There are certain human environmental perturbations so major that they are capable of destabilizing the earth’s normal function at a global scale (1). These so-called planetary boundary threats include climate change, ozone depletion, and ocean acidification. Emerging as a novel addition to this list is the vast quantity of discarded plastic waste that is accumulating in the oceans on an unprecedented scale, where it breaks down to form microscopic and nanoscopic fragments, or microplastics. Microplastics (particles with a diameter <1 mm, with no lower limit) derive from progressive fragmentation of larger plastic items, or may be manufactured to be of a small size, for use in personal care products, medicines, and industry (2). They reach the seas through beach littering, road runoff, sewage, and illegal dumping activities. Microplastics are ubiquitous in marine waters, from deep ocean sediments to polar icecaps, a result of the estimated 8 million tons of plastic that enters the oceans each year (3). Despite calls for plastic to be reclassified as hazardous (4), legislation to restrict marine debris accumulation is hindered by a lack of evidence that it causes ecological harm. In PNAS, Sussarellu et al. (5) provide an important starting point for assembling this evidence: Using an integrative approach, they show that ingestion of microplastics during gametogenesis has impacts on feeding and reproduction in oysters, with negative impacts on adult fecundity and offspring quality, both of which are key components of an organism’s individual fitness.
The results of Sussarellu et al. (5) are important because they support an emerging paradigm that microplastics can reduce reproductive output and fitness in marine species by altering their food consumption and energy allocation. Marine plastic debris is a global threat because of its abundance, persistence, and mobility across scales, with subsequent widespread distribution and potential geophysical and biological impacts (1). Compelling images of large marine species, such as birds and turtles, entangled in plastic debris are widespread (6), and many hundreds of marine species have been recorded to ingest plastic debris, leading to physical injury and death. As plastic polymers degrade to form microplastics, their impacts become more subtle. Microplastics are a cause for concern because their size range overlaps with the preferred particle size ingested by animals at the base of the marine food web. Detritus, suspension, and filter feeders can readily ingest them, leading to uptake and trophic transfer of the plastics themselves and any chemicals they contain or have absorbed from seawater. Many of these species are important to fisheries or perform vital ecosystem functions.
The impacts of plastic ingestion in laboratory studies include gut blockage and physical injury, oxidative stress, altered feeding behavior (7, 8), and reduced energy allocation (9), with knock-on effects for growth and reproduction (5). Transfer to tissues of plastics associated with chemicals, many of which possess endocrine-disrupting activity (10), adds to the potential toxicity of ingested particles through activation of signal transduction pathways relevant to hormone action.
Sussarellu et al. (5) studied oysters, a keystone species of high ecological and economic performance. In shallow coastal waters, oysters typically form reefs, filtering vast quantities of water and improving water quality and biodiversity. Adult oysters were exposed to microscopic polystyrene at environmentally relevant concentrations for 2 mo during a critical point in the reproductive cycle when adults were growing their gametes. Exposed oysters had altered rates of feeding and absorption efficiency from food and reduced fecundity (number of eggs produced), oocyte quality, and sperm swimming speed. Importantly, these impacts had clear carryover effects on offspring quality, measured as reduced growth in their larval progeny.
This reallocation of energy reserves from reproduction to maintenance, with resulting reductions in reproductive success, is a recurring theme emerging from chronic exposure studies with microplastics (6). Sediment-dwelling worms exposed to sediments contaminated with PVC microparticles had increased gut transit times and reduced lipid accumulation (8). Similarly, planktonic copepods exposed to micropolystyrene for prolonged periods had reduced food consumption, resulting in reduced reproductive output (9). They also showed a downward shift in their preference for algal prey, suggesting altered feeding behavior postcapture or postingestion.
The cultured oysters showed a high capacity to ingest micropolystyrene with surprisingly high efficiency, clearing up to 70% of the 6-μm beads supplied to each tank each day (roughly 9.6 mg/mL, or 100 beads per milliliter). Oysters in the wild are evidently capable of ingesting microplastics with similar efficiency. A recent study of oysters cultured in the northeast Atlantic Ocean being sold for human consumption found them to contain an average of 0.47 ± 0.16 microplastics per gram−1 wet weight of tissue, with the most abundant particles and fibers in the size range of 11–15 μm (29.6%) and 16–20 μm (33.3%) (11). Based on this finding, an average dietary portion of six oysters (100 g) would contain around 50 plastic particles. Even higher concentrations of microplastic fibers were reported in wild and farmed mussels (12), up to 178 fibers per farmed mussel, presumably due to the presence of ropes and aquaculture-related paraphernalia.
In Sussarellu et al.’s study (5), there was no apparent translocation of the 2- to 6-μM- diameter microplastics across the gut, although translocation of microplastics occurs in other bivalves (11, 13). In laboratory studies, early-life-stage oysters showed enhanced uptake of nanopolystyrene compared with micropolystyrene (14), which would tend to favor uptake across both gut barrier and cell membranes. However, detecting the uptake of nanoparticles in the wild remains beyond the limits of what is technically possible, despite recent advances (15).
Sussarellu et al. (5) found that stress responses were activated in exposed oyster digestive tissues, with dynamic energy budget models predicting diversion of energy allocation from reproductive output to structural growth and maintenance. There was reduced activity in the insulin pathway in gonadal tissues, suggesting that the typical mobilization of resources that accompanies gametogenesis was not occurring. Oysters are broadcast spawners, which release their eggs and sperm into seawater, where external fertilization occurs. Reduced sperm swimming speeds, together with smaller, fewer eggs, will reduce fertilization success (16). Studies of other stressors, such as ocean acidification, show that carryover effects in oyster larvae can persist through to later life, reducing settlement success, population growth, and productivity (17).
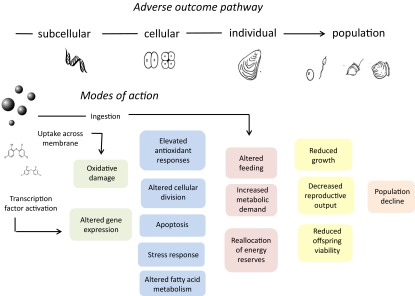
In Fig. 1, we have incorporated these results, supported by previous findings, within a tentative adverse outcome pathway (AOP) scheme. AOPs are extremely useful in deducing the key events linking an apical end point, such as reduced reproductive output, with a perturbation, such as particle ingestion, because they describe generalized motifs of biological response, or key events that are not necessarily specific to any one chemical or substance. For example, applying the AOP concept to growth retardation in fish allowed Groh et al. (18) to distinguish the mode of action of cadmium, which reduced growth through increased metabolic demand, from the mode of action of pyrethroid pesticides and selective serotonin reuptake inhibitors, which reduced food intake through changes in behavior and appetite (18). In relation to microplastics, the situation is further complicated by their potential to associate with chemical contaminants and the as-yet-unknown extent to which these absorbed contaminants are transferred from the ingested particle into the organism’s tissues.
Fig. 1.
Tentative AOP scheme for microplastics exposure of aquatic species showing potential pathways linking ingestion, uptake across membranes, and chemical release with adverse outcomes of growth inhibition and reproductive decline.
The wider implications of these findings relate to the similarity in mode of action between the microplastics themselves and the chemicals that are associated with them. Persistent, bioaccumulative, and toxic organic contaminants that associate with microplastics in the ocean include polychlorinated biphenyls, polyaromatic hydrocarbons, and polybrominated diphenylethers, all of which possess endocrine-disrupting activity (10). This list of contaminants includes a subgroup, termed obesogens, that enhance weight change by shifting energy balance in favor of fat storage in adipocytes and altering basic metabolic rate (19). Obesogenic effects are not limited to vertebrates. The biocide tributyltin (TBT) is a high-affinity ligand for the peroxisome proliferation-activated receptor gamma and its heterodimer partner retinoid receptor X, which regulate lipid metabolism in vertebrates. Water fleas exposed to TBT showed disrupted lipid metabolism, with reduced transfer of triacylglycerols from adults to eggs, prompting their accumulation in the adults. Similar to the microplastics-exposed oysters of Sussarellu et al. (5), the life history of the progeny of females exposed to TBT showed reduced fitness responses, lower survival, and production of fewer eggs (20). Thus, a situation could well arise where significant potentiation of the mode of action of microplastics and the contaminants they are associated with could occur.
Strategies for buffering marine biodiversity against global threats, such as climate change and ocean acidification, include reducing additional stressors, such as pollution and overfishing (21). Because plastic waste is one of the most prevalent of marine pollutants, reducing plastics input should be a high priority. Given the impossibility of removing all microplastics contamination from the oceans, the impetus is on all of us—governments, scientists, and individuals—to reduce our utterly ridiculous levels of plastics consumption and waste before we induce permanent alterations to our fragile marine ecosystem.
Footnotes
The authors declare no conflict of interest.
See companion article on page 2430.
References
- 1.Steffen W, et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science. 2015;347(6223):1259855. doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- 2.Andrady A. Persistence of plastic litter in the oceans. In: Bergmann M, Gutow L, Klages M, editors. Marine Anthropogenic Litter. Springer; Cham, Switzerland: 2015. pp. 57–72. [Google Scholar]
- 3.Jambeck JR, et al. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 4.Rochman CM, et al. Policy: Classify plastic waste as hazardous. Nature. 2013;494(7436):169–171. doi: 10.1038/494169a. [DOI] [PubMed] [Google Scholar]
- 5.Sussarellu R, et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci USA. 2016;113:2430–2435. doi: 10.1073/pnas.1519019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gall SC, Thompson RC. The impact of debris on marine life. Mar Pollut Bull. 2015;92(1-2):170–179. doi: 10.1016/j.marpolbul.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Watts AJR, Urbina MA, Corr S, Lewis C, Galloway TS. Ingestion of Plastic Microfibers by the Crab Carcinus maenas and Its Effect on Food Consumption and Energy Balance. Environ Sci Technol. 2015;49(24):14597–14604. doi: 10.1021/acs.est.5b04026. [DOI] [PubMed] [Google Scholar]
- 8.Wright SL, Rowe D, Thompson RC, Galloway TS. Microplastic ingestion decreases energy reserves in marine worms. Curr Biol. 2013;23(23):R1031–R1033. doi: 10.1016/j.cub.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 9.Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol. 2015;49(2):1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, et al. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cauwenberghe L, Janssen CR. Microplastics in bivalves cultured for human consumption. Environ Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Mathalon A, Hill P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar Pollut Bull. 2014;81(1):69–79. doi: 10.1016/j.marpolbul.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L) Environ Sci Technol. 2008;42(13):5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- 14.Cole M, Galloway TS. Ingestion of Nanoplastics and Microplastics by Pacific Oyster Larvae. Environ Sci Technol. 2015;49(24):14625–14632. doi: 10.1021/acs.est.5b04099. [DOI] [PubMed] [Google Scholar]
- 15.Palchoudhury S, Baalousha M, Lead J. Methods for measuring concentration (mass, surface area and number) of nanoparticles. In: Baalousha M, Lead J, editors. Characterization of Nanomaterials in Complex Environmental and Biological Media. 1st Ed. Elsevier; Amsterdam: 2016. pp. 153–177. [Google Scholar]
- 16.Levitan DR. Optimal egg size in marine invertebrates. Am Nat. 2000;156(2):175–192. doi: 10.1086/303376. [DOI] [PubMed] [Google Scholar]
- 17.Hettinger A, et al. Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology. 2012;93(12):2758–2768. doi: 10.1890/12-0567.1. [DOI] [PubMed] [Google Scholar]
- 18.Groh KJ, et al. Development and application of the adverse outcome pathway framework for understanding and predicting chronic toxicity. Chemosphere. 2015;120:764–777. doi: 10.1016/j.chemosphere.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 19.Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11(11):653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 20.Jordão R, et al. Obesogens beyond Vertebrates: Lipid Perturbation by Tributyltin in the Crustacean Daphnia magna. Environ Health Perspect. 2015;123(8):813–819. doi: 10.1289/ehp.1409163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghedini G, Russell BD, Connell SD. Managing local coastal stressors to reduce the ecological effects of ocean acidification and warming. Water. 2013;5(4):1653–1661. [Google Scholar]