Significance
Our study demonstrated that CD8+CD122+CD49dlow regulatory T cells induced apoptosis in target T cells depending on death receptor Fas (CD95)–FasL (CD178) interaction. This in vitro phenomenon reflected the regulatory activity of these cells in vivo, which was also reproducible in the in vivo experimental system of adoptive T-cell transfer. By using natural mutant mice of Fas or FasL and the gene-targeted mice of IL-10, we confirmed that the regulatory activity of CD8+CD122+CD49dlow cells was mediated by Fas–FasL but not by IL-10. In addition, the necessity of MHC class I molecules on the target T cells was also confirmed by analysis using CD8+ T cells that lack β2-microglobulin and consequently class I MHC.
Keywords: Tregs, Fas/FasL, cytotoxicity T cells, immune homeostasis, central memory phenotype
Abstract
The Fas/FasL (CD95/CD178) system is required for immune regulation; however, it is unclear in which cells, when, and where Fas/FasL molecules act in the immune system. We found that CD8+CD122+ cells, which are mostly composed of memory T cells in comparison with naïve cells in the CD8+CD122− population, were previously shown to include cells with regulatory activity and could be separated into CD49dlow cells and CD49dhigh cells. We established in vitro and in vivo experimental systems to evaluate the regulatory activity of CD122+ cells. Regulatory activity was observed in CD8+CD122+CD49dlow but not in CD8+CD122+CD49dhigh cells, indicating that the regulatory cells in the CD8+CD122+ population could be narrowed down to CD49dlow cells. CD8+CD122− cells taken from lymphoproliferation (lpr) mice were resistant to regulation by normal CD122+ Tregs. CD122+ Tregs taken from generalized lymphoproliferative disease (gld) mice did not regulate wild-type CD8+CD122− cells, indicating that the regulation by CD122+ Tregs is Fas/FasL-dependent. CD122+ Tregs taken from IL-10–deficient mice could regulate CD8+CD122− cells as equally as wild-type CD122+ Tregs both in vitro and in vivo. MHC class I-missing T cells were not regulated by CD122+ Tregs in vitro. CD122+ Tregs also regulated CD4+ cells in a Fas/FasL-dependent manner in vitro. These results suggest an essential role of Fas/FasL as a terminal effector of the CD122+ Tregs that kill activated T cells to maintain immune homeostasis.
Fas (CD95) and its ligand FasL (CD178) compose a unique system that is strongly related to programmed cell death (1). FasL has been considered one of the effector molecules involved in the killing of target cells by cytotoxic T lymphocytes (CTLs) (2). When Fas binds to FasL, it induces downstream signal transduction pathways that subsequently activate cell death induction pathways (3, 4). Thus, the Fas/FasL system appears to act as an effector for CTL-mediated killing of virus-infected or cancer cells, similar to the perforin–granzyme system (5, 6). However, because Fas-mutant (lpr) and FasL-deficient (gld) mice show lymphoproliferative changes, it has been suggested that the Fas/FasL system is important for suppression/regulation of activated effector T cells (7, 8).
Molecular mechanisms after Fas activation have been thoroughly investigated, and the signal transduction pathway has been largely elucidated (9, 10). However, research on the cellular events that use the Fas/FasL system has progressed comparatively slowly. There are only a few relevant reports in this respect, mostly of human CD4+Foxp3+ Tregs that use the Fas/FasL system for their regulatory activity. [In this article, we use the term “Treg(s)” for any kind of T cells that show immune regulatory activity.] To complicate matters further, some contradictory reports claim that such Fas/FasL-engaging Tregs do not exist (8, 11, 12). No reliable reports suggesting that CD8+ T cells use the Fas/FasL system for their regulatory action are available. Therefore, it is not clear precisely which subset of T cells express FasL or where Fas/FasL-dependent CD8+ Tregs, if such cells exist, are located and at which point they function. Thus, the ultimate pathophysiological role of the Fas/FasL system is still unknown.
Regulation of the immune reaction is of pivotal importance for maintaining health in multicellular organisms. In highly developed animals, Tregs, a subset of T lymphocytes especially known as CD4+CD25+Foxp3+ cells, are the main regulators of the immune response (13–16). However, it is not quite clear whether CD8+ regulatory T cells exist; there are only few and contradictory reports on their existence, in contrast to the reports on CD4+ Tregs. A population of predominantly CD8+ suppressor T cells has been described and debated in the 1980s (17, 18). In the 2000s, we found and reported that the cells of central memory phenotype (CD8+CD122+) also possess the regulatory function, and some other reports regarding CD8+ Tregs with some contradictory findings have been published. To date, more than 10 CD8+ Treg populations with different markers have been reported (19).
One of the best characterized CD8+ Treg populations is the CD8+CD122+ Treg (122+ Treg) population. To confirm the existence of CD8+ Tregs, markers that may be possible to distinguish Tregs from other T cells were examined. We previously found that CD49d can separate CD8+CD122+ cells into at least two subsets (CD49dlow and CD49dhigh) (20). To judge which cells have a stronger regulatory activity, we prepared two experimental systems: an in vitro system, based on cell culture, and an in vivo system, based on adoptive transfer of T cells.
Interestingly, CD8+ Tregs express CD122 (IL-2/IL-15 receptor β chain) in contrast to CD4+ Tregs, which express CD25 (IL-2 receptor α chain), indicating the fundamental importance of IL-2 at the development/maturation of both CD4+ and CD8+ Tregs. Suzuki and coworkers generated CD122-deficient mice using gene targeting (21) and used them to identify 122+ Tregs (22). In this study, which is a follow-up study to our previous report, we performed in vitro and in vivo experiments to gain further understanding of these cells. We found that CD49d might be a good marker for classifying CD8+ T cells into naïve, resting T cells (CD62L+CD122−), effector memory T cells (CD62L−), and T cells of central memory phenotype (CD122+CD49dlow), by using multicolor staining of CD62L, CD122, and CD49d. To our knowledge, this is the first report on the role of the Fas/FasL system in the action of CD8+CD122+ Tregs (122+ Tregs) (22–25). Additionally, we show that CD8+ Tregs are included in the CD49dlow population, which corresponds to the central memory T-cell population, and that their mechanism of suppression depends on the cytotoxicity mediated by the Fas and FasL interaction.
Results
CD8+CD122+ Cells Are Divided into Subpopulations Expressing Different Sets of Cell-Surface Markers.
Although it has been shown that the CD8+CD122+ population contains Tregs, CD8+CD122+ cells are generally considered memory T cells. Thus, we needed to identify a better marker to focus on regulatory cells in the CD8+CD122+ population. We chose CD62L and CD49d; CD62L allows separation of effector memory T cells. CD122 is also frequently used as well as CD44 and CD62L for the analysis of memory and regulatory cells (26). We examined the expression of these cell-surface markers in murine splenic T cells (Fig. 1 A and B). At least four different subsets of CD8+ cells could be distinguished by this analysis. Their profiles were CD62L+CD122−, CD62L+CD122+CD49dlow, CD62L+CD122+CD49dhigh, and CD62L−. The first subset is usually referred to as CD122− cells and consists mostly of naïve, resting CD8+ cells (Fig. 1B). The last three subsets could be classified into memory or activated cells and may also contain regulatory cells.
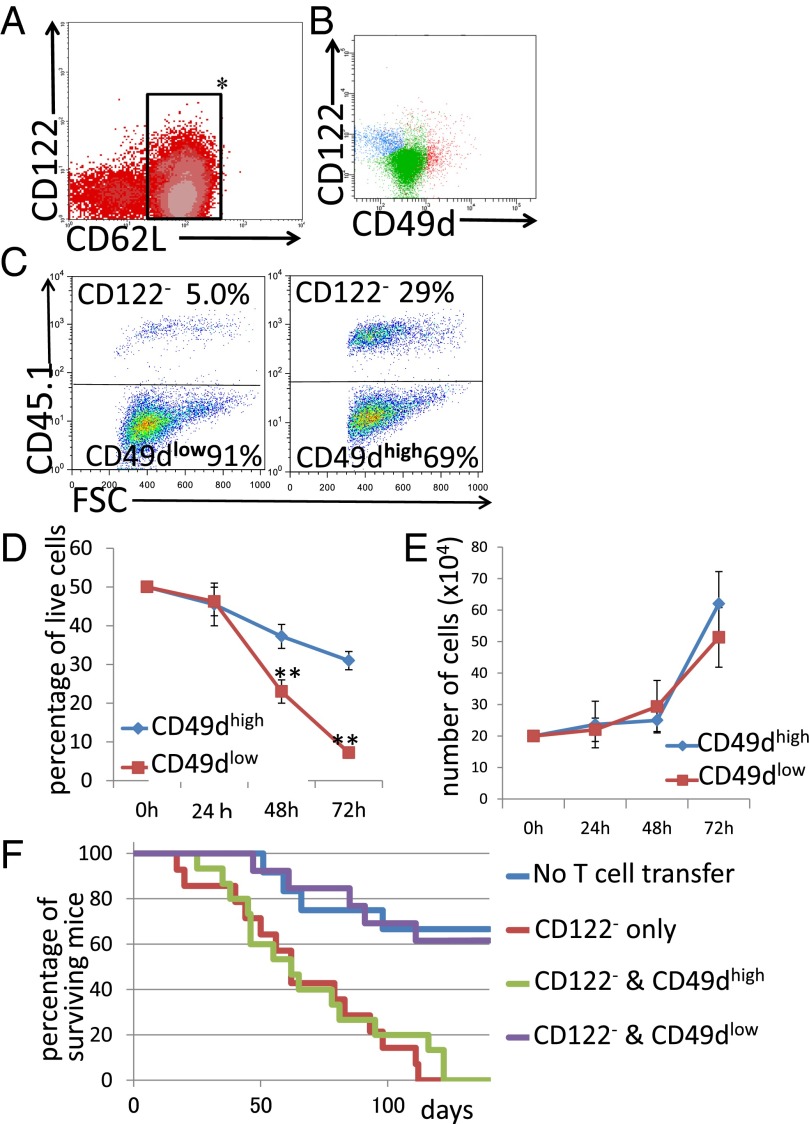
Fig. 1.
(A) Spleen cells were analyzed for the expression of CD8, CD122, CD62L, and CD49d. (B) CD62L+ cells (inside of the rectangle in A) were analyzed for the expression of CD122 and CD49d. (C and D) Analysis of regulatory activity in coculture. (C, Left) CD8+CD122−CD45.1+ cells and (CD8+CD122+)CD49dlowCD45.1− cells. (C, Right) CD8+CD122−CD45.1+ cells and (CD8+CD122+)CD49dhighCD45.1− cells. Representative results obtained after 72 h of coculture are shown. (D) The percentages of CD8+CD122− on total live cells obtained from three independent experiments are shown. **Significantly different at the same time point (P < 0.01, Student’s t test). (E) Cell growth analysis of CD49dlow and CD49dhigh T cells. For all time points, the values obtained for CD49dlow (red line) and CD49dhigh T cells (blue line) did not significantly differ (P > 0.05, Student’s t test). (F) Analysis of adoptive T-cell transfer to RAG-2−/− mice. Transfer of a mixture of CD49dlow and CD122− T cells (purple line) effectively prolonged survival of the mice; survival of these mice was significantly higher than that of mice receiving CD122− cells alone (red line; log-rank test, P = 0.0003; Wilcoxon test, P = 0.0014). In contrast, transfer of a mixture of CD49dhigh and CD122− T cells did not significantly affect survival (green line) compared with that of mice receiving CD122− cells alone (log-rank test, P = 0.5271; Wilcoxon test, P = 0.8964).
CD8+CD122+CD49dlow Cells Show Regulatory Activity in Vitro and in Vivo but CD8+CD122+CD49dhigh Cells Do Not.
We found that CD8+CD122+ cells can be divided into two populations by their expression level of CD49d. Then, we performed an in vitro experiment in which candidate Tregs were cocultured with their target cells. As the mechanism of regulation by CD8+ Tregs is thought to involve elimination of the target cells (27), candidate Tregs should reduce the number of target (regulated) cells during coculture. Representative results after 72 h of coculture are presented in Fig. 1 C and D. When CD49dlow cells (CD45.1−) were mixed with CD8+CD122− cells (CD45.1+) at a 1:1 ratio at the beginning of coculture, the percentage of CD8+CD122−CD45.1− cells markedly decreased during coculture in a time-dependent manner (Fig. 1C). When CD122− cells were cocultured with CD49dhigh cells, the decrease in the percentage of CD122− cells was much less marked than when they were cocultured with CD49dlow cells (Fig. 1 C and D). These results of relative change between two different cells cocultured were the reflection of the absolute number of live cells, which is presented in Fig. S1A.
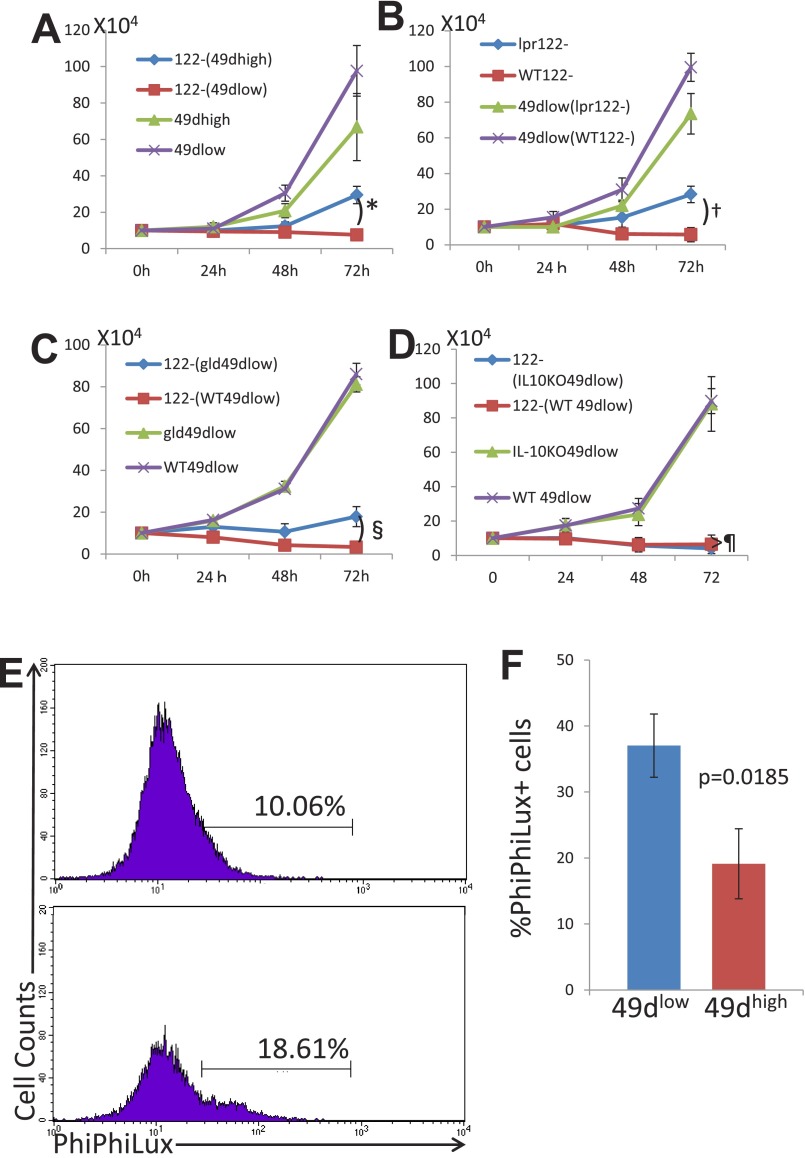
Fig. S1.
(A) Experiment was performed as described for Fig. 1D, and the data were exchanged to absolute numbers of cells by calculating the total numbers of cells obtained by counting cells in a counting chamber times the percentages of the cell of interest obtained by the flow-cytometric analysis. 122−(49dhigh), CD8+CD122− cells cocultured with CD8+CD122+CD49dhigh cells; 122–(49dlow), CD8+CD122− cells cocultured with CD8+CD122+CD49dlow cells. *Significantly different (P = 0.0096, Student’s t test). (B) Experiment was performed as described in Fig. 2B, and the data were exchanged to absolute numbers of cells as in A. 49dlow(lpr122–), CD8+CD122+CD49dlow cells cocultured with C57BL/6lpr/pr mice-derived CD8+CD122− cells; 49dlow(WT122–), CD8+CD122+CD49dlow cells cocultured with wild-type C57BL/6 mice-derived CD8+CD122− cells. †Significantly different (P = 0.0175, Student’s t test). (C) Experiment was performed as described in Fig. 3D, and the data were exchanged to absolute numbers of cells as in A. 122–(gld49dlow), CD8+CD122− cells cocultured with C57BL/6gld/gld mice-derived CD8+CD122+CD49dlow cells; 122–(WT49dlow), CD8+CD122− cells cocultured with wild-type C57BL/6 mice-derived CD8+CD122+CD49dlow cells. §Significantly different (P = 0.0165, Student’s t test). (D) Experiment was performed as described in Fig. 5B, and the data were exchanged to absolute numbers of cells as in A. 122–(IL-10KO49dlow), CD8+CD122− cells cocultured with C57BL/6IL-10KO mice-derived CD8+CD122+CD49dlow cells; 122–(WT49dlow), CD8+CD122− cells cocultured with wild-type C57BL/6 mice-derived CD8+CD122+CD49dlow cells. ¶Not significantly different (P = 0.5071, Student’s t test). (E) Coculture assay was performed as described in Fig. 1, and after 48 h of coculture, cells were subjected to the assay for PhiPhiLux. Cells were treated with the PhiPhiLux system according to the protocol provided by the manufacturer and subsequently analyzed by FACSCalibur flow cytometer. Cells stained with anti–CD45.1-PE (originally CD122−) are shown as histogram analysis. Percentages of cells positive for PhiPhiLux are shown. Data are representative of three independent experiments. (F) Analysis of PhiPhiLux, indicating activity of caspase 3, is shown as a bar graph. Statistically significant difference was observed between the cells cocultured with different cells indicated in the panel. The data are averages of three independent experiments.
These results indicate that CD8+CD122− cells reduce their number by some effect of CD8+CD122+ cells, and such an effect is much stronger in CD49dlow cells than in CD49dhigh cells. However, this interpretation should be adapted only if the proliferation speed of CD49dlow cells and CD49dhigh cells is identical. Therefore, we examined the growth of separately cultured CD49dlow and CD49dhigh cells. As a result, both cells grew at virtually the same rate (Fig. 1E, not significantly different, as determined by Student’s t test, P > 0.1 for any culture duration), supporting that CD49dlow cells, but not CD49high cells, possess an activity to reduce the number of cocultured CD8+CD122− cells, which is possibly achieved by the elimination of target cells (i.e., cytotoxic activity).
For the in vivo experiment, we essentially followed the method established by Rifa’i et al. (22), with minor modifications. We performed T-cell adoptive transfer into RAG-2−/− mice and followed their survival. The CD49dhigh cells did not support survival of RAG-2–deficient mice that had received CD8+CD122− cells. In contrast, the CD49dlow cells clearly reversed the survival of mice to the level of those that had not received T cells (Fig. 1F, blue line). Together with the results of the in vitro experiment, these results confirmed that CD8+ Tregs are encompassed in the CD49dlow subset. Thus, in the following text, CD49dlow cells are described as “CD8+ Tregs” to reduce the text volume.
CD8+CD122+CD49dlow Tregs Cannot Eliminate or Suppress Fas-Mutated CD8+CD122− Cells from lpr Mice.
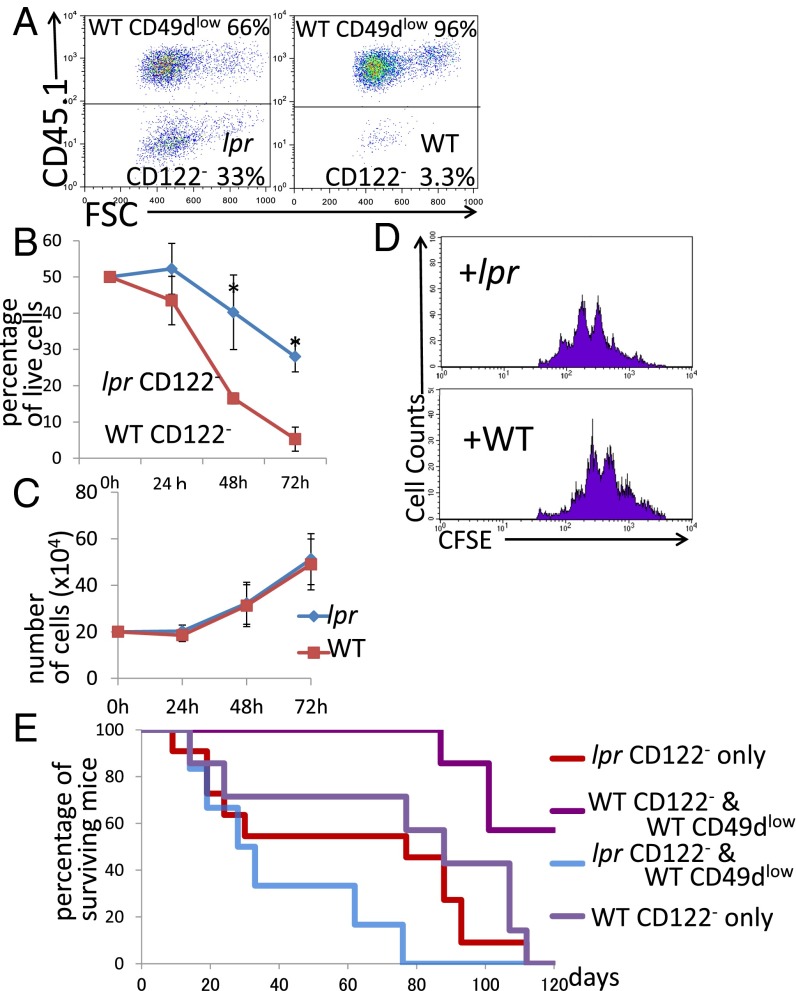
We tested the regulatory activity of CD8+ Tregs under Fas-related conditions. CD8+ Tregs, taken from wild-type mice, were cocultured with CD8+CD122− cells, taken from Fas-mutant lpr mice, and the percentage of target CD8+CD122− cells among total live cells was determined at various time points. The ratio of CD8+CD122− cells from lpr mice to wild-type CD8+ Tregs decreased as coculture continued (Fig. 2 A and B). However, the rate and extent of decrease were significantly higher when CD8+CD122− cells and CD8+ Tregs, both taken from wild-type mice, were cocultured (Fig. 2 A and B). These changes in the ratio of cell number were also observed in the data of absolute cell numbers (Fig. S1B). The proliferation rate of CD8+CD122− cells from lpr mice was not different from that of CD8+CD122− cells from wild-type mice (Fig. 2C). In addition, we examined the proliferation of cocultured cells by the dilution of prelabeled CFSE and further confirmed that any types of cells used in this study show a similar pattern of CFSE peaks (Fig. 2D). This result confirmed that the difference observed between CD8+CD122− cells from lpr mice and those from wild-type mice in the coculture experiment was not due to a difference in proliferation rate but due to resistance to apoptosis of the lpr mice-derived CD8+CD122− cells. In the in vivo experiment, RAG-2–deficient mice that had received CD8+CD122− cells taken from lpr mice died, similar to those injected with CD8+CD122− cells taken from wild-type mice. Mice that had received a mixture of CD8+CD122− cells from lpr mice and CD8+ Tregs obtained from wild-type mice died, similar to those that had received CD8+CD122− cells from lpr mice alone (Fig. 2E). Together, these findings indicated that Fas-mutant CD8+CD122− cells are resistant to regulation by CD8+ Tregs, which have a marked effect on wild-type CD8+CD122− cells and prolong the survival of CD8+CD122− cell-recipient mice.
Fig. 2.
(A and B) Analysis of regulatory activity in coculture of CD49dlow cells (CD45.1) and CD8+CD122− cells (CD45.2) derived from lpr mice and wild-type mice. (A) Representative results after 72 h of coculture obtained from three independent experiments are shown. (Left) CD122− cells (lpr) cocultured with CD49dlow cells. (Right) CD122− cells (WT) cocultured with CD49d− cells. (B) The percentages of CD8+CD122− cells among total live cells are shown. *Significantly different (P < 0.05, Student’s t test). (C) Absolute numbers of CD122− T cells derived from WT mice (red line) and lpr mice (blue line) in simple culture were determined. At all time points, the values obtained for lpr mice-derived cells and WT mice-derived cells were not significantly different (P > 0.05, Student’s t test). (D) CD8+CD122− T cells (WT or lpr) labeled with CFSE were cocultured for 48 h and analyzed. The dilution patterns of CFSE are shown as representative results of three independent experiments. (E) Analysis of adoptive T-cell transfer of CD122− T cells from WT or lpr mice mixed with CD49dlow T cells of WT mice into RAG-2–deficient mice. Addition of WT mice-derived CD49dlow T cells to lpr mice-derived CD122− cells (blue line) did not affect survival; survival of these mice was not significantly different from that of mice receiving lpr mice-derived CD122− cells alone (red line; log-rank test, P = 0.0878; Wilcoxon test, P = 0.2537).
FasL-Mutant CD8+ Tregs Cannot Eliminate or Suppress Conventional CD8+CD122− T Cells.
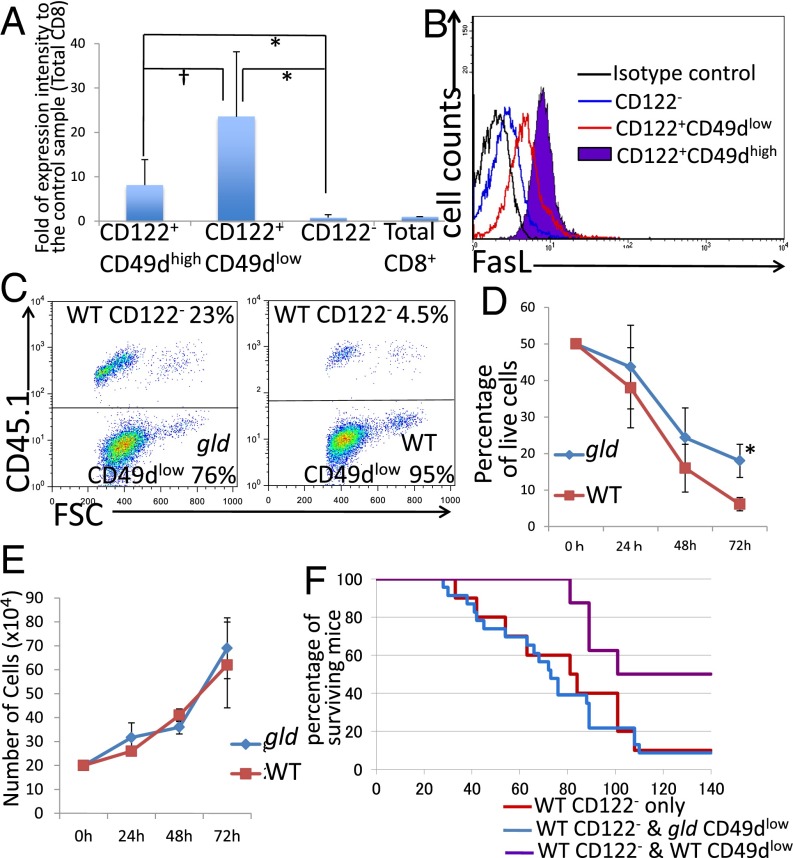
First, the expression of FasL in CD8+ Tregs was confirmed by real-time PCR (Fig. 3A). Furthermore, the existence of the FasL product on the cell surface was confirmed by flow cytometry (Fig. 3B). This result indicated that FasL is a candidate mediator of killing of Fas-expressing target cells.
Fig. 3.
(A) Analysis of RNA expression level of FasL in each population of CD8+ T cells by real-time PCR. For each sample, FasL mRNA levels were normalized to β-actin and are expressed as fold change relative to the control sample (1.00). †Not significantly different (P > 0.05, Student’s t test); **significantly different (P < 0.01, Student’s t test). (B) Analysis of FasL expression of flow cytometory. (C and D) Analysis of coculture of CD8+CD122−CD45.1+ T cells and CD49dlow from gld or WT mice. (C) Representative results obtained from three independent experiments. (D) *Significantly different (P < 0.05, Student’s t test). (E) Cell growth analysis of CD49dlow T cells from gld mice and WT mice. At all time points, the results obtained from two different mice were not significantly different (P > 0.05, Student’s t test). (F) Analysis of adoptive T-cell transfer of CD122− T cells (WT) mixed with CD49dlow T cells (WT or gld) into RAG-2–deficient mice. Addition of gld mice-derived CD49dlow T cells to CD122− cells (blue line) had no significant effect on survival compared with transfer of CD122− cells alone (red line; log-rank test, P = 0.7608; Wilcoxon test, P = 0.6859).
Next, we examined the activity of FasL-mutant CD8+ Tregs. CD8+ Tregs taken from gld mice were cocultured with CD8+CD122− cells taken from wild-type mice, and the percentage of target cells (CD8+CD122−) among CD8+ Tregs was determined at various time points. The percentage of wild-type CD8+CD122− cells among CD8+ Tregs from gld mice decreased over time; however, the rate and extent of decrease were significantly lower than when both types of cells were taken from wild-type mice (Fig. 3 C and D). The difference was also observed in the absolute cell number (Fig. S1C). The proliferation rate of CD8+ Tregs taken from gld mice was not different from that of wild-type CD8+ Tregs (Fig. 3E). This result confirmed that an insufficient decrease of cells cocultured with CD8+ Tregs taken from gld mice, compared with those cocultured with wild-type CD8+ Tregs, was not due to a relative decrease in the proliferation rate of CD8+ Tregs taken from gld mice but rather due to insufficient elimination of CD8+CD122− cells by FasL-mutated CD8+ Tregs.
In the in vivo experiment, RAG-2–deficient mice that had received a mixture of wild-type CD8+CD122− cells and CD8+ Tregs from gld mice died at the same rate as those that had received wild-type CD8+CD122− cells alone (Fig. 3F). Thus, CD8+ Tregs taken from FasL-mutant gld mice demonstrated no ability to control CD8+CD122− cells and could not prolong the survival of mice that received these cells.
CD8+ Tregs Induce Apoptosis in Target-Activated CD8+CD122− T Cells.
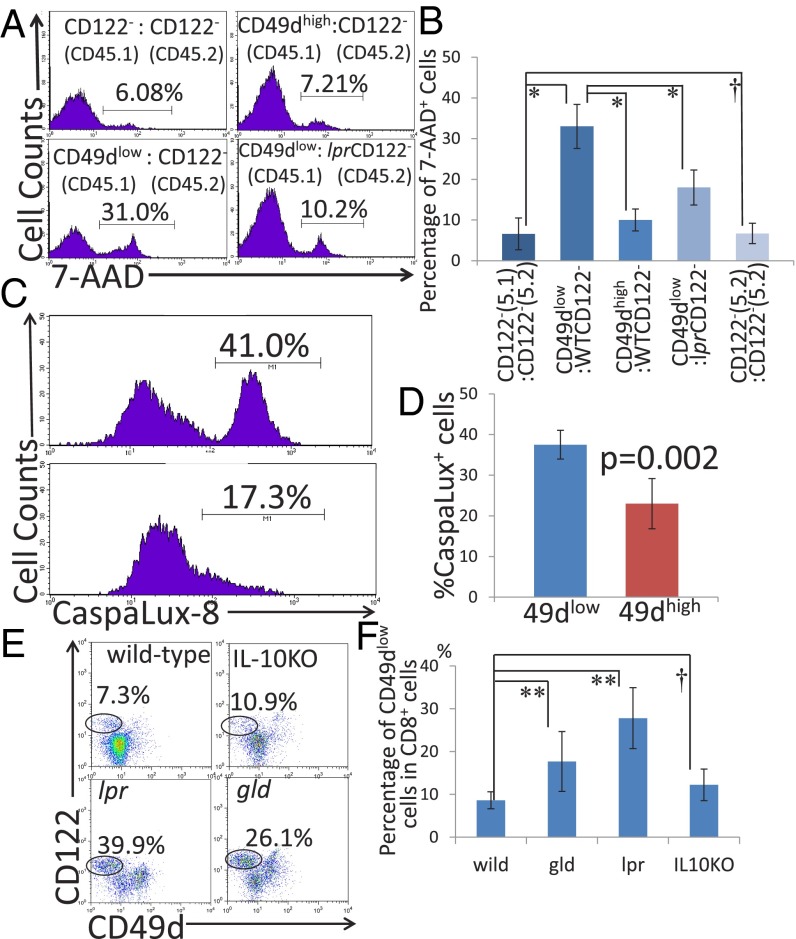
To confirm the cytotoxic mechanisms underlying the regulatory action of CD8+ Tregs, we examined cell death during coculture by staining the cells with annexin V and 7-amino actinomycin D (7-AAD) (Fig. 4 A and B). Although we could not detect annexin V+7-AAD− cells, we observed annexin V+7-AAD+ cells. CD8+CD122− cells cocultured with CD8+ Tregs showed a higher percentage of 7-AAD+ cells than those cocultured with CD49high cells, indicating that CD8+ Tregs induced apoptosis in CD8+CD122− T cells. CD8+CD122− cells taken from lpr mice did not show an increase in 7-AAD–stained cells after coculture with CD8+ Tregs, indicating that Fas-mutant CD8+CD122− cells are resistant to apoptosis induced by CD8+ Tregs (Fig. 4B).
Fig. 4.
(A) Result of 7-AAD analysis after 48 h of coculture. Percentages of 7-AAD–positive cells among total cultured cells are shown in the histogram. (B) Percentages of 7-AAD–positive cells in coculture. *Significantly different (P < 0.01, Student’s t test); †not significantly different (P > 0.05, Student’s t test). (C and D) Analysis of caspase 8 activity in CD122− (CD45.1+) cells using CaspaLux8 after coculture with CD49dlow or CD49dhigh (CD45.1−) cells. (C) Representative results obtained from three independent experiments. (D) Percentages of cells positive for CaspaLux8 in CD122− CD45.1+ cells are shown. The data are averages of three independent experiments. (E) Spleen cells taken from WT, IL-10–deficient, lpr, and gld mice were analyzed by flow cytometry. Representative results obtained from three independent experiments for each mouse strain are shown. (F) Percentages of CD49dlow cells in total CD8+ cells in each group are shown. Statistically significant differences between the WT and each mutant strain were determined. **Significantly different (P < 0.01, Student’s t test); †not significantly different (P > 0.05, Student’s t test).
CD8+CD122+CD49dlow T Cells Induce Apoptosis via Caspase 8 Pathway.
Judging from the experimental results both in vitro and in vivo, it is strongly suspicious that the regulatory mechanism performed by the CD49dlow cells is elimination of cells by using the Fas/FasL system. To prove the involvement of the Fas/FasL system in our T-cell coculture assay, we introduced the assay system of PhiPhiLux and CaspaLux. It is well known that caspases are the key molecule of apoptosis, and caspase 8 is especially directly linked to the Fas-derived signal. When caspase 8 is active, Caspalux-8, a substrate for caspase 8, is digested and becomes a fluorescent substance that can be detected by flow cytometry. As a result, more caspase 8-active cells were observed in the cells cocultured with CD49dlow cells (Fig. 4 C and D), indicating that Fas and its downstream caspase, caspase 8, was activated during the process of apoptosis induction.
CD8+ Tregs Are Increased in Fas/FasL-Mutant Mice but Not in IL-10–Deficient Mice.
We demonstrated that CD8+ Tregs, but not CD49dhigh cells, display regulatory activity that can eliminate cocultured CD8+CD122− cells in vitro and prolong survival of CD8+CD122− T-cell–recipient RAG-deficient mice in vivo. The cause of death in CD8+CD122− T-cell–recipient RAG-deficient mice was not evident but might involve failure of multiple organs, including bone marrow, lung, liver, etc. Additionally, we found that lack of Fas/FasL function affects the regulatory activity of CD8+ Tregs both in vitro and in vivo. Therefore, we expected some changes in CD8+regs of Fas/FasL-mutant mice. The absolute number of CD8+ Tregs and their percentage on total CD8+ cells were significantly increased in lpr and gld mice but not in IL-10–deficient mice, compared with wild-type mice (Fig. 4 E and F). This finding indicates the existence of a negative feedback mechanism that controls the activity of CD8+ Tregs.
CD8+ Tregs Cannot Eliminate or Suppress MHC Class I-Deficient CD8+CD122− Cells.
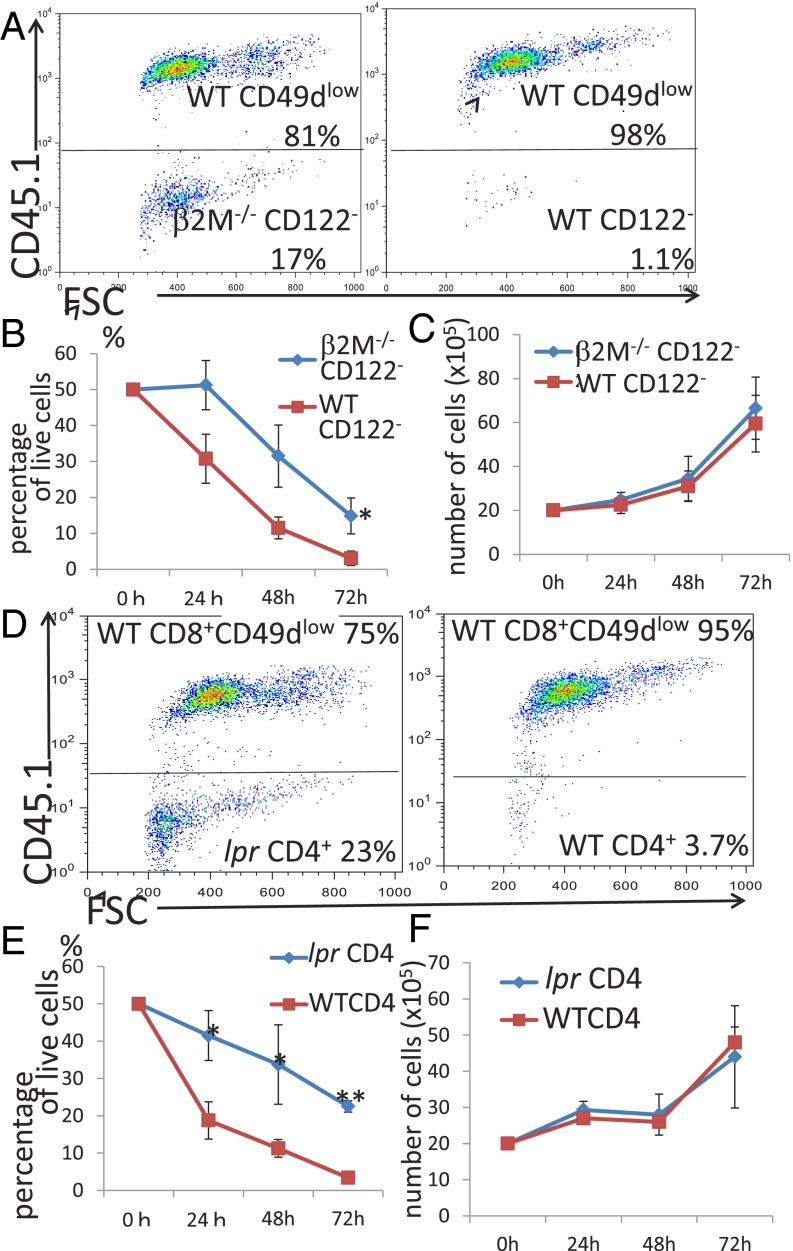
Next, we asked whether recognition of target cells by CD8+ Tregs depends on T-cell receptor (TCR)–MHC class I interaction. To address this question, we used β2-microglobulin–deficient instead of MHC class I-deficient mice; β2-microglobulin–deficient mice have very few CD8+ cells because of a lack of positive selection of CD8+ T cells. This problem can be addressed by generating wild-type mice with bone marrow reconstituted with MHC class I-deficient bone marrow. When CD8+CD122− cells, taken from the bone marrow-reconstituted mice, were cocultured with CD8+ Tregs (Fig. 5 A and B). The rate and extent of reduction were lower than when they were cocultured with wild-type CD8+CD122− cells (Fig. 5 A and B). The proliferation of CD8+CD122− cells from mice in which the bone marrow had been reconstituted with that of β2-microglobulin–deficient mice was not significantly different from that of CD8+CD122− cells taken from wild-type mice (Fig. 5C). This result confirmed that the recovery of the decrease in CD8+CD122− cells from the bone marrow-reconstituted mice, compared with that in cells from wild-type mice, was not due to a difference in the proliferation rate between the CD8+CD122− cells taken from the two different types of mice. Taken together, these results strongly indicate that CD8+ Tregs recognize their target cells by interaction between a MHC class I molecule (plus an antigen) and the TCR.
Fig. 5.
(A and B) Analysis of coculture of CD49dlow cells (CD45.1) with CD8+CD122− cells (CD45.2) derived from mice in which the bone marrow had been replaced with that from β2M (β2-microglobulin)-deficient mice. (A, Left) CD49dlow (CD45.1+) cocultured with CD122−(β2MKO) for 72 h. (A, Right) CD49dlow (CD45.1+) cocultured with CD122− (WT) for 72 h. Representative results after 72 h of coculture obtained from three independent experiments are shown. (B) The percentages of CD8+CD122− cells among total live cells are shown in three independent experiments. *Significantly different (P < 0.05, Student’s t test). (C) Absolute numbers of CD8+CD122− T cells derived from WT mice or from mice (β2MKO) in simple culture. At all time points, no significant differences were found (P > 0.05, Student’s t test). (D and E) Analysis of coculture of CD49dlow T cells (CD45.1+) with CD4+CD25− T cells derived from lpr or WT mice. (D, Left) CD49dlow(CD45.1+) cocultured with CD4+ (lpr) for 72 h. (D, Right) CD49dlow(CD45.1+) cocultured with CD4+ (WT) for 72 h. Representative results after 72 h of coculture obtained from three independent experiments are shown. (E) Percentages of CD4+ cells among total live cells are shown. Data were obtained from three independent experiments. *P < 0.05, Student’s t test; **P < 0.01, Student’s t test. (F) CD4+CD25− T cells derived from WT and lpr mice were harvested, and the absolute numbers of live cells were determined. Average values of three independent experiments are shown. At all time points, the values were not significantly different (P > 0.05, Student’s t test).
CD8+ Tregs Can Eliminate CD4+ Cells in a Fas-Dependent Manner.
One remaining question was whether, besides CD8+ cells, CD8+ Tregs can also suppress and/or eliminate CD4+ cells. CD4+ cells taken from wild-type and lpr mice were cocultured with CD8+ Tregs, and the numbers of surviving cells were determined using flow cytometry. CD8+ Tregs were indeed able to reduce the number of CD4+ cells. This activity was evident for wild-type as well as lpr mouse-derived CD4+ cells; however, the activity of lpr-derived cells was significantly lower than that of wild-type CD4+ cells, indicating that it is Fas-dependent (Fig. 5 D and E). The proliferation rate of CD4+ cells from lpr mice and that of wild-type cells was not significantly different (Fig. 5F), indicating that it is Fas-dependent. The proliferation rates of CD4+ cells from lpr mice and that of wild-type cells were not significantly different (Fig. 5F), indicating that the difference in the reduction speed in coculture was not due to a difference in proliferation rate per se but was due to the elimination of activated CD4+ cells by CD8+ Tregs.
Discussion
The precise cellular events involved in the process of immune regulation related to Fas/FasL systems are largely unknown. Because Fas/FasL-mutated animals are prone to develop autoimmune diseases rather than immunodeficiency and FasL is known to be expressed in CD8+ cytotoxic T cells, it has been predicted that these cells are involved in immune regulation. Additionally, activated CD8+ T cells have been reported to kill clonally established T-cell lines; however, there is no firm evidence that the Fas/FasL system on particular CD8+ T cells in fact regulates the immune response (28). This is the first report describing that CD8+ Treg cells directly control CD8+CD122− T cells both in vivo and in vitro by using the Fas/FasL system.
CD8+CD122+ Tregs were first reported by Rifa’i et al. (22). However, studies of CD8+CD122+ Tregs have been complicated by the concept of memory T cells marked as CD8+CD122+. Some investigators have tried to separate Tregs from memory T cells by staining the CD8+CD122+ cells with a third marker, such as PD-1 (29), whereas such trials have not succeeded well in persuading other investigators. However, our experimental results obtained in the former studies (20–30) and the present studies have definitely shown the regulatory activity in this T-cell subset. Our current interpretation is that the regulatory and the memory functions are compatible. These cells with regulatory activity may belong to CD8+ memory T cells, possibly recognize self-antigens by their TCR, and induce apoptosis of Fas-expressing activated T cells. Based on our new understanding, it should be possible that cells with memory phenotype (CD8+CD122+) possess the regulatory activity at the same time.
Rifa’i et al. and Endharti et al. (31, 32) reported the regulatory activity of CD8+CD122+ T cells in both in vivo and in vitro experiments. We first examined which subset of the CD8+CD122+ cells possessed regulatory activity. Okuno et al. (20) found that CD8+CD122+ cells in murine peripheral immune organs can be divided into at least two populations according to their expression level of CD49d. The current study indicated that the final suppression mechanism used by the CD8+CD122+ Tregs appears to be killing of the activated T cells, which depends on the Fas/FasL pathway, because Fas-deficient T cells derived from lpr/lpr mice were resistant to killing (Fig. 2 A and B) and FasL-deficient Tregs derived from gld/gld mice could not perform this regulatory activity (Fig. 3 C and D).
The molecular mechanism underlying immune suppression by CD8+ Tregs and the chemical pathway involved in the regulatory effect of these cells are still largely unknown. We previously reported that CD8+CD122+ Tregs produce IL-10 and that the cytokine could be the effector molecule of these CD8+ Tregs. Although both the mechanism of recognition of target cells and the effector molecule of regulation by CD8+ Tregs remain to be elucidated, this study yielded some important clues: Activation of Fas via interaction with FasL is largely associated with the killing of T cells that are no longer needed and rather harmful for survival. Another important molecular interaction is that of MHC class I and TCR. β2-microglobulin–deficient cells are neither regulated in the previous study (33) nor in the present study, suggesting the importance of specific recognition between “the regulatory cells” and “the regulated cells.” Another important molecule involved in the action of CD8+ Tregs might be Qa-1, a nonclassical MHC class I molecule (MHC-Ib), as H. Cantor and his colleague found and reported a CD8+ T-cell subset that showed a regulatory activity under a Qa-1–restricted manner (30). In their recent publications, however, the Qa-1–restricted CD8+ Tregs they proposed were apparently shown to be CD122+CD44+ (34, 35). The Qa-1–restricted CD8+ Treg is overlapped with or involved in the CD8+CD122+ Treg reported by H. Suzuki and his colleagues (22). Two CD8+ Tregs that were formerly thought to be distinct cell populations turned out to be the same one, further confirming the existence of CD8+ regulatory T cells, regardless of whether they are the naturally arising type or the induced type. Although the relation between CD4+ Tregs and CD8+ Tregs has not been clarified in detail, both Tregs are indispensable to maintain T-cell homeostasis, and their cooperative work has been suggested in the prevention and cure of an autoimmune disease (31).
This study forms a basis for continued research of immune suppression by CD8+CD122+ Tregs. Elucidation of the effector mechanisms of CD8+ and CD4+ Tregs may pave the way to improve immune regulation by Tregs in clinical practice.
Materials and Methods
Mice.
Animal care was performed according to the guidelines of Nagoya University (Nagoya, Japan). Experimental protocols were approved by the Ethics Committee of the Nagoya University Graduate School of Medicine (Nos. 27015, 27017, and 27365).
Adoptive T-Cell Transfer to RAG-2−/− Mice.
Sorted CD8+CD122− T cells (4 × 105 per recipient mouse) simply or mixed with CD8+CD122+ T cells (49dlow or CD49high) (1 × 105 per recipient mouse) were i.v. transferred into 8–10-wk-old RAG-2–deficient mice that had received a sublethal dose of irradiation (5.5 Gym). The survival of the T-cell–recipient mice was followed up after adoptive T-cell transfer, and the results were analyzed using Kaplan–Meier survival curves.
Activation of CD8+ T Cells.
CD8+ T cells in any desired population were collected from murine spleen and lymph node cells by using a FACS SORP Aria II cell sorter (BD Biosciences) and cultured in an anti-mCD3 antibody-coated 96-well plate for 24 h (1.0 × 105 cells per well).
T-Cell Culture.
Activated T cells as described above were either cocultured or cultured alone in RPMI-1640 media containing 10% (vol/vol) FBS and 20 ng/mL of interleukin-2 (PeproTech Inc.) in a round-bottom 96-well plate for 72 h (0.5 × 105 cells per well).
For other information, see SI Materials and Methods.
SI Materials and Methods
Mice.
Six- to 8-wk-old C57BL/6 mice and gld and lpr mice of a C57BL/6 genetic background were purchased from SLC Japan. Breeding pairs of the C57BL/6CD45.1/CD45.1 congenic strain and RAG-2–deficient mice on a C57BL/6 genetic background (B6.129S6-Rag2tm1FwaN12) were purchased from Taconic. β2-microglobulin–deficient mice and IL-10–deficient mice on a C57BL/6 genetic background (B6.129P2-Il10tm1Cgn/J) were originally obtained from the Jackson Laboratory. All mice were maintained in our animal facility.
Antibodies.
FITC (fluorescein isothiocyanate)-conjugated anti-mouse CD122, PE (R-phycoerythrin)- or APC (allophycocyanin)-conjugated anti-mouse CD49d, PE-conjugated anti-mouse FasL, APC-conjugated anti-mouse CD62L, PE/Cy7-conjugated anti-mouse CD8a, and FITC- or APC-conjugated anti-mouse CD45.1 antibodies were purchased from BioLegend.
Cytotoxicity Assay.
We used APC-conjugated anti-mouse CD45.1 antibody (BioLegend) and annexin V–FITC (BioLegend) with 7-AAD (eBioscience).
Activation of CD4+ Naïve T Cells.
CD4+ T cells in any desired population were collected from murine spleen and lymph node cells by using an FACS Aria II cell sorter (BD Biosciences) and cultured and activated for 24 h in a 96-well plate (1.0 × 105 cells per well) using Dynabeads mouse T activator CD3/CD28 for activation of mouse T cells (In Vitrogen).
Real-Time PCR Primers.
The following real-time PCR primers were used: CD163L1 A5, 5′-gtctggaaactgtcggagat-3′; CD163L1 A3, 5′-agtcttgtgacccctaaggt-3′; FasL A5, 5′-cgtgagttcaccaaccaaagc-3′; FasL A3, 5′-cccagtttcgttgatcacaag-3′; β-actin A5, 5′-agtgtgacgttgacatccgt-3′; and β-actin A3, 5′-gcagctcagtaacagtccgc-3′.
PhiPhiLux and CaspaLax.
PhiPhiLux-G2D2 kit and CaspaLux8-L1D2 kit systems that can detect the activity of caspase 3 and cspase 8, respectively, were purchased from OncoImmunin, Inc.
SI Results
CD8+CD122+CD49dlow Cells Taken from IL-10–Deficient Mice Have a Normal Capacity to Suppress or Eliminate Other Conventional CD8+CD122− T Cells.
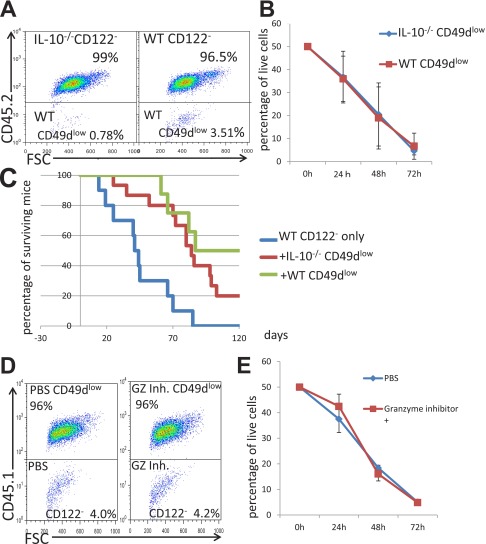
Next, we wanted to clarify whether the cytotoxic mechanism of the CD8+CD122+CD49dlow cells is related to IL-10. Because IL-10 has been shown to be important for the regulatory activity of CD8+CD122+ Tregs (32), we expected that CD8+CD122+CD49dlow cells taken from IL-10–deficient mice would have a reduced ability to regulate other CD8+ T cells. However, we found that CD8+CD122+CD49dlow cells from IL-10–deficient mice had a normal ability to reduce the number of cocultured wild-type CD8+CD122− cells (Fig. S2 A and B). Furthermore, CD8+CD122+CD49dlow cells from IL-10–deficient mice showed apparent activity in supporting the survival of RAG-2–deficient mice when they were transferred (R-phycoerythrin) into mice together with CD8+CD122− cells (Fig. S2C).
Fig. S2.
(A) After 24 h of culture with the stimulation by anti-CD3 Ab and 72 h of coculture of WT CD8+CD122− T cells (CD45.1) and CD8+CD122+CD49dlow T cells (CD45.2), derived from IL-10–deficient mice (Left) or wild-type mice (Right), the cells were stained and analyzed. Representative results obtained after 72 h of coculture are shown. Percentages of cells in the upper or lower rectangle among total remaining live cells are shown. (B) CD8+CD122− T cells (CD45.1) and CD8+CD122+CD49dlow T cells (CD45.2) from WT mice and IL-10–deficient mice were cocultured and analyzed. The percentages of CD8+CD122− cells among total live cells are shown as the averages and SDs obtained from three independent experiments. At all time points, the values were not significantly different (P > 0.05, Student’s t test) between data obtained from IL-10–deficient mice-derived cells and those obtained from WT mice-derived cells. (C) CD8+CD122− T cells (4 × 105 per recipient mouse) obtained from wild-type mice were mixed with CD8+CD122+CD49dlow T cells (1 × 105 per recipient mouse) taken from WT mice and IL-10–deficient mice and were then i.v. transferred into RAG-2–deficient mice. The survival of the T-cell–recipient mice was followed up to 140 d after adoptive T-cell transfer, and the results are presented as Kaplan–Meier curves. Addition of IL-10−/−–derived CD8+CD122+CD49dlow T cells to CD122− cells (red line) led to significantly higher survival of mice compared with transfer of CD122− cells alone (blue line; log-rank test, P = 0.0166; Wilcoxon test, P = 0.0287), but survival of these mice was not significantly different from that obtained by addition of WT CD8+CD122+CD49dlow cells (green line; log-rank test, P = 0.4891; Wilcoxon test, P = 0.3927). (D and E) Analysis of granzyme B activity in coculture using granzyme B Inhibitor I (Calbiochem, Merck Millipore). Representative results of simultaneous experiments with (Right) and without granzyme inhibitor (Left; control) are shown (D). Red line, granzyme inhibitor (+); blue line, granzyme inhibitor (–). At all time points examined, the values in the culture containing the granzyme inhibitor did not differ significantly from that without the granzyme inhibitor at the same time point (P > 0.05, Student’s t test) (E). The data are averages of three independent experiments.
CD8+CD122+CD49dlow T Cells Induce Apoptosis via a Granzyme-Independent Pathway.
Our findings indicated the involvement of the Fas/FasL system in the regulatory mechanism of CD8+CD122+CD49dlow Tregs. Another well-known mechanism of cytotoxicity of CD8+ T cells is perforin/granzyme-induced apoptosis. To test whether this mechanism is involved in the cytotoxic activity of CD8+CD122+CD49dlow Tregs, we added a granzyme inhibitor to the culture medium during coculture of CD8+CD122+CD49dlow Tregs and their target CD8+CD122− cells. The granzyme inhibitor did not affect the elimination of target cells by Tregs, suggesting that the mechanism of regulation by CD8+CD122+CD49dlow Tregs is independent of the perforin/granzyme system (Fig. S2 D and E).
CD163L1, Granzyme B, and FasL Show Characteristic Expression Profiles in Subpopulations of CD8+ Cells.
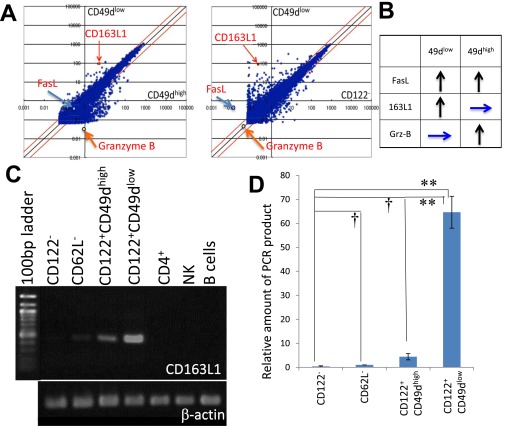
CD49d clearly separated CD8+CD122+ cells into two subpopulations and as such is a good candidate second marker for CD8+ Tregs (20). To identify a better marker or a “master gene” for CD8+ Tregs, we performed microarray analysis. By comparing the expression profile of CD8+CD122+CD49dlow cells with that of CD8+CD122+CD49high cells and that of CD8+CD122− cells, we identified three different expression patterns with higher expression in at least one subpopulation of CD8+CD122+ cells than in CD8+CD122− cells: (i) genes with elevated expression in both CD49dlow and CD49dhigh, (ii) genes with elevated expression in CD49dlow but not in CD49dhigh, and (iii) genes with elevated expression in CD49dhigh but not in CD49dlow cells. We selected one gene from each group [(i) FasL, (ii) CD163L1, (iii) granzyme B], indicated in the scatter plot (Fig. S3A) and interpretation of the results (Fig. S3B). The expression pattern of the unique gene CD163L1 that was highly expressed in CD8+CD122+CD49dlow cells (36), weakly expressed in CD8+CD122+CD49dhigh cells, and not expressed in other cells including CD4+ cells (Fig. S3C) was confirmed by real-time PCR results (Fig. S3D).
Fig. S3.
Search for genes specifically expressed in a subpopulation of CD8+ T cells. (A) Scatter plots comparing gene expression in CD8+CD122+CD49dlow cells (vertical axis) versus CD8+CD122+CD49high cells (horizontal axis in Left panel) and in CD8+CD122− cells (horizontal axis in Right panel). Arrows indicate the positions of CD163L1, Fas, and granzyme B. (B) Relative expression intensity of FasL, CD163L1, and granzyme B in CD8+CD122+CD49dhigh and CD8+CD122+CD49dlow cells compared with that in CD8+CD122− cells is summarized. Upward arrows illustrate higher expression in the indicated cells than in CD122− cells, and horizontal arrows illustrate no difference in expression between the indicated cells and CD122− cells. (C) RT-PCR analysis of CD163L1 in various lymphocytes and CD8+ T-cell subsets. cDNA was prepared by reverse transcription of RNA extracted from the indicated T-cell subsets and PCR-amplified with CD163L1-specific primers. The PCR products were electrophoresed in agarose gels and stained with ethidium bromide. PCR products were normalized by the intensities of β-actin. (D) Quantification of CD163L1 expression in various CD8+ T-cell subsets by real-time PCR analysis. RNA extracted from the indicated T-cell subsets was used to quantify the mRNA levels with SYBR Premix Ex Taq II (TAKARA). The amount of PCR products is shown on a relative scale setting, with that of CD62L− set as 1.00. Differences in expression between CD122− and other cells (CD62L−, CD49dhigh, and CD49dlow) were statistically analyzed. †Not significantly different. **Significantly different (P < 0.01, Student's t test).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525098113/-/DCSupplemental.
References
- 1.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakaki R, Yamada A, Kudo Y, Hayashi Y, Ishimaru N. Mechanism of activation-induced cell death of T cells and regulation of FasL expression. Crit Rev Immunol. 2014;34(4):301–314. doi: 10.1615/critrevimmunol.2014009988. [DOI] [PubMed] [Google Scholar]
- 3.Suda T, Nagata S. Why do defects in the Fas-Fas ligand system cause autoimmunity? J Allergy Clin Immunol. 1997;100(6 Pt 2):S97–S101. doi: 10.1016/s0091-6749(97)70013-7. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S, Suda T. Fas and Fas ligand: Lpr and gld mutations. Immunol Today. 1995;16(1):39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 5.Kreuwel HT, et al. Comparing the relative role of perforin/granzyme versus Fas/Fas ligand cytotoxic pathways in CD8+ T cell-mediated insulin-dependent diabetes mellitus. J Immunol. 1999;163(8):4335–4341. [PubMed] [Google Scholar]
- 6.Aung S, Graham BS. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity in vivo. J Immunol. 2000;164(7):3487–3493. doi: 10.4049/jimmunol.164.7.3487. [DOI] [PubMed] [Google Scholar]
- 7.Salmaso C, et al. Regulation of apoptosis in endocrine autoimmunity: Insights from Hashimoto’s thyroiditis and Graves’ disease. Ann N Y Acad Sci. 2002;966:496–501. doi: 10.1111/j.1749-6632.2002.tb04253.x. [DOI] [PubMed] [Google Scholar]
- 8.Beeston T, Smith TR, Maricic I, Tang X, Kumar V. Involvement of IFN-γ and perforin, but not Fas/FasL interactions in regulatory T cell-mediated suppression of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;229(1-2):91–97. doi: 10.1016/j.jneuroim.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pozzesi N, et al. Role of caspase-8 in thymus function. Cell Death Differ. 2014;21(2):226–233. doi: 10.1038/cdd.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EW, Seo J, Jeong M, Lee S, Song J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012;45(9):496–508. doi: 10.5483/bmbrep.2012.45.9.186. [DOI] [PubMed] [Google Scholar]
- 11.Venet F, et al. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177(9):6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 12.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009;182(3):1469–1480. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Regulatory T cells: History and perspective. Methods Mol Biol. 2011;707:3–17. doi: 10.1007/978-1-61737-979-6_1. [DOI] [PubMed] [Google Scholar]
- 14.Miyara M, Sakaguchi S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol Med. 2007;13(3):108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Wing K, Miyara M. Regulatory T cells—A brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 17.Krzych U, Nanda N, Sercarz E. Specificity and interactions of CD8+ T suppressor cells. Res Immunol. 1989;140(3):302–307, discussion 339–345. doi: 10.1016/0923-2494(89)90067-9. [DOI] [PubMed] [Google Scholar]
- 18.Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: Are they players in autoimmunity? Autoimmun Rev. 2002;1(5):279–283. doi: 10.1016/s1568-9972(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 19.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29(7):337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Okuno Y, et al. CD8+ CD122+ regulatory T cells contain clonally expanded cells with identical CDR3 sequences of the T-cell receptor β-chain. Immunology. 2013;139(3):309–317. doi: 10.1111/imm.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268(5216):1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 22.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200(9):1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YH, et al. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180(2):825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z, et al. Importance of CD80/CD86-CD28 interactions in the recognition of target cells by CD8+CD122+ regulatory T cells. Immunology. 2008;124(1):121–128. doi: 10.1111/j.1365-2567.2007.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Z, et al. Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ Treg. Eur J Immunol. 2009;39(8):2106–2119. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- 26.Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J Exp Med. 2006;203(7):1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Zhou YW, Kato M, Mak TW, Nakashima I. Normal regulatory α/β T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor β in vivo. J Exp Med. 1999;190(11):1561–1572. doi: 10.1084/jem.190.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, et al. CD11c(high)CD8+ regulatory T cell feedback inhibits CD4 T cell immune response via Fas ligand-Fas pathway. J Immunol. 2013;190(12):6145–6154. doi: 10.4049/jimmunol.1300060. [DOI] [PubMed] [Google Scholar]
- 29.Dai H, et al. Cutting edge: Programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185(2):803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 30.Hu D, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5(5):516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 31.Endharti AT, et al. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol. 2011;186(1):41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- 32.Endharti AT, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-γ production and proliferation of CD8+ T cells. J Immunol. 2005;175(11):7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 33.Rifa’i M, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20(7):937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, et al. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA. 2011;108(5):2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350(6258):334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult gammadelta T cells. J Immunol. 2008;181(3):1710–1716. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]