Abstract
Background
Increased plasma free fatty acids (FFA) are considered one of the key elements in the pathogenesis of insulin resistance (IR) and type 2 diabetes (T2DM). We hypothesize that, in diabetic patients undergoing laparoscopic Roux-en-Y gastric bypass (LRYGB), a postoperative decrease in FFA will correlate with improved insulin sensitivity (Si).
Methods
30 obese (body mass index > 35 kg/m2) patients with a diagnosis of T2DM were studied preoperatively and 12 months after LRYGB in a prospective cohort study. Collected data included intravenous glucose tolerance test (IVGTT), total body composition by DEXA and plasma levels of FFA. Insulin sensitivity analysis from the IVGTT was estimated from minimal model analysis. Pre- and postoperative variables were compared using a paired sample t-test. Relationships between changes in variables were determined with Pearson's correlation test.
Results
Twelve months after LRYGB the study population showed a significant decrease in body mass index (BMI) (P = 0.001), FFA (P = 0.03), and total body fat (P = 0.03), with an increase in Si (P = 0.001). Postoperative changes in Si significantly correlated (Pearson's r = −0.53, P=0.01) with change in total body fat, but not with changes in plasma FFA (Pearson's r = −0.22, P= 0.31).
Conclusions
Our study challenges the notion that IR is mediated to a significant degree by changes in plasma FFA concentration. Instead, changes in adiposity and consequently changes in adipokines release can be the key players in determining remission of T2DM after RYGB.
Keywords: Bariatric surgery, Laparoscopic Roux-en-Y gastric bypass, Free Fatty Acid, Insulin sensitivity, Insulin Resistance, Total body fat, adiposity
INTRODUCTION
Obesity often causes IR and has been shown to influence the severity of IR in type 2 diabetes (T2DM)1–3. Levels of plasma free fatty acids (FFA) are considered one of the key elements in the obesity-related pathogenesis of IR and T2DM4. Several studies have shown that increased FFA levels were associated with increased levels of IR in obese individuals, although the interpretation of the causal relationship between these two variables is still debated. For some authors, the increased FFA levels, seen in obese individuals is due to increases in FFA release5 and decreases in FFA clearance6. Increased concentrations of FFA has been proven to induce IR through several mechanisms: reduced glucose transport in skeletal muscle7, increased hepatic glucose production5, inhibition of the insulin receptor substrate-1 signaling pathway8,9 and activation of the proinflammatory nuclear factor-kB pathway10 in insulin-responsive peripheral tissues. On the other hand, for other authors, the increased FFA levels could be the consequence of excessive lipolysis and lipoprotein lipase activity, secondary to the adipose tissue IR and hyperinsulinemia seen in obese individuals11,12.
It has been demonstrated that bariatric surgery can achieve excellent long-term weight loss and improve obesity-related comorbidities, quality of life, and survival13–15. Two large nonblinded, randomized, clinical trials comparing laparoscopic Roux-en-Y gastric bypass (LRYGB) with medical therapy for the treatment of diabetes, showed that bariatric surgery resulted in better glucose control, with rates of hyperinsulinemia and the homeostasis model assessment-estimated insulin resistance index markedly improved as compared with medical therapy alone16,17. Recently, gastric bypass has been shown to increase both Si and pancreatic beta-cell function, whereas, despite comparable weight loss, sleeve gastrectomy has been shown only partially restore the Si with no improvement in the pancreatic beta-cell function18. Insulin sensitivity improves within days after gastric bypass surgery, likely secondary to reduced stimulation of the entero-insular axis by caloric restriction19, 20. However, the mechanisms responsible for the long term improved Si with resolution of T2DM after gastric bypass are still not well understood, but the preferential loss of fat is considered to play a key role20.
Patients undergoing bariatric surgery have been also shown to have decreased FFA levels one-year after operation21,22. Therefore, it could be hypothesized that the decreased FFA levels after bariatric surgery may play a role in mediating the improved Si with resolution of T2DM through insulin receptor signaling pathways.
The aim of our study is to determine if a postoperative decrease in FFA correlates with the improved Si seen in T2DM patients undergoing LRYGB.
MATERIAL AND METHODS
Study design and Data collection
A prospective cohort study was designed. We studied 30 morbidly obese (body mass index [BMI] > 35 kg/m2) patients with a diagnosis of T2DM preoperatively and 12 months after LRYGB as previously described23. Inclusion criteria included clinical diagnosis of T2DM diabetes mellitus according to the American Diabetes Association criteria24 with HbA1c • 10.0%, BMI • 35 kg/m2 in accord with the 1991 NIH obesity surgery consensus conference criteria and stable weight for the previous 3 months25, and age between 18 and 60 years. Exclusion criteria were history of cardiovascular heart disease, malignancy, uncontrolled hypertension, previous esophageal, gastric, pancreatic, small bowel, or large bowel surgery, tobacco use, or significant psychiatric disorder.
Collected data included weight, height, 3 hours frequently sampled intravenous glucose tolerance test (IVGTT), body composition by dual-energy X-ray absorptiometry (DEXA) and plasma levels of FFA. BMI was calculated as weight (kilograms) divided by height (square meters). DEXA was used to study the body composition and total fat and lean mass were measured. We used the minimal model analysis of Bergman to estimate the Si from the 3 hours frequently sampled intravenous glucose tolerance test26. FFA were assayed by an enzymatic colorimetric method.
The LRYGB procedure was performed using a laparoscopic five/six port technique. We transected the jejunum at 50 cm distal to the ligament of Treitz and performed a stapled side-to-side jejunojejunostomy anastomosis, creating a 100 cm Roux limb. Then, we created a 15-ml proximal gastric pouch, and performed an ante-colic, retro-gastric Roux-en-Y gastrojejunostomy with linear stapler technique.
Statistical analysis
Patient's characteristics were summarized using mean ± SD. Pre- and postoperative variables were analyzed by two-sides paired sample Student t test. Pearson's correlation test was used to determine the association between changes in variables. The SPSS statistical software program (version 20) was used for all analyses. P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
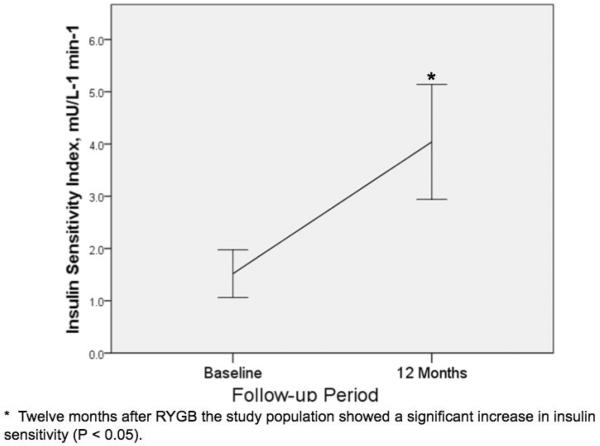
The study's cohort included 30 type 2 diabetic patients (20 female) underwent LRYGB. Mean age was 48 ± 8 years. As shown in Table 1, preoperative BMI was 43.4 ± 4.3 kg/m2 and mean BMI at 12 months was 31.2 ± 4.6 kg/m2. Using a paired T test, twelve months after LRYGB the study population showed a significant decrease in BMI (P = 0.001), FFA (P = 0.03), and total body fat (P = 0.03). As shown in Figure 1, there was a statistically significant increase in Si (P = 0.001) one-year after LRYGB.
Table 1.
Change in Postoperative Variables Correlated to Insulin Sensitivity.
| Paired T-test | Correlation to Insulin Sensitivity | |||
|---|---|---|---|---|
| Preoperative | 12 Months Follow-up | P | ||
| Body Mass Index | 43.4±4.3 | 31.2±4.6 | P = 0.001 | P = 0.12 |
| Total Fat (Kg) | 66.7 ±82.1 | 27.0 ±46.2 | P = 0.03 | P = 0.01 |
| Free Fatty Acids (μmol/L) | 765±251 | 611±197 | P = 0.03 | P = 0.31 |
Fig. 1.
Change in insulin sensitivity after LRYGB
As shown in Table 1, postoperative changes in Si significantly correlated (Pearson's r = −0.53, P=0.01) with change in total body fat, but not with changes in plasma FFA (Pearson's r = −0.22, P= 0.31).
DISCUSSION
We have demonstrated that patients with type 2 diabetic patients have a significant decrease in BMI, FFA and total fat mass and a significant increase in Si twelve months after LRYGB. We also have found a correlation between the increased Si and the decrease in total body fat measured by DEXA one-year after LRYGB. However, interestingly and according to the fact that levels of FFA do not mediate the association between adiposity and Si27, we have found that one-year after LRYGB there is no correlation between the increased Si and the decrease in FFA.
At present, we did not find in the literature any studies reporting the relationship between the decreased FFA levels and the improved Si in the long term after LRYGB.
As pointed out by Miller et al.27, in spite of the fact that FFA have been shown to be associated both with measures of adiposity (BMI, visceral adipose tissue [VAT], and subcutaneous adipose tissue [SAT]) and with Si, this association is not mediated through FFA. They found that adiposity measures explained only approximately 1% of the variance in FFA, arguing that adiposity is not a strong predictor of FFA in our population and that therefore we should not expect circulating levels of FFA to mediate the relationship between adiposity and Si. Moreover, they underlined how levels of obesity may not strongly correlate with levels of circulating FFA due to the influence of several other factors, such as physical activity, dietary intake, overall FFA trafficking and metabolism. Miller et al.27 did not find that increasing levels of FFA mediated the relationship between obesity and increased levels of adiposity and Si, despite demonstrating a very strong association between adiposity and Si. Likewise, we didn't find a correlation between the increased Si and the decrease in FFA after LRYGB, despite demonstrating a correlation between the increased Si and the decrease in total body fat after LRYGB. These results challenge the notion that the decreased levels of FFA after LRYGB mediate the improvement of IR and the resolution of T2DM after LRYGB and indicate that other factors might play a more significant role in the relationship between obesity and Si (i.e. changes in adiposity and changes in gut-hormones).
We have found a significant correlation between the increased Si and the decrease in total body fat measured by DEXA one-year after LRYGB. This correlation underlines the crucial role played by the fat tissue in both the pathogenesis of the obesity-related IR and its resolution after LRYGB, and justifies the relatively recent concept of fat tissue as an endocrine organ28. A correlation between the changes in fat mass and the IR one-year after bariatric surgery had already been pointed out29. Different fat depots have different effects on reducing Si, with intra-abdominal fat having a greater impact than peripheral fat30,31, and with visceral abdominal fat having a greater impact than subcutaneous fat32,33. Kelley et al.33 suggested that plasma FFA levels might be a link mediating the relationship between VAT and skeletal muscle IR in obesity. In this scenario, an increased local production of FFA by VAT would mediate the IR seen in obese individuals: VAT has a high rate of basal lipolysis and respond poorly to lipolysis restraining insulin, resulting in inappropriately high delivery of FFA to the portal circulation that will lead to peripheral IR and beta-cell dysfunction34. As mentioned early, Miller et al.27 did not find that increasing levels of FFA mediate the association between increased levels of adiposity and Si. However, they found that FFA interact with VAT (but not with SAT) to influence levels of Si. They showed that in individuals with lower VAT, those with low FFA levels had improved levels of Si relative to those with high FFA levels. Therefore, Miller et al.27 added another step in our understanding of the role played by FFA in mediating IR in obesity. VAT has been proven to be more insulin resistant than SAT35, such that levels of FFA do not additionally impact IR. Instead, in individuals with low VAT levels, increased FFA levels may additionally contribute to IR through the above-mentioned mechanisms of lipotoxicity. Unfortunately, we didn't include in our study-design a CT scan for differentiating between intra-abdominal fat (VAT and SAT) and peripheral fat. We could have divided our obese patients in those with high VAT and those with low VAT and we could have looked for the presence of a correlation between the decreased FFA levels and the increased Si in the first group after LRYGB.
Other limitations of our study were that we didn't collect data showing the duration of the diabetes prior the enrollment and that we did not perform an insulin suppression test in our obese patients in order to quantify the insulin resistance. It has been demonstrated that circulating plasma FFA levels in obese individuals are not a simple reflection of adiposity, but vary considerably as a function of whether the obese individuals are insulin resistant or insulin sensitive12. Studying metabolic changes in obese individuals after sibutramine-assisted weight loss, McLaughlin et al.12 showed that despite comparable decreases in FFA levels with weight loss in both insulin resistant or insulin sensitive groups, the insulin response fell significantly in association with weight loss only in the insulin resistant group. Based on this observation, if we had found a positive correlation between the decreased FFA levels and the increased Si in the insulin sensitive group after LRYGB, we could have speculated that, at least in insulin sensitive obese individuals, decreased FFA levels after LRYGB play a role in the improvement of Si and T2DM resolution after LRYGB.
In conclusion, our study has demonstrated that one-year after LRYGB there is a correlation between the increased Si and the decrease in total body fat measured by DEXA and that there is no correlation between the increased Si and the decrease in FFA levels. Since we did not divide our obese population based on high/low levels of VAT or based on their response to an insulin suppression test, our findings do not imply that a decrease in FFA level after LRYGB are without a role in mediating the improvement of Si and the resolution of T2DM one-year after LRYGB. However, these data suggest that other factors produced by AT, such as adipokines, might play an important role in mediating obesity-related IR. Therefore, we believe that changes in adiposity, and consequently changes in adipokines, such proinflammatory cytokines, leptin, adiponectin and resistin, are likely the key players in determining remission of IR and type 2 diabetes after LRYGB. Studying the profile of these adipokines after LRYGB was beyond the purpose of our study and further studies in this area are necessary.
Footnotes
DISCLOSURES All the Authors have no conflicts of interest or financial ties to disclose relative to this study.
REFERENCES
- 1.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45(12):1684–93. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PJ, Carlson MG. Impact of obesity on insulin action in NIDDM. Diabetes. 1993;42(3):405–10. [PubMed] [Google Scholar]
- 3.Ludvik B, Nolan JJ, Baloga J, Sacks D, Olefsky J. Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Diabetes. 1995;44(9):1121–5. doi: 10.2337/diab.44.9.1121. [DOI] [PubMed] [Google Scholar]
- 4.Boden G. Obesity and free fatty acids. Endocrinology and metabolism clinics of North America. 2008;37(3):635–46. viii–ix. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10. [PubMed] [Google Scholar]
- 6.Bjorntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta medica Scandinavica. 1969;185(4):351–6. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 7.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. The Journal of clinical investigation. 1999;103(2):253–9. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. The Journal of biological chemistry. 2002;277(52):50230–6. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 9.Guo QY, Gao Y, Cong L. Effects of free fatty acids on insulin signaling proteins in rat islet cells. Zhongguo ying yong sheng li xue za zhi = Zhongguo yingyong shenglixue zazhi = Chinese journal of applied physiology. 2002;18(3):283–6. [PubMed] [Google Scholar]
- 10.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54(12):3458–65. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi F, McLaughlin T, Lamendola C, Reaven GM. Insulin regulation of plasma free fatty acid concentrations is abnormal in healthy subjects with muscle insulin resistance. Metabolism: clinical and experimental. 2000;49(2):151–4. doi: 10.1016/s0026-0495(00)91065-5. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin T, Abbasi F, Lamendola C, Kim HS, Reaven GM. Metabolic changes following sibutramine-assisted weight loss in obese individuals: role of plasma free fatty acids in the insulin resistance of obesity. Metabolism: clinical and experimental. 2001;50(7):819–24. doi: 10.1053/meta.2001.24220. [DOI] [PubMed] [Google Scholar]
- 13.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. The American journal of medicine. 2009;122(3):248–56. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;(2):CD003641. doi: 10.1002/14651858.CD003641.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. The New England journal of medicine. 2012;366(17):1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. The New England journal of medicine. 2012;366(17):1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic Effects of Bariatric Surgery in Patients With Moderate Obesity and Type 2 Diabetes: Analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes care. 2013 doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes care. 2010;33(7):1438–42. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumbs AA, Modlin IM, Ballantyne GH. Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obesity surgery. 2005;15(4):462–73. doi: 10.1381/0960892053723367. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti G, Mingrone G, Marcoccia S, et al. Body composition and energy expenditure after weight loss following bariatric surgery. Journal of the American College of Nutrition. 2000;19(2):270–4. doi: 10.1080/07315724.2000.10718926. [DOI] [PubMed] [Google Scholar]
- 22.Klein S, Mittendorfer B, Eagon JC, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130(6):1564–72. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Khoo CM, Chen J, Pamuklar Z, Torquati A. Effects of Roux-en-Y Gastric Bypass or Diabetes Support and Education on Insulin Sensitivity and Insulin Secretion in Morbidly Obese Patients With Type 2 Diabetes. Annals of surgery. 2013 doi: 10.1097/SLA.0b013e318294d19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 25.NIH conference Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956–61. [PubMed] [Google Scholar]
- 26.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocrine reviews. 1985;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Pereira RI, Langefeld CD, et al. Levels of free fatty acids (FFA) are associated with insulin resistance but do not explain the relationship between adiposity and insulin resistance in Hispanic Americans: the IRAS Family Study. The Journal of clinical endocrinology and metabolism. 2012;97(9):3285–91. doi: 10.1210/jc.2012-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Archives of medical research. 2008;39(8):715–28. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Frige F, Laneri M, Veronelli A, et al. Bariatric surgery in obesity: changes of glucose and lipid metabolism correlate with changes of fat mass. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2009;19(3):198–204. doi: 10.1016/j.numecd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–8. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 31.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. The Journal of clinical endocrinology and metabolism. 2001;86(11):5366–71. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. American journal of physiology Endocrinology and metabolism. 2000;278(5):E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 33.Kelley DE, Williams KV, Price JC, McKolanis TM, Goodpaster BH, Thaete FL. Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2001;86(11):5412–9. doi: 10.1210/jcem.86.11.8027. [DOI] [PubMed] [Google Scholar]
- 34.Raz I, Eldor R, Cernea S, Shafrir E. Diabetes: insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes/metabolism research and reviews. 2005;21(1):3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- 35.Frayn KN. Visceral fat and insulin resistance--causative or correlative? The British journal of nutrition. 2000;83(Suppl 1):S71–7. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]