | C | 13 | 6 | Pakistani | 15 | c.1243C > T | p.Arg415X | 0 | 11 | BCC | 0 | 1 | 3 | 0 | ||
| XP77BR | C | 12 | 4 | Pakistani | 10 | c.1243C > T | p.Arg415X | 0 | 8 | BCC | 1 | 0 | 5 | 0 | ||
| XP58BR | C | 15 | 4 | Pakistani | ND | c.1243C > T | p.Arg415X | 0 | — | 0 | 0 | 4 | 0 | |||
| XP83BR | C | 16 | 9 | Pakistani | 21 | c.1243C > T | p.Arg415X | 0 | — | 0 | 0 | 7 | 0 | |||
| XP62BR | C | 17 | 7 | Pakistani | 6 | c.1243C > T | p.Arg415X | 0 | — | 0 | 0 | 4 | 0 | |||
| XP39BR | C | 17 | 2 | Bangladeshi | 12 | c.2176_2192del17 | p.Glu726fs | 0 | — | 0 | 0 | 5 | 0 | |||
| XP35BR | C | 19 | 4 | Bangladeshi | 13 | c.2176_2192del17 | p.Glu726fs | 0 | — | 0 | 0 | 6 | 0 | |||
| XP28BR | C | 21 | 4 | Bangladeshi | 12 | c.2176_2192del17 | p.Glu726fs | 0 | 8 | BCC | 0 | 8 | 9 | 0 | ||
| XP51BR | C | 23 | 11 | Middle Eastern | 1 | c.2251–1G > C | 0 | 12 | BCC | 0 | 1 | 5 | 0 | |||
| XP6B1 | C | 23 | 5 | Pakistani | 13 | c.1243C > T | p.Arg415X | 0 | 22 | BCC | 0 | 5 | 8 | 0 | ||
| XP1SH | C | 27 | 23 | Caucasian | 6 | c.445_446delGA | p.Glu149fs | c.2336delT | p.Leu779fs | 0 | 3 | BCC | 0 | 5 | 8 | 0 |
| XP22BR | C | 38 | 19 | Middle Eastern | 13 | c.658C > T | p.Arg220X | 0 | 11 | BCC | 0 | 7 | 7 | 0 | ||
| XP21BR | C | Died39 | 6 | Middle Eastern | 15 | c.658C > T | p.Arg220X | 0 | 31 | BCC | 1 | 6 | 7 | 0 | ||
| XP95BR | C | 26 | 20 | Caucasian | 27 | c.2033+5G > A | 0 | 20 | MM | 9 | 0 | 0 | 0 | |||
| XP107BR | C | 37 | 34 | Caucasian | 41 | c.1754A > G | p.Tyr585Cys | 0 | 28 | MIS | 4 | 0 | 1 | 0 | ||
| XP29BR | C | 62 | 46 | Asian | 33 | c.2033+5G > A | 0 | 57 | BCC | 3 | 1 | 2 | 1 | |||
UDS is measured, after a UVC dose of 10 J⋅m−2, as percent of that in normal cells used as control in the same experiment. BCC, basal cell carcinoma; MIS, melanoma in situ; MM, malignant melanoma; ND, not done; NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma; SSS, sunburn severity score (5). Note that the mutation data for the XP-A patients has been presented in ref. 16 and much of the sunburn data have been presented in ref. 5. These data are reproduced here for completeness.
Where no second allele is indicated, the mutation is homozygous.
In these two cases, audiometry and neuro-ophthalmological data were not possible to obtain due to severity of cognitive impairment in each patient, so maximum score from measured parameters is only 5.
In the first subgroup (Table 1, upper group), five of eight patients, XP54PV, XP15BR, XP20BR, XP57BR, and XP111BR, have truncation or frameshift mutations in the XPA gene. One of these patients, XP54PV, developed her first skin cancer aged 8 y; she lived the majority of her childhood in Pakistan and Italy, without significant UVR protection. The remaining four, born and raised in the United Kingdom, have not developed any skin cancers before 30 y of age. They have moderate ocular disease and neurological abnormalities. Neurological severity is quite heterogeneous, although generally progressive with advancing age. XP111BR is homozygous for a stop codon at amino acid (aa)85 and as would be expected from the literature, she has neurological abnormalities, developmental delay, and microcephaly. XP15BR is homozygous for a frameshift mutation at aa90. At age 22, he has a neurology severity score of 5/7. Neither patient has detectable unscheduled DNA synthesis (UDS). XP57BR (p.Met214fs) with a frameshift mutation in exon 5 has severe learning difficulties, negligible speech, and pronounced hearing loss. Her affected brother died at aged 18 y. XP20BR and XP114BR, both with the p.Arg228X mutation reported in several cases in the literature (13), have somewhat slower progression of their neurological problems. There is a large cohort of XP patients with this mutation in Tunisia (14), and their clinical features were described as being less severe than the large cohort of Japanese XP-A patients with a common splicing mutation in intron 3. The latter are expected to have no XPA functional activity.
The XP-A patients in the second subgroup (Table 1, lower group) are quite different. All 10 have the mutation c.555+8A > G. This mutation has previously been reported for a 60-y-old Afghan woman of Punjabi origin, XP40BR (15). It generates a new splice donor site for intron 4, resulting in seven bases from intron 4 being incorporated between exons 4 and 5, resulting in a frameshift at this position. A very low level of normal transcript was noted, which can account for the 5–10% of normal repair in these patients (15). All of the patients in this subgroup have mild dermatological features with no neurological abnormalities and low ocular scores for their age, as described in detail elsewhere (16). This subgroup includes XP1CB, our oldest XP patient, aged 80 y. Despite working outdoors for many years, his first skin cancer was diagnosed at age 46. He has since had multiple skin cancers removed, mainly in the last decade. As previously discussed, it appears that a minimal amount of repair capability in XP-A patients is sufficient to prevent any neurodegeneration, although not to prevent the typical cutaneous features of XP (15, 17).
XP-B (MIM610651).
We are aware of only two XP-B families in the United Kingdom (Table 1). XP1SA and her younger sister, XP33BR (who has not attended our clinic), have been analyzed in detail by Oh et al. (18) They are compound heterozygotes for the missense mutation p.Phe99Ser and nonsense mutation p.Arg425X. The older sister, XP1SA, aged 53 y, had moderate sunburn on minimal sun exposure and developed several skin cancers from the age of 22 y, including a malignant melanoma. She also had moderate neurological abnormalities. As a heavy smoker, she developed a lung cancer, which led to her death at age 55 y, in August 2014. Another (unrelated) individual, XP84BR, is homozygous for the same missense mutation p.Phe99Ser that is heterozygous in both XP1SA and two brothers reported in the literature (XPCS1BA and XPCS2BA) (19, 20). She suffers from severe sunburn on minimal sun exposure and sensorineural hearing loss, but otherwise her symptoms are relatively mild. Other than the hearing loss, she has minimal neurological abnormalities. She has not had any skin cancers. It is likely that XPB protein with this missense mutation retains significant activity.
XP-C (MIM278720).
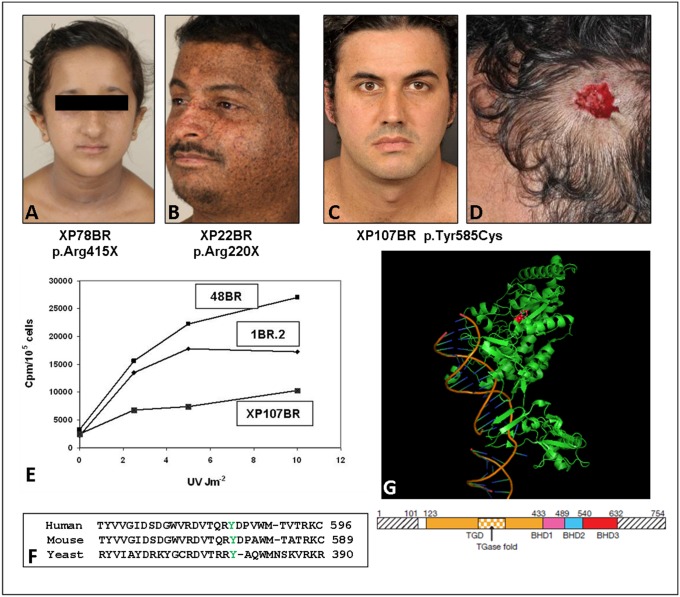
Twenty-eight XP-C patients have attended the clinic (Table 1). Ten belong to an extended consanguineous kindred of Pakistani origin. The XP-C patients comprise the most homogeneous group. None shows any sign of severe sunburn reactions on minimal sun exposure. Most were diagnosed between ages 3 and 7 y because of progressive exposed-site lentigines, especially on the malar area of the face (Fig. 1A). XP21BR and XP22BR spent the first 7 and 2 y of their lives, respectively, in Yemen and were not diagnosed definitively until their late teens. Both have had several skin cancers (Fig. 1B). With three exceptions, the XP-C patients are homozygous for truncation mutations (nonsense, frameshift, or splicing mutations) and UDS levels, as reported in the literature for other XP-C patients, are in the range of 10–20%. Skin cancers are seen relatively early in XP-C patients. However, affected younger siblings, usually diagnosed at birth, have been well protected and therefore have not developed skin cancers.
Fig. 1.
XP-C patients. (A) XP78BR, aged 12 y; note lentigines on face. (B) XP22BR, aged 38 y; note the extensive pigmentary changes. (C) XP107BR, aged 37 y; note the almost complete absence of pigmentary changes, despite not being diagnosed until 34 y of age. (D) Site of excised melanoma from XP107BR. (E) UDS, measured as incorporated 3H-thymidine cpm per 105 cells following UV irradiation of XP107BR with different UVC doses compared with two normal controls: 1BR2 and 48BR. (F) Alignment of XPC protein from human and mouse and yeast Rad4 in the region around Tyr585 (indicated in green) that is mutated in XP107BR. (G) Crystal structure of Rad4 (22) with Tyr379 (corresponding to Tyr585 in human XPC) indicated in red in the structure and the schematic below.
A remarkable and previously unreported observation is that progressive ocular disease in XP-C patients is much more severe than in other groups, with ocular disease scores increasing with advancing age. For example, in their first, second, and third decades, they have mean scores of 3, 4.7, and 7.5, respectively (see Table S2 for more details). If we compare the ocular scores for the non–photo-sensitive groups XP-C, XP-E, and XP-V (Tables 1 and 2) with the number of skin cancers, it is evident that patients in the latter two groups generally have many skin cancers, but relatively low ocular scores. As discussed above, the high incidence of skin cancers is a consequence of late diagnosis in these patients because of the later development of skin signs/symptoms. Despite the lack of protection from UVR, ocular scores are relatively low in these two groups. In contrast, many of the XP-C patients have very high ocular scores despite having had few or no skin cancers. For example, XP73BR and XP94BR, both aged 10, have been well protected and have not developed any skin cancers, yet they have ocular scores of 6 and 7, respectively. In contrast, XP variant, XP63BR, aged 55, has had more than 35 skin cancers removed, yet his ocular score is just 1.
Table S2.
Ocular disease features
| Clinical features | Total | Mild XP-A | % | XP-A | % | XP-B | % | XP-C | % | XP-D | % | XP-E | % | XP-F | % | XP-G | % | XP-V | % | |
| Total cases reported | 89 | 10 | 11 | 8 | 9 | 2 | 2 | 28 | 31 | 14 | 16 | 4 | 4 | 3 | 3 | 8 | 9 | 12 | 13 | |
| Median age as of December 2014 | 35 | 17 | 45 | 14 | 24 | 56 | 23 | 19 | 55 | |||||||||||
| Average ocular score (maximum 12) | 2 | 3 | 4 | 4 | 2 | 3 | 1 | 3 | 2 | |||||||||||
| Maximum ocular score in group | 4 | 6 | 4 | 11 | 4 | 4 | 3 | 4 | 5 | |||||||||||
| Photophobia | 3 | 30 | 6 | 75 | 0 | 0 | 20 | 71 | 12 | 86 | 3 | 75 | 2 | 67 | 3 | 38 | 9 | 75 | ||
| Interpalpebral conjunctival melanosis | 8 | 80 | 5 | 63 | 0 | 0 | 21 | 75 | 1 | 7 | 0 | 0 | 0 | 0 | 2 | 25 | 2 | 17 | ||
| Interpalpebral conjunctival melanosis with fair skin | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 13 | 1 | 8 | ||
| Conjunctival injection | 0 | 0 | 5 | 63 | 1 | 50 | 20 | 71 | 2 | 14 | 3 | 75 | 0 | 0 | 4 | 50 | 3 | 25 | ||
| Conjunctival corkscrew vessels | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 57 | 0 | 0 | 1 | 25 | 0 | 0 | 1 | 13 | 2 | 17 | ||
| Pterygium | 6 | 60 | 3 | 38 | 1 | 50 | 14 | 50 | 7 | 50 | 3 | 75 | 0 | 0 | 1 | 13 | 1 | 8 | ||
| Pingueculae < age 50 y | 2 | 20 | 0 | 0 | 0 | 0 | 9 | 32 | 3 | 21 | 0 | 0 | 0 | 0 | 2 | 25 | 5 | 42 | ||
| Lagophthalmos/ectropion | 1 | 10 | 1 | 13 | 0 | 0 | 6 | 21 | 3 | 21 | 0 | 0 | 1 | 33 | 1 | 13 | 2 | 17 | ||
| Keratopathy (corneal staining) | 1 | 10 | 1 | 13 | 2 | 100 | 9 | 32 | 2 | 14 | 0 | 0 | 1 | 33 | 2 | 25 | 1 | 8 | ||
| Corneal scarring/neovascularization | 0 | 0 | 1 | 13 | 0 | 0 | 8 | 29 | 0 | 0 | 2 | 50 | 0 | 0 | 2 | 25 | 2 | 17 | ||
| Ocular surface cancer | 0 | 0 | 3 | 38 | 1 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Table 2.
XP-D, XP-E, XP-F, XP-G, and XP-V patients
| XP ID no | Group | Age (y) | Age XP diagnosis (y) | Ethnicity | UDS* | Allele 1 | Protein change | Allele 2† | Protein change | SSS | Age first cancer (y) | Type first cancer | No. MM | No. NMSC | Ocular severity score (maxiumum 12) | Neuro severity score (maximum 8) |
| XP109BR | D | 4 | 1 | Caucasian | 32 | c.2047C > T | p.Arg683Trp | c.1381C > G; c.2150C > G | p.Leu461Val; p.Ala717Gly | 3 | — | 0 | 0 | 1 | 0 | |
| XP87BR | D | 8 | 2 | Caucasian | 23 | c.2047C > T | p.Arg683Trp | c.1867dupG | p.Val623fs | 2 | — | 0 | 0 | 3 | 4 | |
| XP70BR | D | 14 | 5 | Caucasian | 10 | c.2047C > T | p.Arg683Trp | c.816–2A > G | 3 | 10 | SCC | 0 | 1 | 4 | 6 | |
| XP16BR24 | D | 21 | 1 | Caucasian | 16 | c.2047C > T | p.Arg683Trp | c.1847G > A | p.Arg616Pro | 3 | 14 | BCC | 0 | 1 | 1 | 5 |
| XP71BR | D | 22 | 14 | Caucasian | 19 | c.2047C > T | p.Arg683Trp | c.816–2A > G | 2 | 10 | BCC | 0 | 4 | 1 | 5 | |
| XP17BR | D | 23 | 2 | Caucasian | 16 | c.2047C > T | p.Arg683Trp | c.1847G > A | p.Arg616Pro | 3 | 21 | BCC | 0 | 2 | 1 | 5 |
| XPJCLO | D | 26 | 1 | Caucasian | 19 | c.2047C > T | p.Arg683Trp | c.1933_1934delCA | p.Gln645fs | 2 | — | 0 | 0 | 1 | 8 | |
| XP135LO-S | D | 34 | 5 | Caucasian | 25 | c.2047C > T | p.Arg683Trp | 3 | 13 | BCC | 0 | 22 | 3 | 5 | ||
| XP135LO | D | 35 | 8 | Caucasian | 25 | c.2047C > T | p.Arg683Trp | 3 | 12 | BCC | 1 | 50 | 3 | 5 | ||
| XP104LO | D | 47 | 3 | Caucasian | 13 | c.2047G > A | p.Arg683Trp | c.718+1C > A | 3 | 23 | BCC | 0 | 4 | 1 | 7 | |
| XP59BR | D | 53 | 9 | Caucasian | 24 | c.2047C > T | p.Arg683Trp | c.1381C > G; c.2150C > G | p.Leu461Val; p.Ala717Gly | 2 | 36 | BCC | 5 | 23 | 4 | 3 |
| XP67BR | D | 65 | 55 | Caucasian | 75 | c.1532G > A | p.Arg511Gln | c.1381C > G; c.2150C > G | p.Leu461Val; p.Ala717Gly | 2 | 27 | BCC | 0 | >40 | 3 | 2 |
| XP30BR | D | 21 | 4 | Mixed | 36 | c.2048G > A | p.Arg683Gln | c.1827delC | p.Phe610fs | 3 | — | 0 | 0 | 1 | 0 | |
| XP97BR | D | 34 | 29 | Haan Chinese | 53 | c.2048G > A | p.Arg683Gln | c.1378–26_1383del32 | 3 | — | 0 | 0 | 3 | 0 | ||
| XP115BR | E | 30 | 29 | Pakistani | 63 | c.1149delG | p.Met383fs | 1 | — | 0 | 0 | 0 | 0 | |||
| XP105BR | E | 49 | 46 | Caucasian | 75 | c.1070C > T | p.Pro357Leu | c.716G > T | p.Arg239Ile | 1 | 15 | MM | 35 | >120 | 4 | 0 |
| XP98BR | E | 62 | 48 | Caucasian | 68 | c.161G > A | p.Trp54X | 1 | 15 | BCC | 2 | >200 | 4 | 0 | ||
| XP100BR | E | 62 | 59 | Caucasian | 53 | c.457–2A > C | Splice | 0 | 9 | BCC | 0 | >100 | 4 | 0 | ||
| XP72BR | F | 18 | 9 | Caucasian | 36 | c.1135C > T | p.Pro379Ser | 3 | — | 0 | 0 | 0 | 0 | |||
| XP32BR37 | F | 23 | 7 | Caucasian | 16 | c.1135C > T | p.Pro379Ser | c.1765C > T | p.Arg589Trp | 3 | — | 0 | 0 | 1 | 0 | |
| XP24BR37 | F | 48 | 30 | Caucasian | 4 | c.1765C > T | p.Arg589Trp | c.2395C > T | p.Arg799Trp | 2 | — | 0 | 0 | 3 | 6 | |
| XP104BR | G | 6 | 2 | Pakistani | 0 | c.136delC | p.His46fs | 3 | — | 0 | 0 | 3 | 5 | |||
| XP118BR | G | 10 | 9 | Caucasian | 3 | c.2383G > A | p.(Ala795Thr) | c.1842delT | p.(Leu615fs) | 3 | — | 0 | 0 | 1 | 0 | |
| XP55BR | G | 15 | 4 | Somali | 1 | c.264+1delG | Splice | 1 | — | 0 | 0 | 4 | 4 | |||
| XP56BR | G | 18 | 7 | Somali | 1 | c.264+1delG | Splice | 0 | — | 0 | 0 | 4 | 4 | |||
| XP34BR | G | 20 | 2 | Caucasian | 3 | c.2453C > T | p.Ala818Val | c.2586_2587delTA | p.Thr863fs | 3 | — | 0 | 0 | 4 | 4 | |
| XP120BR | G | 36 | 36 | Caucasian | ND | c.2453C > T | p.Ala818Val | c.1753G > T | p.Glu585TER | 3 | — | 0 | 0 | 1 | 4 | |
| XP119BR | G | 38 | 38 | Caucasian | ND | c.2453C > T | p.Ala818Val | c.1753G > T | p.Glu585TER | 3 | 20 | BCC | 0 | 1 | 3 | 4 |
| XP101BR | G | 68 | 64 | Caucasian | 14 | c.869T > A | p.Ile290Asn | 2 | — | 0 | 0 | 2 | 6 | |||
| XP1NO | V | 20 | 16 | Caucasian | 144 | c.149dupT | p.Ser51fs | 0 | 16 | SCC | 0 | 5 | 1 | 0 | ||
| XP5B1 | V | 25 | 10 | Caucasian | 111 | c.364A > C | p.Thr122Pro | c.1222_1225delACTT | p.Thr408fs | 0 | — | 0 | 0 | 1 | 0 | |
| XP1CH | V | 26 | 23 | Caucasian | 99 | c.738delC | p.Phe247fs | c.1066C > T | p.Arg356X | 0 | 22 | MIS | 1 | 0 | 2 | 0 |
| XP110BR | V | 27 | 24 | Caucasian | 87 | c.738delC | p.Phe247fs | c.1066C > T | p.Arg356X | 0 | 23 | MM | 3 | 0 | 3 | 0 |
| XP136LO | V | 54 | 53 | Caucasian | 91 | c.25G > T >G | p.Val9Phe | c.490+3A | Splice | 0 | 42 | BCC | 12 | 11 | 0 | 0 |
| XP63BR | V | 55 | 45 | Caucasian | 130 | c.225_227delTCT | p.Leu77del | c.207delG | p.Lys70fs | 0 | 38 | BCC | 15 | >20 | 1 | 1 |
| XP64BR | V | 55 | 45 | Mixed | 144 | c.437dupA | p.Tyr146X | 0 | — | 0 | 0 | 3 | 0 | |||
| XP115LO25 | V | 62 | 24 | Middle Eastern | 100 | c.1117C > T | p.Gln373X | 0 | 21 | SCC | 17 | 16 | 5 | 0 | ||
| XP85BR | V | 62 | 56 | Caucasian | 90 | c.790G > C | p.Ala264Pro | c.490+3A > G | Splice | 0 | 44 | BCC | 0 | 120 | 3 | 0 |
| XP36BR | V | 67 | 44 | Caucasian | 99 | c.332G > A | p.Arg111His | c.1222_1225delACTT | p.Thr408fs | 0 | 33 | MM | 10 | >10 | 4 | 0 |
| XP36BR-S | V | 71 | 48 | Caucasian | 99 | c.332G > A | p.Arg111His | c.1222_1225delACTT | p.Thr408fs | 0 | 44 | MM | 13 | 21 | 1 | 0 |
| XP6DU | V | 75 | 72 | Caucasian | 100 | c.225_227delTCT | p.Leu77del | c.207delG | p.Lys70fs | 1 | 39 | MM | >8 | >30 | 2 | 0 |
UDS is measured, after a UVC dose of 10 J⋅m−2, as percent of that in normal cells used as control in the same experiment. Other abbreviations as in Table 1.
Where no second allele is indicated, the mutation is homozygous.
Although none of the XP-C patients has the progressive neurodegeneration found in other XP complementation groups, we observed intracranial lesions in 4 of 28 patients after routine baseline MRI scans. XP21BR developed a glioblastoma multiforme at age 38 y, which led to death less than a year later. XP28BR had a dysembryonic neuroepithelial tumor. His younger brother XP39BR had an MRI scan at age 16 y to investigate for early morning headaches. This scan showed a right temporal lobe cyst with encroachment on the orbit; he is under neurosurgical follow-up. Another patient, XP1SH, was noted on MRI to have an intraventricular mass with features most in keeping with a benign subependymoma. There have been other reports in the literature associating XP-C with the development of intracranial lesions (21).
Three of our XP-C patients (XP95BR, XP107BR, and XP29BR) fall into a distinct subgroup (bottom of Table 1) with a later presentation of cutaneous features and significantly lower ocular disease scores with respect to age. XP107BR, a Spaniard, first attended the clinic at age 33 y, with an unusual distribution of lentigines limited to his upper back and scalp but sparing his face and forearms, despite excess UVR exposure in his youth (Fig. 1C). He developed his first melanoma at age 28 y on his scalp (despite a thick head of hair) and has subsequently developed three further melanomas at this same site (Fig. 1D). His elder brother also had several malignant melanomas on his scalp, one of which metastasized, leading to his death. UDS in his skin fibroblasts was ∼40% of normal after a UVR dose of 10 Jm−2 (Fig. 1E), and mutation analysis revealed a homozygous missense p.Tyr585Cys mutation in XPC in both brothers. The UDS level suggests that the XPC protein was partially functional. Tyr585 is conserved in all orthologs of XPC including yeast Rad4 (Fig. 1F). It is located in the inside of the protein (Fig. 1G) and is not directly involved in binding DNA (22). It is likely that this mutation destabilizes the protein, resulting in partial loss of function. The reason why this might result in these very specific clinical features remains obscure.
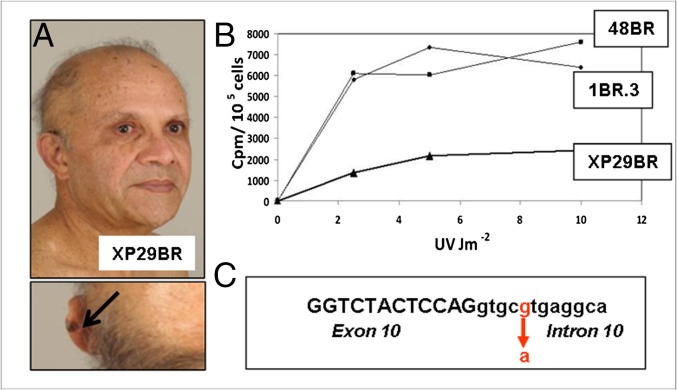
Another patient, XP29BR (Fig. 2A), also has unusually mild cutaneous features. He was not diagnosed until the age of 45 y, when he presented with exaggerated pigmentary changes and multiple dysplastic naevi, but no skin cancers. Fibroblasts showed about 33% UDS (Fig. 2B) and rather mild hypersensitivity to UVR-induced cell killing. Some 15 y later (Fig. 2A), he developed a few skin cancers including a malignant melanoma on his ear, which was completely excised. Molecular analysis of his genomic DNA revealed a homozygous mutation, c.2033+5G > A (intron 10). This mutation in the splice donor site of intron 10 (Fig. 2C) resulted in more or less equal amounts of two major abnormally spliced products in the mRNA, neither of which would be expected to generate functional protein. As the mutation confers only a modest change on the splice donor site, we suggest that the splicing mutation may, as with the milder XP-A patients described above, permit the read-through of a small amount of normal mRNA, which is sufficient to alleviate the clinical features, although we do not have direct evidence to support this suggestion. XP95BR has the identical mutation to XP29BR. She was diagnosed aged 20 y, but she has not adhered to strict UVR protection. In the last 6 y, she has had nine malignant melanomas excised. Her presentation may be more severe than that of XP29BR partly due to her ethnicity; she is white, whereas XP29BR is of Indian origin, or perhaps due to comparatively higher childhood UVR exposure, or there may be other factors within her genetic background that increased her susceptibility to pigmentary changes and skin cancer.
Fig. 2.
XP29BR. (A) Patient XP29BR, with a melanoma on his left ear (arrow). (B) UDS, measured as incorporated 3H-thymidine cpm per 105 cells following UV irradiation of XP29BR with different UVC doses compared with normal fibroblasts 1BR.3 and 48BR. (C) Sequence around the exon 10/intron 10 boundary showing the position of the mutation.
XP-D (MIM278730).
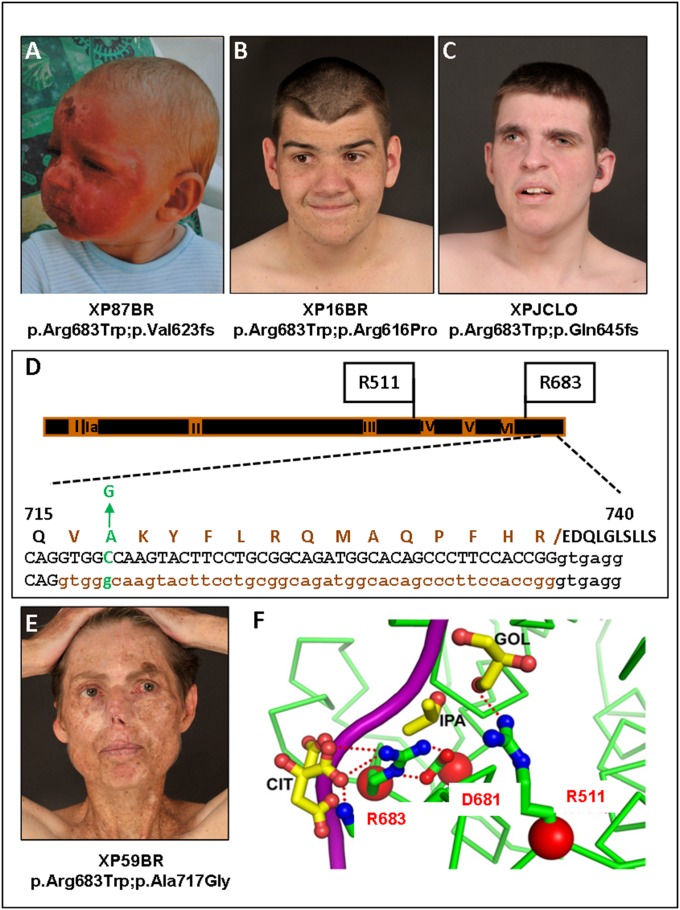
Fourteen XP-D patients have attended the clinic (Table 2). Most presented initially with severe prolonged sunburn on minimal sun exposure (Fig. 3A) in their first year of life. Several patients have been extremely well UVR protected from a very young age and have developed minimal cutaneous features even by the age of 25 y (Fig. 3 B and C). Eleven of 14 patients have severe neurodegeneration (as reflected by increasing neurological scores with advancing age).
Fig. 3.
XP-D patients. (A) Severe sunburn after minimal sun exposure in XP87BR before XP diagnosis (see ref. 5 for another photograph of this patient). (B) XP16BR and (C) XPJCLO showing minimal pigmentary skin changes due to early diagnosis and excellent UVR protection. (D) Scheme of XPD protein, showing the seven helicase domains, I, Ia, II–VI, and the positions of the mutations in the XPD patients. Below is an expanded view of aa715–740 showing the p.Ala717Gly alteration and the deletion of aa716-730 resulting from the c.C2150 > G mutation. Top line: WT protein sequence with p.Ala717Gly indicated in green and the deleted 15 aa resulting from abnormal splicing of intron 22 indicated in brown; middle and bottom lines: WT and mutant DNA with exon 22 sequence in caps, intron 22 in lowercase, with abnormally spliced-out bases in brown. (E) XP59BR, showing multiple surgical scars as a result of poor UVR protection (see ref. 5 for another photograph of this patient). (F) Crystal structure of XPD around Arg683, showing projected hydrogen bonding with Asp681 and DNA (magenta). (Modified from ref. 33.)
All but one of the XP-D patients (XP67BR) is mutated at Arg683 in one allele (Fig. 3D). Strikingly, in the cohort of 11 patients in which Arg683 is replaced with tryptophan (Trp), there is a progression of neurological disease in all patients except for XP59BR, who showed no significant neurological abnormalities before the age of 50 y. XP59BR, although having minimal neurological abnormalities, has not followed UVR protection advice and has subsequently developed many skin cancers as an adult (Fig. 3E).
The differences in progression of neurological disease between XP-D patients with compound heterozygous mutations, including p.Arg683Trp on one allele, have been discussed in the literature. They could be mediated by the other allele, as suggested by Ueda et al. (23), or there could be a more complex effect of the genetic background. XP109BR, aged 4 y, has the same genotype as XP59BR. It will be interesting to observe if he will show a similar neurological phenotype. In several cases, the second allele (e.g., Arg616Pro) has either been demonstrated to be (24), or is by its nature, nonfunctional.
Investigation of the clinical records of XP59BR revealed that the fibroblast culture designated XP102LO (25), whose cells have been widely used in XPD research, is derived from an earlier biopsy from the same patient. She has the common p.Arg683Trp alteration in one allele, and the second allele has two alterations previously reported as a combination in the same allele (24, 26–28), namely p.Leu461Val, a very conservative change, and an in-frame deletion of aa716–730 (Fig. 3D), resulting from splicing-out of the last 45 bases of exon 22. This deletion is caused by the mutation c.2150C > G, generating a new splice donor site (Fig. 3D), and the resulting protein with these two alterations is nonfunctional (24, 29). However, as with other examples discussed above, it is possible that there is a small amount of read-through of the normal splice site, generating full-length XPD protein with p.Ala717Gly resulting from the c.2150C > G mutation. This may have residual activity and protect from neurological problems. Very recently published data suggest that this is indeed likely to be the case (30). Two other XP-D patients expressing this allele had relatively mild neurological features. A small amount of full-length mRNA with the c.2150C > G mutation (p.Ala717Gly) was detected (30).
The ocular problems of the XP-D patients are less severe than those of the XP-C group (Tables 1 and 2). Even XP59BR, despite her poor protection and multiple skin cancers, has an ocular score of just 4, compared with scores of 5–9 in our older XP-C patients.
Two XP-D patients, XP30BR and XP97BR (bottom two XP-D rows in Table 2), in whom Arg683 is mutated to glutamine rather than tryptophan, have no neurological abnormalities at ages 21 and 34 y, respectively. There are several reports in the literature of patients with the p.Arg683Gln mutation (31). In most patients described in detail in these reports, neurological abnormalities were either absent or mild and late onset. These milder features may reflect the fact that glutamine, like arginine, is hydrophilic, whereas tryptophan is hydrophobic and is likely to have a greater disruptive effect on the structure of the XPD protein (32). In particular, Arg683 forms a hydrogen bond with Asp681 and interacts with DNA (Fig. 3F) (33). These interactions are maintained to some degree if Arg683 is mutated to glutamine but not if arginine is replaced with tryptophan (32).
XP-E (MIM278740).
We have seen four XP-E patients (Table 2). Three are of white origin and middle aged (49–62 y). In keeping with other XP-E patients described in the literature, their UDS level is about 50% of normal. None suffer severe sunburn on minimal sun exposure. XP100BR, aged 62 y, was diagnosed clinically with XP at the age of 9 y in Zimbabwe, when she developed her first basal cell carcinoma (BCC). She spent the rest of her life in the United Kingdom with reasonable UVR protection. She has had hundreds of BCCs removed from her head and neck but has not developed any squamous cell carcinomas (SCCs) or melanomas. XP105BR has lived all her life in South Africa; she developed her first skin cancer [malignant melanoma (MM)] at age 15 y and has since had more than 30 further MMs and more than 120 nonmelanoma skin cancers (BCCs and SCCs) removed. XP98BR is 62 y of age; cellular diagnosis was made at age 48 y, although clinical diagnosis was suspected much earlier. She developed her first skin cancer (BCC) at age 15 y and has subsequently developed hundreds of BCCs, MMs, and SCCs. These findings of multiple skin cancers are similar to those reported by Oh et al. (34) The fourth patient, XP115BR, age 30 y and of Pakistani origin, is minimally affected, with scattered lentigines on her face and hypopigmented macules on her forearms. She has not developed any skin cancers. On initial examination, XP was not expected, but her UDS was ∼50% of normal, and mutation analysis revealed a frameshift at Met383 in the 427 aa XPE/DDB2 protein. This mutation is more C-terminal than any of those previously reported (34), but nevertheless, it is expected to disrupt the protein structure (35) and therefore abolish function. Indeed, missense changes or single nucleotide deletions in the C-terminal region of XPE/DDB2 have been shown to drastically reduce the cellular protein levels (36), indicating the importance of this region for the correct structure of the XPE protein.
As expected from the literature, none of the XP-E patients has any significant neurological abnormalities. Interestingly, if we compare the ocular and skin lesions, the XP-C cohort has smaller numbers of skin cancers but quite severe ocular problems. In contrast, in the XP-E cohort, there are many more skin cancers, but the ocular lesions are significantly less severe than in the XP-C cohort.
XP-F (MIM278760).
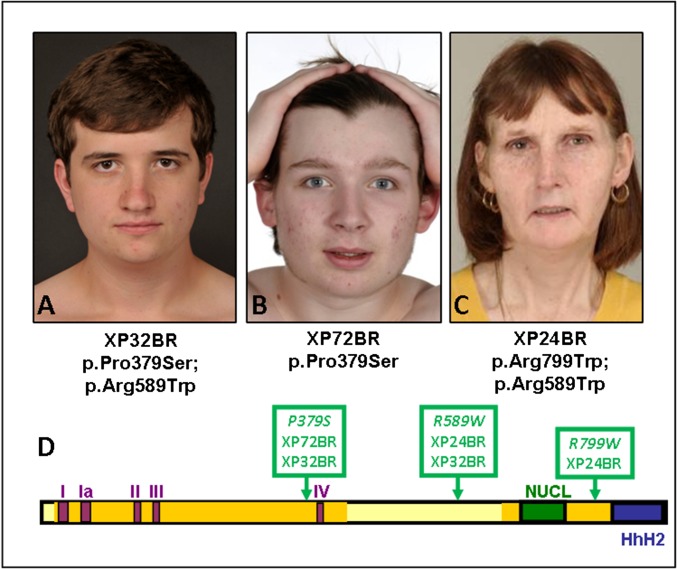
Three XP-F patients (Table 2) have attended the clinic, and all have severe sunburn on minimal sun exposure. XP32BR was diagnosed at age 7 y because of severe sunburn reactions from infancy. At age 23 y, he is surprisingly free of any cutaneous features of XP, despite his pale complexion (Fig. 4A) and low UDS level (18% of normal). He has no neurological abnormalities. He is a compound heterozygote for two missense mutations, p.Pro379Ser and p.Arg589Trp (Fig. 4D), and the properties of his cells in response to UVR have been described (37). XP72BR had several episodes of severe sunburn as a child and was diagnosed at age 9 y. At age 18 y, he has remarkably few lentigines (Fig. 4B), and his UDS level is about 35% of normal. He is homozygous for p.Pro379Ser, the same site mutated in one allele of XP32BR. Neither of these patients has any neurological or ocular abnormalities. Remarkably, the Pro379Ser mutation is listed in the SNP database with an allele frequency of 0.3%. If this is indeed the case, the frequency of individuals homozygous for this mutation would be ∼10 per million, roughly five times the incidence of known XP cases in the United Kingdom (1). This estimate implies there might be a significant cohort of UVR-sensitive individuals not recognized as having XP, but homozygous for this mutation.
Fig. 4.
XP-F patients. (A) XP32BR. (B) XP72BR. (C) XP24BR. Note the very mild skin changes despite only moderate UVR protection. (D) Scheme of XPF protein, showing the five motifs of the disrupted helicase domains (I, Ia, II, III, and IV), the nuclease domain (NUCL), and the two Helix-hairpin-helix (HhH) domains.
XP24BR, age 48 y, has had severe sunburn reactions from early infancy. She uses only moderate UVR protection, but despite barely detectable UDS, her skin features are fairly mild, with only a few lentigines on her face and shoulders, within the normal range of her skin type. She has no significant ocular disease. She is compound heterozygote for two missense mutations described in several other XP-F patients (Fig. 4D), and her cellular features have been described (37). The most prominent clinical features, which have developed only in the last few years, are peripheral sensory neuropathy, progressive cerebellar ataxia, dysarthria, sensorineural hearing loss, and progressive cognitive decline, as well as sunken eyes and loss of subcutaneous fat. These features may all be characteristic of late-onset Cockayne syndrome (CS) (Fig. 4C). We recently described three early-onset CS patients with mutations in XPF or its partner protein ERCC1 (38).
It is remarkable that, despite only moderate UVR protection, none of the XP-F patients has developed any visible actinic damage or skin cancers.
XP-G (MIM278780).
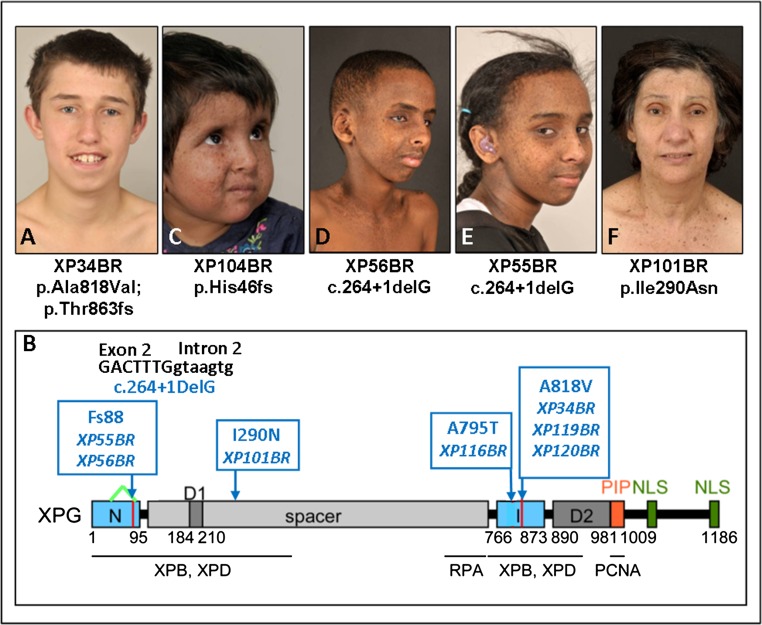
XP-G patients described in the literature span a wide range of clinical phenotypes from mild XP to severe combined XP-CS phenotype. Of the seven XP-G patients that we have seen (Table 2), six fit into this range. XP34BR developed severe sunburn on minimal sun exposure at age 4 wk and has since been adequately UVR protected (Fig. 5A). Because of his UVR protection, he has minimal skin changes. He has moderate learning difficulties. Another pair of siblings of Celtic origin, XP119BR and XP120BR, age 36 and 38 y, respectively, have only recently been diagnosed, although they have suffered from severe sunburn reactions on minimal sun exposure since birth, and they developed exposed-site lentigines from 2 y of age. [Interestingly, XP119BR is the only XP-G patient who has developed a BCC (on her face at age 20 y).] They were only referred for diagnosis in their mid-30s, when both began to develop neurological problems, (more pronounced in the younger brother). The clinical features of these three patients are consistent with molecular analysis. All have a frameshift or truncation mutation in one allele and the missense mutation p.Ala818Val in the other allele (Table 2). The XPG nuclease contains two domains (N and I) required for nuclease activity, separated by a large spacer domain (Fig. 5B), whose function is unclear, although it is required for interaction with transcription factor IIH (TFIIH) (39). Ala-818 is in the nuclease I domain and mutation at this site is likely to inactivate nuclease activity of the protein without affecting its overall structure. A similar mutation 26 aa away, p.Ala792Val, also resulted in a mild phenotype in XP124LO and 125LO (39, 40).
Fig. 5.
XP-G patients. (A) XP34BR. (B) Structure of the XPG protein (39), showing the N and I domains required for nuclease activity, the spacer region, nuclear localization signals (NLS), and binding sites for other proteins. Sites of the mutations in the patients are indicated in blue, and the deletion of aa16-88 in XP55BR and XP56BR is indicated by a green inverted V. (C) XP104BR. (D) XP56BR. (E) XP55BR. (F) XP101BR.
XP104BR is a severely affected 6-y-old child with severe sunburn on minimal sun exposure observed at age 2 wk. She has extensive freckling (Fig. 5C) and gross developmental delay. Her UDS level was undetectable, consistent with a homozygous frameshift mutation at His46.
XP55BR and XP56BR are teenage siblings of Somali origin (Fig. 5 D and E). They have lentigines at exposed sites but have been well protected from UVR exposure. Both siblings appear cachectic with sunken eyes and thin long limbs. Neurological examination revealed evidence of cognitive impairment, peripheral neuropathy, and cerebellar dysfunction. They developed sensorineural hearing loss in early childhood. MRI brain scans in both siblings revealed evidence of bilateral globus pallidus and posterior periventricular white matter calcification. These features are typical of CS. These siblings are homozygous for a −1-bp deletion at the boundary of exon 2 and intron 2, resulting in a frameshift mutation at aa 88 and abnormal splicing of exon 3 (Fig. 5B). Despite the similarity in the mutation to XP104BR and other severely affected XP-CS patients described in the literature (41) (homozygous frameshift or nonsense mutation close to the N terminus, presumably resulting in no functional protein, no detectable UDS), these siblings have much less severe neurological abnormalities than would be expected from patients with apparently similar truncation mutations (41–43). Sequencing of the cDNA reveals an in-frame 216-nucleotide deletion from G48 in the middle of exon 1 to G263 at the end of exon 2 (Fig. 5B) as a minor product. The encoded protein would be completely missing aa16–88, containing most of the N domain, consistent with the undetectable level of UDS. However, the rest of the 1,186 aa protein would be intact and this might account for the clinical features being less severe than anticipated from a frameshift so close to the N terminus.
The most unusual XP-G patient is XP101BR (Fig. 5F), a 68-y-old woman of Greek Cypriot parentage. She has severe sunburn on minimal sun exposure and rarely ventures outdoors. Her skin changes are relatively mild with minimal exposed-site lentigines and she has not developed any skin cancers. Neurologically, she had normal developmental milestones, living a fully active and independent life for more than 50 y. However, over the last 10 y, she has shown cognitive decline and behavioral changes similar to those seen in Alzheimer’s patients, as well as impaired balance and sensorineural hearing loss. Three of her six siblings in Cyprus are reported to have similar cognitive changes in association with hypersensitivity to sunlight, whereas the other three are completely normal, suggesting linkage with XP. The parents were not known to be related, but originated from the same village in Cyprus, consistent with the patient’s homozygosity for a missense mutation in XPG. p.Ile290Asn is located in the spacer region of the protein, between the two nuclease domains (Fig. 5B). One affected sister of XP101BR is confirmed to be homozygous, whereas an unaffected brother is heterozygous for the same mutation. Although little is understood about the detailed function of the spacer region, Ile290 is conserved in mammals, fish, frog, and chicken and is in the region thought to be involved in interaction with TFIIH (44).
XP-V (MIM278750).
These individuals have normal sunburn reactions for skin type and have no neurological abnormalities. Typically they are not diagnosed until their second or third decade at the earliest. At this point, they have accumulated years of UVR-induced mutations, leading to development of multiple skin malignancies at a later age (Table 2). As with the XP-E patients, XP-V patients develop hundreds of skin cancers, but ocular disease is relatively mild compared with that found in XP-C patients. XP-V patients display a range of mutations in the POLH gene (Table 2), both missense and truncations, but the cellular and clinical phenotypes are quite similar in most cases.
SI Methods
Clinical Methods.
Phenotypic variables collected for each XP patient included data on ethnicity (assigned using the Office for National Statistics breakdown of Ethnic Categories for classification from the 2001 census) and age of molecular diagnosis of XP (either from UDS measurement or mutation identification).
Data were collected for dermatology, ophthalmology, and neurology. Dermatological data included information on age of development of first skin cancer, type of skin cancer [BCC, SCC, or MM/melanoma in situ (MIS)], presence of lentigines at exposed sites, and presence of hypopigmented macules.
Disease Severity Scoring Systems.
We previously described an SSS to differentiate between sunburn reactions on minimal sun exposure (5). We subsequently developed two scoring systems for severity of ocular and neurological disease.
Ophthalmology severity score (maximum 12 points).
Points were allocated for:
Photophobia (1 point)
Interpalpebral conjunctival melanosis (2 points awarded if patient has fair skin)
Conjunctival injection (1 point)
Conjunctival corkscrew vessels (1 point)
Pterygia (1 point)
Pingueculae occurring under age 50 y (1 point)
Lagophthalmos/ectropion (1 point)
Keratopathy (1 point)
Corneal scarring/neovascularisation (1 point)
Ocular surface cancer (2 points)
Note that (i) photophobia, a common recognizable feature in XP patients, is a symptom, and the others are all signs; and (ii) whereas racial pigmentation is very common in Afro-Caribbean individuals (95%), it is less common in Asians (30%) and rare in whites (5%), where conjunctival pigmentation/melanosis can frequently be dysplastic. The scoring has therefore been slightly weighted to reflect this.
Neurology severity score (maximum 8 points).
Points were allocated for:
Peripheral (motor or sensory) neuropathy (1 point)
Cerebellar signs (1 point)
Sensorineural hearing loss (1 point)
Impaired cognition (1 point)
Dysphagia/percutaneous, endoscopic gastrostomy feeding (1 point)
Abnormal pupillary response/abnormal eye movements (1 point)
Assisted gait (1 point) (or wheelchair-bound 2 points)
Note that (i) this severity score is based on the neurological systems affected in XP; and (ii) a functional score to reflect baseline mobility is also included.
Mutation Analysis.
Mutations in cDNA were analyzed from cellular RNA using RT-PCR followed by Sanger sequencing. All mutations were confirmed using Sanger sequencing of genomic DNA from blood. In later experiments, mutations were analyzed in genomic DNA by massively parallel Illumina sequencing, using a platform containing the coding exons and splice sites (−30/+20 bp) of 14 NER-related genes (XPA, XPB-ERCC3, XPC, XPD-ERCC2, XPE-DDB2, XPF-ERCC4, XPG-ERCC5, XPV-POLH, ERCC1, DDB1, TTDA, CSA-ERCC8, CSB-ERCC6, and UVSSA1).
Discussion
The literature on XP suggests that there are gradations in severity of the clinical features, dependent on the complementation group. Patients in groups XP-A, -D, and -G are considered to be the most severe (12), with early-onset neurological degeneration and abnormally severe sunburn reactions, whereas XP-C is considered to be intermediate, and XP-E and variant are the least severely affected. Although in some respects this is correct, especially in conditions in which UVR protection and early diagnosis is difficult, we found that early diagnosis and rigorous UVR protection can have profound effects on clinical features. For example, most of our cohort of XP-D patients with very early sunburn episodes were diagnosed in the first year of their lives and have therefore exercised rigorous UVR protection. As a consequence, subsequent skin damage in these individuals is barely detectable. In marked contrast, the groups conventionally described as milder (XP-E and variant) generally are not diagnosed until they are adults, due to subtlety and later onset of skin alterations. During this undiagnosed period, they accumulate large quantities of precarcinogenic lesions, and as a consequence, they may develop hundreds of skin cancers, even if they are well protected after they have been diagnosed. However, there is an additional management problem; whereas it is relatively easy to persuade parents of a child who has an extreme sunburn reaction to protect their child from all UVR exposure, it is much more difficult to persuade an adult, who has had a hitherto normal lifestyle, to instigate rigorous UVR protection.
A striking aspect of our analysis of the UK cohort of more than 80 XP patients has been the unanticipated heterogeneity of clinical features, not only between, but also within, complementation groups. We highlighted here and elsewhere a cohort of mild XP-A patients originating from the Indian subcontinent, in which the mutation causes aberrant splicing, but we can attribute the mild phenotype to a low level of read-through from the normal splice site (16). Likewise the absence of neurological abnormalities in XP-D patient XP59BR with a splicing mutation most likely results from a small amount of read-through from the normal splice site. We also discussed a late-onset XP-C patient, XP29BR, and XP-G siblings (XP55BR and XP56BR) with milder than expected phenotypes. In all these cases, the mutation generates aberrant splice products, and we speculated that small amounts of either normal read-through, or of splicing that results in in-frame products, might alleviate the anticipated phenotype. These findings highlight the importance of sequencing both genomic DNA and cDNA and of carrying out functional DNA repair assays to obtain accurate genotype-phenotype relationships.
A remarkable and unexpected finding is the specific propensity to ocular surface problems in the XP-C group compared with XP-E and XP-V patients, who have many more skin cancers. Very little is known about the etiology of these ocular surface problems, and there are no studies on the control of cell cycle, DNA replication, or response to DNA damage in ocular surface cells. One possible explanation for the lack of ocular damage in XP-V patients may lie in the different nature of the defect in this group, which is only manifest when cells replicate their DNA. It may be that dividing cells in the ocular surface proliferate more slowly than those in the skin. If this is the case, there would be more time before DNA replication for NER (which is unaffected in XP-V and only reduced to 50% of normal in XP-E) to repair DNA damage in the eye than in the skin. This hypothesis is entirely speculative and much more work needs to be done to understand the nature and origin of UVR-induced ocular surface problems.
XP-F is one of the rarer XP groups and only about 30 cases have been described in the literature (45, 46). All three of our patients have severe sunburn on minimal sun exposure, but they have minimal exposed-site lentigines and no skin cancers. In fact, very few XP-F patients have developed skin cancers [see table 3 in Gregg et al. (45), but note that contrary to what is indicated in this table, XP24BR and XP32BR have not had any skin cancers]. The few XP-F patients that have developed skin cancers did so as adults (47, 48), mostly above the age of 40 y (46, 48–50). Nevertheless it appears that, whereas XP-C patients have pigmentary changes and skin cancers without abnormally severe sunburn reactions, XP-F patients, in direct contrast, have severe sunburn reactions with only modest pigmentary changes and relatively few skin cancers. These observations provide further insight into the function of the XPF protein, and it will be of interest to determine in future studies whether XPF mutations do in some ways protect against the anticipated skin cancers.
Among our XP-G patients, as with XP-F, we observed neurological abnormalities of varying severity associated with acute sunburn reactions, but just one skin cancer and little evidence of premalignant skin lesions. A survey of the literature does indeed suggest that skin cancers are rare in this group (51). Many of the severely affected cases with XP-CS died at a very young age, before any cancers had time to develop. Three Japanese XP-G patients developed skin cancers, but only at ages 40, 54, and 32 y, respectively (52–54).
In summary, detailed clinical and molecular analysis, together with functional assay of a large cohort of XP patients by the same multidisciplinary team, has enabled us to provide improved clinical management of patients with this DNA repair disorder. Furthermore, insights gained from previously unreported genotype-phenotype relationships have enabled us in many cases to make prognostic predictions about the likelihood of the development of neurological abnormalities, which is a major concern to patients who control their skin problems by rigorous photo-protection.
The importance of early diagnosis of XP cannot be overemphasized, particularly for patients with normal sunburn reactions for skin type (XP-C, -E, and variant groups), in whom overt skin signs may not become apparent for several years. XP should be considered as a differential diagnosis in any patient who has unusually early or exposed-site patterns of freckling, so that UVR protection can be instigated as early as possible, to improve morbidity and mortality from skin cancer.
Methods
Systematic analysis of all patients’ notes was undertaken to assess more than 60 different genotypic and phenotypic variables. The clinical studies have been carried out in great detail; however, to simplify the data for the reader and to make objective comparisons between the severity of different phenotypic data, we used three severity scoring systems (described in SI Methods). For cellular analyses, UDS and UV+caffeine sensitivity for XP-V cells were carried out with cultured skin fibroblasts as described previously (11, 55). Mutations in cDNA and genomic DNA were analyzed as described in SI Methods. Informed consent was obtained from all patients, and this study was performed in accordance with protocols approved by the Research Ethics Committee of Guy’s and St Thomas’ Foundation Trust (reference 12/LO/0325).
Acknowledgments
We are grateful to all the patients and their families and to Gail Norbury and Tom Callup for help in establishing the laboratory service at Guy’s Hospital. The National XP Clinic in the United Kingdom is funded by the National Health Service England Highly Specialised Services. This work was also supported by Medical Research Council Fellowship Grant MR/M001210/1 (to M. Sethi), British Skin Foundation Innovative Project Award Grant 5042i (to H. Fassihi), the UK National Institute for Health Research Biomedical Research Centre based at Guy's and St Thomas' Foundation Trust and King's College London, and Associazione Italiana per la Ricerca sul Cancro Grant IG 13537 (to M. Stefanini).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519444113/-/DCSupplemental.
References
- 1.Kleijer WJ, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7(5):744–750. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Hirai Y, et al. Heterozygous individuals bearing a founder mutation in the XPA DNA repair gene comprise nearly 1% of the Japanese population. Mutat Res. 2006;601(1-2):171–178. doi: 10.1016/j.mrfmmm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Zghal M, et al. Xeroderma pigmentosum: Manifestations cutanées, oculaires et neurologiques à partir de 49 patients tunisiens. Tunis Med. 2005;83(12):760–763. [PubMed] [Google Scholar]
- 4.Bradford PT, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi M, et al. Patients with xeroderma pigmentosum complementation groups C, E and V do not have abnormal sunburn reactions. Br J Dermatol. 2013;169(6):1279–1287. doi: 10.1111/bjd.12523. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85(11):1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann AR, et al. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci USA. 1975;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masutani C, et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399(6737):700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285(5425):263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 10.Arlett CF, Harcourt SA, Broughton BC. The influence of caffeine on cell survival in excision-proficient and excision-deficient xeroderma pigmentosum and normal human cell strains following ultraviolet-light irradiation. Mutat Res. 1975;33(2-3):341–346. doi: 10.1016/0027-5107(75)90209-2. [DOI] [PubMed] [Google Scholar]
- 11.Broughton BC, et al. Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients. Proc Natl Acad Sci USA. 2002;99(2):815–820. doi: 10.1073/pnas.022473899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews AD, Barrett SF, Robbins JH. Xeroderma pigmentosum neurological abnormalities correlate with colony-forming ability after ultraviolet radiation. Proc Natl Acad Sci USA. 1978;75(4):1984–1988. doi: 10.1073/pnas.75.4.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriwaki S, Kraemer KH. Xeroderma pigmentosum: Bridging a gap between clinic and laboratory. Photodermatol Photoimmunol Photomed. 2001;17(2):47–54. doi: 10.1034/j.1600-0781.2001.017002047.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishigori C, et al. High prevalence of the point mutation in exon 6 of the xeroderma pigmentosum group A-complementing (XPAC) gene in xeroderma pigmentosum group A patients in Tunisia. Am J Hum Genet. 1993;53(5):1001–1006. [PMC free article] [PubMed] [Google Scholar]
- 15.Sidwell RU, et al. A novel mutation in the XPA gene associated with unusually mild clinical features in a patient who developed a spindle cell melanoma. Br J Dermatol. 2006;155(1):81–88. doi: 10.1111/j.1365-2133.2006.07272.x. [DOI] [PubMed] [Google Scholar]
- 16.Sethi M, et al. A distinct genotype of XP complementation group A: Surprisingly mild phenotype higly prevalent in Northern India/Pakistan/Afghanistan. J Invest Dermatol. December 29, 2015 doi: 10.1016/j.jid.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 17.States JC, McDuffie ER, Myrand SP, McDowell M, Cleaver JE. Distribution of mutations in the human xeroderma pigmentosum group A gene and their relationships to the functional regions of the DNA damage recognition protein. Hum Mutat. 1998;12(2):103–113. doi: 10.1002/(SICI)1098-1004(1998)12:2<103::AID-HUMU5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Oh KS, et al. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): Xeroderma pigmentosum without and with Cockayne syndrome. Hum Mutat. 2006;27(11):1092–1103. doi: 10.1002/humu.20392. [DOI] [PubMed] [Google Scholar]
- 19.Scott RJ, et al. Xeroderma pigmentosum-Cockayne syndrome complex in two patients: Absence of skin tumors despite severe deficiency of DNA excision repair. J Am Acad Dermatol. 1993;29(5 Pt 2):883–889. doi: 10.1016/0190-9622(93)70263-s. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen W, et al. Clinical heterogeneity within xeroderma pigmentosum associated with mutations in the DNA repair and transcription gene ERCC3. Am J Hum Genet. 1994;54(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- 21.DiGiovanna JJ, Patronas N, Katz D, Abangan D, Kraemer KH. Xeroderma pigmentosum: Spinal cord astrocytoma with 9-year survival after radiation and isotretinoin therapy. J Cutan Med Surg. 1998;2(3):153–158. doi: 10.1177/120347549800200308. [DOI] [PubMed] [Google Scholar]
- 22.Min JH, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449(7162):570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 23.Ueda T, Compe E, Catez P, Kraemer KH, Egly JM. Both XPD alleles contribute to the phenotype of compound heterozygote xeroderma pigmentosum patients. J Exp Med. 2009;206(13):3031–3046. doi: 10.1084/jem.20091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor EM, et al. Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc Natl Acad Sci USA. 1997;94(16):8658–8663. doi: 10.1073/pnas.94.16.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawsey SA, Magnus IA, Ramsay CA, Benson PF, Giannelli F. Clinical, genetic and DNA repair studies on a consecutive series of patients with xeroderma pigmentosum. Q J Med. 1979;48(190):179–210. [PubMed] [Google Scholar]
- 26.Takayama K, et al. Defects in the DNA repair and transcription gene ERCC2(XPD) in trichothiodystrophy. Am J Hum Genet. 1996;58(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- 27.Takayama K, et al. Defects in the DNA repair and transcription gene ERCC2 in the cancer-prone disorder xeroderma pigmentosum group D. Cancer Res. 1995;55(23):5656–5663. [PubMed] [Google Scholar]
- 28.Botta E, et al. Analysis of mutations in the XPD gene in Italian patients with trichothiodystrophy: Site of mutation correlates with repair deficiency, but gene dosage appears to determine clinical severity. Am J Hum Genet. 1998;63(4):1036–1048. doi: 10.1086/302063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubaele S, et al. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol Cell. 2003;11(6):1635–1646. doi: 10.1016/s1097-2765(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 30.Horibata K, et al. Constructive rescue of TFIIH instability by an alternative isoform of XPD derived from a mutated XPD allele in mild but not severe XP-D/CS. J Hum Genet. 2015;60(5):259–265. doi: 10.1038/jhg.2015.18. [DOI] [PubMed] [Google Scholar]
- 31.Nakano E, et al. Differences in clinical phenotype among patients with XP complementation group D: 3D structure and ATP-docking of XPD in silico. J Invest Dermatol. 2014;134(6):1775–1778. doi: 10.1038/jid.2014.14. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi T, Uchiyama M, Fukuro S, Tanaka K. Mutations in the XPD gene in xeroderma pigmentosum group D cell strains: Confirmation of genotype-phenotype correlation. Am J Med Genet. 2002;110(3):248–252. doi: 10.1002/ajmg.10465. [DOI] [PubMed] [Google Scholar]
- 33.Fan L, et al. XPD helicase structures and activities: Insights into the cancer and aging phenotypes from XPD mutations. Cell. 2008;133(5):789–800. doi: 10.1016/j.cell.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh KS, Emmert S, Tamura D, DiGiovanna JJ, Kraemer KH. Multiple skin cancers in adults with mutations in the XP-E (DDB2) DNA repair gene. J Invest Dermatol. 2011;131(3):785–788. doi: 10.1038/jid.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scrima A, et al. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell. 2008;135(7):1213–1223. doi: 10.1016/j.cell.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapić-Otrin V, et al. True XP group E patients have a defective UV-damaged DNA binding protein complex and mutations in DDB2 which reveal the functional domains of its p48 product. Hum Mol Genet. 2003;12(13):1507–1522. doi: 10.1093/hmg/ddg174. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad A, et al. Mislocalization of XPF-ERCC1 nuclease contributes to reduced DNA repair in XP-F patients. PLoS Genet. 2010;6(3):e1000871. doi: 10.1371/journal.pgen.1000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashiyama K, et al. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. Am J Hum Genet. 2013;92(5):807–819. doi: 10.1016/j.ajhg.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schärer OD. XPG: Its products and biological roles. Adv Exp Med Biol. 2008;637:83–92. doi: 10.1007/978-0-387-09599-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nouspikel T, Clarkson SG. Mutations that disable the DNA repair gene XPG in a xeroderma pigmentosum group G patient. Hum Mol Genet. 1994;3(6):963–967. doi: 10.1093/hmg/3.6.963. [DOI] [PubMed] [Google Scholar]
- 41.Nouspikel T, Lalle P, Leadon SA, Cooper PK, Clarkson SG. A common mutational pattern in Cockayne syndrome patients from xeroderma pigmentosum group G: Implications for a second XPG function. Proc Natl Acad Sci USA. 1997;94(7):3116–3121. doi: 10.1073/pnas.94.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Vermeulen W, Jaeken J, Jaspers NG, Bootsma D, Hoeijmakers JH. Xeroderma pigmentosum complementation group G associated with Cockayne syndrome. Am J Hum Genet. 1993;53(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeken J, et al. Clinical and biochemical studies in three patients with severe early infantile Cockayne syndrome. Hum Genet. 1989;83(4):339–346. doi: 10.1007/BF00291378. [DOI] [PubMed] [Google Scholar]
- 44.Thorel F, et al. Definition of a short region of XPG necessary for TFIIH interaction and stable recruitment to sites of UV damage. Mol Cell Biol. 2004;24(24):10670–10680. doi: 10.1128/MCB.24.24.10670-10680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst) 2011;10(7):781–791. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumura Y, Nishigori C, Yagi T, Imamura S, Takebe H. Characterization of molecular defects in xeroderma pigmentosum group F in relation to its clinically mild symptoms. Hum Mol Genet. 1998;7(6):969–974. doi: 10.1093/hmg/7.6.969. [DOI] [PubMed] [Google Scholar]
- 47.Kondo S, et al. Late onset of skin cancers in 2 xeroderma pigmentosum group F siblings and a review of 30 Japanese xeroderma pigmentosum patients in groups D, E and F. Photodermatol. 1989;6(2):89–95. [PubMed] [Google Scholar]
- 48.Sijbers AM, et al. Homozygous R788W point mutation in the XPF gene of a patient with xeroderma pigmentosum and late-onset neurologic disease. J Invest Dermatol. 1998;110(5):832–836. doi: 10.1046/j.1523-1747.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- 49.Kondo S, et al. Assignment of three patients with xeroderma pigmentosum to complementation group E and their characteristics. J Invest Dermatol. 1988;90(2):152–157. doi: 10.1111/1523-1747.ep12462130. [DOI] [PubMed] [Google Scholar]
- 50.Nishigori C, Fujisawa H, Uyeno K, Kawaguchi T, Takebe H. Xeroderma pigmentosum patients belonging to complementation group F and efficient liquid-holding recovery of ultraviolet damage. Photodermatol Photoimmunol Photomed. 1991;8(4):146–150. [PubMed] [Google Scholar]
- 51.Schäfer A, et al. Characterization of three XPG-defective patients identifies three missense mutations that impair repair and transcription. J Invest Dermatol. 2013;133(7):1841–1849. doi: 10.1038/jid.2013.54. [DOI] [PubMed] [Google Scholar]
- 52.Ichihashi M, Fujiwara Y, Uehara Y, Matsumoto A. A mild form of xeroderma pigmentosum assigned to complementation group G and its repair heterogeneity. J Invest Dermatol. 1985;85(3):284–287. doi: 10.1111/1523-1747.ep12276776. [DOI] [PubMed] [Google Scholar]
- 53.Yoneda K, et al. Xeroderma pigmentosum complementation group G in association with malignant melanoma. Eur J Dermatol. 2007;17(6):540–541. doi: 10.1684/ejd.2007.0275. [DOI] [PubMed] [Google Scholar]
- 54.Moriwaki S, et al. Xeroderma pigmentosum complementation group G patient with a novel homozygous missense mutation and no neurological abnormalities. Exp Dermatol. 2012;21(4):304–307. doi: 10.1111/j.1600-0625.2012.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehmann AR, Stevens S. A rapid procedure for measurement of DNA repair in human fibroblasts and for complementation analysis of xeroderma pigmentosum cells. Mutat Res. 1980;69(1):177–190. doi: 10.1016/0027-5107(80)90187-6. [DOI] [PubMed] [Google Scholar]