Significance
Decades of research into the cause of chronic insomnia have identified hyperarousal as the key factor, but mechanisms underlying hyperarousal have remained elusive. The present findings suggest that hyperarousal can result from an inadequate resolution of emotional distress, which, in turn, is likely due to restless rapid-eye-movement sleep.
Keywords: insomnia, REM sleep, hyperarousal, self-conscious emotion, shame
Abstract
The mechanisms underlying hyperarousal, the key symptom of insomnia, have remained elusive, hampering cause-targeted treatment. Recently, restless rapid-eye-movement (REM) sleep emerged as a robust signature of sleep in insomnia. Given the role of REM sleep in emotion regulation, we hypothesized that restless REM sleep could interfere with the overnight resolution of emotional distress, thus contributing to accumulation of arousal. Participants (n = 1,199) completed questionnaires on insomnia severity, hyperarousal, self-conscious emotional distress, and thought-like nocturnal mentation that was validated to be a specific proxy for restless REM sleep (selective fragmentation: R = 0.57, P < 0.001; eye movement density: R = 0.46, P < 0.01) in 32 polysomnographically assessed participants. The experience of distress lasting overnight increased with insomnia severity (β = 0.29, P < 10−23), whereas short-lasting distress did not (β = −0.02, P = 0.41). Insomnia severity was associated with hyperarousal (β = 0.47, P < 10−63) and with the thought-like nocturnal mentation that is specifically associated with restless REM sleep (β = 0.31, P < 10−26). Structural equation modeling showed that 62.4% of the association between these key characteristics of insomnia was mediated specifically by reduced overnight resolution of emotional distress. The model outperformed all alternative mediation pathways. The findings suggest that restless REM sleep reflects a process that interferes with the overnight resolution of distress. Its accumulation may promote the development of chronic hyperarousal, giving clinical relevance to the role of REM sleep in emotion regulation in insomnia, depression, and posttraumatic stress disorder.
Both insomnia and affective disorders are among the most prevalent and burdening health concerns facing our society. Targeted prevention of affective disorders in people at risk, as well as identification of mechanisms of conversion, could be the most viable approach to mitigate their increasing global burden (1). For the prevention of new-onset or recurrent affective disorder, insomnia may be the major risk factor that can be targeted best (2, 3). About 13% of people with insomnia develop major depression disorder (MDD) within a year (2). Moreover, remission rates after cognitive behavioral therapy are 21% lower for depressed patients with abnormal sleep compared with patients with relatively intact sleep (4). It therefore appears highly relevant to understand the mechanisms involved in the role of insomnia in disturbed emotion regulation (2). The present study addresses the roles of restless rapid-eye-movement (REM) sleep (5) and chronic physiological arousal (6), which are characteristic of both insomnia and MDD.
Although sleep contributes to the more robust consolidation of emotional memories, relative to neutral memories (7, 8), their later recall is not associated with anywhere near the same magnitude of subjective emotional distress, autonomic arousal, and amygdala activation (7, 9–11). REM sleep plays an important role in this process (10, 12–14), although not excluding a role for nonrapid-eye-movement (NREM) sleep (15). Naps containing REM sleep were shown to resolve emotional reactivity, whereas naps without REM sleep did not (12). Furthermore, high-frequency electroencephalographic activity during REM sleep, an index of fragmented REM sleep, hinders resolution of emotional distress (10).
These findings are of particular interest, because metaanalysis suggests disruption of sleep continuity to be among the most robust polysomnographic signatures in insomnia (16). Two studies suggest that this fragmentation is most pronounced in REM sleep and may contribute to the development of the sleep characteristics of MDD, like increased eye movement density (5, 17). We use the term “restless REM sleep” here to refer to REM sleep with a high number of phasic events, including arousals and eye movements. These phasic events commonly occur not only in insomnia (5) but also in depression (18) and posttraumatic stress disorder (PTSD) (19, 20). If restless REM sleep interferes with the overnight resolution of emotional distress, it could contribute to its accumulation, appearing as a chronically hyperaroused state. To our knowledge, the present study is the first to investigate whether chronic interference with this nocturnal process due to restless REM sleep would indeed result in slower resolving of emotional distress and whether hyperarousal, in fact, represents the resulting accumulation of this distress. Of note, hyperarousal is not only present across waking and sleep in people suffering from MDD (21) but is also of key importance in the pathophysiology of primary insomnia (6, 22–24) and a premorbid characteristic of people vulnerable to insomnia (5, 25).
Until now, studies on the role of REM sleep in emotion regulation focused on basic emotions, such as fear, anger, and happiness. In psychological and psychiatric practice, however, self-conscious emotions, which include pride, guilt, embarrassment, humiliation, and shame, are more clinically relevant. The present study focused on shame, because it may interfere the most with healthy psychological functioning (26) and was shown to be predictive of developing depression (27) and PTSD symptoms, including hyperarousal (28). By obstructing effective coping mechanisms, shame often hinders therapeutic progress, to the point that it may even lead to a negative therapeutic outcome (29).
Based on the hypothesis outlined above, the first prediction is that insomnia will not only be associated with indicators of hyperarousal and restless REM sleep but also with increased reporting of slow-resolving emotional distress. More specifically, this association should be the case primarily for emotional distress lasting a day or longer, but not necessarily so for emotional distress that is resolved before the end of the day because the latter does not involve a contribution of sleep. The second prediction is that an indicator of consolidated REM sleep will be associated with successful overnight resolution of emotional distress, whereas restless REM sleep will interfere with this nocturnal process and result in increased reporting of long-lasting distress. The third prediction is that the more frequently people report long-lasting distress, the more likely it is that they accumulate this long-lasting distress into a state of chronic hyperarousal. The encompassing fourth prediction is that the association between the two most typical findings in insomnia, restless REM sleep and hyperarousal, is meditated by a slower resolution of distress induced by an emotional experience. To allow us to address these hypotheses in a large sample, we first validated a nocturnal mentation questionnaire proxy variable selectively associated with restless REM sleep in 32 people who were polysomnographically assessed for two nights. This proxy variable was subsequently assessed through the Internet in 1,199 participants of the Netherlands Sleep Registry (NSR), along with questionnaires on the subjective duration of emotional distress, insomnia severity, hyperarousal, and major life events, as well as a structured interview on health. Predicted associations between the variables were evaluated using multiple regression analyses and structural equation modeling.
Results
Given the limited feasibility of polysomnography (PSG) in a large sample, we first validated a questionnaire proxy variable for restless REM sleep. To do so, we systematically evaluated the association of all items of the NSR’s extended implementation of the Dream Recall Frequency Scale (DRFS; Methods) (30) with the REM sleep arousal index (31) obtained from the second of two nights of PSG in 32 people [19 female and 13 male, mean (SD) of 35.8 (9.0) y of age], of whom 16 had been diagnosed with Insomnia Disorder (ID). Metaanalysis showed that variables reflecting disruption of sleep continuity are the most robust PSG signatures in insomnia (16), especially fragmentation of REM sleep (5, 17). Although less specific to insomnia, fragmentation occurs in NREM sleep as well (5). Therefore, to quantify REM sleep-specific arousal density, we calculated the log-transformed ratio between REM and NREM arousal density indices derived according to the criteria of the American Sleep Disorders Association (31). As reported before (32), REM arousal density was associated with items on thought-like rather than dream-like nocturnal mental content. A three-item “nocturnal mentation” score (Methods) correlated strongly with the REM sleep-specific arousal density index [R(31) = 0.57, P < 0.001]; with the nonspecific REM arousal index [R(31) = 0.41, P = 0.02]; and, moreover, with the eye movement density during REM [R(31) = 0.46, P = 0.01] previously proposed to be consequential to REM sleep fragmentation and involved in the development of depression (5). The nocturnal mentation score did not correlate with NREM arousals [R(31) = −0.18, P = 0.33] or with other sleep parameters previously suggested to affect dream recall (33–37), notably the total duration of REM sleep [R(31) = 0.18, P = 0.32] and REM sleep continuity specifically in the morning as derived from the arousal density of the last REM epoch [R(31) = 0.06, P = 0.73]. Therefore, the nocturnal mentation score appeared useful for subsequent use as a proxy measure specifically associated with restless REM sleep in the sample of 1,199 Internet-assessed participants.
Participants of the NSR [n = 1,199; 891 female and 308 male, mean (SD) of 52.1 (13.4) y of age] completed Internet questionnaires. A dedicated assessment of the subjective duration of distress after a shameful experience (SDDS) was complemented by assessments on nocturnal mentation (30), insomnia severity (38), hyperarousal (39), and major life events (40), as well as an Internet-implemented structured interview on health (41) (Methods). We designed the SDDS assessment to address a range, including short-lasting distress (i.e., within a day) and long-lasting distress (i.e., across one or more nights; Methods), systematically. According to our hypothesis, consolidated REM sleep will aid in the resolution of emotional distress. Therefore, restless REM sleep should selectively increase the reporting of long-lasting emotional distress but not necessarily the frequency of reporting emotional distress resolved within a day (i.e., without sleep). Factor analysis with Varimax rotation was therefore conducted to investigate whether the eight SDDS items (Methods) indeed represented a latent structure of separate long-lasting and short-lasting distress. As predicted, these two factors were identified, representing the items “a whole day” up to “more than a week” vs. “a few minutes” up to “an hour,” respectively. All questions loaded well above 0.40 on only one factor and well below 0.40 on the other, except for the question “half a day,” which loaded 0.68 and 0.58 on both factors. The question may have been ambiguous (12 h of wakefulness or including sleep) and was discarded. The two-factor structure was confirmed in a second factor analysis excluding this question. Eigenvalues were well over Kaiser’s criterion (3.64 and 2.25, respectively) (Table 1). Sampling of the two factors was adequate according to the Kaiser–Meyer–Olkin (KMO) measure (KMO = 0.75). Individual KMO values for items were >0.64, and Bartlett’s test indicated sufficiently strong correlations across the eight time scales of experiencing emotional distress [χ2(28) = 7,567.74, P = 0.00]. Simple averages over the items included in each factor will henceforth be reported.
Table 1.
Factor analysis distinguishes short-lasting emotional distress from distress lasting overnight
| Frequency of emotional distress lasting: | Factor | |
| Long-lasting | Short-lasting | |
| A few minutes | −0.10 | 0.90 |
| A quarter of an hour | 0.07 | 0.96 |
| An hour | 0.33 | 0.85 |
| A whole day | 0.81 | 0.36 |
| Two–three days | 0.91 | 0.19 |
| A week | 0.94 | 0.01 |
| More than a week | 0.87 | −0.13 |
| Eigenvalue | 3.64 | 2.25 |
| Variance explained, % | 52.0 | 32.2 |
| Cronbach’s α | 0.91 | 0.90 |
SDDS items and factor loadings (indicated in bold if above 0.40).
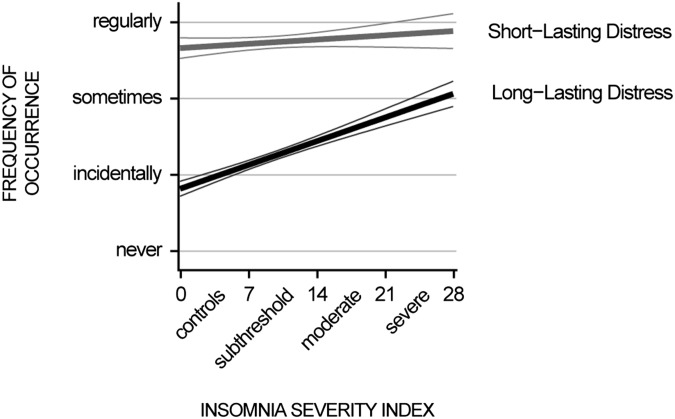
Aforementioned hypotheses were evaluated with multiple regression analyses and structural equation modeling. The significance threshold was Bonferroni-corrected for the total number of regression models and set to P < 0.003. In search for support of the hypothesis that restless REM sleep, a key characteristic of insomnia, should selectively increase long-lasting (overnight) distress, but not short-lasting distress, we found that insomnia severity was specifically associated with how often emotional distress lasted overnight [β = 0.29, T(1,194) = 10.28, P < 10−23; Fig. 1], but not with how often the distress resolved within a day; that is, without sleep [β = −0.02, T(1,194) = −0.82, P = 0.41; all coefficients adjusted for age and gender]. The dissociation indicates a selective insufficiency of overnight resolution of emotional distress in insomnia. As expected, insomnia severity was also strongly associated with nocturnal mentation [β = 0.31, T(1,195) = 11.15, P < 10−26] and with hyperarousal [β = 0.47, T(1,195) = 18.01, P < 10−63; all coefficients adjusted for age and gender]. Next, we evaluated whether the association between nocturnal mentation and hyperarousal might be mediated by insufficient overnight resolution of distress.
Fig. 1.
Disturbed emotion regulation in insomnia specifically concerns overnight dissolving of distress. Least-squares regression lines (thick lines) and 95% confidence interval bounds (thin lines) of the association between insomnia severity and the frequency of occurrence of short-lasting (gray) and long-lasting (black) distress after a shameful experience are shown.
As predicted, the more frequently people reported nocturnal mentation, the more they experienced long-lasting distress [β = 0.27, T(1,194) = 9.65, P < 10−20]. The association of nocturnal mentation with short-lasting distress was weak and did not reach significance after Bonferroni correction for multiple comparisons [β = 0.06, T(1,194) = 2.06, P = 0.04]. After demonstrating this indirect support for a possible role of restless REM sleep in the insufficiency of overnight resolution of distress, we addressed whether the resulting accumulation of unresolved distress might contribute to hyperarousal, assessed with the Self-Reported Hyperarousal Scale (39). Indeed, the more people experienced distress to last overnight, the higher were their hyperarousal ratings [β = 0.47, T(1,194) = 18.12, P < 10−64]. Short-lasting distress was not associated with hyperarousal [β = 0.02, T(1,194) = 0.68, P = 0.50].
To evaluate whether these associations might be secondary to confounding by individual differences in exposure to major life events or neurological, mental, or sleep disorders other than insomnia, participants completed the NSR implementations of both the Life Experiences Survey (LES) (40) and the Duke Structured Interview for Sleep Disorders (DSISD) (41). The DSISD is a validated tool with acceptable reliability and validity for sleep disorder diagnosis (42) according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-Text Revision) and International Classification of Sleep Disorders-2 (ICSD-2). Its NSR implementation also includes sections to query all current and past health issues according to the categories of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). Participants had a lifetime experience of a mean (SD) of 14.9 (4.5) major life events. A neurological, mental, or sleep disorder other than insomnia could not be excluded in 513 participants (details are provided in Supporting Information). Adjusting the regression models with covariates for the number of major life events and dummy-coded possible presence of these disorders only marginally affected the estimated coefficients and their significance (Supporting Information).
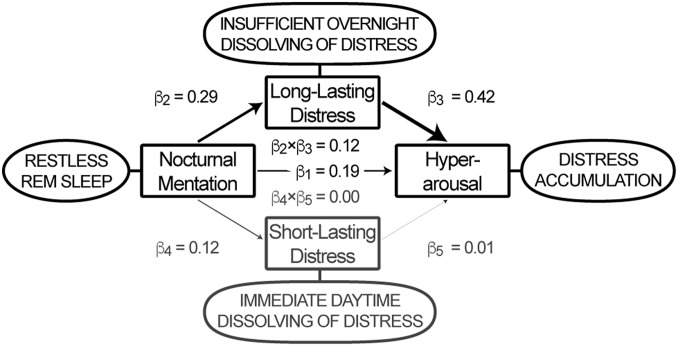
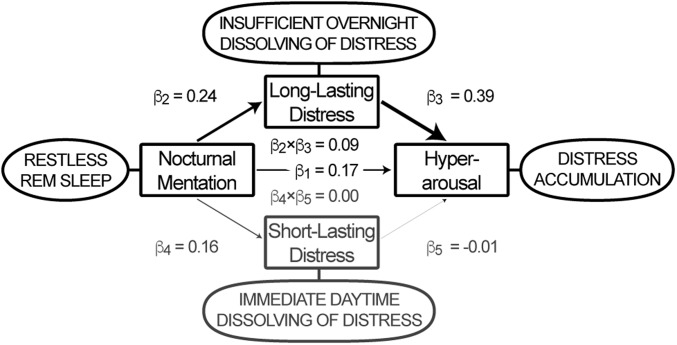
Finally, we used structural equation modeling to evaluate the most likely direction of mediation between the three apparent key characteristics of insomnia. We hypothesized that the thought-like nocturnal mental content that we validated to be specifically associated with fragmented REM sleep and increased eye movement density could reflect a process that interferes with the overnight resolution of distress, and that the consequent accumulation of distress contributes to the development of chronic hyperarousal. Indeed, the association between nocturnal mentation and hyperarousal was, in large part (62.4%), mediated selectively by long-lasting distress [β2 × β3 = 0.12, T(1,194) = 8.70, P < 10−16] but not short-lasting distress [β4 × β5 = 0.00, T(1,194) = 0.28, P = 0.77; Fig. 2]. Alternative models interchanging the independent, mediator, and dependent variables were rejected based on their Bayesian information criterion (BIC), adjusted R2, or inferior mediation effect (Table S1). To evaluate possible confounding, a sensitivity analysis evaluated the mediation model parameters in the subset of 686 participants who were unlikely to suffer from any neurological, mental, or sleep disorder other than insomnia (details are provided in Supporting Information): The partial mediation effect of long-lasting distress in this subsample remained selective and highly significant (Fig. S1).
Fig. 2.
Structural equation model. The thought-like nocturnal mentation that is specifically associated with fragmented REM sleep and increased eye movement density appears to reflect a process that interferes with overnight dissolving of distress, resulting in its accumulation as indexed by hyperarousal. The model, including data of all 1,199 participants, suggests that 62.4% of the association between thought-like nocturnal mentation and hyperarousal [β1 = 0.19, T(1,194) = 7.45, P < 10−11] is mediated by long-lasting distress [β2 × β3 = 0.12, T(1,194) = 8.70, P < 10−16], without involvement of individual differences in the frequency of experiencing short-lasting distress [β4 × β5 = 0.00, T(1,194) = 0.28, P = 0.77].
Table S1.
Mediation effects and variance explained by three-variable structural equation models interchanging the roles of nocturnal mentation, long-lasting distress, and hyperarousal as dependent, independent, and mediator variables
| Independent | Mediator | Dependent | Mediation effect | Adjusted R2 | BIC |
| Nocturnal mentation | Long-lasting distress | Hyperarousal | 0.12 (62.4%) | 0.28 | 3,035 |
| Nocturnal mentation | Hyperarousal | Long-lasting distress | 0.13 (88.4%) | 0.26 | 3,066 |
| Hyperarousal | Long-lasting distress | Nocturnal mentation | 0.09 (36.9%) | 0.13 | 3,261 |
| Hyperarousal | Nocturnal mentation | Long-lasting distress | 0.05 (11.3%) | 0.26 | 3,066 |
| Long-lasting distress | Hyperarousal | Nocturnal mentation | 0.11 (61.7%) | 0.13 | 3,261 |
| Long-lasting distress | Nocturnal mentation | Hyperarousal | 0.06 (13.3%) | 0.28 | 3,035 |
The primary model (bold font) outperformed all five alternative structural equation models. Four models explained less variance as indicated by the adjusted R2 and BIC; in the last model, these values were comparable but the mediation effect was half of our proposed model and accounted for only 13.3% of the association between independent and dependent variables.
Fig. S1.
Structural equation model restricted to 686 participants. The thought-like nocturnal mentation that is specifically associated with fragmented REM sleep and increased eye movement density appears to reflect a process that interferes with overnight dissolving of distress, resulting in its accumulation as indexed by hyperarousal. The model with restricted inclusion of only the 686 participants who were free of any neurological, mental, or sleep disorder other than insomnia suggests that 53.5% of the association between thought-like nocturnal mentation and hyperarousal [β1 = 0.17, T(681) = 4.98, P < 10−5] is mediated by long-lasting distress [β2 × β3 = 0.09, T(681) = 5.54, P < 10−6], without involvement of individual differences in the frequency of experiencing short-lasting distress [β4 × β5 = 0.00, T(681) = −0.38, P = 0.74].
Discussion
Recent studies indicate that unperturbed REM sleep beneficially contributes to resolving the distress associated with previous emotional experiences, both subjectively and objectively, at the level of limbic brain reactivity (10, 12–14). We hypothesized that this beneficial effect may be attenuated in conditions characterized by restless REM sleep (5, 17). Because hyperarousal resembles a state of chronic distress, the present study sought indirect support for the possibility that restless REM sleep interferes with the proper overnight resolving of distress after an emotional experience, and thus contributes to its accumulation into a state of chronic hyperarousal in insomniacs (6, 23).
The first prediction was a slower resolution of emotional distress in insomnia. Indeed, the more severe the insomnia people suffered, the more they experienced selectively long-lasting, but not short-lasting, emotional distress. This finding is compatible with the hypothesis that the overnight resolution of emotional distress is compromised in insomnia, resulting in less emotional depotentiation. The second prediction was that restless REM sleep is positively associated with reporting of long-lasting distress. To address this question in a large population-based sample, we first showed in 32 people assessed with PSG that restless REM sleep specifically correlates with the frequency of experiencing thought-like rather than dream-like nocturnal mentation, as has been noted before to be characteristic of insomnia (32). Indeed, thought-like nocturnal mentation was highly significantly associated with experiencing distress lasting overnight, but not with short-lasting distress resolving during the daytime. The data also supported the third prediction of the model, that the more frequently people report long-lasting distress, the more likely they are to accumulate this long-lasting distress in a state of chronic hyperarousal. The last prediction was that the association between the two typical characteristics of insomnia, restless REM sleep and hyperarousal, is mediated by a slower resolution of distress after an emotional experience. Indeed long-lasting distress accounted for 62.4% of the association of the thought-like nocturnal mentation that is specifically related to restless REM sleep with hyperarousal. The model outperformed all five alternative mediation models between the three variables of interest: four models explained less variance as indicated by the adjusted R2 and BIC; in one model, these values were comparable but the mediation effect was half the size of the proposed model and accounted for only 13.3% of the association between independent and dependent variables. The findings support the hypothesis that the thought-like nocturnal mentation is specifically associated with fragmented REM sleep. Furthermore, increased eye movement density may reflect a process that interferes with overnight resolution of emotional distress, in turn, promoting the development of a chronic state of hyperarousal.
Several limitations of the present study warrant further detailed investigations. First, inherent to the large sample size, restless REM sleep was not directly quantified but approximated by means of a validated questionnaire rating of thought-like nocturnal mentation. Nonetheless, fragmentation of sleep due to arousals, particularly from REM sleep, is a robust finding in people with insomnia (5, 17), and both the current study and others could show that the probability of recalling nocturnal mental content increases in proportion to the number of arousals (5, 35, 43), especially from REM sleep (44). Secondly, NREM sleep has also been implicated in the resolution of emotional distress (11, 15). Studies using PSG in larger samples are needed to reveal the involvement of different sleep stages in the resolution of emotional distress. A third limitation regards the observational nature of the present study. Even though none of the alternative mediation models outperformed the model proposed here on the basis of recent findings on the role of REM sleep in emotion regulation, a more definite conclusion will require studies using experimental manipulation of emotions and sleep. Finally, whereas there was good reason to focus first on distress induced by shame in our innovative approach to the role of sleep in self-conscious emotions rather than the basic emotions usually studied (27, 28, 45), our findings should not be interpreted as supporting a unique role for shame or self-conscious emotions. Future studies could address whether the duration of distress elicited by other self-conscious and basic emotions has a similar two-factor structure and, if so, whether long-lasting distress from such experiences shows a similar strong association with nocturnal mentation, hyperarousal, and insomnia severity.
Notwithstanding these limitations, this large sample study generated a number of important findings. First, the present study is the first, to our knowledge, to addresses the role of sleep in the overnight resolution of distress related to self-conscious emotions, thus showing that the role of sleep in emotion regulation extends beyond the basic emotions that are usually studied. Self-conscious emotions are highly relevant in clinical psychiatry and psychology (29, 46). For example, in sexually abused youth, shame contributes to risk of depression (27) and the maintenance of PTSD symptoms, including hyperarousal (28).
Second, the findings underscore the importance of discriminating short-lasting and long-lasting distress in studies of emotion regulation. Whereas by far the most studies have focused on responses during emotional experiences, it has been recognized already in early studies that the duration of subsequent prolonged responses, in a time window that can extend across sleep, is most relevant to disease. A slow return to baseline after an emotional experience may undermine health through chronic arousal and is indeed prominent in mental disorders (cf. 47–49). Based on these systematic reviews, and on our specific prediction of differential categories of short-lasting (within a day) and long-lasting (across one or more nights) distress, we generated questions to cover this range. Factor analysis confirmed that the resulting SDDS scale indeed covered separate diurnal and overnight constructs, both measured with excellent internal consistency (α ≥ 0.90). The discriminant validity of the factors was supported by their different associations with nocturnal mentation, hyperarousal, and insomnia severity. Moreover, the extensive structured interview on comorbidity allowed us to confirm that people familiar with a mental disorder diagnosis scored higher on long-term distress [T(1,197) = 10.55, P < 0.001] but not on short-term distress [T(1,197) = 1.32, P = 0.19], compared with those people without such a diagnosis. Importantly, our results were not confounded by these diagnoses, as demonstrated both by multiple regression models, including diagnoses as covariates, as well as by a sensitivity analysis providing a structural equation model that included only participants without a disorder. In summary, these assessments, analyses, and results support the internal and external validity of the SDDS scale, providing a simple instrument for future studies on individual differences in immediate and prolonged emotional stress responses.
Third, a valuable contribution of the PSG study is that it provided a questionnaire proxy specifically associated with restless REM sleep that can easily be implemented in future population-scale studies. Arousals from all sleep stages elicit reports of mental activity that may differ depending on the assessment methodology (33–37). We therefore specifically queried both mentation with dream-like qualities and more thought-like mentation without such qualities. We showed in the polysomnographic part that thought-like mentation specifically reflects fragmentation of REM sleep. Thought-like mentation was also related to increased eye movement density, which was previously proposed to be consequential to REM sleep fragmentation and involved in the development of depression (5). Moreover, the frequency of experiencing such thought-like mental content did not reflect fragmentation of NREM sleep, the duration of REM sleep, or the consolidation of the final REM epoch.
Finally, and most importantly, the present findings show that the overnight resolution of distress from shame is compromised in people with insomnia; that this deficit contributes to hyperarousal; and that this deficit seems, in part, to be due to a process that is reflected in a high density of arousals and eye movements in REM sleep and concomitant thought-like nocturnal mentation. These findings reveal important associations between key characteristics of both insomnia and depression (5, 6, 21–25). Although the present study focused on insomnia, the role of restless REM sleep in emotion regulation appears highly relevant to other realms of psychiatry. Restless REM sleep is present in MDD (18) and PTSD (19, 20). Importantly, insomnia impedes the effectiveness of depression intervention (4), whereas treatment of insomnia improves remission from MDD (3). Patients suffering from significant emotional distress and co-occurring sleep disturbances might benefit from targeted interventions to either restore consolidated REM sleep or, if not feasible, prevent the occurrence of fragmented REM sleep.
Methods
Participants.
Two nights of PSG and the extended NSR Internet version of the DRFS (30) were assessed in 32 people [19 female and 13 male, mean (SD) of 35.8 (9.0) y of age], of whom half had no sleep complaints and the other half had previously been diagnosed with ID. Sleep recordings were made in the Department of Psychiatry and Psychotherapy, University Medical Center of Freiburg, and the questionnaire was administered through the Internet. The polysomnographic study adhered to strict exclusion criteria on disorders and use of drugs, including hypnotics [selection criteria and procedures according to Feige et al. (17)].
Large-scale questionnaire data were obtained through the Internet from volunteers of the NSR. The NSR is a national platform that recruits volunteers by advertising in media (Internet, television, radio, magazines, and newspapers) and through flyers distributed in health care institutions and conventions. People are asked to fill out questionnaires regularly to help create a psychometric database to facilitate research on factors that discriminate people with insomnia from people without sleep complaints. Continued commitment of unpaid volunteers is supported by newsletters, reminder emails, and an occasional voucher lottery. The present study included data of the 1,199 participants who completed all surveys listed below. The sample consisted of 308 males and 891 females, with a mean (SD) age of 52.1 (13.4) y. The Medical Ethical Committee of the Academic Medical Center of the University of Amsterdam, as well as the Central Committee on Research Involving Human Subjects, The Hague, The Netherlands, approved unsigned informed consent because volunteers participated anonymously without revealing their full name and address and were not exposed to any intervention or behavioral constraint.
PSG.
In the 32 subjects assessed with PSG, two nights were recorded between 11:00 PM and 7:00 AM. Recordings included electroencephalography (C3–A2, C4–A1), electrooculography (EOG; horizontal and vertical), electromyography (submental and tibialis anterior), breathing effort, nasal airflow, and oximetry. The first night served for adaptation and screening to exclude sleep apnea and periodic limb movements. The second night was scored visually by experienced raters according to standard criteria for sleep staging and NREM and REM arousal density quantification (31, 50). Rapid eye movements defined by fast EOG excursions (≥70 μV⋅s−1) were counted to calculate their density in REM sleep.
Questionnaires.
Insomnia Severity Index.
The severity of insomnia complaints was assessed using the validated Insomnia Severity Index (ISI) (38). It contains seven questions regarding sleep difficulties, satisfaction with sleep, worries about sleep, and adverse consequences of sleep problems for daily functioning and quality of life. Answer options on a five-point Likert-type scale are as follows: “none,” “mild,” “moderate,” “severe,” and “very severe.” Sum scores ranging from 0 to 28 indicate insomnia severity according to the usual cutoff scores: no insomnia, ISI ≤ 7; subthreshold insomnia, 7 < ISI ≤ 14; moderate clinical insomnia, 14 < ISI ≤ 21; and severe clinical insomnia, ISI > 21. According to these criteria, insomnia was not present in 561 participants, was subthreshold in 336, was moderate in 229, and was severe in 73.
Hyperarousal.
The severity of reactive and introspective hyperarousal was acquired using the validated Self-Reported Hyperarousal Scale (39). The items describe arousal-related experiences that have to be rated with respect to how well they apply using a five-point Likert-type scale (“never,” “seldom,” “sometimes,” “often,” and “almost always”). Reactive and introspective hyperarousal comprises 12 items of which the sum score can range between 0 and 48.
Deriving a proxy variable specific for restless REM sleep.
The extended DRFS implemented in the NSR includes 10 items: five that ask about the frequency of (i) recalling dreams during the day, (ii) experiencing anxious dreams, (iii) nocturnal awareness of dreams, (iv) experiencing daytime mood effects of dreams, and (v) sharing dreams; these items are complemented by five additional items (vi–x) that ask about thought-like rather than dream-like nocturnal mental content. People with insomnia were found to be more likely to experience the former during REM sleep (32). Answer options on a nine-point Likert-type scale are “never,” “less than once a year,” “about once a year,” “about two to four times a year,” “about once a month,” “two to three times a month,” “about once a week,” “several times a week,” and “almost daily,” and they yield a score between 0 and 8. Spearman correlations were used to evaluate the association of each item with the standard nonspecific PSG REM arousal index (31), as well as with a REM sleep-specific arousal density index calculated as the log-transformed ratio between REM and NREM arousal density indices, and with the density of eye movements during REM sleep. Items viii–x correlated most strongly with these restless REM sleep indices and were averaged into a nocturnal mentation score.
SDDS.
To quantify the SDDS assessment systematically, participants were first introduced to examples of shameful experiences by filling out the Compass of Shame Scale (CoSS) (51), which sketches four shame-eliciting scenarios and makes an inventory of responses. The CoSS was implemented immediately before the SDDS assessment to facilitate reminiscences of personal shameful experiences. The SDDS questionnaire asks how often it occurred that the emotional distress of a personal shameful experience lasted for eight distinct durations. Specifically, the questions were first introduced as follows: “You just indicated your reaction to four situations. Through these questions, you may have remembered some unpleasant situations of your own. After these situations, you may have experienced intense feelings for a short or a long time. Try to remember how long such feelings could last.” Participants were then asked the following: “An intense feeling elicited by the sort of situations just described could last…” followed by eight durations (“...a few minutes,” “...a quarter of an hour,” “...an hour,” “...half a day,” “...a whole day,” “...two–three days,” “...a week,” and “...more than a week”). Each of these eight questions had to be answered on a six-point Likert-type scale (“never,” “once at most,” “sometimes,” “regularly,” “often,” and “very often”). The sequence of asking all participants to fill out the CoSS immediately before this assessment of distress duration served two aims. First, the fixed sequence was used to limit unexplained variance by promoting a similar self-oriented mindset focused on the evaluation of shameful experiences in all participants. Second, the sequence was used to help participants understand the questions addressed by the SDDS and to facilitate recalling their own distress from shame by providing examples.
Major life events, neurological and mental disorders, and sleep disorders other than insomnia.
Given the average age and SD of our large sample (52.1 ± 13.4 y), two-thirds of the participants in our sample are in the age range of about 45–64 y. In this age range, people without any morbidity are not representative; in fact, 30% can be expected to have multimorbidity (52). To allow for analyses on possible confounding by individual differences in exposure to major life events and neurological, mental, or sleep disorders other than insomnia, we only included NSR participants who had completed the NSR implementations of the LES (40) and the extended DSISD (41).
Statistical Analyses.
Factor analysis on questions about the duration of emotional distress.
Factor analyses with Varimax rotation were conducted to investigate whether the eight “duration of emotional distress” items showed a two-factor structure. An initial factor analysis was used to obtain the number of factors and to select items with factor loading above 0.40 on only one of the factors (53). The factor structure was verified using a second factor analysis that included only the discriminating items. Individual scores on the resulting factors were calculated as the average of the items included in each factor.
Associations of insomnia with nocturnal mentation, distress duration, and hyperarousal.
Linear regression analysis was applied to evaluate the expected associations between insomnia severity, nocturnal mentation, hyperarousal, and the duration of distress factor scores. Age and gender were included as covariates in the regression analyses in case they were significantly associated with the outcome measure. To evaluate confounding by individual differences in exposure to major life events and neurological, mental, or sleep disorders other than insomnia, extended regression models included these variables as covariates. A total of 15 independent regression analyses were evaluated (including regression analyses of the structural equation modeling). The a priori significance threshold was therefore Bonferroni-corrected and set to P < 0.003. Standardized β-coefficients are reported.
Structural equation modeling.
Regression analyses were complemented by structural equation modeling to evaluate whether long-lasting distress mediates the association of hyperarousal with thought-like nocturnal mentation that is specifically associated with fragmented REM sleep. Mediation directionality of the model was investigated by comparing its adjusted R2 and BIC with alternative models rearranging the roles of the three variables of interest as independent, dependent, or mediator variables. A sensitivity analysis evaluated the structural equation model parameters in the subset of 686 participants who were unlikely to suffer from any neurological, mental, or sleep disorder other than insomnia.
Evaluation of Confounding by Individual Differences in Exposure to Major Life Events and Neurological, Mental, or Sleep Disorders Other than Insomnia
Two approaches were taken to evaluate possible confounding of the reported associations and models by common causes related to individual differences in exposure to major life events or neurological, mental, or sleep disorders other than insomnia. As queried using the NSR implementation of the 30-item LONGSCAN version of the LES (40), participants had a lifetime experience of an average (SD) of 14.9 (4.5) major life events. As queried using the NSR implementation of the DSISD (41) that also includes sections to query all current and past disorders according to the categories of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), a neurological, mental, or sleep disorder other than insomnia could not be excluded in 513 participants: a neurological disorder in n = 13, a mental disorder in n = 87, another sleep disorder in n = 318, both a neurological and a mental disorder in n = 2, both a neurological disorder and another sleep disorder in n = 8, both a mental disorder and another sleep disorder in n = 82, and disorders in all three categories in n = 3.
First, all regression equations evaluating bivariate associations were run once more with inclusion of covariates for the number of major life events and the dummy-coded presence of mentioned disorder categories. All significant associations remained highly significant, whereas nonsignificant associations remained nonsignificant. In detail, the adjusted β-coefficients and their significance were as follows. Insomnia severity remained specifically and very significantly associated with nocturnal mentation [β = 0.25, T(1,191) = 9.16, P < 10−18], with hyperarousal [β = 0.41, T(1,191) = 15.08, P < 10−46] (all coefficients also adjusted for age and gender in both measures), and specifically with emotional distress lasting overnight [β = 0.23, T(1,190) = 8.08, P < 10−14], but not with distress resolved within a day (i.e., without sleep) [β = −0.03, T(1,190) = −1.00, P = 0.32] (all coefficients also adjusted for age and gender). The more frequently people reported nocturnal mentation, the more frequently they experienced long-lasting distress [β = 0.23, T(1,190) = 7.94, P < 10−14], but not short-lasting distress [β = 0.05, T(1,190) = 1.85, P = 0.06]. The more people experienced distress that lasted overnight, the higher were their hyperarousal ratings [β = 0.40, T(1,190) = 15.33, P < 10−47]. Short-lasting distress was not associated with hyperarousal [β = 0.02, T(1,190) = 0.79, P = 0.43]. In summary, associations found remained highly significant when adjusting for individual differences in exposure to major life events or neurological, mental, or sleep disorders other than insomnia.
Second, to evaluate possible confounding, a sensitivity analysis evaluated the structural equation model parameters in the subset of 686 participants who were unlikely to suffer from any neurological, mental, or sleep disorder other than insomnia [511 female and 175 male; mean (SD) of 50.9 (14.1) y of age; 398 people without sleep complaints and 182 subthreshold, 83 moderate, and 23 severe insomniacs). As shown in Fig. S1 below, the partial mediation effect of long-lasting distress in this subsample remained selective and highly significant [53.5%; β2 × β3 = 0.09, T(681) = 5.54, P < 10−6), supporting the robustness of the findings and making it unlikely that they result from spurious correlations due to common underlying confounders.
Acknowledgments
We thank Frits Bienfait for initiating the Neuropsychoanalysis Fund. The authors acknowledge and thank the Netherlands Sleep Registry participants who took the time to take part in the surveys on which this publication is based. We thank anonymous reviewers for valuable suggestions. This work was supported by ZONMW Neuropsychoanalysis Fund Grant 16.561.0001 (to F.S. and E.J.W.V.S.), VICI Grant 453.07.001 (to E.J.W.V.S.), and VIDI Grant 452.12.014 and Aspasia Grant 015.009.016 (to S.v.d.S.), all part of the Netherlands Organization of Scientific Research (NWO); by the Grant 253/2012 of the Bial Foundation (to L.M.T.); and by the European Research Council ERC-ADG-2014-671084 INSOMNIA Grant (to E.J.W.V.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data are available on the authors’ institutional web site (dx.doi.org/10.17026/dans-zs4-9f2m).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522520113/-/DCSupplemental.
References
- 1.Cuijpers P, Beekman AT, Reynolds CF., 3rd Preventing depression: A global priority. JAMA. 2012;307(10):1033–1034. doi: 10.1001/jama.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baglioni C, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Manber R, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thase ME, et al. Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. Am J Psychiatry. 1997;154(4):502–509. doi: 10.1176/ajp.154.4.502. [DOI] [PubMed] [Google Scholar]
- 5.Riemann D, et al. REM sleep instability--a new pathway for insomnia? Pharmacopsychiatry. 2012;45(5):167–176. doi: 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14(1):9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Sterpenich V, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5(11):e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner U, Kashyap N, Diekelmann S, Born J. The impact of post-learning sleep vs. wakefulness on recognition memory for faces with different facial expressions. Neurobiol Learn Mem. 2007;87(4):679–687. doi: 10.1016/j.nlm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 10.van der Helm E, et al. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pace-Schott EF, et al. Napping promotes inter-session habituation to emotional stimuli. Neurobiol Learn Mem. 2011;95(1):24–36. doi: 10.1016/j.nlm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21(1):115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spoormaker VI, et al. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp. 2012;33(10):2362–2376. doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosales-Lagarde A, et al. Enhanced emotional reactivity after selective REM sleep deprivation in humans: An fMRI study. Front Behav Neurosci. 2012;6:25. doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talamini LM, Bringmann LF, de Boer M, Hofman WF. Sleeping worries away or worrying away sleep? Physiological evidence on sleep-emotion interactions. PLoS One. 2013;8(5):e62480. doi: 10.1371/journal.pone.0062480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baglioni C, et al. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18(3):195–213. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Feige B, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17(2):180–190. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 18.Duncan WC, Jr, Pettigrew KD, Gillin JC. REM architecture changes in bipolar and unipolar depression. Am J Psychiatry. 1979;136(11):1424–1427. doi: 10.1176/ajp.136.11.1424. [DOI] [PubMed] [Google Scholar]
- 19.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 20.Germain A. Sleep disturbances as the hallmark of PTSD: Where are we now? Am J Psychiatry. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nofzinger EA, et al. A comparison of regional cerebral metabolism across waking and NREM sleep between primary insomnia and major depression. Sleep. 2005;28(Abstr Suppl):A232–A233. [Google Scholar]
- 22.Nofzinger EA, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 23.Riemann D, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Edinger JD, Means MK, Krystal AD. Does physiological hyperarousal enhance error rates among insomnia sufferers? Sleep. 2013;36(8):1179–1186. doi: 10.5665/sleep.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Mendoza J, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4):397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 26.Schalkwijk F. The Conscience and Self-Conscious Emotions in Adolescence: An Integrative Approach. Routledge Hove; New York: 2015. [Google Scholar]
- 27.Stuewig J, McCloskey LA. The relation of child maltreatment to shame and guilt among adolescents: Psychological routes to depression and delinquency. Child Maltreat. 2005;10(4):324–336. doi: 10.1177/1077559505279308. [DOI] [PubMed] [Google Scholar]
- 28.Feiring C, Taska LS. The persistence of shame following sexual abuse: A longitudinal look at risk and recovery. Child Maltreat. 2005;10(4):337–349. doi: 10.1177/1077559505276686. [DOI] [PubMed] [Google Scholar]
- 29.van Es SM, et al. Predicting adherence to prophylactic medication in adolescents with asthma: An application of the ASE-model. Patient Educ Couns. 2002;47(2):165–171. doi: 10.1016/s0738-3991(01)00195-1. [DOI] [PubMed] [Google Scholar]
- 30.Schredl M. Reliability and stability of a dream recall frequency scale. Percept Mot Skills. 2004;98(3 Pt 2):1422–1426. doi: 10.2466/pms.98.3c.1422-1426. [DOI] [PubMed] [Google Scholar]
- 31.Bonnet M, et al. EEG arousals: Scoring rules and examples: A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 32.Ermann M, Peichl J, Pohl H, Schneider MM, Winkelmann Y. Spontanerwachen und Träumen bei Patienten mit psychovegetativen Schlafstörungen. Psychother Psychosom Med Psychol. 1993;43(9-10):333–340. German. [PubMed] [Google Scholar]
- 33.Antrobus J. Dreaming: Cognitive processes during cortical activation and high afferent thresholds. Psychol Rev. 1991;98(1):96–121. doi: 10.1037/0033-295x.98.1.96. [DOI] [PubMed] [Google Scholar]
- 34.Casagrande M, Bertini M, Testa P. Changes in cognitive asymmetries from waking to REM and NREM sleep. Brain Cogn. 1995;29(2):180–186. doi: 10.1006/brcg.1995.1275. [DOI] [PubMed] [Google Scholar]
- 35.Schredl M, Schäfer G, Weber B, Heuser I. Dreaming and insomnia: Dream recall and dream content of patients with insomnia. J Sleep Res. 1998;7(3):191–198. doi: 10.1046/j.1365-2869.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen T, Kuiken D, Hoffmann R, Moffitt A. REM and NREM sleep mentation differences: A question of story structure? Sleep Hypn. 2001;3:9–17. [Google Scholar]
- 37.Nielsen TA. 2000. A review of mentation in REM and NREM sleep: “Covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci 23(6):851–866, discussion 904–1121.
- 38.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 39.Pavlova M, et al. Self-reported hyperarousal traits among insomnia patients. J Psychosom Res. 2001;51(2):435–441. doi: 10.1016/s0022-3999(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 40.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 41.Edinger J, et al. ICSD-2 Sleep Disorder Diagnoses. 2nd Ed (ICSD-2) Duke University Medical Center; Durham, NC: 2004. The Duke Structured Interview Schedule for DSM-IV-TR and International Classification of Sleep Disorders. [Google Scholar]
- 42.Edinger JD, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Arch Gen Psychiatry. 2011;68(10):992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- 43.Koulack D, Goodenough DR. Dream recall and dream recall failure: An arousal-retrieval model. Psychol Bull. 1975;83(5):975–984. [Google Scholar]
- 44.Nielsen TA. Disturbed dreaming in medical conditions. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Ed. Elsevier Saunders; Philadelphia: 2005. pp. 936–945. [Google Scholar]
- 45.Vandekerckhove M, Cluydts R. The emotional brain and sleep: An intimate relationship. Sleep Med Rev. 2010;14(4):219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Schalkwijk F. 2011. Emoties bij jongeren: theorie en diagnostiek van het geweten [Emotions of the Young: Theory and Diagnostics of the Conscious] (Boom, Amsterdam), 1st Ed, pp 368. Dutch. [Google Scholar]
- 47.Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: Conceptual siblings separated at birth? J Psychosom Res. 1997;42(2):117–135. doi: 10.1016/s0022-3999(96)00240-1. [DOI] [PubMed] [Google Scholar]
- 48.Brosschot JF. Markers of chronic stress: Prolonged physiological activation and (un)conscious perseverative cognition. Neurosci Biobehav Rev. 2010;35(1):46–50. doi: 10.1016/j.neubiorev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Koenigsberg HW. Affective instability: Toward an integration of neuroscience and psychological perspectives. J Pers Disord. 2010;24(1):60–82. doi: 10.1521/pedi.2010.24.1.60. [DOI] [PubMed] [Google Scholar]
- 50.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Department of Health, Education, and Welfare; Bethesda: 1968. [Google Scholar]
- 51.Elison J, Pulos S, Lennon R. Shame-focused coping: An empirical study of the compass of shame. Soc Behav Pers. 2006;34(2):161–168. [Google Scholar]
- 52.Barnett K, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 53.Stevens JP. Applied Multivariate Statistics for the Social Sciences. 5th Ed Routledge; London: 2009. [Google Scholar]