Abstract
Background
Influenza vaccination is recommended for vulnerable individuals, including active drug users, to prevent influenza complications and decrease influenza spread. Recent studies suggest that opioids negatively regulate immune responses in experimental models, but the extent to which opioid use will affect the humoral responses to influenza vaccine in humans is unknown. This information is critical in maximizing vaccination efforts.
Objective
To determine whether there is a difference in antibody response after influenza vaccination in heroin or methadone users compared to control subjects.
Methods
We studied active heroin users, subjects on methadone maintenance treatment (MMT) and subjects that did not use any drugs before and 1 and 4 weeks after vaccination with trivalent influenza vaccine (TIV). We measured hemagglutination inhibition and microneutralization titers, and we compared geometric mean titers (GMT), and rates of seroprotection and seroconversion for each of the vaccine strains among the 3 groups of subjects.
Results
Heroin users, subjects on MMT and non-user controls mount a similarly robust serologic response to TIV. GMT and rates of seroprotection and seroconversion were not significantly different among groups.
Conclusion
Our results suggest that opioid use do not significantly alter antibody responses to influenza vaccine supporting the vaccination effort in these populations.
Keywords: Influenza vaccination, Hemagglutination inhibition, Microneutralization, Heroin, Methadone, Opioid users
1. Background
Vaccination is the most widely used strategy to prevent influenza infection and is especially recommended for high-risk groups, such as opioid users. The US Public Health Service has identified heroin users as a population in whom influenza transmission during a pandemic is likely to be especially problematic [1]. In addition to requiring culturally competent outreach efforts and often having poor access to adequate housing and hygiene, opioid use itself may place this population at increased risk for contracting (and transmitting) influenza. Furthermore, opioid users may have more severe influenza complications, as suggested by experimental studies showing that morphine-treated rats had reduced inflammatory lung responses and decreased viral clearance after influenza infection [2,3]. Epidemiological data also show that individuals using opioids have an increased incidence of pneumonia [4]. Recent studies, in fact, suggest that the immune response to infection or vaccination can be modulated by opioids, either by direct binding to the μ-opioid receptors (MOR) present on the leukocytes, or indirectly [5–7]. In animals models, heroin and its metabolite, morphine, negatively affect innate immunity [8–11], and antibody production [12]. In addition, opioid administration increases the susceptibility and severity of bacterial and viral infections [13–15].
Studies in humans also support the role of opioids in the regulation of both innate and acquired immunity [5,15–19], it is unclear, however, whether all opioids share the same immunosuppressive properties. In fact some study suggests that while heroin negatively affects both innate and acquired immunity, patients on long-term methadone treatment seem to have preserved immune functioning [20]. The clinical relevance of this immunomodulation remains uncertain [21].
Although some studies have shown decreased antibody responses among illicit drug users to some vaccines, there is a lack of definitive studies regarding the immunogenicity of vaccination strategies in people who inject drugs for diseases that are highly prevalent or difficult to control, such as influenza [22]. One study evaluated the response to influenza vaccine in former drug users who also have HIV, but not in active users [23]. Some studies found that people using heroin had decreased rates of seroconversion to hepatitis B vaccine compared to individuals who did not use opioids [24–26], although others suggested that the antibody responses were normal after boosting the vaccines with 3 doses [27–29]. Likewise, antibody responses of heroin users after a single dose of hepatitis A virus vaccine are low [30]. These studies have limitations because they used heterogeneous samples that include individuals of various ages with comorbid chronic infections, such as HIV, that can also affect the immune response, vaccines that require booster administrations to achieve protection, and used historic controls for comparing antibody responses.
The present study was designed to ascertain whether opioid use impairs the humoral response to a single dose of influenza vaccine and to determine whether the response to vaccine was different in subjects using heroin compared to those on methadone maintenance therapy (MMT). This information will be useful for the design and implementation of vaccination strategies for seasonal influenza and pandemic influenza preparedness for people who use heroin.
2. Methods
2.1. Participants and study design
We performed a prospective observational study during the influenza season 2010–2011, to compare antibody responses to influenza vaccination among healthy adults aged 18–40 years of any race or sex who used heroin or methadone and healthy adults with the same characteristics but not using opioids. Active heroin users, defined as individuals using intravenous heroin at least once week for the previous 6 months or longer, were recruited from among participants of the Swan Project, a community-based study of young people who injected illicit drugs [31]. Subjects on MMT were recruited from the outpatient methadone program at New York Presbyterian Hospital and were defined as individuals on a stable dose of methadone receiving treatment in the clinic for at least 3 months. Healthy non-user controls were enrolled through flyers and magazine advertisement. Urine toxicology tests were performed at each visit for each subject to confirm patient drug use status. Exclusion criteria included obesity (body mass index > 40), history of acute hypersensitivity to eggs or egg products, acute illnesses at enrollment, history of Guillain-Barré syndrome, pregnancy, HIV infection, blood transfusion in the past 3 months, use of cytokine-based therapies or therapies causing immunosuppression, advanced liver or kidney disease and previous receipt of the influenza vaccine for the season. Information including demographic characteristics, medical history, use of medications, history of alcohol and drug use, history of influenza vaccination or disease were collected at enrollment. HCV status was obtained by self-report. Opioid users enrolled from Swan and MMT participants had been recently tested for HCV antibody and RNA and given their results. Venous blood and urine specimens were obtained from each subject prior to vaccination and at 1 and 4 weeks following vaccination for antibody titers and for toxicology screening respectively. Subjects were asked about symptoms that would be consistent with influenza or influenza-like infection at each visit. Subjects received an incentive of $20 at each study visit to cover transportation and a snack. This study was performed in accordance with the human experimentation guidelines of the United States Department of Health and Human Services and was approved by the Institutional Review Board at Weill Cornell Medical College. Written informed consent was obtained from all study participants.
2.2. Vaccine
Subjects received a single intramuscular dose of the currently licensed inactivated trivalent vaccine (Fluzone, Sanofi) for the year 2010–2011 from a single lot. Vaccines were supplied in prefilled syringes containing 7.5 μg of hemagglutinin (HA) from each of the 3 recommended influenza strains for the 2010–2011 Northern Hemisphere influenza season, which included A/California/07/2009 (H1N1, pandemic influenza vaccine strain), A/Victoria/210/2009, (H3N2) and B/Brisbane/60/2008 and administered intramuscularly in the patient’s deltoid.
2.3. Hemagglutination inhibition and microneutralization assays
Serum antibody titers against each of the 3 influenza vaccine strains were determined using hemagglutination inhibition (HI) and microneutralization assays (MN). Patient sera were pre-treated overnight at 37 °C with receptor destroying enzyme (RDE, Vibrio cholera filtrate; Sigma–Aldrich, MO) [32] and subsequently heated at 56 °C for 45 min to remove non-specific HA inhibitors.
HI assays were performed on RDE-treated serum samples following standard methods [33]. Briefly, 2-fold serial dilutions of RDE-treated serum samples were prepared in saline solution and then incubated in a 96-well V-bottom plate (Nunc) at a 1:1 ratio, with 8 hemagglutination assay (HA) units of each of the 3 influenza virus strains present in the 2010–2011 influenza vaccine. After 30 min at 37 °C, turkey red blood cells (Lampire Biological) were added to each well at a final concentration of 0.5% and incubated for 40 min at 4 °C. Serum samples were tested in triplicate, starting at an initial dilution of 1:10. HI titer was expressed as the reciprocal of the highest dilution of serum able to inhibit HA. Titers below the lower limit of detection were considered as half the lower limit and were assigned a titer of 5.
MN titers were determined as previously described [34]. Briefly, 2-fold serial dilutions of RDE-treated sera were mixed with 200 plaque forming units (PFU) of each of the 3 strains of influenza virus and incubated for 45 min at 37 °C. MDCK cells were then inoculated with the virus-serum mixtures. Following a 1 h incubation at 37 °C, inocula were removed. Cells were washed once with PBS and then incubated in the presence of TPCK-treated trypsin in OPTI-MEM reduced serum media (Gibco) at 37 °C. Four days post-inoculation, supernatants were collected and tested for virus growth by hemagglutination assay. MN titers from duplicate samples were used to calculate the GMT. The initial dilution tested was 1:20 and samples that were not neutralizing at this dilution were assigned a titer of 1:10. Results were expressed as GMT against each of the 3 vaccine viruses.
The mean fold-increase (MFI) in HI titers following immunization was calculated as the geometric mean ratio of the mean fold increase for each subject between post- and prevaccination titers.
2.4. Statistical methods
Demographics (age, gender, race/ethnicity) and antibody response (HI, MN) to all 3 influenza strains (H3N2, H1N1 and B) were compared among non-user controls, heroin users and methadone users and between non-user controls and all opioid users grouped together. GMTs were calculated for HI and MN assays using log conversion. GMTs, rates of seroconversion and seroprotection were compared across groups at each time point. Comparisons between groups were conducted using chi-square, Kruskal–Wallis and t-test statistics. Wilcoxon signed-rank tests were used to compare paired pre- and post-vaccination values within groups. We conducted separate logistic regression models controlling for age, gender, or race/ethnicity as potential confounders. We also conducted logistic regression analysis testing for association of potential confounders (age, race/ethnicity, and gender) alone with the study endpoints. All analyses were conducted using SAS 9.3 (SAS Institute, Carey, NC).
3. Results
3.1. Study population
HI and MN titers to the vaccine strains were compared between 21 opioid users (10 heroin users and 11 subjects on MMT) and 20 non-drug user controls, all vaccinated during the 2010–2011 influenza season. The demographic characteristics of the study participants are summarized in Table 1. The mean age of opioid users as a whole (heroin or methadone) was 25.8 (±5.8) years which was not significantly different from control subjects (28.5 ± 5.0 years; Table 1) but the mean age of heroin users (21.7 ± 2.3 years) was significantly lower than methadone users (29.5 ± 5.6) and non-user controls (p = 0.01). Controls were less likely to be white (p < 0.01) and more likely to be female (p = 0.04) than opioid users. All subjects reported being healthy and none were taking medication for any chronic or immunosuppressive condition. Hepatitis C (indicated by self-reporting) was overrepresented among methadone and heroin users (Table 1). Four opioid users reported having been infected with hepatitis C virus and one of them reported having cleared the infection. Eight subjects using heroin had unstable living conditions.
Table 1.
Baseline characteristics of the study subjects.
| Characteristic | Heroin users | Methadone users | Control group | p value* | Opioid users (heroin + methadone) | p value** |
|---|---|---|---|---|---|---|
| N of subjects | 10 | 11 | 20 | 21 | ||
| Age – years | <0.01a | 0.12 | ||||
| Mean ± SD | 21.7 ± 2.3 | 29.5 ± 5.6 | 28.5 ± 5.0 | 25.8 ± 5.8 | ||
| Median | 21.5 | 30 | 29 | 23 | ||
| Range | 19–24 | 25–40 | 22–35 | 19–40 | ||
| Sex – no. (%) | 0.02b | <0.01 | ||||
| Male | 9 (90) | 8 (73) | 8 (40) | 17 (81) | ||
| Female | 1 (10) | 3 (27) | 12 (60) | 4 (19) | ||
| Race – no. (%) | 0.11 | 0.04 | ||||
| White | 10 (100) | 8 (73) | 9 (45) | 18 (86) | ||
| Black or African American | 0 | 1 (9) | 6 (30) | 1 (5) | ||
| Asian | 0 | 1 (9) | 4 (20) | 1 (5) | ||
| All other races | 0 | 1 (9) | 1 (5) | 1 (5) | ||
| Ethnicity | 0.99 | 0.96 | ||||
| Non-Hispanic/Latino | 9 (90) | 10 (91) | 18 (90) | 19 (90) | ||
| Hispanic/Latino | 1 (10) | 1 (9) | 2 (10) | 2 (10) | ||
| dPrior influenza vaccination (ever) – no. (%) | 6 (60) | 7 (63.6) | 11 (61)e | 0.98 | 13 (62) | 0.96 |
| dHCV infection | 3 (30)f | 1 (9) | 0 (0) | 0.03c | 4 (19) | 0.04 |
Three-way comparison.
Comparison of all opioid users vs controls.
Age, pairwise comparisons: heroin users vs control group p < 0.01; methadone users vs control group p = 0.60; heroin users vs methadone users p < 0.01.
Sex, pairwise comparisons: heroin users vs control group p < 0.01; methadone users vs control group p = 0.08; heroin users vs methadone users p = 0.31.
HCV infection, pairwise comparisons: heroin users vs control group p < 0.01; methadone users vs control group p = 0.17; heroin users vs methadone users p = 0.22.
By self report.
Unknown for 2 patients in control group.
One subject cleared the infection.
All subjects using heroin reported uninterrupted heroin use for more than 24 months except one who had started using heroin 6–8 months before the study. Seven subjects reported daily heroin use, 2–10 bags per day. Three patients reported using 1–5 bags of heroin for 2–4 days/week, one of them also reported daily prescription opioid use. Six of the 9 patients using heroin had a history of polysubstance use and had urine toxicology positive at least on one occasion for cocaine (n = 6), marijuana (n = 3), or benzodiazepines (n = 4). Four subjects on methadone had toxicology revealing benzodiazepine use and one had an episode of cocaine use. Urine toxicology of the control subjects was negative for opioids, cocaine, and benzodiazepines. The median duration of subjects participating in a methadone maintenance program was 14 months (range 3–68 months).
3.2. Influenza vaccination status
Prior to study vaccination, none of the subjects had received the influenza vaccine for the year of the study or the monovalent pandemic H1N1 influenza vaccine, which became available for the first time the year prior to the study. Eleven of the non-user controls and 13 of the opioid users (6 heroin, 7 methadone) thought that they had received seasonal influenza vaccination in previous years, although none could specify when. Eight of the controls, 6 heroin users and 4 methadone users recalled having influenza-like illness sometimes in their lifetime, but none recalled symptomatic influenza-like disease during the outbreak of pandemic H1N1 the year preceding the study.
3.3. HI and MN GMT responses
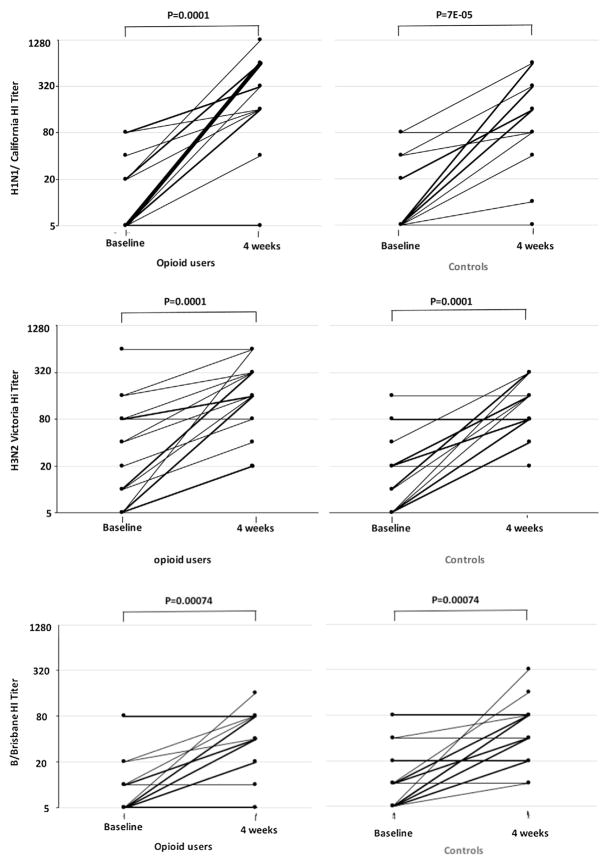
We compared pre- and post-immunization HI and MN titers of all opioid users (heroin and methadone) as a group to non-user controls, and we repeated the analysis separating opioid users in heroin users and subjects on MMT, and comparing their responses to controls. Prior to vaccination there was no difference in HI or MN GMT between opioid users and controls and among the 3 groups (Table 2 and supplemental Table 1), although heroin users had a trend for lower baseline HI GMTs to influenza B (p = 0.07). Following vaccination, HI titers were significantly higher than prevaccine titers for all 3 strains in both opioid users and controls (Fig. 1). When compared among groups (either controls vs all opioid users and controls vs heroin users vs methadone users) there were no significant differences in HI GMTs to any of the influenza strains. MFI ranged from 21.9-fold for A/California/07/2009 to 6.6-fold for H3N2 and 5.3-fold for influenza B in opioid users and from 12.2-, 7.0- and 5.5-folds in non-users controls respectively. These fold increases were not significantly different between groups for any of the influenza strains (Table 2 and supplemental Table 1). Similarly, no significant differences were found in GMT or MFI of the MN titers before and after vaccination for any of the influenza strains (Table 2, supplemental Table 1), although all the groups had significantly increased MN titers in responses to vaccination (not shown).
Table 2.
GMT and mean fold increase (MFI) of HI and MN titers before and after a single dose of influenza vaccine.
| Influenza subtype | HI
|
MN
|
||||||
|---|---|---|---|---|---|---|---|---|
| Heroin users (n = 10) | Methadone users (n = 11) | Non user controls (n = 20) | p value | Heroin users (n = 10) | Methadone users (n = 11) | Non user controls (n = 20) | p value | |
| A/California/7/2009 (H1N1) | ||||||||
| Prevaccine | 7.6 (3.9, 14.8) | 16.6 (8.0, 34.2) | 10.0 (6.2, 16.2) | 0.16 | 19.9 (10.2, 38.8) | 43.04 (20.7, 98.2) | 20.1 (13.0, 30.8) | 0.14 |
| Postvaccine | 278.6 (93.5, 829.7) | 205.9 (72.3, 586.5) | 149.3 (80.9, 275.5) | 0.18 | 416.6 (169.3, 1025.2) | 643.8 (225.6, 1837.8) | 283.2 (117.4, 683.6) | 0.57 |
| MFI | 21.9 (9.4, 51.1) | 8.2 (4.3, 15.7) | 12.2 (7.3, 20.4) | 0.17 | 8.4 (5.4, 13.2) | 6.8 (3.7, 12.6) | 7.2 (5.0, 10.4) | 0.67 |
| A/Victoria/210/2009 (H3N2) | ||||||||
| Prevaccine | 20.0 (7.9, 50.9) | 24.2 (7.7, 75.7) | 16.8 (10.1, 28.2) | 0.94 | 66.0 (21.8, 199.9) | 65.4 (21.2, 202) | 46.3 (21.5, 99.9) | 0.72 |
| Postvaccine | 172.8 (79.7, 374.8) | 124.4 (55.1, 280.6) | 121.3 (83.7, 175.7) | 0.52 | 521.9 (233, 1128) | 513.8 (176.4, 1496.3) | 353.2 (195, 627) | 0.25 |
| MFI | 7.3 (3.4, 15.6) | 6.1 (3.6, 10.5) | 7.0 (4.9, 10.0) | 0.88 | 5.0 (3.3, 7.7) | 5.3 (3.3, 8.5) | 5.6 (4.3, 7.3) | 0.96 |
| B/Brisbane/60/2008 | ||||||||
| Prevaccine | 5.7 (4.7, 7.1) | 12.1 (5.9, 24.9) | 13.2 (8.1, 21.5) | 0.06 | 14.1 (9.3, 21.6) | 20.2 (10.5, 39.1) | 19.4 (10.3, 36.7) | 0.44 |
| Postvaccine | 26.4 (11.7, 59.7) | 29.2 (12.9, 66.1) | 47.6 (31.3, 72.4) | 0.36 | 31.9 (15.9, 64.2) | 36.6 (16.4, 81.6) | 54.4 (31.7, 93.2) | 0.42 |
| MFI | 6.8 (4.0, 11.8) | 4.2 (2.9, 6.1) | 5.5 (3.9, 7.8) | 0.33 | 3.8 (2.8, 5.1) | 3.5 (2.5, 5.1) | 4.3 (3.5, 5.4) | 0.36 |
Data are geometric means (95% confidence interval), unless defined otherwise.
Fig. 1.
HI titers to A/California/07/2009 (H1N1), A/Victoria/210/2009, (H3N2) and B/Brisbane/60/2008 at baseline and 4 weeks after TIV administration to opioid users and non-drug users controls. Lines connect data points from each individual.
3.4. Seroprotection and seroconversion
Similarly to GMT, rates of seroprotection (defined as the proportion of subjects with an HI titer ≥1:40) at baseline and post-vaccination were not statistically different between the opioid users and non-user controls or among the three groups for each of the vaccine strains (Table 3 and supplemental Table 2). In opioid users grouped together these were 14.3%, 43% and 10% (for influenza H1N1, H3N2 and influenza B strains, respectively) vs 20%, 25% and 25% in the non-users control at baseline, and 90.5%, 81%, and 62% vs 90.0%, 95% and 70.0% at 4 weeks post vaccination (supplemental Table 2). Rates of seroconversion, defined as a 4-fold increase in HI titer between pre- and post-immunization serum samples, at 7 or 14 days after immunization were also not significantly different among groups (Table 3) or in opioid users vs controls (supplemental Table 2).
Table 3.
Rates of seroconversion and seroprotection 1 week and 4 weeks after single dose of the influenza vaccine, as measured by HI assay.
| Immunogenicity end point | A/California/7/2009 (H1N1)
|
A/Victoria/210/2009 (H3N2)
|
B/Brisbane/60/2008
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heroin users | Methadone users | Non user controls | p value | Heroin users | Methadone users | Non user controls | p value | Heroin users | Methadone users | Non user controls | p value | |
| No. of subjects | 10 | 11 | 20 | 10 | 11 | 20 | 10 | 11 | 20 | |||
| Baseline | ||||||||||||
| Seroprotection, % (95% CI) | 10.0 (0.3, 44.5) | 27 (6.0,61.0) | 20 (5.7, 43.7) | 0.61 | 40 (12.2, 73.8) | 45 (16.8, 76.6) | 25 (8.7, 49.1) | 0.47 | 0 (–, –) | 18 (2.3, 51.8) | 25 (8.7, 49.1) | 0.23 |
| Postvaccination day 7 | ||||||||||||
| Seroprotection, % (95% CI) | 90.0 (55.5, 99.8) | 82 (48.2, 97.7) | 65.0 (40.8, 84.6) | 0.28 | 90.0 (55.5, 99.8) | 82 (48.2, 97.7) | 80.0 (56.3, 94.3) | 0.79 | 40.0 (12.2, 73.8) | 55 (23.4, 83.3) | 50.0 (28.1, 71.9) | 0.79 |
| Seroconversion, % (95% CI) | 90 (55.5, 99.8) | 55 (23.4, 83.3) | 60 (36.1, 90.9) | 0.17 | 60 (26.2, 87.8) | 45 (16.7, 76.6) | 55 (31.5,76.9) | 0.79 | 40 (12.2, 73.8) | 27 (6, 61.0) | 30 (11.9, 54.3) | 0.80 |
| Postvaccination day 28 | ||||||||||||
| Seroprotection, % (95% CI) | 90.0 (55.5, 99.8) | 91 (58.7, 99.8) | 90.0 (68.3, 98.8) | 0.99 | 80* (44.4, 97.5) | 82 (48.2, 97.7) | 95.0 (75.1, 99.9) | 0.39 | 60.0 (26.2, 87.8) | 64 (35.2, 92.1) | 70.0 (45.7, 88.1) | 0.85 |
| Seroconversion, % (95% CI) | 90 (55.5, 99.8) | 81.2 (48.2, 97.7) | 80 (56.3, 94.3) | 0.79 | 70 (34.8, 93.3) | 73 (39.0, 94.0) | 80 (56.3, 94.3) | 0.81 | 70 (34.8, 93.3) | 45 (16.8, 76.6) | 55 (31.5, 76.9) | 0.52 |
Separate regression analysis adjusted for age, gender, or race/ethnicity found no statistically significant difference in seroprotection, seroconversion, or immune response across opioid use groups (data not shown). In addition, regression analysis testing for association between age, gender, or race/ethnicity alone with immune response found no significant associations.
We also assessed how many individuals had HI antibody titers to A/California/07/2009, before vaccination since this strain appeared for the first time in 2009 [35] and none of the subjects had received the monovalent pH1N1 vaccine. We found that 2/10 (20%) of the heroin users, 7/11 (36%) of the methadone users and 7/20 (35%) of the non-user controls had HI antibody titers ranging from 20 to 80 against pH1N1 at baseline.
4. Discussion
Although active opioid users may be at an increased risk for transmission and complication of influenza, no study has evaluated the humoral responses to the influenza vaccine in this population. Here we report the first study examining the immunogenicity of the influenza vaccine in opioid users. Our study used strict inclusion criteria to avoid decreased responses due to confounding factors such as age or other diseases, opioid use was confirmed (heroin/methadone users) or excluded (controls) by urine toxicology tests at every visit, and included a group of non-heroin user control subjects. Using these rigorous criteria, we found that influenza vaccination in a population of young opioid users (heroin and methadone) gave seroprotection and seroconversion at rates that were not significantly different from those of healthy young controls. The type of opioid use—legal methadone or illegal heroin—also seemed not to affect the responses. Both heroin and methadone users had vaccination responses that were comparable to those of the control subjects.
GMT and fold increase were also similar among groups and were higher for the pandemic H1N1 vaccine strain than for the other vaccine strains, consistent with the higher changes in antibody titers with first-time exposure than with repeated exposure to the same influenza antigen [36,37]. No differences were found when we compared heroin and methadone users to controls.
Nine (42.9%) opioid users and seven (35%) non-user controls had baseline HI antibody titers of 20 or above against pandemic influenza A/California/07/2009 that appeared as a circulating strain for the first time the year before the study. None of the study participants reported having previously received the monovalent vaccine containing the pandemic influenza A/California/07/2009 (available for the first time in the spring preceding the study) nor reported an episode of influenza during the previous season. Cross-reactive antibodies to pH1N1 have been described in about 6–9% of the population aged 18–64 years pre 2009 pandemic vaccination [38]. Higher titers in our patients may be the result of a previous vaccination with a seasonal strain, and/or a mild unrecognized infection with the pandemic strain during the preceding year.
Our study had several limitations. First, the sample size is small and therefore we had limited power to detect small differences in antibody titers between groups in particular in comparing heroin and methadone users and in our study modest differences might have gone undetected. Nonetheless, our pilot data suggest that opioid users might be adequately protected against influenza by vaccination and no consistent pattern of differences emerged among any of the groups in any of our measures.
Our study was also limited to young and healthy subjects and we do not know if opioid use might have a synergistic effect causing decreased responses in patients with other medical comorbidities. In addition, we did not collect long-term follow-up data. Despite these limitations, our results suggest that opioid users achieve protective antibody titers after influenza vaccination similar to those of healthy controls. These findings support the importance of vaccination strategies in this population. Also of importance is the fact that we had little difficulty recruiting or retaining opioid users in our study, although many of them were homeless and participation required four correctly timed follow-up visits. We recruited participants at a program for young people injecting drugs and, as reported in previous studies [39–41] we offered small cash incentives for their participation. This experience suggests that the use of culturally appropriate outreach and small cash incentives to reimburse participants for their time may help in conducting vaccination campaigns or other interventions in this population.
Supplementary Material
Acknowledgments
Financial support
This work was supported by a pilot grant of the National Center for Advancing Translational Science (National Institute of Health Award Number UL1RR024996) to M.S. This work was also partially supported by CRIP (Center for Research in Influenza Pathogenesis), an NIAID funded Center of Excellence for Influenza Research and Surveillance (CEIRS, contract # HHSN272201400008C) to R.A.A. and A.G.-S.
We would like to acknowledge Lola Adekunle, James P. Holahan and Emilie Mankopf for help with patient recruitment and appointment scheduling and Judith Fontana for helpful discussion.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.01.051.
Footnotes
Author contribution
M.S., B.E., A.B.B., A.G-S., Y.J. designed the study. E.M., R.A.A., J.Y. and M.S. performed the research. B.A. and B.E. performed the statistical analysis. E.M. and M.S. wrote the first complete draft and all authors provided contributions and suggestions. All authors read and approved the final version.
Conflicts of interest: All the authors report no potential conflict of interest.
References
- 1.Heffelfinger JD, Patel P, Brooks JT, Calvet H, Daley CL, Dean HD, et al. Pandemic influenza: implications for programs controlling for HIV infection, tuberculosis, and chronic viral hepatitis. Am J Public Health. 2009;99(Suppl 2):S333–9. doi: 10.2105/AJPH.2008.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussons-Read ME, Daniels M, Gilmour MI. Morphine alters the immune response to influenza virus infection in Lewis rats. Adv Exp Med Biol. 1998;437:73–82. doi: 10.1007/978-1-4615-5347-2_9. [DOI] [PubMed] [Google Scholar]
- 3.Coussons-Read ME, Daniels M, Gilmour MI. Morphine reduces pulmonary inflammation in response to influenza infection. Life Sci. 1999;65:1141–52. doi: 10.1016/s0024-3205(99)00348-3. [DOI] [PubMed] [Google Scholar]
- 4.Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Von Korff M, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case–control study. J Am Geriatr Soc. 2011;59:1899–907. doi: 10.1111/j.1532-5415.2011.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16:209–19. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2:14–8. doi: 10.1097/SPC.0b013e3282f5272e. [DOI] [PubMed] [Google Scholar]
- 7.Allolio B, Schulte HM, Deuss U, Kallabis D, Hamel E, Winkelman W. Effect of oral morphine and naloxone on pituitary-adrenal response in man induced by human corticotropin-releasing hormone. Acta Endocrinol (Copenh) 1987;114:509–14. doi: 10.1530/acta.0.1140509. [DOI] [PubMed] [Google Scholar]
- 8.Stoll-Keller F, Schmitt C, Thumann C, Schmitt MP, Caussin C, Kirn A. Effects of morphine on purified human blood monocytes. Modifications of properties involved in antiviral defences. Int J Immunopharmacol. 1997;19:95–100. doi: 10.1016/s0192-0561(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 9.Geber WF, Lefkowitz SS, Hung CY. Duration of interferon inhibition following single and multiple injections of morphine. J Toxicol Environ Health. 1977;2:577–82. doi: 10.1080/15287397709529458. [DOI] [PubMed] [Google Scholar]
- 10.Wan Q, Wang X, Wang YJ, Song L, Wang SH, Ho WZ. Morphine suppresses intracellular interferon-alpha expression in neuronal cells. J Neuroimmunol. 2008;199:1–9. doi: 10.1016/j.jneuroim.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacerdote P, Manfredi B, Gaspani L, Panerai AE. The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in BALB/cJ mice. Blood. 2000;95:2031–6. [PubMed] [Google Scholar]
- 12.Jamali A, Roostaee MH, Soleimanjahi H, Ghaderi Pakdel F, Bamdad T. DNA vaccine-encoded glycoprotein B of HSV-1 fails to protect chronic morphine-treated mice against HSV-1 challenge. Comp Immunol Microbiol Infect Dis. 2007;30:71–80. doi: 10.1016/j.cimid.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998;83:4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang CQ, Li Y, Douglas SD, Wang X, Metzger DS, Zhang T, et al. Morphine withdrawal enhances hepatitis C virus replicon expression. Am J Pathol. 2005;167:1333–40. doi: 10.1016/S0002-9440(10)61220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- 16.Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(Suppl 1):s9–15. [PubMed] [Google Scholar]
- 17.McDonough RJ, Madden JJ, Falek A, Shafer DA, Pline M, Gordon D, et al. Alteration of T and null lymphocyte frequencies in the peripheral blood of human opiate addicts: in vivo evidence for opiate receptor sites on T lymphocytes. J Immunol. 1980;125:2539–43. [PubMed] [Google Scholar]
- 18.Tubaro E, Avico U, Santiangeli C, Zuccaro P, Cavallo G, Pacifici R, et al. Morphine and methadone impact on human phagocytic physiology. Int J Immunopharmacol. 1985;7:865–74. doi: 10.1016/0192-0561(85)90049-9. [DOI] [PubMed] [Google Scholar]
- 19.Novick DM, Ochshorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, et al. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J Pharmacol Exp Ther. 1989;250:606–10. [PubMed] [Google Scholar]
- 20.Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav Immun. 2008;22:606–13. doi: 10.1016/j.bbi.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Garcia JB, Cardoso MG, Dos-Santos MC. Opioids and the immune system: clinical relevance. Rev Bras Anestesiol. 2012;62:709–18. doi: 10.1016/S0034-7094(12)70169-1. [DOI] [PubMed] [Google Scholar]
- 22.Baral S, Sherman SG, Millson P, Beyrer C. Vaccine immunogenicity in injecting drug users: a systematic review. Lancet Infect Dis. 2007;7:667–74. doi: 10.1016/S1473-3099(07)70237-2. [DOI] [PubMed] [Google Scholar]
- 23.Iorio AM, Alatri A, Francisci D, Preziosi R, Neri M, Donatelli I, et al. Immunogenicity of influenza vaccine (1993–94 winter season) in HIV-seropositive and -seronegative ex-intravenous drug users. Vaccine. 1997;15:97–102. doi: 10.1016/s0264-410x(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo JM, Serra MA, Aparisi L, Escudero A, Gilabert MS, Garcia F, et al. Immune response to hepatitis B vaccine in parenteral drug abusers. Vaccine. 1992;10:798–801. doi: 10.1016/0264-410x(92)90516-m. [DOI] [PubMed] [Google Scholar]
- 25.Rumi M, Colombo M, Romeo R, Boschini A, Zanetti A, Gringeri A, et al. Suboptimal response to hepatitis B vaccine in drug users. Arch Intern Med. 1991;151:574–8. [PubMed] [Google Scholar]
- 26.Lum PJ, Ochoa KC, Hahn JA, Page Shafer K, Evans JL, Moss AR. Hepatitis B virus immunization among young injection drug users in San Francisco, Calif: the UFO Study. Am J Public Health. 2003;93:919–23. doi: 10.2105/ajph.93.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quaglio G, Talamini G, Lugoboni F, Lechi A, Venturini L, Jarlais DC, et al. Compliance with hepatitis B vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction. 2002;97:985–92. doi: 10.1046/j.1360-0443.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 28.Lugoboni F, Migliozzi S, Mezzelani P, Pajusco B, Ceravolo R, Quaglio G. Progressive decrease of hepatitis B in a cohort of drug users followed over a period of 15 years: the impact of anti-HBV vaccination. Scand J Infect Dis. 2004;36:131–3. doi: 10.1080/00365540310018833. [DOI] [PubMed] [Google Scholar]
- 29.Lugoboni F, Migliozzi S, Schiesari F, Pauletto N, Bovo GL, Ciaffoni S, et al. Immunoresponse to hepatitis B vaccination and adherence campaign among injecting drug users. Vaccine. 1997;15:1014–6. doi: 10.1016/s0264-410x(96)00290-3. [DOI] [PubMed] [Google Scholar]
- 30.Quaglio G, Pajusco B, Civitelli P, Migliozzi S, Des Jarlais DC, Romano L, et al. Immunogenicity, reactogenicity and adherence with hepatitis A vaccination among drug users. Drug Alcohol Depend. 2004;74:85–8. doi: 10.1016/j.drugalcdep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Edlin BR, Shu MA, Winkelstein E, Des Jarlais DC, Busch MP, Rehermann B, et al. More rare birds, and the occasional swan. Gastroenterology. 2009;136:2412–4. doi: 10.1053/j.gastro.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett CT. Changes in the precordial electrocardiogram due to the position of the exploring electrode. Rocky Mt Med J. 1947;44:107–15. [PubMed] [Google Scholar]
- 33.Wang S, Parker C, Taaffe J, Solorzano A, Garcia-Sastre A, Lu S. Heterologous HA DNA vaccine prime-inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–33. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71:4347–55. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO phasing of pandemic influenza. J Indian Med Assoc. 2009;107:508–9. [PubMed] [Google Scholar]
- 36.Pyhala R, Kumpulainen V, Alanko S, Forsten T. HI antibody kinetics in adult volunteers immunized repeatedly with inactivated trivalent influenza vaccine in 1990–1992. Vaccine. 1994;12:947–52. doi: 10.1016/0264-410x(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 37.Hobson D, Baker FA, Curry RL. Effect of influenza vaccines in stimulating antibody in volunteers with prior immunity. Lancet. 1973;2:155–6. doi: 10.1016/s0140-6736(73)93106-1. [DOI] [PubMed] [Google Scholar]
- 38.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 39.Lorvick J, Thompson S, Edlin BR, Kral AH, Lifson AR, Watters JK. Incentives and accessibility: a pilot study to promote adherence to TB prophylaxis in a high-risk community. J Urban Health. 1999;76:461–7. doi: 10.1007/BF02351503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71:127–31. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 41.Wilcox CE, Bogenschutz MP, Nakazawa M, Woody GE. Compensation effects on clinical trial data collection in opioid-dependent young adults. Am J Drug Alcohol Abuse. 2012;38:81–6. doi: 10.3109/00952990.2011.600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.