Abstract
Nicotinic acetylcholine receptors are ligand-gated ion channels that exogenously bind nicotine. Nicotine produces rewarding effects by interacting with these receptors in the brain’s reward system. Unlike other receptors, chronic stimulation by an agonist induces an upregulation of receptor number that is not due to increased gene expression in adults; while upregulation also occurs during development and adolescence there have been some opposing findings regarding a change in corresponding gene expression. These receptors have also been well studied with regard to human genetic associations and, based on evidence suggesting shared genetic liabilities between substance use disorders, numerous studies have pointed to a role for this system in comorbid drug use. This review will focus on upregulation of these receptors in adulthood, adolescence, and development, as well as the findings from human genetic association studies which point to different roles for these receptors in risk for initiation and continuation of drug use.
Keywords: CHRN genes, review, nicotine-induced receptor upregulation, comorbid drug use, developmental changes and nAChRs
Background and Significance
Drug use
Although alcohol and tobacco use are legal, they contribute to severe and widespread problems. Worldwide, 3.3 million people die each year due to the harmful use of alcohol, representing 5.9% of worldwide deaths. Furthermore, 5.1% of the global burden of disease and injury is attributed to alcohol and recent causal relationships have been established between harmful drinking and occurrence of infectious diseases such as tuberculosis and HIV/AIDS (WHO). As of July 2015, tobacco was estimated to kill up to half of its users (WHO). In the U.S. alone, 1 in 5 deaths are attributable to smoking (CDC), and an additional 6.8 million people suffer from a serious illness caused by smoking (CDC).
Over the years spanning 2005 and 2010 between 3.4 and 6.6% of the adult population aged 15–64 used illicit drugs. Roughly 10 to 13% of these users subsequently developed drug dependence and/or a drug use disorder with high prevalence rates of serious disorders such as HIV, hepatitis C, and hepatitis B. Illicit drug use is responsible for approximately 1 in every 100 deaths among adults (UNODC). In America illicit drug use is increasing; in 2012 9.2% of the population aged 12 or older had used an illicit drug or abused a psychotherapeutic medication in the past month. Finally, 52% of new drug users are under 18, illustrating the importance of studying these behaviors during development since most people use drugs for the first time in their teenage years (NIDA).
Evidence for shared genetic influences between different classes of drugs
Epidemiological and familial studies have shown that comorbidity among substance use disorders (SUDs, i.e. meeting abuse or dependence criteria for more than one legal or illegal drug) is high (Bierut et al., 1998, Kapusta et al., 2007, Kendler et al., 1997, Kessler et al., 1997, Merikangas et al., 1998, Pickens et al., 1995). Converging evidence from twin studies highlights the importance of genetic factors on SUDs with estimates of heritability ranging from 0.30–0.70 (Agrawal & Lynskey, 2008). Furthermore, although genetic factors specific to each substance have been identified, research has indicated that a common genetic factor underlies much of the variation in SUDs in adults (Agrawal et al., 2004, Kendler et al., 2003, Palmer et al., 2015, Palmer et al., 2012, True et al., 1999a, True et al., 1999b, Tsuang et al., 2001, Xian et al., 2008). Although work by Kendler and colleagues has implicated two underlying genetic factors with separate influences on licit and illicit drugs, these factors where shown to be highly correlated (r = 0.82) (Kendler et al., 2007). These results point to a common mechanism in the development of SUDs (Vanyukov et al., 2003).
Similar estimates have been seen for SUDs in adolescence, indicating an underlying genetic liability for substance use (Hopfer et al., 2003). Problem use has been shown to be more heritable than initiation or regular use in adolescents (Rhee et al., 2003) and twin analyses have shown significant genetic correlations for problem use across substances (Young et al., 2006). Substance use is a developmental problem that increases linearly with age (Young et al., 2002) and common genetic factors have been suggested to be particularly important for early onset SUDs that emerge in late adolescence and early adulthood (Iacono et al., 2008, Palmer et al., 2009). Similar to findings in adults, a study by Rhee and colleagues suggested two hypotheses for the comorbidity between alcohol and illicit drug dependence in adolescents: a single general liability, or two highly correlated separate liabilities (Rhee et al., 2006). Finally, tobacco has been shown to pose the greatest substance-specific risk for developing subsequent use problems (Palmer et al., 2009) and as such the remainder of this review will focus specifically on the effects of tobacco and the receptors to which it binds in the brain.
Nicotinic acetylcholine receptors
Physiology
Although there are many compounds in tobacco smoke, nicotine is considered to be the major addictive component of tobacco smoke (Gunby, 1988, Rose, 2006, Stolerman & Jarvis, 1995). Nicotine binds muscle and neuronal nicotinic acetylcholine receptors (nAChRs, encoded by the CHRN genes), members of a family of ligand-gated ion channels, in the peripheral and central nervous systems (Gotti et al., 2009). They are pentameric receptors composed of various subunits ranging from alpha1-alpha10 (α1-α10) and beta1-beta4 (β1-β4) (discussed in more detail below) clustered around a central ion pore. Upon stimulation by nicotine, the five subunits undergo a conformational change causing the central pore to open, allowing extracellular ions to enter the cell. Nicotine is able to cross the blood-brain barrier and bind neuronal nAChRs in the brain (Clarke, 1987), producing rewarding effects by interacting with nAChRs in the brain’s reward system (Changeux, 2010).
Muscle nicotinic receptors
Muscle nAChRs all have the same pentameric stoichiometry of (α1)2β1δγ (fetal-type) or (α1)2β1δγ (adult-type) (Le Novere et al., 2002). Although primarily known to be expressed in muscle tissue, previous work has suggested that subunits of these receptors are also present in mammalian ciliary ganglia (Pugh et al., 1995), vestibular and cochlear hair cells (Scheffer et al., 2007), as well as brain regions such as the cortex, hippocampus, and cerebellum (Ghedini et al., 2010). In addition, a splice variant of the α1 subunit was shown to expressed in numerous locations including brain, kidney, heart, liver, lung, and thymus in humans (Talib et al., 1993).
Neuronal nicotinic receptors
Neuronal nAChRs are comprised of combinations of the α2-α10 and β2-β4 subunits with variable stoichiometry that are expressed in the central and peripheral nervous systems, located both presynaptically and postsynaptically (Millar & Gotti, 2009). All of the subunits are expressed in the mammalian central nervous system with the exception of the α8 subunit, which is only expressed in chicks (Britto et al., 1992, Schoepfer et al., 1990). Neuronal nAChRs can assemble in two ways; they can form homomeric receptors in which all five subunits are the same (α7, α9), or they can form heteromeric receptors in which the five subunits that make up the functional receptor include different α and β subunit combinations, which yield different pharmacological properties.
In the mammalian system, α7 and α9 subunits form homomeric receptors when expressed as isolated subunits in cells (Couturier et al., 1990, Elgoyhen et al., 1994, Schoepfer et al., 1990). The α10 subunit, although it cannot form a functional homomeric receptor, is most closely related to the α9 subunit and forms a functional receptor when coexpressed with the α9 subunit (Elgoyhen et al., 1994, Lustig et al., 2001, Sgard et al., 2002). In the central nervous system, the α9 and α10 subunits have been primarily identified in the inner ear cells, particularly the cochlear and vestibular hair cells (Elgoyhen et al., 1994, Elgoyhen et al., 2001, Luo et al., 1998, Lustig et al., 1999, Lustig et al., 2001, Vetter et al., 1999), as well as the pituitary (Sgard et al., 2002). The α7 subunit, however, is widely expressed throughout the mammalian central nervous system (reviewed in (Millar & Gotti, 2009)).
The rest of the neuronal nAChR subunits form heteromeric receptors consisting of α and β subunits (Anand et al., 1991, Boulter et al., 1987, Deneris et al., 1988, Wada et al., 1989). For heteromeric receptors, subunit composition varies, as does assembly; furthermore, the variable stoichiometry confers differences in calcium permeability and agonist and antagonist sensitivity between different receptors (Luetje & Patrick, 1991) (reviewed in (Millar & Gotti, 2009)). Studies have shown that α2-α4 and β2 and β4 subunits can form functional receptors when expressed as a pair-wise combination of one α subunit and one β subunit; functional expression has been observed for α2β4, α3β2, α3β4, α4β2, and α4β4 combinations (Duvoisin et al., 1989, Papke et al., 1989). The β3 and α5 subunits cannot form functional nAChRs unless expressed in combination with another α and β subunit pair, as neither of these possesses a binding site for an agonist or antagonist. Electrophysiological approaches have been used to distinguish triple pair subunits from pair subunits and subsequently identified receptors composed of the α3β2α5 (Gerzanich et al., 1998, Wang et al., 1996), α3β4α5 (Fucile et al., 1997, Gerzanich et al., 1998, Wang et al., 1996), and α4β2α5 (Ramirez-Latorre et al., 1996) subunits. In addition, the β3 subunit has been shown to co-assemble into a functional receptor composed of α3β3β4 subunits in oocytes (Groot-Kormelink et al., 1998), and may promote expression and stability of human α6 subunits in transfected cell lines (Tumkosit et al., 2006). Expression studies have also demonstrated that the α6 subunit assembles into functional triplet receptors, including α6β3β4 (Kuryatov et al., 2000, Tumkosit et al., 2006), as well as α3α6β4 and α3α6β2 (Fucile et al., 1998, Kuryatov et al., 2000) subunit combinations. Lastly, α6 and β2 combinations have also been observed, particularly as α4α6β2β3 and α6β2β3 receptors (Salminen et al., 2004). While neuronal nAChRs are widespread throughout the central and peripheral nervous systems, α4β2 (Anand et al., 1991, Flores et al., 1992, Wada et al., 1989) receptors are the most abundant heteromeric receptors in the brain and have the highest affinity for nicotine (Wonnacott, 1990).
Upregulation of nicotinic acetylcholine receptors
The response of nicotinic receptors during chronic exposure to agonists has been referred to as a paradox (Wonnacott, 1990), since they do not behave as other receptors during chronic exposure to an agonist. Note that an agonist binds and activates a receptor whereas an antagonist binds and blocks receptor activation. Numerous studies have shown that chronic exposure of a receptor to an antagonist typically leads to upregulation, or an increased number of receptors, while chronic exposure of a receptor to an agonist causes downregulation, or a decreased number of receptors (Creese & Sibley, 1981, Wonnacott, 1990). However, this phenomenon is not observed with some subtypes of nAChRs, most notably, the α4β2 nAChR. Upon extended exposure of nAChRs to an agonist, α4β2 receptor numbers are upregulated. It is important to clarify that for the purposes of this discussion, upregulation refers to an increase in receptor numbers (e.g. not upregulation of mRNA), and the studies mentioned below have focused exclusively on measuring protein levels to assess the number of nAChRs.
Early studies on the brains from rats (Schwartz & Kellar, 1983) and mice (Marks et al., 1983) repeatedly exposed to nicotine showed an increase in [3H]acetylcholine and (−)-[3H]nicotine binding, two radiolabeled nAChR ligands that predominantly measure α4β2 nAChRs. Schwartz and Kellar treated rats with 2 mg/kg of nicotine twice a day for 10 days and observed an increase in [3H]acetylcholine binding in cerebral cortex of rats (Schwartz & Kellar, 1983). Similarly, Marks et al. (1983) chronically infused mice with 5 mg/kg/hr nicotine for 8–10 days (mice are less sensitive to the effects of nicotine than rats are (Matta et al., 2007), accounting for the difference in nicotine treatments in these two experiments) and reported an increase in (−)-[3H]nicotine binding in the cortex, midbrain, hindbrain, hippocampus, and hypothalamus, but not striatum (Marks et al., 1983). These results were confirmed in human post mortem brains in 1988. By measuring (−)-[3H]nicotine binding in smokers compared to nonsmokers, Benwell and colleagues demonstrated that smokers showed higher levels of (−)-[3H]nicotine binding in most grey areas including the hippocampus, cortex, gyrus rectus and median raphe nucleus, with the exception of the medulla oblongata which showed no changes in (−)-[3H]nicotine binding. Importantly, in the areas where smoking was associated with increased (−)-[3H]nicotine binding, radiolabeled ligand binding increased by 50–100% (Benwell et al., 1988). In all three of the above studies, increased radiolabeled ligand binding was confirmed to be due to an increase in receptor number, rather than an increase in binding affinity (Benwell et al., 1988, Marks et al., 1983, Schwartz & Kellar, 1983). Thus, under chronic nicotine exposure, α4β2 nAChRs undergo a nicotine-induced upregulation of receptor numbers at the membrane.
Subsequent studies provided evidence that when nicotine exposure is ceased, decreased binding of (−)-[3H]nicotine occurs after 7–10 days in mice (Marks et al., 1985), 15–20 days in rats (Collins et al., 1990), and between 21 days and 2 months in humans (Breese et al., 1997, Mamede et al., 2007). In humans, nAChR upregulation was additionally shown to be dose-dependent (Breese et al., 1997, Perry et al., 1999). Gopalakrishnan et al. (1997) detected a significant increase in [3H]cytisine binding upon exposure to nicotine at concentrations as low as 100 nM, and a maximal 15-fold increase in binding at concentrations of 10 μM of nicotine in the human embryonic kidney cell line HEK293 that expressed heterologous α4β2 nAChRs (Gopalakrishnan et al., 1997). This is particularly interesting because smokers have an average serum concentration of 100–200 nM nicotine after smoking (Benowitz, 1996, Henningfield et al., 1993), suggesting that nAChR upregulation could occur in smokers in between, as well as during, periods of smoking.
As expected, because at baseline human brains have different levels of nicotine binding in different regions of the brain (Court & Clementi, 1995), the magnitude of increase in nicotine binding sites is not universal among all areas of the brain (Benwell et al., 1988, Perry et al., 1999). Regions of the thalamus appear most resistant to nicotine-induced upregulation of nAChR numbers in rodent models (Kellar et al., 1989, Pauly et al., 1991). In humans, the medulla oblongata, as well as different layers of the cortex and hippocampus are resistant to, or undergo nAChR upregulation at variable rates (Benwell et al., 1988, Perry et al., 1999). Given that nAChR subunits show variable expression in different regions of the brain, this suggests that individual neuronal nicotinic receptor subtypes may undergo differential upregulation according to location in the brain. In addition to upregulation of α4β2 subunit-containing receptors (Flores et al., 1992, Zhang et al., 1995), α3, albeit requiring a higher dose of nicotine, as well as α7, to a lesser extent, subunits are also upregulated (Olale et al., 1997, Peng et al., 1997, Rasmussen & Perry, 2006, Wang et al., 1996). However, not all nAChR subunits undergo nicotine-induced upregulation. For example, it has been reported that α4β2 nAChRs that also contain the α5 subunit (α4β2α5 nAChRs) do not upregulate in response to nicotine treatment (Mao et al., 2008). Autoradiographic studies have demonstrated that chronic nicotine exposure decreases the number of α6-containing receptors (Lai et al., 2005, Marks et al., 2014, Mugnaini et al., 2006, Perry et al., 2007). However, in the presence of β3 subunits this phenomenon is not observed and the α6-containing receptors do not downregulate, suggesting that one role of β3 is to inhibit nicotine-induced downregulation (Perry et al., 2007). Although the typical serum concentration of nicotine in smokers has little effect on muscle nAChRs (Lindstrom, 1996), upregulation of ganglionic α3β4 and α7 subunits, as well as the muscle type nAChR subunits α1, β1, γ, and δ, has been observed on the cell surface after a 48 hour exposure to nicotine at concentrations as low as 1μM (Ke et al., 1998). These findings confirm results from two previous studies on muscle nAChRs (Luther et al., 1989, Siegel & Lukas, 1988). However, earlier studies found that nAChRs in muscle cells are downregulated 40% by chronic exposure to nicotinic agonists (Appel et al., 1981, Gardner & Fambrough, 1979, Noble et al., 1978) at concentrations ranging from 10−3 M to 10 μM (for a review see (Lindstrom, 1996)). Ke et al. (1998) also found that different concentrations of nicotine upregulate different receptor subunits to varying degrees. For example, α3β4 subunits were more prone to upregulation than muscle nAChRs.
Subunit mRNA is unchanged in response to nicotine
Surprisingly, nicotinic receptor subunit mRNA is unchanged in response to nicotine. Early studies by Marks and colleagues showed that while there is an upregulation in α4β2 nAChR binding levels, mRNA for these nAChR subunits as well as for the α2, α3, α5 and β4 subunits remains steady under a nicotine dose of 4 mg/kg/hr (similar to the 5 mg/kg/hr dose originally shown to produce nAChR upregulation in the mouse (Marks et al., 1983)) (Marks et al., 1992). These results were replicated in subsequent studies performed in mouse cell lines (Bencherif et al., 1995, Peng et al., 1994, Zhang et al., 1995), human cell lines (Peng et al., 1997) and rat brain (Bencherif et al., 1995), and are consistent under nicotine concentrations ranging from 10 nM to 5 μM. Ke et al. (1998) expanded the aforementioned work in cultured human cell lines and reported that changes in nAChR numbers are not attributable to changes in CHRNA1, CHRNA3, CHRNA5, CHRNA7, CHRNB2, CHRNB4, CHRND, or CHRNG mRNA levels under exposure to 1 mM nicotine (Ke et al., 1998). It is important to keep in mind that many of the cell lines utilized do not endogenously express nAChRs. Thus, nAChR expression is not controlled by the endogenous regulatory sequences in each cell line, which could confound the results but also clearly demonstrates that native regulatory sequences are not required for upregulation. Although there have been many studies on nAChR subunit mRNA in human brains, these studies have primarily focused on nAChR expression changes in Parkinson’s and Alzheimer’s Diseases, or in aging brains, and to date only one study was conducted to examine the correlation between nAChR subunit protein and mRNA in smokers. In 2003, Mousavi et al. found that protein levels of the α4 and α7 nAChR subunits were significantly increased in the temporal cortex of smokers compared to those of nonsmokers. As in the rodent and cell line studies, there were no differences in CHRNA4 and CHRNA7 mRNA in the temporal cortex of smokers as compared to those of nonsmokers (Mousavi et al., 2003). Thus, all current evidence strongly indicates that the nicotine-induced upregulation of nAChR numbers is independent of transcriptional events.
Upregulation in development
Maternal smoking presents a large public health concern (Mathews, 2001) and research has indicated prenatal nicotine exposure can lead to altered behavior, potentially through interactions with the nAChRs. Numerous studies have found evidence for nAChR (particularly the α4β2-containing and α7 receptors) upregulation in rodents exposed to nicotine during prenatal (Hagino & Lee, 1985, Navarro et al., 1989, Popke et al., 1997, Slotkin et al., 1987, Tizabi & Perry, 2000, Tizabi et al., 1997, van de Kamp & Collins, 1994), neonatal (Eriksson et al., 2000, Huang & Winzer-Serhan, 2006, Miao et al., 1998) and postnatal (Narayanan et al., 2002, Nunes-Freitas et al., 2011) development. Nicotine exposure in each of these studies differed with each experimental objective. The majority of the studies looking at prenatal nicotine exposure used dosages around 6 or 9 mg/kg/day in rats and 2 mg/kg/hr in mice, which is roughly in agreement with what was used previously. However, Hagino and Lee’s 1985 study used a slightly lower dose of 175 μg/0.9μl/hr in rats with similar findings (Hagino & Lee, 1985). The doses used were much more varied in neonatal mice, yet the results were nevertheless consistent. Postnatal rats were treated with 2, 4, or 6 mg/kg/day nicotine, similar to the dose used by Schwartz and Kellar (Schwartz & Kellar, 1983). One study found evidence for downregulation of α7 receptors in rats after exposure to 9 mg/kg/day nicotine during gestation (Tizabi et al., 2000). Concurrent prenatal and postnatal chronic nicotine administration has also induced receptor upregulation in monkeys (Slotkin et al., 2002). Interestingly, nAChR upregulation during rodent fetal development was more long-lasting than in the adult, implying nicotine has some action beyond immediate receptor upregulation (Slotkin et al., 1987, van de Kamp & Collins, 1994). However, the nicotine doses were slightly different than those used in the first studies showing upregulation; 2 mg/kg/hr verses 5 mg/kg/hr nicotine (Marks et al., 1983) in mice and 6 mg/kg/day verses 4 mg/kg/day (Schwartz & Kellar, 1983) in rats. Despite the fact that nAChR upregulation after nicotine exposure in utero does not persist into adolescence or adulthood, prenatal nicotine exposure may, nonetheless, alter the response to nicotine in adolescence or adulthood in rodents (Abreu-Villaca et al., 2004, Gold et al., 2009, Slotkin et al., 2007). Unlike in adulthood, pups born from dams exposed to 2–4 mg/kg/day nicotine have increased nAChR mRNA expression, particularly that of the α7, α4, and β2 subunits (Frank et al., 2001, Lv et al., 2008, Shacka & Robinson, 1998), as well as the α2 subunit (Lv et al., 2008), immediately after nicotine administration is discontinued. Subsequent work has suggested that adolescent rats exposed to 2 mg/kg/day nicotine during gestation show corresponding decreases in nAChR number, as measured by [125I]-epibatidine, and expression of several nAChR subunit mRNAs (Chen et al., 2005). Neonatal nicotine exposure is more similar to nicotine exposure in adult animals in that no change in mRNA expression of the receptors is seen across a range of nicotine concentrations (Huang & Winzer-Serhan, 2006, Miao et al., 1998). Evidence for both receptor upregulation and subunit mRNA expression has also been seen in primary cultures from prenatal human brain exposed to 10 or 100 μM nicotine (Hellstrom-Lindahl et al., 2001). Using a mouse model, work by Picciotto and colleagues have demonstrated that the effects of nicotine exposure during development on later behaviors are attributable to the neuropharmacological effects of nicotine itself, not the effect of nicotine on maternal behaviors (Heath et al., 2010a). Furthermore, the nAChRs are suggested to mediate this effect. Mice developmentally exposed to nicotine though their drinking water (200 μg/ml nicotine) display hypersensitive passive avoidance behavior in adulthood; this behavior is likely driven by α4β2 and α4β2α5 nAChRs on corticothalamic neurons. Specifically, expression of the β2 and α5 subunits is necessary for this behavior (Heath et al., 2010b). The studies above present clear evidence that nicotine exposure during early development acts on and affects both nAChR receptor number and mRNA expression. Although outside the scope of this review, the effect of prenatal nicotine exposure on the central nervous system has been reviewed at length (Dwyer et al., 2009, Pauly & Slotkin, 2008, Winzer-Serhan, 2008). Collectively, long term and widespread consequences of such exposures have been proposed to play a role in various neurobehavioral and physiological disorders (Abbott & Winzer-Serhan, 2012).
Nicotine also produces various effects on the nAChRs when administered in adolescence. Upregulation of α4β2-containing and α7 receptors has been seen in rats administered 2–6 mg/kg/day nicotine in adolescence and periadolescence (Doura et al., 2008), particularly in the midbrain, hippocampus, and cortex (Abreu-Villaca et al., 2003, Trauth et al., 1999). Transient upregulation of α7 receptors in rats after exposure of 2 or 6 mg/kg/day nicotine has been seen also in the striatum (Slotkin et al., 2004). Compared to adults, adolescent upregulation is more persistent (up to 1 month post-treatment), has been seen at doses of nicotine as low as 0.6 mg/kg/day in rats, (Abreu-Villaca et al., 2003), and is more robust, showing increased binding in the midbrain and striatum compared to adults (Levin et al., 2007). Consistent with studies that treated adult rats with 3 or 6 mg/kg/day nicotine (Mugnaini et al., 2006, Perry et al., 2007), downregulation of α6-containing receptors was observed immediately in adolescent rats after discontinuing chronic nicotine treatment of roughly 3 mg/kg/day (Doura et al., 2008). Finally, similar to prenatal exposure, adolescent rat exposure to 6 mg/kg/day nicotine has been shown to alter the activity of the cholinergic system in adulthood (Slotkin et al., 2008). For example, one study found an increase in the mRNA expression of the α5, α6, and β2 subunits of adult rats that self-administered nicotine (roughly 20 μl of 0.04 mg/kg for 1 hour) during adolescence (Adriani et al., 2003).
Proposed mechanisms of upregulation
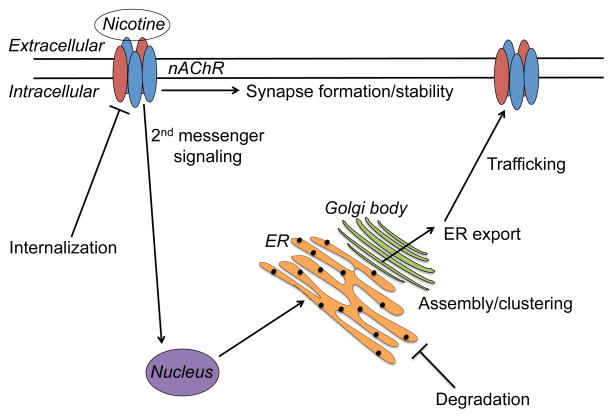
Many studies have been conducted to explore the relation between upregulation of nAChRs and nicotine exposure. Nicotine can pass through the plasma membrane of a cell and has been found in high concentration in cell organelles, as measured in the submaxillary glands of rats by Putney and Borzelleca in 1971 (Putney & Borzelleca, 1971). There are several major models for how nAChR upregulation occurs, some of which are not necessarily mutually exclusive. These include 1) migration from a pool of pre-assembled receptors in the cell, 2) alteration of translation rates via two 2nd messenger pathways, 3) change in stoichiometry of cell surface receptors, 4) increased intracellular assembly of nAChRs in combination with decreased turnover of surface receptors, 5) increased exocytic trafficking of nAChRs to the cell surface, 6) increased subunit maturation and assembly in the endoplasmic reticulum (ER), possibly through the molecular chaperoning of nAChRs by nicotine, and 7) inhibition of subunit degradation leading to new receptors from recycled subunits (Figure 1). It will be important to determine how these different mechanisms might be integrated during development and possibly modified by exposure to nicotine. It is also important to note that the vast majority of studies examining the mechanism of upregulation have focused on α4β2 nAChRs.
Figure 1.
Cellular processes proposed to play a role in nicotine-induced nAChR upregulation. ER: endoplasmic reticulum.
Early work by Bencherif et al. (1995) suggested that the increase in α4β2 nAChR numbers comes from a pool of assembled receptors within the cell as opposed to altered receptor turnover based on the observation that exposure to 1 μM nicotine had no effect on the degradation of nAChRs in an M10 mouse cell line. They hypothesized that the increase in nicotine binding sites is due a migration of intracellular nAChRs to the surface of the cell and observed that nAChR upregulation had an upward limit of 150%, suggesting the intracellular nAChR reservoir is finite. The authors hypothesized that the intracellular reservoir could exist as a result of several different mechanisms and set the stage for subsequent research (Bencherif et al., 1995).
Another theory is that two 2nd messenger pathways influence nAChR upregulation, possibly through altering translation rates. A series of three in vitro studies by Gopalakrishnan et al. (1997) showed that cells treated with 1 μM nicotine and cyclohexamide, a protein synthesis inhibitor, were resistant to nicotine-induced α4β2 nAChR upregulation, cells treated with 1 μM nicotine and the second messenger analog dibutyl cyclic adenosine monophosphate (cAMP) showed enhanced nicotine-induced nAChR upregulation, and cells treated with 1 μM nicotine and protein kinase C (PKC) inhibitors failed to produce nicotine-induced nAChR upregulation. This supports the theory that the nicotine-induced nAChR upregulation could be due to two second messenger pathways, one involving PKC and one involving cAMP, either individually or in conjunction with one another (Gopalakrishnan et al., 1997). A potential role for PKC in α4β2 expression was supported by a study by Nashmi et al. (2003), demonstrating increased trafficking of α4β2 nAChRs to the plasma membrane after the activation of PKC in vitro (Nashmi et al., 2003).
In 2005, Vallejo et al. (2005) proposed that upregulation of α4β2 nAChRs is due to a change in ligand affinity of receptors already present on the cell surface. Using a biotinylation assay they determined that nicotine exposure did not alter the ratio of intracellular to surface receptors. The authors experiments also suggested that nAChR upregulation is independent of intracellular receptor trafficking and concluded that what is termed upregulation is the result of receptors changing from a resting state to an active and high affinity state following chronic nicotine exposure (Vallejo et al., 2005). However, subsequent work has provided evidence contradicting this theory. Marks et al. (2011) showed that mice chronically treated with 1, 2, or 4 mg/kg/hr nicotine show a dose-dependent increase in antibody binding to the α4 and β2 nAChR subunits. This work strongly suggests that nicotine-induced nAChR upregulation reflects increased levels of nAChR protein (Marks et al., 2011) and not a conformational change in existing nAChRs enabling them to bind ligands with higher affinity. It is important to note that these studies had vastly different experimental protocols; Vallejo et al. (2005) used a human cell line with heterologous expression of α4β2 nAChRs exposed to 10 μM nicotine (Vallejo et al., 2005) whereas Marks et al. (2011) exposed mice up to 4 mg/kg/hr nicotine (Marks et al., 2011).
Several studies have provided evidence that upregulation of α4β2 nAChRs is due to mechanisms that increase assembly of intracellular nAChRs and decrease the rate of turnover of surface nAChRs (Kuryatov et al., 2005, Peng et al., 1994, Wang et al., 1998). In a mouse fibroblast cell line, expressing α4 and β2 subunits, exposure of the protein synthesis inhibitor cyclohexamide with 5 μM nicotine showed that (−)-[3H]nicotine binding remained higher compared to control cells for a longer time, indicating that nAChR degradation was prolonged (Peng et al., 1994). This work was replicated with the α3 and β2 subunits at a higher nicotine concentration of 100 μM nicotine in HEK cells, and additionally found a very small increase of β2 subunits within the cell despite the 5 fold increase in α3β2 receptors on the cell surface, suggesting the rate of assembly of the complete nicotinic receptor increases with exposure to nicotine (Wang et al., 1998). Finally, Kuryatov et al (2005) proposed that nicotine binds to the acetylcholine binding site of partially assembled α4β2 nAChRs in the lumen of the ER where a plethora of unassembled nAChR subunits reside. Binding of nicotine to an (α4β2)2 tetramer in the ER would then cause a conformational change that would increase the rate of addition of a 5th subunit to produce a mature nAChR, thus accounting for nicotine-induced nAChR upregulation (Kuryatov et al., 2005).
Another theory is that nAChR upregulation is a consequence of increased nAChR exocytic trafficking to the cell surface. The authors disrupted the secretory pathway with brefeldin A, a compound that disrupts trafficking from the Golgi apparatus, and found that cells exposed to brefeldin A alone show a decrease in nAChR surface numbers and cells exposed to both brefeldin A and nicotine showed no upregulation of surface nAChRs. This result, however, was not seen when the authors measured intracellular nAChRs (Darsow et al., 2005). These results indicated that transport through the secretory pathway is necessary for upregulation of surface nAChRs but there is likely another mechanism responsible for increasing the intracellular concentration of nAChRs. Although a stark contrast to previous findings, this study used a concentration of 500 nM (Darsow et al., 2005), much smaller than the 1–100 μM concentrations of nicotine utilized in the studies described above (Kuryatov et al., 2005, Peng et al., 1994, Wang et al., 1998).
Similar to some of the work described above, several studies have proposed nAChR upregulation is caused by increased subunit maturation and receptor assembly in the ER (Harkness & Millar, 2002, Nashmi et al., 2003, Sallette et al., 2005). Evidence for this theory has come from various studies using transfected α4 and β2 subunits. In one such study, the amount of total β2 subunit protein, but not α4 subunit protein, folded into correct conformation was influenced by both co-assembled partner subunits (the α4 subunit) and by chronic nicotine treatment of 10 μM, suggesting that nAChR upregulation was not due to increase in total protein but how readily the subunits mature and fold in the ER (Harkness & Millar, 2002). Subsequent studies concluded that increased assembly of nAChRs occurs in the soma of neurons after which these nAChRs must be subsequently trafficked to the dendritic processes where there is a larger overall concentration of nAChRs, and that 1 μM nicotine exposure increases the assembly of nAChRs (Nashmi et al., 2003). Additional evidence supporting this theory showed that α4 and β2 subunits undergo complex oligosaccharide glycosylation, a modification known to only occur in the ER and the Golgi apparatus, before they become heteromeric complexes and, furthermore, these glycosylations were found on all pentameric complexes, indicating that the receptor must be completely assembled into a 5-subunit pentameric complex in order to exit the ER and Golgi. Micromolar levels of nicotine exposure were shown to increase the amount of complex oligosaccharides on nAChR subunits (Sallette et al., 2005). In the same vein, a theory has been proposed to account for increased subunit maturation and receptor assembly after nicotine exposure, termed “inside-out pharmacology.” In short, the main posits of this theory are: nicotine and certain other nicotinic ligands enter the ER or Golgi where they bind and stabilize newly forming nAChR pentamers; this “pharmacological chaperoning” of nAChRs by nicotine results in increased trafficking of nAChRs through the secretory pathway; and, once at the plasma membrane, nAChRs remain stabilized by nicotine thus reducing receptor turnover (Henderson & Lester, 2015). In summary, these studies demonstrate several important findings: nAChR subunits undergo maturation and subsequently are assembled into pentamers in the ER of the cell soma and translocated to the cell surface; this process could escalate upon exposure to nanomolar amounts of nicotine; and this phenomenon might in fact be driven by the pharmacological chaperoning of nAChRs by nicotine (Harkness & Millar, 2002, Henderson & Lester, 2015, Nashmi et al., 2003, Sallette et al., 2005).
Yet another theory poses that subunit degradation is blocked and subunits are consequently recycled to form new nicotinic receptors (Christianson & Green, 2004, Ficklin et al., 2005, Rezvani et al., 2007). Treatment with proteasomal inhibitors has been shown to increase nAChR numbers and assembly in several cells lines (Christianson & Green, 2004). Ubiquilin-1, a ubiquitin-like protein that can interact with both the proteasome and ubiquitin ligases in protein degradation, co-immunoprecipitated with the α3 subunit and reduced the number of surface nAChRs when transfected into cells. While neurons not exposed to nicotine and injected with a ubiquilin-1 lentivirus showed no changes in α3-containing nAChR levels, nicotine-induced upregulation of α3-containing nAChRs was abated in neurons exposed to 100 μM nicotine after injection with a ubiquilin-1 lentivirus (Ficklin et al., 2005). Another study showed that nicotine itself can act as a partial proteasome inhibitor: in the prefrontal cortex of mice treated with 0.5 mg/kg of nicotine every 6 hours for 1 day, nicotine caused an increase in ubiquitinated proteins, including the α7 subunit suggesting reduced proteosomal activity. This inhibition of proteosomal activity was not due to a decrease in expression of the proteasome, as nicotine was found to increase expression of proteasomal subunits (Rezvani et al., 2007). Subsequent work by the same group focused on UBXN2A, a protein containing an ubiquitin-like domain that binds to ubiquitin. This protein was also observed to bind to and alter to α3 subunit expression: over expression of UBXN2A resulted in an increase in α3β2 nAChRs, and expression of UBXN2A was found to decrease levels of α3 subunit ubiquitination (Rezvani et al., 2009).
While the above studies have supported various theories of nicotine-induced nAChR upregulation, many of them are not mutually exclusive. It is important to note that there are receptor-specific assembly folding factors that fold individual nAChR subunits present in some mammalian cells lines and not others (Sweileh et al., 2000), and different nAChR upregulation mechanisms may be impacted by cell line specific folding factors. Recently, work by Govind et al. (2012) has proposed that nAChR upregulation is not due to a single process as suggested by some of the above studies, but multiple processes occurring at different rates. By measuring the kinetics of upregulation in cells exposed to 10 μM nicotine, the authors surmised that upregulation occurred at two different rates: an initial fast component of upregulation that saturated after 4 hours, during which binding of conformation-dependent antibodies occurred suggesting the fast component of upregulation results from nicotine-induced conformational changes as previously reported (Vallejo et al., 2005); and a slower component that increased with continued nicotine exposure, during which proteasomal inhibition produced even higher levels of subunits in the cell and the authors concluded was due to decreased proteasome-dependent subunit degradation and increased subunit assembly in line with previous work (Harkness & Millar, 2002, Nashmi et al., 2003, Rezvani et al., 2007, Sallette et al., 2005). These results suggest that there are many mechanisms, each acting at a different rate, that contribute to nAChR upregulation (Govind et al., 2012). Although the findings of several of the studies presented above directly contrast those of other studies, it is important to consider differences in nicotine exposure and experimental procedure as possible explanations. It is possible these mechanisms are differentially regulated during different developmental stages, under different conditions of nicotine exposure, and with additional variation contributed by genetic differences (discussed below).
Nicotinic acetylcholine receptors and human genetic association studies
Drug use phenotypes
There have been numerous associations between the CHRN genes and various drug phenotypes (Table 1), particularly those relating to nicotine. It is worth noting that although other ethnicities have been used and show association with these genes and drug phenotypes, the majority of these associations have been seen in European American (EA) samples. Many of these associations lie within a cluster of genes on chromosome 15q25: CHRNA3, CHRNB4, and CHRNA5. This cluster of genes has been the most consistently replicated association with cigarettes per day (CPD) (Berrettini et al., 2008, Cannon et al., 2014, Caporaso et al., 2009, Furberg et al., 2010, Gabrielsen et al., 2013, Liu et al., 2010, Saccone et al., 2010a, Sarginson et al., 2011, Sorice et al., 2011, Stevens et al., 2008, Thorgeirsson et al., 2010) and nicotine dependence (ND) (Baker et al., 2009, Bierut et al., 2007, Bierut et al., 2008, Broms et al., 2012, Chen et al., 2009, Erlich et al., 2010, Haller et al., 2012, Maes et al., 2011, Saccone et al., 2009a, Saccone et al., 2009b, Sherva et al., 2010, Spitz et al., 2008, Thorgeirsson et al., 2008, Weiss et al., 2008, Wessel et al., 2010), but more recently the genes for other nAChR subunits have been associated with smoking and other drug phenotypes. CHRNB3-A6 on chromosome 8p11 has reached genome-wide significance levels for association with CPD (Thorgeirsson et al., 2010) and ND (Bierut et al., 2007, Culverhouse et al., 2014, Haller et al., 2012, Hoft et al., 2009b, Rice et al., 2012, Saccone et al., 2009a, Saccone et al., 2010b, Saccone et al., 2007, Wang et al., 2014). In addition, evidence for association between common variants in other CHRN genes (CHRND-G, CHRNB1, CHRNA10, CHRNA4, and CHRNB2) and CPD and ND have been reported, although less replicated (Chen et al., 2013, Han et al., 2011, Kamens et al., 2013, Li et al., 2005, Saccone et al., 2009a, Saccone et al., 2010b).
Table 1.
Association of nAChR markers with phenotypes for human drug use.
| Phenotype | Gene(s) | Sample(s) | Reference |
|---|---|---|---|
| NDa | CHRNA4 | Families from Anhui Province, China | (Feng et al., 2004) |
| ADb | CHRNA4 | Unrelated Korean males | (Kim et al., 2004) |
| ND | CHRNA4 | Subjects of European or African ancestry from southern US (TN, MS, or AK) | (Li et al., 2005) |
| Subjective responses to alcohol and tobacco, past 6 month use alcohol | CHRNA4, CHRNB2 | CADDc | (Ehringer et al., 2007) |
| ND | CHRNB3, CHRNA5 | COGENDd | (Bierut et al., 2007, Saccone et al., 2007) |
| Age of initiation of alcohol and tobacco | CHRNA3-B4 | CADD, NYSe | (Schlaepfer et al., 2008) |
| Early subjective response to tobacco | CHRNB3-A6 | CADD, Add Healthf | (Zeiger et al., 2008) |
| CPDg | CHRNA5-A3 | Three European populations | (Berrettini et al., 2008) |
| AD | CHRNA5-A3 | COGAh | (Wang et al., 2009) |
| ND | CHRNA5-A3 | >11,000 Icelandic and European samples | (Thorgeirsson et al., 2008) |
| “Pleasurable buzz” during early experimentation with smoking | CHRNA5 | American Caucasians and African Americans | (Sherva et al., 2008) |
| ND | CHRNA5-A3-B4 | COGA | (Bierut et al., 2008) |
| Age-dependent ND | CHRNA5-A3-B4 | Three American cohorts | (Weiss et al., 2008) |
| ND | CHRNB3-A6 | NYS | (Hoft et al., 2009b) |
| ND | CHRNA5-A3-B4, CHRNB3-A6, CHRND-G | COGEND | (Saccone et al., 2009a) |
| Cocaine dependence | CHRNA5 | FSCDi, COGA | (Grucza et al., 2008) |
| Heavy smoking | CHRNA5-A3-B4 | American Cancer Society CPS-II Cohort, and the CPS-II Nutrition Cohort | (Stevens et al., 2008) |
| Level of response to alcohol | CHRNA5-A3-B4 | San Diego Sibling Pair | (Joslyn et al., 2008) |
| ND | CHRNA5-A3-B4 | Two Texas samples | (Spitz et al., 2008) |
| Smoking cessation | CHRNB2 | Pharmacogenetic trial of bupropion for smoking cessation | (Conti et al., 2008) |
| Smoking cessation | CHRNA5, CHRNA2 | Pharmacogenetic trial of bupropion for smoking cessation | (Heitjan et al., 2008) |
| Smoking cessation during pregnancy | CHRNA5-A3-B4 | European pregnant women | (Freathy et al., 2009) |
| CPD | CHRNA5-A3 | Cancer Genetic Markers of Susceptibility | (Caporaso et al., 2009) |
| Alcohol consumption | CHRNB3-A6 | NYS Family Study | (Hoft et al., 2009a) |
| ND | CHRNA5-A3-B4 | Utah and Wisconsin cohorts | (Baker et al., 2009) |
| ND | CHRNA5-A3-B4 | African Americans subjects from COGEND | (Saccone et al., 2009b) |
| ND, AD | CHRNA5-A3 | Virginia Adult Twin Study | (Chen et al., 2009) |
| Heavy alcohol use | CHRNA6 | Spanish population | (Landgren et al., 2009) |
| CPD | CHRNA3 | Tobacco and Genetics Consortium, ENGAGEj, Ox-GSKk | (Furberg et al., 2010) |
| ND | CHRNB3-A6, CHRNA4, CHRNB1, CHRNA10, CHRND-G | COGEND | (Saccone et al., 2010b) |
| CPD | CHRNA5-A3-B4, CHRNB3-A6 | ENGAGE | (Thorgeirsson et al., 2010) |
| ND in treatment-seeking smokers | Neuronal CHRN genes | Group Health | (Wessel et al., 2010) |
| Smoking initiation, smoking cessation | CHRNA5-A3-B4 | Korean subjects | (Li et al., 2010) |
| Dizziness to tobacco | CHRNB3, CHRNA10 | COGEND | (Ehringer et al., 2010) |
| Smoking quantity | CHRNA5-A3-B4 | Os-GSK | (Liu et al., 2010) |
| Smoking quantity | CHRNA5-A3-B4 | CGASP l | (Saccone et al., 2010a) |
| ND, cocaine dependence, AD | CHRNA5-A3-B4 | Five American cohorts (Uconn, Yale, MUSC, UPENN, McLean) | (Sherva et al., 2010) |
| Opioid dependence severity, ND severity | CHRNA5-A3-B4 | Outpatients in treatment for opioid dependence | (Erlich et al., 2010) |
| Heavy smoking | CHRNA5-A3-B4 | Three German Cohorts (KORA, NCOOP, ESTHER) | (Winterer et al., 2010) |
| ND | CHRNA5 | Yale University Transdisciplinary Tobacco Use Research Center | (Lori et al., 2011) |
| Subjective response to nicotine | CHRNB2 | Daily smokers from the Denver/Boulder area | (Hoft et al., 2011) |
| Smoking quantity, response to smoking cessation therapy | CHRNA5-A3-B4 | Two studies, MT1 and MT2 | (Sarginson et al., 2011) |
| CPD, ND | CHRNA4 | Five American cohorts (Uconn, Yale, MUSC, UPENN, McLean) | (Han et al., 2011) |
| ND | CHRNA5-A3 | Virginia Twin Registry | (Maes et al., 2011) |
| Smoking quantity | CHRNA5-A3-B4 | Three Italian populations | (Sorice et al., 2011) |
| ND | CHRNA4 | Five American cohorts (Uconn, Yale, MUSC, UPENN, McLean) | (Xie et al., 2011) |
| ND | CHRNA5-A3-B4, CHRNB3-A6 | COGEND | (Haller et al., 2012) |
| ND, CPD | CHRNB3 | SAGEm | (Rice et al., 2012) |
| ND, regular drinking | CHRNA5-A3-B4 | Finnish Twin Cohort | (Broms et al., 2012) |
| Objective measures of tobacco exposure | CHRNA5-A3 | Six European studies | (Munafo et al., 2012) |
| Onset of habitual smoking | CHRNA5-A3-B4 | COGA | (Kapoor et al., 2012) |
| Genetic vulnerability to smoking in early-onset smokers | CHRNA5 | Meta-Analysis | (Hartz et al., 2012) |
| Smoking quantity | CHRNA5-A3 | STOMPn Genetics Consortium | (David et al., 2012) |
| General substance use initiation | CHRNA5-A3-B4 | Add Health | (Lubke et al., 2012) |
| Pack years of smoking | CHRNA5-A3 | Chinese Han population of chronic obstructive pulmonary disease patients | (Zhou et al., 2012) |
| ND | CHRNA5-A3-B4, CHRNB3-A6 | COGEND | (Chen et al., 2012a) |
| CPD, age of smoking cessation, response to pharmacologic therapy | CHRNA5-A3-B4 | Atherosclerosis Risk in Communities, University of Wisconsin Transdisciplinary Tobacco Use Research Center | (Chen et al., 2012b) |
| ND | CHRNA4, CHRNB2 | Japanese | (Chen et al., 2013) |
| ND | CHRNA4, CHRNB2 | CADD, GADDo | (Kamens et al., 2013) |
| Alcohol use | CHRNA5-A3-B4 | National FINRISK Study, Health 2000 Survey | (Hallfors et al., 2013) |
| Response to smoking cessation therapies | CHRNA5-A3-B4 | Eight clinical trials for smoking cessation | (Bergen et al., 2013) |
| Smoking quantity, snus consumption | CHRNA5-A3-B4 | Nord-Trøndelag Health Study | (Gabrielsen et al., 2013) |
| ND | CHRNB3 | Subjects of European, African, and Asian ancestry from MSTCCp, SAGE, CGEMSq, and KAREr studies | (Cui et al., 2013) |
| Nicotine intake | CHRNA5-A3-B4 | Alaskan natives | (Zhu et al., 2013) |
| Onset of regular smoking | CHRNA5-A3-B4 | Meta-Analysis | (Stephens et al., 2013) |
| Cocaine use disorder | CHRNB3 | SAGE | (Sadler et al., 2014) |
| CPD | CHRNA5-A3-B4, CHRNB3-A6, CHRNA2 | SECASPs | (Cannon et al., 2014) |
| Frequency of binge drinking | CHRNA4 | SECASP | (Coon et al., 2014) |
| ND | CHRNA2, CHRNA6 | European and African Americans from TN, AR, MI, and MS; SAGE | (Wang et al., 2014) |
| CPD | CHRNB4 | COGEND | (Haller et al., 2014b) |
| Opioid dependence and withdrawal | CHRNA3 | SAGE | (Muldoon et al., 2014) |
| Dizziness at smoking initiation | CHRNA3, CHRNA4, CHRNA6, CHRNA7 | Nicotine Dependence in Teens | (Pedneault et al., 2014) |
| Smoking cessation | CHRNA5-A3-B4 | Two smoking cessation trials in African Americans: the Nicotine Gum Study, and Bupropion Study | (Zhu et al., 2014) |
| Use of smokeless tobacco | CHRNA5-A3 | Two studies conducted in India: the International multicenter oral cancer study, and the Mumbai study | (Anantharaman et al., 2014) |
| Severe withdrawal in a subgroup of smokers with higher lifetime prevalence of depression | CHRNA4 | Treatment-seeking smokers from 5 Hungarian cessation centers | (Lazary et al., 2014) |
| COPD, lung cancer, heavy smoking (pack years) | CHRNA7 | Southern and eastern Han Chinese population | (Yang et al., 2015) |
| AD and cocaine dependence | CHRNA3, CHRNB3 | COGA | (Haller et al., 2014a) |
| ND | CHRNB3-A6 | COGA, COGEND, FSCD | (Culverhouse et al., 2014) |
| CPD | CHRNA5, CHRNB4, CHRNA6 | Virginia Twin Study on Adolescent and Behavioral Development | (Clark et al., 2015) |
| ND | CHRNA5 | Meta-analysis | (Olfson et al., 2015) |
ND: Nicotine Dependence
AD: Alcohol Dependence
CADD: Center on Antisocial Drug Dependence
COGEND: Collaborative Study on the Genetics of Nicotine Dependence
NYS: National Youth Survey
Add Health: National Longitudinal Study of Adolescent Health
CPD: Cigarettes per Day
COGA: Collaborative Study on the Genetics of Alcoholism
FSCD: Family Study of Cocaine Dependence
ENGAGE: European Network of Genomic and Genetic Epidemiology
Ox-GSK: Oxford-GlaxoSmithKline
CGASP: Consortium for the Genetic Analysis of Smoking Phenotypes
SAGE: Study of Addiction: Genetics and Environment
STOMP: Study of Tobacco in Minority Populations
GADD: Genetics of Antisocial Drug Dependence
MSTCC: Mid-South Tobacco Case Control Study
CGEMS: Genome Wide Scan of Lung Cancer and Smoking
KARE: Korea Association Resource study
SECASP: Social and Emotional Contexts of Adolescent Smoking Patterns
Work by Saccone and colleagues have confirmed at least three independent genetic signals in the 15q25 cluster (Saccone et al., 2010a). Twin studies have estimated that ND and CPD are roughly 56–72% and 51–61% heritable in men and women, respectively (Broms et al., 2006, Kendler et al., 1999, Lessov et al., 2004, True et al., 1999b, Vink et al., 2004); however, the most well-replicated of these associations accounts for a very small proportion of the variance in smoking (roughly 1 cigarette per day) (Berrettini & Doyle, 2012, Saccone et al., 2010b). This single nucleotide polymorphism (SNP), rs16969968 (a nonsynonymous SNP residing in CHRNA5, also often tagged by rs1051730), has been consistently associated with ND and smoking behavior (Baker et al., 2009, Berrettini et al., 2008, Bierut et al., 2007, Chen et al., 2012a, Chen et al., 2009, Furberg et al., 2010, Hartz et al., 2012, Liu et al., 2010, Saccone et al., 2010a, Saccone et al., 2007, Sherva et al., 2010, Stevens et al., 2008, Thorgeirsson et al., 2008, Thorgeirsson et al., 2010, Weiss et al., 2008, Winterer et al., 2010). This signal has also been associated with alcohol dependence (AD) (Wang et al., 2009), cocaine dependence (CD) (Grucza et al., 2008, Sherva et al., 2010), opioid dependence (Sherva et al., 2010), and opioid dependence severity (Erlich et al., 2010). Interestingly, studies in EAs have shown that the rs16969968 risk allele for ND is a protective allele for AD (Chen et al., 2009) and CD (Grucza et al., 2008). Furthermore, the finding with CD has been replicated in another EA sample, as well as an African American sample (Sherva et al., 2010). Although the biological mechanisms for the bi-directional effect are unknown, one explanation might be due to the contribution of nAChRs to both excitatory and inhibitory effects on dopaminergic reward pathways (Grucza et al., 2008).
Many of these genes have been associated also with alcohol use. CHRNA5-A3-B4 has been associated with AD (Chen et al., 2009, Sherva et al., 2010, Wang et al., 2009), alcohol use (Broms et al., 2012, Hallfors et al., 2013), age of initiation of alcohol use (Schlaepfer et al., 2008), and level of response to alcohol (Joslyn et al., 2008). CHRNB3-A6 have been associated with alcohol consumption (Hoft et al., 2009a) and heavy alcohol use (Landgren et al., 2009). Finally, CHRNA4 has been associated with AD (Kim et al., 2004) and frequency of binge drinking (Coon et al., 2014). Both CHRNA4 and CHRNB2 have been associated with subjective response to alcohol (Ehringer et al., 2007).
More recently, several groups have used Next Generation sequencing approaches to comprehensively assess the coding regions of CHRN genes. In particular, past evidence for common variant associations in CHRNA4 have been limited. However, sequencing studies have revealed this gene has been technically more challenging to sequence, suggesting there may be genomic features in this region that have impeded traditional SNP assays. In a sample of EAs participating in a smoking cessation trial, Wessel et al. (2010) found evidence for association with ND between common and rare variants in CHRNA5 and CHRNB2, and for rare variants in CHRNA4 (Wessel et al., 2010). At the time, the research team was able to sequence the other CHRN genes using 454 technology, but needed to use Sanger sequencing for CHRNB2 and CHRNA4. In 2011, Xie et al. reported a protective effect of rare alleles in CHRNA4 for ND in a small EA sample (Xie et al., 2011). Some of these variants were shown to lead to significant differences in nAChR expression, subcellular distribution, and sensitivity to nicotine-induced receptor upregulation in HEK 293 cells and Xenopus laevis oocyte studies (Mcclure-Begley et al., 2014). In addition, rare variants in CHRNB4 have been associated with ND (Haller et al., 2012), and rare variants in CHRNB3 and CHRNA3 have been associated with AD and CD (Haller et al., 2014a). More recently, rare variants in CHRNA5 and CHRNB4 (but not CHRNA3) were associated with CPD in an EA sample, as was CHRNA6 (but not CHRNB3) (Clark et al., 2015). Finally, evidence for rare variant associations in both EA and African American samples in the CHRNA5 gene were reported for ND (Olfson et al., 2015). Collectively, the emerging data from rare variant studies suggests there are many different ways in which genetic variation in the CHRN genes may lead to risk for increased smoking. It will be important to draw on these databases of naturally occurring rare variants to perform follow-up functional molecular work and complementary animal studies in order to understand the molecular and pharmacological mechanisms for different kinds of variants.
In addition, recent pharmacogenetic studies have demonstrated that risk haplotype at CHRNA5-A3-B4 is highly predictive of a person’s response to treatment with Varenicline (a nAChR agonist) (Bergen et al., 2013, Chen & Bierut, 2013). To put this in terms of number needed to treat (NNT) for one person to successfully quit smoking, the NNT is 4 among individuals who carry the risk haplotype in the region, whereas among those with the low-risk haplotype the NNT >1000. Thus the collective work of many who have studied the human CHRN genes over the last 10 years is beginning to bear fruit with regard to potential future clinical application.
Age-related effects
Although rs16969968 has been robustly associated with ND and CPD as described above, recent research has indicated that this signal is not associated with age of onset of regular smoking or age of smoking initiation (Furberg et al., 2010, Hartz et al., 2012, Stephens et al., 2013). While two studies, including a large-scale meta-analysis, have found that age of onset of smoking modifies the association between rs16969968 and smoking severity in that that the risk allele for rs16969968 is associated with severity of heavy smoking and ND in smokers who began smoking before age 16 (Hartz et al., 2012, Weiss et al., 2008), research has primarily shown no association between rs16969968 and age of onset of smoking (Hartz et al., 2012, Stephens et al., 2013) and age of initiation of smoking (Furberg et al., 2010, Stephens et al., 2013, Thorgeirsson et al., 2010).
A recent meta-analysis examined two signals independent of rs16969968 (rs578776 and rs1948/rs684513) and found evidence for association with age of onset of smoking but not age of initiation of smoking (Stephens et al., 2013). Although this did not replicate previous associations with rs1948 and rs684513 and early age of tobacco initiation in two samples (Schlaepfer et al., 2008), it is in agreement with two previous genome-wide association studies (Furberg et al., 2010, Thorgeirsson et al., 2010) and one candidate gene study (Winterer et al., 2010) in finding no association with age of smoking initiation. Previous associations have been identified between rs684513 and age of onset of smoking (Broms et al., 2012), heavy smoking (Winterer et al., 2010), and ND (Broms et al., 2012, Lori et al., 2011), and between rs1948 and the tolerance factor of the Nicotine Dependence Syndrome Scale (Broms et al., 2012). In the meta-analysis by Stephens and colleagues in 2013 rs578776 had the strongest association with age of onset of smoking (Stephens et al., 2013), replicating previous work (Broms et al., 2012). This SNP has previously been associated with ND (Broms et al., 2012, Saccone et al., 2010a, Saccone et al., 2009a, Saccone et al., 2009b, Weiss et al., 2008) and heavy smoking (Stevens et al., 2008). Although the evidence for signals in the CHRN gene cluster on chromosome 15q25 remains mixed for association with age of initiation, studies suggest that some of the signals in the cluster that affect onset of smoking may be different from those affecting dependence and heavy smoking.
Conclusion
Chronic exposure by an agonist induces an upregulation of nAChRs that is not due to increased gene expression in adults. Numerous mechanisms have been proposed to be responsible for this phenomenon: increased nAChR subunit maturation and folding, increased receptor trafficking, and decreased subunit degradation, possibly through the pharmacological chaperoning of nAChRs by nicotine; and increased translation and 2nd messenger signaling. While upregulation also occurs during prenatal, neonatal, and postnatal developmental periods, as well as adolescence, there have been conflicting results regarding a change in corresponding gene expression. However, there is strong evidence that nicotine exposure during these influential periods of development contributes to later changes in the cholinergic system. Results from human genetics studies have provided also strong indication that variation with the CHRN genes is associated with SUDs. Most of the human genetic studies on the nAChRs however have focused on specific associations with nicotine behaviors and have shown several well-replicated signals that are associated with smoking quantity and ND. However, recent work has demonstrated that the most well-replicated of these associations with smoking levels and ND is not associated with age of smoking initiation or age of regular smoking. These differences highlight the overall complexity of molecular genetics in context of similarly heterogeneous phenotypes. As human geneticists continue to search for genes associated with drug behaviors, it is clear that careful attention to the phenotype, and developmental stage of subjects, will be an important consideration for obtaining a global view of how genetics influence behavior over the life-course. Although this review has focused on the nAChRs, other genes are likely to contribute at unique stages of development in concert with nicotine and other drug exposures to modify risk for SUDs.
Acknowledgments
The authors were supported by grants DA017637 (J.K.H) and DA036673 (J.A.S.) from the National Institute on Drug Abuse, and AA017889 (M.A.H.) and AA013525 (E.P.R.) from the National Institute on Alcohol Abuse and Alcoholism.
References
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol. 2012;42:279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: comorbid use and abuse/dependence in males and females. Behav Genet. 2004;34:217–228. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991;266:11192–11198. [PubMed] [Google Scholar]
- Anantharaman D, Chabrier A, Gaborieau V, Franceschi S, Herrero R, Rajkumar T, Samant T, Mahimkar MB, Brennan P, McKay JD. Genetic variants in nicotine addiction and alcohol metabolism genes, oral cancer risk and the propensity to smoke and drink alcohol: a replication study in India. PLoS One. 2014;9:e88240. doi: 10.1371/journal.pone.0088240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SH, Blosser JC, McManaman JL, Ashizawa T, Elias SB. The effects of carbamylcholine, calcium, and cyclic nucleotides on acetylcholine receptor synthesis in cultured myotubes. Ann N Y Acad Sci. 1981;377:189–197. doi: 10.1111/j.1749-6632.1981.tb33732.x. [DOI] [PubMed] [Google Scholar]
- Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, Piper ME, Matsunami N, Smith SS, Coon H, McMahon WM, Scholand MB, Singh N, Hoidal JR, Kim SY, Leppert MF, Cannon DS. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif M, Fowler K, Lukas RJ, Lippiello PM. Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. J Pharmacol Exp Ther. 1995;275:987–994. [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, Liu J, Lee W, Edlund CK, Hall S, Kwok PY, Benowitz NL, Baker TB, Tyndale RF, Lerman C, Swan GE. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23:94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH, Doyle GA. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Mol Psychiatry. 2012;17:856–866. doi: 10.1038/mp.2011.122. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Britto LR, Keyser KT, Lindstrom JM, Karten HJ. Immunohistochemical localization of nicotinic acetylcholine receptor subunits in the mesencephalon and diencephalon of the chick (Gallus gallus) J Comp Neurol. 1992;317:325–340. doi: 10.1002/cne.903170402. [DOI] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- Broms U, Wedenoja J, Largeau MR, Korhonen T, Pitkaniemi J, Keskitalo-Vuokko K, Happola A, Heikkila KH, Heikkila K, Ripatti S, Sarin AP, Salminen O, Paunio T, Pergadia ML, Madden PA, Kaprio J, Loukola A. Analysis of detailed phenotype profiles reveals CHRNA5-CHRNA3-CHRNB4 gene cluster association with several nicotine dependence traits. Nicotine Tob Res. 2012;14:720–733. doi: 10.1093/ntr/ntr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DS, Mermelstein RJ, Hedeker D, Coon H, Cook EH, McMahon WM, Hamil C, Dunn D, Weiss RB. Effect of neuronal nicotinic acetylcholine receptor genes (CHRN) on longitudinal cigarettes per day in adolescents and young adults. Nicotine Tob Res. 2014;16:137–144. doi: 10.1093/ntr/ntt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, Chen C, Jacobs K, Wheeler W, Landi MT, Ziegler RG, Hunter DJ, Chanock S, Hankinson S, Kraft P, Bergen AW. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Department of Health and Human Services. Smoking and Tobacco Use—Fact Sheet: Health Effects of Cigarette Smoking. Updated January 2008. Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm.
- CDC, Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Department of Health and Human Services. Tobacco Use: Targeting the Nation’s Leading Killer—At a Glance. 2009 Available at: http://www.cdc.gov/chronicdisease/resources/publications/aag/pdf/tobacco.pdf.
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Chen H, Parker SL, Matta SG, Sharp BM. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur J Neurosci. 2005;22:380–388. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Chen HI, Shinkai T, Utsunomiya K, Yamada K, Sakata S, Fukunaka Y, Hwang R, De Luca V, Ohmori O, Kennedy JL, Chuang HY, Nakamura J. Possible association of nicotinic acetylcholine receptor gene (CHRNA4 and CHRNB2) polymorphisms with nicotine dependence in Japanese males: an exploratory study. Pharmacopsychiatry. 2013;46:77–82. doi: 10.1055/s-0032-1323678. [DOI] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Grucza R, Wang JC, Johnson EO, Breslau N, Hatsukami D, Smith SS, Saccone N, Saccone S, Rice JP, Goate AM, Bierut LJ. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine Tob Res. 2012a;14:425–433. doi: 10.1093/ntr/ntr231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, Gogarten SM, Johnson EO, Saccone NL, Wang JC, Weiss RB, Goate AM, Bierut LJ. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012b;169:735–742. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Bierut LJ. Genomics and personalized medicine: and smoking cessation treatment. J Food Drug Anal. 2013;21:S87–S90. doi: 10.1016/j.jfda.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JC, Green WN. Regulation of nicotinic receptor expression by the ubiquitin-proteasome system. Embo J. 2004;23:4156–4165. doi: 10.1038/sj.emboj.7600436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL, McClay JL, Adkins DE, Aberg KA, Kumar G, Nerella S, Xie L, Collins AL, Crowley JJ, Quakenbush CR, Hillard CE, Gao G, Shabalin AA, Peterson RE, Copeland WE, Silberg JL, Maes H, Sullivan PF, Costello EJ, van den Oord EJ. Deep Sequencing of Three Loci Implicated in Large-Scale Genome-Wide Association Study Smokig Meta-Analyses. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB. Nicotine and smoking: a perspective from animal studies. Psychopharmacology (Berl) 1987;92:135–143. doi: 10.1007/BF00177905. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull. 1990;25:373–379. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, Bergen AW, Swan GE, Tyndale RF, Benowitz NL, Lerman C. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon H, Piasecki TM, Cook EH, Dunn D, Mermelstein RJ, Weiss RB, Cannon DS. Association of the CHRNA4 neuronal nicotinic receptor subunit gene with frequency of binge drinking in young adults. Alcohol Clin Exp Res. 2014;38:930–937. doi: 10.1111/acer.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Clementi F. Distribution of nicotinic subtypes in human brain. Alzheimer Dis Assoc Disord. 1995;9(Suppl 2):6–14. doi: 10.1097/00002093-199501002-00003. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Creese I, Sibley DR. Receptor adaptations to centrally acting drugs. Annu Rev Pharmacol Toxicol. 1981;21:357–391. doi: 10.1146/annurev.pa.21.040181.002041. [DOI] [PubMed] [Google Scholar]
- Cui WY, Wang S, Yang J, Yi SG, Yoon D, Kim YJ, Payne TJ, Ma JZ, Park T, Li MD. Significant association of CHRNB3 variants with nicotine dependence in multiple ethnic populations. Mol Psychiatry. 2013;18:1149–1151. doi: 10.1038/mp.2012.190. [DOI] [PubMed] [Google Scholar]
- Culverhouse RC, Johnson EO, Breslau N, Hatsukami DK, Sadler B, Brooks AI, Hesselbrock VM, Schuckit MA, Tischfield JA, Goate AM, Saccone NL, Bierut LJ. Multiple distinct CHRNB3-A6 variants are genetic risk factors for nicotine dependence in African Americans and European Americans. Addiction. 2014;109:814–822. doi: 10.1111/add.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Booker TK, Pina-Crespo JC, Heinemann SF. Exocytic trafficking is required for nicotine-induced up-regulation of alpha 4 beta 2 nicotinic acetylcholine receptors. J Biol Chem. 2005;280:18311–18320. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- David SP, Hamidovic A, Chen GK, Bergen AW, Wessel J, Kasberger JL, Brown WM, Petruzella S, Thacker EL, Kim Y, Nalls MA, Tranah GJ, Sung YJ, Ambrosone CB, Arnett D, Bandera EV, Becker DM, Becker L, Berndt SI, Bernstein L, Blot WJ, Broeckel U, Buxbaum SG, Caporaso N, Casey G, Chanock SJ, Deming SL, Diver WR, Eaton CB, Evans DS, Evans MK, Fornage M, Franceschini N, Harris TB, Henderson BE, Hernandez DG, Hitsman B, Hu JJ, Hunt SC, Ingles SA, John EM, Kittles R, Kolb S, Kolonel LN, Le Marchand L, Liu Y, Lohman KK, McKnight B, Millikan RC, Murphy A, Neslund-Dudas C, Nyante S, Press M, Psaty BM, Rao DC, Redline S, Rodriguez-Gil JL, Rybicki BA, Signorello LB, Singleton AB, Smoller J, Snively B, Spring B, Stanford JL, Strom SS, Swan GE, Taylor KD, Thun MJ, Wilson AF, Witte JS, Yamamura Y, Yanek LR, Yu K, Zheng W, Ziegler RG, Zonderman AB, Jorgenson E, Haiman CA, Furberg H. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl Psychiatry. 2012;2:e119. doi: 10.1038/tp.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Connolly J, Boulter J, Wada E, Wada K, Swanson LW, Patrick J, Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988;1:45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK, Hopfer CJ, Krauter K, Lessem J, Rhee SH, Schlaepfer I, Smolen A, Stallings MC, Young SE, Zeiger JS. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, McQueen MB, Hoft NR, Saccone NL, Stitzel JA, Wang JC, Bierut LJ. Association of CHRN genes with “dizziness” to tobacco. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:600–609. doi: 10.1002/ajmg.b.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Ankarberg E, Fredriksson A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Res. 2000;853:41–48. doi: 10.1016/s0006-8993(99)02231-3. [DOI] [PubMed] [Google Scholar]
- Erlich PM, Hoffman SN, Rukstalis M, Han JJ, Chu X, Linda Kao WH, Gerhard GS, Stewart WF, Boscarino JA. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum Genet. 2010;128:491–499. doi: 10.1007/s00439-010-0876-6. [DOI] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficklin MB, Zhao S, Feng G. Ubiquilin-1 regulates nicotine-induced up-regulation of neuronal nicotinic acetylcholine receptors. J Biol Chem. 2005;280:34088–34095. doi: 10.1074/jbc.M506781200. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Frank MG, Srere H, Ledezma C, O’Hara B, Heller HC. Prenatal nicotine alters vigilance states and AchR gene expression in the neonatal rat: implications for SIDS. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1134–1140. doi: 10.1152/ajpregu.2001.280.4.R1134. [DOI] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, Smith GD, Frayling TM, Hattersley AT. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo AM, Alema S, Ballivet M, Eusebi F. Alpha 5 subunit forms functional alpha 3 beta 4 alpha 5 nAChRs in transfected human cells. Neuroreport. 1997;8:2433–2436. doi: 10.1097/00001756-199707280-00005. [DOI] [PubMed] [Google Scholar]
- Fucile S, Matter JM, Erkman L, Ragozzino D, Barabino B, Grassi F, Alema S, Ballivet M, Eusebi F. The neuronal alpha6 subunit forms functional heteromeric acetylcholine receptors in human transfected cells. Eur J Neurosci. 1998;10:172–178. doi: 10.1046/j.1460-9568.1998.00001.x. [DOI] [PubMed] [Google Scholar]
- Furberg H, Kim Y, Dackor J, Boerwinkle E, Franceschini N, Ardissino D, Bernardinelli L, Mannucci PL, Mauri F, Merlini PA, Absher D, Assimes TL, Fortmann SP, Iribarren C, Knowles JW, Quertermous T, Ferrucci L, Tanaka T, Bis JC, Furberg CD, Haritunians T, McKnight B, Psaty BM, Taylor KD, Thacker EL, Almgren P, Groop L, Ladenvall C, Boehnke M, Jackson AU, Mohlke KL, Stringham HM, Tuomilehto J, Benjamin EJ, Hwang SJ, Levy D, Preis SR, Vasan RS, Duan J, Gejman PV, Levinson DF, Sanders AR, Shi J, Lips EH, McKay JD, Agudo A, Barzan L, Bencko V, Benhamou S, Castellsague X, Canova C, Conway DI, Fabianova E, Foretova L, Janout V, Healy CM, Holcátová I, Kjaerheim K, Lagiou P, Lissowska J, Lowry R, Macfarlane TV, Mates D, Richiardi L, Rudnai P, Szeszenia-Dabrowska N, Zaridze D, Znaor A, Lathrop M, Brennan P, Bandinelli S, Frayling TM, Guralnik JM, Milaneschi Y, Perry JR, Altshuler D, Elosua R, Kathiresan S, Lucas G, Melander O, O’Donnell CJ, Salomaa V, Schwartz SM, Voight BF, Penninx BW, Smit JH, Vogelzangs N, Boomsma DI, de Geus EJ, Vink JM, Willemsen G, Chanock SJ, Gu F, Hankinson SE, Hunter DJ, Hofman A, Tiemeier H, Uitterlinden AG, van Duijn CM, Walter S, et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen ME, Romundstad P, Langhammer A, Krokan HE, Skorpen F. Association between a 15q25 gene variant, nicotine-related habits, lung cancer and COPD among 56,307 individuals from the HUNT study in Norway. Eur J Hum Genet. 2013;21:1293–1299. doi: 10.1038/ejhg.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Fambrough DM. Acetylcholine receptor degradation measured by density labeling: effects of cholinergic ligands and evidence against recycling. Cell. 1979;16:661–674. doi: 10.1016/0092-8674(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Ghedini PC, Honda L, Avellar MC, Souccar C. Presence of mRNA of muscle nicotinic acetylcholine receptor subunits and an epsilon-subunit splice variant in the mouse brain. Brain Res Bull. 2010;81:453–457. doi: 10.1016/j.brainresbull.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Gold AB, Keller AB, Perry DC. Prenatal exposure of rats to nicotine causes persistent alterations of nicotinic cholinergic receptors. Brain Res. 2009;1250:88–100. doi: 10.1016/j.brainres.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan M, Molinari EJ, Sullivan JP. Regulation of human alpha4beta2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Mol Pharmacol. 1997;52:524–534. [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci. 2012;32:2227–2238. doi: 10.1523/JNEUROSCI.5438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit beta3 into a functional nicotinic receptor. J Biol Chem. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, Fox L, Bertelsen S, Kramer J, Hesselbrock V, Tischfield J, Nurnberger JI, Jr, Almasy L, Porjesz B, Kuperman S, Schuckit MA, Edenberg HJ, Rice JP, Goate AM, Bierut LJ. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunby P. Surgeon General emphasizes nicotine addiction in annual report on tobacco use, consequences. Jama. 1988;259:2811. [PubMed] [Google Scholar]
- Hagino N, Lee JW. Effect of maternal nicotine on the development of sites for [(3)H]nicotine binding in the fetal brain. Int J Dev Neurosci. 1985;3:567–571. doi: 10.1016/0736-5748(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Haller G, Druley T, Vallania FL, Mitra RD, Li P, Akk G, Steinbach JH, Breslau N, Johnson E, Hatsukami D, Stitzel J, Bierut LJ, Goate AM. Rare missense variants in CHRNB4 are associated with reduced risk of nicotine dependence. Hum Mol Genet. 2012;21:647–655. doi: 10.1093/hmg/ddr498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller G, Kapoor M, Budde J, Xuei X, Edenberg H, Nurnberger J, Kramer J, Brooks A, Tischfield J, Almasy L, Agrawal A, Bucholz K, Rice J, Saccone N, Bierut L, Goate A. Rare missense variants in CHRNB3 and CHRNA3 are associated with risk of alcohol and cocaine dependence. Hum Mol Genet. 2014a;23:810–819. doi: 10.1093/hmg/ddt463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller G, Li P, Esch C, Hsu S, Goate AM, Steinbach JH. Functional characterization improves associations between rare non-synonymous variants in CHRNB4 and smoking behavior. PLoS One. 2014b;9:e96753. doi: 10.1371/journal.pone.0096753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallfors J, Loukola A, Pitkaniemi J, Broms U, Mannisto S, Salomaa V, Heliovaara M, Lehtimaki T, Raitakari O, Madden PA, Heath AC, Montgomery GW, Martin NG, Korhonen T, Kaprio J. Scrutiny of the CHRNA5-CHRNA3-CHRNB4 smoking behavior locus reveals a novel association with alcohol use in a Finnish population based study. Int J Mol Epidemiol Genet. 2013;4:109–119. [PMC free article] [PubMed] [Google Scholar]
- Han S, Yang BZ, Kranzler HR, Oslin D, Anton R, Gelernter J. Association of CHRNA4 polymorphisms with smoking behavior in two populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:421–429. doi: 10.1002/ajmg.b.31177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness PC, Millar NS. Changes in conformation and subcellular distribution of alpha4beta2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. J Neurosci. 2002;22:10172–10181. doi: 10.1523/JNEUROSCI.22-23-10172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Short SE, Saccone NL, Culverhouse R, Chen L, Schwantes-An TH, Coon H, Han Y, Stephens SH, Sun J, Chen X, Ducci F, Dueker N, Franceschini N, Frank J, Geller F, Gubjartsson D, Hansel NN, Jiang C, Keskitalo-Vuokko K, Liu Z, Lyytikainen LP, Michel M, Rawal R, Rosenberger A, Scheet P, Shaffer JR, Teumer A, Thompson JR, Vink JM, Vogelzangs N, Wenzlaff AS, Wheeler W, Xiao X, Yang BZ, Aggen SH, Balmforth AJ, Baumeister SE, Beaty T, Bennett S, Bergen AW, Boyd HA, Broms U, Campbell H, Chatterjee N, Chen J, Cheng YC, Cichon S, Couper D, Cucca F, Dick DM, Foroud T, Furberg H, Giegling I, Gu F, Hall AS, Hallfors J, Han S, Hartmann AM, Hayward C, Heikkila K, Hewitt JK, Hottenga JJ, Jensen MK, Jousilahti P, Kaakinen M, Kittner SJ, Konte B, Korhonen T, Landi MT, Laatikainen T, Leppert M, Levy SM, Mathias RA, McNeil DW, Medland SE, Montgomery GW, Muley T, Murray T, Nauck M, North K, Pergadia M, Polasek O, Ramos EM, Ripatti S, Risch A, Ruczinski I, Rudan I, Salomaa V, Schlessinger D, Styrkarsdottir U, Terracciano A, Uda M, Willemsen G, Wu X, Abecasis G, Barnes K, Bickeboller H, Boerwinkle E, Boomsma DI, et al. Increased genetic vulnerability to smoking at CHRNA5 in early-onset smokers. Arch Gen Psychiatry. 2012;69:854–860. doi: 10.1001/archgenpsychiatry.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Horst NK, Picciotto MR. Oral nicotine consumption does not affect maternal care or early development in mice but results in modest hyperactivity in adolescence. Physiol Behav. 2010a;101:764–769. doi: 10.1016/j.physbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, King SL, Gotti C, Marks MJ, Picciotto MR. Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through alpha4/beta2/alpha5 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2010b;35:2324–2338. doi: 10.1038/npp.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitjan DF, Guo M, Ray R, Wileyto EP, Epstein LH, Lerman C. Identification of pharmacogenetic markers in smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:712–719. doi: 10.1002/ajmg.b.30669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Seiger A, Kjaeldgaard A, Nordberg A. Nicotine-induced alterations in the expression of nicotinic receptors in primary cultures from human prenatal brain. Neuroscience. 2001;105:527–534. doi: 10.1016/s0306-4522(01)00209-3. [DOI] [PubMed] [Google Scholar]
- Henderson BJ, Lester HA. Inside-out neuropharmacology of nicotinic drugs. Neuropharmacology. 2015;96:178–193. doi: 10.1016/j.neuropharm.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Huizinga D, Menard S, Ehringer MA. SNPs in CHRNA6 and CHRNB3 are associated with alcohol consumption in a nationally representative sample. Genes Brain Behav. 2009a;8:631–637. doi: 10.1111/j.1601-183X.2009.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Schlaepfer IR, Huizinga D, Ehringer MA. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology. 2009b;34:698–706. doi: 10.1038/npp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft NR, Stitzel JA, Hutchison KE, Ehringer MA. CHRNB2 promoter region: association with subjective effects to nicotine and gene expression differences. Genes Brain Behav. 2011;10:176–185. doi: 10.1111/j.1601-183X.2010.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Winzer-Serhan UH. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain Res. 2006;1113:94–109. doi: 10.1016/j.brainres.2006.06.084. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci U S A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Corley RP, McQueen MB, Stallings MC, Hopfer CJ, Crowley TJ, Brown SA, Hewitt JK, Ehringer MA. Nominal association with CHRNA4 variants and nicotine dependence. Genes Brain Behav. 2013;12:297–304. doi: 10.1111/gbb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Wang JC, Bertelsen S, Bucholz K, Budde JP, Hinrichs A, Agrawal A, Brooks A, Chorlian D, Dick D, Hesselbrock V, Foroud T, Kramer J, Kuperman S, Manz N, Nurnberger J, Jr, Porjesz B, Rice J, Tischfield J, Xuei X, Schuckit M, Edenberg HJ, Bierut LJ, Goate AM. Variants located upstream of CHRNB4 on chromosome 15q25.1 are associated with age at onset of daily smoking and habitual smoking. PLoS One. 2012;7:e33513. doi: 10.1371/journal.pone.0033513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta ND, Plener PL, Schmid R, Thau K, Walter H, Lesch OM. Multiple substance use among young males. Pharmacol Biochem Behav. 2007;86:306–311. doi: 10.1016/j.pbb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Ke L, Eisenhour CM, Bencherif M, Lukas RJ. Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes. I. Dose- and time-dependent effects of nicotine treatment. J Pharmacol Exp Ther. 1998;286:825–840. [PubMed] [Google Scholar]
- Kellar KJ, Giblin BA, Lumpkin MD. Regulation of brain nicotinic cholinergic recognition sites and prolactin release by nicotine. Prog Brain Res. 1989;79:209–216. doi: 10.1016/s0079-6123(08)62480-2. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. Br J Psychiatry. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kim JW, Song JY, Park S, Lee HJ, Chung JH. Association of polymorphisms in nicotinic acetylcholine receptor alpha 4 subunit gene (CHRNA4), mu-opioid receptor gene (OPRM1), and ethanol-metabolizing enzyme genes with alcoholism in Korean patients. Alcohol. 2004;34:115–120. doi: 10.1016/j.alcohol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39:2570–2590. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment decreases striatal alpha 6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- Landgren S, Engel JA, Andersson ME, Gonzalez-Quintela A, Campos J, Nilsson S, Zetterberg H, Blennow K, Jerlhag E. Association of nAChR gene haplotypes with heavy alcohol use and body mass. Brain Res. 2009;1305(Suppl):S72–79. doi: 10.1016/j.brainres.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Lazary J, Dome P, Csala I, Kovacs G, Faludi G, Kaunisto M, Dome B. Massive withdrawal symptoms and affective vulnerability are associated with variants of the CHRNA4 gene in a subgroup of smokers. PLoS One. 2014;9:e87141. doi: 10.1371/journal.pone.0087141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, Heath AC, Madden PA. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Li MD, Yoon D, Lee JY, Han BG, Niu T, Payne TJ, Ma JZ, Park T. Associations of variants in CHRNA5/A3/B4 gene cluster with smoking behaviors in a Korean population. PLoS One. 2010;5:e12183. doi: 10.1371/journal.pone.0012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. Neuronal nicotinic acetylcholine receptors. Ion Channels. 1996;4:377–450. doi: 10.1007/978-1-4899-1775-1_10. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, Wareham NJ, Zhao JH, Loos RJ, Barroso I, Khaw KT, Grundy S, Barter P, Mahley R, Kesaniemi A, McPherson R, Vincent JB, Strauss J, Kennedy JL, Farmer A, McGuffin P, Day R, Matthews K, Bakke P, Gulsvik A, Lucae S, Ising M, Brueckl T, Horstmann S, Wichmann HE, Rawal R, Dahmen N, Lamina C, Polasek O, Zgaga L, Huffman J, Campbell S, Kooner J, Chambers JC, Burnett MS, Devaney JM, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein S, Wilson JF, Wild SH, Campbell H, Vitart V, Reilly MP, Li M, Qu L, Wilensky R, Matthai W, Hakonarson HH, Rader DJ, Franke A, Wittig M, Schafer A, Uda M, Terracciano A, Xiao X, Busonero F, Scheet P, Schlessinger D, St Clair D, Rujescu D, Abecasis GR, Grabe HJ, Teumer A, Volzke H, Petersmann A, John U, Rudan I, Hayward C, Wright AF, Kolcic I, Wright BJ, Thompson JR, Balmforth AJ, Hall AS, Samani NJ, Anderson CA, Ahmad T, Mathew CG, Parkes M, Satsangi J, Caulfield M, Munroe PB, Farrall M, Dominiczak A, Worthington J, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori A, Tang Y, O’Malley S, Picciotto MR, Wu R, Conneely KN, Cubells JF. The galanin receptor 1 gene associates with tobacco craving in smokers seeking cessation treatment. Neuropsychopharmacology. 2011;36:1412–1420. doi: 10.1038/npp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubke GH, Stephens SH, Lessem JM, Hewitt JK, Ehringer MA. The CHRNA5/A3/B4 gene cluster and tobacco, alcohol, cannabis, inhalants and other substance use initiation: replication and new findings using mixture analyses. Behav Genet. 2012;42:636–646. doi: 10.1007/s10519-012-9529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Bennett T, Jung HH, Ryan AF. Developmental expression of alpha 9 acetylcholine receptor mRNA in the rat cochlea and vestibular inner ear. J Comp Neurol. 1998;393:320–331. [PubMed] [Google Scholar]
- Lustig LR, Hiel H, Fuchs PA. Vestibular hair cells of the chick express the nicotinic acetylcholine receptor subunit alpha 9. J Vestib Res. 1999;9:359–367. [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10) Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- Luther MA, Schoepfer R, Whiting P, Casey B, Blatt Y, Montal MS, Montal M, Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989;9:1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Mao C, Zhu L, Zhang H, Pengpeng H, Xu F, Liu Y, Zhang L, Xu Z. The effect of prenatal nicotine on expression of nicotine receptor subunits in the fetal brain. Neurotoxicology. 2008;29:722–726. doi: 10.1016/j.neuro.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Chen X, Chen J, Prescott CA, Kendler KS. A twin association study of nicotine dependence with markers in the CHRNA3 and CHRNA5 genes. Behav Genet. 2011;41:680–690. doi: 10.1007/s10519-011-9476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Salminen O, Paley MA, Wageman CR, McIntosh JM, Whiteaker P. α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration. J Neurochem. 2014;130:185–198. doi: 10.1111/jnc.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, Collins AC, Lindstrom JM. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther. 2011;337:187–200. doi: 10.1124/jpet.110.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Mathews TJ. Smoking during pregnancy in the 1990s. Natl Vital Stat Rep. 2001;49:1–14. [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McClure-Begley TD, Papke RL, Stone KL, Stokes C, Levy AD, Gelernter J, Xie P, Lindstrom J, Picciotto MR. Rare human nicotinic acetylcholine receptor alpha4 subunit (CHRNA4) variants affect expression and function of high-affinity nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2014;348:410–420. doi: 10.1124/jpet.113.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Miao H, Liu C, Bishop K, Gong ZH, Nordberg A, Zhang X. Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. J Neurochem. 1998;70:752–762. doi: 10.1046/j.1471-4159.1998.70020752.x. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Mousavi M, Hellstrom-Lindahl E, Guan ZZ, Shan KR, Ravid R, Nordberg A. Protein and mRNA levels of nicotinic receptors in brain of tobacco using controls and patients with Alzheimer’s disease. Neuroscience. 2003;122:515–520. doi: 10.1016/s0306-4522(03)00460-3. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [(125)I]Y0-alpha-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137:565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, McIntosh JM, Dierssen M, Miles MF, Chen X, De Biasi M, Damaj MI. The alpha3beta4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br J Pharmacol. 2014;171:3845–3857. doi: 10.1111/bph.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Timofeeva MN, Morris RW, Prieto-Merino D, Sattar N, Brennan P, Johnstone EC, Relton C, Johnson PC, Walther D, Whincup PH, Casas JP, Uhl GR, Vineis P, Padmanabhan S, Jefferis BJ, Amuzu A, Riboli E, Upton MN, Aveyard P, Ebrahim S, Hingorani AD, Watt G, Palmer TM, Timpson NJ, Davey Smith G. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst. 2012;104:740–748. doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Birru S, Vaglenova J, Breese CR. Nicotinic receptor expression following nicotine exposure via maternal milk. Neuroreport. 2002;13:961–963. doi: 10.1097/00001756-200205240-00012. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. Assembly of alpha4beta2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. 1989;251:894–900. [PubMed] [Google Scholar]
- NIDA, National Institute on Drug Abuse. Nationwide Trends. Retrieved from http://www.drugabuse.gov/publications/drugfacts/nationwide-trends on April 9, 2015.
- Noble MD, Brown TH, Peacock JH. Regulation of acetylcholine receptor levels by a cholinergic agonist in mouse muscle cell cultures. Proc Natl Acad Sci U S A. 1978;75:3488–3492. doi: 10.1073/pnas.75.7.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Freitas AL, Ribeiro-Carvalho A, Lima CS, Dutra-Tavares AC, Manhaes AC, Lisboa PC, Oliveira E, Gaspar de Moura E, Filgueiras CC, Abreu-Villaca Y. Nicotine exposure during the third trimester equivalent of human gestation: time course of effects on the central cholinergic system of rats. Toxicol Sci. 2011;123:144–154. doi: 10.1093/toxsci/kfr147. [DOI] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283:675–683. [PubMed] [Google Scholar]
- Olfson E, Saccone NL, Johnson EO, Chen LS, Culverhouse R, Doheny K, Foltz SM, Fox L, Gogarten SM, Hartz S, Hetrick K, Laurie CC, Marosy B, Amin N, Arnett D, Barr RG, Bartz TM, Bertelsen S, Borecki IB, Brown MR, Chasman DI, van Duijn CM, Feitosa MF, Fox ER, Franceschini N, Franco OH, Grove ML, Guo X, Hofman A, Kardia SL, Morrison AC, Musani SK, Psaty BM, Rao DC, Reiner AP, Rice K, Ridker PM, Rose LM, Schick UM, Schwander K, Uitterlinden AG, Vojinovic D, Wang JC, Ware EB, Wilson G, Yao J, Zhao W, Breslau N, Hatsukami D, Stitzel JA, Rice J, Goate A, Bierut LJ. Rare, low frequency and common coding variants in CHRNA5 and their contribution to nicotine dependence in European and African Americans. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Brick L, Nugent NR, Bidwell LC, McGeary JE, Knopik VS, Keller MC. Examining the role of common genetic variants on alcohol, tobacco, cannabis and illicit drug dependence: genetics of vulnerability to drug dependence. Addiction. 2015;110:530–537. doi: 10.1111/add.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, Hopfer CJ, Hewitt JK. Genetic etiology of the common liability to drug dependence: evidence of common and specific mechanisms for DSM-IV dependence symptoms. Drug Alcohol Depend. 2012;123(Suppl 1):S24–32. doi: 10.1016/j.drugalcdep.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 1989;3:589–596. doi: 10.1016/0896-6273(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Pedneault M, Labbe A, Roy-Gagnon MH, Low NC, Dugas E, Engert JC, O’Loughlin J. The association between CHRN genetic variants and dizziness at first inhalation of cigarette smoke. Addict Behav. 2014;39:316–320. doi: 10.1016/j.addbeh.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol. 1997;51:776–784. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994;46:523–530. [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates alpha6- and beta3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Pickens RW, Svikis DS, McGue M, LaBuda MC. Common genetic mechanisms in alcohol, drug, and mental disorder comorbidity. Drug Alcohol Depend. 1995;39:129–138. doi: 10.1016/0376-8716(95)01151-n. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Tizabi Y, Rahman MA, Nespor SM, Grunberg NE. Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacol Biochem Behav. 1997;58:843–849. doi: 10.1016/s0091-3057(97)98985-1. [DOI] [PubMed] [Google Scholar]
- Pugh PC, Corriveau RA, Conroy WG, Berg DK. Novel subpopulation of neuronal acetylcholine receptors among those binding alpha-bungarotoxin. Mol Pharmacol. 1995;47:717–725. [PubMed] [Google Scholar]
- Putney JW, Jr, Borzelleca JF. On the mechanisms of 14C-nicotine distribution in rat submaxillary gland in vitro. J Pharmacol Exp Ther. 1971;178:180–191. [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Perry DC. An autoradiographic analysis of [125I]alpha-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci Lett. 2006;404:9–14. doi: 10.1016/j.neulet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Pan Y, Dani JA, Lindstrom J, Garcia Gras EA, McIntosh JM, De Biasi M. UBXD4, a UBX-containing protein, regulates the cell surface number and stability of alpha3-containing nicotinic acetylcholine receptors. J Neurosci. 2009;29:6883–6896. doi: 10.1523/JNEUROSCI.4723-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Neale MC, Stallings MC. Comorbidity between alcohol dependence and illicit drug dependence in adolescents with antisocial behavior and matched controls. Drug Alcohol Depend. 2006;84:85–92. doi: 10.1016/j.drugalcdep.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rice JP, Hartz SM, Agrawal A, Almasy L, Bennett S, Breslau N, Bucholz KK, Doheny KF, Edenberg HJ, Goate AM, Hesselbrock V, Howells WB, Johnson EO, Kramer J, Krueger RF, Kuperman S, Laurie C, Manolio TA, Neuman RJ, Nurnberger JI, Porjesz B, Pugh E, Ramos EM, Saccone N, Saccone S, Schuckit M, Bierut LJ. CHRNB3 is more strongly associated with Fagerstrom test for cigarette dependence-based nicotine dependence than cigarettes per day: phenotype definition changes genome-wide association studies results. Addiction. 2012;107:2019–2028. doi: 10.1111/j.1360-0443.2012.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliovaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang BZ, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PA, Nothen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010a;6:e1001053. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009a;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, Johnson EO, Rice JP, Goate AM, Bierut LJ. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010b;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009b;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler B, Haller G, Agrawal A, Culverhouse R, Bucholz K, Brooks A, Tischfield J, Johnson EO, Edenberg H, Schuckit M, Saccone N, Bierut L, Goate A. Variants near CHRNB3-CHRNA6 are associated with DSM-5 cocaine use disorder: evidence for pleiotropy. Sci Rep. 2014;4:4497. doi: 10.1038/srep04497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, Murphy GM., Jr Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- Scheffer D, Sage C, Plazas PV, Huang M, Wedemeyer C, Zhang DS, Chen ZY, Elgoyhen AB, Corey DP, Pingault V. The alpha1 subunit of nicotinic acetylcholine receptors in the inner ear: transcriptional regulation by ATOH1 and co-expression with the gamma subunit in hair cells. J Neurochem. 2007;103:2651–2664. doi: 10.1111/j.1471-4159.2007.04980.x. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol. 2002;61:150–159. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Robinson SE. Exposure to prenatal nicotine transiently increases neuronal nicotinic receptor subunit alpha7, alpha4 and beta2 messenger RNAs in the postnatal rat brain. Neuroscience. 1998;84:1151–1161. doi: 10.1016/s0306-4522(97)00564-2. [DOI] [PubMed] [Google Scholar]
- Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, Anton RF, Oslin D, Farrer LA, Gelernter J. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology. 2010;35:1921–1931. doi: 10.1038/npp.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel HN, Lukas RJ. Nicotinic agonists regulate alpha-bungarotoxin binding sites of TE671 human medulloblastoma cells. J Neurochem. 1988;50:1272–1278. doi: 10.1111/j.1471-4159.1988.tb10604.x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res Bull. 2008;76:152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Seidler FJ. Administration of nicotine to adolescent rats evokes regionally selective upregulation of CNS alpha 7 nicotinic acetylcholine receptors. Brain Res. 2004;1030:159–163. doi: 10.1016/j.brainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL. Development of [3H]nicotine binding sites in brain regions of rats exposed to nicotine prenatally via maternal injections or infusions. J Pharmacol Exp Ther. 1987;242:232–237. [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res Dev Brain Res. 2002;133:175–179. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Seidler FJ. Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res Bull. 2007;74:91–103. doi: 10.1016/j.brainresbull.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Sorice R, Bione S, Sansanelli S, Ulivi S, Athanasakis E, Lanzara C, Nutile T, Sala C, Camaschella C, D’Adamo P, Gasparini P, Ciullo M, Toniolo D. Association of a variant in the CHRNA5-A3-B4 gene cluster region to heavy smoking in the Italian population. Eur J Hum Genet. 2011;19:593–596. doi: 10.1038/ejhg.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz MR, Amos CI, Dong Q, Lin J, Wu X. The CHRNA5-A3 region on chromosome 15q24–25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst. 2008;100:1552–1556. doi: 10.1093/jnci/djn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SH, Hartz SM, Hoft NR, Saccone NL, Corley RC, Hewitt JK, Hopfer CJ, Breslau N, Coon H, Chen X, Ducci F, Dueker N, Franceschini N, Frank J, Han Y, Hansel NN, Jiang C, Korhonen T, Lind PA, Liu J, Lyytikainen LP, Michel M, Shaffer JR, Short SE, Sun J, Teumer A, Thompson JR, Vogelzangs N, Vink JM, Wenzlaff A, Wheeler W, Yang BZ, Aggen SH, Balmforth AJ, Baumeister SE, Beaty TH, Benjamin DJ, Bergen AW, Broms U, Cesarini D, Chatterjee N, Chen J, Cheng YC, Cichon S, Couper D, Cucca F, Dick D, Foroud T, Furberg H, Giegling I, Gillespie NA, Gu F, Hall AS, Hallfors J, Han S, Hartmann AM, Heikkila K, Hickie IB, Hottenga JJ, Jousilahti P, Kaakinen M, Kahonen M, Koellinger PD, Kittner S, Konte B, Landi MT, Laatikainen T, Leppert M, Levy SM, Mathias RA, McNeil DW, Medland SE, Montgomery GW, Murray T, Nauck M, North KE, Pare PD, Pergadia M, Ruczinski I, Salomaa V, Viikari J, Willemsen G, Barnes KC, Boerwinkle E, Boomsma DI, Caporaso N, Edenberg HJ, Francks C, Gelernter J, Grabe HJ, Hops H, Jarvelin MR, Johannesson M, Kendler KS, Lehtimaki T, Magnusson PK, Marazita ML, Marchini J, Mitchell BD, Nothen MM, et al. Distinct loci in the CHRNA5/CHRNA3/CHRNB4 gene cluster are associated with onset of regular smoking. Genet Epidemiol. 2013;37:846–859. doi: 10.1002/gepi.21760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Sweileh W, Wenberg K, Xu J, Forsayeth J, Hardy S, Loring RH. Multistep expression and assembly of neuronal nicotinic receptors is both host-cell- and receptor-subtype-dependent. Brain Res Mol Brain Res. 2000;75:293–302. doi: 10.1016/s0169-328x(99)00302-2. [DOI] [PubMed] [Google Scholar]
- Talib S, Okarma TB, Lebkowski JS. Differential expression of human nicotinic acetylcholine receptor alpha subunit variants in muscle and non-muscle tissues. Nucleic Acids Res. 1993;21:233–237. doi: 10.1093/nar/21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Perry DC. Prenatal nicotine exposure is associated with an increase in [125I]epibatidine binding in discrete cortical regions in rats. Pharmacol Biochem Behav. 2000;67:319–323. doi: 10.1016/s0091-3057(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Popke EJ, Rahman MA, Nespor SM, Grunberg NE. Hyperactivity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Pharmacol Biochem Behav. 1997;58:141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Russell LT, Nespor SM, Perry DC, Grunberg NE. Prenatal nicotine exposure: effects on locomotor activity and central [125I]alpha-BT binding in rats. Pharmacol Biochem Behav. 2000;66:495–500. doi: 10.1016/s0091-3057(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- True WR, Heath AC, Scherrer JF, Xian H, Lin N, Eisen SA, Lyons MJ, Goldberg J, Tsuang MT. Interrelationship of genetic and environmental influences on conduct disorder and alcohol and marijuana dependence symptoms. Am J Med Genet. 1999a;88:391–397. doi: 10.1002/(sici)1096-8628(19990820)88:4<391::aid-ajmg17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999b;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Tumkosit P, Kuryatov A, Luo J, Lindstrom J. Beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic alpha6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol Pharmacol. 2006;70:1358–1368. doi: 10.1124/mol.106.027326. [DOI] [PubMed] [Google Scholar]
- UNODC, United Nations Office on Drugs and Crime. World Drug Report. 2012. (United Nations publication, Sales No. E.12.XI.1) [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kamp JL, Collins AC. Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacol Biochem Behav. 1994;47:889–900. doi: 10.1016/0091-3057(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Vink JM, Beem AL, Posthuma D, Neale MC, Willemsen G, Kendler KS, Slagboom PE, Boomsma DI. Linkage analysis of smoking initiation and quantity in Dutch sibling pairs. Pharmacogenomics J. 2004;4:274–282. doi: 10.1038/sj.tpj.6500255. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Wang F, Nelson ME, Kuryatov A, Olale F, Cooper J, Keyser K, Lindstrom J. Chronic nicotine treatment up-regulates human alpha3 beta2 but not alpha3 beta4 acetylcholine receptors stably transfected in human embryonic kidney cells. J Biol Chem. 1998;273:28721–28732. doi: 10.1074/jbc.273.44.28721. [DOI] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, ADvdV, Xu Q, Seneviratne C, Pomerleau OF, Pomerleau CS, Payne TJ, Ma JZ, Li MD. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Hum Genet. 2014;133:575–586. doi: 10.1007/s00439-013-1398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, Piper ME, Fiore MC, Scholand MB, Connett JE, Kanner RE, Gahring LC, Rogers SW, Hoidal JR, Leppert MF. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J, McDonald SM, Hinds DA, Stokowski RP, Javitz HS, Kennemer M, Krasnow R, Dirks W, Hardin J, Pitts SJ, Michel M, Jack L, Ballinger DG, McClure JB, Swan GE, Bergen AW. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerstrom test for nicotine dependence. Neuropsychopharmacology. 2010;35:2392–2402. doi: 10.1038/npp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, World Health Organization. Alcohol: Fact sheet. Updated May 2014. Available at: http://www.who.int/mediacentre/factsheets/fs349/en/
- WHO, World Health Organization. Tobacco: Fact sheet N°339. Updated July 2015. Available at: http://www.who.int/mediacentre/factsheets/fs339/en/
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, Breitling LP, Nitz B, Raum E, Muller H, Gallinat J, Gal A, Heim K, Prokisch H, Meitinger T, Hartmann AM, Moller HJ, Gieger C, Wichmann HE, Illig T, Dahmen N, Rujescu D. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1448–1458. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Krauthammer M, Cosgrove KP, Oslin D, Anton RF, Farrer LA, Picciotto MR, Krystal JH, Zhao H, Gelernter J. Rare nonsynonymous variants in alpha-4 nicotinic acetylcholine receptor gene protect against nicotine dependence. Biol Psychiatry. 2011;70:528–536. doi: 10.1016/j.biopsych.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Lu X, Qiu F, Fang W, Zhang L, Huang D, Xie C, Zhong N, Ran P, Zhou Y, Lu J. Duplicated copy of CHRNA7 increases risk and worsens prognosis of COPD and lung cancer. Eur J Hum Genet. 2015;23:1019–1024. doi: 10.1038/ejhg.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ, Hewitt JK, Hopfer CJ, Lessem J, McQueen MB, Rhee SH, Ehringer MA. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum Mol Genet. 2008;17:724–734. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gong ZH, Hellstrom-Lindahl E, Nordberg A. Regulation of alpha 4 beta 2 nicotinic acetylcholine receptors in M10 cells following treatment with nicotinic agents. Neuroreport. 1995;6:313–317. doi: 10.1097/00001756-199501000-00022. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yang J, Li D, Xiao J, Wang B, Wang L, Ma C, Xu S, Ou X, Feng Y. Association of IREB2 and CHRNA3/5 polymorphisms with COPD and COPD-related phenotypes in a Chinese Han population. J Hum Genet. 2012;57:738–746. doi: 10.1038/jhg.2012.104. [DOI] [PubMed] [Google Scholar]
- Zhu AZ, Renner CC, Hatsukami DK, Benowitz NL, Tyndale RF. CHRNA5-A3-B4 genetic variants alter nicotine intake and interact with tobacco use to influence body weight in Alaska Native tobacco users. Addiction. 2013;108:1818–1828. doi: 10.1111/add.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, David SP, Ahluwalia JS, Benowitz NL, Tyndale RF. Association of CHRNA5-A3-B4 SNP rs2036527 with smoking cessation therapy response in African-American smokers. Clin Pharmacol Ther. 2014;96:256–265. doi: 10.1038/clpt.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]