Abstract
Exposure to ambient air pollutants increases risk for adverse cardiovascular health outcomes in adults. We aimed to evaluate the contribution of prenatal air pollutant exposure to cardiovascular health, which has not been thoroughly evaluated. The Testing Responses on Youth (TROY) study consists of 768 college students recruited from the University of Southern California in 2007–2009. Participants attended one study visit during which blood pressure, heart rate and carotid artery arterial stiffness (CAS) and carotid artery intima-media thickness (CIMT) were assessed. Prenatal residential addresses were geocoded and used to assign prenatal and postnatal air pollutant exposure estimates using the U.S. Environmental Protection Agency’s Air Quality System (AQS) database. The associations between CAS, CIMT and air pollutants were assessed using linear regression analysis. Prenatal PM10 and PM2.5 exposures were associated with increased CAS. For example, a 2 SD increase in prenatal PM2.5 was associated with CAS indices, including a 5% increase (β = 1.05, 95% CI 1.00–1.10) in carotid stiffness index beta, a 5% increase (β = 1.05, 95% CI 1.01–1.10) in Young’s elastic modulus and a 5% decrease (β = 0.95, 95% CI 0.91–0.99) in distensibility. Mutually adjusted models of pre- and postnatal PM2.5 further suggested the prenatal exposure was most relevant exposure period for CAS. No associations were observed for CIMT. In conclusion, prenatal exposure to elevated air pollutants may increase carotid arterial stiffness in a young adult population of college students. Efforts aimed at limiting prenatal exposures are important public health goals.
Introduction
The negative health effects of air pollution exposure on cardiovascular risk are well documented in adults [1,2,3]. Long-term exposures have been associated with measures of atherosclerosis, including carotid intima-media thickness (CIMT) and arterial stiffness (CAS),[2,4,5,6,7,8] both of which predict future cardiovascular events in adults [9,10]. Changes in CAS, in particular, may reflect both the structural and functional health of the arterial vasculature [11]. Whether changes in CAS in children predict adult cardiovascular risk remains unknown, although recent evidence suggests blood pressure and CAS are highly related[12] and childhood blood pressure tracks closely with adult blood pressure, increasing later cardiovascular risk [13]. CAS, in fact, may be viewed as an early biomarker of endothelial function in which observed abnormalities reflect changes in the integrity of the vascular structure prior to manifestation of symptomatic cardiovascular events [14]. Plenty of evidence exists linking endothelial dysfunction to the later development of clinical vascular disease [14]. Thus, use of these surrogate vascular markers, which represent some of the best early biomarkers of adverse outcomes available in youth, may help to develop a better understanding of early vascular changes and their correlates and may also facilitate identification of children at risk for cardiovascular disease later in life [15]. Given that atherosclerosis has its origins in early life[16] an that an adverse intrauterine environment contributes to the early development of atherosclerosis, with a long latency period between exposures and adult CVD,[17] we hypothesized that exposure to air pollutants early in life may be associated with early biomarkers of cardiovascular phenotypes such as CIMT and CAS. Prenatal exposure to air pollutants may disrupt biological mechanisms that regulate fetal growth and development, which in turn may make children more susceptible to the development of cardiovascular pathologies and disease later in life. In the few studies conducted in healthy populations of children or young adults, childhood or recent exposures to air pollutants have been associated with CAS and CIMT but prenatal exposures have not been evaluated [6,18,19]. Animal models of prenatal exposure to pollutants and to tobacco smoke have demonstrated increased cardiac oxidative stress and atherogenesis in adult mice [20,21]. Pollutants such as PM2.5 have also been associated with systemic inflammation, oxidative stress, and endothelial injury in children and young adults [22,23,24]. To address this lack of knowledge, we investigated the association between prenatal trimester-specific and postnatal exposures to PM10, PM2.5, NO2 and O3 with CAS and CIMT in a population of University of Southern California (USC) college students.
Methods
The Testing Responses on Youth (TROY) study consists of 768 college students recruited from USC in 2007–2009. The primary purpose of the TROY study is to assess lifetime histories of air pollution exposure in relation to early determinants of atherosclerosis. Participants were eligible for study inclusion if they were non-tobacco smokers, were born in the United States or moved to the United States within the first six months of life, and provided written informed consent to participate.
Participants attended a study visit during which CAS, CIMT, systolic and diastolic blood pressure, and heart rate were assessed by a single physician-imaging specialist from the USC Atherosclerosis Research Unit Core Imaging and Reading Center. Self-administered questionnaires were completed to gather information about health and socio-demographic characteristics as described previously [6,25]. Participants also provided a 12-hr fasting blood sample for lipid and biomarker analyses (see online supplement for further details).
The study protocol was approved by the institutional review board for human studies at the University of Southern California, and written consent was provided by the study subjects.
High-resolution B-mode ultrasound images of the right common carotid artery (CCA) were obtained with a portable Biosound MyLab 25 ultrasound system attached to a 10-MHz linear array transducer and read by a single physician-imaging specialist. Blood pressure and heart rate were measured immediately after the ultrasound examination by standard techniques after the subject was recumbent for at least ten minutes. Blood pressure was measured three times in one-minute intervals, using an OMRON blood pressure monitor with automatic cuff inflation and deflation. As previously described (Patents 2005, 2006, 2011)[6,26,27], the jugular vein and carotid artery were imaged transversely with the jugular vein stacked above the carotid artery and CIMT was measured. Media-adventitia to media-adventitia arterial dimensions were measured for calculation of the carotid arterial stiffness variables in the same arterial segment along the same 1 cm electronic ruler used to measure the CIMT using an in-house developed software package (Patents 2005, 2006, 2011) [26,27,28,29]. The lumen diameters measured during peak systole and end diastole were used to calculate three measures of arterial stiffness: distensibility, Young’s elastic modulus (YEM) and stiffness index beta (C-beta) according to standard formula (see online supplement for details) [27,30]. Duplicate scans were performed on 87 subjects and the correlation coefficients for minimum arterial diameter, maximum arterial diameter, and CIMT were 0.95, 0.95, and 0.98, respectively.
Participants completed a detailed lifetime residential history. Participant residence addresses within the U.S. were standardized and their locations were geocoded using the Tele Atlas Geocoding Service (Tele Atlas Inc., Menlo Park, California, www.na.teleatlas.com). Of the 2,598 residential locations reported, 98.3% (2,553) were U.S. residences that were successfully geocoded.
Prenatal ambient air pollution concentrations were estimated for each subject’s reported birth residence based on average monthly air pollutant exposure data and trimesters defined as follows: first trimester from 0 to 13 weeks post-conception, second trimester from 14 to 26 weeks, and third trimester from 27 to delivery. Because we previously reported an association between early childhood, elementary school and lifetime air pollution exposures with CIMT in this cohort [6], we also investigated these postnatal exposure windows with CAS. Postnatal exposure corresponding to the early childhood (0–5), elementary school years (6–12) and postnatal exposure (from birth to date of CIMT measurement) were calculated by averaging exposures across the relevant residential histories for those time periods as described previously [6]. Briefly, ambient air pollution concentrations were estimated for each subject’s residence within the U.S. from the time the subject occupied that residence to the participant’s CIMT measurement. Move-in and move-out dates were provided for each residence, and ambient air quality data was spatially interpolated to those locations for the relevant time periods using inverse distance-squared weighting (IDW2) [31,32]. The data from up to four air quality measurement stations were included in each interpolation. Due to the regional nature of O3, NO2, PM10, and PM2.5 concentrations, a maximum interpolation radius of 50 km was used for all pollutants. However, when a residence was located within 5 km of one or more stations with valid observations, the interpolation was based solely on the nearby values. A leave one out evaluation of the spatial mapping method produced r2 of 0.76, 0.73, 0.53, and 0.46 for monthly ozone, NO2, PM2.5, and PM10 concentrations using data from California (representing 85% of the population).
Air pollutant estimates were derived from the U.S. Environmental Protection Agency’s Air Quality System (AQS) database for the years 1980 through 2009. Hourly concentrations of O3 and NO2, and daily concentrations of PM10 and PM2.5 measured in all 50 states for January 1980 through 2009 were downloaded from AQS. The PM data were primarily limited to those collected with Federal Reference Method (FRM) monitors and Federal Equivalent Method (FEM). Non-FEM PM2.5 data were used when no FEM measurements were available. Automated quality control checks on the concentration ranges and persistence were applied to the AQS data. The AQS data were augmented in southern California with O3, NO2, PM10, and PM2.5 data from the Children’s Health Study (CHS) for 1994–2009 [33,34]. National-scale PM10 data were filled in using adjusted total suspended particulates (TSP) data for 1981–1987. Pre-1999 PM2.5 data for southern California were filled in with 1994–1998 estimated PM2.5 concentrations developed for the CHS.
In order to assign a postnatal exposure estimate, data were required to be 75% complete for O3 and NO2 and 12% for PM to account for the one-in-six day sampling. As a result, of 768 initial study subjects, a range of 23 up to 113 subjects could be missing trimester-specific concentrations of specific pollutants (see online supplement for more details).
Means and standard deviations of subjects’ health and anthropometric characteristics at the time of carotid ultrasound measurement as well as the distributions of prenatal and postnatal air pollutants were calculated. Air pollutants were treated as continuous variables and were scaled to a 2 standard deviation (SD) difference in level for testing associations with CAS and CIMT. The associations between CAS and CIMT and prenatal and postnatal air pollutants were assessed using linear regression analysis. Non-linear associations were evaluated using penalized splines in the GAM function of the R statistical package[35] but all associations were found to be linear. Arterial stiffness metrics were log-transformed to achieve normality. The exponentiated regression model coefficient can be interpreted as a fold-change in CAS per 2SD change in level of pollutant. Variables evaluated for confounding but not selected as confounders based on whether they changed the effect estimate of interest by greater than 10% included diastolic and systolic blood pressure, hsCRP, LDL-C, HDL-C, prenatal tobacco smoke exposure, second hand tobacco smoke exposure during childhood and homeostatic model assessment (HOMA) of insulin sensitivity and beta cell function. A final multivariate model adjusted for age, sex, race/ethnicity, maternal education, BMI, height, insulin, triglycerides, birth season and geographic region at birth for all CAS models. The final model for CIMT analysis was adjusted for age, sex, race/ethnicity, maternal education, BMI, systolic blood pressure, second hand smoke, hsCRP, LDL-C and HDL-C to be comparable to previously published results [6]. Regression procedures were conducted in SAS v9.3 (Cary, NC). [36] All statistical testing was conducted with a two-sided alpha level of 0.05.
We conducted a series of sensitivity analyses to evaluate whether exclusion of subjects by the following criteria affected our results: 1) preterm birth, 2) reported smoking of alternative tobacco products, 3) high cholesterol or high blood pressure, 4) family history of hypertension or high cholesterol, 5) family history of heart attack, heart failure or stroke, and 6) non-California born subjects; 7) poor air quality codes.
Results
Baseline characteristics of the 768 study participants are shown in Table 1 and S1 Table in the online supplement. All participants were college students who were on average 20±1.5 years of age; the sample included more females (59%) than males (41%). Only one participant had high blood pressure (defined as > 120/80 mmHg) and family history of heart disease (5.5%) was rare in this population. C-beta, YEM, and Distensibility were log-normally distributed with geometric means (SD) of 6.2 (1.3), 2621.9 mmHg (1.4), and 30.2 x 10−6 x m2/N (1.3), respectively. These three CAS measurements were also highly correlated with one another but not with CIMT (S2 Table).
Table 1. Demographic characteristics of TROY participants (N = 768)*.
| N | % | |
|---|---|---|
| Male sex | 317 | 41.3 |
| Race/ethnicity | ||
| Non Hispanic White | 344 | 44.8 |
| Black | 38 | 5 |
| Asian | 161 | 21 |
| Hispanic White | 132 | 17.2 |
| Other | 93 | 12.1 |
| BMI† | ||
| Underweight | 31 | 4 |
| Normal | 574 | 74.7 |
| Overweight | 133 | 17.3 |
| Obese | 30 | 3.9 |
| Current exposure to second-hand smoke‡ | 296 | 38.5 |
| Second-hand smoke exposure during childhood | 61 | 7.9 |
| Ever smoked something other than cigarettes | ||
| Yes | 175 | 22.8 |
| Don't know | 1 | 0.1 |
| Mother's Education | ||
| High school or less | 83 | 10.8 |
| Some college | 177 | 23.1 |
| College grad/some grad school | 503 | 65.5 |
| Unknown | 5 | 0.7 |
| Family history of heart disease§ | ||
| Yes | 42 | 5.5 |
| Don't know | 26 | 3.4 |
* TROY participants were non-smokers (of cigarettes).
† Underweight was defined as BMI < 18.5, normal weight as 18.5 ≤ BMI <25, overweight as25≤ BMI <30, and obese as BMI ≥ 30.
‡Current second hand smoke exposure locations: Home, dormitory room, workplace, school or places other than home or school.
§History of heart attack, heart failure, or stroke.
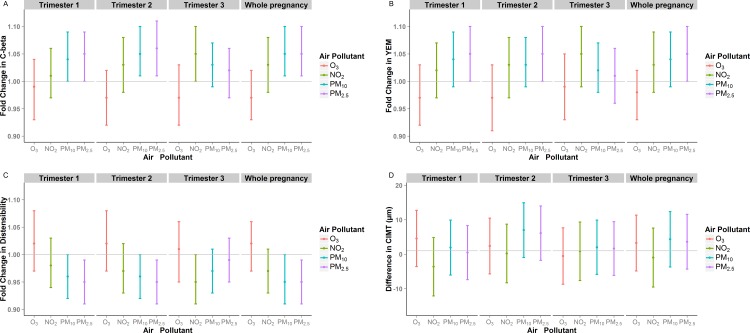
Prenatal air pollutants had a range of distribution across trimesters (S3 Table). In general, NO2, PM10 and PM2.5 were highly correlated within trimester but less so across trimesters (S4 Table). O3 was not highly correlated with the other pollutants. Prenatal exposures to PM10, and PM2.5 were associated with increased CAS (Fig 1, S5 Table). For example, a 2SD higher level of PM2.5 during pregnancy was associated with a 5% higher C-Beta (β = 1.05, 95% CI 1.01–1.10), a 5% higher YEM (β = 1.05, 95% CI 1.01–1.10), and a 5% decrease in distensibility (β = 0.95, 95% CI 0.91–0.99). Prenatal O3 showed no association with CAS and prenatal NO2 was marginally associated. A multi-pollutant model which included both O3 and PM10 as representative of the suite of correlated pollutants did not alter interpretation of the results (Table 2). Prenatal air pollutants were not associated with CIMT (Fig 1, S5 Table).
Fig 1.
The association between prenatal air pollutant exposures and A) C-beta, B) YEM, C) Distensibility, and D) CIMT, by trimester and whole pregnancy.
Table 2. Results from a multi-pollutant model* of PM10 and O3 (N = 673).
| Trimester 1 | Trimester 2 | Trimester 3 | Whole pregnancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Pollutant per 2SD change | fold change in outcome | 95% CI | fold change in outcome | 95% CI | fold change in outcome | 95% CI | fold change in outcome | 95% CI | ||||
| C-beta | O3 (ppb) | 0.97 | 0.92 | 1.02 | 0.95 | 0.9 | 1.01 | 0.96 | 0.9 | 1.01 | 0.96 | 0.91 | 1 |
| PM10 (μ/m3) | 1.06 | 1.01 | 1.11 | 1.07 | 1.02 | 1.12 | 1.04 | 1 | 1.09 | 1.07 | 1.02 | 1.12 | |
| YEM | O3 (ppb) | 0.96 | 0.9 | 1.01 | 0.96 | 0.9 | 1.02 | 0.98 | 0.92 | 1.04 | 0.96 | 0.91 | 1.01 |
| PM10 (μ/m3) | 1.06 | 1.01 | 1.11 | 1.05 | 0.99 | 1.1 | 1.04 | 0.99 | 1.09 | 1.06 | 1.01 | 1.11 | |
| Distensibility | O3 (ppb) | 1.04 | 0.98 | 1.1 | 1.04 | 0.98 | 1.1 | 1.02 | 0.96 | 1.08 | 1.03 | 0.99 | 1.08 |
| PM10 (μ/m3) | 0.94 | 0.9 | 0.99 | 0.95 | 0.91 | 0.99 | 0.96 | 0.92 | 1.01 | 0.94 | 0.9 | 0.99 | |
*adjusted for sex, age, ethnicity, maternal education, BMI, height, insulin, triglycerides, birth season and geographic region
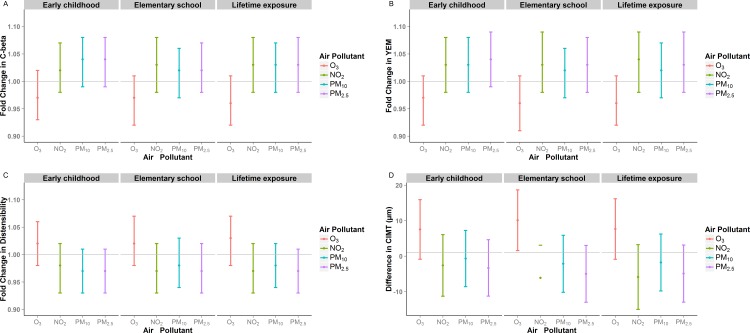
Because we previously reported an association between early childhood, elementary school and postnatal air pollution exposures (notably O3) with CIMT in this cohort,[6] we also evaluated these time periods of exposure for CAS (Fig 2, S6 Table). We observed non-significant associations that were similar in magnitude to the effects observed with prenatal exposures.
Fig 2.
The association between postnatal air pollutant exposures and A) C-beta, B) YEM, C) Distensibility, and D) CIMT, by early childhood, elementary school and postnatal exposure.
We also sought to evaluate the relative contributions of prenatal and postnatal exposures on CAS and CIMT. Prenatal and postnatal NO2 and PM10 levels were highly correlated, whereas O3 and PM2.5 were moderately correlated (S7 Table). Results from models that mutually adjusted for prenatal and postnatal PM2.5 suggested that the effects on CAS were due to the prenatal rather than postnatal exposure (Table 3). In models that mutually adjusted for prenatal and postnatal O3 on CIMT, our previously reported findings of an association with postnatal O3 remained robust (S8 Table) whereas prenatal O3 had no effect on CIMT [6]. Mutually adjusted models of O3 on CAS showed no associations (data not shown).
Table 3. The association between prenatal and postnatal PM2.5 (μ/m3) exposures and CAS*(N = 724).
| Trimester 1 | Trimester 2 | Trimester 3 | Whole pregnancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Pollutant per 2SD unit change | fold change in outcome | 95% CI | fold change in outcome | 95% CI | fold change in outcome | 95% CI | fold change in outcome | 95% CI | ||||
| C-beta | Prenatal PM2.5 | 1.05 | 0.99 | 1.11 | 1.06 | 1 | 1.13 | 0.99 | 0.94 | 1.05 | 1.07 | 0.99 | 1.15 |
| Lifetime PM2.5 | 1 | 0.94 | 1.06 | 0.99 | 0.94 | 1.05 | 1.04 | 0.98 | 1.1 | 0.98 | 0.91 | 1.05 | |
| YEM | Prenatal PM2.5 | 1.05 | 0.99 | 1.12 | 1.04 | 0.98 | 1.11 | 0.98 | 0.93 | 1.04 | 1.05 | 0.97 | 1.14 |
| Lifetime PM2.5 | 1 | 0.94 | 1.06 | 1.01 | 0.94 | 1.07 | 1.04 | 0.98 | 1.11 | 0.99 | 0.91 | 1.07 | |
| Distensibility | Prenatal PM2.5 | 0.95 | 0.9 | 1 | 0.94 | 0.89 | 1 | 1.01 | 0.96 | 1.06 | 0.94 | 0.87 | 1 |
| Lifetime PM2.5 | 1 | 0.95 | 1.06 | 1.01 | 0.95 | 1.06 | 0.96 | 0.91 | 1.02 | 1.02 | 0.95 | 1.10 | |
*adjusted for sex, age, ethnicity, maternal education, BMI, height, insulin, triglycerides, birth season and geographic region
Sensitivity analyses were conducted to evaluate several exclusion criteria. Removal of 68 participants who reported a family history of heart disease did not affect our results, nor did removal of 354 participants who reported a family history of hypertension or high cholesterol or removal of 40 subjects with high cholesterol or high blood pressure. Excluding the 118 participants who were born preterm or the 175 participants who reported smoking alternative tobacco products did not alter our results. Restriction of the population to participants from southern California (n = 549) on whom we had supplemental air monitoring data also did not alter our results (S9 Table). Further restriction of the population to participants who lived within 5 km from an air pollution monitor did not alter our results, though the sample size was small (S10 Table).
Discussion
Prenatal exposure to PM2.5 and PM10 was associated with higher CAS but not CIMT in a population of college students. These results lend further evidence in support of the developmental origins of disease hypothesis for atherosclerosis [37,38,39].
Several studies in adults have demonstrated associations between long-term air pollutant exposures, particularly PM2.5, with CAS and CIMT [2,3,4,5,7,8,40,41], While most of these are cross-sectional in nature, longitudinal evidence is beginning to emerge [3]. PM2.5 is also associated with plaque burden and vascular dysfunction in murine models of atherosclerosis [3]. A few studies have demonstrated associations between air pollutants and CAS or CIMT in children or young adults [6,18,19]. Ianuzzi et al evaluated 52 Italian children and found that children living closer to a main road had higher CAS than those living farther away [19]. Lenters et al observed a 37.6% increase in augmentation index and a 4% increase in pulse wave velocity, another indicator of arterial stiffness, in response to a 25 μg/m3 increase in NO2, estimated from subjects’ residential addresses [18]. Our observed effects of a 5% increase in CAS per 2 SD (15.4 μg/m3 for PM2.5) change in pollutant level are slightly smaller in magnitude to changes in CAS observed for passive tobacco smoke exposure[30] and are comparable to an aging effect of 2.5 to 10 years during childhood [42].
In our previous report in this same study population, we observed that childhood exposure to O3 was associated with increased CIMT. Herein, we extend these findings to suggest that prenatal exposures ambient pollutants (PM10, PM2.5) are also important, exhibiting increases on CAS but not CIMT. One explanation for this observation may be that CAS, as a biomarker of endothelial function, reflects functionality of the arterial vasculature that may be a more sensitive marker for early subclinical cardiovascular changes in response to chronic environmental exposures whereas CIMT, a structural change, may take longer to demonstrate measurable differences.
While our observed associations were stronger and statistically significant for prenatal exposures to PM10 and PM2.5, these pollutants were correlated between prenatal and postnatal exposure periods, limiting our ability to conclude with certainty which time period confers the most risk. Nevertheless, we evaluated prenatal and postnatal PM2.5 and O3 in mutually adjusted models (pollutants with the least amount of correlation). We found that the effects of PM2.5 on CAS were likely due to the prenatal rather than postnatal exposure, whereas the opposite was true for O3, and remained consistent with previously published results [6]. The observed effects of prenatal PM2.5 may occur through altered fetal growth and development. High prenatal PM exposure has been associated with lower birth weight [43] and patent ductus arteriosus [44]. PM constituents, particularly transition metals, could generate oxidative stress leading to DNA damage in the placenta, affecting the growing embryo [45]. PM may also bind receptors for placental growth factors resulting in decreased fetal–placental exchange of oxygen and nutrients, upregulate systemic pro-inflammatory mediators or alter hemodynamic responses with negative downstream consequences [45].
One of the strengths of this large study is the availability of prenatal and cumulative postnatal air pollutant exposure histories for participants. However, because we calculated air pollutant exposure estimates using existing pollutant databases acquired over twenty years prior to CAS assessment, measurement error may be of concern. To counter this, we only assigned exposure when we had relevant measurement data. Moreover, sensitivity analyses restricting the dataset to participants with only the highest quality data (i.e. in southern California for which we had additional monitoring data), as well as restricting to subjects within 5km of a monitor, yielded similar results, thereby strengthening our conclusions. A lack of monitoring data for PM2.5 in early years resulted in a smaller sample size for those analyses. In addition, imputation of PM2.5 values based on historical PM10/PM2.5 ratios may have increased measurement error. However, the pattern of results for both pollutants as well as for NO2 were similar, suggesting that errors specific to lack of PM2.5 data did not affect our results.
A general limitation to this study is the lack of information on the mothers at the time of pregnancy, including general health, habits, and occupation which could lead to unmeasured confounding. In cases where we knew maternal information, such as for preterm delivery and maternal history of cardiovascular disease or hypertension, we conducted sensitivity analyses to evaluate potential effects and found no changes to our conclusions. Traffic-related noise is another environmental stressor relevant to pregnant women and a likely contributor to vascular pathologies for which we had no data available. However, as reviewed by Tetrault et al, correlations between noise and traffic related pollution are rather modest [46]. Thus, our results are unlikely to be confounded by unmeasured exposure to night time noise. We studied a population of non-smoking university students who may be on average healthier and socio-economically advantaged relative to the general population. Therefore, the results of this study may not be generalizable to all individuals.
Recent guidelines for prevention of hypertension have suggested the use of vascular parameters aimed at evaluating the mechanical and functional properties of peripheral arteries in order to identify vulnerable individuals [9]. CAS is included in this list, and is considered a subclinical target in evaluating hypertensive patients [9]. Given that children rarely present with overt cardiovascular disease, use of these early vascular biomarkers in children and young adults may help to develop a better understanding of pathological vascular changes associated with air pollution exposures as well as facilitate identification of children at risk for cardiovascular disease later in life [15].
In conclusion, the atherogenic process has important determinants early in life. We present evidence that prenatal exposures to PM2.5 and PM10 are associated with CAS in a healthy population of college students. The implications of such early vascular changes with respect to adult cardiovascular disease remain unclear and require investigation. Nevertheless, regulation of air pollutants and efforts that focus on limiting prenatal and childhood exposures continue to be important public health goals to potentially reduce the atherosclerosis burden and its consequences.
Supporting Information
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
The minimal set of data underlying the current analyses has been provided as a supplemental file.
Funding Statement
This work was supported by National Institutes of Health 1K01ES017801, http://www.nih.gov/, CVB; National Institutes of Health 5R01ES014708, EA; National Institutes of Health 5P30ES007048, FG; National Institutes of Health 5P01ES009581, FG; National Institutes of Health R826708-01 and RD831861-01. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Fred Lurmann is employed by Sonoma Technology Inc (STI). STI was paid to conduct the air pollution exposure assignments used in the analyses in this manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. One of the authors.
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. (2004) Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S (2010) Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep 12: 291–300. 10.1007/s11883-010-0122-7 [DOI] [PubMed] [Google Scholar]
- 3.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. (2015) Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36: 83–93b. 10.1093/eurheartj/ehu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta AJ, Zanobetti A, Koutrakis P, Mittleman MA, Sparrow D, Vokonas P, et al. (2014) Associations between short-term changes in air pollution and correlates of arterial stiffness: The Veterans Affairs Normative Aging Study, 2007–2011. Am J Epidemiol 179: 192–199. 10.1093/aje/kwt271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu CF, Kuo IC, Su TC, Li YR, Lin LY, Chan CC, et al. (2010) Effects of personal exposure to particulate matter and ozone on arterial stiffness and heart rate variability in healthy adults. Am J Epidemiol 171: 1299–1309. 10.1093/aje/kwq060 [DOI] [PubMed] [Google Scholar]
- 6.Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, et al. (2012) Childhood air pollutant exposure and carotid artery intima-media thickness in young adults. Circulation 126: 1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunzli N, Jerrett M, Garcia-Esteban R, Basagana X, Beckermann B, Gilliland F, et al. (2010) Ambient air pollution and the progression of atherosclerosis in adults. PLoS One 5: e9096 10.1371/journal.pone.0009096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer M, Moebus S, Mohlenkamp S, Dragano N, Nonnemacher M, Fuchsluger M, et al. (2010) Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol 56: 1803–1808. 10.1016/j.jacc.2010.04.065 [DOI] [PubMed] [Google Scholar]
- 9.Bianchini E, Giannarelli C, Bruno RM, Armenia S, Landini L, Faita F, et al. (2013) Functional and structural alterations of large arteries: methodological issues. Curr Pharm Des 19: 2390–2400. [DOI] [PubMed] [Google Scholar]
- 10.Chirinos JA (2012) Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res 5: 243–255. 10.1007/s12265-012-9359-6 [DOI] [PubMed] [Google Scholar]
- 11.Herouvi D, Karanasios E, Karayianni C, Karavanaki K (2013) Cardiovascular disease in childhood: the role of obesity. Eur J Pediatr 172: 721–732. 10.1007/s00431-013-1932-8 [DOI] [PubMed] [Google Scholar]
- 12.Stabouli S, Papakatsika S, Kotronis G, Papadopoulou-Legbelou K, Rizos Z, Kotsis V (2015) Arterial stiffness and SBP variability in children and adolescents. J Hypertens 33: 88–95. 10.1097/HJH.0000000000000369 [DOI] [PubMed] [Google Scholar]
- 13.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, et al. (2008) Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52: 433–451. 10.1161/HYPERTENSIONAHA.108.190329 [DOI] [PubMed] [Google Scholar]
- 14.Glasser SP, Dudenbostel T (2011) The Global Burden of Cardiovascular Disease: The Role of Endothelial Function and Arterial Elasticity in Cardiovascular Disease as Novel and Emerging Biomarkers. Curr Cardiovasc Risk Rep 5: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeal CJ, Wilson DP, Christou D, Bush RL, Shepherd LG, Santiago J, et al. (2009) The use of surrogate vascular markers in youth at risk for premature cardiovascular disease. J Pediatr Endocrinol Metab 22: 195–211. [DOI] [PubMed] [Google Scholar]
- 16.Barker DJ (2008) Human growth and cardiovascular disease. Nestle Nutr Workshop Ser Pediatr Program 61: 21–38. 10.1159/0000113163 [DOI] [PubMed] [Google Scholar]
- 17.Kelishadi R, Poursafa P (2014) A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care 44: 54–72. 10.1016/j.cppeds.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 18.Lenters V, Uiterwaal CS, Beelen R, Bots ML, Fischer P, Brunekreef B, et al. (2010) Long-term exposure to air pollution and vascular damage in young adults. Epidemiology 21: 512–520. 10.1097/EDE.0b013e3181dec3a7 [DOI] [PubMed] [Google Scholar]
- 19.Iannuzzi A, Verga MC, Renis M, Schiavo A, Salvatore V, Santoriello C, et al. (2010) Air pollution and carotid arterial stiffness in children. Cardiol Young 20: 186–190. 10.1017/S1047951109992010 [DOI] [PubMed] [Google Scholar]
- 20.Fetterman JL, Pompilius M, Westbrook DG, Uyeminami D, Brown J, Pinkerton KE, et al. (2013) Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoE(-/-) mice. PLoS One 8: e66835 10.1371/journal.pone.0066835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damaceno-Rodrigues NR, Veras MM, Negri EM, Zanchi AC, Rhoden CR, Saldiva PH, et al. (2009) Effect of pre- and postnatal exposure to urban air pollution on myocardial lipid peroxidation levels in adult mice. Inhal Toxicol 21: 1129–1137. 10.3109/08958370902798430 [DOI] [PubMed] [Google Scholar]
- 22.Calderon-Garciduenas L, Villarreal-Calderon R, Valencia-Salazar G, Henriquez-Roldan C, Gutierrez-Castrellon P, Torres-Jardon R, et al. (2008) Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol 20: 499–506. 10.1080/08958370701864797 [DOI] [PubMed] [Google Scholar]
- 23.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS (2009) Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 203: 311–319. 10.1016/j.atherosclerosis.2008.06.022 [DOI] [PubMed] [Google Scholar]
- 24.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS (2007) The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 176: 370–376. [DOI] [PubMed] [Google Scholar]
- 25.Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, et al. (2011) Carotid artery intima-media thickness in college students: race/ethnicity matters. Atherosclerosis 217: 441–446. 10.1016/j.atherosclerosis.2011.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, et al. (2002) Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 106: 1453–1459. [DOI] [PubMed] [Google Scholar]
- 27.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN (2001) Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 154: 185–193. [DOI] [PubMed] [Google Scholar]
- 28.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. (2001) Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 135: 939–953. [DOI] [PubMed] [Google Scholar]
- 29.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, et al. (1994) Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis 111: 1–11. [DOI] [PubMed] [Google Scholar]
- 30.Mack WJ, Islam T, Lee Z, Selzer RH, Hodis HN (2003) Environmental tobacco smoke and carotid arterial stiffness. Prev Med 37: 148–154. [DOI] [PubMed] [Google Scholar]
- 31.Rivera-Gonzalez LO, Zhang Z, Sanchez BN, Zhang K, Brown DG, Rojas-Bracho L, et al. (2015) An assessment of air pollutant exposure methods in Mexico City, Mexico. J Air Waste Manag Assoc 65: 581–591. 10.1080/10962247.2015.1020974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannam K, McNamee R, De Vocht F, Baker P, Sibley C, Agius R (2013) A comparison of population air pollution exposure estimation techniques with personal exposure estimates in a pregnant cohort. Environ Sci Process Impacts 15: 1562–1572. 10.1039/c3em00112a [DOI] [PubMed] [Google Scholar]
- 33.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, et al. (1999) A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med 159: 768–775. [DOI] [PubMed] [Google Scholar]
- 34.Peters JM, Avol E, Navidi W, London SJ, Gauderman WJ, Lurmann F, et al. (1999) A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med 159: 760–767. [DOI] [PubMed] [Google Scholar]
- 35.Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Amer Statist Ass 99: 673–686. [Google Scholar]
- 36.SAS/STAT (2002) Version 9 [program] 9.0 ed. Cary, NC: SAS Institute. [Google Scholar]
- 37.Okada T (2010) Developmental origins of cardiovascular disease: cholesterol metabolism and higher carotid artery intima-media thickness in young adults born small for gestational age. Circ J 74: 2299–2300. [DOI] [PubMed] [Google Scholar]
- 38.Barker DJ (2007) The origins of the developmental origins theory. J Intern Med 261: 412–417. [DOI] [PubMed] [Google Scholar]
- 39.Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, et al. (2007) Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatr Res 61: 625–629. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Sun Z, Ruan Y, Yan J, Mukherjee B, Yang F, et al. (2014) Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension 63: 871–877. 10.1161/HYPERTENSIONAHA.113.02588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez L, Wolf K, Hennig F, Penell J, Basagana X, Foraster M, et al. (2015) Air Pollution and Atherosclerosis: A Cross-Sectional Analysis of Four European Cohort Studies in the ESCAPE Study. Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernhall B, Agiovlasitis S (2008) Arterial function in youth: window into cardiovascular risk. J Appl Physiol (1985) 105: 325–333. [DOI] [PubMed] [Google Scholar]
- 43.Bell ML, Belanger K, Ebisu K, Gent JF, Lee HJ, Koutrakis P, et al. (2010) Prenatal exposure to fine particulate matter and birth weight: variations by particulate constituents and sources. Epidemiology 21: 884–891. 10.1097/EDE.0b013e3181f2f405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strickland MJ, Klein M, Correa A, Reller MD, Mahle WT, Riehle-Colarusso TJ, et al. (2009) Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am J Epidemiol 169: 1004–1014. 10.1093/aje/kwp011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kannan S, Misra DP, Dvonch JT, Krishnakumar A (2006) Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect 114: 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tetreault LF, Perron S, Smargiassi A (2013) Cardiovascular health, traffic-related air pollution and noise: are associations mutually confounded? A systematic review. Int J Public Health 58: 649–666. 10.1007/s00038-013-0489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The minimal set of data underlying the current analyses has been provided as a supplemental file.