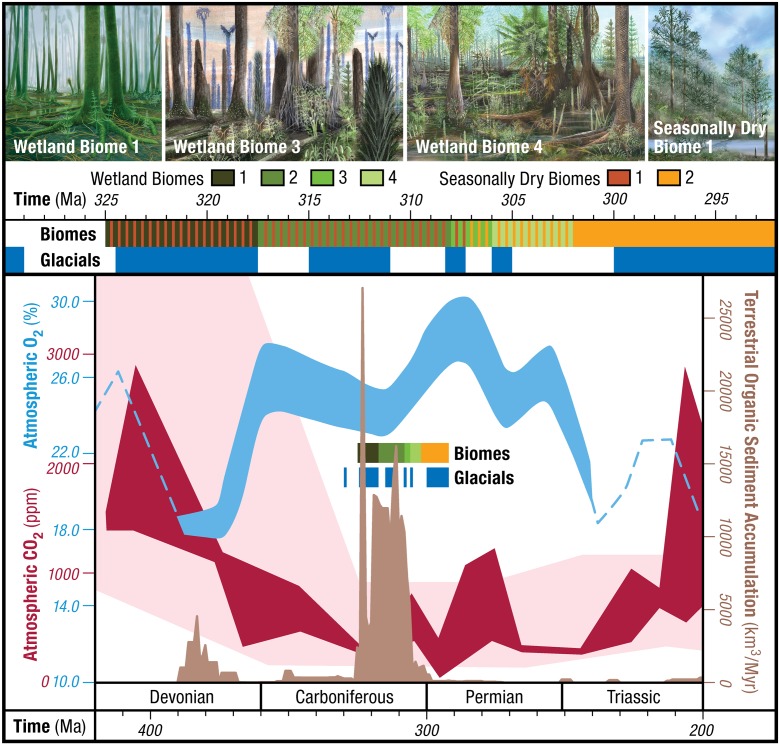
The highest rates of global organic carbon burial (up to 6.5 × 1018 mol/Myr) over the past half billion years occurred during the Carboniferous–Permian (330–260 Myr), in large part because of the accumulation and burial of peat in broad tropical lowland basins (1). Atypical rates of organic carbon sequestration led to low atmospheric pCO2 and anomalously high pO2 (Fig. 1), which in turn triggered the longest-lived and perhaps most severe icehouse of the Phanerozoic (1, 2) and, possibly, unusual physiological innovations, such as insect flight and gigantism (3). This unique atmospheric composition arose with the radiation of the Earth’s most expansive wetland tropical forests (4), introducing into the terrestrial realm a vast supply of biodegradably resistant organic matter. A hypothesized temporal gap of ∼120 Myr between the emergence of lignin biosynthesis by plants and the evolution of lignin-degrading fungi has been argued to have created a window of opportunity for substantially increased organic carbon sequestration in the Carboniferous (4–6). This long-standing evolutionary lag paradigm becomes even more compelling with a recent fungal genome study suggesting that Agaricomycetes fungi, with the enzymatic capacity to efficiently degrade lignin, did not evolve until the early Permian (7). In PNAS, Nelsen et al. (8) invoke multiple lines of evidence to refute this paradigm, documenting: (i) the low lignin content of some of the most important Carboniferous peat-forming plants, (ii) the lack of correspondence between peak coal accumulation rates in North America and anticipated periods of abundant lignin production in late Paleozoic tropical forests, (iii) that lignin resistance to decay was not limited to Agaricomycetes fungi, and (iv) phylogenomic data that close the hypothesized evolutionary gap.
Fig. 1.
Late Paleozoic atmospheric pCO2 and pO2 (17–18), North American terrestrial organic sediment accumulation (8), and temporal distribution of tropical biomes (see text for details and references). Upper section is a detailed inset of the biome-glaciation history shown in the Lower section. Glaciation history from ref. 2. WB 1 owned, commissioned by, and courtesy of the City and County of Denver, K. Johnson, and J. Vriesen. WB 3 and 4, and Seasonally Dry Biome 1 courtesy of Mary Parrish (National Museum of Natural History, Smithsonian Institution).
There is a long-invoked but incorrect (9) perception that lycopsid-dominated forests were the main source vegetation of Carboniferous coals because of the abundance and resistance to decomposition of lycopsid periderm (bark) (e.g., refs. 1, 4, and 5). Thus, lycopsid dominance supposedly was essential for formation of the economically important Carboniferous coals. In what initially appears to support this perception, Nelsen et al. (8) use spatiotemporal trends in Phanerozoic organic-rich terrestrial sediments to demonstrate peak North American coal production during intervals when lycopsids dominated Euramerican tropical forests. Most dominant Carboniferous wetland plants, however, produced little wood, and the arborescent lycopsids were no exception (10). The towering lycopsid stature was made possible by unlignified periderm rather than lignin-rich wood. Therefore, they contributed less lignin to peat accumulations than woody plants, which were episodically abundant, although rarely dominant, in Pennsylvanian–early Permian tropical lowland basins (9, 11). Nelsen et al.’s (8) scientific twist to the hypothesized lycopsid–lignin–coal linkage—that is, documenting coincidence in peak coal production and lycopsid dominance, when lignin would have contributed minimally to peat accumulation—weakens the argument that the emergence of lignin synthesis was one criteria for intensified terrestrial organic carbon sequestration in the Carboniferous.
Large-scale peat accumulation further requires suppression of organic matter decay, of which woody tissue, in the absence of efficient lignin-degrading fungi, has been considered the source (5). Nelsen et al. (8) challenge this view by documenting fungal and bacterial degradation of Paleozoic lignified tissues and, conversely, evidence for abundant unlignified organic matter in many peats. Nelson et al.’s study, building on decades of paleobotanical research, thus unequivocally demonstrates that pre-Permian lignin resistance to decay, and thus preferential biochemical composition of vegetation, was not the key to high rates of peat accumulation 325–300 Myr ago. Intriguingly, lignin’s legacy in Carboniferous peat accumulation may be moot: Nelsen et al. (8) point out that the evolution of Agaricomycetes fungi, based on reassessment of phylogenomic evidence (7), is permissible as far back as the Devonian (420–359 Myr), thus potentially closing the evolutionary lignin–fungal gap. If correct, then a closer temporal association between the onset of lignin biosynthesis and the evolution of lignin-degrading fungi suggests rapid evolutionary innovation in response to a novel resource. Nelsen et al.’s collective findings (8) further resolve a mass balance conundrum regarding the Carboniferous–Permian atmosphere. In a world with hypothesized abundant lignin, the absence of fungal-mediated degradation, in concert with widespread peat accumulation and burial, would have depleted atmospheric CO2 within a fraction of the proposed ∼120 Myr evolutionary gap. Large-magnitude CO2 degassing from volcanism, for which there is no clear evidence, would be needed to maintain the requisite long-term balance in total CO2 inputs and outputs to and from the atmosphere (1).
If abundant lignin production wasn’t the primary control on peat and coal accumulation in the Carboniferous, then what was? Nelsen et al. (8) invoke the coupled influence of climate and tectonics on peat accumulation, noting that major floral transitions do not coincide with changes in coal accumulation rates (figure 1 in ref. 8). Euramerican tropical forests underwent repeated ecosystem restructuring following their mid-Carboniferous expansion that involved quantitative changes in species composition and vegetational architecture. A closer look at the tropical forest dynamics reveals repeated shifts between wetland and interspersed seasonally dry biomes (Fig. 1) that occurred in-step with climate change over a hierarchy of time-scales (105 to 107 yr) (4, 11–13). Several of the floral transitions are accompanied by variations in the regional extent, thickness, and quality of coals, linked to climate through the obligate water-logged environment of peat accumulation (13). Within the age uncertainty, transitions between biomes appear to coincide with turnovers between glacial and interglacial conditions (Fig. 1). A shift from lycopsid dominance [wetland biome (WB) 1] to woody cordaitalean–lycopsid forests (WB 2) overlaps with initiation of the main phase of Pennsylvanian glaciation, with further quantitative changes in abundance and new floral dominants at the onset (∼311 Myr) of long-term waning of Gondwanan ice sheets and increased seasonality (2).
The contribution of Nelsen et al., published in PNAS, extends beyond shattering the lignin–fungal evolution linkage by introducing potentially complex interactions between late Paleozoic tropical vegetation and the environment.
Two subsequent biome turnovers, involving the loss of cordaitaleans (WB 2–3) (14) and many lycopsid taxa (WB 3–4) (9), appear to be contemporaneous with the return of short-lived glaciations (Fig. 1). Ensuing warmings saw the rise of water-stress–tolerant tree ferns (WB 3) and the stepped emergence of marattialean tree ferns as swamp-community dominants (WB 4). A permanent shift to seasonally dry woodland flora [cordaitaleans and conifers; dryland biome (DB) 2] occurred across the Carboniferous–Permian boundary (299 Myr) coincident with widespread aridification (12). Thick, regionally extensive coals formed under humid climates, whereas shifts to water-stress–tolerant vegetation coincide with thinner, less-extensive coals of decreased quality (13).
Superimposed eccentricity scale (105 yr), glacial–interglacial fluctuations (Fig. 1) drove shifts in the tropical lowlands between glacial floras characteristic of swamp habitats and interglacial floras of seasonally dry habitats rich with woody cordaitaleans (DB 1) and conifers (DB 2) (11). Peat accumulation on the 105-yr-scale was also episodic, restricted to late glacial everwet periods (11). Perhaps the most compelling evidence for a dominant climate influence on peat accumulation is the North China peats that accumulated through the Permian. These wetlands, inhabited by lycopsids, tree ferns, and cordaitaleans (15), prevailed despite widespread tropical aridity and the early Permian evolution of lignin-degrading fungi. Late Paleozoic climate models provide unique insight into a “climate window of opportunity” by documenting that this isolated area of eastern equatorial Pangaea is the sole tropical region that remains everwet under inferred rising CO2 of early Permian terminal deglaciation (16). The contribution of Nelsen et al. (8), published in PNAS, extends beyond shattering the lignin–fungal evolution linkage by introducing potentially complex interactions between late Paleozoic tropical vegetation and the environment. Their findings provide the stimulus for further investigation of the role of vegetation–climate–CO2 feedbacks in carbon sequestration, the evolution of atmospheric composition, and climate dynamics during the Earth’s penultimate icehouse.
Footnotes
The author declares no conflict of interest.
See companion article on page 2442.
References
- 1.Berner RA. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature. 2003;426(6964):323–326. doi: 10.1038/nature02131. [DOI] [PubMed] [Google Scholar]
- 2.Montañez IP, Poulsen CJ. The Late Paleozoic Ice Age: An evolving paradigm. Annu Rev Earth Planet Sci. 2013;41:629–656. [Google Scholar]
- 3.Dudley R. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol. 1998;201(Pt 8):1043–1050. doi: 10.1242/jeb.201.8.1043. [DOI] [PubMed] [Google Scholar]
- 4.Cleal CJ, Thomas BA. Palaeozoic tropical rainforests and their effect on global climates: Is the past the key to the present? Geobio. 2005;3:13–31. [Google Scholar]
- 5.Robinson JM. Lignin, land plants, and fungi: Biological evolution affecting Phanerozoic oxygen balance. Geology. 1990;18(7):607–610. [Google Scholar]
- 6.Raven JA, Andrews M. Evolution of tree nutrition. Tree Physiol. 2010;30(9):1050–1071. doi: 10.1093/treephys/tpq056. [DOI] [PubMed] [Google Scholar]
- 7.Floudas D, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336(6089):1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 8.Nelsen MP, DiMichele WA, Peters SE, Boyce CK. Delayed fungal evolution did not cause the Paleozoic peak in coal production. Proc Natl Acad Sci USA. 2016;113:2442–2447. doi: 10.1073/pnas.1517943113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips TL, Peppers RA, DiMichele WA. Stratigraphic and interregional changes in Pennsylvanian coal-swamp vegetation: Environmental inferences. Int J Coal Geol. 1985;5(1-2):43–109. [Google Scholar]
- 10.Boyce CK, Abrecht M, Zhou D, Gilbert PUPA. X-ray photoelectron emission spectromicroscopic analysis of arborescent lycopsid cell wall composition and Carboniferous coal ball preservation. Int J Coal Geol. 2010;83(2-3):146–153. [Google Scholar]
- 11.DiMichele WA. Wetland-dryland vegetational dynamics in the Pennsylvanian ice age tropics. Int J Plant Sci. 2014;175(2):123–164. [Google Scholar]
- 12.Tabor NJ, DiMichele WA, Montañez IP, Chaney DS. Late Paleozoic continental warming of a cold tropical basin and floristic change in western Pangea. Int J Coal Geol. 2013;119:177–186. [Google Scholar]
- 13.Cecil CB, DiMichele WA, Elrick SD. Middle and Late Pennsylvanian cyclothems, American Midcontinent: Ice-age environmental changes and terrestrial biotic dynamics. C R Geosci. 2014;346:159–168. [Google Scholar]
- 14.Pfefferkorn HW, Thomson MC. Changes in the dominance patterns in Upper Carboniferous plant-fossil assemblages. Geology. 1982;10:641–644. [Google Scholar]
- 15.Wang J, Pfefferkorn HW, Zhang Y, Feng Z. Permian vegetational Pompeii from Inner Mongolia and its implications for landscape paleoecology and paleobiogeography of Cathaysia. Proc Natl Acad Sci USA. 2012;109(13):4927–4932. doi: 10.1073/pnas.1115076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poulsen CJ, Pollard D, Montañez IP, Rowley D. Late Paleozoic tropical climate response to Gondwanan deglaciation. Geology. 2007;35(9):771–774. [Google Scholar]
- 17.Royer DL. 2014. Atmospheric CO2 and O2 during the Phanerozoic: Tools, patterns, and impacts. Treatise on Geochemistry, Vol. 6, 2nd Ed, eds Holland H, Turekian K (Elsevier, Amsterdam, The Netherlands) pp 251–267.
- 18.Glasspool IJ, Scott AC, Waltham D, Pronina N, Shao L. The impact of fire on the Late Paleozoic Earth system. Front Plant Sci. 2015;6:756. doi: 10.3389/fpls.2015.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]