Significance
Oocyte fitness to support embryo development and pregnancy is dependent on an elaborate cross-talk with the surrounding environment of the ovarian follicle. Here, we show that this cross-talk continues during the periovulatory period when a new set of bioactive molecules is secreted by the oocyte in mice and humans. This shift in pattern of secretion is dependent on oocyte maturation and on paracrine factors secreted by somatic cells at the time of ovulation. These changes in pattern of secretion may be used to assess oocyte quality.
Keywords: oocyte maturation, mRNA translation, oocyte secreted factors, interleukin-7
Abstract
The differentiation of the female gamete into a developmentally competent oocyte relies on the protected environment of the ovarian follicle. The oocyte plays a key role in establishing this microenvironment by releasing paracrine factors that control the functions of surrounding somatic cells. Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are secreted during follicle growth and play pivotal roles in this local regulation. The current view is that the function of these secreted factors declines in the periovulatory period when the oocyte reenters the meiotic cell cycle. Here, we provide evidence that oocyte reentry into meiosis is instead associated with a shift in the pattern of secretion with a new set of bioactive molecules synthesized before ovulation. Using interleukin 7 (IL7) as a prototypic secreted factor, we show that its secretion is dependent on activation of mRNA translation in synchrony with the cell cycle and that its translation is under the control of somatic cells. IL7 is part of a local feedback loop with the soma because it regulates cumulus cell replication. Similar conclusions are reached when IL7 secretion is measured in human follicular fluid during in vitro fertilization cycles. IL7 concentration in the follicular fluid correlates with the oocyte ability to reach the MII stage of maturation. These findings are consistent with the hypothesis that a new set of local factors is secreted by the oocyte during ovulation. These dynamic secretions are likely critical for promoting the final stages of maturation and oocyte developmental competence.
The differentiation of mammalian oocytes and the acquisition of developmental competence require a unique microenvironment generated by bidirectional regulation integrating the gamete function with that of surrounding somatic cells (1). Although the property of the oocyte to secrete factors that inhibit luteinization was suggested almost four decades ago (2), its critical role in directing somatic cell differentiation has been recognized more recently with the molecular identification of oocyte-secreted factors (OSFs), their receptors, and associated signals (3). In addition to the members of the TGFβ superfamily, which have been extensively studied, other factors secreted by the oocyte, such as FGF members, regulate cumulus cell function (4, 5). Specifically, these OSFs are essential for ovulation and fertility because they stimulate granulosa cell proliferation (6), function as antiapoptotic agents (7), and regulate cumulus expansion (8) and the metabolism of somatic cells surrounding the oocyte (9).
The oocyte/somatic cell dialogue is bidirectional because oocyte maturation and ovulation are regulated by a number of local factors secreted by somatic cells. A surge in luteinizing hormone (LH) induces reprogramming of the mural granulosa cells in the follicle and the expression of epidermal growth factor (EGF)-like peptides, including amphiregulin (AREG), epiregulin (EREG), and betacellulin (10). AREG and EREG are needed to propagate the LH stimulus from the mural granulosa cells to the cumulus cells, which are insensitive to direct LH stimulation (10).
Oocytes remain arrested at the prophase I of meiosis, the chromosomes are decondensed, and the chromatin is transcriptionally active throughout follicle growth. Toward the end of the folliculogenesis when the oocyte is fully grown, the transcriptional activity ceases and the chromatin in the germinal vesicle (GV) becomes condensed (11). After the ovulatory stimulus, meiosis resumes, and the oocyte nucleus undergoes the changes required for the correct ploidy of the gamete. These final stages of oocyte maturation, as well as fertilization and early embryo development, occur in the absence of transcription (12). Cell cycle progression and genome reprogramming rely on translation of stored maternal mRNAs until the embryo genome becomes activated at the early two-cell stage in mice or at four to eight cells in humans (12). The translation of stored mRNAs is, therefore, highly regulated, and the oocyte developmental competence depends almost exclusively on posttranscriptional events. Here, we have investigated how translational regulations contribute to oocyte secretion.
Functional and genetic studies have been used to characterize the secretory products of the oocyte (13, 14). Previous systematic approaches to identify these secreted factors have relied on mining of established databases (15). Proteomics profiling of the oocytes has also been used (16). In most cases, the sensitivity of the approach was insufficient to provide a genome-wide assessment of oocyte secretion. Oocyte transcriptomics had the potential to identify secretory products. However, given the fact that transcription is absent during the oocyte maturation, this approach cannot capture the dynamic nature of the synthesis/secretion by the oocyte.
It is generally accepted that the major secretory activity of the oocyte takes place during the growth phase of the follicle. Conversely, there is little information regarding the regulation of oocyte secretion during the final stages of oocyte maturation, and it is believed that oocyte secretion ceases as the oocyte reenters meiosis (3). Here, we have revisited this concept and hypothesized that translation and secretion of oocyte factors during oocyte maturation are actually highly dynamic and synchronized with the meiotic cell cycle. We have also explored whether somatic cells affect oocyte secretion via translational regulation in the oocyte, supporting the concept that these regulated translations/secretions are part of cross-talks or feedbacks between the gamete and surrounding cumulus cells. Such regulations may be crucial for oocyte final differentiation to sustain fertilization and early embryo development.
Results
Translational Regulation of mRNA Coding for Secreted Proteins During in Vivo Oocyte Maturation.
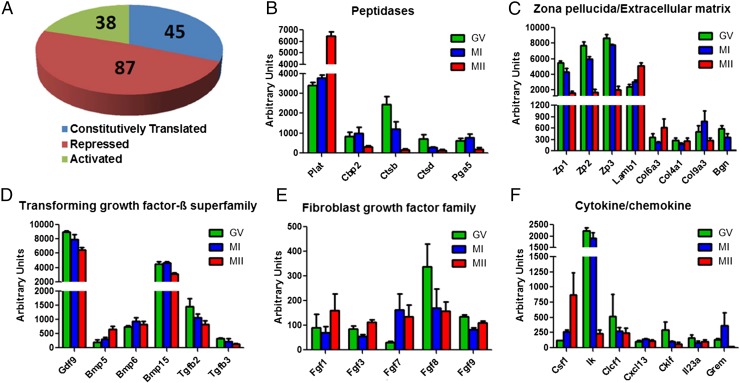
Association of mRNA with the polysomes is an established approach to assess translation (17). This strategy was used to explore the pattern of translation of secreted proteins during oocyte maturation in a genome-wide fashion. We used two datasets generated by our laboratory, one assessing polysome association at GV, MI, and MII (18) and a second set comparing GV and MII (19). Of the ∼7,600 transcripts present in the oocyte, 170 were classified as transcripts coding for secreted proteins (see Materials and Methods for the criteria used). Inspection of the data indicated that the analysis indeed captures the pattern of secretion by the oocyte because growth differentiation factor 9 (GDF9), bone morphogenetic protein 5 (BMP5), plasminogen activator (PLAT), and zona pellucida glycoproteins (ZP1-3), all established oocyte secretory products, were among the transcripts identified. By comparing the polysome association during different stages of maturation, we identified three classes of transcripts of secreted proteins with distinct patterns of ribosome recruitment from GV to MII oocytes [false discovery rate (FDR) of <5%, q < 0.05] (Fig. 1A). Although no significant changes were observed for 45 transcripts, suggesting constitutive translation, one additional group of transcripts decreased (87 transcripts) and another increased (38 transcripts) in the polysome fraction during oocyte maturation (Fig. 1A and Dataset S1), suggesting that synthesis, and possibly secretion, of these proteins is dynamic. Consistent with previous reports (20), Plat translation increased approximately twofold (Fig. 1B). The translation of all three zona pellucida glycoproteins decreased dramatically during oocyte maturation, indicating that the turnover of the zona decreases as the oocyte matures (Fig. 1C). In addition to peptidases and extracellular matrix components, several additional classes of bioactive secretory products were identified. Consistent with the established production of members of the TGFβ superfamily by the murine oocyte, we identified Gdf9, Bmp5, Bmp6, Bmp15, Tgfb2, and Tgfb3 as translated during oocyte maturation (Fig. 1D). Significant changes were observed in the pattern when comparing GV and MII: Gdf9, Bmp15, and Tgfb3 translation decreased during oocyte maturation whereas translation of Bmp5 increased, and Bmp6 remained constant, suggesting a shift in the pattern of secretion of these factors around the time of ovulation (Fig. 1D). Among the FGF family, Fgf1, Fgf3, Fgf7, Fgf8, and Fgf9 transcripts were present in the polysome fraction, with Fgf8 being the most abundant (Fig. 1E). Although Fgf7 translation increased and Fgf8 translation showed a decrease of ∼50% (although statistically borderline) during oocyte maturation, the rest of the FGF family’s association with polysomes did not change between GV and MII (Fig. 1E). In addition, the translation of multiple cytokines/chemokines was dynamic during oocyte maturation. Whereas the translation of Csf1 increased, Ik and Grem1 translation decreased during the transition from GV to MII (Fig. 1F).
Fig. 1.
Translational regulation of mRNA coding for secreted proteins during in vivo oocyte maturation. (A) Pie chart reporting different classes of transcripts for secreted proteins according to their polysome recruitment from GV to MII. The number of transcripts constitutively translated, those decreased (repressed) and increased (activated), is reported. See Dataset S1 for the full list. FDR < 5% and q < 0.05 were used in this analysis. Secreted proteins were grouped according to function: (B) peptidases, (C) zona pellucida and extracellular matrix proteins, (D) transforming growth factor β superfamily, (E) fibroblast growth factor family, and (F) cytokines/chemokines. Error bars correspond to the SEM, and data are from three biologically different samples. The dataset in ref. 18 was used.
This initial survey opens the possibility that the oocyte secretion pattern varies in phase with progression through the meiotic cell cycle and that different sets of signals are exchanged with the surrounding cellular environment while differentiating into a developmentally competent egg. To solidify this concept, we further analyzed the translation and secretion of some of these transcripts. Interleukin 7 (IL7), a cytokine that is expressed by the oocyte compared with other cells in the follicle (21), was selected as the prototype of transcripts that are increasingly translated during oocyte maturation (Fig. 2A and Dataset S1).
Fig. 2.
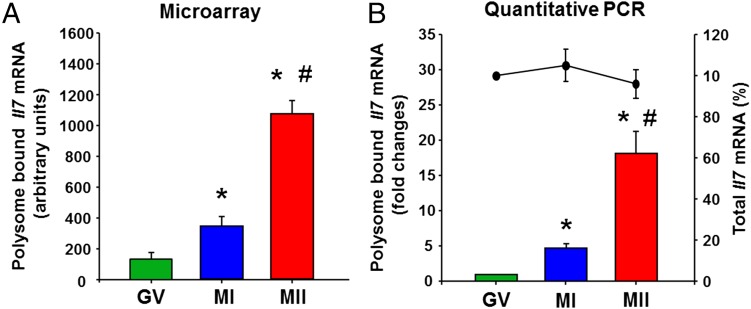
Il7 mRNA translation during in vivo oocyte maturation. (A) Microarray analysis of Il7 mRNA in the polysome fraction during oocyte maturation. *P < 0.05 vs. GV; #P < 0.05 vs. MI, ANOVA. (B) Quantitative PCR analysis of Il7 mRNA in polysome fractions (bar graph) and whole-cell lysates (line plot) during oocyte maturation. *P < 0.05 vs. GV; #P < 0.05 vs. MI, ANOVA. Error bars correspond to the SEM, and data are from three experiments from biologically distinct samples.
By using quantitative RT-PCR analysis of mRNAs recovered in the polysome fractions, we confirmed the maturation stage-dependent pattern of Il7 mRNA recruitment to the translating pool of ribosomes, recapitulating the data obtained by microarray hybridization (Fig. 2 A and B). The level of polysome-bound Il7 mRNA increased significantly in the MI oocyte compared with the GV oocyte and further increased with maturation to MII stage (Fig. 2B). Consistent with the absence of transcription in the fully grown oocyte, the total Il7 mRNA level did not change during oocyte maturation (Fig. 2B). Thus, changes in transcript levels in the polysome fraction were due to transfer from or to the polysome fraction or destabilization, but not to de novo RNA synthesis.
Regulation of Il7 mRNA Translation and Protein Synthesis/Secretion.
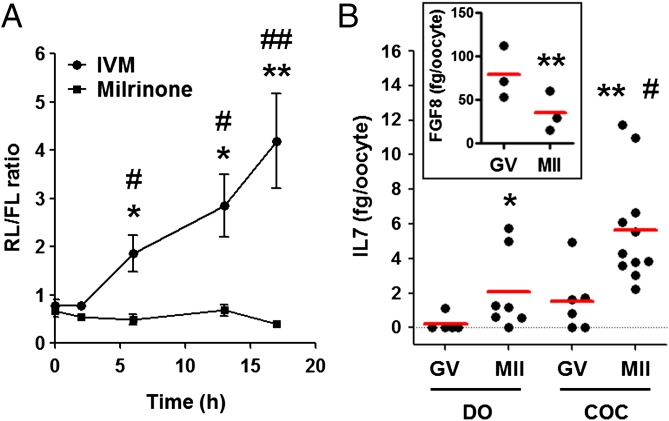
To determine how the translation of Il7 is activated during maturation, we constructed a reporter with the renilla luciferase ORF under the control of Il7 3′ UTR and polyadenylated firefly luciferase reporter as control. When injected into the oocyte, the accumulation of the Il7 luciferase reporter paralleled the mRNA recruitment to the polysome pool during oocyte maturation (Fig. 3A). However, when oocyte maturation was prevented, the luciferase activity did not change (Fig. 3A). This experiment, as well as others reported below, shows that regulations impinging on the 3′ UTR of the mRNA are likely responsible for the regulation. The translation of the reporter indeed reflected the translation of the endogenous Il7 mRNA and de novo IL7 synthesis, and its secretion was confirmed by measuring the levels of IL7 in the spent media of the oocyte culture (Fig. 3B). A significant increase in IL7 protein accumulation was observed in the spent media of either denuded or cumulus enclosed oocytes [cumulus oocyte complexes (COCs)] (Fig. 3B). Moreover, COCs secreted significantly higher amounts of IL7 during maturation compared with denuded oocytes (DOs), implying potential cumulus cell regulation of oocyte IL7 secretion (Fig. 3B). In agreement with translation data (Fig. 1E and Dataset S1), FGF8 secretion measured by ELISA decreased during oocyte maturation (Fig. 3B, Inset), confirming that polysome recruitment predicts pattern of translation.
Fig. 3.
Il7 3′ UTR reporter translation and IL7 protein secretion during oocyte maturation. (A) Ratio of renilla luciferase (RL) to firefly luciferase (FL) activity in oocytes cultured as COCs. Oocytes still in complex with cumulus cells were injected with two reporters, one coding for RL under the control of IL7 3′ UTR and one coding for FL with a polyadenylated 3′ UTR as the control. After 3 h of preincubation in the presence of milrinone (2 μM) to maintain the meiotic arrest, a group of injected COCs were washed free of inhibitor and in vitro matured (IVM) or kept in milrinone to block maturation. Both groups were incubated for 2, 6, 13, or 17 h. At the end of the incubation, oocytes were dissected free of cumulus cells, and luciferase activity was measured in oocyte extracts. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. *P < 0.05 vs. 0 h; **P < 0.01 vs. 0 h; #P < 0.05 and ##P < 0.01 vs. milrinone counterparts, t test and ANOVA. (B) IL7 protein levels in spent media during oocyte maturation. Denuded oocytes (DOs) and COCs were incubated with milrinone (2 μM) or in maturing media for 20 h; culture supernatant was collected, and IL7 levels were measured by ELISA. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples.*P < 0.05 vs. DO GV; **P < 0.05 vs. COC GV; #P < 0.05 vs. DO MII, ANOVA. (Inset) FGF8 secretion during oocyte maturation. COCs were incubated with milrinone (2 μM) or in maturing media for 20 h; culture supernatant was collected, and FGF8 levels were measured by ELISA. **P < 0.01 vs. GV, paired t test.
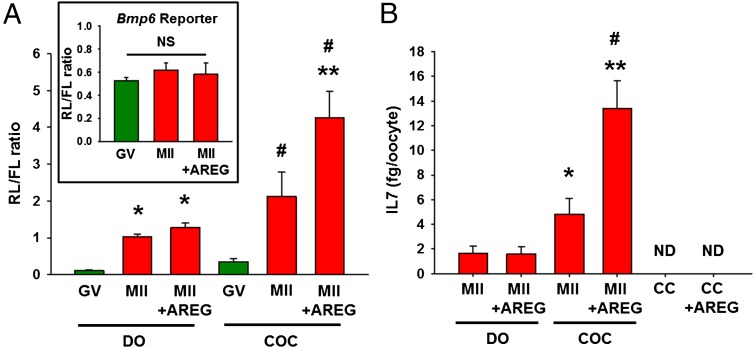
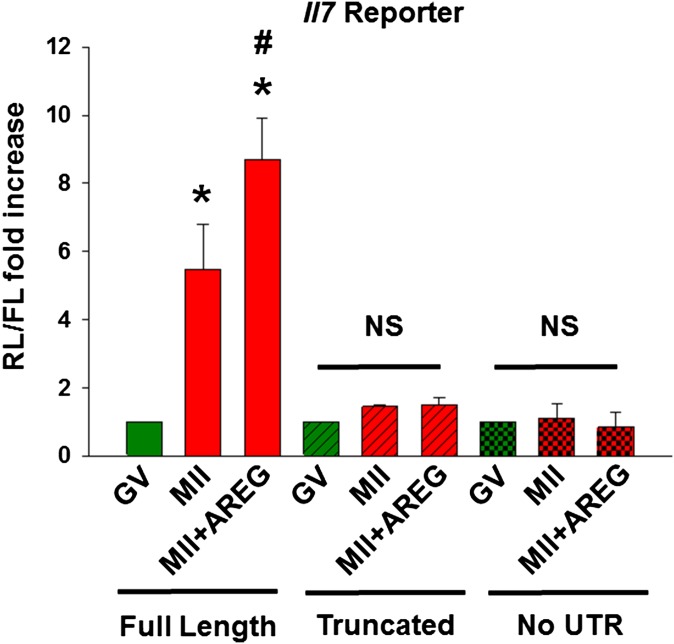
We have shown that activation of translation is at least in part dependent on the progression through the cell cycle but that somatic cell inputs can also affect translation in the oocyte during maturation (19). To distinguish between these two mechanisms, COCs were incubated with or without the somatic cell-derived EGF-like growth factor AREG, which accumulates in response to LH surge and stimulates translation in the oocyte (19). Translation rates of the Il7 reporter significantly increased as the oocytes progressed from GV to MII (Fig. 4A), consistent with recruitment of the endogenous Il7 mRNA to the polysome fraction. However, Il7 reporter translation in COC was further increased by supplementing the medium with AREG. When truncated Il7 3′ UTR reporter or a reporter lacking Il7 3′ UTR were injected to COCs, their translation did not change during oocyte maturation or after the addition of AREG (Fig. S1). This pattern suggests that full-length Il7 3′ UTR is required for the translation of Il7 reporter. Although increase in the translation rate of Il7 reporter was observed with meiotic maturation in DOs, AREG effects were not detected when oocytes were denuded before stimulation (Fig. 4A). This latter finding indicates that the stimulatory effect of AREG on Il7 reporter translation requires somatic cells. As an additional control, we used 3′ UTR reporter of Bmp6, whose polysome recruitment is constant during oocyte maturation (Fig. 1D and Dataset S1). After injection in COCs and consistent with the polysome data, Bmp6 reporter translation did not change with oocyte maturation or supplementing the medium with AREG (Fig. 4A, Inset).
Fig. 4.
Somatic cells regulate Il7 mRNA translation and IL7 protein secretion. (A) Ratio of renilla luciferase (RL) to firefly luciferase (FL) activity in oocytes cultured as denuded oocytes (DOs) or cumulus oocyte complexes (COCs). Oocytes were injected with two reporters and cultured as detailed in Fig. 3 with or without amphiregulin (AREG, 100 nM) for 17 h. At the end of the incubation, luciferase activity was measured in oocyte extracts. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. *P < 0.05 vs. DO GV; #P < 0.05 vs. COC GV; **P < 0.05 vs. COC MII, two way ANOVA. (B) IL7 protein levels in spent media during oocyte maturation. Denuded oocytes (DOs), COCs, and cumulus cells (CCs) were incubated in maturing media with or without amphiregulin (AREG, 100 nM) for 20 h; culture supernatant was collected, and IL7 levels were measured by ELISA. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. ND, not detectable. *P < 0.05 vs. DO MII; **P < 0.05 vs. DO MII+AREG; #P < 0.05 vs. COC MII, ANOVA. (A, Inset) Bmp6 3′ UTR reporter translation during oocyte maturation. Ratio of RL to FL activity in oocytes cultured as COCs. Oocytes still in complex with cumulus cells were injected with two reporters, one coding for RL under the control of Bmp6 3′ UTR and one coding for FL with a polyadenylated 3′ UTR as the control. COCs were cultured as detailed in Fig. 3 with or without amphiregulin (AREG, 100 nM) for 17 h. At the end of the incubation, luciferase activity was measured in oocyte extracts. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. NS, Not significant, two way ANOVA.
Fig. S1.
Full-length Il7 3′ UTR is required for the translation of Il7 reporter. Ratio of renilla luciferase (RL) to firefly luciferase (FL) activity in oocytes cultured as COCs. Oocytes still in complex with cumulus cells were injected with two reporters, one coding for RL either under the control of full-length Il7 3′ UTR or truncated Il7 3′ UTR or reporter lacking Il7 3′ UTR; and one coding for FL with a polyadenylated 3′ UTR as the control. After 3 h of preincubation in the presence of milrinone (2 μM) to maintain the meiotic arrest, a group of injected COCs were washed free of inhibitor and in vitro matured with or without AREG (100 nM) or kept in milrinone to block maturation for 17 h. At the end of the incubation, luciferase activity was measured in oocyte extracts. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. *P < 0.05 vs. GV (full-length), #P < 0.05 vs. MII (full-length). NS, not significant, two way ANOVA.
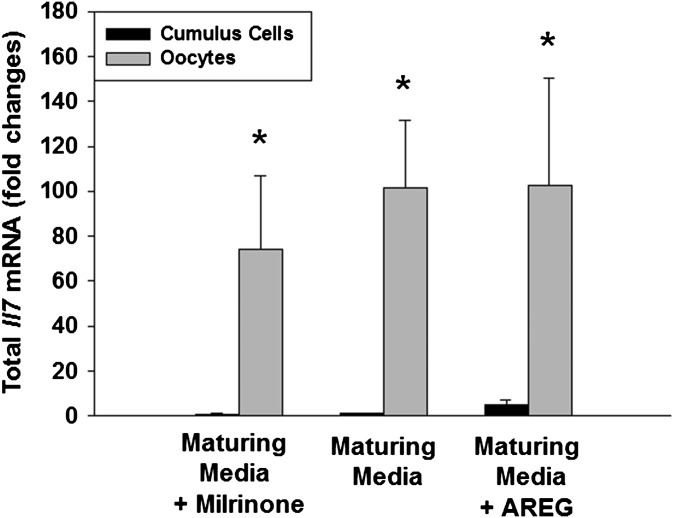
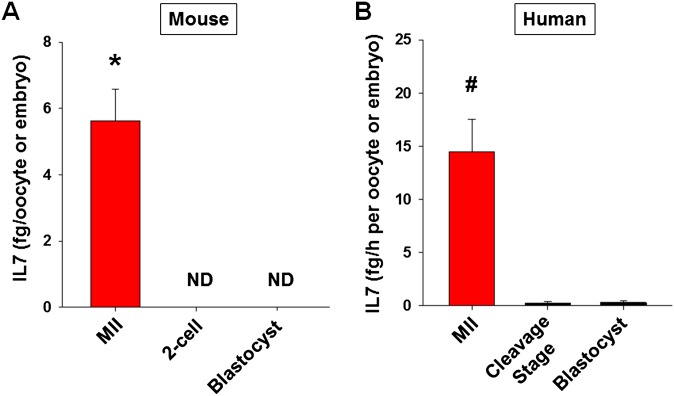
To confirm the above data, GV oocytes obtained from pregnant mare's serum gonadotropin (PMSG)-primed mice were matured in vitro, and the IL7 levels in the culture supernatant were measured by ELISA. AREG enhanced IL7 secretion only in COCs, but not in DOs (Fig. 4B). This result confirmed that the stimulatory effect of AREG on IL7 protein secretion requires somatic cells. Nondetectable levels of secreted IL7 in cumulus cell-only cultures (Fig. 4B) with or without AREG treatment and at least 30-fold higher total Il7 mRNA in oocytes compared with cumulus cells in the same treatment group (Fig. S2) are consistent with the view that IL7 measured in the culture supernatant is secreted only by the oocytes. After fertilization, IL7 secretion by the embryo ceases (Fig. S3A).
Fig. S2.
Il7 mRNA transcription in oocyte and cumulus cells during in vitro maturation. COCs were incubated in maturing media with or without milrinone (2 μM) or amphiregulin (AREG, 100 nM) for 20 h. Oocytes were separated from cumulus cells by brief treatment with 3 μg/mL hyaluronidase. The total RNA from oocytes and cumulus cells was extracted, and quantitative RT-PCR was performed.*P < 0.05 vs. cumulus cell counterpart, ANOVA. Error bars correspond to the SEM, and data are from three experiments from biologically distinct samples.
Fig. S3.
IL7 secretion diminishes in both mouse and human embryo after fertilization. (A) IL7 protein levels in spent media during oocyte maturation and embryo culture in mouse. COCs were incubated in maturing media for 20 h, and culture supernatant was collected for IL7 ELISA. After in vitro fertilization, the spent embryo media were obtained when the embryos reached to two-cell and blastocyst stages to assess secreted IL7 with ELISA. IL7 levels were normalized to the number of oocytes/embryos incubated. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. *P < 0.05 vs. two-cell and blastocyst stages. (B) IL7 protein levels in spent media during COC and embryo culture in human. After oocyte retrieval, COCs were washed and incubated 3 h before cumulus cell stripping for intracytoplasmic sperm injection, and spent media were collected for IL7 ELISA. After fertilization check (day 1), the embryos were cultured to blastocyst stage (day 5). The human embryo media were obtained when the embryos reached to cleavage (day 3) and blastocyst (day 5) stages to assess secreted IL7 with ELISA. IL7 levels were normalized to the number of oocytes/embryos incubated and incubation time. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples. #P < 0.05 vs. cleavage and blastocyst stages, ANOVA.
IL7 Enhances Cumulus Cell Proliferation During Oocyte Maturation.
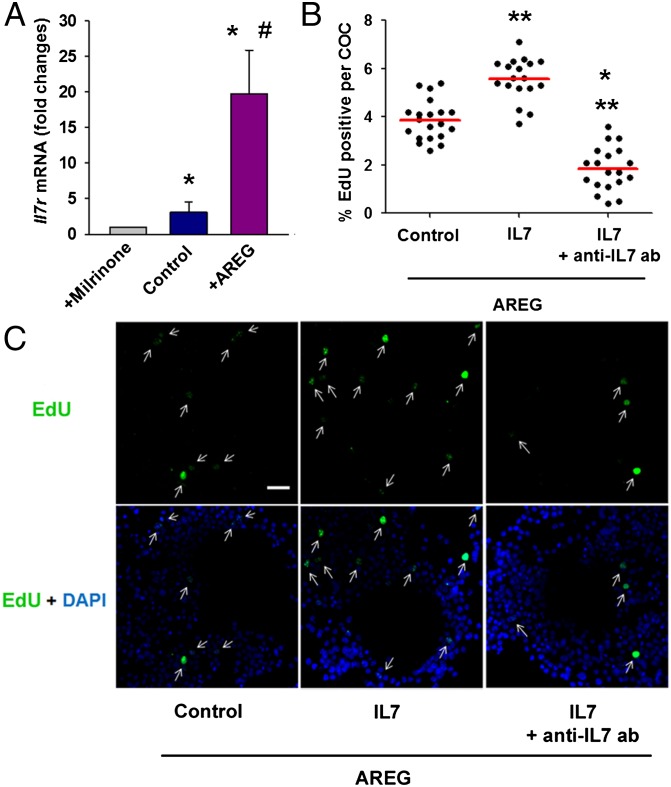
IL7 receptor is a heterodimer membrane receptor and has a specific α-chain (Il7r) and γ-chain (Il2rc) that is shared by the receptors for IL2 and IL4 (21). To determine whether IL7 secreted by the oocyte functions as part of a feedback, we investigated the expression of Il7r in cumulus cells during oocyte maturation. COCs obtained from PMSG-primed mice were matured in vitro, cumulus cells were stripped, and Il7r mRNA levels in cumulus cells were assessed by quantitative RT-PCR. The level of Il7r mRNA in cumulus cells increased with oocyte maturation and was further augmented with addition of AREG (Fig. 5A). Thus, during oocyte maturation, cumulus cells become more sensitive to the IL7 ligand.
Fig. 5.
IL7 enhances cumulus cell proliferation during oocyte maturation. (A) COCs obtained from PMSG-primed mice were matured in vitro, cumulus cells were stripped, and Il7r mRNA levels in cumulus cells were assessed by quantitative RT-PCR. *P < 0.05 vs. maturing media + milrinone; #P < 0.05 vs. maturing media, ANOVA. Error bars correspond to the SEM, and data are from at least three experiments. Cumulus cell proliferation was measured as EdU incorporation during in vitro maturation of COCs with or without recombinant IL7 ± anti-IL7 blocking antibody. (B) Percentage of EdU-positive cells in individual COCs. *P < 0.05 vs. IL7, **P < 0.05 vs. control, ANOVA. The red line represents the average. (C) Representative COCs labeled with EdU (green) and nuclear stained with DAPI (blue). Arrows show EdU-positive cumulus cells. (Scale bar: 20 μm.)
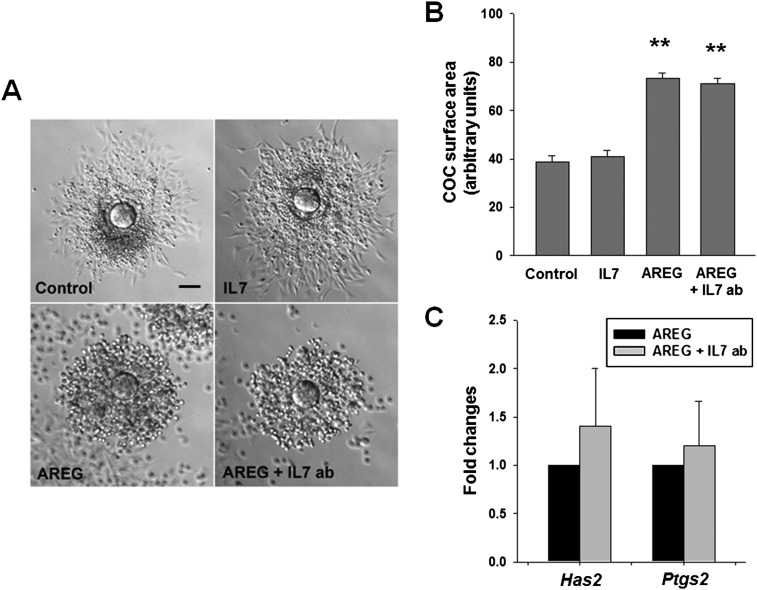
Lastly, we determined whether ligation of the IL7 receptor on cumulus cells has a biological effect on these cells. IL7 is a growth factor that stimulates the proliferation of immune cells (21). During in vitro maturation of COCs obtained from PMSG-primed mice, cell replication measured as EdU incorporation was enhanced by recombinant IL7 and was attenuated by adding IL7 blocking antibody to the culture media (Fig. 5 B and C). Because Il7r mRNA levels were enhanced with AREG, all of the experiments were performed in the presence of this ligand. In contrast, the modulation of IL7 activity did not impact the cumulus cell expansion (Fig. S4 A and B). Moreover, blocking IL7 activity did not change the expression of Has2 and Ptgs2 mRNA in the cumulus cells, both transcripts necessary for cumulus expansion (Fig. S4C).
Fig. S4.
IL7 does not regulate cumulus cell expansion. COCs were incubated in maturing media supplemented with 5% FBS with or without AREG (100 nM) or recombinant IL7 (0.1 ng/mL) or blocking IL7 antibody (10 μg/mL) for 16–18 h. (A) Representative COCs micrographs. (Scale bar: 60 μm.) (B) COCs were scored for cumulus expansion by quantification of COC area. **P < 0.01 vs. control and IL7, ANOVA. Error bars correspond to the SEM (C) Quantitative PCR of transcripts (Has2 and Ptgs2) necessary for cumulus cell expansion. Error bars correspond to the SEM, and data are from at least three experiments from biologically different samples.
IL7 Levels in the Follicular Fluid Increase with Oocyte Maturation and Correlate with Follicular AREG Levels in Human.
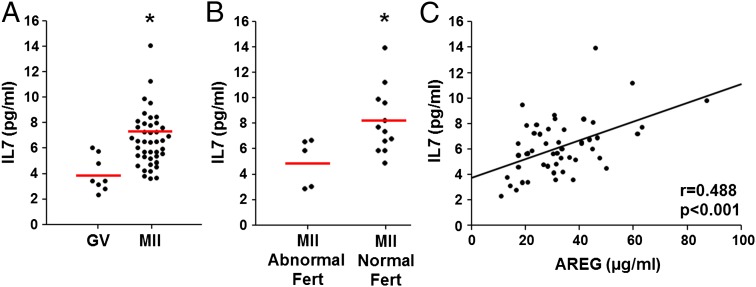
To investigate whether IL7 secretion increases with oocyte maturation in vivo in human as we have shown in the mouse in vitro, follicular fluid was obtained from a single dominant follicle before or after human chorionic gonadotropin (hCG) administration during controlled ovarian stimulation cycles. Eight samples taken before hCG administration were from follicles yielding GV oocytes and 42 samples obtained 36 h after hCG contained MII oocytes. IL7 levels were significantly higher in follicles containing MII oocyte (with normal fertilization) compared with those containing GV oocyte (Fig. 6A). Moreover, in egg donors, oocytes that were normally fertilized (two-pronuclei formation) after intracytoplasmic sperm injection had significantly higher follicular levels of IL7 compared with those with abnormal fertilization (Fig. 6B). Additionally, IL7 levels positively correlated with AREG levels in the follicular fluid (P < 0.001, σ = 0.488), suggesting that AREG may be the factor inducing IL7 secretion from COCs also in human (Fig. 6C). In the culture system, IL7 was detectable in human COC spent media (Fig. S3B). After fertilization, similar to mouse, IL7 secretion diminished in the human embryo (Fig. S3B).
Fig. 6.
IL7 in follicular fluid increases with oocyte maturation and correlates with follicular AREG levels in human. (A) IL7 levels in follicles yielding GV (n = 8) or MII (n = 42) oocyte. *P < 0.01, Student t test. The red line represents the average. (B) Comparison of follicular levels of IL7 in follicles yielding oocytes with normal (n = 12) or abnormal (n = 5) fertilization after intracytoplasmic sperm injection *P < 0.05, Student t test. (C) IL7 levels positively correlated with AREG levels in follicular fluid (n = 50). P < 0.001, σ = 0.488, Spearman’s correlation test.
Discussion
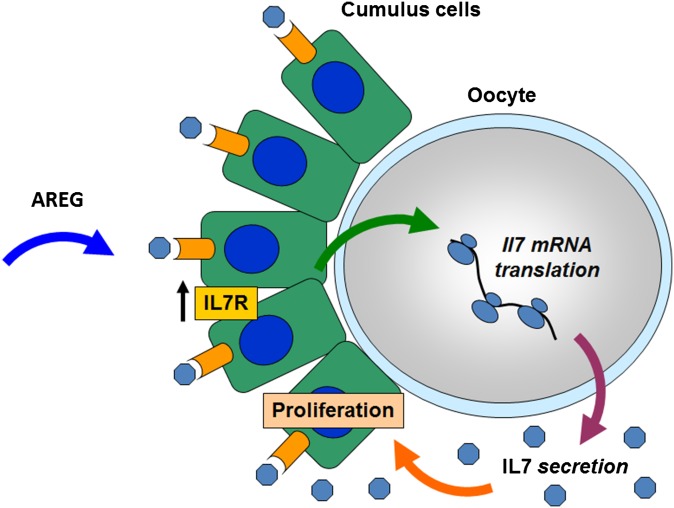
Communication between the oocyte and the surrounding somatic cells is critical for follicle growth, oocyte maturation, and ovulation of a developmentally competent egg. It is generally accepted that oocyte-secreted factors modulate the intrafollicular environment until the formation of a graafian follicle and cease during the periovulatory period. Here, we propose the novel concept that the pattern of oocyte protein secretion during the transition from transcriptionally quiescent oocyte in GV to an MII oocyte ready to be ovulated is instead highly dynamic. Our genome-wide analysis predicts changes in secretion of 125 proteins, including synthesis/secretion of extracellular matrix proteins, cytokines, chemokines, and growth factors. Regulated translation of the maternal mRNAs coding for these putative oocyte-secreted proteins is a molecular mechanism underlying this timed secretion. More importantly, we show that secretion is also sensitive to somatic cues, supporting the concept that these interactions are part of a feedback between the gamete and surrounding cumulus cells, crucial for oocyte development and survival. We propose that cumulus cells respond to ovulatory stimuli by sending signals that promote translation and secretion of bioactive molecules by the oocytes (Fig. S5). The secreted factors, in turn, act on cumulus cells, regulating their function, thus completing a feedback regulatory loop (Fig. S5). This timed oocyte secretion before ovulation likely modulates crucial molecular events in cumulus cells that are necessary for the development of a competent oocyte and are possibly necessary for communication with the other cellular environments the oocyte becomes in contact with after ovulation.
Fig. S5.
Model of bidirectional communication between the oocyte and the surrounding cumulus cells. Amphiregulin (AREG) binds to its receptor on the cumulus cells and indirectly leads to increased translation/secretion of IL7 in the oocyte. Secreted IL7 increases the proliferation of cumulus cells. AREG also causes a significant increase in IL7 receptor (IL7R) expression in cumulus cells, preparing these cells to receive the oocyte IL7 signal.
Our conclusions on dynamic translation are based on genome-wide data documenting polysome recruitment of mRNA coding for secreted protein increases or decreases during the transition from GV to MII. Using IL7 as a prototype of oocyte-secreted factors, we confirm that Il7 mRNA is recruited to the polysomes during maturation and that the translation of reporter constructs containing the 3′ UTR of Il7 mRNA increases three- to fivefold as the oocyte matures. The activation of translation of the endogenous Il7 mRNA is confirmed by the finding that IL7 secretion is marginally detectable in GV oocytes and increases significantly when oocytes reach the MII stage. A similar pattern can be detected in the follicular fluid of human follicles before and after hCG stimulation in vivo. Of note, IL7 secretion becomes undetectable after fertilization, further supporting major fluctuations in the concentration of this interleukin in the environment surrounding the cumulus oocyte complex.
We established significant changes in mRNA association with the polysomes for 125 secreted proteins. These transcripts include proteins involved in the formation/maintenance of the extracellular matrix and the zona pellucida. The translation of all three major components of the zona (ZP1, -2, and -3) are greatly decreased during oocyte maturation, which is consistent with the view that the zona proteins accumulate during oocyte growth but that their synthesis ceases as the oocyte reenters the cell cycle. A classical prototype of translationally induced oocyte secretion is the tissue plasminogen activator PLAT, an endopeptidase that promotes fibrinolysis. In our survey, we found that other transcripts that regulate blood coagulation, such as Tfpi and Serpine2, show an increased polysomal association whereas several activators of blood coagulation and platelet degranulation decreased (Cpb2, Calu, Scg3). This observation, together with the finding that many of the changed transcripts are involved in the extracellular matrix organization, suggests that the oocyte actively participates in the remodeling of its surroundings to facilitate the ovulatory process. We also observed major changes in translation of growth factors that belong to the TGFβ superfamily whereas synthesis of FGF family members seems to be constitutive, except for Fgf7 and Fgf8. Specifically, an increase in Bmp5 and decrease in Tgfb3 mRNA translation, as well as a decrease in Gdf9 and Bmp15, were detected. The decrease in Gdf9 mRNA translation is consistent with a report of GDF9 protein concentration in porcine oocytes during maturation (22) and on the commonly held idea that production of these factors is decreased in MII oocytes (3). In addition to Il7, other transcripts encoding for molecules that have cytokine/chemokine activity, including Csf1 and Tnfsf11, showed an increased polysome association. In contrast, other cytokine mRNAs, such as Il34, Il23a, and Ik, decreased their association with the polysomal fraction. Although the biological significance of these changes needs to be determined, it is likely that the surrounding somatic cells sense and adjust their function to support the maturing oocyte.
In humans, IL7 protein was detectable in follicles where oocytes are still at the GV stage. It is likely that IL7 from the circulation accounts at least in part for the detection in the follicular fluid. It is established that the follicular fluid is transudate from the plasma and to some extent in equilibrium with the circulation system (23). One could envisage that increased secretion by the oocyte before ovulation may lead to increased IL7 in the circulation. However, in our pilot experiments this increase was not detected.
We propose two mechanisms of regulation of translation/synthesis of secreted proteins. One mechanism is dependent on progression through the cell cycle and may involve regulation of RNA binding proteins such as cytoplasmic polyadenylation element binding protein 1 (CPEB1) or deleted in azoospermia-like (DAZL) (24). An additional mechanism involves somatic cell activation that indirectly leads to increased translation in the oocyte. Paracrine factors released at the time of ovulation, such as AREG, likely mediate this regulation. AREG induced activation of EGF receptor signaling in the cumulus cells, which indirectly activates phosphatidylinositol-3-phosphate-kinase (PI3K) in the oocyte and plays a role in the regulation of translation (19).
The importance of bidirectional communications between oocytes and follicular somatic cells for the acquisition of oocyte developmental competence is well-established (3, 25). Decreased proliferation and increased apoptosis in cumulus cells have been associated with immaturity of human oocytes, impaired fertilization (26), suboptimal blastocyst development, and poorer in vitro fertilization (IVF) outcomes (27). IL7 was previously shown to suppress apoptosis of cultured granulosa cells by activating the PI3K/AKT pathway (28). Here, we demonstrated that IL7 stimulates the proliferation of cumulus cells, providing additional evidence that timely oocyte secretion regulates cumulus cell function, and in turn, potentially improves its own developmental competence. It has been reported that a lower IL7 level in pooled follicular fluid samples is associated with lower egg/embryo quality and lower in vitro fertilization success rates (29). In the same vein, we have demonstrated that normally fertilized oocytes had significantly higher follicular levels of IL7 compared with those with no or abnormal fertilization in egg donors. These findings further support the concept of the oocyte determining its own fate by regulating its own microenvironment with secreted factors.
As an extension of the above concepts, noninvasive biomarkers monitoring the oocyte secretion pattern during oocyte maturation may be prognostic of the “fitness” of an oocyte to develop into an embryo that successfully implants and sustains pregnancy. Further studies are required to assess whether these OSFs can be used as a tool to select the most competent oocytes in assisted reproductive technologies.
Materials and Methods
Human follicular fluid samples and COC/embryo culture media were obtained from patients undergoing controlled ovarian stimulation/in vitro fertilization treatments at the University of California, San Francisco (UCSF) Center for Reproductive Health. The study was approved by the UCSF Committee on Human Research, and informed consent was obtained from all patients involved in this study. C57BL/6 female mice (22–24 d old) were used in animal experiments. All animal experiments were approved by the UCSF Animal Care and Use Committee.
Refer to SI Materials and Methods for the rest of the materials and methods.
SI Materials and Methods
Assessment of Translation of Secreted Factors During Oocyte Maturation.
Two datasets previously generated by our laboratory were used. One dataset probed polysome association at GV, MI, and MII (n = 3) (18), and a second dataset compared GV and MII (n = 6) (19). The initial screening was performed by identifying the proteins with signal peptides using the Ensembl Genome Browser (www.ensembl.org/index.html). Additional screening was performed by manually assessing the properties of encoded proteins using the Uniprot database (www.uniprot.org). The transcripts with low signals (<100 arbitrary units) were excluded from the analysis.
Oocyte Isolation and in Vitro Maturation Experiments.
C57BL/6 female mice (22–24 d old) were used in the experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee. COCs were isolated as described previously (18). Forty-eight hours after pregnant mare's serum gonadotropin (PMSG) (5 IU) injection, mice were killed, and both ovaries were obtained. Cumulus–oocyte complexes were collected after puncturing the dominant follicles. For some experiments, oocytes were denuded by mechanical stripping. The culture medium used for all experiments was Eagle’s minimum essential medium with Earle’s salts (MEMα; GIBCO), supplemented with 0.23 mM pyruvate, 75 μg/mL penicillin, 10 μg/mL streptomycin sulfate, and 3 mg/mL BSA, and buffered with 26 mM sodium bicarbonate. For oocyte isolation and microinjection, the medium was supplemented with 2 μM milrinone (specific PDE3 inhibitor to prevent oocyte maturation) and buffered with 25 mM Hepes (pH 7.2) with sodium bicarbonate reduced to 6 mM.
COCs, DOs, and cumulus cells were incubated in culture media with or without 2 μM milrinone or 100 nM AREG for 24 h. After the incubation, culture supernatant was collected for IL7 ELISA, and the oocytes were separated from cumulus cells for RNA isolation.
RNA Isolation and Reverse Transcription.
After in vivo or in vitro maturation experiments, oocytes were separated from cumulus cells by brief treatment with 3 μg/mL hyaluronidase. The total RNA from oocytes and cumulus cells was extracted by an RNeasy Plus Micro kit (Qiagen). The quality and concentration of RNA were determined by measuring the absorbance at 260 and 280 nm. After incubation of RNA at 65 °C for 5 min, total RNA was reverse transcribed by using a Sensiscript RT Kit (Qiagen) with random hexamers.
Polysome-bound RNA was prepared by the sucrose density method as previously described (18). Briefly, 48 h after PMSG injection, C57BL/6 female mice (22–24 d old) were stimulated with human CG (hCG) for 0 h, 4 h, or 14 h, and GV, MI, and MII stage oocytes were collected. Approximately 500–600 oocytes were lysed with 13 PLB [30 mM Tris⋅HCl at pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1% Triton, 1 mM DTT, 0.25 mM Na3VO4, 20 mM β-glycerophosphate, 30 U/mL RNase inhibitor (USB Chemical), and 10 mg/mL cycloheximide, plus protease inhibitor mixture (Roche)]. Oocyte lysates were centrifuged at 12,000 × g for 10 min at 4 °C. Supernatants were loaded on a 10-mL 15–50% (wt/vol) sucrose gradient and centrifuged at 100,000 × g for 110 min at 4 °C. RNAs from the polysome were precipitated with ethanol overnight and purified with an RNeasy Plus Micro kit (Qiagen). Polysome-bound RNAs were reverse-transcribed and linearly amplified with the WT-Ovation FFPE RNA Amplification System, version 2 (NuGEN).
Real-Time Quantitative PCR.
Real-time quantitative PCR was performed using Power SYBR PCR master mix with the ABI 7900 Real-Time PCR system (Applied Biosystems). All primers were designed in two exons flanking introns to avoid amplification of genomic DNA. A dissociation curve analysis was performed at the end of the amplification to verify the specificity of the primers. The polysome data were corrected for oocyte number, and the total transcripts data were normalized to housekeeping genes (GAPDH and L19). REST 2009 software was used to analyze the data. The primers used in real-time quantitative PCR were as follows:
| Name | Accession No. | Forward primer (5′–3′) | Reverse primer (5′–3′) |
| Il7 | NM_008371 | GCCTGTCACATCATCTGAG | AAAGTTTGGTTCATTATTCGGG |
| Il7r | NM_008372 | CAAGAATCAAGGAGGATGGGA | GAGACTAGGCCATACGACAG |
| Has2 | NM_008216 | CGAGTCTATGAGCAGGAGCTG | GTGATTCCGAGGAGGAGAGACA |
| Ptgs | NM_011198 | CCCTTCCTCCCGTAGCAGAT | TGAACTCTCTCCGTAGAAGAACCTTT |
| Gapdh | NM_001289726 | TGGAGAAACCTGCCAAGTATG | GGTCCTCAGTGTAGCCCAAG |
| L19 | NM_009078 | CTGAAGGTCAAAGGGAATGTG | GGACAGAGTCTTGATGATCTC |
Reporter mRNA Preparation, Microinjection, and Luciferase Assay.
The Renilla luciferase (RL) reporter plasmids were constructed from a pRL-TK vector (Promega). The 1,862-base pair (bp) construct for Il7 3′ UTR (654–2516) and the 660 bp construct for Bmp6 3′ UTR were generated by PCR from GV oocyte cDNA using primers CAATAATTCTAGAAGAGACTACTGAGAGAAATAAAAACTT (forward), CACCTGGATCCGCCAAAATCAGAATATTGTTC (reverse) for IL7; and TGAACAATAATT CTAGAGTTGAAGCTGGTGTGTG (forward), CCACCTGGATCCGAATTCTGTGAG (reverse) for BMP6. IL7 and BMP6 constructs were subcloned into pRL-TK downstream of the coding sequence for renilla luciferase. The truncated form of Il7 3′ UTR was obtained by restriction digestion of the pRL-TK full-length Il7 3′ UTR plasmid with HindIII to obtain a 528-bp 3′ UTR or with XBaI to completely remove the 3′ UTR. The reporter contained a T7 promoter allowing in vitro transcription to synthesize mRNAs (Ambion). The firefly luciferase control mRNA was polyadenylated by a Poly(A) tailing kit (Ambion). COCs or DOs were injected with Il7 3′ UTR reporter mRNA (25 ng/µL) together with 25 ng/µL polyadenylated firefly luciferase RNA as a normalizing RNA, and incubated in MEMα/BSA containing 2 µM milrinone at 37 C˚ with 5% (vol/vol) CO2 for 3 h before treatments. After washing, COCs and DOs were either matured in MEMα/BSA with or without 100 nM AREG, or arrested in GV stage with 2 µM milrinone. After 17 h of treatment in different conditions, oocytes were denuded and collected. Luciferase activity in the oocyte extracts was measured using the Dual Luciferase Reporter Assay Kit (Promega), and luminescence was detected by a SpectraMaxL Luminometer (Molecular Devices). Data were reported as the ratio of renilla luciferase to firefly luciferase.
In Vitro Fertilization in Mouse.
C57BL/6 female mice (22–24 d old) were injected with 5 IU of PMSG followed 46–48 h later by 5 IU of hCG to induce superovulation. Thirteen to 15 h after hCG administration, COCs were isolated from ampulla of the tube and incubated 4–6 h in human tubal fluid medium (MR-070-D; Millipore) with capacitated (1 h) cauda epididymal sperm from C57BL/6 males. Fertilized zygotes were washed and cultured to the blastocyst stage in potassium simplex optimization medium (KSOM) (containing amino acids and 0.2 mM pyruvate, 10 mM lactate, 0.2 mM glucose, and 1 mM glutamine) (MR-106-D; Millipore), at 37 °C under Ovoil (10029; Vitrolife) with 5% (vol/vol) CO2 and 5% (vol/vol) O2 in a modular humidified chamber. The spent embryo media were obtained when the embryos reached to two-cell and blastocyst stages to assess the IL7 with ELISA.
Collection of Human Follicular Fluid and Culture Media.
Human follicular fluid samples and COC/embryo culture media were obtained from patients undergoing controlled ovarian stimulation/in vitro fertilization treatments at the UCSF Center for Reproductive Health. The study was approved by the institutional review board, and informed consent was obtained from all patients involved in this study. Each patient (n = 47) had one follicle aspirate collected at the time of oocyte retrieval 36 h after human CG (hCG) 10,000 IU s.c. injection from mature-sized follicles (≥16 mm diameter). Seventeen out of 47 patients were anonymous egg donors. Details regarding the stimulation parameters, oocyte retrieval, and follicular fluid preparation and storage have been described previously (30). Pre-hCG follicular fluid was also obtained from mature-sized follicles (≥16 mm diameter). Eight samples taken before hCG administration were obtained from the patients who forgot to get hCG injection before oocyte retrieval or who had a single lead follicle during ovarian stimulation that was aspirated to allow salvage of the cycle. For the comparison, the samples were age-matched. The maturity of the oocytes was assessed by stripping the cumulus cells 2–3 h after the egg retrieval.
After oocyte retrieval (day 0), COCs obtained from five oocyte donors were washed and incubated 2–3 h before cumulus cell stripping for intracytoplasmic sperm injection, and spent media was collected for IL7 ELISA. The fertilization check was performed 18 h after intracytoplasmic sperm injection. After fertilization check (day 1), the normally fertilized embryos (i.e., embryos with two pronuclei) were cultured to blastocyst stage (day 5) in Global media (LifeGlobal). The human embryo media were obtained when the embryos reached to cleavage (day 3) and blastocyst (day 5) stages to assess secreted IL7 with ELISA. IL7 levels were normalized to the number of oocytes/embryos incubated and incubation time.
ELISA.
COCs, DOs, and cumulus cells were incubated with milrinone (2 μM) or in maturing media with or without AREG (100 nM) for 20 h, and spent media were collected. Up to 200 COCs/DOs were cultured per treatment group. Mouse embryo culture media were obtained as detailed in In Vitro Fertilization in Mouse. IL7 and FGF8 levels were measured by ELISA according to instructions provided by the manufacturer (for IL7, M7000, R&D Systems; and for FGF8, LS-F8646, LifeSpan Biosciences). IL7 and FGF8 levels were normalized to the number of oocytes/embryos incubated. IL7 ELISA (HS750; R&D Systems), in human follicular fluid and embryo media, and AREG ELISA (DAR00; R&D Systems), in human follicular fluid, were performed according to instructions provided by the manufacturer.
Proliferation Assay.
COCs were incubated in maturing media supplemented with AREG (100 nM) with or without recombinant IL7 (0.1 ng/mL; R&D Systems) or blocking IL7 antibody (10 μg/mL, AB-407-NA; R&D Systems) for 20 h. EdU was added during the last 4 h of the incubation. Immunofluorescence was performed according to instructions provided by the manufacturer (Click-iT EdU Alexa Fluor 488; Life Technologies). Nuclei were stained with DAPI, and COCs were imaged on a Leica TCS SP5 confocal microscope. Total and EdU-positive cumulus cells per COC were determined using Volocity 6.2 (Improvision).
Assessment of Cumulus Expansion.
COCs were incubated in maturing media supplemented with 5% (vol/vol) FBS with or without AREG (100 nM) or recombinant IL7 (0.1 ng/mL; R&D Systems) or blocking IL7 antibody (10 μg/mL; R&D Systems) for 16–18 h. COCs were scored for cumulus expansion by quantification of COC area using QCapture ProVersion 5.1 (Media Cybernetics).
Statistical Analysis.
Normality of the data distribution was as determined by Kolmogorov–Smirnov test. The statistical analyses for parametric data were performed by using t test, Mann–Whitney rank sum test, and ANOVA, followed by pair-wise multiple comparisons with the Holms–Sidak method or ANOVA on ranks followed by pair-wise multiple comparisons with Dunn’s method as appropriate. Correlation analysis was performed by using Spearman’s test. Stata 12.1 software (StataCorp) was used for analysis. Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH Cooperative Agreement P50HD055764-09, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (M.C.). H.C. was supported by Grant T32 HD007263-28 from NICHD. F.F. was supported by FP7-PEOPLE-2013-IOF GA 624874 MateRNA. A.M.Z. was supported by the Eunice Kennedy Shriver NICHD-sponsored Women's Reproductive Health Research (WRHR) Career Development Program K12 HD001262-12, and by the National Center for Advancing Translational Sciences, NIH, through Grant UCSF-CTSI UL1 TR000004.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519990113/-/DCSupplemental.
References
- 1.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science. 2002;296(5576):2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 2.Nekola MV, Nalbandov AV. Morphological changes of rat follicular cells as influenced by oocytes. Biol Reprod. 1971;4(2):154–160. doi: 10.1093/biolreprod/4.2.154. [DOI] [PubMed] [Google Scholar]
- 3.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 4.Chaves RN, de Matos MH, Buratini J, Jr, de Figueiredo JR. The fibroblast growth factor family: Involvement in the regulation of folliculogenesis. Reprod Fertil Dev. 2012;24(7):905–915. doi: 10.1071/RD11318. [DOI] [PubMed] [Google Scholar]
- 5.Emori C, Sugiura K. Role of oocyte-derived paracrine factors in follicular development. Anim Sci J. 2014;85(6):627–633. doi: 10.1111/asj.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilchrist RB, et al. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119(Pt 18):3811–3821. doi: 10.1242/jcs.03105. [DOI] [PubMed] [Google Scholar]
- 7.Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118(Pt 22):5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 8.Dragovic RA, et al. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology. 2005;146(6):2798–2806. doi: 10.1210/en.2005-0098. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: Energy metabolism. Dev Biol. 2005;279(1):20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356(1-2):65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De La Fuente R, et al. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol. 2004;275(2):447–458. doi: 10.1016/j.ydbio.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Susor A, Jansova D, Anger M, Kubelka M. Translation in the mammalian oocyte in space and time. Cell Tissue Res. 2016;363(1):69–84. doi: 10.1007/s00441-015-2269-6. [DOI] [PubMed] [Google Scholar]
- 13.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9(1):131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 14.Yan C, et al. Oosp1 encodes a novel mouse oocyte-secreted protein. Genesis. 2001;31(3):105–110. doi: 10.1002/gene.10010. [DOI] [PubMed] [Google Scholar]
- 15.Taft RA, Denegre JM, Pendola FL, Eppig JJ. Identification of genes encoding mouse oocyte secretory and transmembrane proteins by a signal sequence trap. Biol Reprod. 2002;67(3):953–960. doi: 10.1095/biolreprod.102.005546. [DOI] [PubMed] [Google Scholar]
- 16.Peng Q, et al. Secretome profile of mouse oocytes after activation using mass spectrum. J Assist Reprod Genet. 2012;29(8):765–771. doi: 10.1007/s10815-012-9789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mašek T, Valášek L, Pospíšek M. Polysome analysis and RNA purification from sucrose gradients. Methods Mol Biol. 2011;703:293–309. doi: 10.1007/978-1-59745-248-9_20. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25(7):755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol. 2013;15(12):1415–1423. doi: 10.1038/ncb2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stutz A, et al. Masking, unmasking, and regulated polyadenylation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev. 1998;12(16):2535–2548. doi: 10.1101/gad.12.16.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dooms H. Interleukin-7: Fuel for the autoimmune attack. J Autoimmun. 2013;45:40–48. doi: 10.1016/j.jaut.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Li HK, et al. Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim Reprod Sci. 2008;103(3-4):312–322. doi: 10.1016/j.anireprosci.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82(6):1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 24.Richter JD. CPEB: A life in translation. Trends Biochem Sci. 2007;32(6):279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: Paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Høst E, Gabrielsen A, Lindenberg S, Smidt-Jensen S. Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injection. Fertil Steril. 2002;77(3):511–515. doi: 10.1016/s0015-0282(01)03006-0. [DOI] [PubMed] [Google Scholar]
- 27.Corn CM, Hauser-Kronberger C, Moser M, Tews G, Ebner T. Predictive value of cumulus cell apoptosis with regard to blastocyst development of corresponding gametes. Fertil Steril. 2005;84(3):627–633. doi: 10.1016/j.fertnstert.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, et al. Oocyte-expressed interleukin 7 suppresses granulosa cell apoptosis and promotes oocyte maturation in rats. Biol Reprod. 2011;84(4):707–714. doi: 10.1095/biolreprod.110.086504. [DOI] [PubMed] [Google Scholar]
- 29.Ostanin AA, Aizikovich BI, Aizikovich IV, Kozhin AY, Chernykh ER. Role of cytokines in the regulation of reproductive function. Bull Exp Biol Med. 2007;143(1):75–79. doi: 10.1007/s10517-007-0021-2. [DOI] [PubMed] [Google Scholar]
- 30.Rosen MP, et al. The effect of follicular fluid hormones on oocyte recovery after ovarian stimulation: FSH level predicts oocyte recovery. Reprod Biol Endocrinol. 2009;7:35. doi: 10.1186/1477-7827-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.