Significance
Bordetella bronchiseptica isolates from diverse hosts, including humans, display potent cytotoxicity against a broad range of mammalian cells, which is dependent on type III secretion system (T3SS) effector BteA. In contrast, neither laboratory nor clinical isolates of Bordetella pertussis have been observed to display T3SS-dependent cytotoxicity, despite the fact that T3SS genes are present, intact, and nearly identical to their B. bronchiseptica counterparts. We have characterized a regulatory node, involving a T3SS-exported anti-σ factor, BtrA, that controls virulence gene expression in Bordetella species. Of particular relevance to human disease, deletion of btrA in B. pertussis derepresses T3SS gene expression and confers readily detectable BteA-dependent cytotoxicity. These observations warrant a reassessment of type III secretion in the pathogenesis and prevention of pertussis.
Keywords: virulence gene regulation, ECF sigma factor, T3SS, Bordetella, host adaptation
Abstract
The BvgAS phosphorelay regulates ∼10% of the annotated genomes of Bordetella pertussis and Bordetella bronchiseptica and controls their infectious cycles. The hierarchical organization of the regulatory network allows the integration of contextual signals to control all or specific subsets of BvgAS-regulated genes. Here, we characterize a regulatory node involving a type III secretion system (T3SS)-exported protein, BtrA, and demonstrate its role in determining fundamental differences in T3SS phenotypes among Bordetella species. We show that BtrA binds and antagonizes BtrS, a BvgAS-regulated extracytoplasmic function (ECF) sigma factor, to couple the secretory activity of the T3SS apparatus to gene expression. In B. bronchiseptica, a remarkable spectrum of expression states can be resolved by manipulating btrA, encompassing over 80 BtrA-activated loci that include genes encoding toxins, adhesins, and other cell surface proteins, and over 200 BtrA-repressed genes that encode T3SS apparatus components, secretion substrates, the BteA effector, and numerous additional factors. In B. pertussis, BtrA retains activity as a BtrS antagonist and exerts tight negative control over T3SS genes. Most importantly, deletion of btrA in B. pertussis revealed T3SS-mediated, BteA-dependent cytotoxicity, which had previously eluded detection. This effect was observed in laboratory strains and in clinical isolates from a recent California pertussis epidemic. We propose that the BtrA-BtrS regulatory node determines subspecies-specific differences in T3SS expression among Bordetella species and that B. pertussis is capable of expressing a full range of T3SS-dependent phenotypes in the presence of appropriate contextual cues.
The evolution of the human respiratory pathogen Bordetella pertussis from its broad host range Bordetella bronchiseptica-like ancestor was accompanied by genome reduction, a proliferation of pseudogenes, and host restriction (1–3). This transition also correlates with a systematic pattern of transcriptional diversity in the BvgAS virulence regulon that we hypothesized was due to functional alterations in regulatory factors that control clusters of loci in a hierarchical network (4). In B. bronchiseptica and B. pertussis, the transmembrane BvgS histidine kinase and BvgA response regulator coordinate expression of nearly all known colonization and virulence genes, an array of transcriptional regulators, and numerous genes of unknown function (3–6). BvgAS establishes a spectrum of phenotypic phases in response to environmental cues, functioning as a rheostat that controls the infectious cycle (7, 8). The Bvg+ phase is characterized by high level BvgAS activity, expression of virulence factors [including pertussis toxin (9), adenylate cyclase toxin (10, 11), filamentous hemagglutinin (12), fimbriae (13), the Bordetella secretion complex (Bsc) type III secretion system (T3SSBsc) (14–16)], and the ability to survive and replicate on respiratory epithelia (17). In the Bvg phase, the BvgAS phosphorelay is quiescent, Bvg+ phase genes are silent, and “virulence-repressed genes” are maximally transcribed (4, 17, 18). These genes include flagella and motility loci in B. bronchiseptica and genes with unknown function in B. pertussis. The roles of BvgAS-regulated genes that are differentially expressed between the Bvg+ and Bvg− extremes of the regulatory spectrum are largely unresolved (7, 19).
In vitro, T3SSBsc is a particularly conspicuous virulence determinant owing to its ability to induce rapid cytotoxicity in a diverse array of eukaryotic cells, ranging from mammalian to yeast (15, 20). In vivo studies with B. bronchiseptica show that T3SSBsc plays an immunomodulatory role that facilitates persistence in the lower respiratory tract (21, 22). Remarkably, only a single effector, BteA, has been definitively identified as a translocated substrate (15, 16). In B. bronchiseptica, BteA is necessary and sufficient for cytotoxicity in vitro, and mutations in bteA recapitulate phenotypes associated with deleting the T3SS ATPase gene, bscN (14–16).
As a result of their close evolutionary relationship, comparative studies exploiting the experimental tractability of B. bronchiseptica are relevant for understanding B. pertussis (3, 4, 7, 16, 23–25). B. bronchiseptica isolates from diverse hosts, including humans, readily display T3SS activity in vitro (20). In contrast, there are no reports of T3SS-dependent cytotoxicity by B. pertussis, despite the fact that T3SS loci are highly conserved, are functionally interchangeable, and bear the hallmarks of positive selection (3, 16, 25). Recent studies are beginning to shed light on this paradox. Although T3SS activity is not observed with laboratory-adapted strains, Bsp22, a highly expressed secretion substrate that polymerizes to form a flexible filament connecting the needle to the translocon, has been detected in culture supernatants of B. pertussis clinical isolates (24, 26). Furthermore, mutations in the B. pertussis bscN allele result in elevated production of proinflammatory cytokines and accelerate clearance from the lungs of aerosol-infected mice (24). A hypothesis that could explain species-specific differences is that the regulatory module in the BvgAS regulon that includes T3SS loci has diverged with host specificity. Indeed, T3SS activity is controlled at multiple levels. bteA and bsc apparatus genes are transcriptionally activated by an extracytoplasmic function (ECF) sigma factor, BtrS, which is encoded by a BvgAS-activated gene (25). In addition, the partner-switching proteins BtrU, BtrV, and BtrW mediate a cycle of serine phosphorylation and dephosphorylation events that gate secretion through the T3SS apparatus (25, 27).
Here, we characterize a regulatory node involving a T3SS-exported anti-σ factor, BtrA (20, 28), and demonstrate its activity as a secreted BtrS antagonist that differentially controls expression of nearly 300 genes that define six distinct regulatory modules within the BvgAS virulence regulon. In B. bronchiseptica, the relative levels of BtrA and BtrS determine the magnitude of T3SS activity. In both laboratory and recent clinical isolates of B. pertussis, deletion of btrA derepressess T3SS gene expression and confers readily observable, BteA-dependent cytotoxicity in vitro. Our results show that B. pertussis is capable of expressing the full range of T3SS-dependent phenotypes and that the BtrA regulatory node is a key determinant of species-specific differences in expression. These observations provide the impetus and foundation for a comprehensive assessment of T3SSBsc in the pathogenesis and prevention of human pertussis.
Results
BtrA Is a T3SS Substrate That Regulates Multiple Virulence Genes.
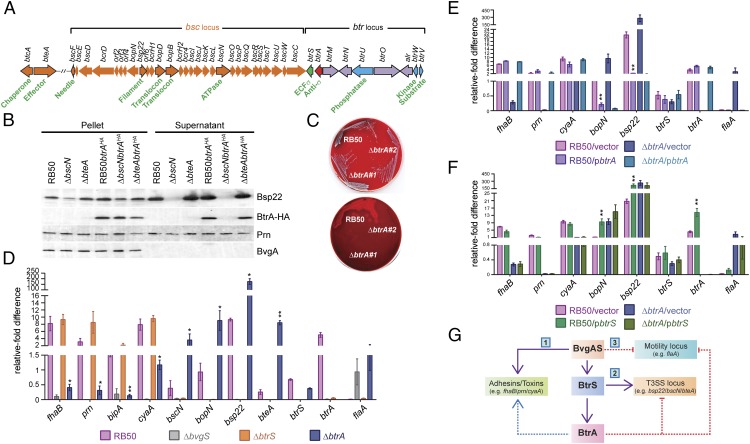
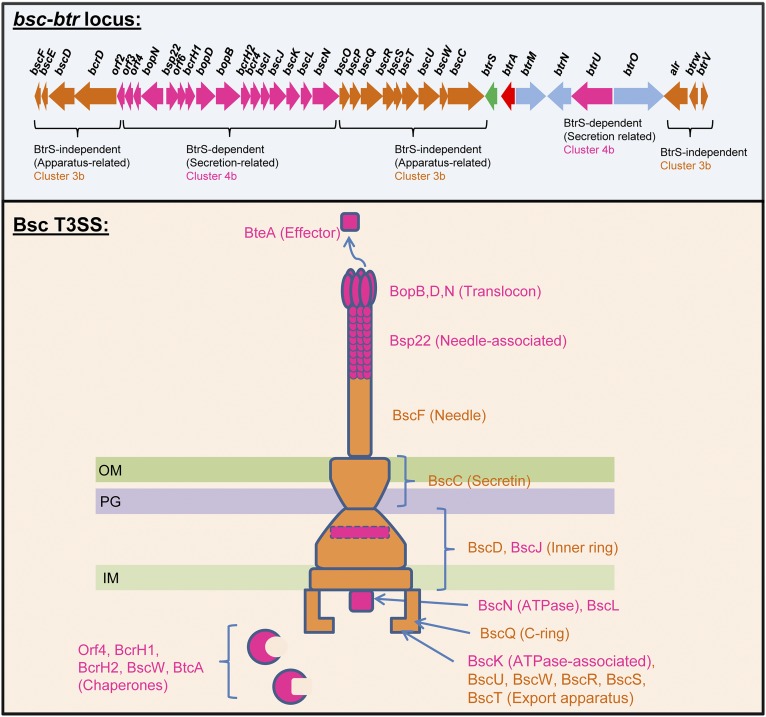
The bsc locus, which includes T3SS apparatus, translocon, and chaperone genes, is flanked by a cluster of regulatory loci that encode BtrS and the BtrUWV partner-switching module (25, 27) (Fig. 1A). Recent studies implicate BtrA (also called BspR), which is encoded directly upstream of btrS, as an exported substrate of the Bsc T3SS, illustrated in Fig. 1B using B. bronchiseptica strain RB50 grown in vitro under conditions permissive for type III secretion (20, 28). BtrA and Bsp22, a known T3SS substrate (26), are exported into supernatants in a manner dependent on the BscN ATPase. Fractionation controls included pertactin (Prn), an OM autotransporter that releases an N-terminal domain after cleavage (29), and BvgA, a cytoplasmic response regulator. These results confirm that BtrA is an exported T3SS substrate in RB50.
Fig. 1.
BtrA differentially regulates three distinct modules of the BvgAS regulon. (A) The bsc-btr locus gene organization. Orange ORFs designate T3SSBsc loci (14), blue ORFs encode partner-switching proteins (27), and purple ORFs are uncharacterized. btrA and btrS are red and green, respectively. Select ORFs are highlighted in black outline with functions shown. (B) BtrA secretion characterized by immunoblot analysis of culture supernatant or pellet fractions from the indicated strains. Blots were probed with antisera against BvgA, Prn, Bsp22, or the HA tag. (C) Colony morphology and hemolysis phenotypes of RB50 vs. independently isolated ∆btrA derivatives on Bordet–Gengou agar scanned with reflective mode (Top) or transmission mode (Bottom). RB50∆btrA#1 was used in all subsequent studies. (D–F) qRT-PCR with RNA from indicated strains. Fold differences in transcript levels were calculated relative to recA expression. Colored bars represent average values from three independent experiments, and error bars represent ± SEM. (G) Summary of results showing that BtrA positively regulates adhesin and toxin genes (i), negatively controls flaA expression (ii), and represses a subset of T3SS genes (iii). Student's t test was used for statistical analysis, and genes showing significant differences between RB50 vs. RB50∆btrA (D), RB50/vector vs. RB50/pbtrA (E), or RB50/vector vs. RB50/pbtrS (F) are labeled with an * (P < 0.05) or ** (P < 0.01). See Fig. S1 for additional supporting data.
Next, we generated an RB50 derivative with an in-frame deletion in btrA. A readily apparent phenotype was observed in which independently constructed mutants formed small, weakly hemolytic colonies on blood agar in comparison with RB50 (Fig. 1C). Relative growth rates in shake flask cultures mirrored differences in colony size because the ∆btrA mutant grew more slowly and reached lower levels in stationary phase than the WT parental strain (Fig. S1A). Because hemolytic activity, colony morphology, and growth rate are all BvgAS-regulated phenotypes, we compared the effects of deleting btrA on expression of a panel of genes chosen to represent different modules of the BvgAS virulence regulon.
Fig. S1.
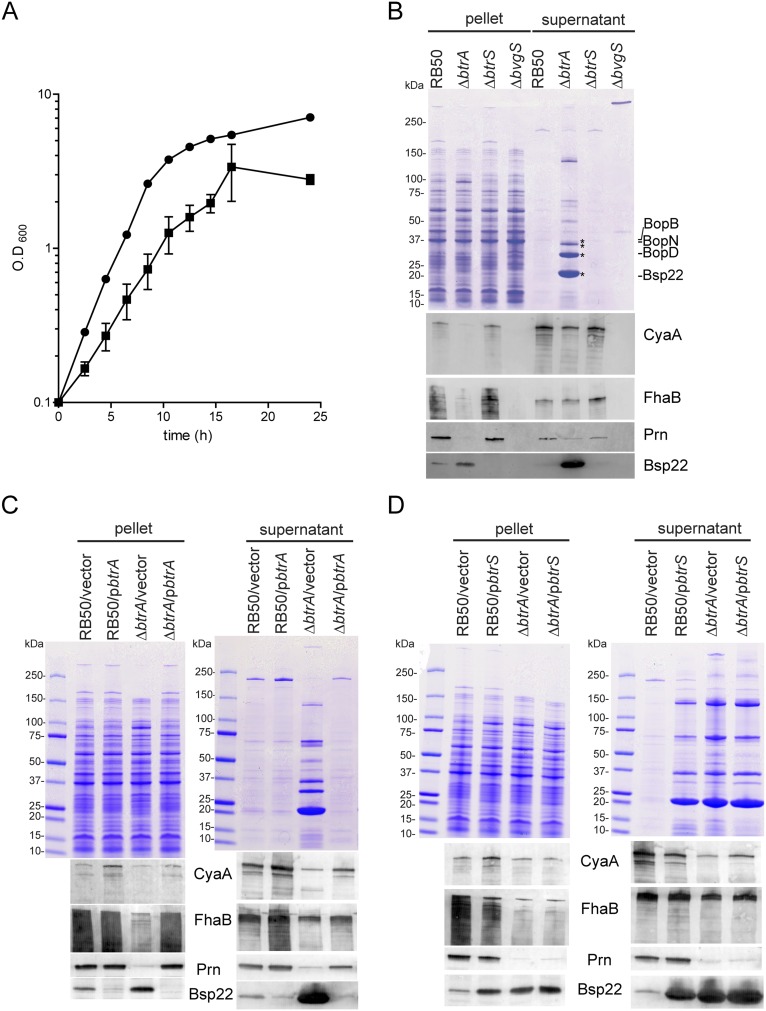
Effects of BtrA on growth and on protein expression and secretion. (A) Growth in baffled 250-mL Erlenmeyer flasks containing Stainer–Scholte medium incubated at 37 °C with shaking. Doubling times, calculated from cfu on Bordet–Gengou agar, were 1.89 ± 0.17 h for RB50 and 6.78 ± 1.35 h for the ∆btrA derivative. Each point on the curve is an average from triplicate experiments. Error bars represent ±SEM. B. bronchiseptica RB50 is represented with circles and ∆btrA with squares. (B–D) Protein expression and secretion from midlog phase deletion mutants (B), pbtrA complemented strains (C), or pbtrS complemented strains (D) grown in Stainer–Scholte medium. (Top) Coomassie brilliant blue-stained SDS/PAGE showing pellet and culture supernatant fraction profiles. (Bottom) Immunoblot analysis of culture supernatant or pellet fractions from the indicated strains. Blots were probed with antisera against CyaA, FhaB, Prn, and Bsp22. Asterisks indicate T3SSBsc secretion substrates.
As shown in Fig. 1D, transcript levels of adhesin and toxin genes (fhaB, prn, bipA, and cyaA), T3SS loci (bopN, bsp22, and bteA), btrS, and btrA are highly dependent on BvgS whereas the flagellin locus (flaA) is Bvg-repressed (3). Expression of T3SS genes additionally requires the BtrS sigma factor, and the same is true for btrA. Deletion of btrA resulted in a marked increase in T3SS-associated transcripts, with induction ratios ranging from 10-fold (bscN) to 34-fold (bteA). In contrast, toxin and adhesin genes showed the opposite effect and were down-regulated 7-fold (cyaA) to 20-fold (fhaB) in ∆btrA mutants. Interestingly, flaA expression was increased in the ∆btrA strain to levels similar to the ∆bvgS mutant. As shown in Fig. S1B, these transcriptional effects are reflected at the levels of protein expression and secretion. Complementation with btrA reversed the effects of the ∆btrA deletion on transcription (Fig. 1E), protein expression, and secretion (Fig. S1C). In WT RB50, overexpression of btrA decreased expression of T3SS-associated genes but did not confer increased expression of toxin or adhesin loci, suggesting that BtrA levels in RB50 are sufficient for maximal expression under laboratory conditions. Together, these data confirm and extend previously published results (28) and show that BtrA differentially regulates three distinct modules of the BvgAS regulon: (i) Bvg+ phase adhesin and toxin genes, which require BtrA for full level expression, (ii) Bvg+ phase T3SS loci that require BtrS and are repressed by BtrA, and (iii) the Bvg phase flagellin locus, which is down-regulated by BtrA (Fig. 1G).
BtrA Is a Secreted BtrS Antagonist.
The observations that BtrA and BtrS have opposing effects on T3SS gene expression and that BtrA is itself a T3SS substrate prompted the hypothesis that BtrA antagonizes BtrS. As shown in Fig. 1F, overexpression of btrS in RB50 resulted in elevated levels of btrA, bsp22, and bopN transcription. For the T3SS apparatus components bsp22 and bopN, BtrS overexpression mimicked the effects of deleting btrA. In contrast, cyaA, fhaB, and flaA transcript levels were relatively insensitive to increased BtrS. Corroborating results were obtained by monitoring protein expression and secretion (Fig. S1D). These data show that high level expression of T3SS genes can be achieved by eliminating BtrA or by overexpressing BtrS, consistent with an antagonistic interaction.
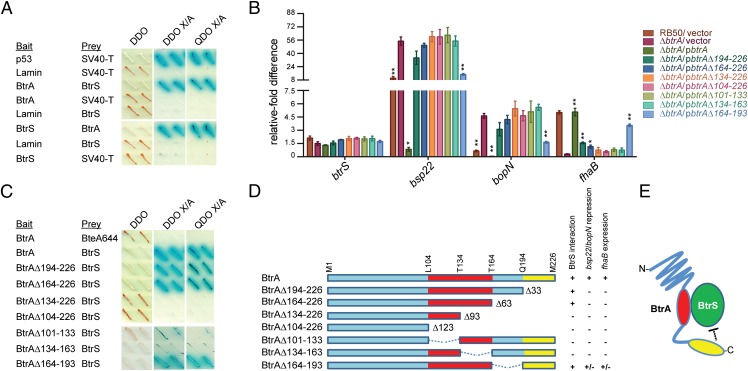
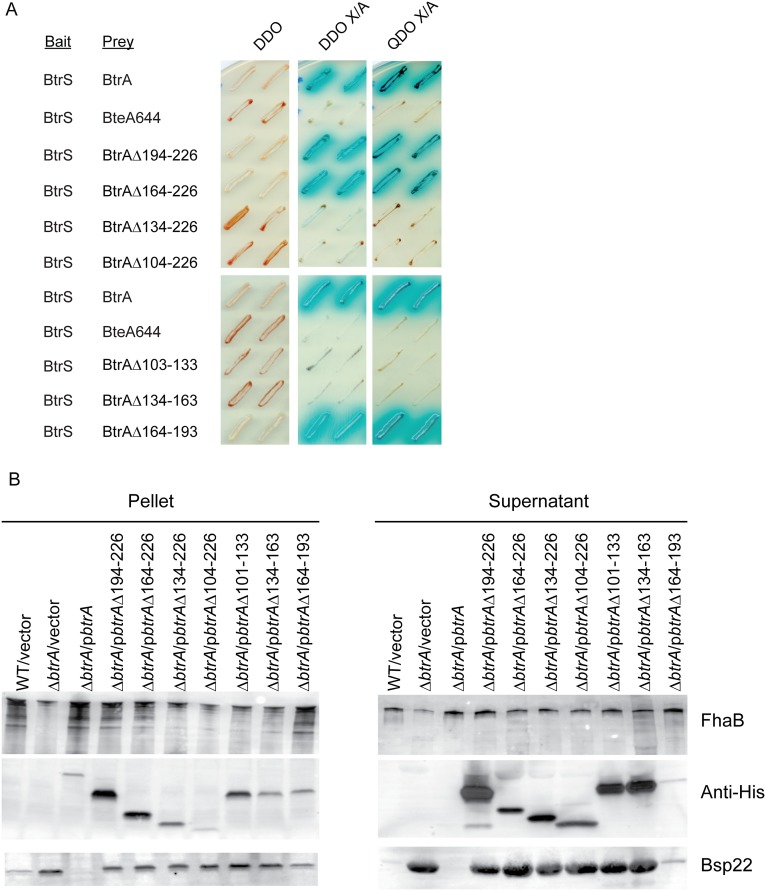
Because ECF sigma factors are often controlled by antagonistic binding partners (i.e., anti-σ factors), we used a multireadout yeast two-hybrid system to determine whether BtrA and BtrS interact (Fig. 2A and Fig. S2A). BtrA fused to the Gal4 DNA binding domain strongly interacted with a BtrS-Gal4 activation domain fusion protein, and identical results were observed when bait and prey inserts were swapped. No interactions were detected with negative control constructs encoding fusions with noncytotoxic BteA (BteA644) or Lamin. To determine the relationships between BtrS binding and gene regulation, we examined the ability of a series of truncated BtrA derivatives to interact with BtrS, repress bsp22 and bopN expression, and up-regulate the expression of fhaB. As shown in Fig. 2 B and D, removal of 33, 63, 93, or 123 residues from the BtrA C terminus eliminated the ability to repress bsp22 and bopN and to activate fhaB in the ∆btrA background. The ∆93 and ∆123 truncations also eliminated BtrS binding (Fig. 2C and Fig. S2A), as did two internal deletions. All mutant BtrA proteins were expressed and secreted at levels that corresponded to their ability to repress T3SS gene expression (Fig. 2B and Fig. S2B).
Fig. 2.
BtrA binds and antagonizes BtrS. (A) Yeast two-hybrid analysis of BtrA–BtrS interactions. Yeast diploids were grown on double dropout (DDO) medium lacking leucine and tryptophan, DDO containing the chromogenic substrate X-α-gal and aureobasidin A (DDO X/A), and on quadruple dropout (QDO X/A) medium containing X-α-gal and aureobasidin A but lacking leucine, tryptophan, histidine, and adenine. Binding interactions result in growth on high stringency QDO X/A medium, resistance to aureobasidin A, and α-galactosidase activity indicated (blue). Interaction of SV40 large T antigen with p53 or Lamin served as positive or negative controls, respectively. Noncytotoxic BteA644 also served as a negative control. Representative results from two independent experiments are shown. All interactions were confirmed by swapping bait and prey protein fusions (Fig. S2). (B) qRT-PCR with RNA from indicated strains. Deletion endpoints correspond to amino acid residues in BtrA as shown in D. Fold differences in transcript levels were calculated relative to recA expression. Colored bars represent average values from three independent experiments, and error bars represent ± SEM. Student's t test was used for statistical analysis. For each gene, strains showing significant differences from ∆btrA/vector are labeled with * (P < 0.01) or ** (P < 0.001). (C) Yeast two-hybrid analysis of interactions between BtrS and BtrA deletion mutants. (D) Ability of BtrA variants to bind BtrS, repress T3SS genes, and activate fhaB expression. (E) Model of BtrA—BtrS interactions occurring in the cytoplasm, which are disrupted by BtrA secretion; see Results for details. Fig. S2 contains additional supporting data.
Fig. S2.
Effect of BtrA truncations and internal deletions on BtrS binding and T3S. (A) Yeast two-hybrid interaction of BtrA and BtrS proteins fused to GAL4 activation domain (prey) and a Gal4 DNA-binding domain (bait), respectively. Yeast diploids were grown on double dropout (DDO) medium lacking leucine and tryptophan, DDO containing the chromogenic substrate X-α-gal and aureobasidin A (DDO X/A), and on quadruple dropout (QDO X/A) medium containing X-α-gal and aureobasidin A but lacking leucine, tryptophan, histidine, and adenine. Binding interactions result in yeast growth on high stringency QDO X/A medium, resistance to aureobasidin A, and α-galactosidase activity (blue). Interaction of noncytotoxic BteA644 served as negative control. (B) Expression and secretion of virulence gene products by indicated strains in midlog phase growth in Stainer–Scholte media. Blots were probed with antisera against FhaB, His-tag (BtrA), or Bsp22.
As summarized in Fig. 2E, our data resolve three functional regions of BtrA: a C-terminal domain required for transcriptional repression and activation, a central region required for BtrS binding, and N-terminal sequences that mediate recognition as a T3SS substrate. BtrS binding is likely required for transcriptional control, but it is clearly not sufficient. A simple hypothesis for the ability of BtrA to activate expression of fhaB and coregulated loci invokes the existence of a BtrS-activated repressor specific for this subset of genes. This model accounts for the observations that BtrS binding is required for both activation and repression by BtrA, and it is supported by RNA-seq analysis.
Effects of BtrA on Cytotoxicity and Virulence.
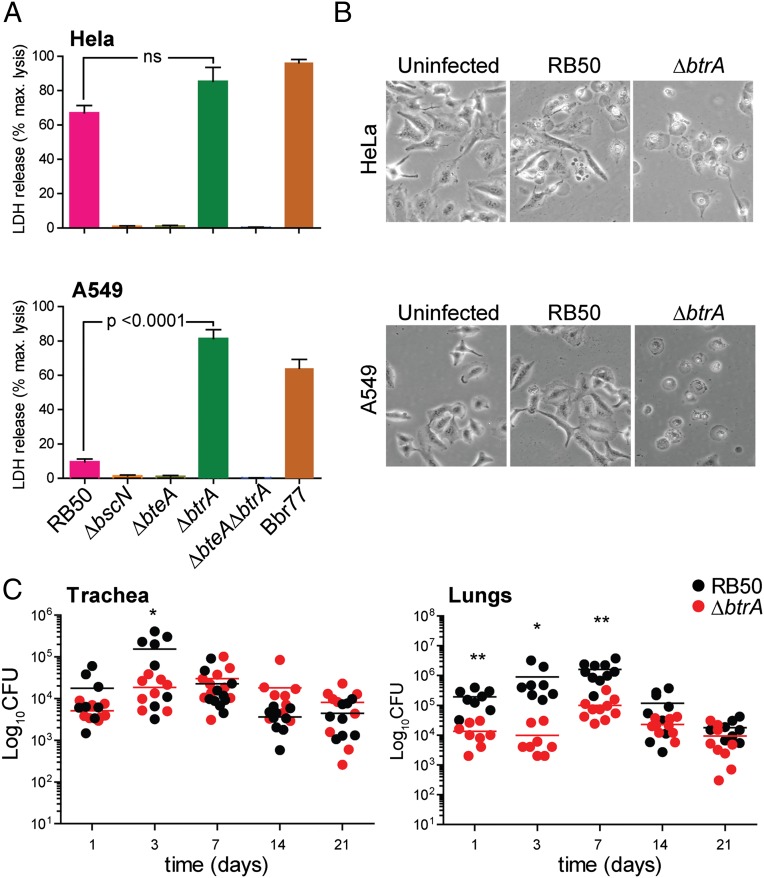
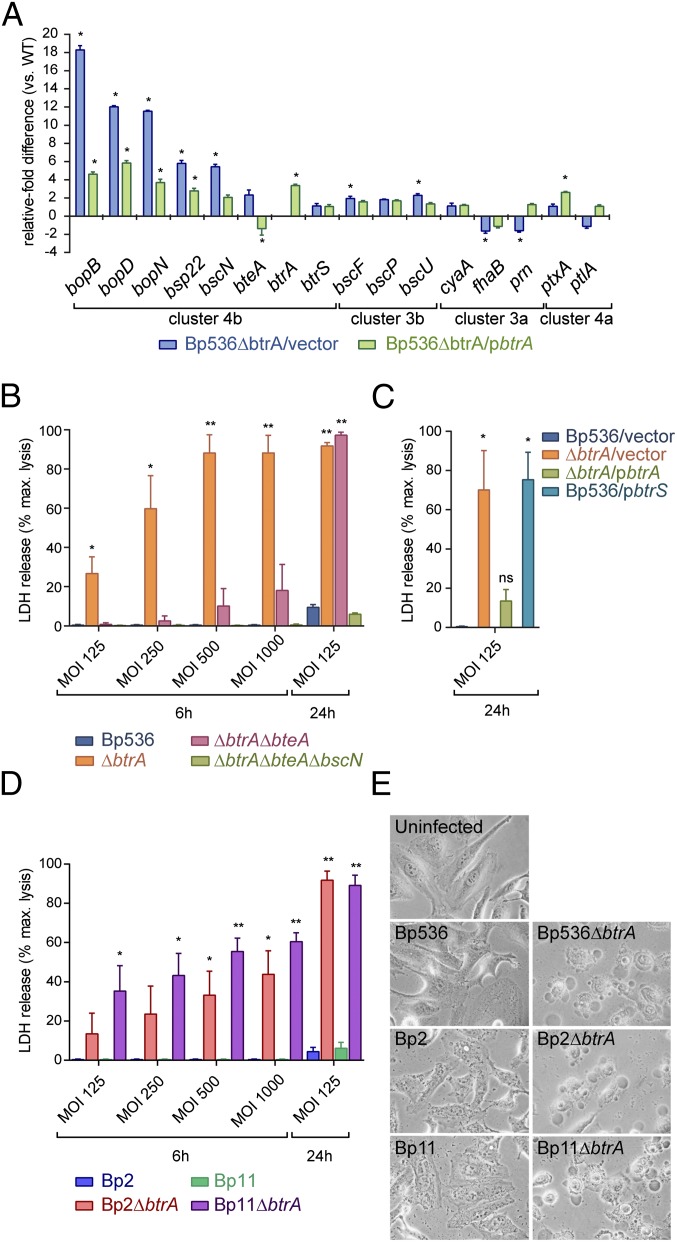
B. bronchiseptica isolates have T3SS-dependent cytotoxic effects on a broad range of cell types (14, 16, 20, 30). Most respond like HeLa cells (Fig. 3A), which are efficiently killed by RB50 in a BscN- and BteA-dependent manner. In contrast, human pneumocyte-derived A549 cells are unusually resistant to RB50 cytotoxicity through an unknown mechanism (20) (Fig. 3B). Resistance was reversed, however, by deleting btrA, which resulted in high level BteA-dependent cytotoxicity against HeLa as well as A549 cells. The levels of cell death observed with RB50∆btrA are similar to strain Bbr77, which represents a clade of hypercytotoxic B. bronchiseptica isolates from human respiratory infections (20).
Fig. 3.
Hypercytotoxicity and virulence phenotypes of RB50∆btrA. (A) HeLa cells (Top) or A549 cells (Bottom) were infected with indicated strains at an MOI of 50:1 for 3 h, and cytotoxicity was measured by lactate dehydrogenase (LDH) release. Bars represent averages from three independent experiments, and error bars indicate ± SEM. (B) HeLa or A549 cells were infected with indicated strains at an MOI of 50:1 for 2 h and examined by phase-contrast microscopy at 20× magnification on a Carl Zeiss Axiovert 40 CFL microscope. Images are representative of three independent experiments. (C) Four-week-old female C57BL/6NCr (B6) mice were intranasally inoculated with 5 × 105 cfu of RB50 or RB50∆btrA and killed on indicated days. The number of colony-forming units (CFU) per lung or trachea is shown for each animal. Statistical significance (t test) is indicated with * (P < 0.05) or ** (P < 0.001). Fig. S3 contains additional data.
We next used a murine intranasal challenge model to examine the effects of deleting btrA on respiratory colonization and virulence. Groups of C57BL/6NCr mice were intranasally infected with WT or ΔbtrA strains using a protocol that results in infection of the entire respiratory tract by RB50, followed by gradual clearance from the lungs and trachea (31). Bacterial numbers in the trachea were indistinguishable throughout the course of the experiment (Fig. 3C). In lungs, the ∆btrA mutant showed a modest 1- to 2-log decrease in infection levels until the peak at day 7, followed by clearance with similar kinetics as the WT strain RB50. All animals seemed healthy during the course of the experiment.
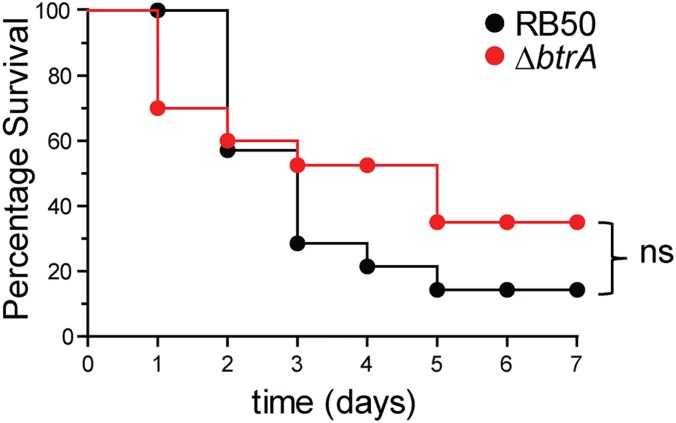
Surprised by relative lack of a correlation between hypercytotoxicity in vitro (Fig. 3B) and the infection results shown in Fig. 3C, we compared RB50 and the ∆btrA mutant in an acute respiratory infection model in which the majority of animals become moribund within 1 wk postinoculation (20, 31). Again, the ∆btrA mutant was no more virulent than the WT strain (Fig. S3). Because eliminating BtrA results in increased T3SS activity and decreased adhesin and toxin expression, these opposing effects and other changes may account for the lack of an overt phenotype. An alternative possibility is that the system is normally derepressed in vivo through active secretion of BtrA, making the ∆btrA allele superfluous. In either case, it is important to understand the extent to which BtrA differentially regulates gene expression in Bordetella.
Fig. S3.
Survival of mice inoculated with WT B. bronchiseptica RB50 or ΔbtrA mutant. Groups of 12 mice (4-wk-old female C57BL/6NCr) were intranasally inoculated with 7.5 × 106 cfu as described in SI Materials and Methods. The animals were killed when moribund. No statistically significant (log-rank test) differences were observed between the two groups.
BvgAS, BtrS, and BtrA Regulatory Networks.
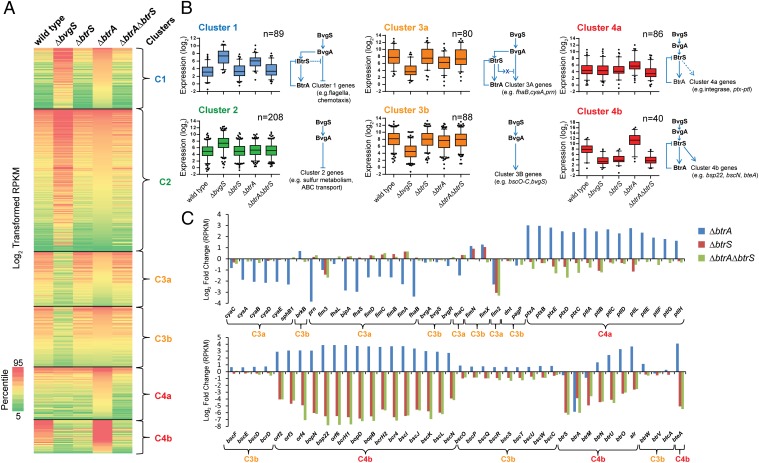
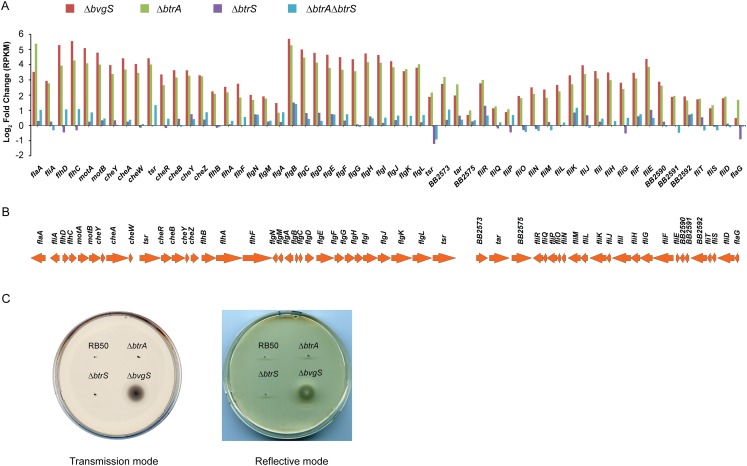
We compared the transcriptomes of RB50 and isogenic ∆bvgS, ∆btrS, ∆btrA, and ∆btrA∆btrS derivatives using RNA-seq. The transcriptional data were used to generate nonhierarchical clusters, from which four broad groups of coexpressed gene classes were derived (Fig. 4). Bvg-repressed genes (n = 297) were found in clusters 1 and 2 whereas Bvg-activated genes (n = 242) grouped in clusters 3 and 4. Genes influenced by deletion of btrA (n = 294) were divided between clusters 1, 3, and 4. Network models consistent with observed regulatory profiles are shown in Fig. 4B.
Fig. 4.
BtrA, BtrS, and BvgS regulatory networks identified using RNA-seq. (A) Heat map of log2 transformed reads per kilobase of transcript per million mapped reads (RPKM) values showing expression levels of specific transcripts (rows) in samples from WT RB50 or ∆bvgS, ∆btrS, ∆btrA, or ∆btrA∆btrS derivatives (columns). Genes with at least plus or minus threefold change in expression relative to WT were included in the analysis. Nonhierarchical clustering of RPKM values revealed four coexpressed gene clusters (C1–C4). Map colors indicate the percentile score of expression level. RNA-seq alignment statistics, datasets, and comparative analyses are listed in Table S4 and Dataset S1. (B) Box-and-whisker plots showing the distribution of transcripts within each gene cluster. Boxes depict data between the 25th and 75th percentiles, with horizontal lines within boxes representing the median value. Whiskers show 5th and 95th percentiles, with outliers as dots. The projected regulatory network for each cluster is drawn next to the respective plot. Arrowheads indicate transcriptional activation, and bars indicate repression. For cluster 3a, “X” designates a predicted BtrS-activated repressor that acts on a subset of BvgAS-regulated genes. (C) Log2-fold changes in expression levels of selected genes in deletion mutants compared with WT RB50. (Top) Genes are ordered by relative position in the RB50 genome. (Bottom) T3SS genes ordered by relative position. Curly brackets designate coexpression clusters.
Cluster 1 contains 89 genes that are coregulated with the flagellin locus flaA. These loci are minimally expressed in RB50, highly activated in the Bvg phase (∆bvgS), and activated in the ∆btrA strain. Although deleting btrS alone has little effect, combining the ∆btrS and ∆btrA alleles reverses the ∆btrA phenotype. Coexpressed genes in cluster 1 encode a large subset of motility and chemotaxis proteins, along with an assortment of predicted surface and regulatory factors (Dataset S1).
Cluster 2 includes 208 Bvg-repressed genes that are maximally expressed in the ∆bvgS strain with no apparent involvement of BtrA or BtrS. These repressed genes include loci encoding ABC transporters, a sulfate transport system, urea uptake and metabolism (32), a host of diverse metabolic functions, and chemotaxis and motility genes distinct from those in cluster 1. The distribution of motility factors between clusters 1 and 2 accounts for the lack of motility in soft agar by the ∆btrA strain, in which only cluster 1 genes are derepressed, compared with active motility in the ∆bvgS mutant in which all chemotaxis and motility loci are actively transcribed (Fig. S4 and Dataset S1).
Fig. S4.
Expression of motility and chemotaxis-related genes. (A) Log2-transformed RPKM values of flagellar and chemotaxis locus genes from the indicated strains. (B) Genomic organization of flagella and chemotaxis locus in B. bronchiseptica RB50. (C) Soft-agar motility assay. Bacteria were stab inoculated onto a semisolid Stainer–Scholte motility agar (0.4% agar) for 20 h at 37 °C.
The 168 Bvg-activated genes in cluster 3 split into two groups. Cluster 3a loci encode many of the canonical Bordetella virulence determinants and their accessory factors, including FHA (fhaB and -C), adenylate cyclase toxin (cyaA to -E), pertactin (prn), and fimbria (fimA to -D). In addition to their dependency on BvgS, these genes are down-regulated in the ∆btrA strain, and this phenotype is reversed in the ∆btrA∆btrS double mutant. In contrast, cluster 3b genes are Bvg-activated with little if any effect of BtrA or BtrS. The differential effects of BtrA/BtrS on cluster 3a vs. 3b loci are readily apparent in Fig. 4C. Cluster 3b includes numerous T3SS loci that are BtrS-independent. These loci encode structural components of the secretion apparatus (bscC to -F, bscO to -W), proteins involved in controlling secretion activity (btrW, btrV), and other T3SS-associated factors (Dataset S1).
Cluster 4 contains 126 genes that require BtrS for expression and are negatively regulated by BtrA. This cluster divides into two subgroups that differ in their basal levels of expression. As shown in Fig. 4B, cluster 4a loci are poorly transcribed in vitro by WT or ∆bvgS strains whereas cluster 4b loci are expressed by RB50 at relatively high levels and down-regulated in the ∆bvgS mutant. In both cases, transcript levels are substantially increased by deletion of btrA, and this effect is reversed in a ∆btrA∆btrS double mutant (Fig. 4 B and C). Cluster 4a includes 86 genes predicted to encode several autotransporters and ABC transport systems, regulatory proteins, and various metabolic functions. Surprisingly, this cluster also includes the complete set of genes required for pertussis toxin production (ptxA to -E) and secretion (ptlA to -L), which were previously thought to be quiescent in B. bronchiseptica. Cluster 4b includes T3SS loci that encode extracellular components of the translocation needle (bscI to -K, bsp22), the membrane-penetrating translocon and other secreted apparatus components (bopB, -D, and -N), the BscN ATPase, the BtrU phosphatase, and the BteA effector (Dataset S1).
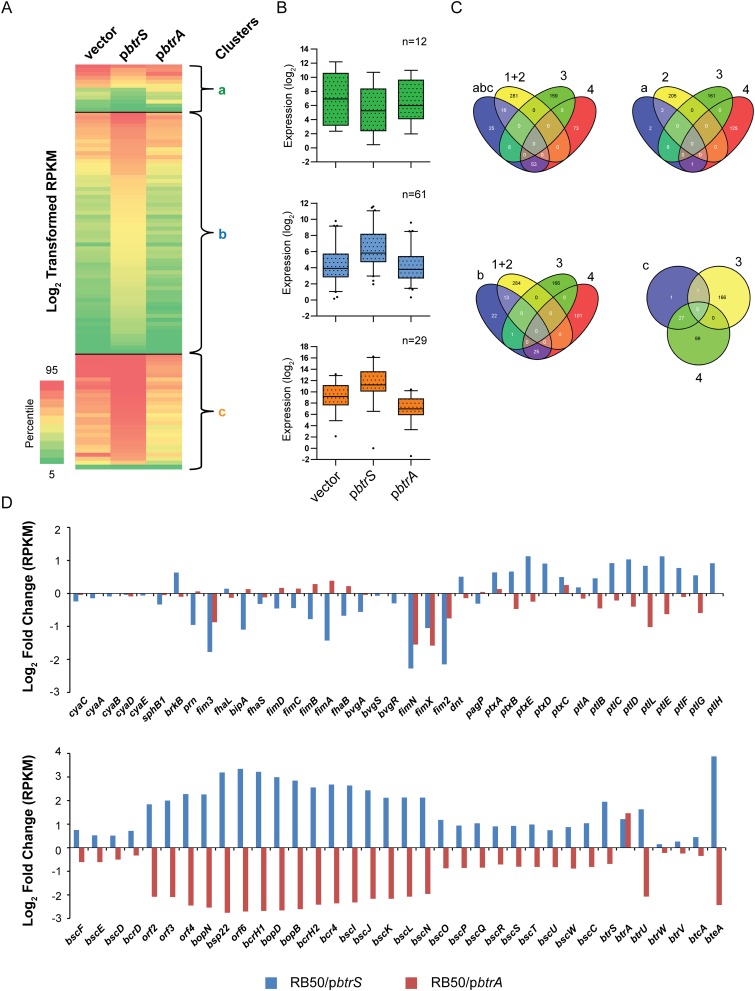
As shown in Fig. S5, the effects of overexpressing btrA or btrS corroborate results obtained with deletion mutants. Taken together, transcriptional profiling experiments reveal an unexpected diversity of expression patterns among BvgAS-regulated genes revealed by manipulating BtrA. Given its pervasive effects on gene expression and the observation that the BtrA proteins of B. bronchiseptica and B. pertussis are 93% identical, we tested the possibility that BtrA regulates virulence genes in B. pertussis.
Fig. S5.
Overexpression of btrA or btrS corroborate regulatory networks predicted by RNA-seq analysis of deletion mutants. (A) Heat map of log2-transformed RPKM values showing expression levels of specific transcripts (rows) in samples from WT RB50/vector, RB50/pbtrS, or RB50/pbtrA (columns). Genes with at least plus or minus threefold perturbation in expression relative to vector-only were analyzed. Nonhierarchical clustering of RPKM values was performed to derive coexpressed gene clusters (clusters a–c). Colors on map indicate percentile relative expression levels. (B) Box-and-whisker plots showing distribution of transcripts within each cluster. Boxes depict data between the 25th and 75th percentiles, with horizontal lines within boxes representing the median value. Whiskers show 5th and 95th percentiles, with outliers as dots. (C) Venn diagrams showing the number of genes that overlap between null-mutants (Fig. 4) and after overexpression of btrA and btrS. Areas within Venn diagrams are not scalable. The Venn diagrams were created using Venny (bioinfogp.cnb.csic.es/tools/venny/). Of the 90 genes that displayed significantly increased transcription after overexpression of BtrS, 29 were down-regulated by BtrA overexpression and all were categorized as BtrS-activated and BtrA-repressed in the analysis in Fig. 4 (clusters 4a and 4b). Of the 80 loci with decreased expression in the ∆btrA strain (cluster 3a), none were further induced in RB50 by overexpressing BtrA. Although results from deletion vs. overexpression strains are congruent, complete correspondence is not expected. If the levels of BtrA in RB50 exceed the threshold for maximal activation or repression of a given gene, for example, overexpression will have no effect as observed with cluster 3a genes. See also Dataset S1. (D) Log2-fold change in gene expression of known virulence-related genes compared with WT.
BtrA Controls T3SS-Mediated Cytotoxicity in B. pertussis.
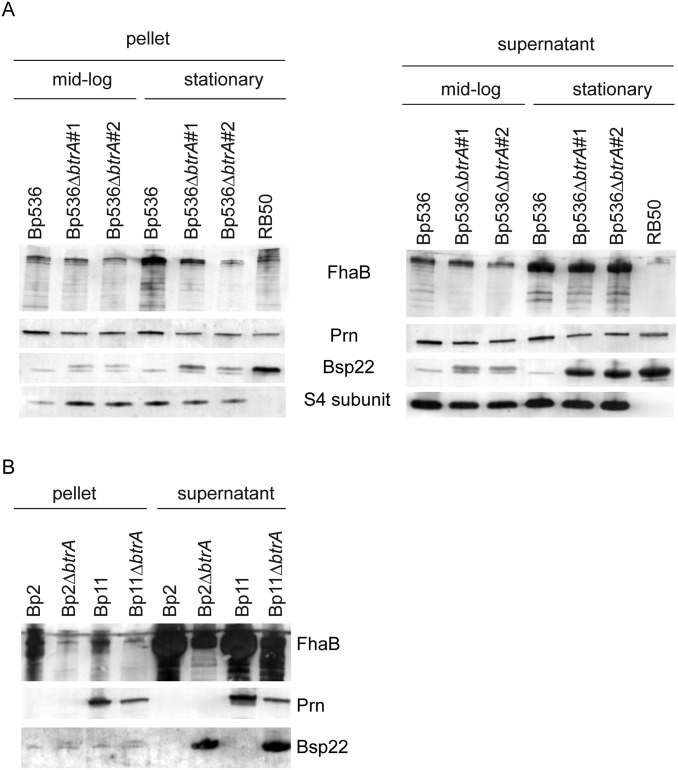
As shown in Fig. 5A, deletion of btrA in B. pertussis strain Bp536 derepresses bopB, bopD, bopN, bsp22, bscN, and bteA transcription, and these effects were partially reversed by complementation. Other T3SS genes, fhaB, cyaA, prn, ptxA, and ptlA, were unaffected by btrA deletion or overexpression. Protein expression and secretion assays (Fig. S6A) mirror results obtained by quantitative real-time polymerase chain reaction (qRT-PCR). We conclude that Bp536 has retained BtrA-mediated control of cluster 4b genes encoding T3SS substrates and components whereas BtrA regulation of virulence genes in clusters 3a and 4a has either been lost or is inapparent in vitro. Cluster 3b genes are BtrA-independent in both B. bronchiseptica and B. pertussis.
Fig. 5.
BtrA-dependent regulation of BteA-mediated cytotoxicity in laboratory and clinical B. pertussis isolates. (A) qRT-PCR analysis of transcripts from indicated strains. Fold differences in transcript levels were calculated relative to Bp536/vector (WT) using the ΔΔCt method. Statistical significance is indicated with * (P < 0.05). Colored bars represent average values obtained from three independent experiments, and error bars represent ± SEM. (B–D) HeLa cells were infected with bacterial strains at different MOIs for 6 or 24 h, and cytotoxicity was measured by LDH release. Error bars represent ±SEM. Statistical significance (t test) is indicated with * (P < 0.05), or ** (P < 0.001). (E) HeLa cells were infected with indicated strains at an MOI of 250:1 for 5 h and examined by phase-contrast microscopy at 40× magnification on a Carl Zeiss Axiovert 40 CFL microscope. Images are representative of three independent experiments.
Fig. S6.
Protein expression and secretion by B. pertussis strains. (A and B) Immunoblot analysis of cell pellet and culture supernatant fractions from the indicated strains prepared from midlog or stationary phase cultures in Stainer–Scholte medium. Blots were probed with antisera specific for FhaB, CyaA, Prn, Bsp22, or pertussis toxin subunit S4.
Having observed that BtrA functions in B. pertussis, we tested the hypothesis that the lack of discernable T3SS-mediated cytotoxicity in vitro reflects an alteration in regulation as opposed to an inability to deploy a functional secretion apparatus. Indeed, Fig. 5B shows a marked, dose-dependent increase in cytotoxicity conferred by deleting btrA, which is reversed by complementation. Reasoning that the cryptic nature of T3SS cytotoxicity is likely due to BtrS antagonism by BtrA, we overexpressed BtrS in Bp536 and, as predicted, observed a level of cell death that was nearly identical to the ∆btrA mutant (Fig. 5C).
As previously seen with B. bronchiseptica (Fig. 3A) (16), high level cytotoxicity by B. pertussis required both the BteA effector and the BscN ATPase (Fig. 5B). We were intrigued, however, by the ability to detect toxicity by ∆btrA∆bteA double mutants at high multiplicities of infection (MOIs) or longer time points, but not by ∆btrA∆bteA∆bscN triple mutants (Fig. 5B). Although levels were low and did not reach statistical significance at 6 h, they were highly significant at 24 h, providing evidence for the presence of a BteA-independent, T3SS-dependent activity that confers cytotoxic effects after prolonged incubation.
To further assess the relevance of our results, we determined whether low passage clinical isolates representing currently circulating strains also display enhanced cytotoxicity after btrA deletion. B. pertussis strains Bp2 and Bp11 were isolated from lethal infections in infants during the 2010 pertussis epidemic in California, and, although challenging to grow and manipulate, we were able to delete btrA in both strains. This deletion resulted in levels of cytotoxicity similar to those seen with Bp536 (Fig. 5D), and a corresponding increase in Bsp22 secretion (Fig. S6B). Cells exposed to B. pertussis isolates with derepressed T3SSs displayed blebbing and other morphological changes characteristic of those seen with WT B. bronchiseptica (Fig. 5E) (30).
Our data show that BtrA is active in B. pertussis, that B. pertussis is capable of expressing a fully functional Bsc T3SS, and that the lack of cytotoxic activity in vitro is due to BtrA-mediated repression. Although BteA clearly functions as a cytotoxic effector, the observation of BteA-independent, T3SS-dependent cytotoxicity raises the possibility that additional effectors await discovery. The implications of these findings and other results are discussed below.
Discussion
Through its function as a secreted BtrS antagonist, BtrA establishes a feedback loop that couples the activity of the T3SS apparatus with expression of T3SS genes encoding the BteA effector, other secretion substrates, and interacting factors. In B. bronchiseptica, BtrA is also required for high level transcription of genes encoding adenylate cyclase toxin, FHA, fimbria, Prn, SphB1, and numerous other products, establishing a reciprocal relationship between their expression and T3SS activity. Reciprocal control of adhesins and toxins vs. T3SS genes could reflect differential requirements during temporally or spatially distinct stages of infection. An alternative hypothesis is that it functions to facilitate immune evasion. It has previously been suggested that T3SSBsc may target antigen presenting cells (APCs) that extravagate to the respiratory surface during infection (22, 33). If true, simultaneous down-regulation of major antigen loci and up-regulation of T3SS genes may combine to suppress antigen processing and the generation of protective immunity. Although both regulatory functions are observed in B. bronchiseptica, adhesin and toxin gene expression have apparently been uncoupled from BtrA control in B. pertussis. This observation correlates with the long term and often asymptomatic association between B. bronchiseptica and natural hosts, in contrast to the acute and immunizing nature of human pertussis (3, 34).
Considerable evidence supports the conclusion that BtrA functions as a BtrS antagonist. For T3SS genes in cluster 4b, deletions in btrA or btrS have opposing transcriptional effects (Fig. 4), and btrS overexpression in a WT background mimics the effects of deleting btrA (Fig. 1 E and F and Fig. S5D). BtrA interacts directly with BtrS using a central domain, and regulation of gene expression requires C-terminal sequences in addition to BtrS binding (Fig. 2). The BtrA N terminus confers recognition as a secretion substrate, thereby coupling T3SS activity to transcriptional control (Fig. S2B), and it has also been shown to mediate nuclear localization after translocation into host cells (35). Secreted antagonists regulate flagellar genes in Salmonella (36, 37) and T3SS loci in Pseudomonas aeruginosa (38), Yersinia spp. (39), Shigella (40), and other bacteria (41). A unique feature of btrA, however, is the extent and diversity of expression profiles it controls.
Six coexpressed clusters of BtrA-regulated genes can be resolved by comparing WT B. bronchiseptica with ∆bvgS, ∆btrA, ∆btrS, and ∆btrA ∆btrS derivatives (Fig. 4B). In several cases, expression profiles predict the existence of new, unidentified control factors. For example, high level expression of cluster 3a genes (e.g., fhaB, cyaA, and prn) is clearly dependent on btrA. This requirement is relieved, however, by simultaneously eliminating btrA and btrS, indicating a negative role for BtrS. Because sigma factors are not generally associated with direct repression, we predict the existence of a BtrS-activated negative regulator (Fig. 4B). Based on its expression profile, this postulated control factor is likely encoded by a cluster 4 gene.
T3SS loci are distributed between cluster 3b, which contains BvgAS-activated genes that are BtrA- and BtrS-independent, and cluster 4b, which includes loci that require BvgAS and BtrS for transcription and are negatively controlled by BtrA. As shown in Fig. S7, cluster 3b encodes components of the secretion apparatus, up to and including the BscF needle. Cluster 4b encodes secreted substrates, such as the BteA effector, translocon components, and the Bsp22 filament protein, along with multiple chaperones and the BscN ATPase complex. We have previously shown that the BtrU, -W, and -V partner-switching proteins operate at a posttranslational level to “gate” the secretion apparatus, controlling its function in response to unknown queues (27). Coupling transcription of genes encoding secreted factors and ancillary systems to T3SS activity via negative control by BtrA presumably ensures the availability of an adequate pool of T3SS substrates to meet demand. Although clusters 3b and 4b together include all known T3SS genes, they also include numerous coregulated loci with unexplored roles in Bordetella–host interactions.
Fig. S7.
T3SSBsc gene expression patterns inferred from RNA-seq analysis. Known or predicted annotated functions and locations of T3SSBsc gene products are indicated. IM, inner membrane; OM, outer membrane; PG, peptidoglycan.
We identified two surprising consequences of deleting btrA in B. bronchiseptica. First, ∆btrA strains display high level, BtrS-dependent expression of flagellar and chemotaxis genes in cluster 1, which were previously assumed to be exclusively expressed in the Bvg- phase (4, 42). A regulatory scheme that can account for these observations is shown in Fig. 4B although the advantage of activating these loci in the Bvg+ phase is unclear given the deleterious effect of flagellar expression on respiratory infection (17). A second surprise was the observation that deletion of btrA activates expression of ptxA to -E and ptlA to -H genes in cluster 4a, which are homologs of the B. pertussis loci that encode pertussis toxin and the T4SS that exports it (3). Although all genes in cluster 4 require BvgAS and BtrS for transcription, cluster 4a genes seem to require substantially higher levels of BtrS for expression than cluster 4b genes and therefore appear quiescent in vitro.
Of the estimated 5,072 genes in RB50, 12% (n = 591) are BvgAS-regulated as determined by RNA-seq (Fig. 4). One half of all BvgAS-regulated genes are also controlled by BtrA, and these genes partition into BtrA-repressed (n = 215) and BtrA-activated (n = 80) loci. The pervasive and complex nature of BtrA-mediated transcriptional control in B. bronchiseptica was unexpected, particularly in light of the relatively modest effects of deleting btrA in murine models of infection. Increased expression of cluster 4 genes and decreased transcription of cluster 3a loci could have net effects that are nearly compensatory during short-term infection, or the ∆btrA allele may mimic expression levels that normally occur in vivo. Longer time points, competition formats, and other approaches may clarify subtle phenotypes in vivo. In contrast, derepression of T3SS genes by deleting BtrA conferred an increase in BteA-dependent cytotoxicity that was readily apparent, even in cells that are nearly refractory to killing by the WT strain (Fig. 3A). The magnitude of this effect and the extent to which BtrA controls gene expression in B. bronchiseptica prompted us to explore the possibility that differential regulation might explain the enigma of why T3SS-dependent cytotoxicity has never been reported in B. pertussis (3, 25).
B. pertussis T3SS loci are clearly responsive to BtrA, and the dichotomy between BtrA-regulated expression of secretion substrates and associated factors vs. BtrA-independent expression of apparatus genes is also conserved. Most importantly, deletion of btrA derepressed T3SS activity in B. pertussis, providing the first clear evidence, to our knowledge, for cytotoxicity dependent on the BteA effector and the BscN ATPase (Fig. 5 B–E). Overexpression of btrS in Bp536 mimicked the effects of deleting btrA, suggesting that BtrA functions by antagonizing BtrS as it does in B. bronchiseptica. Because Bp536 is a derivative of Tohama I (43), which was isolated in the early 1950s and has been passaged in laboratories ever since, it was essential to determine whether BtrA also controls cytotoxicity in currently circulating strains. Indeed, deletion of btrA in two B. pertussis isolates from infants that died of pertussis during the 2010 California epidemic derepressed cytotoxicity to a similar extent as observed in Bp536, thereby demonstrating the relevance of our observations to understanding human disease. In contrast to B. bronchiseptica, BtrA has little effect on expression of virulence genes that encode CyaA, FHA, Prn, or pertussis toxin in B. pertussis Bp536. As indicated in the regulatory model for genes in cluster 3a (Fig. 4B), BtrA control could be uncoupled in a single step by mutation of a predicted BtrS-activated negative regulator. Indeed, our previously reported comparative microarray analysis showed that numerous transcriptional regulators that are expressed and BvgAS-regulated in B. bronchiseptica are missing, not transcribed, or present as pseudogenes in B. pertussis (4).
BtrA establishes a critical regulatory loop in both B. pertussis and B. bronchiseptica. We hypothesize that differences in T3SS activity between these subspecies reflects differential control over the intracellular level of BtrA, which is itself determined by secretion activity. The BtrU, -W, and -V partner-switching proteins likely play a pivotal role (27), and experiments are underway to understand signals and signaling mechanisms that control their interactions and, consequently, the activity of the secretory apparatus. Regardless of the basis for in vitro differences from B. bronchiseptica, the observation that B. pertussis isolates are fully capable of expressing BteA-mediated cytotoxicity in vitro warrants renewed consideration of the potential role of type III secretion in the pathogenesis and prevention of human pertussis.
Materials and Methods
Bacterial cultures and mammalian cells were grown as previously described (20) and are listed in Table S1 along with plasmids used in the study. Mutants were constructed using allelic exchange as previously described (14, 44). Immunoblotting, cytotoxicity assays, microscopy, and animal experiments were performed as previously described (20). All animals were maintained in University of California, Los Angeles animal research facilities according to National Institutes of Health and University of California Institutional Animal Care and Use Committee (IACUC) guidelines. The animal experiments were approved by the UCLA Chancellor's Animal Research Committee. Yeast two-hybrid assays were performed per the manufacturer’s instructions (Clontech). RNA extraction and processing, quantitative RT-PCR, RNA-seq library preparation, Illumina sequencing, and the data analysis pipeline are described in SI Materials and Methods. Oligonucleotides used for PCR, RT-PCR, and sequencing are listed in Tables S2 and S3. The RNA-seq alignment statistics, dataset, and comparative analyses are listed in Table S4 and Dataset S1. RNA-seq data reported in this paper have been deposited in the Sequence Read Archive, www.ncbi.nlm.nih.gov/sra (submission no. SRP064665; NCBI BioProject no. PRJNA296526).
SI Materials and Methods
Bacterial Strains and Growth Conditions.
Strains and plasmids used in this study are listed in Table S1. Bordetella strains were grown in Stainer–Scholte (SS) liquid medium (45) at 37 °C, with aeration to midexponential growth phase or overnight as indicated, or on Bordet–Gengou (BG) agar (Becton Dickinson Microbiology Systems) supplemented with defibrinated sheep blood at a concentration of 7.5% (Bordetella bronchiseptica) or 15% (Bordetella pertussis) and incubated at 37 °C for 2–3 d (B. bronchiseptica) or 5–7 d (B. pertussis). Antibiotics were added at the following final concentrations: ampicillin (Ap), 100 μg/mL; chloramphenicol (Cm), 25 μg/mL; streptomycin (Sm), 20 μg/mL; kanamycin (Km), 50 μg/mL; and gentamicin (Gm), 20 μg/mL.
Plasmid Construction.
E. coli strains DH5α or Top10 were used as hosts for plasmid construction as detailed below. PCR amplifications were performed using chromosomal DNA isolated from RB50 unless otherwise indicated, using PCR primers listed in Table S2.
pUAB50, which harbors btrA tagged with a hexa-histidine coding sequence at the 3′ end plus an additional 60 bp upstream of the start codon, was constructed by ligating a KpnI- and HindIII-treated 761-bp PCR fragment into KpnI- and HindIII-digested pBBR1MCS.
pUAB56 is a derivative of pBBR1MCS that lacks the lac promotor region, which was removed by treating pBBR1MCS with SphI and KpnI and religating after T4 DNA polymerase treatment.
pUAB60, harboring the btrA (ΔK7-R218) deletion allele (designated ∆btrA in the main text and figures) with 5′ and 3′ flanking regions intact, was constructed by PCR amplifying a fragment beginning 543 bp upstream of the chromosomal btrA gene with primers del-bb1639-F1 and del-bb1639-ovlp-R2. A second fragment, which includes 487 bp downstream of btrA, was amplified using primers del-bb1639-ovlp-F2 and del-bb1639 Xma R3. The upstream and downstream PCR products were used for overlap PCR amplification with primers del-bb1639-F1 and del-bb1639 Xma. The 955-bp product containing the ∆btrA allele with 5′ and 3′ flanking sequences was digested with KpnI and XmaI before ligation into KpnI- and XmaI-digested pRE112.
pUAB68, which harbors the btrS allele plus an additional 58 bp upstream of the start codon, was constructed by ligating a KpnI- and SacI-treated 667-bp PCR fragment into KpnI- and SacI-digested pBBR1MCS.
pUAB70, which harbors btrA plus an additional 200 bp upstream of the start codon, was constructed by ligating a HindIII-treated 877-bp PCR fragment into HindIII-digested pUAB56. The btrA locus in pUAB70 is cloned in the opposite direction of the cat gene.
pUAB74 and pUAB76, which harbor the entire btrA gene fused to GAL4 DNA binding domain (GAL4-BD) or GAL4 transcriptional activation domain (GAL4-AD) coding sequences, respectively, were constructed by ligating an EcoRI- and BamHI-treated 681-bp PCR fragment into EcoRI- and BamHI-digested pGBKT7 and pGADT7 AD, respectively.
pUAB75 and pUAB77, which harbor the btrS gene fused to the GAL4-BD or GAL4-AD, respectively, were constructed by ligating an NdeI- and BamHI-treated 603-bp PCR fragment into NdeI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB80 harboring btrA tagged at the 3′ end with HA (btrA-HA), with 5′ and 3′ flanking regions, was constructed by amplifying a 354-bp fragment containing sequences upstream of the btrA fusion site with primers bb1639-HA-F1and bb1639-HA-R1. A second fragment consisting of 358 bp downstream of the fusion site was amplified using bb1639-HA-F2 and bb1639-HA-R2. Upstream and downstream PCR products were used for overlap PCR amplification with primers bb1639-HA-F1 and bb1639-HA-R2. The 713-bp product containing the HA fusion with 5′ and 3′ flanking sequences was digested with KpnI and XmaI before ligation into KpnI- and XmaI-digested pRE112.
pUAB103, which harbors btrAΔQ194-M226 tagged with a hexa-histidine (His6) coding sequence at the 3′ end plus an additional 60 bp upstream of the start codon, was constructed by ligating a KpnI- and HindIII-treated 662-bp PCR fragment amplified using pUAB50 as template DNA into KpnI- and HindIII-digested pBBR1MCS.
pUAB104, which harbors btrAΔT164-M226 with His6 at the 3′ end plus an additional 60 bp upstream of the start codon, was constructed by ligating a KpnI- and HindIII-treated 572-bp PCR fragment amplified using pUAB50 as template DNA into KpnI- and HindIII-digested pBBR1MCS.
pUAB105, which harbors btrAΔT134-M226 with His6 at the 3′ end plus an additional 60 bp upstream of the start codon, was constructed by ligating a KpnI- and HindIII-treated 482-bp PCR fragment into KpnI- and HindIII-digested pBBR1MCS.
pUAB106, which harbors btrAΔL104-M226 with His6 at the 3′ end plus an additional 60 bp upstream of the start codon, was constructed by ligating a KpnI- and HindIII-treated 392-bp PCR fragment amplified using pUAB50 as template DNA into KpnI- and HindIII-digested pBBR1MCS.
pUAB127 and pUAB152, which harbor btrAΔQ194-M226 fused to GAL4-BD or GAL4-AD coding sequences, respectively, were constructed by ligating EcoRI- and BamHI-treated 588-bp PCR fragments into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB128 and pUAB153, which harbor btrAΔT164-M226 fused to GAL4-BD or GAL4-AD coding sequences, respectively, were constructed by ligating EcoRI- and BamHI-treated 498-bp PCR fragments into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB129 and pUAB154, which harbor btrAΔT134-M226 fused to GAL4-BD or GAL4-AD coding sequences, respectively, were constructed by ligating EcoRI- and BamHI-treated 408-bp PCR fragments into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB130 and pUAB155, which harbor btrAΔL104-M226 fused to GAL4-BD or GAL4-AD coding sequences, respectively, were constructed by ligating EcoRI- and BamHI-treated 498-bp PCR fragments into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB146, which harbors a B. pertussis ∆btrA deletion allele with 5′ and 3′ flanking sequences, was constructed by PCR amplifying a 548-bp fragment that includes sequences upstream of btrA using primers del-BP2233-SacI-F1 and del-BP2233-ovlp-R2 with chromosomal DNA prepared from Bp536. A second fragment, consisting of 484 bp downstream of btrA was amplified from Bp536 chromosomal DNA using del-BP2233-ovlp-F2 and del-BP2233-BamHI-R1. Upstream and downstream PCR products were used for overlap PCR amplification with del-BP2233-SacI-F1 and del-BP2233-BamHI-R1 primers. The 984-bp product containing 5′ and 3′ sequences flanking the ∆btrA deletion was digested with SacI and BamHI before ligation into SacI- and BamHI-digested pSS4245.
pUAB163, which harbors a B. pertussis ∆btrA deletion allele with 5′ and 3′ flanking sequences, was constructed by PCR amplifying a 548-bp fragment that includes sequences upstream of btrA using primers del-BP2233-EcoR1-F1 and del-BB2233-ovlp-R2 with chromosomal DNA prepared from Bp536. A second fragment, consisting of 484 bp downstream of btrA was amplified from Bp536 chromosomal DNA using del-bb2233-ovlp-F2 and del-bb2233 HindIII R1. Upstream and downstream PCR products were used for overlap PCR amplification with del-BP2233-EcoR1-F1 and del-bb2233 HindIII R1 primers. The 984-bp product containing 5′ and 3′ sequences flanking the ∆btrA deletion was digested with EcoRI and HindIII before ligation into EcoRI- and HindIII-digested pSORTP1.
pUAB181, which harbors a ∆btrA internal deletion allele (ΔA101-V133) tagged with His6 at the 3′ end, was constructed by PCR amplifying a 392-bp fragment upstream of the deletion using primers BB1639upstream KpnI and 181-ovlp-R. A second fragment consisting of 336 bp downstream of the deletion was amplified using 181-ovlp-F and BB1639c 6XHis HindIII. Upstream and downstream PCR products were used for overlap PCR amplification with primers BB1639upstream KpnI and BB1639c 6XHis HindIII. The 672-bp product containing the internal deletion was digested with KpnI and HindIII before ligation into KpnI- and HindIII-digested pBBR1MCS.
pUAB182, which harbors a ∆btrA internal deletion allele (ΔT134-D163) tagged with His6 at the 3′ end, was constructed by PCR amplifying a 494-bp fragment upstream of the deletion using primers BB1639upstream KpnI and 182-ovlp-R. A second fragment consisting of 244 bp downstream of the deletion was amplified using 182-ovlp-F and BB1639c 6XHis HindIII. Upstream and downstream PCR products were used for overlap PCR amplification with primers BB1639upstream KpnI and BB1639c 6XHis HindIII. The 681-bp product containing the internal deletion was digested with KpnI and HindIII before ligation into KpnI- and HindIII-digested pBBR1MCS.
pUAB183, which harbors a ∆btrA internal deletion allele (ΔT164-M193) tagged with His6 at the 3′ end, was constructed by PCR amplifying a 585-bp fragment upstream of the deletion using primers BB1639upstream KpnI and 183-ovlp-R. A second fragment consisting of 151 bp downstream of the deletion was amplified using 183-ovlp-F and BB1639c 6XHis HindIII. Upstream and downstream PCR products were used for overlap PCR amplification with primers BB1639upstream KpnI and BB1639c 6XHis HindIII. The 681-bp product containing the internal deletion was digested with KpnI and HindIII before ligation into KpnI- and HindIII-digested pBBR1MCS.
pUAB185, which harbors a ∆bteA deletion allele with 5′ and 3′ flanking sequences, was constructed by PCR amplifying a 550-bp fragment upstream of bteA using primers del-BP0500-EcoR1-F1 and del-BP0500-ovlp-R2. A second fragment consisting of 545 bp downstream of bteA was amplified using del-BP0500-ovlp-F2 and del-BP0500-HindIII-R1. Upstream and downstream PCR products were used for overlap PCR amplification with primers del-BP0500-EcoR1-F1 and del-BP0500-HindIII-R1. The 1,045-bp product containing the deletion fragment with 5′ and 3′ flanking sequences was digested with EcoRI and HindIII before ligation into EcoRI- and HindIII-digested pSORTP1.
pUAB186 and pUAB190, which harbor the btrAΔA101-V133 allele fused to GAL4-BD or GAL4-AD sequences, respectively, were constructed by ligating an EcoRI- and BamHI-treated 600-bp PCR fragment amplified using DNA from plasmid pUAB181, into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB187 and pUAB191, which harbor the btrAΔT134-D163 allele fused to GAL4-BD or GAL4-AD sequences, respectively, were constructed by ligating an EcoRI- and BamHI-treated 609-bp PCR fragment amplified using DNA from plasmid pUAB182, into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB188 and pUAB192, which harbor the btrAΔT164-M193 allele fused to GAL4-BD or GAL4-AD sequences, respectively, were constructed by ligating an EcoRI- and BamHI-treated 609-bp PCR fragment amplified using DNA from plasmid pUAB183, into EcoRI- and BamHI-digested pGBKT7 or pGADT7 AD, respectively.
pUAB198, which harbors a B. pertussis ∆bscN deletion allele with 5′ and 3′ flanking sequences, was constructed by PCR amplifying a fragment containing 548 bp upstream of bscN using primers del-BP2245-EcoR1-F1 and del-BP2245-ovlp-R2. A second fragment consisting of 615 bp downstream of bscN was amplified using del-BP2245-ovlp-F2 and del-BP2245-HindIII-R1. Upstream and downstream PCR products were used for overlap PCR amplification with primers del-BP2245-EcoR1-F1 and del-BP2245 HindIII R1. The 1,105-bp product containing the deletion fragment with 5′ and 3′ flanking sequences was digested with EcoRI and HindIII before ligation into EcoRI- and HindIII-digested pSORTP1.
SDS/PAGE and Immunoblotting.
For SDS-polyacrylamide gel electrophoresis (SDS/PAGE) sample preparation, bacteria were grown in SS media and harvested by centrifugation at 10,000 × g at 4 °C for 10 min. Supernatants containing secreted proteins were filtered through 0.2-µm membranes and then precipitated with 15% (vol/vol) trichloroacetic acid (TCA) for 1 h on ice. Samples were centrifuged at 15,000 × g for 15 min at 4 °C, TCA was removed, and pellets were resuspended in 1× SDS-loading dye with 25 mM freshly prepared DTT. To neutralize the acidic pH of the samples, a few crystals of Tris-base were added. Protein pellets (normalized to culture OD600) were dissolved by shaking for 30 min at room temperature, or boiled for 10 min, before fractionation on various fixed percentage or gradient precast SDS-polyacrylamide gels (Bio-Rad). After electrophoresis, proteins were stained with Coomassie or transferred onto poly(vinylidene difluoride) (PVDF) membranes (Immobilon P; EMD Millipore) using a semidry blot apparatus. BvgA and BvgS were detected using polyclonal mouse sera at dilutions of 1:1,000 (43). Pertactin (Prn) was probed using monoclonal mouse antibody at a dilution of 1:1,000 (46). Bsp22 was detected using polyclonal mouse serum at a dilution of 1:10,000 (25). HA-tags were detected using monoclonal mouse (HA.11) antibody at a dilution of 1:5,000 (Covance). CyaA was probed using monoclonal mouse antibody (9D4) at a dilution of 1:1,000 (Santa Cruz Biotechnology). FHA (FhaB) was probed using polyclonal rabbit serum at a dilution of 1:10,000 (12). Immunodetection was carried out by chemifluorescence using horseradish peroxidase-labeled goat anti-mouse IgG and the ECL plus detection substrate (GE Healthcare). Chemifluorescent signals were visualized using a Typhoon scanner (GE Healthcare).
Allelic Exchange.
Unmarked, in-frame deletions were introduced into btrA in B. bronchiseptica RB50 and derivatives by allelic exchange as described by Yuk et al. (14), using the pRE112-based suicide plasmid pUAB60, and btrA-HA (hemagglutinin epitope tagged) strains were constructed using suicide plasmid pUAB80 (Table S1). Unmarked, in-frame deletions were introduced into B. pertussis Bp536 by allelic exchange as described (44), using pUAB163, pUAB185, or pUAB198 to exchange ∆btrA, ∆bteA, or ∆bscN mutations, respectively. btrA deletion in clinical B. pertussis isolates was introduced by allelic exchange using the pSS4245-based plasmid pUAB146 as described by Inatsuka et al. (47).
Yeast Two-Hybrid Analysis.
Bait vector pGBKT7 and prey vector pGADT7 AD were used for yeast two-hybrid analysis using the Clontech Matchmaker Gold Yeast 2-Hybrid system (Clontech). Full-length or truncated btrA or full-length btrS coding sequences were cloned into the EcoRI and BamHI sites, or NdeI and BamHI sites, respectively, of pGBKT7 and pGADT7 AD. pGBKT7-p53, which encodes the GAL4 DNA-BD fused with murine p53, and pGADT7 AD-SV40, which encodes the GAL4 AD fused with SV40 large T-antigen, were used as positive control bait and prey plasmids, respectively. pGADT7 AD (empty vector) was used as a negative control prey plasmid. pGADT7 AD- and pGBKT7-derived plasmids were transformed into Y187 and Y2HGold yeast strains, respectively (Clontech Yeastmaker Yeast Transformation System 2). Transformants were selected on Leu− or Trp− minimal media plates after growth at 30 °C for 3–5 d. Prey strains were mated with bait strains to generate diploid yeast cells, which were selected on Leu−Trp− minimal media plates (DDO) and then patched onto Leu−Trp− minimal media plates with X-α-Galactosidase (40 μg/mL) and Aureobasidin A (125 ng/mL) (DDO X/A). Blue diploid cells appearing after 24–72 h at 30 °C indicated positive binding interactions between bait and prey fusion proteins. To confirm results, diploid cells were patched onto higher stringency His−Ade−Leu−Trp− minimal media plates supplemented with X-α-Galactosidase (40 μg/mL) and Aureobasidin A (125 ng/mL) (QDO X/A) (48, 49).
Cell Lines.
Cell lines were obtained from the American Type Culture Collection (ATCC). Human cervical adenocarcinoma, HeLa (ATCC CCL-2) cells were maintained in Dulbecco's Modified Eagle Medium; human lung carcinoma, A-549 (ATCC CCL-185) cells were maintained in Ham’s F-12K medium (F-12K) supplemented with 10% FBS at 37 °C with 5% CO2.
Cytotoxicity Assays.
Bordetella were grown in SS media overnight and then subcultured in SS media to an OD600 of ∼0.5. Bacteria were added to semiconfluent cell monolayers in 12-well tissue culture plates, which were centrifuged for 5 min at 60 × g and incubated at 37 °C with 5% CO2. Lactate dehydrogenase (LDH) release into culture media, measured using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega), was used as a surrogate marker for cell death. All assays were conducted in triplicate in at least three independent experiments.
Respiratory Infection Models.
WT female C57BL/6NCr (B6) mice, 4–6 wk of age, were purchased from Charles River Breeding Laboratories. Animals were lightly sedated with isoflurane (Novation Laboratories) before intranasal inoculation with 5 × 105 cfu of RB50 or RB50∆btrA in a total volume of 40 µL of PBS (Mediatech Inc.). Bacteria were cultured overnight in SS media and then subcultured in SS media to an OD600 of ∼0.5 before diluting into PBS, and inocula were confirmed by plating. To measure colonization levels, mice were killed at indicated time points, and bacterial numbers in the lungs and tracheas were quantified by plating dilutions of tissue homogenates on BG plates with antibiotics, after incubation at 37 °C for 2 d. The mean ± SE was determined for each group, and the statistical significance between groups was calculated by Student's two-tailed t test. A significance level was set at P ≤ 0.05. For survival curves, groups of 12 mice were inoculated with 7.5 × 106 cfu, and the percent survival was monitored over a 30-d period. Mice with lethal bordetellosis, indicated by ruffled fur, labored breathing, and diminished responsiveness, were euthanized to alleviate unnecessary suffering (31). The statistical significance between the two groups was calculated by log-rank test. All mice were maintained in UCLA animal research facilities according to National Institutes of Health and University of California Institutional Animal Care Committee guidelines, and were housed under specific pathogen-free conditions with free access to food and water. All experiments were approved by the UCLA Chancellor's Animal Research Committee.
Total RNA Extraction, RT-PCR, and Quantitative RT-PCR.
Total RNA was extracted from midlog phase bacterial cultures (∼2 × 109 cells) using the RiboPure-Bacteria Kit (Ambion, Life Technologies). After high-speed pulse centrifugation at 4 °C, bacterial pellets were flash-frozen in dry ice-ethanol slurry and kept at −80 °C until RNA extraction. All RNA samples were assessed for quality by agarose gel electrophoresis, and mRNA integrity was determined using a Bioanalyzer 2100 (Agilent Technologies). RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific), and the absence of genomic DNA contamination was confirmed by PCR.
RNA was reverse transcribed into cDNA using 1 µg of RNA, 200 ng of random hexamers, and SuperScript III (Invitrogen). PCR was performed according to the manufacturer's instructions (0.5 U of iproof polymerase, 200 µM each of the four dNTPs, and 1 µM each primer) and supplemented with 3% dimethyl sulphoxide (DMSO). Primer pairs for measuring gene expression are listed in Table S3. Cycling parameters were as follows: one cycle of 99 °C for 1 min, 25–27 cycles of 98 °C for 10 s, 55 °C or 60 °C for 20 s, 72 °C for 30 s, and a final incubation at 72 °C for 5 min. Quantitative RT-PCR (qRT-PCR) measurements were conducted using an iCycler iQ Real-Time PCR detection system (Bio-Rad) unless otherwise indicated. The iQ SYBR Green Supermix kit from Bio-Rad was used for all qRT-PCRs according to the manufacturer's instructions, with 10% of the total cDNA as template, primers at 300 nM each, and supplemented with 5% DMSO. Primers were designed per the manufacturer's guidelines to generate amplicons of 100–150 bp. PCR conditions were 3 min at 95 °C, followed by 40 cycles of 45 s at 60 °C. Melt-curve analysis was performed immediately after the amplification protocol under the following conditions: 1 min denaturation at 95 °C, 1 min annealing at 55 °C, 80 cycles of 0.5 °C increments (10 s each) beginning at 55 °C (data collection step). The critical threshold (CT, the first cycle in which fluorescence is determined to be above background with statistical significance) was determined and normalized to the signal output for the control gene recA for each cDNA sample. All real-time PCRs were performed in duplicate in three independent experiments with mean values and SDs calculated. The ΔCT method was used to evaluate changes in gene expression (Figs. 1 and 2), and the ΔΔCT method (50) was used to normalize gene expression levels to WT Bp536 carrying an empty vector (Fig. 5).
Quantitative RT-PCR Using Fluidigm.
Preamplified cDNA was diluted 1:5 with TE buffer and used for real-time PCR. Gene expression was analyzed using a BioMark 48·48 Dynamic Array (Fluidigm). The PCR profile included a 10 min hot start at 95 °C, followed by 40 cycles of a two-step program: 15 s at 95 °C (denaturation) and 60 s at 60 °C (annealing and extension). Data were analyzed using BioMark Real-Time PCR Analysis Software v2.0 (Fluidigm). The statistical significance of differences in values between indicated groups was determined using t tests. Differences were considered statistically significant if P < 0.05. Data are expressed as mean ± SEM. Gene expression data in Fig. 2B and Fig. 5A were obtained using this method.
Ribosomal RNA Depletion.
Before RNA-Seq, ribosomal RNA (rRNA) depletion was performed twice on 8–10 µg of isolated total RNA using magnetic beads (MICROBExpress; Ambion) according to the manufacturer's protocol.
RNA-Seq Library Preparation.
Libraries for Illumina sequencing were prepared with the TruSeq RNA sample preparation kit version 2.0 rev. A (Illumina Inc.) according to the manufacturer's high sample protocol. Bypassing the initial poly(A) selection steps, 9.5 μL of enriched mRNA (350-500 ng, rRNA-depleted) was mixed with 9.5 μL of 2xEPF buffer before cDNA priming/library fragmentation. All cDNA libraries were uniquely indexed and were generated from two biological replicates per group. Libraries were constructed at the UCLA Neuroscience Genomics Core.
Illumina Sequencing and Data Analysis Pipeline.
cDNA libraries were sequenced using an Illumina HiSeq 2000 system (Illumina Inc.) according to the manufacturer's protocols at the UCLA Neuroscience Genomics Core. All samples were multiplexed on two lanes of the flow cell. Sequencing mode (single or paired-end) and read lengths for each experiment are listed in Table S4. Raw RNA-seq reads were processed with Illumina quality control tools using default settings. Filtered short reads were aligned using Burrows–Wheeler Aligner (51) to the B. bronchiseptica RB50 reference genome (RefSeq version NC_002729), with default parameters (<5% of read length or four mismatches allowed per 100-bp read). Additional quality control was performed after the alignment to examine the level of read duplication, mismatch rate, mapping rate to the whole genome, repeats, and key transcriptomic regions. Next, rRNA-specific reads were filtered from uniquely aligned reads from known gene regions for differential gene analysis. The uniquely mapped read data output was processed using custom scripts in Perl and R, and then normalized using the Bioconductor package EdgeR ver. 2.2.5 using the trimmed mean of M-values method (52) whereby scale factors between samples are estimated and used for the statistical analysis. The percentage of covered transcriptome was determined using BEDTools (53). Gene expression was measured as total read counts per gene, with the number of reads aligned to a given gene normalized using EdgeR (52). Normalized expression values were calculated as reads per kilobase per million mapped reads (RPKM) (54). In our study, differentially expressed genes with a false discovery rate less than 0.01 were considered significant (55). The gene cluster analysis was performed by applying k-means clustering to mean-centered log2 transformed (RPKM) expression values. The selection of genes for different clusters was based on expression difference of at least threefold between the isogenic strains included in the analysis.
Supplementary Material
Acknowledgments
We thank all members of the J.F.M. laboratory, especially Atish Ganguly and Diego Arambula, for helpful discussions and feedback. We thank Maria L. Tondella (Centers for Disease Control and Prevention) for B. pertussis clinical isolates, and Joe De Young (UCLA Neuroscience Genomics Core) for next-generation sequencing services. We also thank Prabhat Purbey for help with RNA-seq and Baochen Shi for help with NCBI data submission. This work was supported by the M. Philip Davis endowment and by NIH Grant R01 AI061598 (to J.F.M.). The UCLA NINDS Informatics Center for Neurogenetics and Neurogenomics is supported by NIH Grant P30 NS062691.
Footnotes
The authors declare no conflict of interest.
Data deposition: RNA-seq data reported in this paper have been deposited in the Sequence Read Archive, www.ncbi.nlm.nih.gov/sra (submission no. SRP064665; National Center for Biotechnology Information BioProject no. PRJNA296526).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600320113/-/DCSupplemental.
References
- 1.Preston A, Parkhill J, Maskell DJ. The bordetellae: Lessons from genomics. Nat Rev Microbiol. 2004;2(5):379–390. doi: 10.1038/nrmicro886. [DOI] [PubMed] [Google Scholar]
- 2.Diavatopoulos DA, et al. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005;1(4):e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: Current and future challenges. Nat Rev Microbiol. 2014;12(4):274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings CA, Bootsma HJ, Relman DA, Miller JF. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol. 2006;188(5):1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aricó B, et al. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhl MA, Miller JF. Integration of multiple domains in a two-component sensor protein: The Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15(5):1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter PA, Miller JF. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol. 1997;24(4):671–685. doi: 10.1046/j.1365-2958.1997.3821741.x. [DOI] [PubMed] [Google Scholar]
- 8.Cotter PA, Jones AM. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 2003;11(8):367–373. doi: 10.1016/s0966-842x(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 9.Carbonetti NH. Contribution of pertussis toxin to the pathogenesis of pertussis disease. Pathog Dis. 2015;73(8):ftv073. doi: 10.1093/femspd/ftv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eby JC, et al. Quantification of the adenylate cyclase toxin of Bordetella pertussis in vitro and during respiratory infection. Infect Immun. 2013;81(5):1390–1398. doi: 10.1128/IAI.00110-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masin J, Osicka R, Bumba L, Sebo P. Bordetella adenylate cyclase toxin: A unique combination of a pore-forming moiety with a cell-invading adenylate cyclase enzyme. Pathog Dis. 2015;73(8):ftv075. doi: 10.1093/femspd/ftv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inatsuka CS, Julio SM, Cotter PA. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc Natl Acad Sci USA. 2005;102(51):18578–18583. doi: 10.1073/pnas.0507910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willems RJ, van der Heide HG, Mooi FR. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992;6(18):2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuk MH, Harvill ET, Miller JF. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28(5):945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]
- 15.Panina EM, et al. A genome-wide screen identifies a Bordetella type III secretion effector and candidate effectors in other species. Mol Microbiol. 2005;58(1):267–279. doi: 10.1111/j.1365-2958.2005.04823.x. [DOI] [PubMed] [Google Scholar]
- 16.French CT, et al. The Bordetella type III secretion system effector BteA contains a conserved N-terminal motif that guides bacterial virulence factors to lipid rafts. Cell Microbiol. 2009;11(12):1735–1749. doi: 10.1111/j.1462-5822.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akerley BJ, Cotter PA, Miller JF. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80(4):611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 18.Merkel TJ, Stibitz S. Identification of a locus required for the regulation of bvg-repressed genes in Bordetella pertussis. J Bacteriol. 1995;177(10):2727–2736. doi: 10.1128/jb.177.10.2727-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones AM, Boucher PE, Williams CL, Stibitz S, Cotter PA. Role of BvgA phosphorylation and DNA binding affinity in control of Bvg-mediated phenotypic phase transition in Bordetella pertussis. Mol Microbiol. 2005;58(3):700–713. doi: 10.1111/j.1365-2958.2005.04875.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahuja U, et al. Phenotypic and genomic analysis of hypervirulent human-associated Bordetella bronchiseptica. BMC Microbiol. 2012;12:167. doi: 10.1186/1471-2180-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuk MH, Harvill ET, Cotter PA, Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol. 2000;35(5):991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- 22.Skinner JA, Pilione MR, Shen H, Harvill ET, Yuk MH. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J Immunol. 2005;175(7):4647–4652. doi: 10.4049/jimmunol.175.7.4647. [DOI] [PubMed] [Google Scholar]
- 23.Martínez de Tejada G, Miller JF, Cotter PA. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol. 1996;22(5):895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- 24.Fennelly NK, et al. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect Immun. 2008;76(3):1257–1266. doi: 10.1128/IAI.00836-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattoo S, Yuk MH, Huang LL, Miller JF. Regulation of type III secretion in Bordetella. Mol Microbiol. 2004;52(4):1201–1214. doi: 10.1111/j.1365-2958.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 26.Medhekar B, Shrivastava R, Mattoo S, Gingery M, Miller JF. Bordetella Bsp22 forms a filamentous type III secretion system tip complex and is immunoprotective in vitro and in vivo. Mol Microbiol. 2009;71(2):492–504. doi: 10.1111/j.1365-2958.2008.06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak NA, Mattoo S, Foreman-Wykert AK, Whitelegge JP, Miller JF. Interactions between partner switcher orthologs BtrW and BtrV regulate type III secretion in Bordetella. J Bacteriol. 2005;187(16):5665–5676. doi: 10.1128/JB.187.16.5665-5676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurushima J, Kuwae A, Abe A. The type III secreted protein BspR regulates the virulence genes in Bordetella bronchiseptica. PLoS One. 2012;7(6):e38925. doi: 10.1371/journal.pone.0038925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotto JW, et al. Biochemical and immunological properties of two forms of pertactin, the 69,000-molecular-weight outer membrane protein of Bordetella pertussis. Infect Immun. 1993;61(5):2211–2215. doi: 10.1128/iai.61.5.2211-2215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockbauer KE, Foreman-Wykert AK, Miller JF. Bordetella type III secretion induces caspase 1-independent necrosis. Cell Microbiol. 2003;5(2):123–132. doi: 10.1046/j.1462-5822.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 31.Harvill ET, Cotter PA, Miller JF. Pregenomic comparative analysis between bordetella bronchiseptica RB50 and Bordetella pertussis tohama I in murine models of respiratory tract infection. Infect Immun. 1999;67(11):6109–6118. doi: 10.1128/iai.67.11.6109-6118.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMillan DJ, Shojaei M, Chhatwal GS, Guzmán CA, Walker MJ. Molecular analysis of the bvg-repressed urease of Bordetella bronchiseptica. Microb Pathog. 1996;21(5):379–394. doi: 10.1006/mpat.1996.0069. [DOI] [PubMed] [Google Scholar]
- 33.Legarda D, Klein-Patel ME, Yim S, Yuk MH, Diamond G. Suppression of NF-kappaB-mediated beta-defensin gene expression in the mammalian airway by the Bordetella type III secretion system. Cell Microbiol. 2005;7(4):489–497. doi: 10.1111/j.1462-5822.2004.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe A, Nishimura R, Tanaka N, Kurushima J, Kuwae A. The Bordetella Secreted Regulator BspR is translocated into the nucleus of host cells via Its N-terminal moiety: Evaluation of bacterial effector translocation by the Escherichia coli type III secretion system. PLoS One. 2015;10(8):e0135140. doi: 10.1371/journal.pone.0135140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes KT, Gillen KL, Semon MJ, Karlinsey JE. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262(5137):1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 37.Kutsukake K. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243(6):605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 38.Rietsch A, Vallet-Gely I, Dove SL, Mekalanos JJ. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2005;102(22):8006–8011. doi: 10.1073/pnas.0503005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng LW, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182(11):3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botteaux A, Sory MP, Biskri L, Parsot C, Allaoui A. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol Microbiol. 2009;71(2):449–460. doi: 10.1111/j.1365-2958.2008.06537.x. [DOI] [PubMed] [Google Scholar]
- 41.Kodama T, et al. Transcription of Vibrio parahaemolyticus T3SS1 genes is regulated by a dual regulation system consisting of the ExsACDE regulatory cascade and H-NS. FEMS Microbiol Lett. 2010;311(1):10–17. doi: 10.1111/j.1574-6968.2010.02066.x. [DOI] [PubMed] [Google Scholar]
- 42.Akerley BJ, Monack DM, Falkow S, Miller JF. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol. 1992;174(3):980–990. doi: 10.1128/jb.174.3.980-990.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stibitz S, Yang MS. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173(14):4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stibitz S, Black W, Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- 45.Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 46.Brennan MJ, et al. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inatsuka CS, et al. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect Immun. 2010;78(7):2901–2909. doi: 10.1128/IAI.00188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 49.Chien CT, Bartel PL, Sternglanz R, Fields S. The two-hybrid system: A method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinlan AR, Hall IM. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 55.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.