Significance
Current tools for bacterial genome engineering suffer from major limitations. They have been optimized for a few laboratory model strains, lead to the accumulation of numerous undesired, off-target modifications, and demand extensive modification of the host genome prior to large-scale editing. Herein, we address these problems and present a simple, all-in-one solution. By utilizing a highly conserved mutant allele of the bacterial mismatch-repair system, we were able to gain unprecedented precision in the control over the generation of desired modifications in multiple bacterial species. These results have broad implications with regards to both biotechnological and clinical applications.
Keywords: genome engineering, synthetic biology, recombineering, off-target effects, methyl-directed mismatch repair
Abstract
Currently available tools for multiplex bacterial genome engineering are optimized for a few laboratory model strains, demand extensive prior modification of the host strain, and lead to the accumulation of numerous off-target modifications. Building on prior development of multiplex automated genome engineering (MAGE), our work addresses these problems in a single framework. Using a dominant-negative mutant protein of the methyl-directed mismatch repair (MMR) system, we achieved a transient suppression of DNA repair in Escherichia coli, which is necessary for efficient oligonucleotide integration. By integrating all necessary components into a broad-host vector, we developed a new workflow we term pORTMAGE. It allows efficient modification of multiple loci, without any observable off-target mutagenesis and prior modification of the host genome. Because of the conserved nature of the bacterial MMR system, pORTMAGE simultaneously allows genome editing and mutant library generation in other biotechnologically and clinically relevant bacterial species. Finally, we applied pORTMAGE to study a set of antibiotic resistance-conferring mutations in Salmonella enterica and E. coli. Despite over 100 million y of divergence between the two species, mutational effects remained generally conserved. In sum, a single transformation of a pORTMAGE plasmid allows bacterial species of interest to become an efficient host for genome engineering. These advances pave the way toward biotechnological and therapeutic applications. Finally, pORTMAGE allows systematic comparison of mutational effects and epistasis across a wide range of bacterial species.
Recent advances in genome engineering technologies are transforming basic research and industrial biotechnology through the previously unprecedented ability to engineer biological traits. Techniques incorporating zinc-finger nucleases, transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR) RNA-guided nucleases have allowed efficient targeted modification of a host of model organisms (1), promising better understanding of biological processes and more efficient production of bioproducts. Although directed nucleases have widened the range of bacterial species into which individual genomic modifications can be introduced, there seems to be a technical limit when it comes to using these techniques for simultaneous modification of multiple loci (2, 3). Among others, multiplex genome editing is required for explicit genotype-phenotype mapping, as well as modification of protein complexes and biosynthetic pathways (4).
Currently, the only genome engineering method in bacteria that enables rapid, automated and high-throughput genome editing is multiplex automated genome engineering (MAGE) (5). MAGE uses recombineering (6) to simultaneously incorporate multiple single-strand DNA (ssDNA) oligonucleotides (oligos), and thereby rapidly creates desired allele combinations and combinatorial genomic libraries. From accelerated optimization of biosynthetic pathways (5, 7–9) to the construction of a so-called “genomically recoded organism” (10–12), MAGE has allowed genome-engineering endeavors of unparalleled complexity in Escherichia coli. Functionality of ssDNA-mediated recombineering has been described in various other species, including lactic acid bacteria (13), Corynebacterium glutamicum (14), and Bacillus subtilis (15). However, portability remains seriously limited as these efforts require prior optimization for each individual target species (Table S1).
Table S1.
Comparison of various genome engineering methods, based partly on ref. 57
| Method | Multiplexability | Ease-of-use | Off-target mutagenesis | Portability |
| Zinc-finger nucleases (ZFNs) (1, 57) | Low; limited to one or two simultaneous targets. | Low; limited in targetable sites, specific and active ZFNs are difficult and expensive to design. | Moderate; application of separated FokI domain - ZF fusions can make ZFNs less prone to off-site cleavage. Specificity can also be enhanced by increasing the length of target sequence (increasing the number of ZF modules). | High; ZFN-based genome editing has been demonstrated in viruses, bacteria, nematodes, plants, insects, mammals and other higher order eukaryotes. |
| TALE-nucleases (TALENs) (1, 57) | Low; limited to one or two simultaneous targets. | Moderate; one-to-one correspondence between TALE modules and the four DNA bases makes specific TALEN design easy. The chemical synthesis of TALE DNA arrays can be challenging due to repetitive sequence motifs. | Low; TALENs have fewer off-target effects than corresponding ZFNs and strategies applied for increasing ZFNs specificity can also be applied here. | High; TALEN-based genome editing has been demonstrated in viruses, bacteria, yeast, nematodes, plants, insects, mammals, and other higher order eukaryotes. |
| RNA-guided endonucleases (RGENs) (1, 57) | Moderate; limited to ∼five simultaneous targets. | High; implementation and design of RGENs with desired sequence specificities is simple, new RGENs can be easily prepared by expressing guide DNA sequences from an expression vector or by direct in vitro transcription. | Variable; RGENs have more off-target effects than corresponding ZFNs and TALENs due to shorter target sequences, but application of paired-nickases and the truncation of guide RNAs have been shown to improve specificity. | High; RGEN based genome editing has been demonstrated in viruses, bacteria, yeast, nematodes, plants, insects, mammals, and other higher order eukaryotes. |
| MuGENT (37) | Moderate; has been demonstrated for up-to five simultaneous targets. | High; generation of cassettes for allelic replacement are more time and resource consuming compared with the direct chemical synthesis of ssDNA oligos for MAGE, number of cycles are limited by the number of coselection markers available, iterative genome editing cycles are time consuming (∼1 wk per cycle). | Variable; dependence on MMR functionality and in turn vulnerability to off-target mutagenesis is species dependent. | Moderate; demonstrated only in Vibrio cholerae and Streptococcus pneumoniae, transformation procedure is species specific. |
| MAGE (5, 7) | High; microarray oligonucleotide (MO)-MAGE (36) is amenable to simultaneous targeted mutagenesis of thousands of chromosomal loci. | High; implementation, design and synthesis of recombineering ssDNA oligonucleotides are easy and computer programs (optMAGE, MODEST, MERLIN) (35, 58) are available to aid design. Has also been demonstrated to be automatable. | High; extensive off-target mutagenesis is due to the MMR deficient background necessary for efficient MAGE. Various strategies have been developed to suppress off-target activity. | Low; demonstrated only in E. coli, similar approach (YOGE) has been developed for S. cerevisiae (40). |
| pORTMAGE | High; easily adaptable to the (MO)-MAGE protocol allowing thousands of simultaneous targets. | High; same as MAGE with the added benefit of requiring only a single plasmid transformation to prepare target cell. | Low; no observable off-target events after 24 pORTMAGE cycles. | Moderate to high; demonstrated thus far in enterobacterial species, potentially applicable to a wider range of bacteria and eukaryotes. |
Even in E. coli, for efficient and unbiased incorporation of mutations by MAGE, extensive modifications—expression of the λ Red recombinase enzymes, as well as inactivation of the native methyl-directed mismatch repair (MMR) system—need to be made to the host strain. Additionally, because of the inactivation of the MMR machinery (16) necessary for MAGE, there is a nearly two orders-of-magnitude increase in the background mutation rate during the process, leading to the accumulation of undesired, off-target mutations (17). These off-target mutations could in turn interfere with the phenotypic effects of the engineered modifications. Recently, we attempted to address this issue by replacing wild-type mutL and mutS with heat-sensitive mutants, and limiting the inactivation of MMR to a short period of the MAGE cycle. Although we managed to reduce the number of off-target mutations by 85% (18), modification of the parental strain was still a prerequisite. This issue was also recently addressed by the so-called transient-mutator MAGE technique (19), which allows for flexible, plasmid-based modification of bacterial chromosomes. However, transient-mutator MAGE was only demonstrated to work in E. coli, therefore portability across species remained a formidable challenge.
Here, we developed a generalized strategy for bacterial genome editing that overcomes these limitations of MAGE and expands multiplex recombineering to other bacterial species. Key to this process is the temperature-controlled expression of a dominant-negative mutator allele of the E. coli MMR protein MutL (20), enabling transient suppression of DNA repair during oligonucleotide integration. MutL is a component of the MutHLS complex responsible for methyl-directed mismatch repair, acting to recruit the MutH endonuclease to sites of DNA damage (21). Importantly, this particular mutator allele cannot be complemented by the native MutL protein (20). Therefore, in contrast to traditional MAGE, no prior disruption of the genomic copy is needed.
In this work, a set of plasmids (dubbed pORTMAGE) expressing the λ Red recombinase enzymes, as well as the dominant-negative mutator allele of MutL, all under the control of the cI857 temperature-sensitive repressor, were constructed. During each MAGE cycle, expression of the synthetic operon is induced by a single temporal temperature shift. Lowered MMR activity is only necessary during a brief period of each MAGE cycle (i.e., during allelic replacement). Because our protocol allows for rapid switching between mutator and nonmutator states, it minimizes the time the bacterial population spends susceptible to the accumulation of off-target mutations. As the standard MAGE protocol already incorporates a shift in temperature during induction of the λ Red recombinase enzymes, the temperature shift required during pORTMAGE is entirely compatible with the established procedure (5).
Additionally, because of the highly conserved nature of MutL (22), expression of this E. coli mutL allele can suppress mismatch repair in distant relatives of E. coli, diverged from it ∼100–200 million y ago (23). Thus, pORTMAGE simultaneously allows genome editing and mutant library generation in several biotechnologically and clinically relevant bacterial species. As a proof-of-concept study, we demonstrate that pORTMAGE is a valuable tool to study clinically relevant issues, such as the phenotypic impact of antibiotic resistance conferring mutations across bacterial species.
Results
Characterization of the MutL E32K Dominant-Negative Mutator.
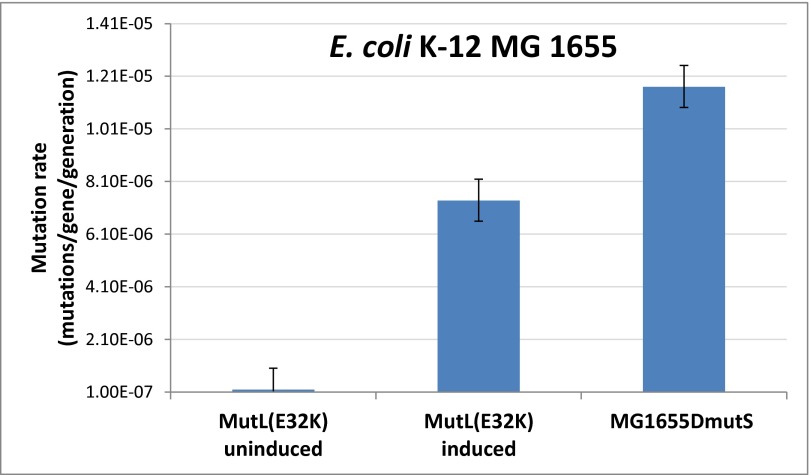
First, we characterized the effect of the previously described (20) dominant-negative mutator allele MutL E32K on the functionality of the MMR system in wild-type E. coli K-12 MG1655. Using standard techniques (SI Materials and Methods), we cloned the mutant allele into the inducible expression vector pZA31tetR (24, 25). Induced expression resulted in an over 30-fold increase in mutation rate in the wild-type as measured by a rifampicin resistance assay and subsequent fluctuation analysis (26) (Fig. S1). This approached the mutation rate of the MG1655ΔmutS deletion strain lacking functional MMR machinery, and showed that the wild-type copy of MutL was not able to suppress the effect of the dominant-negative mutant allele. We conclude that this construct enables controlled disruption of the MMR machinery from an extrachromosomal expression system.
Fig. S1.
Mutation rate measurement of E. coli K-12 MG1655 (MG) harboring the AhTC inducible pZA31tetR-mutLE32K plasmid for MutL(E32K) expression, as well as the MG1655ΔmutS strain for comparison. A rifampicin resistance assay was used to calculate mutation rates, as described in SI Materials and Methods. Error bars represent 95% confidence intervals based on two independent measurements of 10 parallel samples each. DmutS denotes deletion of the mutS gene.
pORTMAGE Allows Highly Efficient and Unbiased Allelic Replacement in E. coli.
We rationalized that temperature-controlled expression of a dominant-negative mutator allele of MutL (18) would allow a transient switch from a nonmutator to mutator phenotype of the host cell. At nonpermissive temperatures (30–34 °C), the mutant protein is not expressed, allowing the native MMR system to function properly, thus limiting the number of background mutations to wild-type level. Moreover, as the particular E32K mutator allele cannot be complemented by the native MutL protein, no disruption of the genomic copy is needed.
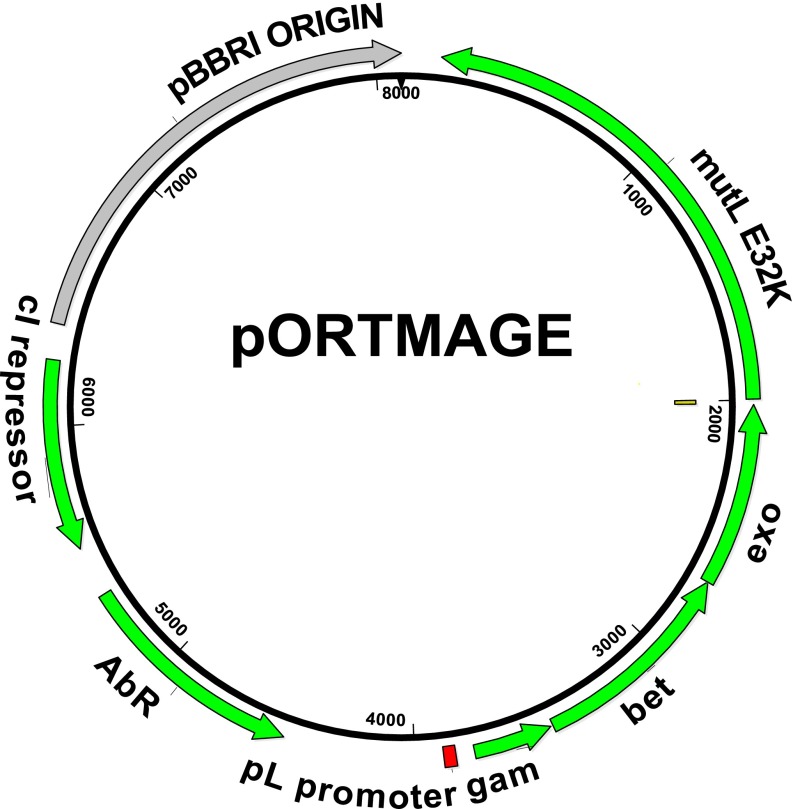
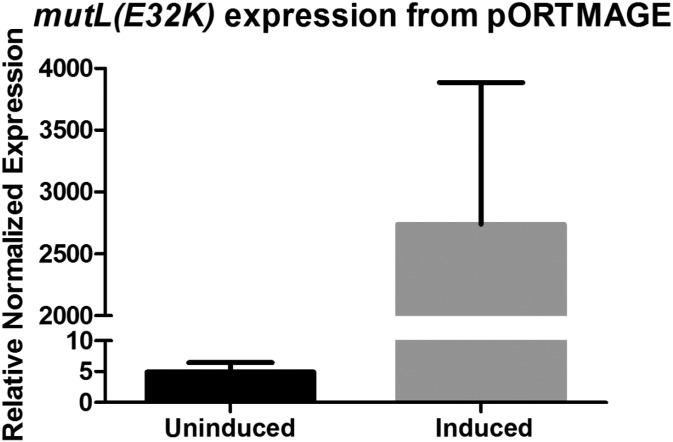
For ease of use, all required genetic parts for MAGE were assembled on a single plasmid with a broad host range origin of replication (pBBR1) (27), resulting in the pORTMAGE plasmid (Fig. 1). In this arrangement, expression of the mutL E32K gene, as well as the three λ Red recombinase enzymes (exo, bet, and gam) were under the control of the cI857 temperature-sensitive λ repressor. Quantitative real-time RT-PCR (qPCR) testing showed that temperature induced transcription of the operon resulted in a 320- to 770-fold increase in mutL expression (Fig. S2).
Fig. 1.
General map of the pORTMAGE plasmid. Expression of the mutL E32K gene [along with the three λ Red recombinase enzymes (exo, bet, and gam)] is controlled by the cI857 temperature-sensitive λ repressor.
Fig. S2.
Relative expression of mutL(E32K) from pORTMAGE measured with qPCR. For details, see SI Materials and Methods. Values represent the mean of six parallel samples each, error bars represent SEM.
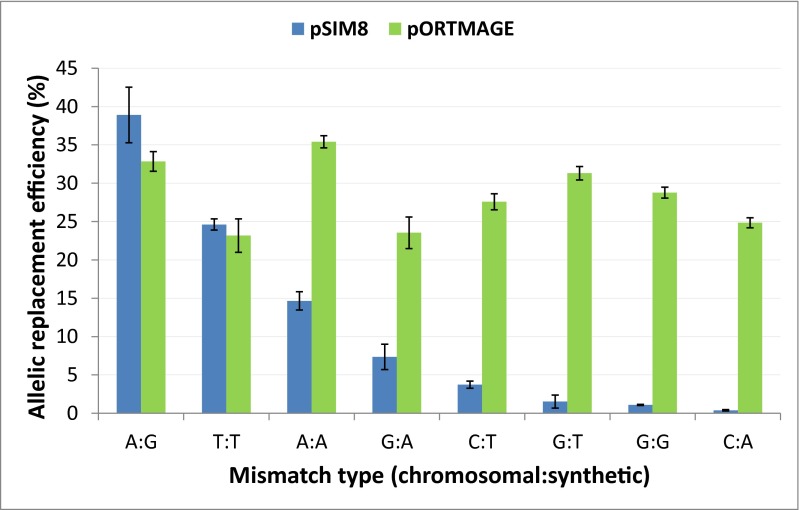
To investigate the effect of the expression of the dominant-negative MutL allele on allelic replacement frequency of ssDNA oligos, we used a previously characterized test system (28). We introduced a diverse set of single-base pair mismatches (A:A, G:G, T:T, G:A, G:T, C:A, and C:T) at specific genomic locations within lacZ. Because these mutations introduce premature stop codons within lacZ, the frequency of allelic replacement could be easily measured by a colorimetric assay (SI Materials and Methods). Two separate oligos were used to generate a G:A mismatch at different positions to show that repair of a specific mismatch type is also context-dependent, as demonstrated previously (28). We found that in all cases, pORTMAGE allowed highly efficient and unbiased oligo incorporation, comparable to the capacity of the traditional MAGE protocol on a mismatch repair knockout background (18, 28). Indeed, allelic replacement frequency greater than 20% was observed in all instances, even in the cases of mismatches that are otherwise well recognized and almost completely corrected by the wild-type MMR system (Fig. S3).
Fig. S3.
Allelic replacement frequencies of oligos generating various types of single-base pair modifications in the chromosome in MG1655(pSIM8) and MG1655(pORTMAGE). The frequency of allelic replacement was estimated by the number of mutant cells per total colony forming units. The values are the means of two independent measurements each; error bars represent SEMs. For details, see SI Materials and Methods.
Efficient Multiplex Genome Editing Is Coupled with Low Off-Target Mutation Rate.
Using pORTMAGE, we carried out multiplex genome editing to investigate allelic replacement frequency and off-target mutagenesis simultaneously. Three strains were compared: a mutator strain carrying the control pSIM8 plasmid (27) expressing the λ Red recombinases [ΔmutS(pSIM8)], the wild-type nonmutator strain carrying pORTMAGE [MG1655(pORTMAGE)], and the wild-type strain carrying the control plasmid [MG1655(pSIM8)].
In 24 cycles of MAGE, six different loci were subsequently targeted (four cycles each). These loci are widely distributed across the genome and were targeted by oligos that introduce various types of mismatches into them (see ref. 18 for details). Clones carrying a particular modification were verified by capillary sequencing, followed by MAGE cycles targeting the next locus. Allelic replacement frequencies were determined at each locus either by colorimetric assay or allele-specific PCR (SI Materials and Methods).
As expected, allelic replacement frequency with MG1655(pSIM8) was very low in all cases. MG1655(pORTMAGE) generally displayed a high level of replacement, approaching the values observed with traditional MAGE using ΔmutS(pSIM8) (Table 1). In some cases (e.g., araB T50A and cycA AA139TG), the values observed with MG1655(pORTMAGE) were significantly lower than with ΔmutS(pSIM8) but still over an order-of-magnitude higher than that of MG1655(pSIM8).
Table 1.
Allelic replacement frequency of all six used oligos in MG1655(pSIM8), ΔmutS(pSIM8), and MG1655(pORTMAGE) after four genome editing cycles each
| Strain | Allelic replacement frequency (%) | |||||
| lacZ A652T | malK C252G | araB T50A | hisB C166T | rpsL A128G | cycA AA139TG | |
| MG1655(pORTMAGE) | 54.58 | 61.56 | 39.76 | 22.92 | 33.76 | 22.92 |
| ΔmutS(pSIM8) | 51.32 | 60.23 | 62.87 | 39.58 | 38.91 | 41.67 |
| MG1655(pSIM8) | 45.31 | 51.82 | 1.56 | <0.1 | 0.72 | 1.04 |
Each oligo name consists of the targeted gene and the introduced nucleotide modification.
Next, we investigated the accumulation of off-target mutations. After 24 cycles of MAGE, we selected one independently edited clone each derived from MG1655(pORTMAGE), ΔmutS(pSIM8), and MG1655(pSIM8), respectively. To infer the number of off-target mutations, the genomes of the starting and the MAGEderived clones were sequenced using the IonTorrent PGM system. MG1655(pSIM8) accumulated only two off-target mutations. In sharp contrast, ΔmutS(pSIM8) carried 84 different off-target genomic mutations, a figure that is in line with previous reports (17, 18). Remarkably, we failed to find any off-target mutation in MG1655(pORTMAGE). For a complete list of all off-target mutations, see Dataset S1.
Taken together, these results demonstrate that high allelic replacement frequency in pORTMAGE is coupled with an exceptionally low off-target mutation rate.
pORTMAGE Allows Rapid Genome Editing Across a Range of Bacterial Species.
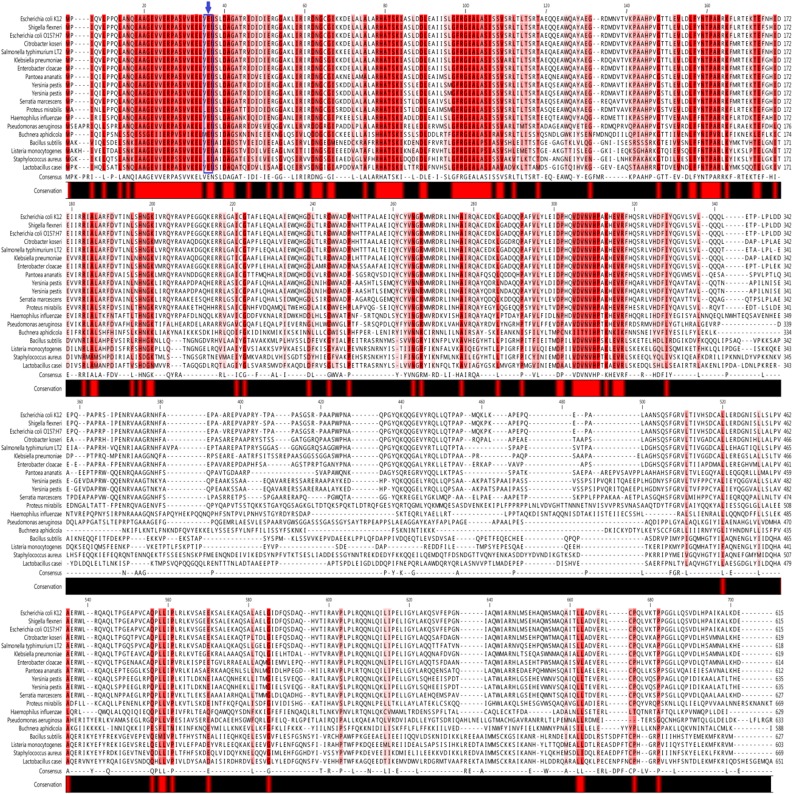
We first tested the impact of the dominant mutL allele on mutation rates in several enterobacterial species with biotechnological or clinical relevance. We selected as models the clinically important Salmonella enterica serovar Typhimurium (strain LT2), the fish pathogen Edwardsiella tarda (strain ATCC15947), the opportunistic pathogen Escherichia hermanii (strain HNCMB35034), as well as the biotechnologically relevant organisms Citrobacter freundii (strain ATCC8090), and E. coli BL21(DE3). Phylogenetic comparison of MutL sequences (Fig. S4) showed that glutamic acid is conserved in all investigated species at the position of the mutation in the E. coli dominant-negative allele E32K (22). We therefore assumed that the mutant allele would have similar phenotypic effect in these species. In agreement with expectation, overproduction of the E. coli mutant MutL showed similar, at least an order-of-magnitude increase in mutation rates in all other investigated species (Fig. S5), indicating the feasibility of the pORTMAGE strategy in other species.
Fig. S4.
Sequence alignment of MutL proteins from different species. Regions of high homology are shown in red, position 32 (carrying the dominant mutation) is highlighted in blue.
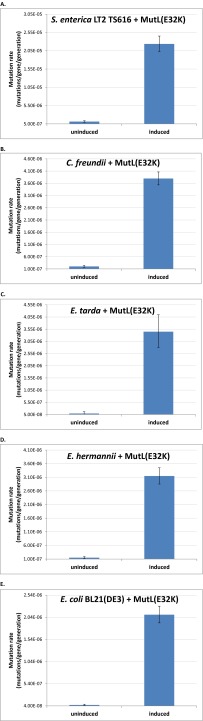
Fig. S5.
Mutation rate measurement of candidate species from Enteriobacteriaceae harboring the AhTC inducible pZA31tetR-mutLE32K plasmid for MutL(E32K) expression. Uninduced and induced mutation rates for (A) TS616 derivative of S. enterica serovar Typhimurium LT2, (B) C. freundii (strain ATCC8090), (C) Edwardsiella tarda (strain ATCC15947), (D) Escherichia hermannii (strain HNCMB35034), and (E) E. coli strain BL21(DE3). A rifampicin resistance assay was used to calculate mutation rates as described in SI Materials and Methods. Error bars represent 95% confidence intervals based on two independent measurements of 10 parallel samples each.
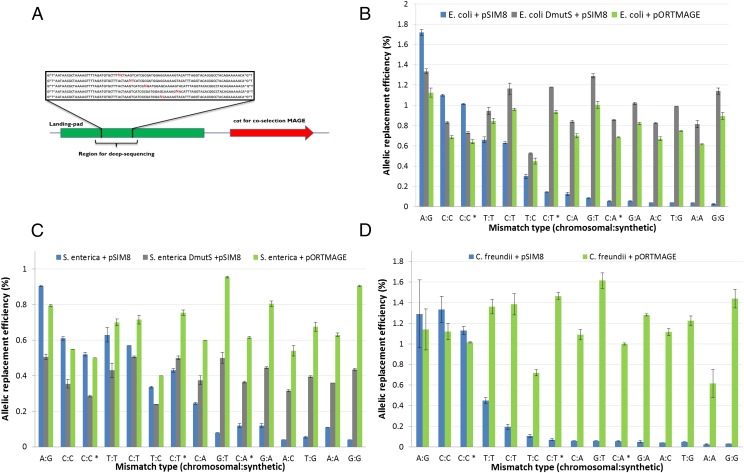
Next, we compared the efficacy of pORTMAGE in E. coli K-12 MG1655, and distant relatives S. enterica and C. freundii. To broaden the potential applications of pORTMAGE, we engineered three modified pORTMAGE plasmids with elevated MutL expression and different antibiotic markers (SI Materials and Methods). To characterize the performance of pORTMAGE in a uniform manner across species, we constructed a landing pad sequence integrated into the host genome, and used it as the target sequence (Fig. 2A; see also Dataset S2 for all bacterial strains used in the study and the corresponding genotype information, and Dataset S3 for the complete nucleotide sequence). A set of five oligos (90 nucleotides in length each), introducing all possible single-base mismatches at given positions were designed. Each oligo carried a degenerate base at one of four specific positions representing all four nucleotides, plus an additional oligo to assay positional differences of the same target nucleotide. One MAGE cycle was carried out using these oligos pooled together.
Fig. 2.
(A) General map of the landing pad sequence inserted into the various strains. The green region represents a target sequence for allelic replacement by a set of five oligos shown in the targeting box. Degenerate bases are shown for each oligo in red. The cat gene conferring resistance to chloramphenicol was included in the landing pad to allow for cos-MAGE to be performed (see SI Materials and Methods for details). Allelic replacement frequency of the various test oligonucleotides targeting the tetR landing pad sequence in (B) E. coli K-12 MG1655, (C) TS616 derivative of S. enterica serovar Typhimurium LT2, and (D) C. freundii (strain ATCC8090). The values are the means of two independent measurements using Illumina deep-sequencing; error bars represent SEMs. An asterisk denotes oligos generating the same mismatch as another oligo to demonstrate context dependency of allelic replacement. DmutS denotes deletion of the mutS gene.
As in the previous section, we compared the performances of the wild-type strain carrying the control plasmid, the wild-type nonmutator strain carrying a pORTMAGE plasmid, and the mutator ΔmutS strain carrying the control pSIM8 plasmid. To measure allelic replacement frequencies within the population after one MAGE cycle, we performed deep-sequencing of the targeted landing pad sequence using an Illumina MiSeq set-up.
Replacement efficiency in the wild-type control carrying pSIM8 varied substantially across the three investigated species (Fig. 2 B–D), suggesting natural variation in mismatch repair. In addition, mutations introducing the same mismatch at different positions showed a difference in some cases compared with each other, indicating a context dependency of the efficiency of mismatch repair. Despite these differences, allelic replacement frequency with pORTMAGE was in several cases two orders-of-magnitude higher and largely unbiased in all three species compared with the wild-type control (Fig. 2 B–D). Moreover, in E. coli K-12 MG1655 (Fig. 2B) and S. enterica (Fig. 2C), allelic replacement frequencies with pORTMAGE approached the values obtained with ΔmutS for all incorporated mismatch types. In C. freundii, the pORTMAGE plasmid showed the same robust performance as in E. coli and S. enterica (Fig. 2D).
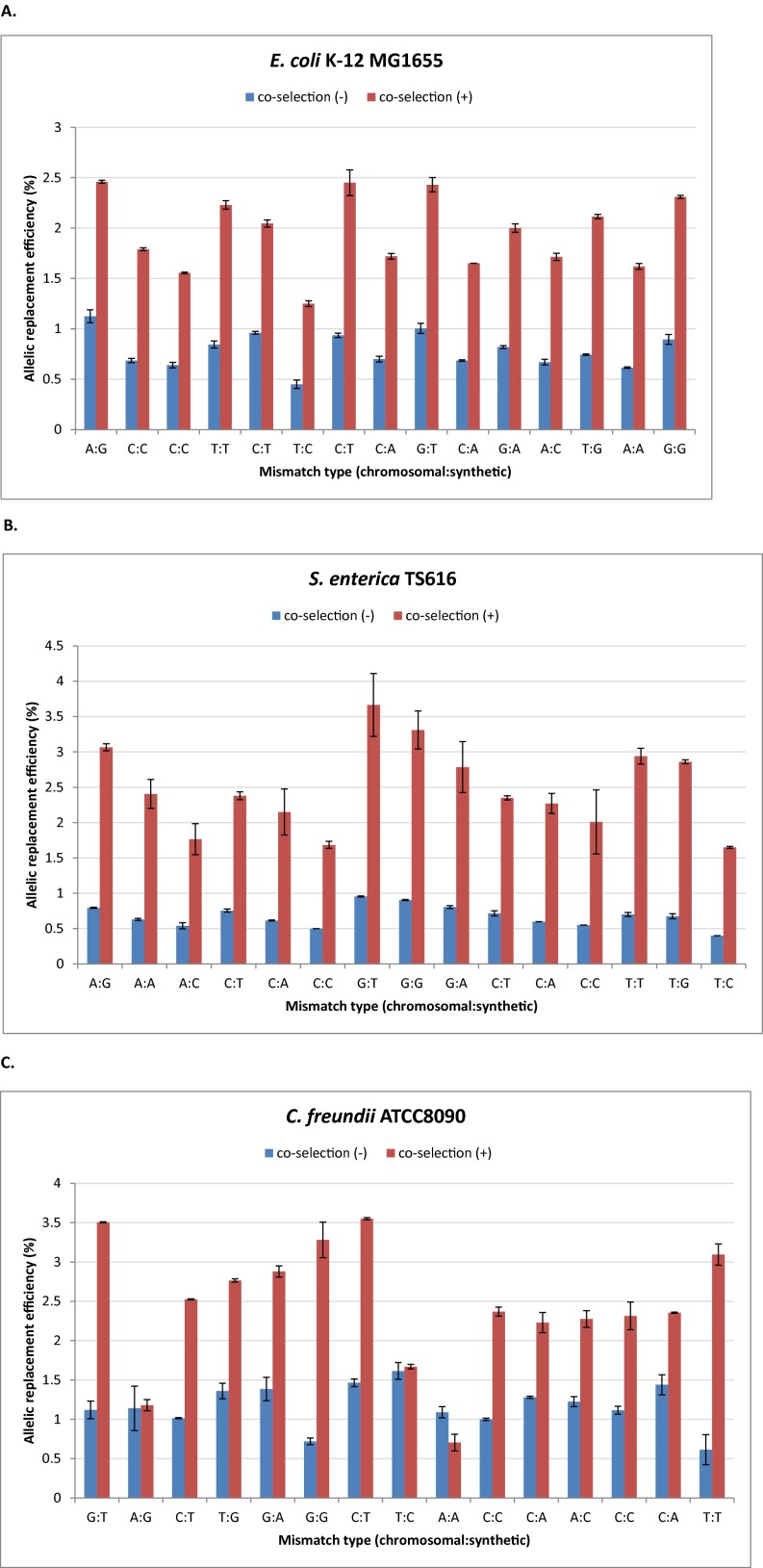
Finally, we investigated the impact of pORTMAGE on replacement efficiency using an established protocol (cos-MAGE) that includes enrichment of the desired genetic modifications using a selectable marker gene (7). Such a coselection procedure is biotechnologically relevant, as it aids incorporation of hard-to-engineer genomic modifications. The landing pad sequence was designed to contain an inactivated cat gene. Accordingly, a single MAGE cycle was carried out using an oligo repairing the inactivated cat gene, followed by selection for the appropriate genetic marker (chloramphenicol resistance). We found that pORTMAGE substantially increased allelic replacement frequencies in all investigated species (Fig. S6).
Fig. S6.
Effects of coselection on allele replacement efficiency. (A) E. coli K-12 MG1655(pORTMAGE) allelic replacement frequency with and without coselection. (B) S. enterica TS616(pORTMAGE) allelic replacement frequency with and without coselection. (C) C. freundii ATCC8090(pORTMAGE) allelic replacement frequency with and without coselection. The values are the means of two independent measurements using Illumina deep-sequencing, error bars represent SEMs.
pORTMAGE Allows Efficient Generation of Mutant Libraries in Various Bacterial Species.
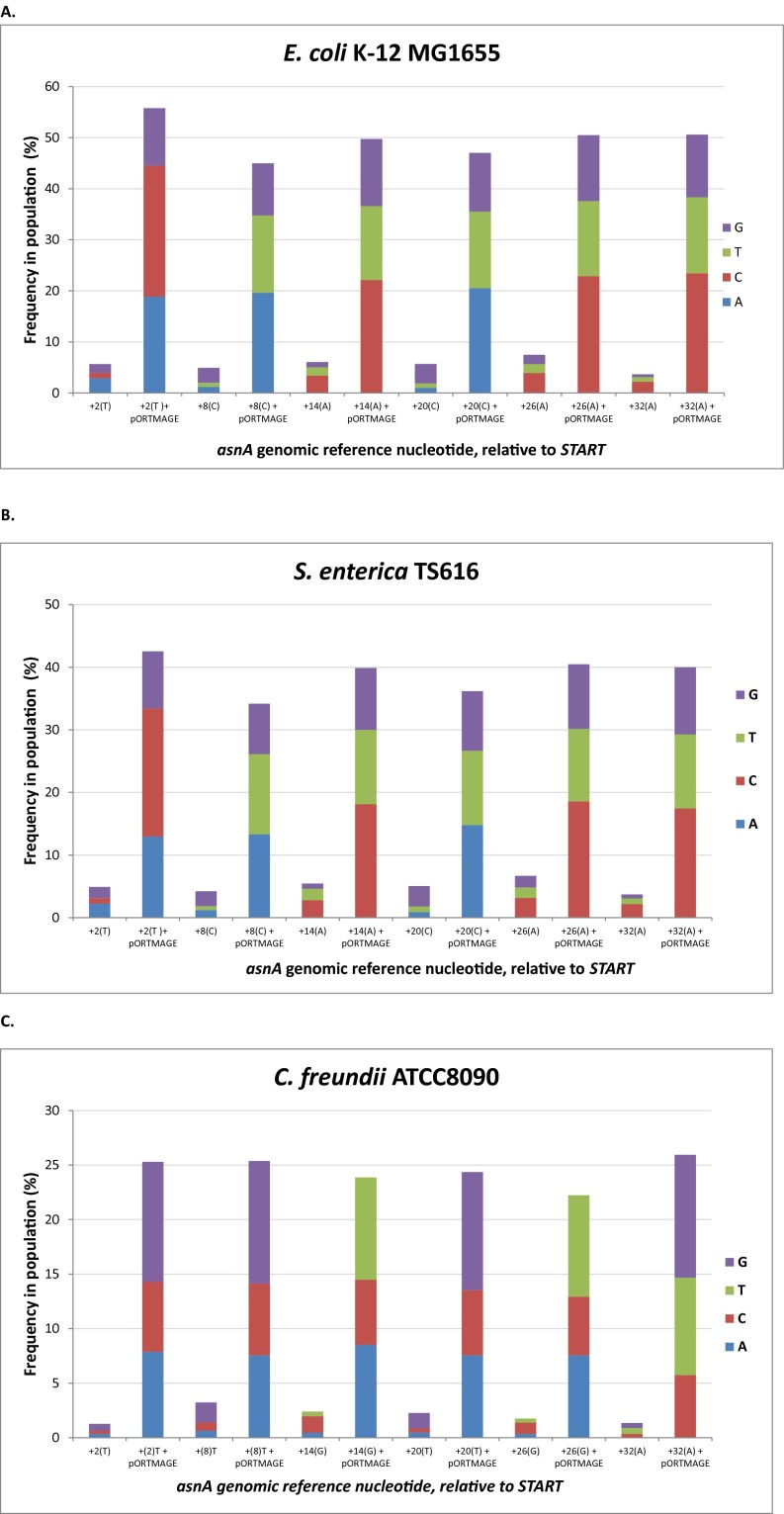
Next, using pORTMAGE, we introduced sequence diversity at specific genetic loci. We randomized six individual bases within the asnA gene in three species (E. coli, S. enterica, and C. freundii). Using organism-specific 90-base oligos carrying six randomized positions, we carried out five cycles of MAGE. Genomic DNA was isolated, the target region was amplified by PCR, and finally, the fragments were subjected to Illumina deep-sequencing. In all three species (Fig. S7), allelic replacement frequencies with pORTMAGE were at least an order-of-magnitude higher at all six positions, compared with the frequencies obtained with the control pSIM8 plasmid. Additionally, pORTMAGE substantially reduced the biases in the incorporation frequency of certain nucleotides, allowing for a more uniform distribution of the mutants in the population. Using pORTMAGE, we obtained a bias-free mutant library at the target locus for each species (Fig. S7), suggesting that all possible ∼4,000 variants were represented at a reasonable quantity.
Fig. S7.
Sequence randomization of 6 nucleotides of the asnA gene of (A) E. coli K-12 MG1655, (B) TS616 derivative of S. enterica serovar Typhimurium LT2, and (C) C. freundii (strain ATCC8090). A comparison is shown using the control pSIM8 (first stacked column for each position) versus pORTMAGE (indicated with “+pORTMAGE” for the given stacked column). The frequencies of each nucleotide at each of the mutated genomic positions are represented in each stacked column. The genomic positions of each randomized nucleotide relative to the first nucleotide of the gene, as well as the wild-type allele (shown in parentheses) are indicated.
Application of pORTMAGE to Study Antibiotic Resistance in S. enterica.
It has long been suggested that because of the prevalence of epistatic interactions, mutations beneficial in one genetic background are frequently neutral or even deleterious in another (29). This issue is especially relevant in the context of antibiotic resistance, as this phenomenon could contribute to the observed differences in the molecular mechanisms underlying antibiotic resistance in related microbial species. Better understanding of this problem demands genome editing applicable in a range of species. Here, we demonstrate that pORTMAGE is an exceptionally effective tool for studying the phenotypic effects of individual mutations.
We introduced 10 mutations individually into the genomes of S. enterica and E. coli (for the list of mutations, see Table 2). These mutations have previously been detected in an experimental evolution study (30) and confer resistance to one or multiple antibiotics. The corresponding genes are not only clinically relevant, but also show 79–99% sequence identities between the two species. The nucleotide sequences subjected to editing by pORTMAGE were fully conserved in most cases and were mutated to the same residue in all cases.
Table 2.
Relative MIC values of mutant strains of E. coli and S. enterica
| Gene | Mutation | Antibiotic | E. coli relative MIC | S. enterica relative MIC |
| mprA | Arg110Leu | NIT | 1.20 | 1.73 |
| marR | Val84Glu | AMP | 2.20 | 1.15 |
| soxR | Leu139* | ERY | 2.31 | 1.73 |
| phoQ | Gly384Cys | NIT | 1.20 | 0.83 |
| trkH | Thr350Lys | STR | 3.18 | 1.59 |
| gyrA | Ser83Leu | CPR | 16.00 | 16.00 |
| gyrA | Ser83Leu | NAL | 97.66 | 610.35 |
| fis | Thr70Pro | ERY | 1.44 | 1.44 |
| acrR | Gln78* | ERY | 1.20 | 1.00 |
| ompC | Met1 | NIT | 1.20 | 1.20 |
| ycbZ | Ser438Arg | ERY | 1.23 | 1.51 |
The sensitivity of each mutant strain was measured against the antibiotic against which the specific mutation formed during laboratory adaptation (30). The measured MIC for each strain was then compared with the MIC of the wild-type strain, resulting in the relative MIC value. The antibiotic abbreviations are as follows: AMP, ampicillin; CPR, ciprofloxacin; ERY, erythromycin; NAL, nalidixic acid; NIT, nitrofurantoin; STR, streptomycin.
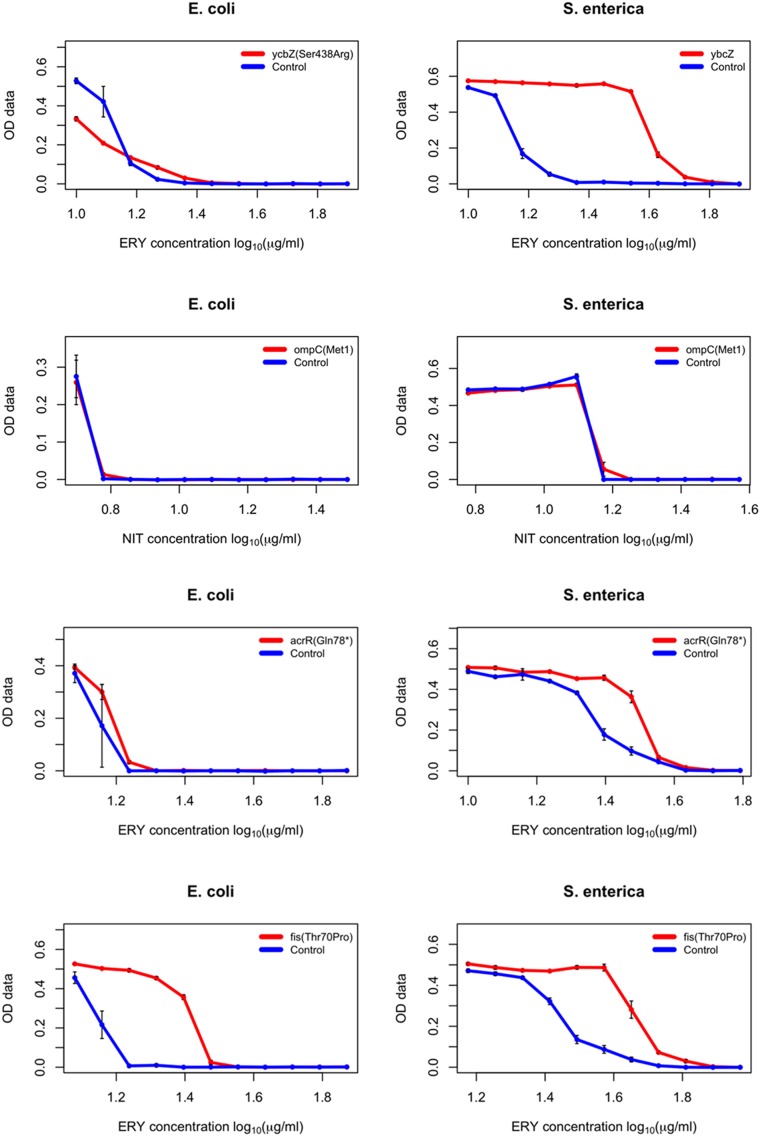
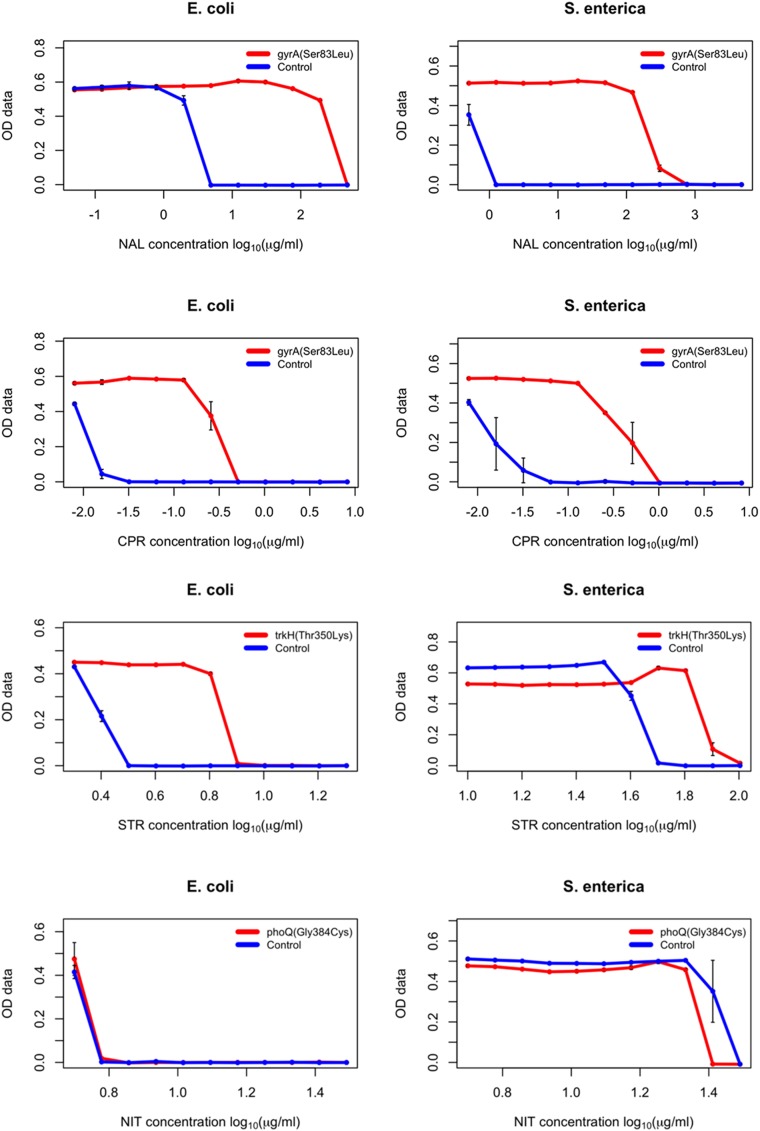
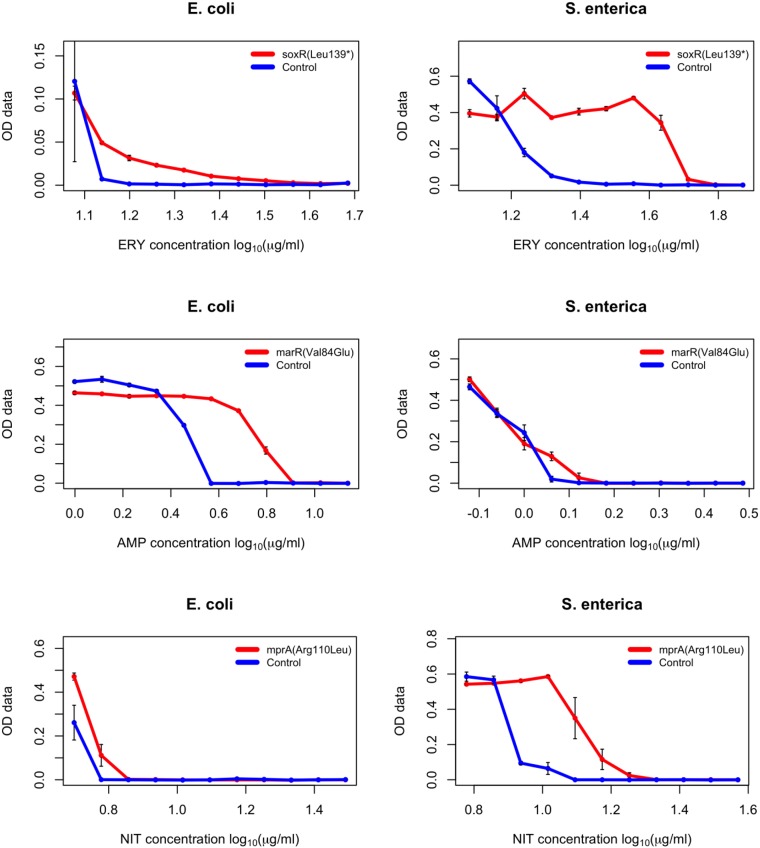
The workflow consisted of the introduction of each mutation into the receiver strain by a single MAGE cycle, selection of the desired mutation by high-resolution melting (HRM) analysis, and subsequent confirmation of the mutations by capillary-sequencing. The complete workflow took as few as 4 d for completion. As a measure of the effect of each individual mutation, the sensitivity of each mutant was measured against the antibiotic that it arose against during the course of laboratory evolution (30). Using a well-established protocol (31), the minimum inhibitory concentration (MIC) value of each antibiotic against the given mutant was determined compared with the wild-type strain (relative MIC value = MIC of the mutant/MIC of the wild-type). Finally, we compared the relative MIC changes conferred by each individual mutation in S. enterica and E. coli (Table 2 and Fig. S8).
Fig. S8.
Dose–response curves of the generated E. coli and S. enterica mutants (specific mutation indicated in the chart legends) each compared with the wild-type ancestral strain (E. coli BW25113 and S. enterica serovar Typhimurium LT2, respectively) to determine relative MIC values of given antibiotics (indicated at the bottom of each chart). Each strain was measured in three replicates, error bars represent SEMs. OD data indicated on the y axis represents optical density measurements taken at 600 nm.
We found that despite over 100 million y of divergence between the two species, mutational effects remained generally conserved. In most cases, the investigated mutations resulted in a small, but significant decline in antibiotic susceptibilities in both species (Table 2).
There were a few notable exceptions to this trend. For example, the S83L amino acid substitution in the major target protein (GyrA) confers resistance to nalidixic acid and other gyrase inhibitor drugs (32). The very same mutation is regularly observed in antibiotic-resistant laboratory and clinical E. coli strains (30, 33). Surprisingly, we found that the level of resistance conferred by S83L is at least six-times higher in S. enterica than in E. coli (Table 2). Another interesting case is MarR, a central regulator of the Mar (multiple anbiotic resistance) regulon that coordinates the expression of a global network of at least 80 chromosomal genes (34). The Val84Glu mutation in this gene confers a twofold decrease in ampicillin susceptibility in E. coli, whereas mutating to the same residue barely had any phenotypic effect in S. enterica (Table 2). Clearly, the genetic and molecular mechanisms underlying these differences in mutational effects between species deserve future investigations. Our main aim here was to demonstrate how unique pORTMAGE is to study these clinically important issues.
SI Materials and Methods
Strains and Reagents.
All bacterial strains used in the study and the corresponding genotype information are listed in Dataset S2.
Unless otherwise noted, cultures were grown in Lysogeny-Broth-Lennox (LBL) media (10 g of tryptone, 5 g of yeast extract, 5 g of sodium chloride per 1 L of water) for cell manipulations. AraB, LacZ, and MalK activities were assayed on supplemented MacConkey agar (peptone 20 g, bile salts 1.5 g, sodium chloride 5 g, agar 13.5 g, neutral red 0.03 g, and Crystal violet 1.0 mg per 1 L of water) with either 1% arabinose for AraB, 1% of lactose for LacZ, or 1% maltose for MalK added. Terrific-broth (TB) was applied as recovery media during recombineering (yeast extract 24 g, tryptone 12 g, K2HPO4 9.4 g, KH2PO4 2 g per 1 L of water).
DNA modification enzymes were purchased from Thermo Scientific. Gibson Assembly Master Mix (E2611S) was purchased from New England Biolabs. Polymerase chain reactions (PCR) for cloning purposes and double-stranded DNA (dsDNA) recombineering were performed with Phusion High-fidelity DNA Polymerase (Thermo Scientific). PCR amplifications for screening purposes were performed with Taq DNA Polymerase (Thermo Scientific). Zymo Research DNA Clean and Concentrator Kit was used to concentrate DNA for and after Gibson assembly or conventional T4 DNA ligase cloning and before electroporation of cloned plasmid constructs and linear DNA fragments for dsDNA recombineering.
Oligonucleotides.
A full list of DNA oligonucleotides used in this work is provided in Dataset S2. All oligos were ordered with standard desalting from IDT (Integrated DNA Technologies). Design of recombineering oligos followed the general guidelines for MAGE (4).
Plasmid Construction.
All plasmids used in the study are listed in Dataset S2. pSIM6 and pSIM8 were a gift of Donald L. Court, National Cancer Institute, Frederick, MD. pZA31tetR and pZA31YFPtetR were donated by Tamás Fehér, Biological Research Centre of the Hungarian Academy of Sciences, Szeged, Hungary.
pORTMAGE1 and derivatives were constructed by introducing the Escherichia coli MutL E32K mutant into the broad host range (pBBR1 origin of replication) plasmid pSIM8. First, the native mutL from E. coli K-12 MG1655 was cloned into the λ Red operon, downstream of the exo gene and upstream of the tL terminator to yield pSIM8-mutL. Assembly of pSIM8-mutL was verified by PCR and subsequent sequencing with LExoF and tL3R primers. Introduction of the dominant MutL E32K mutation into pSIM8-mutL was achieved by whole-plasmid amplification mutagenesis. Briefly, PCRs in 50-μL total volume were cycled 35 times at 98 °C 10 s, 56 °C 30 s, and 72 °C for 5 min using the mutL32F and mutL32R primers. The product was treated with 1 U of DpnI for 60 min at 37 °C and was cleaned and concentrated into 12 μL deionized water. Recircularization of the plasmid was performed at 18 °C overnight by T4 DNA Ligase and subsequent transformation into E. coli K12 MG1655 electrocompetent cells. Correct clones were verified by colony-PCR and subsequent sequencing with LExoF and tL3R primers.
pORTMAGE2 was constructed by introducing the strong, consensus (in bold) ribosomal binding site (5′-GAGAGGAGGTATATAC) upstream of the mutL E32K allele in pORTMAGE1 by whole-plasmid amplification mutagenesis.
To broaden the applicability of pORTMAGE toward genome engineering in certain antibiotic-resistant species and isolates, we constructed kanamycin and chloramphenicol resistance-marker variants of the pORTMAGE2 plasmid (pORTMAGE3 and -4, respectively), as well as Standard European Vector Architecture (SEVA) format-based derivatives (49). A kanamycin resistant variant of pSIM8 and the kanamycin and chloramphenicol resistant variants of pORTMAGE2 were constructed by replacing the AmpR gene with either KanR or cat resistance marker using Gibson-assembly. The plasmids were linearized by PCR amplification with pL32K frame_1 and pL32K frame_2 primer pairs and the KanR or cat gene was amplified using the Gibson Kan_Fw or Gibson Chlo_Fw and Gibson Kan_rev or Gibson Chlo_rev primer pair, respectively, with flanking homology to the plasmid terminal sequence. Assembly reactions were performed in Gibson Assembly Master Mix (New England Biolabs) at 50 °C for 60 min. Purified products were transformed into E. coli DH5α electrocompetent cells. Successful assemblies were verified by colony PCR.
pORTMAGE Cycling Protocol.
Freshly streaked individual colonies from LBL agar plates were inoculated into 2-mL LBL aliquots in the presence of 100 μg/mL ampicillin (or 50 μg/mL kanamycin for pSIM8KAN or pORTMAGE3 in Citrobacter freundii) and incubated in a shaking incubator at 30 °C, 250 rpm. Overnight cultures were diluted 1:100 in antibiotic supplemented LBL media and were grown at 30 °C at 250 rpm in a shaking incubator. Each MAGE cycle consisted of the following steps: Upon reaching OD600 = 0.4–0.6, cells were transferred to a 42 °C shaking water bath to induce λ Red protein expression for 15 min at 250 rpm. Cells were then immediately chilled on ice for at least 10 min. Cells were made electrocompetent by washing and pelleting twice in 10 mL of ice-cold dH2O at 2,490 × g for 8 min in a chilled Eppendorf 5702 R centrifuge with swing-out rotor. Electrocompetent cells were suspended in 160 μL of dH2O and were kept on ice until electroporation. Unless otherwise noted, 40-μL cell suspension was mixed with 1 μL of 100 μM oligonucleotide in single-plex experiments. For six-plex allelic replacements, 6 μL of oligo mixture was added, containing each oligo at 2.5 μM final concentration. Electroporation was done on a BTX (Harvard Apparatus) CM-630 Exponential Decay Wave Electroporation System in 1-mm gap VWR Signature Electroporation cuvettes (1.8 kV, 200 Ω, 25 μF). Immediately after electroporation, 1 mL TB medium was added and cells were transferred to 5 mL room-temperature TB media to allow for recovery. After 60 min at 30 °C 250 rpm, 5 mL antibiotic supplemented LBL media was added and cells were allowed to reach midlogarithmic state under continuous agitation. At this point, cells were either subjected to additional MAGE cycles or analyzed for phenotype or genotype.
To assay the performance of recombineering in E. coli K-12 MG1655, single MAGE cycles were performed in E. coli K-12 MG1655(pSIM8), E. coli K-12 MG1655(pORTMAGE2), and E. coli K-12 MG1655 ΔmutS(pSIM8) using oligonucleotides introducing a diverse set of single-base pair mismatches (A:A, G:G, T:T, G:A, G:T, C:A, and C:T) at lacZ (Dataset S2).
For the determination of the off-target effects during long-term application of MAGE, E. coli K-12 MG1655(pSIM8), E. coli K-12 MG1655(pORTMAGE1), and E. coli K-12 MG1655 ΔmutS(pSIM8) cells were applied. During the MAGE cycling, six different loci were subsequently targeted (four cycles individually targeting each locus). These loci are distributed widely across the genome and were targeted by oligos that introduce various types of mismatches into them. Allelic replacement frequencies for all three strains were measured at each locus either by colorimetric assay or allele-specific PCR, as described previously (18). Selected clones carrying the desired modifications were verified by capillary sequencing, after which the MAGE cycles targeting a new locus were continued. Notably, after screening 760 colonies of the E. coli K-12 MG1655(pSIM8) strain, we were not able to isolate one of the desired modifications (hisB) by itself, just in the presence of an undesired 6-bp insertion. After all six desired modifications in each strain [with the exception of the hisB mutation in MG1655(pSIM8)] were identified, genomic DNA (gDNA) was isolated (GenElute Bacterial Genomic DNA kit, Sigma-Aldrich, Cat no. NA2110) according to the manufacturer’s protocol for whole-genome resequencing.
Whole-Genome Resequencing Using the Ion Torrent PGM System.
Fragment libraries were constructed from purified genomic DNA using NEBNext Fast DNA Fragmentation & Library Prep Set for Ion Torrent (New England Biolabs) according to the manufacturer’s instructions. Briefly, genomic DNA was enzymatically digested and the fragments were end-repaired. Ion Xpress Barcode Adaptors (Life Technologies) were than ligated and the template fragments size-selected using AmPure beads (Agencourt). Adaptor ligated fragments were than PCR amplified, cleaned-up using AmPure beads, quality-checked on D1000 ScreenTape and Reagents using TapeStation instrument (Agilent), and finally quantitated using Ion Library TaqMan Quantitation Kit (Life Technologies). The library templates were prepared for sequencing using the Life Technologies Ion OneTouch reagents according to the manufacturer’s protocol (Life Technologies). Template-positive beads were deposited onto the Ion 318 chips (Life Technologies); finally, sequencing was performed with the Ion PGM Hi-Q Sequencing Kit (Life Technologies).
The PGM sequencing data were processed using Ion Torrent Suite to perform signal processing and base calling. Read mapper module of Torrent Suite (tmap) was used to align raw reads to the E. coli K-12 MG1655 genome sequence (U00096.3). The Torrent Variant caller (tvc) module of Torrent Suite was subsequently applied to detect single nucleotide mutations as well as small indel variants. Variant caller was programmed to run in high stringency mode requesting at least 12× read coverage and at least 66% mutation frequency. Only those variants that were supported by sequencing on both strands were taken into account. BAM alignment files were imported in CLC Genomics Workbench Tool and variant regions were manually inspected in all strains.
Measurement of Allelic-Replacement Frequency by High-Throughput Sequencing.
MAGE performance in E. coli, Salmonella enterica, and C. freundii was assayed by high-throughput sequencing of allele-compositions at the MAGE-targeted landing-pad region using an Illumina MiSeq sequencer. pSIM8, (pSIM8KAN for C. freundii ATCC8090 asnA::TET-CAT_OFF), pORTMAGE2 (pORTMAGE3 for C. freundii ATCC8090 asnA::TET-CAT_OFF) harboring E. coli K-12 MG1655 asnA::TET-CAT_OFF, S. enterica serovar Typhimurium TS616 asnA::TET-CAT_OFF, E. coli K-12 MG1655 asnA::TET-CAT_OFF ΔmutS, and S. enterica serovar Typhimurium TS616 asnA::TET-CAT_OFF ΔmutS cells with the landing pad sequence in their genome were subjected to MAGE.
λ Red expressing electrocompetent cells were prepared from each query strain, as described above in the general MAGE workflow, and 40 μL of cell suspension was subjected to electroporation. Six-plex single MAGE cycles were carried out on each strain by using equimolar concentrations (1.25 μM each) of each of the five landing-pad targeting oligonucleotides listed in Dataset S2. Coselection MAGE oligo CAT_ON, which restores the nonsense mutation in the CAT gene, was added at a molar ratio of 1:50 to the oligo mixture. After electroporation, 1 mL of room-temperature TB media was added, of which 100 μL was transferred to glass flasks and allowed to recover for 24 h in 500 μL TB media under vigorous shaking at 30 °C. Selection of cells with restored chloramphenicol resistance (CATON) during coselection MAGE was performed by 1:100 dilution of the stationary phase culture in 10 mL 20 μg/mL chloramphenicol supplemented LBL media. Coselected cultures were allowed to grow at 30 °C 250 rpm for 24 h to reach stationary phase. gDNA was extracted from unselected and coselected cultures (GenElute Bacterial Genomic DNA kit, Sigma-Aldrich, Cat no. NA2110), according to the manufacturer’s protocol.
Determination of allelic replacement frequency at the landing-pad region was based on the high-throughput sequencing of a 436-bp PCR amplicon, which covers the MAGE targeted landing-pad region. Of the isolated gDNA samples, 200 ng were used as a template to PCR amplify the corresponding region using the tetDS1 and tetDS2 primers. Reaction mixtures consisted of 1 U/50 μL Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Scientific) in Phusion HF buffer (1.5 mM MgCl2 in the final concentration), 200 μM dNTPs, and 0.5 μM sample-specific primer pairs. PCRs in 150-μL total volumes were divided into 50-μL separate volumes and were cycled 26 times at 98 °C 10 s, 57 °C 30 s, and 72 °C for 30 s, with a final extension of 3 min at 72 °C. Products were assayed with agarose gel electrophoresis and using the D1000 ScreenTape and Reagents on TapeStation instrument (Agilent) to ensure maximum sample quality.
Sequencing libraries from the 436-bp-long amplicons were prepared using the NEBNext DNA Library Prep Master Mix Set for Illumina and NEBNext Multiplex Oligos for Illumina (Index Primers Sets 1 and 2) (New England Biolabs). The libraries were prepared according to manufacturer’s instructions, with the following modifications: the initial fragmentation step was skipped and all purifications were performed by using Agencourt AMPure XP beads (Beckman Coulter). Libraries were quality-checked and quantified on TapeStation using D1000 ScreenTape and Reagents (Agilent) and Qubit using dsDNA Assay (LifeTechnologies). Final libraries were sequenced using MiSeq Reagent Kit v2 for a 250-bp paired-end sequencing run using MiSeq sequencer (all from Illumina). CLC Genomics Workbench Tool (v8.0.1) was used to process PE Illumina raw reads (read length 250 bp). Reads were first trimmed with an error probability threshold of 0.05 (Phred score of 13). Overlapping read pairs were identified and merged yielding one 436-bp-long template read from each combined paired-end pair. Those paired-end pairs that could not be merged were removed. Template reads were strictly mapped against their corresponding reference sequence, keeping only those reads that displayed at least 90% sequence identity to the reference over at least 80% of their lengths, ensuring that only high-quality reads were subjected to downstream analysis. Read-mapping data were exported from CLC Genomics Workbench Tool in binary alignment (bam) format and “pysamstats” (https://github.com/alimanfoo/pysamstats) Python script was subsequently used to extract coverage and base count information for each mapping position. Coverage for each mapping position was above 120,000 in all samples.
Allelic replacement frequency at each MAGE oligo targeted nucleotide position was quantified by measuring the distribution of nucleotide variants for each mapping position. The quality of each Illumina run was also assayed in each run for each sample by measuring the sequencing noise. We relied on control regions to assess PCR amplification and sequencing bias at positions that were not targeted by MAGE oligos. For these control regions, we calculated the frequency of alleles that were not wild-type to yield the error rate. This strict quality control and the removal of low-quality reads resulted in a reproducibly constant base-call false error rate at each locus in all experiments, which was consistent with previously reported values from the literature for Illumina MiSeq Reagent Kit v2 for 250-bp paired-end sequencing runs (51). The background false-error rate was applied as the detection threshold in all experiments.
MAGE performance for library generation at asnA in E. coli K-12 MG1655, S. enterica serovar Typhimurium TS616, and C. freundii ATCC8090 was measured by targeting endogenous asnA by a species-specific recombineering oligo (for further details, see Dataset S2) carrying six randomized nucleotide positions. For comparing wild-type and pORTMAGE efficiency in library generation, E. coli K-12 MG1655(pSIM8), S. enterica serovar Typhimurium TS616(pSIM8), C. freundii ATCC8090(pSIM8KAN), and E. coli K-12 MG1655(pORTMAGE2), S. enterica serovar Typhimurium TS616(pORTMAGE2), and C. freundii ATCC8090(pORTMAGE3) were used. gDNA from each cell populations after five subsequent MAGE cycles was extracted and amplified according to the protocol described for the landing-pad assay. Primers used for amplification of asnA are listed in Dataset S2. Allelic replacement frequency at asnA in each species was assayed and quantified by Illumina high-throughput sequencing as described above.
Allelic replacement frequency in E. coli K-12 MG1655 at the lacZ, malK, araB, rpsL, cycA, and hisB loci was assayed exactly as described previously (18).
Integration of Landing Pad Sequence for Allelic-Replacement Frequency Measurement.
Allelic-replacement frequency in E. coli K-12 MG1655, S. enterica serovar Typhimurium TS616, and C. freundii ATCC8090 was measured at an engineered landing pad by high-throughput sequencing. The landing pad was inserted into the endogenous asnA open-reading-frame by dsDNA recombineering. AsnA is one of two asparagine synthetase enzymes in enterobacteria (52) and is located in conserved genomic location in both species. The inactivation of it did not cause a fitness defect in rich media. Landing-pad recombineering cassettes with genomic overhangs was generated by PCR from the previously described pZA31YFPtetR plasmid (25) using the corresponding primer combinations (Dataset S2). PCR products were cleaned and concentrated. Insertion of the landing pad into the genome of candidate strains was performed by standard dsDNA λ Red recombineering. Background strains for coselection MAGE were prepared by inactivating the cat marker within the landing pad, through the integration of a premature stop codon by ssDNA mediated allelic replacement with the CAT_OFF synthetic oligonucleotide, as described previously (7). Clones with an inactive cat marker were identified by replica plating and screening colonies for sensitivity to chloramphenicol.
Permanent mismatch repair deactivation was achieved in E. coli K-12 MG1655 asnA::TET-CAT_OFF and S. enterica TS616 asnA::TET-CAT_OFF by endogenous mutS removal by replacing the mutS ORF with kanamycin resistance cassette by dsDNA recombineering (53). For primers used in the PCR based generation of cassettes, see Dataset S2. Disruption of MMR activity by mutS deletion yielded E. coli K-12 MG1655 asnA::TET-CAT_OFF ΔmutS and S. enterica TS616 asnA::TET-CAT_OFF ΔmutS, respectively (Dataset S3).
Introduction of Antibiotic Resistance-Associated Mutations in S. enterica.
Mutations associated with in vitro development of antibiotic resistance in E. coli were previously identified by adaptive laboratory evolution followed by subsequent genome-resequencing (30). From the observed set of mutations, 11 were subjected for analysis.
MAGE oligonucleotides, each carrying one of the 11 marked mutations, were designed using the MODEST tool (35) with the S. enterica serovar Typhimurium LT2 genome sequence as the target. A single MAGE cycle was performed with each of the 11 MAGE oligos separately in S. enterica serovar Typhimurium LT2(pORTMAGE3) according to the general MAGE cycling protocol. The only exception was the recovery of cell populations, which took place in 600 μL TB media without any antibiotics added. Upon reaching stationary phase, cells were diluted and plated to LBL agar plates. Presence of each mutation was verified by HRM colony-PCRs in Luminaris HRM Master Mix (Thermo Scientific) according to the manufacturer’s guidelines and by subsequent capillary-sequencing. HRM PCR and sequencing primers are listed in Dataset S2 and were designed using DNASTAR Lasergene 11.1.
MIC Measurements.
Measurement of MIC values was performed using a well-established protocol (31). Briefly, a standard linear broth dilution technique (54) was used. To maximize reproducibility and accuracy, we used a robotic liquid handling system (Hamilton Star Workstation) to prepare 12-step linear dilutions automatically in 96-well microtiter plates. Approximately 106 bacteria per milliliter were inoculated into each well with a 96-pin replicator, and were propagated at 30 °C shaken at 300 rpm (three replicates per strain/antibiotic concentration). After 24-h of incubation, raw OD values were measured in a Biotek Synergy 2 microplate reader. MIC was defined by a cut-off OD value (i.e., mean + 2 SDs of OD values of bacteria-free wells containing only growth medium). As a measure of the effect of each individual mutation, the MIC value of a given antibiotic against the corresponding mutant was determined compared with the wild-type strain (relative MIC value = MIC of the mutant/MIC of the wild-type ancestor). The sensitivity of each mutant was measured against the antibiotic that it arose against during laboratory adaptation (30).
Verification of MutL E32K Induction by qPCR.
Relative induction level of MutL from pORTMAGE1 compared with the genomic copy was determined by quantitative reverse-transcription Real-Time PCR (qRT-PCR). Quantitation of rrsA expression level was applied as reference-standard across all samples (55). Total RNA was extracted from samples using NucleoSpin RNA extraction kit (Macherey-Nagel) according to the manufacturer’s protocol. Purity of total RNA was determined as 260 nm/280 nm absorbance ratio with expected values between 1.8 and 2.0 by the NanoDrop 1000 spectrophotometer (Thermo Scientific). RNA integrity was confirmed by gel electrophoresis using 1% agarose with GelRed staining. Total RNA was DNase I treated to eliminate genomic DNA and 100 ng of RNA was subjected to analysis per PCR in 25-μL volume.
qRT-PCRs were performed in a Bio-Rad CFX96 Real Time System (Bio-Rad Laboratories). The PCR mixtures consisted of 0.5 μL RNA sample, corresponding to ∼100 ng total RNA, 0.2 μM of the mutLQPCR5-mutLQPCR6 primer pair or 0.2 μM of the rrsAQ1-rrsAQ2 reference primer pair and Verso one-step RT-qPCR Master Mix with low ROX reference dye (Thermo Scientific) in a final volume of 25 μL. The assay included “nontemplate” and “non-RT” controls to detect reagent contamination and presence of gDNA. RT reaction was performed at 50 °C for 15 min and the thermal profile of the PCR procedure repeated for 40 cycles was: 95 °C for 10 min; 20-s denaturation at 95 °C, 20-s annealing at 60 °C and 60 s at 72 °C coupled to data collection at the end of each amplification step. Dissociation curve consisted of 10-s incubation at 95 °C, 5-s incubation at 65 °C, and ramp up to 95 °C. Melting curves were used to validate product specificity. All samples were amplified in triplicates from the same total RNA preparation and the mean value was used for further analysis. Cycle threshold (Ct) values were determined using Bio-Rad CFX96 software and the single-threshold mode.
mutL expression level was measured by relative mRNA quantitation. Relative expression change of the gene of interest was calculated using Bio-Rad CFX96 software based on the mean of technical replicates and was normalized to the expression level of the rrsA control.
Mutation Rate Measurement.
Measurement of mutation rates and the effect of dominant mutL allele on methyl-directed mismatch repair were based on rifampicin resistance fluctuation analysis. Query strains harboring the anhydrotetracycline (AhTC) inducible pZA31tetR-mutLE32K plasmid for mutLE32K expression were inoculated into 1 mL 20 μg/mL chloramphenicol containing LBL media, separately either in the presence or in the absence of 100 ng/mL AhTC inducer. After overnight growth at 30 °C 250 rpm, ∼104 cells from each culture were transferred into 10 separate vials of antibiotic-supplemented LBL media, with or without 100 ng/mL AhTC, respectively. Upon reaching stationary phase, appropriate dilutions were spread onto nonselective agar plates as well as LBL agar plates containing 100 μg/mL rifampicin. Plates were incubated at 37 °C and total colony count was determined after 24 h. The mutation rate was calculated by using the Ma-Sandri-Sarkar maximum-likelihood method and the FALCOR web tool (56).
Discussion
Among the currently available bacterial genome-editing tools, oligonucleotide-mediated recombineering is probably the most cost-effective and versatile choice (Table S1). Bacterial recombineering has been optimized toward multiplexing and automation, resulting in MAGE (5). Additionally, oligonucleotides can now be designed (35) and synthesized (36) with great ease at large quantities, allowing for genome-engineering undertakings of unprecedented complexity (10–12). However, multiplex bacterial genome engineering has been optimized for a few laboratory model strains, demands extensive prior modification of the host strain, and leads to the accumulation of numerous off-target modifications (Table S1). Accumulation of unwanted mutations in the targeted cells can mask the effect of the intentionally introduced modifications. Furthermore, for inactivation of the MMR system, the host has to be modified beforehand, greatly limiting the ease of use and narrowing the applicable range of organisms. Although a method expanding multiplex genome editing has recently been described [MuGENT (37)], it is applicable only to naturally transformable bacteria (for other potential limitations, see Table S1). Building on prior works, our study addresses the above mentioned three major problems—ease of use, off-target mutagenesis, and portability across species—in a single framework.
We developed pORTMAGE, an all-in-one plasmid set easily applicable to a range of Gram-negative bacterial species. The main results are as follows. First, transient expression of a dominant mutator allele of the MutL MMR protein allows for a controllable switch between mutator and nonmutator phenotype in a range of clinically and biotechnologically relevant species. Second, by implementing this advance, highly efficient and precise allelic replacement was achieved in selected enterobacterial species. Third, efficient multiplex genome editing was coupled with low off-target mutation rate. Fourth, pORTMAGE allowed rapid generation of large, unbiased sequence libraries at desired positions in these species.
Finally, we used pORTMAGE to introduce 10 antibiotic-resistance mutations into the genomes of S. enterica and E. coli, two species that diverged from each other more than 100 million y ago. The complete workflow took 4 d for completion. To the best of our knowledge, this is a significant improvement over traditional gene-editing protocols applicable to S. enterica, as they are either slow, have relatively low replacement efficiency, or are prone to off-target mutagenesis. These advances allowed us to address the extent of conservation of the molecular mechanisms underlying antibiotic resistance. We found that despite over 100 million y of divergence between the two species, mutational effects remained generally conserved. In most cases, the investigated mutations resulted in a small, but significant decline in antibiotic susceptibilities in both species. However, the phenotypic effects of certain canonical mutations varied extensively between the two species. For example, the level of resistance conferred by the canonical S83L mutation in gyrase A is at least six-times higher in S. enterica than in E. coli (Table 2). One possible interpretation of this result is that epistatic interactions within and across genes shape the antibiotic resistance profile. Clearly, pORTMAGE facilitates future studies in this direction.
In all, by combining efficient allelic replacement with low background mutation rate and portability, pORTMAGE offers a convenient tool to refactor complex cellular traits and systematically engineer metabolic pathways in a diverse set of enterobacterial species. pORTMAGE also paves the way toward exploring mutational effects and epistasis, which could potentially be exploited for the development of novel antimicrobial strategies.
However, pORTMAGE has two potential limitations (Table S1). First, an important unresolved issue is the extent to which the applicability of pORTMAGE demands sequence similarity of MutL between the host organism and E. coli. We suspect that pORTMAGE does not require high sequence conservation, as long as the functional role of MutL in the mismatch-repair system remains unchanged. Indeed, despite substantial differences in MutL sequences between Pseudomonas aeruginosa and E. coli, the P. aeruginosa copy is able to complement that of E. coli (38). In fact, even the human mismatch-repair protein MutL homolog 1 (hMLH1) functionally interacts with the E. coli MMR machinery and was able to induce a dominant mutator state in E. coli (39). Because inactivation of the MMR machinery greatly improves allele replacement efficiency in organisms ranging from yeast (40) to human cell lines (41, 42), pORTMAGE could inspire future development of general genome editing tools.
Another potential problem is that, similarly to other recombinase-based genome-editing tools, pORTMAGE heavily relies on the use of specific enzymes and expression vectors. The expanding repertoire of characterized recombinases and expression systems (15, 43–46) will presumably allow for broad use in the near future.
We anticipate that our work will have implications for clinical and biotechnological problems as well. For example, pORTMAGE may be used in engineering of attenuated bacterial pathogens for vaccine development (47). It may be also useful for metabolic engineering attempts in previously untapped species. For example, C. freundii is an efficient host for the production of valuable bioproducts (48), but optimization of metabolic pathways remained challenging in this species. Because C. freundii is amenable to pORTMAGE, we expect rapid future development in this area. Other species, such as environmental Pseudomonas strains, have great potential to serve as chassis for industrial biotechnology (49) but are thus far lacking in robust techniques allowing for efficient multiplex genome editing. Finally, pORTMAGE could also open a new avenue of research in diverse fields such as functional genomics and evolutionary biology (50). To our knowledge, for the first time, pORTMAGE allows systematic comparison of mutational effects and epistasis across a wide range of bacterial species.
Materials and Methods
Detailed descriptions of the methodology, including (i) strains and reagents, (ii) oligonucleotides, (iii) plasmid construction, (iv) pORTMAGE cycling protocol, (v) whole-genome resequencing, (vi) high-throughput sequencing for allelic replacement frequency measurement, (vii) integration of landing pad sequence into host strains, (viii) construction of antibiotic resistance associated mutants, (ix) MIC measurement, (x) qPCR measurement, and (xi) mutation rate measurement can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Donald L. Court for providing the λ Red recombinase expression plasmids; Tamás Fehér for donating pZA31tetR and pZA31YFPtetR; and Andrea Tóth for her technical assistance. This work was supported by grants from the European Research Council (to C.P.), the Wellcome Trust (to C.P.), and the Lendület Program of the Hungarian Academy of Sciences (to C.P.); Hungarian Scientific Research Fund Grants OTKA PD 109572 (to B.C.) and OTKA PD 106231 (to K.U.); Hungarian Academy of Sciences Postdoctoral Fellowship Program Grant SZ-039/2013 (to B. Bogos); the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (I.N.); and a PhD fellowship from the Boehringer Ingelheim Fonds (to Á.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520040113/-/DCSupplemental.
References
- 1.Esvelt KM, Wang HH. Genome-scale engineering for systems and synthetic biology. Mol Syst Biol. 2013;9:641. doi: 10.1038/msb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, et al. Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab Eng. 2015;31:13–21. doi: 10.1016/j.ymben.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, et al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microiol. 2015;81(7):2506–2514. doi: 10.1128/AEM.04023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher RR, Li Z, Lewis AO, Isaacs FJ. Rapid editing and evolution of bacterial genomes using libraries of synthetic DNA. Nat Protoc. 2014;9(10):2301–2316. doi: 10.1038/nprot.2014.082. [DOI] [PubMed] [Google Scholar]
- 5.Wang HH, et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460(7257):894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Court DL, Sawitzke JA, Thomason LC. Genetic engineering using homologous recombination. Annu Rev Genet. 2002;36(1):361–388. doi: 10.1146/annurev.genet.36.061102.093104. [DOI] [PubMed] [Google Scholar]
- 7.Wang HH, et al. Genome-scale promoter engineering by coselection MAGE. Nat Methods. 2012;9(6):591–593. doi: 10.1038/nmeth.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raman S, Rogers JK, Taylor ND, Church GM. Evolution-guided optimization of biosynthetic pathways. Proc Natl Acad Sci USA. 2014;111(50):17803–17808. doi: 10.1073/pnas.1409523111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoval NR, et al. Strategy for directing combinatorial genome engineering in Escherichia coli. Proc Natl Acad Sci USA. 2012;109(26):10540–10545. doi: 10.1073/pnas.1206299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lajoie MJ, et al. Genomically recoded organisms expand biological functions. Science. 2013;342(6156):357–360. doi: 10.1126/science.1241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovner AJ, et al. Recoded organisms engineered to depend on synthetic amino acids. Nature. 2015;518(7537):89–93. doi: 10.1038/nature14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandell DJ, et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature. 2015;518(7537):55–60. doi: 10.1038/nature14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Pijkeren JP, Britton RA. Precision genome engineering in lactic acid bacteria. Microb Cell Fact. 2014;13(Suppl 1):S10. doi: 10.1186/1475-2859-13-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder S, Siedler S, Marienhagen J, Bott M, Eggeling L. Recombineering in Corynebacterium glutamicum combined with optical nanosensors: A general strategy for fast producer strain generation. Nucleic Acids Res. 2013;41(12):6360–6369. doi: 10.1093/nar/gkt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z, et al. A high-efficiency recombineering system with PCR-based ssDNA in Bacillus subtilis mediated by the native phage recombinase GP35. Appl Microbiol Biotechnol. 2015;99(12):5151–5162. doi: 10.1007/s00253-015-6485-5. [DOI] [PubMed] [Google Scholar]
- 16.Costantino N, Court DL. Enhanced levels of λ Red-mediated recombinants in mismatch repair mutants. Proc Natl Acad Sci USA. 2003;100(26):15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs FJ, et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333(6040):348–353. doi: 10.1126/science.1205822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyerges Á, et al. Conditional DNA repair mutants enable highly precise genome engineering. Nucleic Acids Res. 2014;42(8):e62. doi: 10.1093/nar/gku105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lennen RM, et al. Transient overexpression of DNA adenine methylase enables efficient and mobile genome engineering with reduced off-target effects. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronshtam A, Marinus MG. Dominant negative mutator mutations in the mutL gene of Escherichia coli. Nucleic Acids Res. 1996;24(13):2498–2504. doi: 10.1093/nar/24.13.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polosina YY, Mui J, Pitsikas P, Cupples CG. The Escherichia coli mismatch repair protein MutL recruits the Vsr and MutH endonucleases in response to DNA damage. J Bacteriol. 2009;191(12):4041–4043. doi: 10.1128/JB.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ban C, Yang W. Crystal structure and ATPase activity of MutL: Implications for DNA repair and mutagenesis. Cell. 1998;95(4):541–552. doi: 10.1016/s0092-8674(00)81621-9. [DOI] [PubMed] [Google Scholar]
- 23.Baumler DJ, Ma B, Reed JL, Perna NT. Inferring ancient metabolism using ancestral core metabolic models of enterobacteria. BMC Syst Biol. 2013;7(1):46. doi: 10.1186/1752-0509-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25(6):1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehér T, et al. Competition between transposable elements and mutator genes in bacteria. Mol Biol Evol. 2012;29(10):3153–3159. doi: 10.1093/molbev/mss122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster PL. Methods for determining spontaneous mutation rates. Methods Enzymol. 2006;409:195–213. doi: 10.1016/S0076-6879(05)09012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Wang HH, Xu G, Vonner AJ, Church G. Modified bases enable high-efficiency oligonucleotide-mediated allelic replacement via mismatch repair evasion. Nucleic Acids Res. 2011;39(16):7336–7347. doi: 10.1093/nar/gkr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehner B. Molecular mechanisms of epistasis within and between genes. Trends Genet. 2011;27(8):323–331. doi: 10.1016/j.tig.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Lázár V, et al. Genome-wide analysis captures the determinants of the antibiotic cross-resistance interaction network. Nat Commun. 2014;5:4352. doi: 10.1038/ncomms5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lázár V, et al. Bacterial evolution of antibiotic hypersensitivity. Mol Syst Biol. 2013;9(1):700. doi: 10.1038/msb.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullen ME, Wyke AW, Kuroda R, Fisher LM. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz J, et al. High frequency of mutations at codon 83 of the gyrA gene of quinolone-resistant clinical isolates of Escherichia coli. J Antimicrob Chemother. 1995;36(4):737–738. doi: 10.1093/jac/36.4.737. [DOI] [PubMed] [Google Scholar]
- 34.Alekshun MN, Levy SB. The mar regulon: Multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 1999;7(10):410–413. doi: 10.1016/s0966-842x(99)01589-9. [DOI] [PubMed] [Google Scholar]
- 35.Bonde MT, et al. MODEST: A web-based design tool for oligonucleotide-mediated genome engineering and recombineering. Nucleic Acids Res. 2014;42(Web Server issue):W408–W415. doi: 10.1093/nar/gku428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonde MT, et al. Direct mutagenesis of thousands of genomic targets using microarray-derived oligonucleotides. ACS Synth Biol. 2015;4(1):17–22. doi: 10.1021/sb5001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalia AB, McDonough E, Camilli A. Multiplex genome editing by natural transformation. Proc Natl Acad Sci USA. 2014;111(24):8937–8942. doi: 10.1073/pnas.1406478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacquelín DK, Filiberti A, Argaraña CE, Barra JL. Pseudomonas aeruginosa MutL protein functions in Escherichia coli. Biochem J. 2005;388(Pt 3):879–887. doi: 10.1042/BJ20042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quaresima B, et al. Human mismatch-repair protein MutL homologue 1 (MLH1) interacts with Escherichia coli MutL and MutS in vivo and in vitro: A simple genetic system to assay MLH1 function. Biochem J. 2003;371(Pt 1):183–189. doi: 10.1042/BJ20021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiCarlo JE, et al. Yeast oligo-mediated genome engineering (YOGE) ACS Synth Biol. 2013;2(12):741–749. doi: 10.1021/sb400117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rios X, et al. Stable gene targeting in human cells using single-strand oligonucleotides with modified bases. PLoS One. 2012;7(5):e36697. doi: 10.1371/journal.pone.0036697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu K, Stewart AF, Porter ACG. Stimulation of oligonucleotide-directed gene correction by Redβ expression and MSH2 depletion in human HT1080 cells. Mol Cells. 2015;38(1):33–39. doi: 10.14348/molcells.2015.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datta S, Costantino N, Zhou X, Court DL. Identification and analysis of recombineering functions from Gram-negative and Gram-positive bacteria and their phages. Proc Natl Acad Sci USA. 2008;105(5):1626–1631. doi: 10.1073/pnas.0709089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopes A, Amarir-Bouhram J, Faure G, Petit M-A, Guerois R. Detection of novel recombinases in bacteriophage genomes unveils Rad52, Rad51 and Gp2.5 remote homologs. Nucleic Acids Res. 2010;38(12):3952–3962. doi: 10.1093/nar/gkq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y, Kragelund BB, White MF, Peng X. Functional characterization of a conserved archaeal viral operon revealing single-stranded DNA binding, annealing and nuclease activities. J Mol Biol. 2015;427(12):2179–2191. doi: 10.1016/j.jmb.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Kushwaha M, Salis HM. A portable expression resource for engineering cross-species genetic circuits and pathways. Nat Commun. 2015;6:7832. doi: 10.1038/ncomms8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranallo RT, Barnoy S, Thakkar S, Urick T, Venkatesan MM. Developing live Shigella vaccines using λ Red recombineering. FEMS Immunol Med Microbiol. 2006;47(3):462–469. doi: 10.1111/j.1574-695X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang C, et al. Fed-batch fermentation of recombinant Citrobacter freundii with expression of a violacein-synthesizing gene cluster for efficient violacein production from glycerol. Biochem Eng J. 2011;57:55–62. [Google Scholar]
- 49.Nikel PI, Martínez-García E, de Lorenzo V. Biotechnological domestication of pseudomonads using synthetic biology. Nat Rev Microbiol. 2014;12(5):368–379. doi: 10.1038/nrmicro3253. [DOI] [PubMed] [Google Scholar]
- 50.Pál C, Papp B, Pósfai G. The dawn of evolutionary genome engineering. Nat Rev Genet. 2014;15(7):504–512. doi: 10.1038/nrg3746. [DOI] [PubMed] [Google Scholar]
- 51.Schirmer M, et al. Insight into biases and sequencing errors for amplicon sequencing with the Illumina MiSeq platform. Nucleic Acids Res. 2015;43(6):e37. doi: 10.1093/nar/gku1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felton J, Michaelis S, Wright A. Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J Bacteriol. 1980;142(1):221–228. doi: 10.1128/jb.142.1.221-228.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 55.Zhou K, et al. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol Biol. 2011;12:18. doi: 10.1186/1471-2199-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall BM, Ma C-X, Liang P, Singh KK. Fluctuation analysis CalculatOR: A web tool for the determination of mutation rate using Luria-Delbruck fluctuation analysis. Bioinformatics. 2009;25(12):1564–1565. doi: 10.1093/bioinformatics/btp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H, Kim J-S. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15(5):321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 58.Wang HH, Church GM. Multiplexed genome engineering and genotyping methods applications for synthetic biology and metabolic engineering. Methods Enzymol. 2011;498:409–426. doi: 10.1016/B978-0-12-385120-8.00018-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.