Abstract
Objective
This pilot study evaluated the efficacy and safety of prolonged-release oxycodone/naloxone (OXN-PR) in older subjects with chronic pain and mild-to-moderate cognitive impairment.
Methods
This was a prospective, observational, open-label study of 45-day duration. Patients with moderate-to-severe chronic pain and naïve to strong opioids were recruited from nursing homes and Alzheimer’s disease centers. OXN-PR was initiated at low doses (5 mg od or bid) and increased to a maximum of 20 mg bid. The primary efficacy endpoint was a pain intensity reduction of ≥30% from baseline (T0) to 15 days after OXN-PR initiation, as assessed by a numerical rating scale or the Pain Assessment in Advanced Dementia scale. Other assessments included the Barthel activities of daily living index, Neuropsychiatric Inventory, Bowel Function Index, and adverse events.
Results
The analysis included 53 patients (mean age, 83.0 years; mean Mini-Mental State Examination score, 18.6) with severe pain (median Numerical Rating Scale/Pain Assessment in Advanced Dementia 6) and substantial impairment in daily functioning (mean Barthel index, 32.2). The primary endpoint was achieved by 92.4% of patients. OXN-PR significantly reduced mean pain intensity from baseline to study end (numerical rating scale, 6.6±1.0 vs 2.3±1.1, P<0.0001; Pain Assessment in Advanced Dementia, 6.9±1.6 vs 0.9±0.8, P<0.0001). Substantial improvements from T0 to T45 in daily functioning (mean Barthel index, 32.2±16.8 vs 53.7±23.9, P<0.0001) and neuropsychiatric symptoms (mean Neuropsychiatric Inventory, 25.5±27.3 vs 8.8±9.0, P<0.0001) were also reported. OXN-PR was well tolerated and did not worsen bowel function.
Conclusion
In this pilot study, OXN-PR was effective in improving pain and other symptoms associated with dementia, with a favorable safety and tolerability profile. Large-scale trials in people with dementia are needed to improve clinical guidance for the assessment and treatment of pain in these fragile individuals.
Keywords: dementia, Alzheimer’s disease, oxycodone/naloxone, elderly, cognitive impairment
Introduction
Chronic pain is often unrecognized and undertreated in older people, especially in those living in residential care, despite the high prevalence of this symptom in the aging population.1–3 Several factors contribute to the inadequate management of pain in this vulnerable population, including educational deficiencies on the part of health professionals, poor pain communication between patients and nursing staff, and fear of adverse events (AEs) associated with analgesic drugs.3,4 As advancing age is also associated with an increase in the prevalence of dementia, pain and cognitive impairment often coexist in elderly patients.5,6 Evidence from the literature indicates that cognitive impairment is a risk factor for pain undertreatment.1,5,7,8
A number of studies demonstrate, however, that communicative elderly patients with mild-to-moderate cognitive impairment are able to report their own pain symptoms and pain intensity.8–10 As pointed out in the recent guidelines of the British Geriatrics Society, before measuring pain in older subjects, the extent of cognitive impairment should be considered.3 Approaches for recognizing and evaluating pain in older persons with severe cognitive impairment unable to provide pain information by self-report are also available.4
Current treatment recommendations for chronic pain in elderly patients recommend acetaminophen as first-line pharmacotherapy in the treatment of persistent pain, particularly musculoskeletal pain.4 Conventional and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs (NSAIDs) are rarely an option because of a high risk of potentially serious and life-threatening side effect, and should be considered with extreme caution and in highly selected patients.3,4 All patients with moderate-to-severe pain should be considered for opioid therapy, in the absence of contraindications.3,4,11 Oxycodone is one of the strong opioid options currently available for the treatment of patients with moderate-to-severe pain, including elderly patients.3 Despite proven analgesic efficacy, oxycodone, as well as other opioids, is commonly associated with dose-limiting bowel dysfunction, in particular, persistent constipation that utterly impacts the patients’ quality of life.12 In older subjects, more prone to developing constipation than the general population, this symptom may occur more frequently and be more bothersome. The oral prolonged-release oxycodone/naloxone (OXN-PR) combination was developed to improve the gastrointestinal tolerability of opioids.13 The efficacy and safety of OXN-PR in chronic nonmalignant pain have been established in a number of clinical trials.14–17 Recent observations have suggested the effectiveness and tolerability of OXN-PR also in elderly patients.18,19
Data on the use of strong opioids for the treatment of pain in the presence of cognitive impairment are currently lacking. We report here the results of a pilot, observational study conducted to evaluate the efficacy and tolerability of oral OXN-PR in elderly patients with chronic pain and mild-to-moderate cognitive impairment.
Methods
Study design and patients
This was a prospective, observational, multicenter, open-label, pilot study of 45-day duration.
The primary objective of the study was to evaluate the analgesic efficacy and tolerability of oral OXN-PR in elderly patients with mild-to-moderate cognitive impairment. Secondary objectives included the evaluation of the effects of OXN-PR on activities of daily living, mental status and behavioral disturbances, and bowel function. Safety and tolerability of OXN-PR were also evaluated. During the treatment period, patients were visited four times, at T0 (baseline, beginning of treatment and observation) and at 7, 15, and 45 days from treatment beginning (T7, T15, and T45, respectively). Data from T0 to T45 were recorded in patients’ charts specifically designed for the study.
Residents were screened and enrolled from two nursing homes and two Alzheimer’s disease clinics in Northern Italy between September 2013 and December 2014. The inclusion criteria were: age >65 years; mild-to-moderate cognitive impairment (Mini-Mental State Examination [MMSE] score 15−24/30);20 moderate-to-severe pain not responding to acetaminophen and/or NSAIDs; and naïve to strong opioids (World Health Organization-step III analgesics).21 The exclusion criteria were: inability to swallow tablets; known or active psychiatric disease; cancer; hypersensitivity to study medication or to its excipients; severe respiratory depression with hypoxemia and/or hypercapnia; severe pulmonary obstructive disease; severe bronchial asthma; paralytic ileus; moderate-to-severe liver insufficiency (total bilirubin ≥1.5 mg/dL); moderate-to-severe renal insufficiency (glomerular filtration rate <30 mL/min/1.73 m, according to the Cockcroft–Gault equation); and any contraindications for opioids.
The study was approved by the Azienda Sanitaria Locale Bergamo institutional review board (ASL-BG N.0086418.25-06-2013). All patients, or caregivers for patients with moderate cognitive impairment, provided a written informed consent for the collection and analysis of demographic and clinical data. Care was taken to ensure the anonymity of the collected data.
Treatment
Treatment with OXN-PR (Targin® tablets, Mundipharma Pharmaceuticals, Milan, Italy) was initiated at T0 (baseline). Previous analgesics, if any, were discontinued. Adjuvants (antidepressants and antiepileptic agents) and other medications used for the treatment of underlying conditions were maintained, with no change in their dosages. The starting doses of OXN-PR were individually determined for each patient by the treating pain physician according to patient needs and previous analgesic therapy. In opioid-naïve subjects, OXN-PR was started at a low dose (oxycodone/naloxone, 5/2.5 mg od or bid) according to current recommendations for opioid therapy in older individuals.3,4 In patients who had previously received weak opioids, the starting OXN-PR dose was determined using conventional conversion tables for opioid dosages. All patients received their first OXN-PR tablet at 8 pm of T0. After 3 days, if analgesia was insufficient, caregivers were instructed to increase the OXN-PR daily dose by adding one 5/2.5 mg OXN-PR tablet od or bid. In the following visits, OXN-PR doses could be titrated according to patient needs by the treating pain physician. The maximum OXN-PR dose allowed in this study was 20/10 mg bid to be reached after the T15 visit. At any time during the study, if pain control with OXN-PR was insufficient, oral acetaminophen (500 mg od or bid) was allowed as rescue medication. If rescue acetaminophen was required at doses higher than 500 mg bid, caregivers were instructed to contact the referring physicians in advance of the next follow-up visit. In case of satisfactory analgesia, adjuvants could be gradually discontinued. A standard prophylactic laxative regimen with emollients or nonabsorbed osmotic agents was recommended, as needed.
Assessments
Demographic information, clinical and pain characteristics, as well as presence of constipation, were recorded at study entry (T0).
Pain intensity (the primary efficacy measure) was measured at all four study visits, as mean of the lowest, average, and highest 24-hour intensities. In the presence of mild cognitive impairment (MMSE score ≥18 and <24), pain was assessed using a verbally administered 11-point numerical rating scale (NRS), with 0 indicating no pain, 1–3 mild pain, 4–6 moderate pain, and 7–10 severe pain.22 In case of moderate cognitive impairment or dementia (MMSE <18 and ≥15) not allowing pain assessment with the NRS, the Pain Assessment in Advanced Dementia (PAINAD) scale was employed, with a total score ranging from 0 to 10 and a classification of pain intensities similar to that of the NRS.23 The PAINAID scale is designed for nonverbal elderly patients with dementia. Pain is assessed by trained personnel, after observing patient behavior (breathing, negative vocalization, facial expression, body language, and consolability) for 5 minutes. The use of rescue medication was also recorded throughout the 45-day observation period as an indirect measure of the analgesic efficacy of OXN-PR. Furthermore, the efficacy of analgesic treatment was also assessed by investigating patient perception of treatment effectiveness at T7, T15, and T45, based on a 7-point patient global impression of change scale. The 7-point scale ranged from +3 (very much improved) to −3 (very much worse). Patients were assisted by caregivers during patient global impression of change evaluation, if needed.
The study also investigated the effects of treatment with OXN-PR on neuropsychiatric symptoms and psychopathology of dementia at all study visits using the Neuropsychiatric Inventory (NPI), a questionnaire developed to characterize the neuropsychiatric symptom profile in neurological diseases (maximum score, 144; NPI <20, mild symptoms; NPI ≥20 and <50, moderate symptoms; NPI ≥50, severe symptoms).24
Changes in bowel function during treatment with OXN-PR were also recorded. To this purpose, the Bowel Function Index (BFI), a patient-reported, 3-item questionnaire, was administered at every visit and completed with the assistance of caregivers, if needed.25 The BFI is a validated measure of general bowel function during the previous 7 days, with the total score ranging from 0 (no symptoms) to 100 (most severe symptoms). In patients with chronic pain, normal bowel function is defined as a BFI score ≤29, and changes in BFI score ≥12 points are considered as clinically meaningful.25 As an indirect measure of bowel function, the frequency of laxative use during treatment was also recorded.
Daily functioning and comorbidities were assessed at baseline and study end (T45). Daily functioning, that is, patient degree of independence from any help and performances over the preceding 24–48 hours, was evaluated using the Barthel activities of daily living index.26 The maximum score is 100 (scores ranging between 80 and 100 indicate that the patient is independent; 60–79, patient needs minimal help with activities of daily living; 40–59, patient is partially dependent; 20–39, patient is very dependent; <20, patient is totally dependent).27 Comorbidities were assessed using the Cumulative Index Illness Rating Scale, which rates 14 body systems on a 5-point severity scale and was adapted to be used in geriatrics (Cumulative Illness Rating Scale-[CIRS]-Geriatric version).28,29 Mean number of affected organ systems (CIRS-comorbidity index) and mean severity score (CIRS-severity index) were recorded.
Safety evaluations were performed at baseline and at T7, T15, and T45 visits by recording the presence and intensity of symptoms commonly related to opioid treatment (sweating, nausea/vomiting, drowsiness, dizziness, mouth dryness, pruritus, tremor) that occurred and/or worsened in intensity and/or frequency before and after the first intake of OXN-PR treatment, and any other adverse drug reaction reported during the observation; the potential correlation between the AE and OXN-PR was judged by the pain physician. Symptom intensity was graded as mild (not requiring any specific treatment or ONX-PR dose reduction), moderate (not allowing any OXN-PR dose increase or requiring OXN-PR dose reduction), or severe (requiring OXN-PR discontinuation).
Statistical analysis
The primary efficacy measure of the study, the response rate to OXN-PR at T15, was defined as the proportion of patients who achieved a ≥30% reduction in pain intensity (NRS or PAINAD scores) from baseline to 15 days of treatment with OXN-PR. Secondary efficacy measures included the absolute and percent changes in NRS, NPI, BFI, and CIRS scores from T0 to T7–T45 as well as the proportions of patients reporting satisfaction with pain relief throughout the observation.
To determine the size of the sample to be included in the observation, it was estimated that clinically relevant observations could be made on a population composed of at least 70% of responders. Given the short duration of the observation and the low doses of OXN-PR planned for this study, the lower limit of the observed patient proportion was set at 50%. With a power of 80% and a significance level of 0.05, a minimum of 39 patients was therefore needed. The minimum number of patients to be enrolled was set at 47 to allow for incomplete data (~10%) and premature drug discontinuation (~10%).
Data were analyzed by descriptive statistics. Normality distributions of continuous variables were assessed by the Shapiro–Wilk test. The significance of differences between pairs of continuous variables was evaluated by the Student’s t-test or the Wilcoxon test, as appropriate. Changes in continuous variables over time were evaluated by an analysis of variance with a post hoc Bonferroni’s correction to adjust for multiple comparisons or by the Kruskal–Wallis test. Changes in categorical variables over time were compared using the Cochran’s Q test. The analyses were performed on the intention-to-treat population, which consisted of all enrolled patients who initiated treatment with OXN-PR treatment and had at least one postbaseline assessment. Linear interpolation was used to impute intermittent missing scores and the last observation carried forward method was used to impute missing scores after early discontinuation. The corresponding last observation carried forward data set built the basis for all primary and secondary endpoint analyses. A P-value <0.05 was considered statistically significant. Analyses were performed using the STATISTICA software, version 8.0 (StatSoft® Inc., Tulsa, OK, USA).
Results
Overall, 147 elderly patients were screened. Of these, 54 met the inclusion criteria and were enrolled in the study (Figure 1). One patient moved to another nursing home shortly after enrollment and was lost to follow-up. The remaining 53 patients (36% of the screened population) constituted the intention-to-treat population included in the analysis. Treatment with OXN-PR was discontinued prematurely by five patients because of the achievement of satisfactory pain control. No patient discontinued treatment due to lack of efficacy or tolerability/safety issues.
Figure 1.
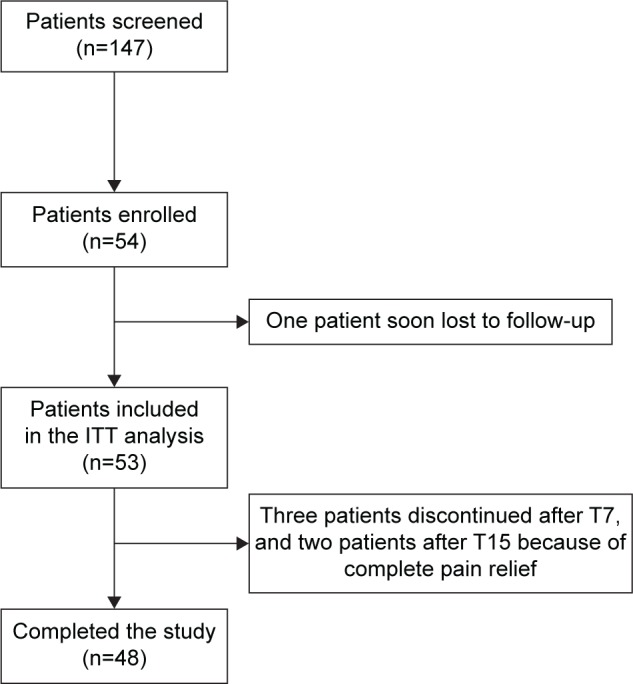
Patient disposition.
Abbreviations: ITT, intention-to-treat; T7 and T15, respectively, 7- and 15-day treatment with prolonged-release oxycodone/naloxone.
Demographic and baseline clinical and pain characteristics of the intention-to-treat population are shown in Table 1. Two-thirds of the patients were older than 80 years (median age 83 [range: 66–97] years). Mean (± standard deviation) MMSE score at baseline was 18.6 (±2.6) confirming the presence of mild-to-moderate cognitive impairment, in agreement with the inclusion criteria. Cognitive impairment was moderate (MMSE <18 and ≥15) in 23 patients (43.4%). At baseline, 29 (54.7%) patients reported severe pain (NRS or PAINAD score >6) despite analgesic and adjuvant therapies. The median NRS was 7 (range: 4–10), as was the median PAINAD (range: 4–9). Pain was mainly nociceptive (88.7% of patients), which caused by osteoarthritis, bone fractures, or recent arthroplasty (94.3% of patients). Acetaminophen was the predominant current analgesic therapy (18/53, 34%), followed by weak opioids (6/53, 11.3%) and NSAIDs (5/53, 9.4%). A total of 22 patients (41.5%) did not receive any analgesic therapy. Adjuvants were used by 18 patients (34.0%) and rescue analgesics by 17 patients (32.1%). The mean Barthel index (32.2) suggested a high degree of dependence in the performance of daily activities, while the mean NPI score (25.5) was indicative of moderate neuropsychiatric symptoms. As expected for an elderly population, patients were affected by multiple comorbidities (mean CIRS-comorbidity index, 3.9) of moderate severity (mean CIRS-severity index, 2.1).
Table 1.
Demographic and baseline characteristics of the intention-to-treat study population (n=53)
| Characteristic | All patients (n=53) |
|---|---|
| Age, years | 83.0±5.9 |
| >80 years, n (%) | 34 (64.1) |
| Females/males, n (%) | 35/18 (66.0/34.0) |
| Pain intensity | |
| NRS | 6.6±1.0 |
| PAINAD | 6.9±1.6 |
| Severe pain,a n (%) | 29 (54.7) |
| Nociceptive | 47 (88.7) |
| Mixed | 3 (5.6) |
| Neuropathic, peripheral | 2 (3.8) |
| Neuropathic, central | 1 (1.9) |
| Cause of pain, n (%) | |
| Osteoarthritis | 25 (47.2) |
| Bone fractures | 19 (35.8) |
| Arthroplasty | 6 (11.3) |
| Diabetic polyneuropathy | 2 (3.8) |
| Stroke | 1 (1.9) |
| Analgesic therapy, n (%) | |
| No analgesics | 22 (41.5) |
| Acetaminophen | 18 (34.0) |
| NSAIDs | 7 (13.2) |
| Codeine | 3 (5.6) |
| Tramadol | 3 (5.6) |
| Adjuvants, n (%) | 18 (34.0) |
| Rescue analgesics,b n (%) | 17 (32.1) |
| MMSE | 18.6±3.0 |
| NPI | 25.5±27.3 |
| Barthel index | 32.2±16.8 |
| CIRS-G Severity Index | 2.1±0.7 |
| CIRS-G Comorbidity Index | 3.9±1.7 |
| BFI | 25.6±19.7 |
| Use of laxatives, n (%) | 21 (39.6) |
Notes: All values are expressed as mean ± standard deviation or n (%).
Severe pain defined by NRS or PAINAD score >6.
Oral acetaminophen, 500–1,000 mg, taken in addition to current analgesics.
Abbreviations: BFI, Bowel Function Index; CIRS-G, Cumulative Illness Rating Scale-Geriatric version; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; NRS, numerical rating scale; PAINAD, Pain Assessment in Advanced Dementia; NSAIDs, nonsteroidal anti-inflammatory drugs.
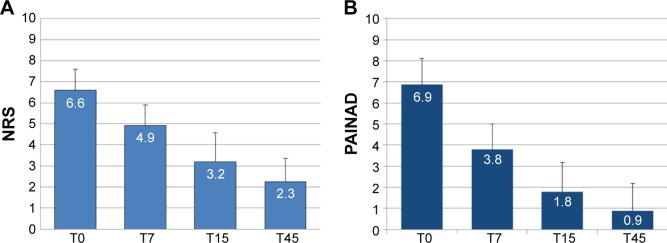
Patients initiated treatment with OXN-PR at a mean (± standard deviation) daily dose of 10.8±4.9 mg of oxycodone (median 10 [range: 5–20] mg). Daily OXN-PR doses increased significantly but modestly (Figure 2), and remained below 20 mg throughout the observation in more than half of the population (n=30, 56.6%); only seven (13.2%) patients required daily OXN-PR doses >20 mg. The treatment with OXN-PR was associated with substantial and statistically significant decreases in pain intensity, as assessed by NRS or PAINAD depending on the cognitive ability of patients, from baseline to all time points considered (Figure 3). At T15, the primary study endpoint (ie, reduction from baseline to T15 in mean pain intensity ≥30%) was achieved by 49 (92.4%) patients. Mean pain intensity decreased from 6.6±1.0 at baseline to 2.3±1.1 at T45 as assessed by the NRS, and from 6.9±1.6 to 0.9±0.8 in patients assessed using the PAINAD (Figure 3). The mean percentage change in pain intensity at T45 versus baseline was −47.5%±28.1% (median −50% [range: −100% to 4.8%]). Of note, a tendency toward a decreased use of rescue medication and adjuvants over the 45 days of treatment with OXN-PR was observed (Table 2).
Figure 2.
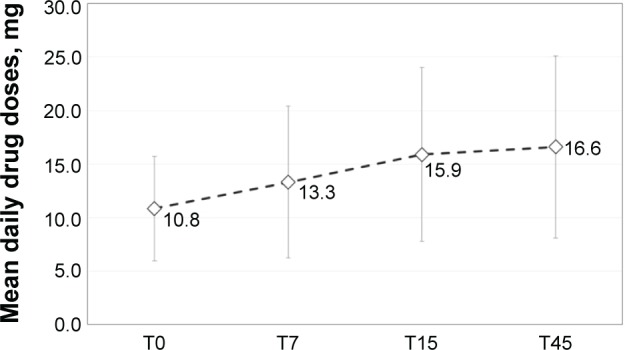
Changes in mean daily prolonged-release oxycodone/naloxone (OXN-PR) doses during the 45-day treatment.
Notes: Mean and standard deviation values are shown. Oxycodone doses (mg per day) are reported. P<0.001 for T0 versus T45. T0, baseline and OXN-PR initiation; T7, T15, T45, respectively, 7-, 15-, and 45 days after beginning of OXN-PR treatment.
Figure 3.
Changes in pain intensity scores during treatment with prolonged-release oxycodone/naloxone (OXN-PR).
Notes: (A) Pain assessed using a numerical rating scale (NRS) (n=30); P<0.001 for T7 versus T0, T15 versus T7, and T45 versus T15. (B) Pain assessed using the Pain Assessment in Advanced Dementia (PAINAD) questionnaire in patients whose pain could not be assessed using the NRS (n=23, see text for further explanations); P<0.001 for T7 versus T0, and T15 versus T7; P<0.01 for T45 versus T15. T0, baseline (OXN-PR initiation); T7, T15, T45, respectively, 7-, 15-, and 45 days after beginning of OXN-PR treatment.
Table 2.
Changes in secondary efficacy variables during treatment with prolonged-release oxycodone/naloxone (OXN-PR)
| Variable | Baseline | T7 | T15 | T45 | P-value |
|---|---|---|---|---|---|
| Rescue analgesics | 17 (32.1) | 16 (30.2) | 14 (26.4) | 12 (22.6) | 0.24a |
| Adjuvants | 18 (34.0) | 14 (26.4) | 15 (28.3) | 13 (24.5) | 0.34a |
| NPI | 25.5±27.3 | 20.6±23.0 | 11.6±11.3c | 8.8±9.0 | <0.0001b |
| BFI | 25.6±19.7 | 24.5±17.9 | 19.7±16.2c | 18.1±16.9 | <0.0001b |
| Laxatives | 21 (39.6) | 30 (56.6)c | 32 (60.4) | 31 (58.5) | 0.047a |
Notes: All values are expressed as mean ± standard deviation or n (%). T45 versus baseline.
Chi-square for trend;
analysis of variance;
P<0.05 versus previous observation.
Abbreviations: BFI, Bowel Function Index; NPI, Neuropsychiatric Inventory; T7, T15, and T45, respectively, 7-, 15-, and 45-day treatment with OXN-PR.
The treatment with OXN-PR was not associated with a worsening in the number and severity of existing comorbidities, as shown by clinically irrelevant changes in the CIRS-Geriatric version scores from baseline to T45 (mean CIRS-comorbidity index score from 3.9±1.7 to 3.7±1.6; mean CIRS-severity index from 2.1±0.7 to 2.0±0.8; both not statistically significant).
A marked improvement in the ability to perform daily activities was found at T45, as shown by a substantial increase in the Barthel index, from 32.2±16.8 (marked dependence) at baseline to 53.7±23.9 (partial dependence) at T45 (P<0.0001). The proportion of totally or very dependent patients (Barthel index 0–39) significantly and substantially decreased from 71.7% at baseline to 28.3% at T45 (P=0.001) (Figure 4).
Figure 4.
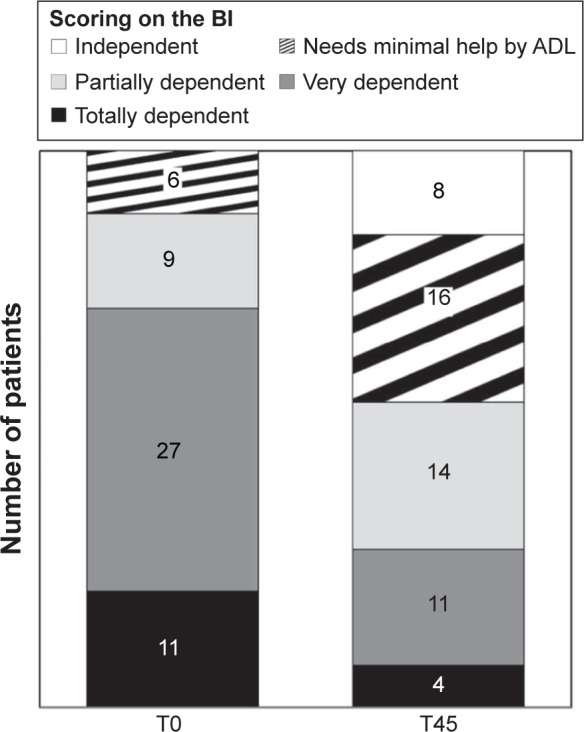
Pattern of physical disabilities at baseline (T0) and after the 45-day treatment with prolonged-release oxycodone/naloxone (T45), as measured by the Barthel activities of daily living (ADL) index.
Note: Differences between T0 and T45 were statistically significant (P<0.001 for all comparisons).
Abbreviation: BI, Barthel activities of daily living index.
The NPI score, as well, significantly improved after OXN-PR (P<0.001) (Table 2). The proportion of patients with moderate or severe symptoms (NPI score ≥50) noticeably decreased from 39.6% at baseline to 11.3% at T45 (P=0.001).
At baseline, the mean BFI value in our patients was normal (25.6±19.7, normal values <29/100), and 39.4% of subjects were already on laxatives. Although BFI values at entry were abnormal in 26 (49.0%) subjects, only five (9.4%) patients had BFI values >60/100, thus indicating severe constipation. According to MMSE score (15–17 or ≥18), BFI values were not dissimilar between patients with mild (24.0±20.8, range: 0–70) or moderate cognitive impairment (27.5±18.5, range: 0–70) (P=0.53).
The treatment with OXN-PR was associated with a progressive and significant improvement in bowel function from baseline (P<0.001, Table 2). On the other hand, there was a moderate but significant (P=0.04) increase in laxative consumption during the first week of treatment, after which laxative use remained stable (Table 2).
Overall, patient satisfaction with OXN-PR treatment was high. The proportion of patients perceiving a substantial improvement in their health status significantly increased during treatment with OXN-PR (Figure 5). At T7, T15, and T45, respectively, 22.6% (12/53), 56.6% (30/53), and 67.9% (36/53) of patients found their condition much or very much improved with OXN-PR compared with the previous treatment.
Figure 5.
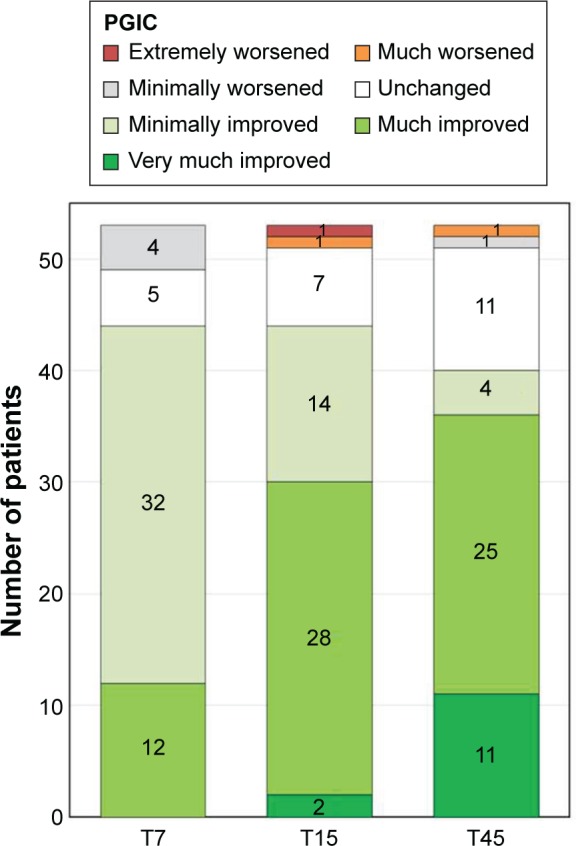
Patients’ perception of change in their health status (PGIC) during treatment with prolonged-release oxycodone/naloxone (OXN-PR).
Note: T7, T15, and T45: 7, 15, and 45 days, respectively, after beginning of OXN-PR treatment.
Abbreviation: PGIC, patient global impression of change.
Treatment with OXN-PR was generally well tolerated. Prevalence of common opioid-related symptoms (ie, nausea, tremor, drowsiness, dizziness, itching, and dry mouth) at baseline and different time points of the observation is shown in Table 3; the most frequently reported were drowsiness (five patients, 9.4%) and nausea (three patients, 5.7%), which were all of mild intensity. Neither severe symptoms nor other adverse drug reactions were reported during the 45-day treatment, and no patient discontinued OXN-PR therapy or required a reduction of the OXN-PR dose due to side effects. According to MMSE score (15–17 or ≥18), more side effects were reported during the observation by patients with mild cognitive impairment (14 AEs reported by four patients, range: 1–8 for each) than in moderate cognitive impairment (one AE reported by only one patient). Given the limited number of patients reporting side effects, the different proportions (13.3% vs 7.7%) in mild versus moderate cognitive impairment did not achieve statistical significance (odds ratio 1.85; 95% confidence interval 0.18, −18.3).
Table 3.
Summary of patients affected by disorders commonly related to opioids at baseline and during treatment with prolonged-release oxycodone/naloxone (OXN-PR)
| Symptoms | Patients, n (%)
|
||||
|---|---|---|---|---|---|
| Baseline | T7 | T15 | T45 | P-valuea | |
| Nausea and vomiting | 0 | 2 | 0 | 1 | NS |
| Pruritus | 1 | 0 | 0 | 0 | NS |
| Drowsiness | 2 | 2 | 2 | 1 | NS |
| Mouth dryness | 2 | 2 | 0 | 0 | NS |
| Vertigo | 1 | 0 | 0 | 0 | NS |
| Hyperhidrosis | 3 | 0 | 1 | 0 | NS |
Note:
Cochran’s Q test for changes in frequencies from day 0 to day 45.
Abbreviations: NS, not statistically significant; T7, T15, and T45, respectively, 7-, 15-, and 45-day treatment with OXN-PR.
Discussion
This pilot study investigated for the first time the efficacy and safety of OXN-PR in elderly patients with chronic pain and cognitive impairment. Similar to what has been described in several reports on nursing home residents with chronic pain, the patients of the present study appeared to receive inadequate analgesic treatment, if any, at baseline despite their pain being mostly of severe intensity. Indeed, ~40% of patients did not receive any analgesic treatment at study entry. The predominant analgesic used at baseline was acetaminophen, a drug indisputably insufficient to control severe pain.30 The switch to low-dose OXN-PR was associated with a marked improvement in pain control, with the majority of patients (92.4%) achieving the primary efficacy endpoint of ≥30% decrease in pain intensity after 15 days of treatment with OXN-PR. The observed reduction in pain intensity was also reflected in the fact that in almost 70% of patients, the health status was described as much or very much improved after 45 days of OXN-PR treatment. Not surprisingly, the significant improvement in pain relief was also accompanied by a marked increase in patient autonomy in activities of daily living. A significant improvement was observed also in neuropsychiatric symptoms.
Cognitive impairment remains a major risk factor for pain undertreatment.5 Data from a survey conducted among nursing home residents with cognitive impairment showed that 62% of them reported pain and that pain control was generally not adequate.1 A retrospective analysis involving over 4,000 cancer patients aged ≥65 years and admitted to nursing homes identified low cognitive performance as an independent predictor of lack of analgesic prescription.7 Dementia was found to correlate with a lower prevalence of reported pain and analgesic use in a survey conducted among nursing home residents aged ≥75 years, highlighting the fact that the inability to communicate and remember constitutes a major barrier in the assessment and appropriate management of pain.8
Although standardized tools for pain assessment in older adults with dementia are still lacking,31 the awareness of the problems related to pain management in this complex patient population has significantly increased in recent years. According to the latest international guidelines for the treatment of persistent pain in older persons, the presence of mild-to-moderate cognitive impairment does not preclude pain assessment that can be made using simple questions and tools developed specifically for this purpose.3,4
All patients with moderate-to-severe pain, or with pain causing functional impairment or reducing quality of life, should be considered for opioid therapy.3,4 In older people, opioids may be associated with a lower risk than NSAIDs.3 In recent years, the efficacy of weak opioids (or step-II analgesics according to the analgesic ladder proposed by the World Health Organization [eg, codeine or tramadol])21 has been called into question. As an alternative to step-II analgesics, low-dose strong opioids (or step-III analgesics according to the World Health Organization ladder) are increasingly recommended by current guidelines on pain management in various settings, including geriatric patients.11 Opioid-related side effects, in particular gastrointestinal effects, should be anticipated and prevented with suitable prophylaxis.
OXN-PR was developed to improve the gastrointestinal tolerability of opioids and is currently listed among the options for the prevention and management of opioid-related constipation by guidelines for the treatment of pain.3,11,32 In the present population of elderly patients, the treatment with OXN-PR was not associated with a worsening in bowel function, as shown by BFI scores that slightly improved over the 45 days of treatment. The increase of laxative consumption recorded mostly during the first week of treatment with OXN-PR was moderate, although statistically significant, and was likely prompted by the standard prophylactic laxative regimen recommended at study entry, in accordance with current guidelines on opioid therapy initiation. In fact, after the first week of treatment, laxative use did not change significantly, despite the gradual increase in OXN-PR doses.
The efficacy and tolerability of OXN-PR in elderly patients without cognitive impairment were recently investigated in two studies.18,19 Gatti et al,19 in an observational study involving 1,051 patients with chronic nonmalignant pain and constipation, found that the subgroup of patients aged >75 years had greater benefits from the treatment with OXN-PR in terms of magnitude of pain relief, decreased use of rescue medications and laxatives, more marked improvement in bowel function, and greater satisfactions with treatment. In a recent prospective observational study investigating the efficacy and safety of low-dose OXN-PR in older patients (mean age, 82 years), over 70% of patients responded to treatment (≥30% reduction in mean pain intensity from baseline to 4 weeks, without deterioration of bowel function).18 OXN-PR was associated with an improvement in daily functioning, as shown by significant increases in the Barthel activities of daily living index, without changes in cognitive status and bowel function. The treatment with OXN-PR was well tolerated and only one patient (1.9%) discontinued OXN-PR due to an adverse drug reaction (drowsiness).
As for the treatment of chronic pain in elderly patients with cognitive impairment, there is a lack of studies – in particular, large randomized controlled studies with pain intensity as the main outcome – investigating opioids and other analgesic therapies in this complex population. In patients with dementia, pain can manifest as agitation or aggression, and an association has been suggested between pain and neuropsychiatric symptoms of dementia.5,33 As shown by our findings, as well as by previous studies, pain treatment can indeed improve neuropsychiatric symptoms. Effective pain management is increasingly regarded as an important component of the treatment of agitation in patients with dementia, which may result in a reduction of unnecessary prescriptions of psychotropic drugs in this vulnerable population.34 A small study in older nursing home residents (median age, 85 years) with advanced dementia and severe agitation, despite the treatment with psychotropic drugs, tested the hypothesis that unrecognized pain in dementia may contribute to agitation and that low-dose long-acting opioids may reduce agitation by reducing pain.35 The study found that, compared with placebo, opioid treatment was associated with decreased levels of agitation, without an increase in sedation. The first large randomized controlled trial in nursing home residents with moderate-to-severe dementia and behavioral disturbances (n=352), designed to determine whether pain treatment could reduce agitation, found that daily pain treatment according to a stepwise protocol with acetaminophen, morphine, buprenorphine transdermal patch, or pregabalin significantly reduced agitation after 8 weeks compared with conventional treatment (psychotropic drugs).34 Treatment of pain was also significantly beneficial for the overall severity of neuropsychiatric symptoms. As most patients in the pain treatment group received only acetaminophen, the observed effect was unlikely to be due to sedation caused by stronger analgesics. A secondary analysis found that mood symptoms of dementia, including depression, also responded to pain treatment.36
The 45-day treatment with low-dose OXN-PR was well tolerated by the elderly patients of the present study, affected on average by multiple comorbidities. In addition, our patients were all naïve to strong opioids (a requirement for inclusion in the analysis), most of them (89%) were naïve to any opioid, and more than 40% were not receiving any analgesic therapy at baseline. Despite these characteristics, the switch to OXN-PR, or its initiation, was easy to perform and well tolerated. This is noteworthy especially considering that the fear of opioid-related AEs, including respiratory depression, functional and cognitive impairment, and development of drug dependence, is one of, and perhaps, the major reason for the persistent reluctance to prescribe strong opioids to older individuals. Clearly, long-term studies are necessary for a thorough evaluation of OXN-PR safety in chronic pain management. The reported AEs in the present study, the majority of which were already present at baseline and may have been related to previous treatments, were all of mild intensity and did not require any treatment or change in OXN-PR dose. This may be a consequence of the low OXN-PR dosages used. Notably, the frequency of nausea/vomiting, a common opioid-related side effect, was low. A possible explanation is that the combination of oxycodone with the opioid antagonist naloxone improves not only constipation but also other opioid-related gastrointestinal effects, as suggested by other authors.37
Limitations
This study had several limitations, including the open-label, observational design, lack of a control group, small sample size, and short treatment duration. Assessing pain and other patient-reported variables was challenging in subjects with mild-to-moderate cognitive impairment, and might constitute an additional important limitation of this investigation. In particular, the findings based on BFI and patient global impression of change assessments require careful interpretation, as these evaluation tools have been standardized in subjects with no impairment of cognitive function. In the present study, these assessments relied on patient self-report and/or feelings of their caregivers. With regard to bowel function, the use of laxatives, which is an indirect but more objective measure of bowel function, increased during the first week of treatment and was stable afterward, suggesting no deterioration of bowel function in line with the BFI findings. As for pain intensity assessment, the significant reduction in the use of rescue medication suggested an improvement of analgesia, in line with the NRS and PAINAD assessments of pain intensity. Despite these limitations, the results of this pilot study conducted to explore the effectiveness of OXN-PR, as well as to test the feasibility of the assessment of treatment outcomes in cognitively impaired older individuals, are encouraging both in terms of treatment outcomes and study feasibility and deserve to be further analyzed in more adequately designed trials.
Conclusion
In this pilot study in elderly residents of nursing homes with mild-to-moderate cognitive impairment, OXN-PR was effective on pain symptoms with a favorable safety and tolerability profile. Adequate pain control was associated with substantially improved daily functioning and neuropsychiatric symptoms. OXN-PR appears therefore as an interesting option for analgesia in patients affected with dementia. Large-scale trials in older patients with dementia are needed to improve clinical guidance for the assessment and treatment of pain in these fragile subjects.
Acknowledgments
Editorial assistance was provided by Ray Hill, an independent medical writer, on behalf of HPS Publishing & Services Srl. This assistance was funded by Mundipharma Pharmaceuticals Srl, Italy. The study was independently completed with no funding.
Footnotes
Author contributions
EP, ER, MC, VG, GB, SF, and CL designed the study and were responsible for data collection. CM was the project statistician and oversaw data analysis and interpretation. All authors met ICMJE criteria and those who fulfilled the criteria are listed as authors. All authors had access to the study data, provided direction and formal review of the manuscript, and made the final decision about where to publish these data.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10(8):591–598. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 2.Gibson SJ, Lussier D. Prevalence and relevance of pain in older persons. Pain Med. 2012;13(Suppl 2):S23–S26. doi: 10.1111/j.1526-4637.2012.01349.x. [DOI] [PubMed] [Google Scholar]
- 3.Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1–i57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 4.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 5.Achterberg WP, Pieper MJ, van Dalen-Kok AH, et al. Pain management in patients with dementia. Clin Interv Aging. 2013;8:1471–1482. doi: 10.2147/CIA.S36739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dementia: A public health priority. World Health Organization; 2012. [Accessed February 9, 2016]. Available from: http://apps.who.int/iris/bitstream/10665/75263/1/9789241564458_eng.pdf. [Google Scholar]
- 7.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic assessment of geriatric drug use via epidemiology. JAMA. 1998;279(23):1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 8.Mäntyselkä P, Hartikainen S, Louhivuori-Laako K, Sulkava R. Effects of dementia on perceived daily pain in home-dwelling elderly people: a population-based study. Age Ageing. 2004;33(5):496–499. doi: 10.1093/ageing/afh165. [DOI] [PubMed] [Google Scholar]
- 9.Monroe TB, Misra SK, Habermann RC, Dietrich MS, Cowan RL, Simmons SF. Pain reports and pain medication treatment in nursing home residents with and without dementia. Geriatr Gerontol Int. 2014;14(3):541–548. doi: 10.1111/ggi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shega JW, Paice JA, Rockwood K, Dale W. Is the presence of mild to moderate cognitive impairment associated with self-report of non-cancer pain? A cross-sectional analysis of a large population-based study. J Pain Symptom Manage. 2010;39(4):734–742. doi: 10.1016/j.jpainsymman.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106(5):835–842. doi: 10.1038/ajg.2011.30. [DOI] [PubMed] [Google Scholar]
- 13.Reimer K, Hopp M, Zenz M, et al. Meeting the challenges of opioid-induced constipation in chronic pain management – a novel approach. Pharmacology. 2009;83(1):10–17. doi: 10.1159/000165778. [DOI] [PubMed] [Google Scholar]
- 14.Ueberall MA, Mueller-Schwefe GH. Safety and efficacy of oxycodone/naloxone vs. oxycodone vs. morphine for the treatment of chronic low back pain: results of a 12 week prospective, randomized, open-label blinded endpoint streamlined study with prolonged-release preparations. Curr Med Res Opin. 2015;31(7):1413–1429. doi: 10.1185/03007995.2015.1047747. [DOI] [PubMed] [Google Scholar]
- 15.Simpson K, Leyendecker P, Hopp M, et al. Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin. 2008;24(12):3503–3512. doi: 10.1185/03007990802584454. [DOI] [PubMed] [Google Scholar]
- 16.Vondrackova D, Leyendecker P, Meissner W, et al. Analgesic efficacy and safety of oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J Pain. 2008;9(12):1144–1154. doi: 10.1016/j.jpain.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein O, Leyendecker P, Hopp M, et al. Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: a randomised controlled trial. Expert Opin Pharmacother. 2009;10(4):531–543. doi: 10.1517/14656560902796798. [DOI] [PubMed] [Google Scholar]
- 18.Guerriero F, Sgarlata C, Marcassa C, Ricevuti G, Rollone M. Efficacy and tolerability of low-dose oral prolonged-release oxycodone/naloxone for chronic nononcological pain in older patients. Clin Interv Aging. 2015;10:1–11. doi: 10.2147/CIA.S72521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatti A, Casali M, Lazzari M, et al. Prolonged-release oxycodone/naloxone in nonmalignant pain: single-center study in patients with constipation. Adv Ther. 2013;30(1):41–59. doi: 10.1007/s12325-012-0074-0. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva: World Health Organization; 1996. [Google Scholar]
- 22.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 23.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4(1):9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 24.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 25.Rentz AM, Yu R, Muller-Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12(4):371–383. doi: 10.3111/13696990903430481. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney FI, Barthel DW. Function evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 27.Sinoff G, Ore L. The Barthel activities of daily living index: self- reporting versus actual performance in the old-old (> or =75 years) J Am Geriatr Soc. 1997;45(7):832–836. doi: 10.1111/j.1532-5415.1997.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 28.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 29.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 30.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtner V, Dowding D, Esterhuizen P, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014;14:138. doi: 10.1186/1471-2318-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol. 2012;23(Suppl 7):vii139–vii154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 33.Flo E, Gulla C, Husebo BS. Effective pain management in patients with dementia: benefits beyond pain? Drugs Aging. 2014;31(12):863–871. doi: 10.1007/s40266-014-0222-0. [DOI] [PubMed] [Google Scholar]
- 34.Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manfredi PL, Breuer B, Wallenstein S, Stegmann M, Bottomley G, Libow L. Opioid treatment for agitation in patients with advanced dementia. Int J Geriatr Psychiatry. 2003;18(8):700–705. doi: 10.1002/gps.906. [DOI] [PubMed] [Google Scholar]
- 36.Husebo BS, Ballard C, Fritze F, Sandvik RK, Aarsland D. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. Int J Geriatr Psychiatry. 2014;29(8):828–836. doi: 10.1002/gps.4063. [DOI] [PubMed] [Google Scholar]
- 37.Schutter U, Grunert S, Meyer C, Schmidt T, Nolte T. Innovative pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: a large observational study under conditions of daily practice. Curr Med Res Opin. 2010;26(6):1377–1387. doi: 10.1185/03007991003787318. [DOI] [PubMed] [Google Scholar]