Significance
Here, we report a previously unidentified alternative pathway mediated by mitogen-activated protein kinase kinase kinase 2 (MEKK2) for the activation of β-catenin in osteoblasts that is distinct from the classical glycogen synthase kinase 3β (GSK3β)-mediated degradation pathway. The GSK3β pathway regulates β-catenin stability via β-catenin ubiquitination, and its inhibition prevents β-catenin degradation. The MEKK2 pathway instead rescues ubiquitinated β-catenin from degradation via deubiquitination. This pathway plays a critical role in controlling bone mass in vivo, and targeting this pathway may offer an attractive approach for the therapeutic regulation of β-catenin activity.
Keywords: osteoblasts, beta-catenin, bone, MEKK2, MAPK
Abstract
Proper tuning of β-catenin activity in osteoblasts is required for bone homeostasis, because both increased and decreased β-catenin activity have pathologic consequences. In the classical pathway for β-catenin activation, stimulation with WNT ligands suppresses constitutive phosphorylation of β-catenin by glycogen synthase kinase 3β, preventing β-catenin ubiquitination and proteasomal degradation. Here, we have found that mitogen-activated protein kinase kinase kinase 2 (MAP3K2 or MEKK2) mediates an alternative pathway for β-catenin activation in osteoblasts that is distinct from the canonical WNT pathway. FGF2 activates MEKK2 to phosphorylate β-catenin at serine 675, promoting recruitment of the deubiquitinating enzyme, ubiquitin-specific peptidase 15 (USP15). USP15 in turn prevents the basal turnover of β-catenin by inhibiting its ubiquitin-dependent proteasomal degradation, thereby enhancing WNT signaling. Analysis of MEKK2-deficient mice and genetic interaction studies between Mekk2- and β-catenin–null alleles confirm that this pathway is an important physiologic regulator of bone mass in vivo. Thus, an FGF2/MEKK2 pathway mediates an alternative nonclassical pathway for β-catenin activation, and this pathway is a key regulator of bone formation by osteoblasts.
Tight control of the activity of the β-catenin transcription factor has emerged as a key determinant of osteoblast differentiation and activity. Removal of β-catenin in mature osteoblasts leads to osteopenia associated with increased osteoclast activity (1, 2). Similarly, deletion of β-catenin in the mesenchymal progenitors of osteoblasts leads to a substantial reduction in the mineralization of both intramembranous and endochondral bones (3, 4). At a cellular level, deletion of β-catenin activity reduces osteoblast mineralization capacity in vitro. Thus, maintaining β-catenin activity is critical for the development and maintenance of bone mass. However, increased β-catenin activity also has deleterious effects. Activating β-catenin in osteoblasts by conditional deletion of the third exon containing the inhibitory glycogen synthase kinase 3β (GSK3β) phosphorylation sites leads to both osteoblast-intrinsic osteopetrosis and the development of spontaneous acute myeloid leukemia resulting from altered hematopoietic niche function (1, 5). Taken together, these findings emphasize that β-catenin activity in osteoblasts must be tailored carefully to maintain homeostasis of skeletal and hematopoietic systems. However, how this tailoring is accomplished in osteoblasts has yet to be fully elucidated.
β-Catenin activity is regulated primarily by stimulation with a subset of the WNT family of ligands, the so-called “canonical” WNTs. Activation of these ligands leads to the suppression of a constitutively active destruction complex containing the adaptor proteins anaphase-promoting complex (APC) and Axin alongside the kinases GSK3β and casein kinase 1α (CK1α) (6). CK1α phosphorylates β-catenin S45, priming subsequent GSK3β phosphorylation of S33, S37, and T41. GSK3β-phosphorylated β-catenin is recognized by the F-box–containing ubiquitin E3 ligase β-transducin repeat-containing protein (β-TrCP), leading to its ubiquitination and rapid degradation by the 26S proteasome (7). Release of this constitutive degradation of β-catenin by WNT stimulation allows β-catenin accumulation in the nucleus and transcriptional activity.
Mitogen-activated protein kinase kinase kinase 2 (MAP3K2, MEKK2) is a member of the MEK kinase (MEKK) group of MAP3Ks, and early overexpression studies demonstrated that MEKK2 has the ability to activate a number of downstream MAPK pathways, including ERK1/2, JNK, p38, and ERK5 (8–11). However, mice with a germline deletion of MEKK2 display intact ERK, p38, and JNK activation in T cells (12). Instead, MEKK2 collaborates with the highly homologous MEKK3 to modulate responses to TGF-β by phosphorylating the regulatory linker sequences in mothers against decapentaplegic homolog 2 (SMAD2) and SMAD3 (13). In contrast to these findings in T cells, JNK activation was impaired in MEKK2-deficient mouse embryonic fibroblasts (MEFs) (14). Knockdown of MEKK2 in HEK293 cells attenuated the activation of ERK, but not of JNK or p38, in response to hyperosmotic stress (15). Thus, as has been observed previously for other MAP3Ks in osteoblasts, the pathways engaged by MEKK2 are highly context dependent and vary by both cell type and stimulus.
Here we present evidence that MEKK2 mediates an alternative pathway for the stabilization of β-catenin in osteoblasts. MEKK2-deficient mice show a significant decrease in both bone mass and osteoblast activity. Through an unbiased mass spectrometry-based affinity purification analysis, we found that β-catenin is a substrate of MEKK2, with MEKK2-mediated phosphorylation of β-catenin acting to stabilize β-catenin through the recruitment of the deubiquitinase (DUB) ubiquitin-specific peptidase 15 (USP15), which acts downstream of FGF2. Thus, MEKK2 mediates a previously unidentified β-catenin pathway that is a key determinant of bone formation in vivo.
Results
MEKK2-Deficient Mice Display Reduced Bone Mass and Impaired Osteoblast Activity.
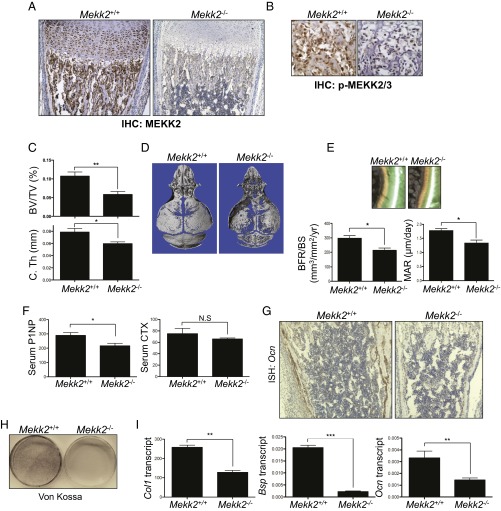
We initially identified MEKK2 as a positive regulator of osteoblast differentiation in a shRNA screen of ∼1,500 human kinases, phosphatases, and receptors using a human mesenchymal stromal cell (hMSC) system (16). To explore the contribution of MEKK2 to osteoblast biology, expression of active MEKK2 was examined in osteoblasts in vivo. Immunohistochemistry for MEKK2 and phospho-MEKK2 were performed, demonstrating that MEKK2 was both expressed and present in the active phosphorylated state in osteoblasts in vivo (Fig. 1 A and B). Ablation of MEKK2 and phospho-MEKK2 staining in Mekk2−/− femurs confirmed the specificity of the immunohistochemical stain. Next, the contribution of MEKK2 to the in vivo regulation of bone mass was assessed in WT and Mekk2−/− mice by micro-CT analysis (Fig. 1 C and D). Bone mass was reduced significantly in the long bones and calvaria of Mekk2−/− mice, suggesting that MEKK2 is important for both endochondral and intramembranous ossification. Accordingly, dynamic histomorphometry revealed a defect in osteoblast activity, as indicated by decreased mineral apposition rate (MAR) (Fig. 1E). The bone formation rate (BFR) also was reduced without a statistically significant alteration in osteoblast or osteoclast numbers (Fig. 1E and Fig. S1 A and B). Consistent with the reduction in BFR, serum levels of the marker of total osteoblast synthetic capacity, procollagen type I N-terminal peptide (P1NP), were reduced, whereas levels of crosslinked collagen type I C-terminal telopeptide (CTX), a marker of total osteoclast resorption activity, showed no significant difference (Fig. 1F). Similarly, levels of the secreted regulator of osteoclast differentiation, osteoprotegrin (OPG), were unchanged in Mekk2−/− mice (Fig. S1C). Moreover, expression of an osteoblast marker gene, osteocalcin (Ocn), was markedly decreased in vivo (Fig. 1G). Although MEKK2 is expressed in chondrocytes of the growth plate, significant alterations in growth plate morphology or in the growth of Mekk2−/− mice were not detected (Fig. 1A). Thus, MEKK2 regulates bone homeostasis in vivo by promoting osteoblast activity without affecting the development of osteoclasts and chondrocytes at a phenotypically detectable level.
Fig. 1.
MEKK2 promotes osteoblast activity in vivo and in vitro. (A and B) Immunohistochemistry for MEKK2 (A) and phospho-MEKK2/3 (B) at 40× and 100× magnification, respectively on 1-wk-old mouse tibias. (C) Quantification of BV/TV and cortical thickness (C.Th) in the femur of 4-wk-old Mekk2+/+ and Mekk2−/− mice. All error bars indicate SEM. P < 0.05 was considered statistically significant by two-tailed, unpaired Student’s t test. *P < 0.05 and **P < 0.01; n = 5 mice per group. (D) 3D reconstructions of calvaria of 4-wk-old Mekk2+/+ and Mekk2−/− mice. (E) Histomorphometric analysis of tibias from 8-wk-old Mekk2+/+ and Mekk2−/− mice; n = 5 mice per group. (Upper) Photomicrographs of dual-labeled trabecular bones. (Lower) Quantification of BFR and MAR. *P < 0.05. (F) Serum levels of P1NP and CTX in 8-wk-old Mekk2+/+ and Mekk2−/− mice; n = 5 mice per group. *P < 0.05; N.S, not significant. (G) In situ hybridization for Ocn on tibias from 1-wk-old Mekk2+/+ and Mekk2−/− mice. (Magnification: 40×.) (H and I) Primary calvarial osteoblasts were isolated and placed under differentiation conditions for 21 d. (H) Von Kossa staining was performed to determine osteoblast mineralization activity. (I) After 7 d of culture, the expression of osteoblast marker genes was analyzed by RT-PCR. **P < 0.01; ***P < 0.001.
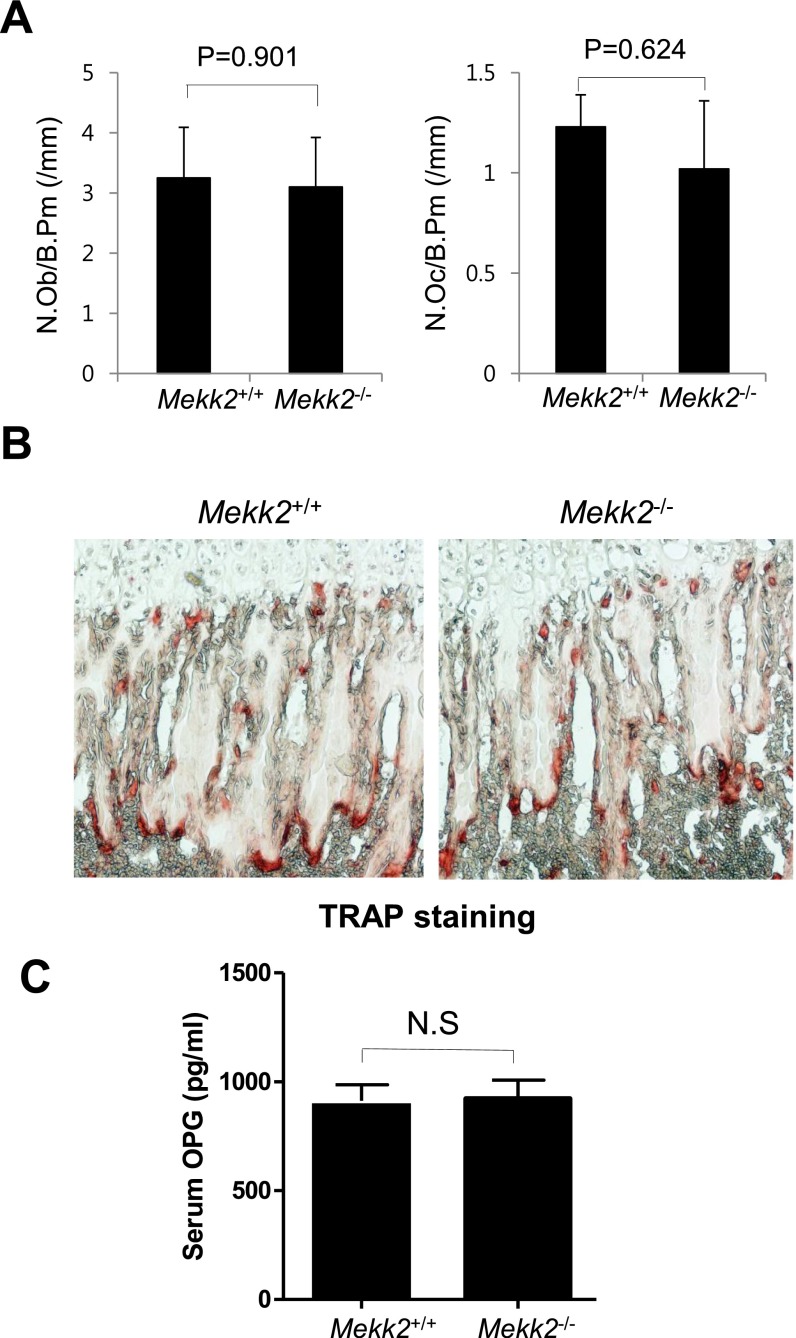
Fig. S1.
Characterization of the skeletal phenotype of Mekk2−/− mice. (A) Histomorphometric analysis of tibias from 8-wk-old Mekk2+/+ and Mekk2−/− mice. N.Ob/B.Pm, number of osteoblasts per bone perimeter. (B) TRAP staining was performed on femurs from 4-wk-old Mekk2+/+ and Mekk2−/− mice. (C) Serum was collected from 4-wk-old Mekk2+/+ and Mekk2−/− mice, and an ELISA was run to determine OPG levels. N.S, not significant.
To examine the role of MEKK2 in osteoblast differentiation in vitro, calvarial osteoblasts (COBs) were isolated from WT and Mekk2−/− pups and cultured under osteoblast differentiation conditions. As observed by Von Kossa staining, mineralization activity was reduced significantly in Mekk2−/− COBs (Fig. 1H), and Mekk2−/− COBs displayed a decrease in expression of osteoblast markers such as collagen type 1, α1 (Col1a1), bone sialoprotein (Bsp), and Ocn (Fig. 1I). Taken together with the osteoporotic phenotype of Mekk2−/− mice, these results indicate that MEKK2 is critical for osteoblast mineralization activity in vitro and in vivo.
MEKK2 Regulates β-Catenin Stability in Osteoblasts.
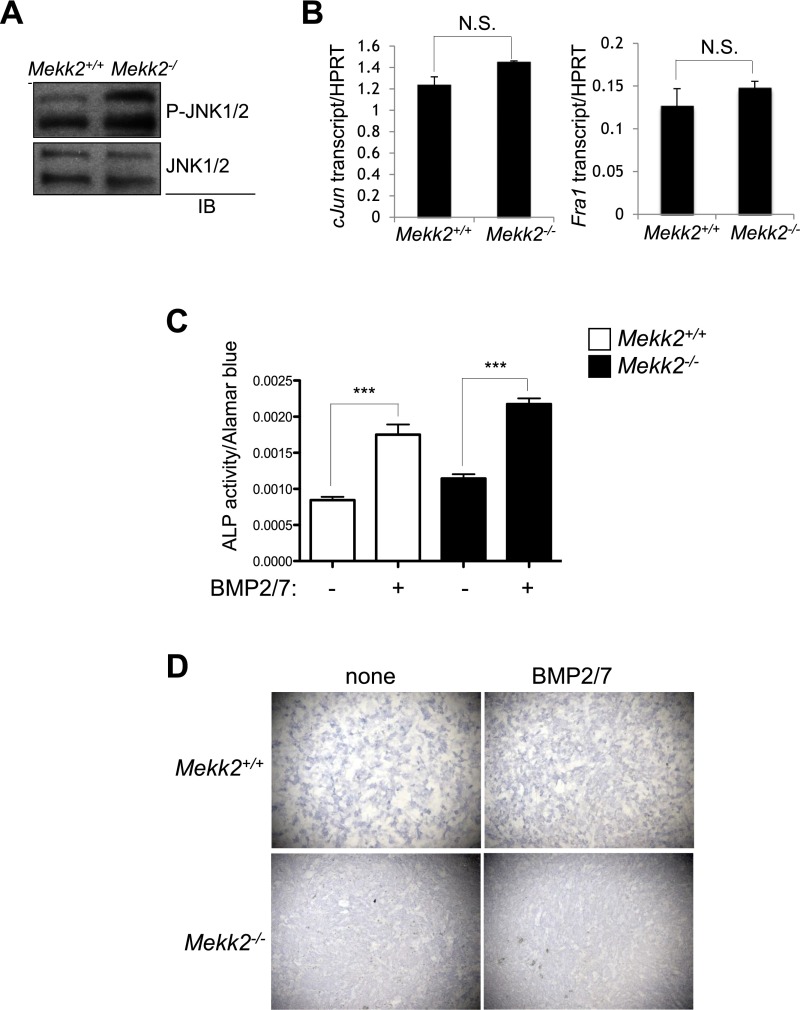
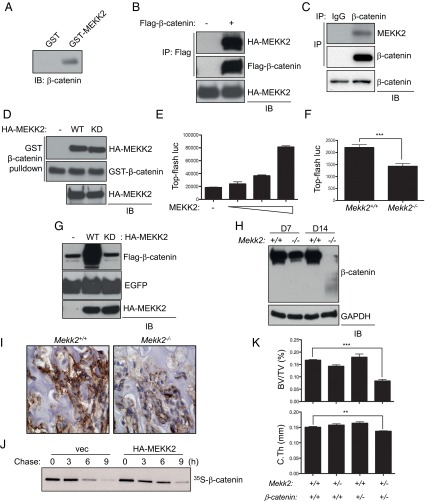
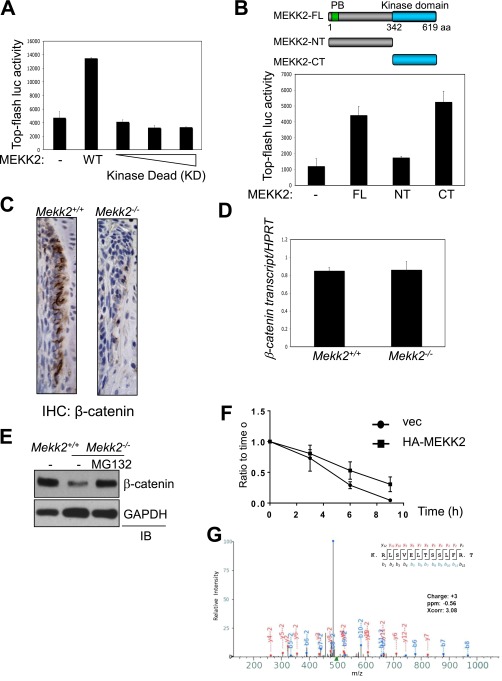
A previous study reported that the E3 ubiquitin ligase SMAD-specific E3 ubiquitin protein ligase 1 (SMURF1) suppresses osteoblast differentiation by downregulating MEKK2-mediated JNK MAPK activation (8, 17). However, in contrast to previous observations that RNAi-mediated knockdown of MEKK2 decreased JNK activation in osteoblasts (17), phosphorylation levels of JNK1 and -2 and the expression of well-studied transcriptional targets regulated by active JNK, Jun and Fos-related antigen 1 (Fra1) (18), were normal or slightly increased in Mekk2−/− COBs (Fig. S2 A and B). Additionally, although a SMURF1/MEKK2/JNK pathway was described as being critical for responses to bone morphogenetic protein (BMP) stimulation in osteoblasts in vitro (17), responses to BMPs were unaltered in Mekk2−/− COBs (Fig. S2 C and D). Thus, under the conditions examined, MEKK2 is dispensable for JNK activation in osteoblasts. Therefore we investigated alternative mechanisms by which MEKK2 controls osteoblast differentiation by identifying MEKK2-interacting proteins in mature osteoblasts. To investigate these mechanisms in a large-volume homogenous culture of osteoblasts with mineralization capacity, we used the ST2 osteoblast cell line stably expressing WNT 10b to drive osteoblast differentiation via activation of WNT signaling in this study (19). After lysates of WNT10b-expressing ST2 osteoblasts were incubated with GST-MEKK2, GST-MEKK2–binding proteins were immunoprecipitated with anti-GST antibody–conjugated agarose, and the eluates were subjected to mass spectrometry. This approach identified β-catenin, a key transcriptional regulator of osteoblast differentiation, as a putative binding partner of MEKK2 (Fig. 2A). This interaction was confirmed by coimmunoprecipitation both with exogenously expressed proteins in HEK293 cells and with endogenous proteins in immortalized COBs (Fig. 2 B and C). Moreover, this interaction occurs directly and does not require MEKK2 kinase activity, as evidenced by the coimmunoprecipitation of purified WT and kinase-dead (KD) MEKK2 with GST–β-catenin (Fig. 2D).
Fig. S2.
MEKK2 is dispensable for JNK activation and responses to BMPs in osteoblasts. (A) Primary COBs were isolated from Mekk2+/+ and Mekk2−/− pups, cultured under osteoblast differentiation conditions for 7 d, and immunoblotted with the indicated antibodies. (B) Alternatively, expression of Jun and Fra1 was analyzed by RT-PCR analysis. P = N.S., not significant. (C and D) Primary COBs were cultured under osteoblast differentiation conditions in the presence or absence of 100 ng/mL of BMP2/7 for 7 d, and BMP-induced osteoblast differentiation was determined by ALP activity (C) and ALP staining (D). In C, P = N.S. for all comparisons between Mekk2+/+ and corresponding Mekk2−/− samples. P values for two-way t tests of samples ± BMP2/7 are indicated: ***P < 0.001.
Fig. 2.
MEKK2 increases β-catenin stability. (A) GST or GST-MEKK2 was incubated with lysates of WNT10-expressing ST2 cells, immunoprecipitated with anti-GST antibody–conjugated agarose, and immunoblotted with an anti–β-catenin antibody. (B) HEK293 cells were transfected with vector or Flag–β-catenin along with HA-MEKK2, and lysates were immunoprecipitated with anti-Flag antibody–conjugated agarose and immunoblotted for the indicated antibodies. (C) COBs were lysed, immunoprecipitated with anti–β-catenin or an IgG control and protein G-conjugated Dynabeads, and immunoblotted with anti-MEKK2 antibody. (D) GST–β-catenin was incubated with purified HA-MEKK2 (WT or KD mutant), immunoprecipitated with anti-GST antibody–conjugated agarose, and immunoblotted with the indicated antibodies. Input indicates loading controls for purified HA-MEKK2. (E) C3H10T1/2 cells were transfected with different amounts of MEKK2 along with TOPflash-luciferase and Renilla. Luciferase activity was measured after 48 h of transfection and normalized to Renilla. P < 0.01 by one-way ANOVA. (F) Primary COBs isolated from Mekk2+/+ and Mekk2−/− mice were transfected with TOPflash luciferase and Renilla and were cultured under osteoblast differentiation conditions for 6 d. Luciferase activity was normalized to Renilla. ***P < 0.001. (G) HEK293 cells were transfected with vector or HA-MEKK2 (WT or KD mutant) along with Flag–β-catenin and EGFP, and lysates were immunoblotted with the indicated antibodies. EGFP was used as a transfection control. (H) Mekk2+/+ and Mekk2−/− COBs were cultured under osteoblast differentiation conditions for 7 or 14 d, and lysates were blotted with the indicated antibodies. (I) Immunohistochemistry for β-catenin on tibias from 1-wk-old Mekk2+/+ and Mekk2−/− mice. (J) HEK293 cells were transfected with vector or HA-MEKK2 along with Flag–β-catenin, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography. (K) Quantification of BV/TV and cortical thickness in the femur of the following strains: Mekk2+/+;Ctnnbfl/fl (+/+), Mekk2+/−;Ctnnbfl/fl (+/+), Mekk2+/+;Ctnnbfl/+;Prx1 (+/−), and Mekk2+/−;Ctnnbfl/+;Prx1 (+/−) mice. (n = 5 mice per group). In both the cortical thickness and BV/TV comparisons, P < 0.05 by two-way ANOVA for both the Mekk2 and Ctnnb groups and also for the interaction between these groups. **P < 0.01, ***P < 0.001, Bonferroni-corrected Student’s t tests.
To determine the significance of this interaction, the effect of MEKK2 on β-catenin transcriptional activity was analyzed using a β-catenin–responsive reporter gene. Overexpression of MEKK2 resulted in a dose-dependent increase in β-catenin activity (Fig. 2E), whereas β-catenin activity was reduced in primary Mekk2−/− COBs at 7 d of differentiation (Fig. 2F). This induction of β-catenin activity required MEKK2 kinase activity (Fig. S3A); a C-terminal fragment of MEKK2 containing the kinase domain was sufficient to increase β-catenin activity (Fig. S3B). The effect of MEKK2 on β-catenin transcriptional activity mirrored changes in β-catenin protein abundance, because overexpression of MEKK2 increased β-catenin protein levels, and this effect was ablated in a KD MEKK2 mutant (Fig. 2G). Likewise, in Mekk2−/− COBs, protein levels of β-catenin were markedly reduced at 7 d of differentiation and were nearly undetectable after 14 d of differentiation (Fig. 2H). This reduction in β-catenin levels also was observed in osteoblasts in Mekk2−/− long bones (Fig. 2I and Fig. S3C). The ability of MEKK2 to increase β-catenin levels resulted from posttranslational regulation of β-catenin stability, because β-catenin transcript levels were not altered by the absence of MEKK2 (Fig. S3D), and the low β-catenin levels in Mekk2−/− COBs could be rescued by blocking proteasomal degradation of β-catenin with the proteasome inhibitor MG132 (Fig. S3E). Next, a pulse-chase assay was performed to test directly the ability of MEKK2 to stabilize β-catenin (Fig. 2J and Fig. S3F). Enforced expression of MEKK2 increased the half-life of β-catenin from ∼4.5 h to 6 h, demonstrating that MEKK2 functions as a positive regulator of β-catenin stability. Last, we sought to confirm the in vivo significance of this effect by testing whether heterozygous Mekk2- and β-catenin–null alleles display a genetic interaction. Mice with either one null allele of Mekk2 (Mekk2+/−) or β-catenin (β-catenin+/−) or both (Mekk2+/−; β-catenin+/−) were bred, and bone mass was examined by micro-CT analysis (Fig. 2K). Although heterozygosity for either the β-catenin– or Mekk2-null allele alone was insufficient to render mice osteopenic, mice heterozygous for both genes developed an ∼50% reduction in trabecular bone mass. Thus, Mekk2- and β-catenin–null alleles display a positive epistatic interaction, supporting the notion that control of β-catenin stability by MEKK2 is critical for the regulation of bone mass in vivo.
Fig. S3.
MEKK2 regulates β-catenin activity and stability. (A) C3H10T1/2 cells were transfected with different amounts of MEKK2 (KD) or MEKK2 (WT) along with TOPflash-luciferase and Renilla. Luciferase activity was measured 48 h after transfection and normalized to Renilla. (B, Upper) A diagram showing the truncated mutants of MEKK2. (Lower) C3H10T1/2 cells were transfected with MEKK2 along with TOPflash-luciferase and Renilla. CT, C-terminal; FL, full length; NT, N-terminal. (C) Immunohistochemistry for β-catenin on tibias from 1-wk-old Mekk2+/+ and Mekk2−/− mice. (Magnification: 40×.) (D and E) Primary COBs were isolated from Mekk2+/+ and Mekk2−/− pups and were cultured under osteoblast differentiation conditions for 7 d. (D) Expression of β-catenin was analyzed by RT-PCR. (E) Cells were treated with 10 μM MG132 for 8 h and immunoblotted with anti–β-catenin antibody. (F) HEK293 cells were transfected with vector (vec) or HA-MEKK2 along with Flag–β-catenin, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography and ImageJ analysis. (G) Mass spectra data demonstrating identification of serine 675 as the site of MEKK2-mediated phosphorylation in β-catenin.
MEKK2 Stabilizes β-Catenin via Phosphorylation of S675.
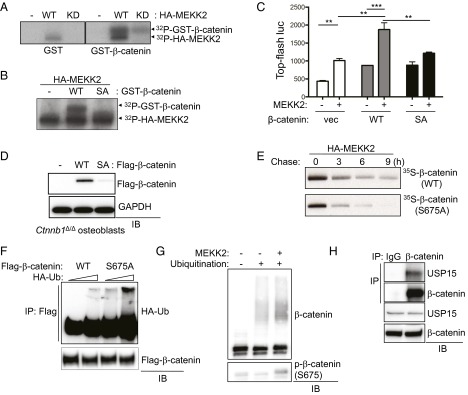
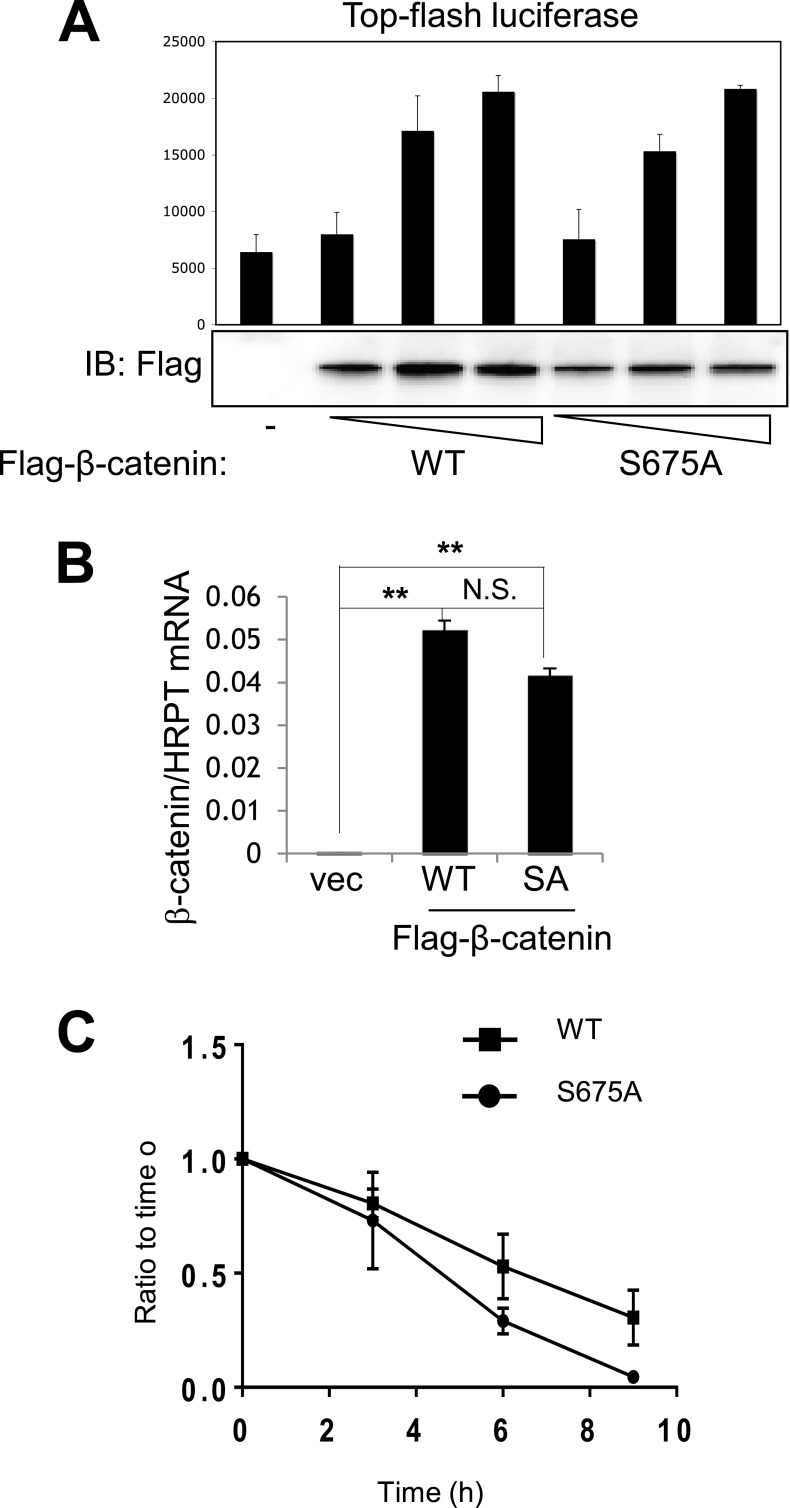
Because regulation of β-catenin stability requires MEKK2 kinase activity, we examined the ability of MEKK2 to phosphorylate β-catenin. An in vitro kinase assay using purified proteins revealed that MEKK2 phosphorylated GST–β-catenin and that this activity was greatly reduced in the MEKK2 with the kinase-inactive mutation (Fig. 3A). To identify the site of phosphorylation, phospho-mass spectrometry was performed using the coimmunoprecipitates of Flag–β-catenin and HA-MEKK2 (WT or KD mutant). This investigation identified serine 675 (human NP_001895.1 p.Ser675, mouse NP_031640.1 p.Ser675) as the site of phosphorylation by WT-MEKK2 but not by the KD mutant (Fig. S3G). β-Catenin bearing a mutation of serine 675 to alanine (S675A) was largely refractory both to MEKK2-mediated phosphorylation and to the MEKK2-induced increase in β-catenin transcriptional activity (Fig. 3 B and C). Next, after compensating for the reduced stability of β-catenin S675A, we assessed whether this construct had any further effect on β-catenin transcriptional activity. By greatly increasing the amount of input DNA for Flag–β-catenin S675A, largely equivalent levels of expression of WT and S675A β-catenin were achieved (Fig. S4A). When equivalent protein levels were present, the S675A mutant displayed transcriptional activity comparable to that of WT β-catenin. Thus, the effects of MEKK2-mediated S675 phosphorylation on β-catenin activity appear to be limited to protein stability. To assess the effect of the S675 phosphorylation on β-catenin stability during osteoblast differentiation, Flag–β-catenin (WT or S675A) was reconstituted in primary β-catenin–deficient COBs via lentivirus-mediated delivery (Fig. 3D). At 7 d of differentiation, the stability of β-catenin (S675A) protein was decreased significantly compared with WT β-catenin. This effect was the result of posttranslational destabilization, because transcript levels of S675A were comparable to those of WT β-catenin (Fig. S4B). Likewise, in the presence of MEKK2, S675A β-catenin was relatively unstable, with a reduction in half-life from ∼4.5 h in WT β-catenin to 3 h for S675A β-catenin (Fig. 3E and Fig. S4C). Thus, MEKK2-induced phosphorylation of S675 is crucial for β-catenin stability.
Fig. 3.
MEKK2 stabilizes β-catenin via phosphorylation of S675. (A) GST or GST–β-catenin was incubated with purified HA-MEKK2 (WT or KD mutant), and MEKK2 kinase activity was analyzed by an in vitro kinase assay. (B) Purified HA-MEKK2 was incubated with GST or GST–β-catenin [WT or S675A (SA) mutant], and MEKK2 kinase activity was analyzed by in vitro kinase assay. (C) Vector or β-catenin (WT or S675A mutant) was transfected into C3H10T1/2 cells along with TOPflash-luciferase and Renilla in the absence or presence of MEKK2. Luciferase activity was measured after 48 h of transfection and normalized to Renilla. P < 0.05 by one-way ANOVA. P < 0.05; P values for Bonferroni-corrected Student’s t tests: **P < 0.01; ***P < 0.001. (D) β-Catenin–deficient COBs were reconstituted with Flag–β-catenin WT or S675A mutant via lentivirus-mediated delivery and cultured under osteoblast differentiation conditions for 7 d. Lysates were immunoblotted with the indicated antibodies. (E) HEK293 cells were transfected with Flag–β-catenin WT or S675A mutant along with HA-MEKK2, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography. (F) HEK293 cells were cotransfected with Flag–β-catenin WT or S675A mutant along with different amounts of HA-ubiquitin and were treated with 10 µM MG132 for 8 h before lysis. Ubiquitinated β-catenin was immunoprecipitated by anti-Flag antibody–conjugated agarose and immunoblotted with anti-HA antibody. (G) An in vitro ubiquitination assay of β-catenin was performed using recombinant proteins. Activated His–β-catenin was incubated with the recombinant SCFSKP1 complex, ubiquitin, ATP, E1, and E2 in the presence or absence of recombinant MEKK2. Ubiquitinated β-catenin was immunoprecipitated by Ni-NTA agarose, subjected to SDS/PAGE, and immunoblotted with an anti-ubiquitin antibody. S675 phosphorylation of β-catenin by MEKK2 was confirmed by immunoblotting. (H) WT immortalized COBs were lysed, immunoprecipitated with anti–β-catenin or an IgG control and protein G-conjugated Dynabeads, and immunoblotted with the indicated antibodies.
Fig. S4.
MEKK2 regulates β-catenin stability via S675 phosphorylation. (A) C3H10T1/2 cells were transfected with different amounts of β-catenin (WT or S675A mutant) along with TOPflash-luciferase and Renilla. Luciferase activity was measured 48 h after transfection and normalized to Renilla. Expression of β-catenin was analyzed by immunoblotting with anti–β-catenin antibody. (B) β-Catenin–deficient COBs were reconstituted with Flag–β-catenin WT or S675A mutant via lentivirus-mediated delivery and were cultured under osteoblast differentiation conditions for 7 d. Transcript levels of Flag–β-catenin were measured by RT-PCR. P values for Bonferroni-corrected two-way Student’s t tests are indicated: **P < 0.01; N.S., not significant. (C) HEK293 cells were transfected with Flag–β-catenin (WT or S675A mutant) along with HA-MEKK2, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography and analysis in ImageJ.
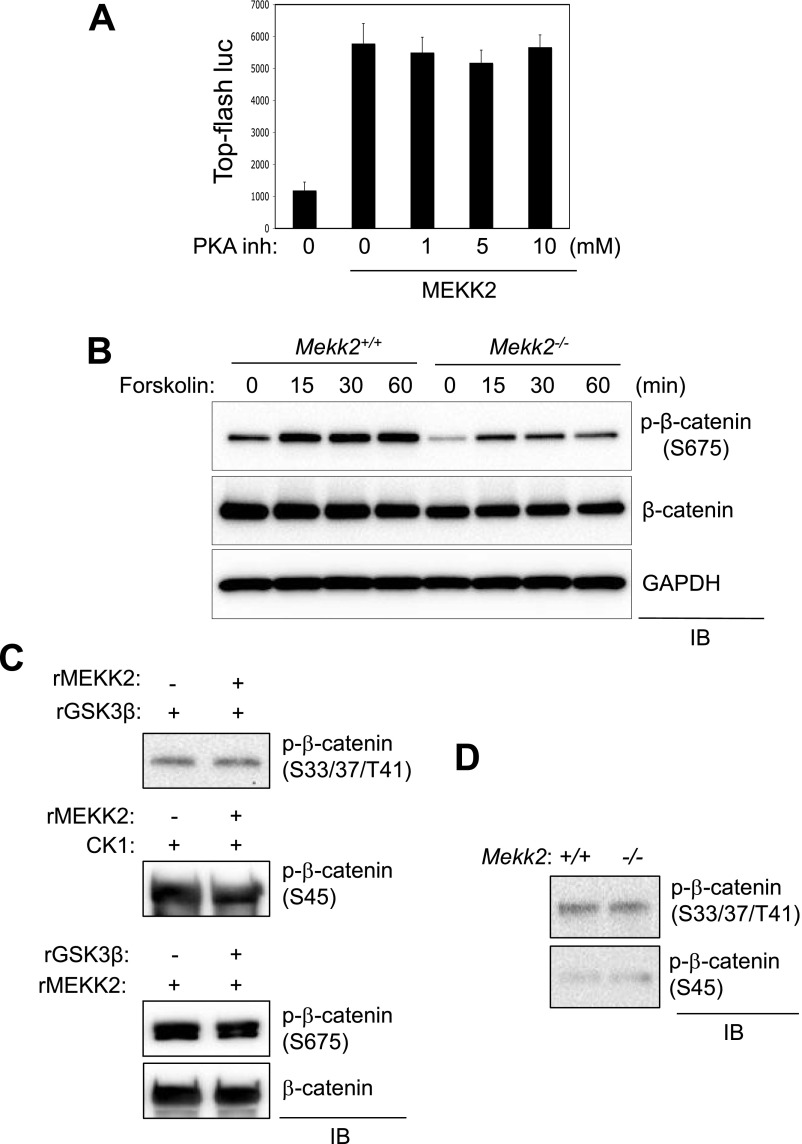
Because a previous study reported that protein kinase A (PKA) is able to phosphorylate β-catenin at serine 675 and 552 (20), we assessed whether PKA activity is required for the ability of MEKK2 to up-regulate β-catenin activity. MEKK2-induced β-catenin activation was not affected by the PKA inhibitor H-89 (Fig. S5A), and MEKK2 was not required for PKA to phosphorylate β-catenin S675, because the PKA activator forskolin still was able to induce S675 phosphorylation in the absence of MEKK2 (Fig. S5B). Thus, the functional interaction between MEKK2 and β-catenin is independent of PKA activity. In addition to PKA, CK1 and GSK3β also have been identified as β-catenin kinases that regulate its stability via proteasome-dependent degradation (21). As shown by an in vitro kinase assay, MEKK2-mediated phosphorylation of S675 did not influence the phosphorylation of β-catenin by CK1 and GSK3β, and basal phosphorylation at the CK1-mediated S45 phosphorylation site or the GSK3β-mediated S33/S37/T41 phosphorylation site was largely intact in Mekk2−/− COBs (Fig. S5 C and D). Thus, MEKK2 operates independently from the previously known β-catenin kinases PKA, CK1, and GSK3β.
Fig. S5.
MEKK2 functions independently from other known β-catenin kinases in osteoblasts. (A) C3H10T1/2 cells were transfected with a construct encoding MEKK2 along with TOPflash-luciferase and Renilla. Twelve hours after transfection, cells were incubated with different concentrations of the PKA inhibitor H-89, and luciferase activity was measured and normalized to Renilla. (B) Primary COBs isolated from Mekk2+/+ and Mekk2−/− pups were stimulated with 10 μM forskolin at different time points, and lysates were immunoblotted with the indicated antibodies. (C) GST–β-catenin was incubated with recombinant GSK3β (Top) or CK1 (Middle) in the presence or absence of MEKK2, and β-catenin phosphorylation by GSK3β or CK1 was analyzed by immunoblotting with antibodies specific to phospho-β-catenin. (Bottom) GST–β-catenin was incubated with recombinant MEKK2 in the absence or presence of GSK3β, and MEKK2-induced phosphorylation of β-catenin was analyzed by immunoblotting with an anti–phospho-β-catenin (S675) antibody. (D) Primary COBs isolated from Mekk2+/+ and Mekk2−/− pups were lysed and immunoblotted with the indicated antibodies.
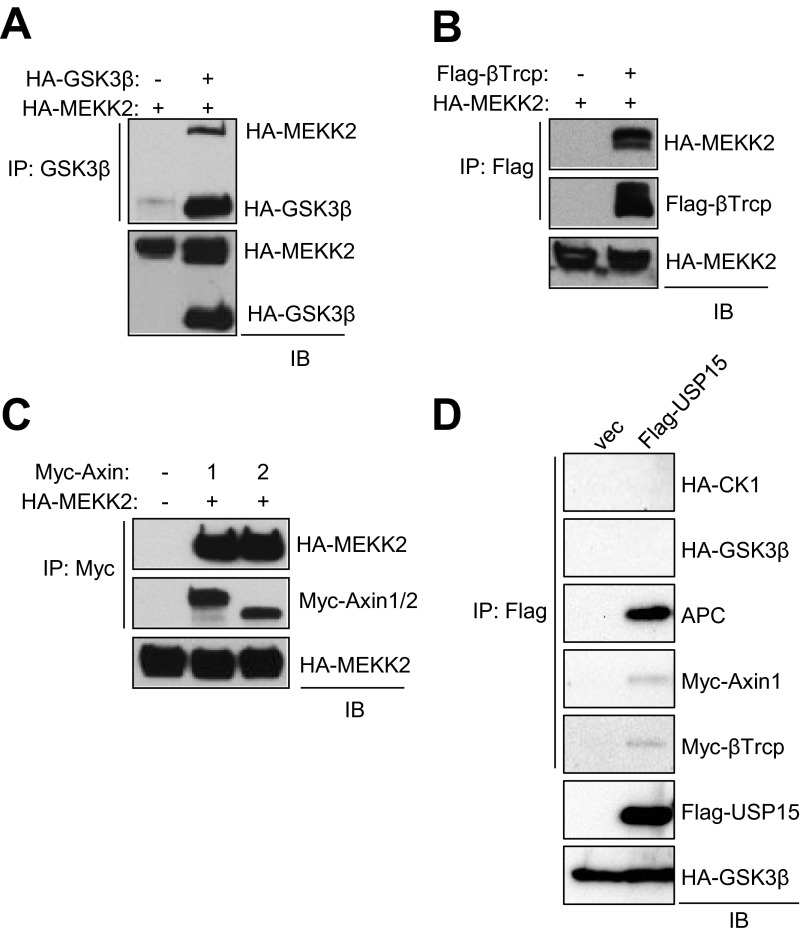
It has been well established that β-catenin stability is regulated mainly by ubiquitin-mediated proteasomal degradation (22). Because we had observed that the S675A mutation renders β-catenin unstable in osteoblasts (Fig. 3 D and E), we examined the effect of MEKK2-mediated S675 phosphorylation on β-catenin ubiquitination. As seen in Fig. 3F, the levels of β-catenin ubiquitination were increased significantly in the S675A mutant. However, MEKK2-mediated S675 phosphorylation did not impair the addition of ubiquitin to β-catenin in a cell-free assay using S-phase kinase-associated protein 1 (SKP1) as an E3 ligase, suggesting that MEKK2 instead may regulate the deubiquitination of β-catenin (Fig. 3G). To understand how S675 phosphorylation influences β-catenin and proteasomal turnover, mass spectrometry was performed to identify proteins selectively recruited to S675-phosphorylated β-catenin using coimmunoprecipitates of Flag–β-catenin (WT or S675A) and MEKK2. This approach revealed that the DUB USP15 binds preferentially to WT but not to S675A β-catenin; this finding was confirmed by coimmunoprecipitation of overexpressed proteins in HEK293 cells (Fig. S6) and by coimmunoprecipitation of endogenous proteins in osteoblasts (Fig. 3H). Next, we performed a series of coimmunoprecipitation experiments to assess whether MEKK2 or USP15 is part of the destruction complex regulating β-catenin turnover (Fig. S7). Intriguingly, MEKK2 interacts with several components of this complex, including the ubiquitin E3 ligase β-TrCP, Axin1/2, and GSK3β, but not with APC and CK1. Interaction with USP15 occurs only with APC, Axin1, and β-TrCP. Thus, MEKK2 and USP15 associate with the destruction complex regulating β-catenin turnover, suggesting that MEKK2-mediated regulation of β-catenin may occur in association with components of the Axin complex.
Fig. S6.
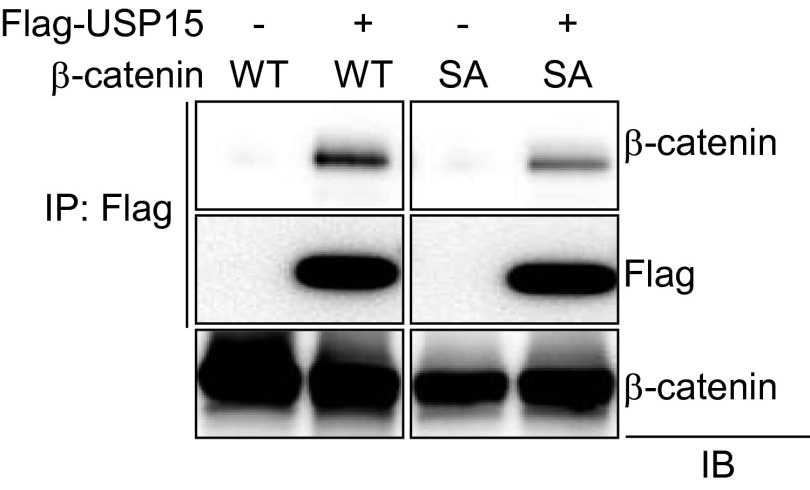
β-Catenin interacts with USP15. HEK293 cells were transfected with vector or β-catenin (WT or S675A mutant) along with Flag-USP15, immunoprecipitated with anti-Flag antibody–conjugated agarose, and immunoblotted with the indicated antibodies.
Fig. S7.
MEKK2 and USP15 interact with components of the Axin complex. HEK293 cells were transfected with the indicated constructs, immunoprecipitation was performed as indicated, and the subsequent immunocomplex was immunoblotted with the indicated antibodies.
USP15 Promotes β-Catenin Stability and Osteoblast Differentiation.
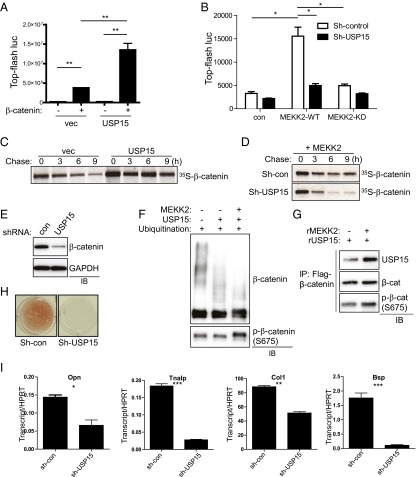
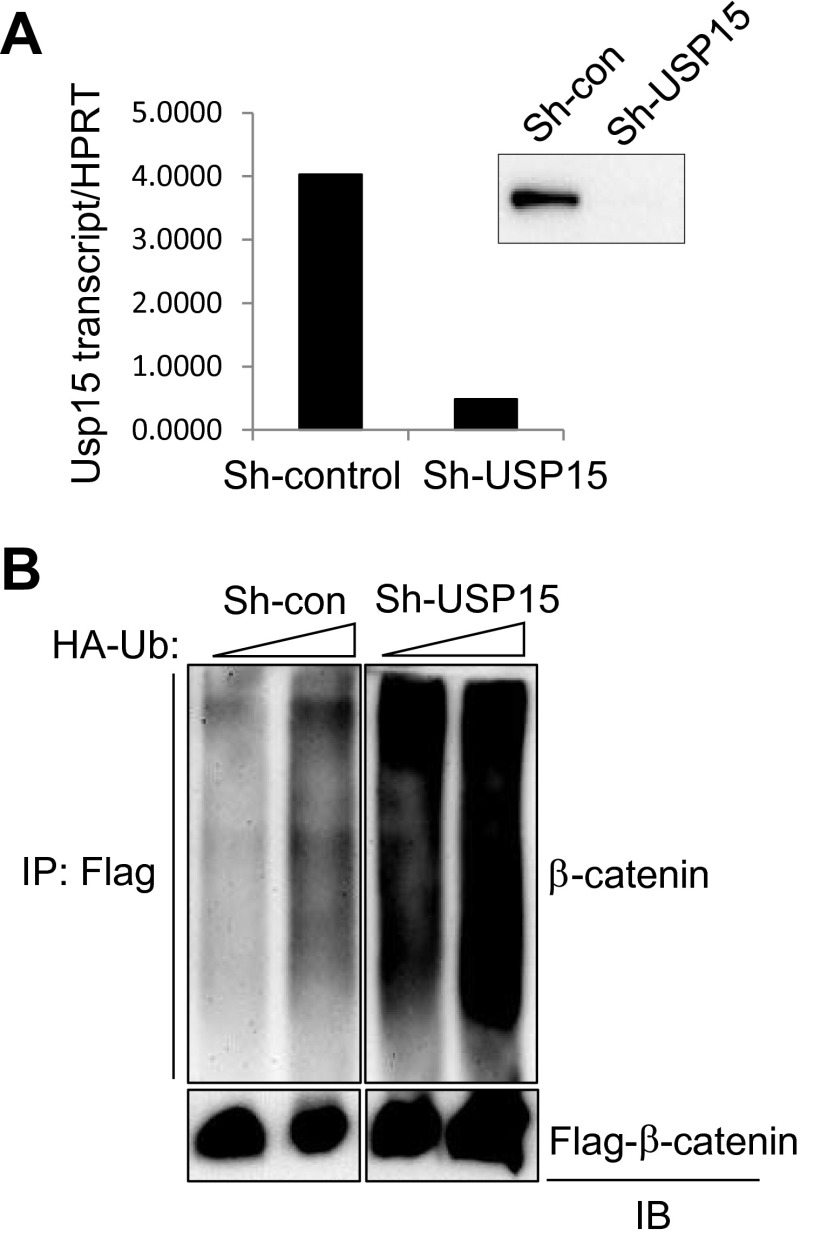
To determine if USP15 is required for the regulation of β-catenin activity by MEKK2, we investigated the effect of USP15 on MEKK2-induced β-catenin activation (Fig. 4 A and B and Fig. S8A). The addition of USP15 to the β-catenin construct induced a further increase in β-catenin transcriptional activity, and knockdown of USP15 decreased MEKK2-induced β-catenin activation. To test directly whether USP15 stabilizes β-catenin, we performed a pulse-chase experiment. Overexpression of USP15 increased the stability of β-catenin (Fig. 4C), whereas MEKK2-mediated β-catenin stabilization was reduced markedly by the knockdown of USP15 (Fig. 4D). Accordingly, β-catenin levels were decreased in USP15-deficient osteoblasts after 6 d of differentiation (Fig. 4E). Accompanying ubiquitination analysis demonstrated that USP15 knockdown increased total levels of ubiquitinated β-catenin (Fig. S8B). As expected, the addition of USP15 alone induced deubiquitination of β-catenin, and the addition of MEKK2 further promoted β-catenin deubiquitination by enhancing the interaction between ubiquitinated β-catenin and USP15 (Fig. 4 F and G). Thus, MEKK2-mediated phosphorylation is sufficient to promote USP15-mediated deubiquitination of β-catenin without the presence of additional factors. These results support the idea that MEKK2-mediated S675 phosphorylation enhances the binding affinity of β-catenin to USP15, and USP15 in turn deubiquitinates constitutively ubiquitinated β-catenin, preventing its proteasome-dependent degradation. If MEKK2 mediates its effects on osteoblast differentiation by promoting the action of USP15 on β-catenin, then the loss of USP15 should impair osteoblast differentiation. Accordingly, osteoblast differentiation was blocked by knockdown of USP15, as shown by a nearly complete loss of mineralization activity and loss of the characteristic osteoblast transcriptional signature, such as expression of Opn, Alp, Col1a1, and Bsp (Fig. 4 H and I).
Fig. 4.
USP15 promotes β-catenin stability and osteoblast differentiation. (A) USP15 or a vector control was transfected into HEK293 cells along with TOPflash-luciferase and Renilla in the absence or presence of β-catenin. Luciferase activity was measured 48 h after transfection and normalized to Renilla. P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t test: **P < 0.01. (B) C3H10T1/2 cells were infected with lentivirus expressing control or Usp15 shRNAs, and the resulting cells were transfected with Flag–β-catenin, TOPflash-luciferase, and Renilla in the presence or absence of MEKK2 WT or KD mutant. Luciferase activity was measured 48 h after transfection and normalized to Renilla. P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t tests: *P < 0.05. (C) HEK293 cells were transfected with Flag–β-catenin along with vector or USP15, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography. Numbers indicate the ratio of the densitometric measurement of the corresponding band to the value at time = 0. (D) HEK293 cells were infected with lentiviruses expressing control or Usp15 shRNAs. The resulting cells were transfected with Flag–β-catenin and MEKK2, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography. (E) Human MSCs were infected with the indicated lentiviruses and were cultured under osteoblast differentiation conditions for 6 d before immunoblotting. (F) An in vitro ubiquitination assay of β-catenin was performed using recombinant proteins. Activated His–β-catenin was incubated with recombinant SCFSKP1 complex, ubiquitin, ATP, E1, and E2 in the presence or absence of recombinant MEKK2 or USP15. S675 phosphorylation of β-catenin by MEKK2 was confirmed by immunoblotting. (G) An in vitro interaction assay of ubiquitinated β-catenin with USP15 was performed using recombinant proteins. Flag-tagged ubiquitinated β-catenin was incubated with recombinant USP15 in the presence or absence of recombinant MEKK2, immunoprecipitated with anti-Flag antibody–conjugated agarose, and immunoblotted with the indicated antibodies. MEKK2-mediated β-catenin S675 phosphorylation was confirmed by immunoblotting with anti–phospho-β-catenin (S675) antibody. (H and I) Human MSCs were infected with lentiviruses expressing control or Usp15 shRNAs, and the resulting cells were cultured under osteoblast differentiation conditions for 21 d. (H) Alizarin red staining was performed to determine osteoblast mineralization activity. (I) After 10 d of culture, the expression of osteoblast marker genes was analyzed by RT-PCR. For each graph, P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t tests: *P < 0.05; **P < 0.01; ***; P < 0.001.
Fig. S8.
USP15 regulates the ubiquitination of β-catenin. (A) The efficiency of shRNA construct targeting human Usp15 was determined by RT-PCR and immunoblotting. (B) HEK293 cells were infected with lentiviruses expressing control or Usp15 shRNAs and then were transfected with HA-ubiquitin (HA-Ub) and Flag–β-catenin. Ubiquitinated β-catenin was immunoprecipitated with anti-Flag antibody–conjugated agarose and was immunoblotted with anti-HA antibody.
FGF2 Activates MEKK2 to Stabilize β-Catenin in Osteoblasts.
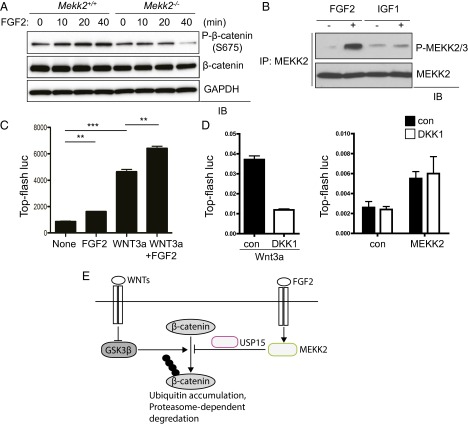
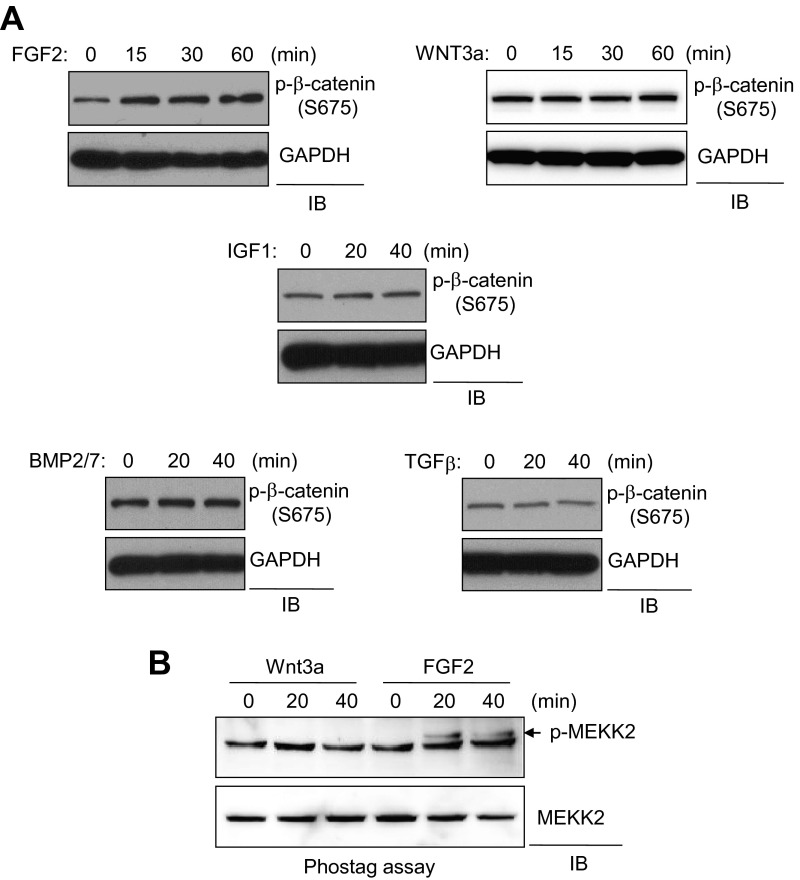
Next, we sought to determine what stimulus activates the MEKK2 pathway in osteoblasts. WT COBs were stimulated with various osteogenic factors, and S675 phosphorylation levels of β-catenin were analyzed by immunoblotting. FGF2, but not WNT3a, insulin-like growth factor 1 (IGF1), BMP2/7, or TGF-β, increased S675 phosphorylation, and this activity was markedly reduced in Mekk2−/− osteoblasts (Fig. 5A and Fig. S9A). We also tested the ability of FGF2, IGF1, or WNT3a to induce phosphorylation of MEKK2, demonstrating that FGF2 stimulation, but not stimulation with IGF1 or WNT3a, promoted the phosphorylation of MEKK2 (Fig. 5B and Fig. S9B). Thus, these results suggest that the FGF2–MEKK2–β-catenin signaling axis is an independent mechanism for the activation of β-catenin, distinct from the canonical WNT3a pathway. To test this hypothesis, osteoblasts were stimulated with FGF2 or WNT3a, either alone or together, and β-catenin activity was analyzed by a luciferase assay (Fig. 5C). The total degree of activation after stimulation with both ligands was simply additive as compared with stimulation using either ligand alone, suggesting the lack of a functional interaction between these two pathways. Moreover, the effect of a soluble antagonist of WNT signaling, Dickkopf-related protein 1 (DKK1) (23), on MEKK2-induced β-catenin activation was assessed to exclude the possibility that MEKK2 activity influences an autocrine pathway for WNT stimulation. Saturating levels of DKK1 largely blocked the ability of exogenous WNT3a to activate β-catenin, but β-catenin activation by MEKK2 was not altered by DKK1 (Fig. 5D). Taken together with our data demonstrating that the β-catenin activation by FGF2 and MEKK2 is distinct from the biochemical pathway engaged by WNT stimulation, these results demonstrate that the FGF2–MEKK2–β-catenin axis is an independent and parallel pathway for the activation of β-catenin in osteoblasts.
Fig. 5.
FGF2 activates MEKK2 to stabilize β-catenin in osteoblasts. (A) Primary COBs isolated from Mekk2+/+ and Mekk2−/− mice were stimulated with FGF2 for the indicated times, and lysates were blotted with the indicated antibodies. (B) Primary WT COBs were stimulated with FGF2 (25 ng/mL) or IGF1 (25 ng/mL) for 15 min. Cell lysates were immunoprecipitated with anti–phospho-MEKK2/3 antibody and protein G-conjugated Dynabeads and were immunoblotted with the indicated antibodies. (C) C3H10T1/2 cells were transfected with TOPflash-luciferase and Renilla, and 24 h after transfection cells were stimulated with the indicated ligands for 24 h. Luciferase activity was measured subsequently and normalized to Renilla. P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t test: **P < 0.01; ***P < 0.001. (D, Left) C3H10T1/2 cells were transfected with TOPflash-luciferase and Renilla and were stimulated with WNT3a in the absence or presence of DKK1 for 24 h. (Right) C3H10T1/2 cells were transfected with TOPflash-luciferase and Renilla along with a construct encoding MEKK2 or a vector control, and then cells were incubated with 1 μg/mL of DKK1. P = N.S. for comparison of DKK1 versus vehicle-treated cells in either the vector control or MEKK2-transfected groups. (E) A diagram of the FGF2/MEKK2/β-catenin pathway in osteoblasts.
Fig. S9.
FGF2 acts upstream of MEKK2 in osteoblasts. (A) Primary WT COBs were stimulated as indicated and immunoblotted with anti–phospho-β-catenin antibody. GAPDH was used for a loading control. (B) Primary WT COBs were stimulated as indicated. Cell lysates were analyzed using a Phos-tag gel (Wako) and immunoblotted with an anti-MEKK2 antibody. The arrow indicates phospho-MEKK2.
Discussion
Our data revealed that MEKK2 promotes osteoblast activity via an unexpected activity to control β-catenin stability. This pathway is biochemically and functionally independent from canonical WNT3a-mediated β-catenin activation. In osteoblasts, FGF2 activates MEKK2, and active MEKK2 in turn phosphorylates β-catenin at S675, resulting in the recruitment of USP15. USP15 removes ubiquitin from β-catenin, preventing its constitutive proteasome-dependent turnover. Thus, whereas canonical WNT stimulation acts by preventing β-catenin ubiquitination, the MEKK2 pathway promotes β-catenin deubiquitination (Fig. 5E) (6, 22). Although the MEKK2 and WNT pathways appear to be distinct in this regard, both pathways are dependent on reversing or preventing the basal ubiquination and degradation of β-catenin to exert their effects. Thus, the profound effect on bone seen in mice with a conditional deletion of β-catenin exon 3, which prevents this basal ubiquination of β-catenin, would be expected to reflect a decoupling from both the WNT and FGF2/MEKK2 pathways (1, 24).
Notably, these findings provide a mechanistic basis for prior observations that β-catenin protein levels and activity are reduced in the absence of FGF2 (25). The phenotype of Mekk2−/− mice also is consistent with the reduced bone mass and decreased BFR seen in adult Fgf2−/− mice, indicating that a FGF2/MEKK2 pathway contributes to the maintenance of adult bone mass (26). We did not observe a clear role for MEKK2 in embryonic bone development, although it is possible that redundancy with other MAP3Ks, such as the closely related MEKK3, may mask such a function.
In contrast to observations that MEKK2 primarily controls the anabolic functions of osteoblasts, several mouse models bearing loss-of-function β-catenin alleles demonstrate that a major function of β-catenin is to control osteoclastogenesis by regulating Opg expression in osteoblasts (1). However, inducible deletion of β-catenin in adult mice using an inducible cre system demonstrated that β-catenin can have direct anabolic effects in osteoblasts (27). This finding suggests that β-catenin is a bona fide regulator of anabolic bone formation, albeit one that may be masked by the gradual engagement of compensatory mechanisms in adults. Thus, the overall balance of anabolic versus anticatabolic activities of β-catenin is likely context dependent, perhaps explaining why Mekk2–/– mice only display an anabolic defect despite β-catenin being a key mediator of the effects of MEKK2 in osteoblasts. Additionally, it is also possible that β-catenin is not the sole effector downstream of MEKK2 in osteoblasts and that these other pathways shift the balance of anabolic versus anticatabolic activities of MEKK2 in adult mice.
Although dysregulated β-catenin signaling contributes to the pathogenesis of diverse disease processes, ranging from subsets of colorectal or ovarian cancer to type II diabetes, there currently are no approved therapeutics to inhibit the WNT pathway (28–31). This absence of therapeutic means to inhibit WNT pathway activity reflects several challenges complicating drug development, including redundancy in WNTs or their cognate receptors, the promiscuous participation of key signaling components such as GSK3β in multiple signaling pathways, or the many clinically undesirable phenotypes induced by ablation of the WNT/β-catenin pathway (32). From this perspective, MEKK2 may present an alternative target to manipulate β-catenin activity, because MEKK2 appears to be both necessary and sufficient to mediate activation of the FGF2–MEKK2–β-catenin signaling axis. Moreover, because Mekk2−/− mice do not display the developmental defects seen with full ablation of the β-catenin pathway (33, 34), inhibition of only MEKK2-mediated β-catenin activation may be better tolerated than full ablation of β-catenin activity. Thus, the MEKK2 pathway may allow fine tuning of β-catenin activity in certain contexts where this fine-tuning is critical, such as regulation of bone mass, and this MEKK2-mediated fine tuning may present an opportunity to target the β-catenin pathway while avoiding some of the challenges involved in inhibiting WNT signaling. Notably, the Food and Drug Administration-approved drugs crizotinib and bosutinib have been demonstrated to display potent off-target activity in inhibiting MEKK2. Our results suggest that further investigation of the influence of these drugs on β-catenin activation is warranted (35).
Materials and Methods
Reagents and Mice.
Anti-HA (agarose conjugated), anti–c-Myc (HRP conjugated), anti-HA (HRP conjugated), and anti-ubiquitin (P4D1) were obtained from Santa Cruz. Anti-Flag (M2; Sigma-Aldrich), anti-ELK1 (Abcam), anti–β-catenin (BD Biosciences), anti–phospho β-catenin (S675) (Cell Signaling), anti-USP15 (Bethyl Laboratories, Inc.), anti-MEKK2 (Bethyl Laboratories, Inc.), and anti-GAPDH (Affinity Bioreagents) were used also. The generation of the anti-phospho MEKK2/3 polyclonal antibody was described previously (9). All mouse strains were housed in a barrier facility under protocols approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Generation of the Mekk2−/− mouse strain was described previously (12). Mouse strains used were backcrossed more than six generations onto the C57BL/6 background. Ctnnb1 floxed allele mice were described previously and were purchased from Jackson Laboratories (36). The PKA inhibitor H-89, dihydrocloride, was obtained from Santa Cruz (37).
Osteoblast Culture and Differentiation Assays.
Primary COBs were isolated from 5-d-old neonates by triple collagenase/dispase II digestion. Cells were cultured in differentiation medium containing ascorbic acid and β-glycerophosphate. hMSCs were cultured and differentiated into osteoblasts as described by the manufacturer (Lonza). Primary COBs or C3H10T1/2 cells (a mouse mesenchymal fibroblast-like cell line obtained from the American Type Culture Collection) were cultured in α-MEM medium (Cellgro) containing 10% FBS, 2 mM l-glutamine, 1% penicillin/streptomycin, 1% Hepes, and 1% nonessential amino acids and differentiated with ascorbic acid and β-glycerophosphate. All cells were routinely tested to be mycoplasma negative.
For Von Kossa staining of extracellular matrix mineralization, cells were fixed with 10% neutral buffered formalin and stained with 2.5% silver nitrate (Sigma-Aldrich). For alkaline phosphatase (ALP) activity, osteoblasts were fixed with 10% neutral formalin buffer and stained with the solution containing Fast Blue and Naphthol (Sigma-Aldrich). Alternatively, osteoblasts were incubated with 10-fold diluted Alamar Blue solution, washed, and incubated with a solution containing 6.5 mM Na2CO3, 18.5 mM NaHCO3, 2 mM MgCl2, and phosphatase substrate (Sigma-Aldrich). ALP activity was measured by luminometer (Thermo Electron Corporation).
Micro-CT Analysis, Histology, in Situ Hybridization, Immunohistochemistry, and Histomorphometry.
Micro-CT analysis was conducted on a Scanco Medical Micro-CT 35 system using the previously described parameters (38). Micro-CT analysis was performed by an investigator blinded to the genotypes of the animals under analysis. Paraffin embedding, in situ hybridization, and immunohistochemistry were performed as previously described (39).
For histomorphometry, 8-wk-old Mekk2−/− mice and their littermate controls were s.c. injected with 25 mg/kg calcein and 40 mg/kg demeclocycline on days 7 and 2, respectively, before necropsy. Tibias were removed and embedded without demineralization in methyl methacrylate. Undecalcified 5-μm sections were cut and mounted unstained to quantify MAR and the BFR/bone surface (BS) ratio. Consecutive sections were stained with toluidine blue to quantify osteoblast and osteoclast number. Histomorphometric analysis was performed using the OsteoMeasure system (Osteometrics, Inc.). A sampling site with an area of ∼1.2 mm2 was established in the secondary spongiosa of tibial metaphysis, and the results were expressed according to standardized nomenclature (40).
In Vitro Kinase Assay, Immunoprecipitation, and Immunoblotting.
Recombinant MEKK2, GSK3β, or CK1 (200 ng) was incubated for 15 min at 30 °C in kinase buffer [20 mM Hepes (pH 7.5), 20 mM MgCl2, 1 mM EDTA, 2 mM NaF, 2 mM glycerophosphate, 1 mM DTT, 10 μM ATP] containing β-catenin protein and 10 μCi of (γ32P) ATP (PerkinElmer). The substrates then were resolved by SDS/PAGE, and phosphorylated proteins were visualized by autoradiography.
Cells were lysed in TNT lysis buffer (10 mM Tris, 50 mM NaCl, 5 mM EDTA, 2 mM NaF, 30 mM peptin, 5 μg/mL aprotinin, 1% Triton X-100), and the lysates were incubated with Flag-, β-catenin–, or MEKK2-conjugated agarose beads overnight at 4 °C. Beads were washed three times with TNT buffer, and immunoprecipitates were eluted with Laemmli sample buffer (Bio-Rad). The eluates were separated by SDS/PAGE and transferred to Immobilon-P membranes (Millipore). Protein levels were normalized by immunoblotting with anti-GAPDH antibody. Membranes were blocked in TTBS buffer [100 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 0.1% Tween-20] with 4% skim milk and then were incubated with the indicated antibodies. Finally, membranes were washed, incubated with HRP-conjugated secondary antibodies, and developed with ECL (Thermo Scientific).
GST Pull Down.
One microgram of GST or the relevant GST-fusion protein was incubated with recombinant proteins for 1 h in IPP-150 buffer (10 mM Tris⋅HCl, 150 mM NaCl, and 0.1% Nonidet P-40), and 20 µL of GST beads were added and incubated at 4 °C for 90 min with rotation. For the mass spectrometry assay, 10 μg of GST or GST-MEKK2 was embedded into a column using anti-GST antibody–conjugated agarose and then was incubated at 4 °C overnight with 2 mg of cell lysates obtained from ST-2 osteoblasts expressing WNT 10b. The columns were washed three times with IPP-150 buffer and eluted with IPP-325 buffer (325 mM NaCl, 0.1% Nonidet P-40, 10 mM Tris⋅HCl) containing glutathione.
Pulse-Chase Assay.
HEK293T cells were maintained at 37 °C, 5% CO2. One day before transfection, cells were plated in a six-well plate with 4.2 × 105 cells/mL. After 48 h of transfection, cells were changed to starvation medium (Cys/Met-free DMEM, 3% dialyzed FBS, Gln, Hepes) and were incubated 1 h at 37 °C, 5% CO2. Then cells were harvested and resuspended with 2 mL starvation medium with 1 mCi 35S (Perkin-Elmer) and were incubated for 1 h on a rocking stand at 37 °C, 5% CO2. After incubation, cells were washed with PBS and centrifuged at 1,300 × g for 5 min. The medium was changed to chase medium (DMEM, 5% FBS, +Cys, +Met), and cells were incubated on a rocking stand for 0, 3, 6, or 9 h at 37 °C, 5% CO2. After incubation, cells were harvested and immunoprecipitated with Flag M2 beads (Sigma), and eluted proteins were detected with autoradiography.
In Vitro Ubiquitination/Deubiquitination Assay.
UbcH3 (500 nM) (Millipore), 0.1 μg SCFβ-TrCP1 (activated; Millipore), 100 nM β-catenin (activated; Millipore), and 2 μM ubiquitin were mixed in a ubiquitination buffer containing 25 mM 3-(N-morpholino)propanesulfonic acid (pH 7.5), 0.01% Tween 20, 5 mM MgCl2, 2 mM ATP, and 1 mM DTT. Phosphorylation was initiated by adding 100 nM recombinant MEKK2 (Invitrogen) and incubation for 1 h at 30 °C. Afterward, 10 nM E1 (Millipore) was added to initiate ubiquitination, and this mixture was incubated for 1 h at 37 °C. Then, 5 mM EDTA was added for 15 min at room temperature to stop the ubiquitination reaction. Finally, 400 nM recombinant USP15 (Enzo Life Sciences) was added for deubiquitination and incubated for 1 h at 37 °C. SDS sample buffer was added to stop the reaction.
Luciferase Reporter Assay.
C3H10T1/2 cells grown on 12-well plates were transiently transfected using Effectene (QIAGEN) with 0.5 μg of TOPflash plasmid and 50 ng of a plasmid expressing Renilla luciferase (Promega). Twelve hours after transfection, 100 ng/mL of Wnt3a (R&D Systems) in serum-free medium was added for 24 h. For inhibition of Wnt signaling, 1 μg/mL DKK1 (R&D Systems) was added 1 h before Wnt3a treatment. Normalized luciferase activity was obtained using the Dual-Luciferase Reporter assay System (Promega).
Statistics.
Where applicable, statistical analysis was performed by first assessing for normality using a Shapiro–Wilk test and equality of variances using an F test followed by a two-tailed, unpaired Student’s t test. When multiple comparisons were performed, one-way or two-way ANOVAs with Bonferroni-corrected Student t tests as posttests were performed. A P value less than 0.05 was considered significant. *P < 0.05, **P < 0.01, ***P < 0.001. In general, animal experiments were powered to detect a 20% difference in bone volume/tissue volume ratio (BV/TV) assuming an SD of 15% of the mean with an α of 0.05 and a β of 0.80. No animals or other experimental samples were excluded from analysis. Except where otherwise indicated, experiments were repeated three times. All images shown are representative.
Acknowledgments
We thank Drs. Antonios Aliprantis, Weiguo Zou, and Marc Wein for helpful discussions; Nicholas Brandy, Yeon-Suk Yang, and Steven Doty for technical support; and the many individuals who provided valuable reagents. M.B.G. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and is supported by NIH Director’s Early Independence Award 1DP5OD021351. J.-H.S. is supported by a Musculoskeletal Transplant Foundation award, and this work is partially supported by National Natural Science Foundation of China Grants 31470845 and 81430033 (to B.S.).
Footnotes
Conflict of interest statement: L.H.G. is on the board of directors of and holds equity in Bristol Myers Squibb Pharmaceutical Company.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600813113/-/DCSupplemental.
References
- 1.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Holmen SL, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280(22):21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 3.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Kode A, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391(6666):493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Yang J, Xia Y, Karin M, Su B. Synergistic interaction of MEK kinase 2, c-Jun N-terminal kinase (JNK) kinase 2, and JNK1 results in efficient and specific JNK1 activation. Mol Cell Biol. 2000;20(7):2334–2342. doi: 10.1128/mcb.20.7.2334-2342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, et al. Identification of MEKK2/3 serine phosphorylation site targeted by the Toll-like receptor and stress pathways. EMBO J. 2006;25(1):97–107. doi: 10.1038/sj.emboj.7600913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blank JL, Gerwins P, Elliott EM, Sather S, Johnson GL. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J Biol Chem. 1996;271(10):5361–5368. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, et al. MEK kinase 2 and the adaptor protein Lad regulate extracellular signal-regulated kinase 5 activation by epidermal growth factor via Src. Mol Cell Biol. 2003;23(7):2298–2308. doi: 10.1128/MCB.23.7.2298-2308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z, et al. Disruption of Mekk2 in mice reveals an unexpected role for MEKK2 in modulating T-cell receptor signal transduction. Mol Cell Biol. 2002;22(16):5761–5768. doi: 10.1128/MCB.22.16.5761-5768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang X, et al. The kinases MEKK2 and MEKK3 regulate transforming growth factor-β-mediated helper T cell differentiation. Immunity. 2011;34(2):201–212. doi: 10.1016/j.immuni.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesavan K, et al. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol. 2004;199(1):140–148. doi: 10.1002/jcp.10457. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama T, et al. CHIP-dependent termination of MEKK2 regulates temporal ERK activation required for proper hyperosmotic response. EMBO J. 2010;29(15):2501–2514. doi: 10.1038/emboj.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou W, et al. The microtubule-associated protein DCAMKL1 regulates osteoblast function via repression of Runx2. J Exp Med. 2013;210(9):1793–1806. doi: 10.1084/jem.20111790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita M, et al. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121(1):101–113. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karin M, Gallagher E. From JNK to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57(4-5):283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(19):14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 20.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25(20):9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruciat CM. Casein kinase 1 and Wnt/β-catenin signaling. Curr Opin Cell Biol. 2014;31:46–55. doi: 10.1016/j.ceb.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 24.Tu X, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci USA. 2015;112(5):E478–E486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fei Y, Xiao L, Doetschman T, Coffin DJ, Hurley MM. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem. 2011;286(47):40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero A, et al. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000;105(8):1085–1093. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Long F. β-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res. 2013;28(5):1160–1169. doi: 10.1002/jbmr.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262(5140):1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 29.Rubinfeld B, et al. Association of the APC gene product with beta-catenin. Science. 1993;262(5140):1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 30.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 31.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 32.Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19(4):634–664. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huelsken J, et al. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148(3):567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad S, Hughes MA, Johnson GL, Scott JE. Development and validation of a high-throughput intrinsic ATPase activity assay for the discovery of MEKK2 inhibitors. J Biomol Screen. 2013;18(4):388–399. doi: 10.1177/1087057112466430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 37.Chijiwa T, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265(9):5267–5272. [PubMed] [Google Scholar]
- 38.Zou W, et al. MLK3 regulates bone development downstream of the faciogenital dysplasia protein FGD1 in mice. J Clin Invest. 2011;121(11):4383–4392. doi: 10.1172/JCI59041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shim JH, et al. Administration of BMP2/7 in utero partially reverses Rubinstein-Taybi syndrome-like skeletal defects induced by Pdk1 or Cbp mutations in mice. J Clin Invest. 2012;122(1):91–106. doi: 10.1172/JCI59466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dempster DW, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]