Abstract
Recent advance of bioinformatics and analytical apparatuses such as next generation DNA sequencer (NGS) and mass spectrometer (MS) has brought a big wave of comprehensive study to biology. Comprehensive study targeting all genes, transcripts (RNAs), proteins, metabolites, hormones, ions or phenotypes is called genomics, transcriptomics, proteomics, metabolomics, hormonomics, ionomics or phenomics, respectively. These omics are powerful approaches to identify key genes for important traits, to clarify events of physiological mechanisms and to reveal unknown metabolic pathways in crops. Recently, the use of omics approach has increased dramatically in fruit tree research. Although the most reported omics studies on fruit trees are transcriptomics, proteomics and metabolomics, and a few is reported on hormonomics and ionomics. In this article, we reviewed recent omics studies of major fruit trees, i.e. citrus, grapevine and rosaceae fruit trees. The effectiveness and prospects of omics in fruit tree research will as well be highlighted.
Keywords: citrus, grapevine, metabolomics, omics, proteomics, rosaceae fruit tree, transcriptomics
Introduction
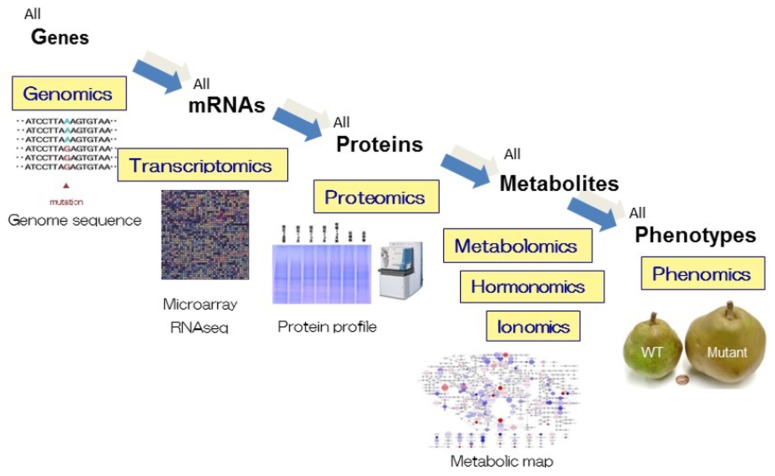
In the last two decades, comprehensive studies called omics, have been applied on model plant study and has contributed enormously in plant science. The conceptual scheme of “omics” is described in Fig. 1. Genome means a full haploid set of chromosomes with its gene set in one organism whereas phenome is the physical appearance (phenotype) of an organism. Transcriptome and proteome respectively describes the entire set of RNAs and proteins derived from genome. Metabolome, hormonome and ionome are all metabolites, hormones and ions present in a biological sample, such as a cell, tissue, organ or organism, respectively. Comprehensive analysis targeting genome, transcriptome, proteome, metabolome, hormonome, ionome and phenome are called genomics, transcriptomics, proteomics, metabolomics, hormonomics, ionomics and phenomics, respectively. Recently, combination and integration of several omics are performed on a single sample or material and these are called multi-omics or integrated-omics. The advance of omics study is supported by the invention and improvement of analytical instruments, including next generation DNA sequencer (NGS) and mass spectrometer (MS). Omics data are analyzed by the use of bioinformatics and various important genes, proteins, metabolites and metabolic pathways have been identified by these approaches.
Fig. 1.
Conceptual scheme of omics study. Comprehensive studies targeting all genes, transcripts (RNAs), proteins, metabolites, hormones, ions and phenotypes are called genomics, transcriptomics, proteomics, metabolomics, hormonomics, ionomics and phenomics, respectively.
For the model plant, Arabidopsis, a precise genome sequence data and gene prediction with substantial annotations are provided and a vast omics data, especially transcriptome, proteome and metabolome data have been collected in databases. On the contrary, availability of this information is limited for horticultural crops. The recent decrease in cost of DNA sequencing has made it possible to sequence various crop genomes as well as making transcriptome data more available and accessible. In addition, proteomics and metabolomics are often applied in various crop studies these days.
Every fruit has a unique and attractive characteristic, i.e. taste, flavor, color, shape and texture. Clarification of fruit developmental physiology, metabolic pathways and identification of key genes of important traits are necessary to improve fruit yield and quality. Fruit trees studies have many disadvantages; such as long juvenility (long life cycle), heterosis, self-incompatibility, polyploidy, large body size (require large cultivation space). Due to these disadvantages, fruit tree research is far less advanced compared to herbaceous crops. These have also hindered comprehensive research activities on fruit tress thereby leaving a vast number of undiscovered genes, metabolites, metabolic pathways and physiological activities in fruit trees. Omics study is the powerful approach that has been used recently by researchers to overcome these shortcomings. In this article, we review recent omics studies in fruit tree research.
Genomics
Development of NGS and technology for assembly of sequence data from NGS has made whole genome sequencing of horticultural crops possible. In 2007, grapevine genome was sequenced firstly in fruit trees and fourthly in plants, i.e. after Arabidopsis, rice and poplar (Jaillon et al. 2007). Nowadays genomes of some horticultural crops, including apple, peach, Japanese apricot, Chinese pear, Valencia orange, mandarin orange, and had already been sequenced (http://www.genome.jp/kegg/catalog/org_list.html). As shown in Table 1, the genome data of rosaceae plants, citrus and grapevine are released from several databases. Comparison of genome sequences among different cultivars is an effective approach to identify genes for cultivar-specific traits. One interesting example is the report by Da Silva et al. (2013). That is, they compared genome sequences between ‘Pinot Noir’ and ‘Tannat’, and found cultivar-specific genes, which contribute to a high accumulation of polyphenolic compounds in Tannat grape berry. In addition to de novo sequencing of genomes of horticultural crops, resequencing of genomes of different cultivars is a promising approach.
Table 1.
Useful web pages for fruit omics study
| Name | Object | Target/Species | URL |
|---|---|---|---|
| Genome Database for Rosaceae | genome | rosaceae plants | https://www.rosaceae.org/ |
| Citrus Genome Database | genome | citrus | https://www.citrusgenomedb.org/ |
| Grape Genome Database | genome | grape | http://genomes.cribi.unipd.it/grape/ |
| Grape Genome Browser | genome | grape | http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/ |
| PlantGDB | genome | various plants (grape, peach, papaya) | http://www.plantgdb.org/ |
| GEO | microarray raw data | all organisms | http://www.ncbi.nlm.nih.gov/geo/ |
| SRA | RNAseq raw data | all organisms | http://www.ncbi.nlm.nih.gov/sra |
| eFP Browser | gene expression | various plants (grape) | http://bar.utoronto.ca/efp_grape/cgi-bin/efpWeb.cgi |
| ATTED-II | coexpression | various plants (grape) | http://atted.jp/ |
| CoP | coexpression | various plants (grape) | http://webs2.kazusa.or.jp/kagiana/cop0911/ |
| MassBank | MS spectrum | all metabolites | http://www.massbank.jp/index.html?lang=en |
| ReSpect | MS spectrum | all metabolites | http://spectra.psc.riken.jp/ |
| KNApSAcK | metabololite | all metabolites | http://kanaya.naist.jp/KNApSAcK_Family/ |
| KEGG | metabolic pathway etc. | various organisms (grape) | http://www.genome.jp/kegg/ |
| PMN/PlantCyc | metabolic pathway | various plants (grape, papaya) | http://www.plantcyc.org/ |
| VitisCyc | metabolic pathway | grape | http://pathways.cgrb.oregonstate.edu/index.html |
| KaPPA-View 4 KEGG | metabolic pathway drafting | various plants (grape) | http://kpv.kazusa.or.jp/kpv4-kegg-1402/ |
| Fruit Omics Database | omics data | fruit trees (pear) | http://www.tr.yamagata-u.ac.jp/~oikawa/oikawa/GLPDB%202/GLPDB_E/index.html |
Transcriptomics
Transcriptomics is the comprehensive study of transcripts, i.e. RNAs, in an organism, organ, tissue or cell. Microarray, also called gene chip or DNA chip was the most popular tool to perform transcriptome analysis. To design microarray, information of predicted genes from genome sequence or expressed sequenced tag (EST) is required. For minor crops, neither genome sequence nor EST is available to design its microarray. However, recently the situation has changed. By the invention of NGS and advance of bioinformatics, RNA sequencing (RNAseq) became a popular approach in transcriptomics. More than 10 million sequence reads from NGS are assembled using reference sequence, such as genome sequence or EST, or de novo. From the number of sequence reads for one gene, its expression level is calculated. In case of de novo assembly, no reference sequence is required; therefore it can be applied on organisms without genome sequence data and EST. Most publications reporting transcriptomics of fruit trees after 2014 used RNAseq (Supplemental Table 1). In fruit trees, there are various target traits for transcriptomics and the major ones are gene expression profile in different tissues and organs, fruit development, ripening and post-harvest physiology, fruit traits (size, color, brix, acidity, firmness, flavor), secondary metabolites (anthocyanin, carotenoid) and response to pathogens, stresses or plant hormones.
Citrus
Several transcriptomics of citrus focusing on citrus greening (huanglongbing (HLB)) disease have been reported (Mafra et al. 2013, Martinelli et al. 2012, 2013) together with combination of proteomics (Fan et al. 2011, Zhong et al. 2015). HLB disease is one of the serious diseases in citrus cultivation and there is no effective countermeasure against this disease. Although the key gene for HLB resistance has not been identified yet, many candidates and regulated genes have been found in these omics studies. To clarify self-incompatibility mechanism, Zhang et al. (2015c) performed RNAseq in lemon and found a putative S-RNase gene that had not been previously reported. Transcriptome and proteome analyses of a spontaneous late-ripening sweet orange mutant were performed (Wu et al. 2014b, Zeng et al. 2012, Zhang et al. 2014b). From these omics studies, the presence of multiple ripening events in citrus or roles of abscisic acid (ABA), sucrose and jasmonic acid (JA) in citrus ripening was suggested. Sun et al. (2012) and Zhang et al. (2011a) reported transcriptomics and Ai et al. (2012) reported multi-omics (transcriptomics and proteomics) data on an early flowering tyrifoliate orange mutant. The target of transcriptomics in Sun et al. (2012) was not mRNA but microRNAs (miRNAs), which are small non-coding RNAs (ncRNAs) that regulate RNA silencing and post-transcriptional genes regulation. RNAseq using NGS is the major approach to analyze ncRNA comprehensively. Nishikawa et al. (2010) performed microarray analysis for the early flowering transgenic tyrifoliate orange expressing Flowering Locus T from citrus (CiFT) and confirmed higher gene expression levels of MADS-box transcription factors related to floral organ formation.
Corky split vein is caused by boron deficiency. Yang et al. (2013) and Lu et al. (2014) reported transcriptomics for boron deficiency in citrus. The former study suggested the involvement of cytokinin signaling pathway in corky split vein. On the other hand, the latter study focused on miRNAs and suggested the involvement of miRNAs in the response to boron deficiency. Recently, single cell omics has become a trend in biology. Voo and Lange (2014) reported a protocol for the isolation of essential oil gland cells of citrus fruit peel for single cell transcriptomics. Transcriptomics of citrus focusing on physiological abscission of fruits, called “self-pruning” (Zhang et al. 2014a), polyploidy (Allario et al. 2011), somatic hybrid (Bassene et al. 2010), response to ethylene in fruit (Patel et al. 2014) or GA treatment on bud (Goldberg-Moeller et al. 2013), red fruit flesh (Bernardi et al. 2010, Yu et al. 2012), fruit skin color (Guo et al. 2015) and flavor (Tietel et al. 2011) also have been reported (Supplemental Table 1).
Grapevine
Fasoli et al. (2012) performed a large scale transcriptome analysis of grapevine. This study determined transcriptomes of 54 samples for various tissues and organs at different developmental stages. The obtained transcriptome data have been registered in eFP Browser (see the chapter “Omics Databases and Tools”) to be used as a valuable tool. Interestingly co-expression analysis of the transcriptome data revealed “woody developmental program”, which is a characteristic of perennial woody plants, and is inactive in vegetative/ green tissues in grapevine. Many transcriptomics of grapevine focused on response to pathogens, including fungus, oomycete, virus and phytoplasma (Abbà et al. 2014, Almagro et al. 2014, Gauthier et al. 2014, Giraud et al. 2012, Li et al. 2015, Malacarne et al. 2011, Vega et al. 2011). Gray mold caused by Botrytis cinerea is one of the most problematic diseases of grapevine. To clarify the resistance mechanism against B. cinerea, multi-omics approaches, such as transcriptomics, proteomics (Dadakova et al. 2015), and metabolomics (Agudelo-Romero et al. 2015), were performed. The latter study provided evidence of a reprogramming of carbohydrate and lipid metabolisms towards synthesis of secondary metabolites involved in plant defense, including resveratrol. The other transcriptomics studies also revealed dynamic changes in gene expressions upon pathogens infection.
Another major targets of grapevine transcriptomics are ripening of berry (Carbonell-Bejerano et al. 2013, Guillaumie et al. 2011, Koyama et al. 2010, Lijavetzky et al. 2012) and syntheses of ripening related secondary metabolites, such as anthocyanins (Ali et al. 2011, Wu et al. 2014a), proanthocyanidins (Carrier et al. 2013) and volatile compounds (Cramer et al. 2014). Contents of anthocyanins, proanthocyanidins and volatile compounds decide color, taste and flavor of grape berry and wine, respectively. Dramatic increase of ABA in the later berry growth is a trigger of ripening and this stage is called “véraison”. Several studies have reported the relationship between ABA, ripening and anthocyanin accumulation (Carbonell-Bejerano et al. 2013, Koyama et al. 2010). On the other hand, Cramer et al. (2014) reports the relation between ethylene signaling and flavor synthetic pathway in berry skin at ripening stage. Another important plant hormone in grape berry growth is gibberellin (GA). The first exogenous application of GA on grape flowers induces seedless fruits and the second application (about 10 days after flowering) induces parthenocarpic fruit set. Transcriptome analyses in grape berries after GA application (Chai et al. 2014, Cheng et al. 2015) and in seedless somatic variant (Nwafor et al. 2014) have been performed. The transcriptomics by Chai et al. (2014) revealed that exogenous GA induced cross talk between auxin, cytokinin, brassinosteroid (BR), ABA and ethylene. Some transcriptomics of grapevine focus on environmental conditions and stresses, such as different growing areas “terroir” (Dal Santo et al. 2013, Sun et al. 2015), cold (Xin et al. 2013), heat (Rocheta et al. 2014), drought (Corso et al. 2015, Perrone et al. 2012), salt (Daldoul et al. 2010) stresses and high light intensity (Carvalho et al. 2011). Other transcriptomics of grapevine focus on different species (Wen et al. 2013), tendril and inflorescence development (Díaz-Riquelme et al. 2014), defoliation (Pastore et al. 2013), bud dormancy (Díaz-Riquelme et al. 2012), graft union formation (Cookson et al. 2013), late harvest technique (Corso et al. 2013), pest damage (Nabity et al. 2013) and so on (Supplemental Table 1).
Rosaceae fruit trees
Peach, cherry, apricot, apple and pear belong to Rosaceae family. Peach, cherry and apricot are called stone fruits, because the fruit has a large and hard seed, and their fruit flesh is derived from pericarp (mesocarp). Whiles apple and pear belong to pome fruits and their fruit flesh is derived from receptacle. Therefore transcriptomes, proteomes and metabolomes of stone fruits and pome fruits might be different. One of the major targets of transcriptomics of rosaceae fruit trees is response to pests, such as infection of fungi (Gusberti et al. 2013) or virus (Chen et al. 2014) in apple, infection of bacteria (Socquet-Juglard et al. 2013) or virus (Herranz et al. 2013, Rubio et al. 2015) in peach, virus infection in plum (Rodamilans et al. 2014) and insect damage in apple (Zhang et al. 2015b). In these studies, induction of known and unknown genes expressions related to pest resistance was detected. In Gusberti et al. (2013), several candidate genes involved in the ontogenic resistance of apple were identified. Most rosaceae fruit trees need cumulative chilling in winter to break bud dormancy in spring. Transcriptomics targeting bud dormancy of Japanese pear (Bai et al. 2013, Liu et al. 2012, Nishitani et al. 2012a), Chinese cherry (Zhu et al. 2015) and peach (Barakat et al. 2012) have been reported. Interestingly, transcriptomics both by Bai et al. (2013) and Nishitani et al. (2012a) revealed decrease in gene expressions for ABA and GA biosynthesis, down-regulation of dormancy-associated MADS-box gene and involvement of epigenetic regulation during endodormancy release. Tree architecture is an important trait in orchard management. One of the characteristic tree architecture of apple is “columnar”. Krost et al. (2012, 2013) performed transcriptome analyses for columnar apples. They couldn’t find the causative gene of columnar, but their transcriptomics provided a molecular explanation for earlier findings on the hormonal state of columnar apple trees.
Abscission of fruits is one of the important traits in fruit tree cultivation. Ferrero et al. (2015) performed transcriptomics of young seeds and discussed its relation to physiological abscission of apple fruits. Zhu et al. (2011) focused on abscission of apple fruits induced by application of a synthetic auxin analogue, naphthaleneacetic acid (NAA). Nishitani et al. (2012b) compared transcriptomes among various parthenocarpic genetic resources of pear and found several phenylpropanoid-related and photosystem-related genes as differently expressed genes. The authors suggested that these genes might be candidates DNA markers for parthenocarpy. Nashima et al. (2013a) performed transcriptome analysis throughout fruit development of European pear and the data is released from “Fruits Omics Database” (Fig. 2). The transcriptome data revealed the dynamic fluctuations of gene expressions during fruit development, including high expression of genes related to stone cell formation at early fruit developmental stages and those related to ripening at ripening stage. Xie et al. (2013) reported transcriptome analysis of Chinese pear and also showed dynamic fluctuations of gene expressions during fruit development. Many transcriptomics studies of rosaceae fruit trees focused on fruit ripening and postharvest physiology, such as in apple (Costa et al. 2010, Gapper et al. 2013, Mellidou et al. 2014), peach (Falara et al. 2011, Lauxmann et al. 2012, Pons et al. 2014) and Chinese pear (Huang et al. 2014, Liu et al. 2013). To prolong storage period of apple fruits, controlled atmosphere (CA) storage and a competitor of ethylene receptor, 1-methylcyclopropene (1-MCP), treatment are applied in the apple industry. Mellidou et al. (2014) focused on CA storage, Costa et al. (2010) focused on 1-MCP treatment and Gapper et al. (2013) focused on both. Interestingly, Huang et al. (2014) and Gapper et al. (2013) suggested the involvement of epigenetic regulation during fruit ripening and storage.
Fig. 2.
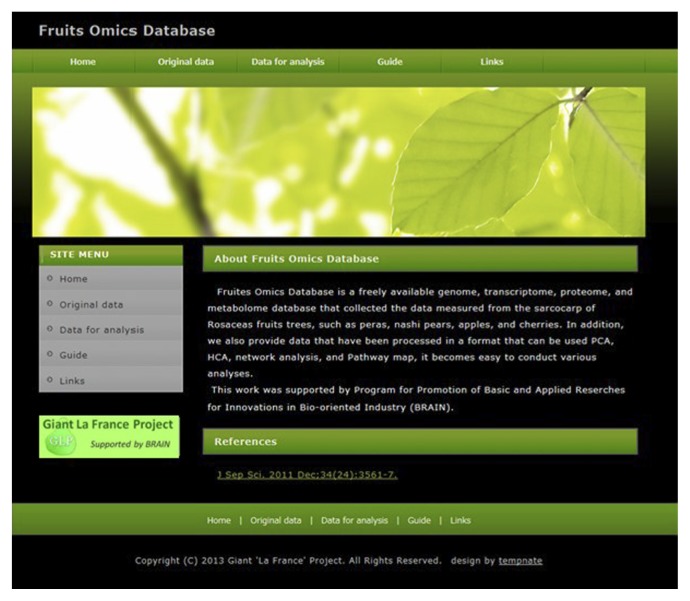
“Fruits Omics Database”. The website, named “Fruits Omics Database”, are collecting transcriptome, proteome, metabolome, hormonome and ionome data of fruits, especially focusing rosaceae fruit trees. At present, the transcriptome, metabolome, hormonome and ionome data of European pear fruits are available. The URL of Fruits Omics Database is http://www.tr.yamagata-u.ac.jp/~oikawa/oikawa/GLPDB%202/GLPDB_E/index.html.
Schaffer et al. (2013) or Wu et al. (2015) compared transcriptomes between normal apple cultivar and its transgenic plants of the MADS box gene, SEP1/2, or the gene encoding the key enzyme for sorbitol synthesis, aldose-6-phosphate reductase (A6PR), respectively. Many other traits of rosaceae fruit trees have been studied by transcriptomic approach. Several transcriptomics studies focused on stress resistances, such as cold (Mousavi et al. 2014, Puig et al. 2015), drought (Wang et al. 2015) and salt (Li et al. 2013) stresses. Li et al. (2012) determined differences between Japanese apricot and apricot transcriptomes. Similarly, Wei et al. (2015) compared difference in transcriptomes between yellow peel and red peel cultivars of cherry, whereas Vimolmangkang et al. (2014) compared difference in transcriptomes in apple skin between in light and dark conditions. Further transcriptome studies will clarify interesting traits of rosaceae fruit trees, such as species- or cultivar-specific traits and a unique sugar metabolism, i.e. sorbitol metabolism, in Rosaceae fruit trees.
Proteomics
Proteomics is the comprehensive study of proteins in an organism, organ, tissue or cell. Peptide sequencing, i.e. Edman sequencing, was the popular method to identify proteins before. However nowadays MS is used for protein identification and quantification in almost all proteomics. To identify proteins by MS, protein sequence database is required. Protein sequence database of allied species or other organisms can be used. However, for efficient and accurate identification, protein sequence database derived from one’s own genome sequence or EST should be available for usage. Normally number of proteins identified in one proteomics is less than 10% of gene number, because of the sensitivity of MS and masking of minor peptides by major ones.
Before MS analysis, proteins are often separated, enriched or labeled depending on purposes. In comparative proteomics, 2D-PAGE are often used. Comparing several 2D-PAGE images, difference in protein amounts among different samples can be determined. iTRAQ labeling is also one of the methods in comparative proteomics (Bindschedler and Cramer 2011) and various studies of fruit trees have used iTRAQ labeling (Ai et al. 2012, Fan et al. 2011, Hu et al. 2015, Palmieri et al. 2012, Wu et al. 2014b, Zheng et al. 2014, Zhong et al. 2015, Zhou et al. 2015). Post translational modifications of proteins, such as acetylation, glycosylation and phosphorylation, can be monitored in proteomics. Protein phosphorylation often causes dynamic change in protein function. Therefore, to identify phosphorylation status of proteins, phosphoproteomics is performed. Phosphoproteomics has also been used in fruit tree proteomics (Margaria et al. 2013, Wang et al. 2014, Zeng et al. 2014) (Supplemental Table 1). Concerning proteomics of fruit trees, one review article has been published (Molassiotis et al. 2013).
Citrus
By proteomic approach, Katz et al. (2010) identified approximately 1,500 proteins in citrus fruit juice sac cells and quantified their amounts at three developmental stages. As a protein database for this proteome analysis, a comprehensive sequence database of citrus genes, ESTs and proteins, named iCitrus, has been established. Peel color of citrus fruit changes from green to yellow or orange during fruit maturation. The green color is derived from chlorophyll in chloroplast and chloroplast change to chromoplast accumulating carotenoids during fruit maturation. Zeng et al. (2011) identified 493 proteins in sweet orange, including proteins involved in carotenoid synthesis and storage in the chromoplast. Comparison of chromoplast proteomes between sweet orange and tomato showed some unique characteristics of the sweet orange chromoplast. The sweet orange chromoplast has more extensive carotenoid synthesis and amino acid synthesis without nitrogen assimilation and lipid metabolism for jasmonic acid (JA) synthesis. Phosphoproteomics of chromoplast for sweet orange have been performed and 109 plastid-localized phosphoproteins were identified during fruit ripening (Zeng et al. 2014). This shows the existence of active protein regulation via posttranslational modifications in the chromoplast. These organelle proteomics showed interesting characters of chromoplast in fruits.
Many citrus species show “biennial bearing”, also called “alternate bearing”, meaning repeating heavy fruit load (on-crop) and low yield (off-crop) in every other year. To clarify the mechanism of biennial bearing, 2D-PAGE images of on-crop and off-crop were compared and then the differentially expressed proteins were identified by proteomic approach (Muñoz-Fambuena et al. 2013a, 2013b). The results showed that the proteins involved in the primary metabolisms, such as carbohydrate metabolism, were more abundant in the buds and leaves of off-crop trees. Shalom et al. (2014) compared transcriptomes of buds between on-crop and off-crop trees. The result revealed up-regulation of genes involved in photosynthesis and ABA metabolism and down-regulation of auxin polar transport in off-crop trees, although their hormone analyses showed the decrease of both ABA and auxin levels in the buds of off-crop trees. Additionally, proteomics of citrus focusing on response to male sterility (Zheng et al. 2014), response to pathogens (Dória et al. 2015, Monavarfeshani et al. 2013, Nwugo et al. 2013, Rani and Podile 2014, Rani et al. 2015), pest damage and methyl JA treatment (Maserti et al. 2011), water deficit (Oliveira et al. 2015), salt stress (Podda et al. 2013, Tanou et al. 2014) and Fe (Muccilli et al. 2013) and Mg (Peng et al. 2015) deficiencies have been also reported (Supplemental Table 1).
Grapevine
Many proteomics of grapevine focused on response to pathogens (Belchí-Navarro et al. 2013, Dadakova et al. 2015, Margaria et al. 2013, Palmieri et al. 2012, Spagnolo et al. 2012, 2014, Yao et al. 2012, Zhao et al. 2011). In these studies, proteins related to defense response, including pathogenesis related (PR) proteins and proteins involved in signal transduction of defense response, were identified. Yao et al. (2012) studied Mn-induced resistance against powdery mildew in grapevine. Their proteome analysis showed induction of PR-like protein and nucleotide-binding site-leucine-rich repeat (NBS-LRR) proteins, which detect pathogens. This suggests that high concentration of Mn triggers defense mechanisms against pathogens in grapevine. Margaria et al. (2013) performed phosphoproteomics in grapevine leaves after phytoplasma infection. In the phosphoproteomics, 15 differentially phosphorylated proteins of healthy and infected plants, including proteins involved in response to stress and antioxidant system, were identified. This shows importance of protein phosphorylation for defense response. One of the interesting reports is the proteomics of grape embryogenic callus in the response to Agrobacterium tumefaciens-mediated transformation (Zhao et al. 2011). PR10 and resistance protein Pto were identified as proteins significantly up-regulated after A. tumefaciens inoculation. In addition, by biochemical measurements, A. tumefaciens transformation induced oxidative burst and modified protein-degradation pathways. The authors suggested that the apoptosis signaling pathway and hypersensitive response are strengthened by A. tumefaciens transformation and these partially explain the low efficiency of grape transformation. Regarding interaction between grapevine and microorganisms, proteomes of non-mycorrhizal and mycorrhizal roots of grapevine were analyzed (Cangahuala-Inocente et al. 2011).
Concerning grape berry ripening, Negri et al. (2011) detected 36 proteins, which amounts changed significantly after véraison, by proteomic approach. Most of them were related to biotic and abiotic stress responses. Thus the authors suggested that such proteins involved in biotic and abiotic stress responses must be most significant biomarkers for berry ripening. Zamboni et al. (2010) performed thorough multi-omics, i.e. a combination of transcriptomics, proteomics and metabolomics, during berry development and postharvest drying “withering”. In this study, the authors identified stage-specific biomarkers for berry development and withering. Metabolomes in berries between red and white grapes (Niu et al. 2013b), among different varieties (Bertazzo et al. 2010), among different terroir and ripening stages (Fraige et al. 2015) have been compared. Other proteomics of grapevine focused on postharvest withering (Di Carli et al. 2011), thermal stresses (George et al. 2015) and light (Nilo-Poyanco et al. 2013, Niu et al. 2013a) (Supplemental Table 1).
Rosaceae fruit trees
Long juvenility is a problematic trait in fruit tree cultivation and breeding. Zeng et al. (2010) and Cao et al. (2011) compared proteomes among juvenile, adult vegetative and reproductive phases in apple. The former report concluded that the transition from vegetative phase to reproductive phase is regulated independently. Bud dormancy is also another problematic trait in fruit tree cultivation in warm area and it is becoming more severe because of global warming. Zhuang et al. (2013) compared proteomes of dormant buds of Japanese apricot at four stages and similarly, vegetative buds and floral buds of peach in post-dormancy were compared by Prassinos et al. (2011). They identified many differentially expressed proteins which may be related to bud dormancy and its release. Self-incompatibility is also another problematic trait in fruit tree cultivation and breeding. Cao et al. (2012) compared proteomes in primary styles of self-incompatible and compatible apricots and found differentially expressed proteins.
Hu et al. (2011) performed proteome analysis of peach endocarp (stone) and mesocarp (fruit flesh) during early fruit development. They found higher expression of enzymes involved in lignin and flavonoid synthetic pathways in the endocarp. Transcriptomes and proteomes of European pear fruits throughout its development were determined by Nashima et al. (2013a) and Reuscher et al. (2016), respectively. In these studies, higher expression of enzymes involved in lignin and flavonoid synthetic pathways in early developmental stages was revealed. The higher expression of enzymes involved in lignin and flavonoid synthetic pathways might be responsible for stone hardening in peach and stone cell formation in pear. Fruit firmness is one of the important traits of fruits. Marondedze and Thomas (2012) compared proteomes of high and low fruit firmness of apple cultivars. The result indicated a lower expression of proteins involved in ethylene biosynthesis in the high firmness cultivar. Several proteomic works for rosaceae fruit trees have focused on fruit ripening and postharvest physiology, which includes; fruit ripening of apricot (D’Ambrosio et al. 2013), fruit softening in melting peach varieties (Nilo et al. 2012), postharvest heat treatment of peach fruits (Bustamante et al. 2012, Jiang et al. 2014, Zhang et al. 2011b) and ethylene treatment of apple fruits (Zheng et al. 2013).
Hu et al. (2015) determined salt tolerance of transgenic apple plants overexpressing MdSOS2L1, a calcineurin B-like protein-interacting proteinkinase. The proteome analysis revealed that enzymes involved in reactive oxygen species (ROS) scavenging, procyanidin biosynthesis and malate metabolism were higher in the MdSOS2L1-overexpressing apple. Other proteomics of rosaceae fruit trees focused on response to pathogens (Clemente-Moreno et al. 2013, Li et al. 2014, Zhang et al. 2015a), Fe deficiency and resupply (Rodríguez-Celma et al. 2013), fruit bagging (Feng et al. 2011), drought stress (Zhou et al. 2015) and early-maturing bud mutant (Liu et al. 2014) (Supplemental Table 1).
Metabolomics
Metabolomics is the comprehensive study of metabolites in an organism, organ, tissue or cell. Wide variety metabolites, whose molecular size, structures and characters are quite different and unique, are present in organisms. Therefore, different analytical apparatuses are applied on metabolomics. Gas chromatography (GC)-MS is one of the most powerful and widely used methods in metabolomics, because chromatographic resolution of GC is very high. Only volatile compounds can be analyzed in GC-MS, therefore derivatization step to volatilize non-volatile compounds is needed before analysis of non-volatile compounds. Liquid chromatography (LC)-MS, which requires no derivatization steps, is the often used method in metabolomics. Capillary electrophoresis (CE)-MS is another popular method in metabolomics. Generally CE has higher separation efficiency than LC, so CE is suitable for separating wider range of metabolites. However, because CE uses electrophoretic technique, CE-MS can analyze only charged compounds excluding neutral compound. In metabolomics, nuclear magnetic resonance (NMR) has been used to detect compounds including H or C atoms. Compared with MS-based techniques, NMR has a wide range of detection of target compounds, although the detection limit and sensitivity are low.
Metabolome analysis does not need genome sequence data or EST data, but needs standard chemical compounds of metabolites or their MS spectrum data. Most organisms use common primary metabolic pathway. Thus primary metabolites can be easily identified in metabolome analysis because standard chemical compounds of primary metabolites or their MS spectrum data are available. On the other hand, each plant species has unique secondary metabolic pathways. Most of plant secondary metabolic pathways are unclear and standard chemical compounds and MS spectrums of most plant secondary metabolites are unavailable. Therefore, in metabolome analysis, most secondary metabolites are detected as unknown metabolite peaks and cannot be identified. Publications on metabolomics of fruit trees are lesser than transcriptomics or proteomics.
Citrus
Metabolomics and transcriptomics have been performed in ponkan fruit growth (Lin et al. 2015) and in postharvest storage of pumelo fruit (Sun et al. 2013). In the former study, sugar accumulation and organic acid degradation according to changes in metabolic enzymes were observed in fruit maturation. In the latter study, increase in succinic acid, γ-aminobutyric acid (GABA) and glutamine and decrease in oxoglutaric acid were observed. Interestingly, both studies suggested the involvement of GABA shunt in organic acid degradation in the fruits. One of the physiological disorders of citrus fruit is “puffing”, i.e. breakdown of albedo (white part under the peel) and separation between pulp and flavedo (outer colored part of the peel). To elucidate the mechanism of puffing, transcriptome and metabolome analyses ware performed (Ibáñez et al. 2014). The multi-omics revealed several alternations of primary metabolism, less citric acid content and down-regulation of GA and cytokinin signaling in puffed fruits. Ballester et al. (2013) determined metabolic profiles of albedo and flavedo in elicited navel orange fruits. The results suggested that phenylpropanoids and their derivatives play an important role in the induction of resistance against Penicillium spp., which are the major postharvest pathogens of citrus fruit (Ballester et al. 2013). Metabolomes of different citrus species and cultivars whose sensitivities against HLB disease are different, were compared. Higher levels of the amino acids, organic acids and galactose in leaves were characteristic of the most sensitive sweet orange varieties (Cevallos-Cevallos et al. 2012). On the other hand, differences in the concentration of phenylalanine, histidine, limonin and synephrine in asymptomatic fruits and symptomatic fruits were observed (Chin et al. 2014). As Chin et al. (2014) suggested, metabolomics has a potential to generate metabolite-based “biomarkers” of important traits of crops, such as HLB resistance in citrus.
Grapevine
Comparing with other fruit trees, much larger number of metabolomics studies has been reported for grapevine. Primarily metabolites, including sugars, organic acids and secondary metabolites, such as anthocyanins, proanthocyanidins and resveratrol, are targets of grapevine metabolomics. Some metabolomics focused on volatile compounds which often relate to flavor (Arbulu et al. 2013). Many studies compared metabolomes among different grapevine cultivars (Degu et al. 2014, Gika et al. 2012, Teixeira et al. 2014), biotypes (Mulas et al. 2011) and wild species (Narduzzi et al. 2015). Degu et al. (2014) compared metabolomes and transcriptomes of two grapevine varieties, i.e. Cabernet Sauvignon and Shiraz. Da Silva et al. (2013) found 1,873 unique genes in the high polyphenol content grapevine cultivar ‘Tannat’, which are not found in the reference genome of grapevine ‘Pinot Noir PN40024’. This study indicates that the reference genome lacks many genes which may relate to varietal phenotypes. Omics-study can reveal differences among cultivars and the information could be useful for crop breeding.
Another major target of metabolomics of grapevine is fruit development. Several studies have compared grapevine metabolomes of before and after véraison showing dramatic shifts of primary and secondary metabolisms after véraison (Dai et al. 2013, Fortes et al. 2011, Suzuki et al. 2015a). Suzuki et al. (2015a) showed that anthocyanin was the most abundant phenolic compounds in grape berry skin at harvest, whereas the amount of catechin was higher before véraison. In addition, using MS/MS fragment spectra database to search for the stage specific unidentified metabolites, arginine was identified as a ripening stage specific one. As mentioned above, secondary metabolisms such as anthocyanin synthetic pathways, are induced by berry maturation as well as environmental stresses. Suzuki et al. (2015b) reported on metabolomics and transcriptomics and demonstrated clear and specific induction of resveratrol synthetic pathway by ultraviolet (UV) irradiation. The authors showed this induction using the metabolic pathway map, in which metabolome data and transcriptome data were projected by using drafting tool of metabolic pathway map (see the chapter “Omics Databases and Tools”). Metabolomics of grapevine on response to pathogen (Agudelo-Romero et al. 2015), insect (Lawo et al. 2011), drought stress (Griesser et al. 2015) or elevated temperature (Sweetman et al. 2014), different terroir (Anesi et al. 2015, Teixeira et al. 2014), polyamine biosynthesis (Agudelo-Romero et al. 2014), grape berry and its wine product differences (Aura et al. 2013) and antioxidant and antiproliferative properties (Pacifico et al. 2011) have been also reported (Supplemental Table 1).
Rosaceae fruit trees
Freeze-drying (FD) is often used for sample preparation to remove water from plant tissues before metabolome analysis. Oikawa et al. (2011) have checked effect of FD using flesh tissue of European pear fruit. The results showed that FD causes significant decrease of some metabolites, such as succinate and choline. Therefore we FD process should be skipped, if the target metabolites will be degraded. For profiling metabolites of apples, Risticevic et al. (2012) have developed and optimized sample preparation procedure for GC-MS, i.e. solid phase microextraction (SPME). Lombardo et al. (2011) determined changes in metabolomes during peach fruit development and showed high levels of bioactive polyphenols in early developmental stages. The authors suggested that the high levels of bioactive polyphenols are used as substrates for phenylpropanoid and lignin synthesis of hardening of stone (seed coat). Oikawa et al. (2015) reported changes in metabolomes of European pear fruits throughout development and the data is released from “Fruits Omics Database” (Fig. 2). The authors quantified over 250 metabolites in pear fruits. Interestingly, changes in each amino acid during fruit development showed completely different pattern. That is, the levels of histidine and phenylalanine were high at bloom stage and then decreased dramatically. Methionine was highest in the middle stage. Proline was highest before boom, decreased at bloom stage and then increased again at ripening stage. Although the meaning of the different fluctuation patterns of amino acids is unclear, they give interesting features of pear fruit development. Tsukaya et al. (2015) compared metabolomes of diploid and tetraploid Arabidopsis thaliana and diploid and tetraploid fruits of European pear. The authors concluded that metabolite content is not universal nor the direct target of polyploidy-dependent changes. Several metabolomics studies focused on flavor and volatile compounds of fruits. Aprea et al. (2011) developed SPME for volatile compounds. They then determined volatile compounds in four different apple varieties and succeeded to identify characteristic markers for each variety. Sánchez et al. (2012, 2014) profiled volatile compounds in peach fruits. The authors called their volatile compound analysis “volatilome”. Their volatilome of F1 segregating population and quantity trait locus (QTL) analysis revealed loci controlling the aroma of peach. Sánchez et al. (2012) identified 110 volatile compounds and quantified. Combination of hierarchical cluster analysis and metabolomic correlation network analysis revealed not only previously known cluster of peach fruit volatiles but also novel one. Leisso et al. (2013) and Lauxmann et al. (2014) performed metabolomics of postharvest storage apple fruits and peach fruits, respectively. The former study determined metabolomes under antioxidant treatment, temperature shifts and peel necrosis. The latter study focused on heat and cold responses.
Other omics studies
Hormonomics is a part of metabolomics. Generally concentrations of plant hormones are very low in all metabolites. Most plant hormones cannot be identified in normal metabolome analysis therefore specialized MS detection system is needed to analyze them. Hormonomics is rarely utilized in fruit tree research and also in general plant science. Only Oikawa et al. (2015) have reported comprehensive plant hormone analysis on fruit trees. Oikawa et al. (2015) determined fluctuations of 15 plant hormones, i.e. salicylic acid, JA, JA isoleucine conjugate, ABA, GA1, GA4, indole acetic acid, brassinolide (BL), castasterone (CS), trans-zeatin, dihydrozeatin, isopentenyladenine, trans-zeatin riboside, dihydrozeatin riboside and isopentenyladenine riboside in the fruit development of European pear using LC-tripleQ MS. The obtained hormonome data are available in “Fruits Omics Database” (Fig. 2). Concentrations of all plant hormones were very high in the youngest fruit and then decreased dramatically. Only concentrations of ABA, BL and CS increased in the ripening stage. Although increase of ethylene and ABA in fruit ripening stage is often observed, that of BL or CS has almost not been previously reported. BRs, including BL and CS, might be related to fruit ripening at least in European pear. Plant hormones control numerous events in plants, including fruit development. Further hormonomics will elucidate unknown functions of plant hormones in fruit tree growth and fruit development.
Ionomics is the comprehensive study of inorganic ions in an organism, organ, tissue or cell. Ionomics studies is also rare in fruit tree research field. Presently, a few ionomics studies of fruit trees have been reported (Parent et al. 2013a, 2013b). Reuscher et al. (2014) reported ionome analysis throughout fruit development of European pear and the data is released from “Fruits Omics Database” (Fig. 2). The authors tried to detect 27 elements, i.e. Ag, Al, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, Ga, In, K, Mg, Mn, Mo, Na, Ni, P, Pb, Rb, S, Sr, Tl, V and Zn in European pear fruits and its giant mutant fruits (Isuzugawa et al. 2014, Nashima et al. 2013b, 2014) using an inductively coupled plasmaatomic emission spectrometry (ICP-AES). Among them, 12 elements, i.e. Ca, K, Mg, P, S, B, Cu, Fe, Mn, Mo, Na and Zn, were detected and quantified in pear fruits. Concentrations of all these elements were high especially in the youngest fruit and decrease dramatically, although the decreasing rates were different among elements. Decreasing rates of B, K and Na were slower than other elements. In comparison between European pear fruits and its giant mutant fruits, concentrations of B and Ca in the giant mutant fruits were lower. This might be a cause of the corky spots in the giant mutant fruit as observed by Isuzugawa et al. (2014). Seventeen elements, i.e. B, C, Cl, Ca, Cu, Fe, H, K, Mg, Mn, Mo, N, Ni, O, P, S, and Zn are necessary for plant growth and excess amount of some elements, such as Al, Cd and Na, has harmful effect on plants and human also. Therefore ionomics is one of the important techniques to monitor plant health as well as food safety for human.
Omics databases and tools
Useful web pages for fruit omics study are listed in Table 1. These web sites provide genome data, microarray raw data, RNAseq raw data, gene expression data, MS spectrum dada, metabolite information, omics data, coexpression analytical tool, metabolic pathway map and its drafting tool and so on. However, presently, most web pages provide limited information support for some plant species.
Genome databases are provided for citrus (Citrus Genome Database), for grapevine (Grape Genome Database, Grape Genome Browser) and for rosaceae plants (Genome Database for Rosaceae; GDR). Plant GDB stores genome data of various plants, including grapevine, peach and papaya. These databases provide information about genome sequence and CDS.
Gene Expression Omnibus (GEO) and Sequence Read Archive (SRA) in National Center for Biotechnology Information (NCBI) store microarray and RNAseq raw data of all organisms, respectively. eFP Browser provides gene expression data and its analytical tool. On demand, eFP Browser displays gene expression patterns in different tissues and organs or induction of gene by different stimuli. Presently, only grape eFP Browser is available for fruit trees. ATTED-II and Co-expression biological Processes (CoP) provide co-regulated gene relationships. Functions of unknown genes could be estimated from gene relationships shown by ATTED-II or CoP. They provide only the systems for grapevine among fruit trees.
MassBank and ReSpect store mass spectrums of chemical compounds. The mass spectrum data is used to estimate metabolites in metabolome analysis. KNApSAcK is the database for various natural chemical compounds and it provides information on metabolites such as molecular weight, structural formula, detected organisms and related articles. Kyoto Encyclopedia of Genes and Genomes (KEGG) is a general database of biological information for all organisms at molecular level derived from omics studies. KEGG PATHWAY Database harbors various metabolic pathway maps. For certain organisms, metabolic pathway maps, in which related proteins, including metabolic enzymes, and their genes are integrated, are provided. For fruit trees, KEGG supports only grapevine metabolic pathway map as a species specified one. Plant Metabolic Network/Plant Metabolic Pathway Database (PMN/PlantCyc) provides metabolic pathway map with enzyme and gene information of various plants, including grapevine and papaya. VitisCyc provides similar metabolic pathway map of grapevine. KaPPA-View 4 KEGG is a drafting tool of metabolic pathway map. Using KaPPA-View 4 KEGG, transcriptome data, proteome data and metabolome data can be projected on a metabolic pathway map easily. KaPPA-View 4 KEGG supports only grapevine among fruit trees. Recently, we have updated the KaPPA-View 4 KEGG system of grapevine to adapt to newer genome data, called V1 (Suzuki et al. 2015b). Using the updated system, Suzuki et al. (2015b) clearly showed the specific induction of resveratrol synthetic pathway in grape berry skin by UV irradiation. This work shows that metabolic pathway map with omics data can reveal interesting metabolic changes in fruit trees.
Fruits Omics Database is a unique database collecting omics data of fruit trees (Fig. 2). Presently, Fruits Omics Database provides transcriptome data (Nashima et al. 2013a), metabolome data (Oikawa et al. 2015), hormonome data (Oikawa et al. 2015) and ionome data (Reuscher et al. 2014) of European pear fruits throughout its developmental stages. Proteome data of European pears (Reuscher et al. 2016) is scheduled to be released in the database.
All these five omics data, i.e. the transcriptome, proteome, metabolome, hormonome and ionome data of European pear fruits, are derived from the same material, thus all the data are comparable each other. This must be the widest multi-omics study using the same material in plant science field. Fruits Omics Database will collect omics data of other fruit trees.
Conclusion
As shown in the many examples introduced in this article, omics is a powerful approach to identify key genes for important traits, to clarify mechanisms of physiological events and to reveal unknown metabolic pathways in fruit trees. Supplemental Table 1 is the list of publications on omics studies of citrus, grapevine and rosaceae fruit trees in the last 5 years. Fruit tree researchers have recognized effectiveness of omics and it is becoming more popular in fruit tree research. However the study is still limited to specific crops, such as citrus, grapevine and rosaceae fruit trees. One of the reasons is lack of fundamental information for omics study, such as genome sequence data, EST and chemical compound information.
As described above, analytical costs of NGS is decreasing dramatically. On the other hand, volume of information from NGS and efficiency and accuracy of bioinformatics are increasing and increasing. Due to these situations, genome sequence data and EST for even minor fruit trees will be provided in the near future. This will accelerate the use of transriptomic and proteomic approaches in fruit tree research. Microarray was an indispensable tool for transcriptomics studies before. However RNAseq has become an alternative approach recently. High-throughputs and accurate de novo assemble technology of more than 10 million sequence reads from NGS has made it possible to perform transcriptomics without genome or EST data. This technology can be applied in fruit trees transcriptomics studies where limited genome sequence or EST data is available.
The bottleneck of metabolomics is lack of standard chemical compounds and their MS spectrum data on metabolites. In MS analysis of plant extract, several thousands of metabolite peaks are detected but a few hundreds of primary metabolites are identifiable (Oikawa et al. 2015). For secondary metabolites, less than a dozen can be identified (Suzuki et al. 2015a, 2015b), because of no existence of standard chemical compounds or their MS spectrum dada. Secondary metabolites often contribute to unique fruit characters, such as taste, flavor, color and functionality. Each plant species has unique secondary metabolic pathway and accumulation. High-throughput technologies are needed to identify and clarify synthetic pathways of secondary metabolites so as to accelerate metabolomics studies in plants.
In the research field of fruit trees, enormous undiscovered treasures, i.e. important genes, metabolites, metabolic pathways and unknown physiological processes, lie under the ground. Treasure hunting using omics technology has been just started.
Supplementary Material
Acknowledgements
We would like to acknowledge Mr. Ofori Peter Amoako for proofreading this manuscript. This work was supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry from the Bio-oriented Technology Research Advancement Institution (BRAIN), by the Cross-ministerial Strategic Innovation Promotion Program (SIP) from the Cabinet Office and by the Grant-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT).
Literature Cited
- Abbà, S., Galetto, L., Carle, P., Carrère, S., Delledonne, M., Foissac, X., Palmano, S., Veratti, F. and Marzachì, C. (2014) RNA-Seq profile of flavescence dorée phytoplasma in grapevine. BMC Genomics 15: 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo-Romero, P., Ali, K., Choi, Y.H., Sousa, L., Verpoorte, R., Tiburcio, A.F. and Fortes, A.M. (2014) Perturbation of polyamine catabolism affects grape ripening of Vitis vinifera cv. Trincadeira. Plant Physiol. Biochem. 74: 141–155. [DOI] [PubMed] [Google Scholar]
- Agudelo-Romero, P., Erban, A., Rego, C., Carbonell-Bejerano, P., Nascimento, T., Sousa, L., Martínez-Zapater, J.M., Kopka, J. and Fortes, A.M. (2015) Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J. Exp. Bot. 66: 1769–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai, X.Y., Lin, G., Sun, L.M., Hu, C.G., Guo, W.W., Deng, X.X. and Zhang, J.Z. (2012) A global view of gene activity at the flowering transition phase in precocious trifoliate orange and its wild-type [Poncirus trifoliata (L.) Raf.] by transcriptome and proteome analysis. Gene 510: 47–58. [DOI] [PubMed] [Google Scholar]
- Ali, M.B., Howard, S., Chen, S., Wang, Y., Yu, O., Kovacs, L.G. and Qiu, W. (2011) Berry skin development in Norton grape: distinct patterns of transcriptional regulation and flavonoid biosynthesis. BMC Plant Biol. 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allario, T., Brumos, J., Colmenero-Flores, J.M., Tadeo, F., Froelicher, Y., Talon, M., Navarro, L., Ollitrault, P. and Morillon, R. (2011) Large changes in anatomy and physiology between diploid Rangpur lime (Citrus limonia) and its autotetraploid are not associated with large changes in leaf gene expression. J. Exp. Bot. 62: 2507–2519. [DOI] [PubMed] [Google Scholar]
- Almagro, L., Carbonell-Bejerano, P., Belchí-Navarro, S., Bru, R., Martínez-Zapater, J.M., Lijavetzky, D. and Pedreño, M.A. (2014) Dissecting the transcriptional response to elicitors in Vitis vinifera cells. PLoS ONE 9: e109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anesi, A., Stocchero, M., Santo, S.D., Commisso, M., Zenoni, S., Ceoldo, S., Tornielli, G.B., Siebert, T.E., Herderich, M., Pezzotti, M.et al. (2015) Towards a scientific interpretation of the terroir concept: plasticity of the grape berry metabolome. BMC Plant Biol. 15: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprea, E., Gika, H., Carlin, S., Theodoridis, G., Vrhovsek, U. and Mattivi, F. (2011) Metabolite profiling on apple volatile content based on solid phase microextraction and gas-chromatography time of flight mass spectrometry. J. Chromatogr. A1218: 4517–4524. [DOI] [PubMed] [Google Scholar]
- Arbulu, M., Sampedro, M.C., Sanchez-Ortega, A., Gómez-Caballero, A., Unceta, N., Goicolea, M.A. and Barrio, R.J. (2013) Characterisation of the flavour profile from Graciano Vitis vinifera wine variety by a novel dual stir bar sorptive extraction methodology coupled to thermal desorption and gas chromatography-mass spectrometry. Anal. Chim. Acta 777: 41–48. [DOI] [PubMed] [Google Scholar]
- Aura, A.M., Mattila, I., Hyötyläinen, T., Gopalacharyulu, P., Cheynier, V., Souquet, J.M., Bes, M., Le Bourvellec, C., Guyot, S. and Orešič, M. (2013) Characterization of microbial metabolism of Syrah grape products in an in vitro colon model using targeted and non-targeted analytical approaches. Eur. J. Nutr. 52: 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, S., Saito, T., Sakamoto, D., Ito, A., Fujii, H. and Moriguchi, T. (2013) Transcriptome analysis of Japanese pear (Pyrus pyrifolia Nakai) flower buds transitioning through endodormancy. Plant Cell Physiol. 54: 1132–1151. [DOI] [PubMed] [Google Scholar]
- Ballester, A.R., Lafuente, M.T., de Vos, R.C.H., Bovy, A.G. and González-Candelas, L. (2013) Citrus phenylpropanoids and defence against pathogens. Part I: metabolic profiling in elicited fruits. Food Chem. 136: 178–185. [DOI] [PubMed] [Google Scholar]
- Barakat, A., Sriram, A., Park, J., Zhebentyayeva, T., Main, D. and Abbott, A. (2012) Genome wide identification of chilling responsive microRNAs in Prunus persica. BMC Genomics 13: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassene, J.B., Froelicher, Y., Dubois, C., Ferrer, R.M., Navarro, L., Ollitrault, P. and Ancillo, G. (2010) Non-additive gene regulation in a citrus allotetraploid somatic hybrid between C. reticulata Blanco and C. limon (L.) Burm. Heredity. 105: 299–308. [DOI] [PubMed] [Google Scholar]
- Belchí-Navarro, S., Almagro, L., Sabater-Jara, A.B., Fernández-Pérez, F., Bru, R. and Pedreño, M.A. (2013) Induction of trans-resveratrol and extracellular pathogenesis-related proteins in elicited suspension cultured cells of Vitis vinifera cv Monastrell. J. Plant Physiol. 170: 258–264. [DOI] [PubMed] [Google Scholar]
- Bernardi, J., Licciardello, C., Russo, M.P., Chiusano, M.L., Carletti, G., Recupero, G.R. and Marocco, A. (2010) Use of a custom array to study differentially expressed genes during blood orange (Citrus sinensis L. Osbeck) ripening. J. Plant Physiol. 167: 301–310. [DOI] [PubMed] [Google Scholar]
- Bertazzo, A., De Rosso, M., Vedova, A.D., Agnolin, F., Flamini, R. and Traldi, P. (2010) Parental relationships among three grape varieties studied by MALDI of grape seed protein profiles. J. Mass Spectrom. 45: 966–970. [DOI] [PubMed] [Google Scholar]
- Bindschedler, L.V. and Cramer, R. (2011) Quantitative plant proteomics. Proteomics 11: 756–775. [DOI] [PubMed] [Google Scholar]
- Bustamante, C.A., Budde, C.O., Borsani, J., Lombardo, V.A., Lauxmann, M.A., Andreo, C.S., Lara, M.V. and Drincovich, M.F. (2012) Heat treatment of peach fruit: modifications in the extracellular compartment and identification of novel extracellular proteins. Plant Physiol. Biochem. 60: 35–45. [DOI] [PubMed] [Google Scholar]
- Cangahuala-Inocente, G.C., Da Silva, M.F., Johnson, J.M., Manga, A., van Tuinen, D., Henry, C., Lovato, P.E. and Dumas-Gaudot, E. (2011) Arbuscular mycorrhizal symbiosis elicits proteome responses opposite of P-starvation in SO4 grapevine rootstock upon root colonisation with two Glomus species. Mycorrhiza 21: 473–493. [DOI] [PubMed] [Google Scholar]
- Cao, X., Gao, Y., Wang, Y., Li, C.M., Zhao, Y.B., Han, Z.H. and Zhang, X.Z. (2011) Differential expression and modification of proteins during ontogenesis in Malus domestica. Proteomics 11: 4688–4701. [DOI] [PubMed] [Google Scholar]
- Cao, X., Feng, J., Wang, D., Sun, J., Lu, X. and Liu, H. (2012) Primary style protein expression in the self-incompatible/compatible apricot by the 2D-DIGE technique. Gene 503: 110–117. [DOI] [PubMed] [Google Scholar]
- Carbonell-Bejerano, P., María, E.S., Torres-Pérez, R., Royo, C., Lijavetzky, D., Bravo, G., Aguirreolea, J., Sánchez-Díaz, M., Antolín, M.C. and Martínez-Zapater, J.M. (2013) Thermotolerance responses in ripening berries of Vitis vinifera L. cv Muscat Hamburg. Plant Cell Physiol. 54: 1200–1216. [DOI] [PubMed] [Google Scholar]
- Carrier, G., Huang, Y.F., Le Cunff, L., Fournier-Level, A., Vialet, S., Souquet, J.M., Cheynier, V., Terrier, N. and This, P. (2013) Selection of candidate genes for grape proanthocyanidin pathway by an integrative approach. Plant Physiol. Biochem. 72: 87–95. [DOI] [PubMed] [Google Scholar]
- Carvalho, L.C., Vilela, B.J., Mullineaux, P.M. and Amâncio, S. (2011) Comparative transcriptomic profiling of Vitis vinifera under high light using a custom-made array and the Affymetrix GeneChip. Mol. Plant 4: 1038–1051. [DOI] [PubMed] [Google Scholar]
- Cevallos-Cevallos, J.M., Futch, D.B., Shilts, T., Folimonova, S.Y. and Reyes-De-Corcuera, J.I. (2012) GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 53: 69–76. [DOI] [PubMed] [Google Scholar]
- Chai, L., Li, Y., Chen, S., Perl, A., Zhao, F. and Ma, H. (2014) RNA sequencing reveals high resolution expression change of major plant hormone pathway genes after young seedless grape berries treated with gibberellin. Plant Sci. 229: 215–224. [DOI] [PubMed] [Google Scholar]
- Chen, S., Ye, T., Hao, L., Chen, H., Wang, S., Fan, Z., Guo, L. and Zhou, T. (2014) Infection of apple by apple stem grooving virus leads to extensive alterations in gene expression patterns but no disease symptoms. PLoS ONE 9: e95239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C., Jiao, C., Singer, S.D., Gao, M., Xu, X., Zhou, Y., Li, Z., Fei, Z., Wang, Y. and Wang, X. (2015) Gibberellin-induced changes in the transcriptome of grapevine (Vitis labrusca × V. vinifera) cv. Kyoho flowers. BMC Genomics 16: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, E.L., Mishchuk, D.O., Breksa, A.P. and Slupsky, C.M. (2014) Metabolite signature of Candidatus Liberibacter asiaticus infection in two citrus varieties. J. Agric. Food Chem. 62: 6585–6591. [DOI] [PubMed] [Google Scholar]
- Clemente-Moreno, M.J., Díaz-Vivancos, P., Rubio, M., Fernández-García, N. and Hernández, J.A. (2013) Chloroplast protection in plum pox virus-infected peach plants by L-2-oxo-4-thiazolidine-carboxylic acid treatments: effect in the proteome. Plant Cell Environ. 36: 640–654. [DOI] [PubMed] [Google Scholar]
- Cookson, S.J., Moreno, M.J.C., Hevin, C., Mendome, L.Z.N., Delrot, S., Trossat-Magnin, C. and Ollat, N. (2013) Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signaling, and secondary metabolism. J. Exp. Bot. 64: 2997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso, M., Ziliotto, F., Rizzini, F.M., Teo, G., Cargnello, G. and Bonghi, C. (2013) Sensorial, biochemical and molecular changes in Raboso Piave grape berries applying “Double Maturation Raisonnée” and late harvest techniques. Plant Sci. 208: 50–57. [DOI] [PubMed] [Google Scholar]
- Corso, M., Vannozzi, A., Maza, E., Vitulo, N., Meggio, F., Pitacco, A., Telatin, A., D’Angelo, M., Feltrin, E., Negri, A.S.et al. (2015) Comprehensive transcript profiling of two grapevine rootstock genotypes contrasting in drought susceptibility links the phenylpropanoid pathway to enhanced tolerance. J. Exp. Bot. 66: 5739–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, F., Alba, R., Schouten, H., Soglio, V., Gianfranceschi, L., Serra, S., Musacchi, S., Sansavini, S., Costa, G., Fei, Z.et al. (2010) Use of homologous and heterologous gene expression profiling tools to characterize transcription dynamics during apple fruit maturation and ripening. BMC Plant Biol. 10: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, G.R., Ghan, R., Schlauch, K.A., Tillett, R.L., Heymann, H., Ferrarini, A., Delledonne, M., Zenoni, S., Fasoli, M. and Pezzotti, M. (2014) Transcriptomic analysis of the late stages of grapevine (Vitis vinifera cv. Cabernet Sauvignon) berry ripening reveals significant induction of ethylene signaling and flavor pathways in the skin. BMC Plant Biol. 14: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadakova, K., Havelkova, M., Kurkova, B., Tlolkova, I., Kasparovsky, T., Zdrahal, Z. and Lochman, J. (2015) Proteome and transcript analysis of Vitis vinifera cell cultures subjected to Botrytis cinerea infection. J. Proteomics 119: 143–153. [DOI] [PubMed] [Google Scholar]
- Dai, Z.W., Léon, C., Feil, R., Lunn, J.E., Delrot, S. and Gomès, E. (2013) Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J. Exp. Bot. 64: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldoul, S., Guillaumie, S., Reustle, G.M., Krczal, G., Ghorbel, A., Delrot, S., Mliki, A. and Höfer, M.U. (2010) Isolation and expression analysis of salt induced genes from contrasting grapevine (Vitis vinifera L.) cultivars. Plant Sci. 179: 489–498. [DOI] [PubMed] [Google Scholar]
- Dal Santo, S., Tornielli, G.B., Zenoni, S., Fasoli, M., Farina, L., Anesi, A., Guzzo, F., Delledonne, M. and Pezzotti, M. (2013) The plasticity of the grapevine berry transcriptome. Genome Biol. 14: r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio, C., Arena, S., Rocco, M., Verrillo, F., Novi, G., Viscosi, V., Marra, M. and Scaloni, A. (2013) Proteomic analysis of apricot fruit during ripening. J. Proteomics 78: 39–57. [DOI] [PubMed] [Google Scholar]
- Da Silva, C., Zamperin, G., Ferrarini, A., Minio, A., Dal Molin, A., Venturini, L., Buson, G., Tononi, P., Avanzato, C., Zago, E.et al. (2013) The high polyphenol content of grapevine cultivar tannat berries is conferred primarily by genes that are not shared with the reference genome. Plant Cell 25: 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degu, A., Hochberg, U., Sikron, N., Venturini, L., Buson, G., Ghan, R., Plaschkes, I., Batushansky, A., Chalifa-Caspi, V., Mattivi, F.et al. (2014) Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 14: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Riquelme, J., Grimplet, J., Martínez-Zapater, J.M. and Carmona, M.J. (2012) Transcriptome variation along bud development in grapevine (Vitis vinifera L.). BMC Plant Biol. 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Riquelme, J., Martínez-Zapater, J.M. and Carmona, M.J. (2014) Transcriptional analysis of tendril and inflorescence development in grapevine (Vitis vinifera L.), PLoS ONE 9: e92339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carli, M., Zamboni, A., Pè, M.E., Pezzotti, M., Lilley, K.S., Benvenuto, E. and Desiderio, A. (2011) Two-dimensional differential in gel electrophoresis (2D-DIGE) analysis of grape berry proteome during postharvest withering. J. Proteome Res. 10: 429–446. [DOI] [PubMed] [Google Scholar]
- Dória, M.S., de Sousa, A.O., Barbosa, C.D.J., Costa, M.G.C., Gesteira, A.D.S., Souza, R.M., Freitas, A.C.O. and Pirovani, C.P. (2015) Citrus tristeza virus (CTV) causing proteomic and enzymatic changes in sweet orange variety “Westin”. PLoS ONE 10: e0130950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falara, V., Manganaris, G.A., Ziliotto, F., Manganaris, A., Bonghi, C., Ramina, A. and Kanellis, A.K. (2011) A β-D:-xylosidase and a PR-4B precursor identified as genes accounting for differences in peach cold storage tolerance. Funct. Integr. Genomics 11: 357–368. [DOI] [PubMed] [Google Scholar]
- Fan, J., Chen, C., Yu, Q., Brlansky, R.H., Li, Z.G. and Gmitter, F.G. (2011) Comparative iTRAQ proteome and transcriptome analyses of sweet orange infected by ‘Candidatus Liberibacter asiaticus’. Physiol. Plant. 143: 235–245. [DOI] [PubMed] [Google Scholar]
- Fasoli, M., Dal Santo, S., Zenoni, S., Tornielli, G.B., Farina, L., Zamboni, A., Porceddu, A., Venturini, L., Bicego, M., Murino, V.et al. (2012) The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 24: 3489–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, S., Chen, X., Zhang, Y., Wang, Y., Song, Y., Chen, X., Li, X., Li, M., Liu, J., Wang, Q.et al. (2011) Differential expression of proteins in red pear following fruit bagging treatment. Protein J. 30: 194–200. [DOI] [PubMed] [Google Scholar]
- Ferrero, S., Carretero-Paulet, L., Mendes, M.A., Botton, A., Eccher, G., Masiero, S. and Colombo, L. (2015) Transcriptomic signatures in seeds of apple (Malus domestica L. Borkh) during fruitlet abscission. PLoS ONE 10: e0120503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes, A.M., Agudelo-Romero, P., Silva, M.S., Ali, K., Sousa, L., Maltese, F., Choi, Y.H., Grimplet, J., Martinez-Zapater, J.M., Verpoorte, R.et al. (2011) Transcript and metabolite analysis in Trincadeira cultivar reveals novel information regarding the dynamics of grape ripening. BMC Plant Biol. 11: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraige, K., González-Fernández, R., Carrilho, E. and Jorrín-Novo, J.V. (2015) Metabolite and proteome changes during the ripening of Syrah and Cabernet Sauvignon grape varieties cultured in a nontraditional wine region in Brazil. J. Proteomics 113: 206–225. [DOI] [PubMed] [Google Scholar]
- Gapper, N.E., Rudell, D.R., Giovannoni, J.J. and Watkins, C.B. (2013) Biomarker development for external CO2 injury prediction in apples through exploration of both transcriptome and DNA methylation changes. AoB Plants 5: plt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, A., Trouvelot, S., Kelloniemi, J., Frettinger, P., Wendehenne, D., Daire, X., Joubert, J.M., Ferrarini, A., Delledonne, M., Flors, V.et al. (2014) The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 9: e88145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, I.S., Pascovici, D., Mirzaei, M. and Haynes, P.A. (2015) Quantitative proteomic analysis of cabernet sauvignon grape cells exposed to thermal stresses reveals alterations in sugar and phenylpropanoid metabolism. Proteomics 15: 3048–3060. [DOI] [PubMed] [Google Scholar]
- Gika, H.G., Theodoridis, G.A., Vrhovsek, U. and Mattivi, F. (2012) Quantitative profiling of polar primary metabolites using hydrophilic interaction ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1259: 121–127. [DOI] [PubMed] [Google Scholar]
- Giraud, E., Ivanova, A., Gordon, C.S., Whelan, J. and Considine, M.J. (2012) Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ. 35: 405–417. [DOI] [PubMed] [Google Scholar]
- Goldberg-Moeller, R., Shalom, L., Shlizerman, L., Samuels, S., Zur, N., Ophir, R., Blumwald, E. and Sadka, A. (2013) Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in citrus buds. Plant Sci. 198: 46–57. [DOI] [PubMed] [Google Scholar]
- Griesser, M., Weingart, G., Schoedl-Hummel, K., Neumann, N., Becker, M., Varmuza, K., Liebner, F., Schuhmacher, R. and Forneck, A. (2015) Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 88: 17–26. [DOI] [PubMed] [Google Scholar]
- Guillaumie, S., Fouquet, R., Kappel, C., Camps, C., Terrier, N., Moncomble, D., Dunlevy, J.D., Davies, C., Boss, P.K. and Delrot, S. (2011) Transcriptional analysis of late ripening stages of grapevine berry. BMC Plant Biol. 11: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F., Yu, H., Xu, Q. and Deng, X. (2015) Transcriptomic analysis of differentially expressed genes in an orange-pericarp mutant and wild type in pummelo (Citrus grandis). BMC Plant Biol. 15: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusberti, M., Gessler, C. and Broggini, G.A.L. (2013) RNA-Seq analysis reveals candidate genes for ontogenic resistance in Malus-Venturia pathosystem. PLoS ONE 8: e78457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz, M.C., Niehl, A., Rosales, M., Fiore, N., Zamorano, A., Granell, A. and Pallas, V. (2013) A remarkable synergistic effect at the transcriptomic level in peach fruits doubly infected by prunus necrotic ringspot virus and peach latent mosaic viroid. Virol. J. 10: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D.G., Ma, Q.J., Sun, C.H., Sun, M.H., You, C.X. and Hao, Y.J. (2015) Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plant. doi: 10.1111/ppl.12354. [DOI] [PubMed] [Google Scholar]
- Hu, H., Liu, Y., Shi, G.L., Liu, Y.P., Wu, R.J., Yang, A.Z., Wang, Y.M., Hua, B.G. and Wang, Y.N. (2011) Proteomic analysis of peach endocarp and mesocarp during early fruit development. Physiol. Plant. 142: 390–406. [DOI] [PubMed] [Google Scholar]
- Huang, G., Li, T., Li, X., Tan, D., Jiang, Z., Wei, Y., Li, J. and Wang, A. (2014) Comparative transcriptome analysis of climacteric fruit of Chinese pear (Pyrus ussuriensis) reveals new insights into fruit ripening. PLoS ONE 9: e107562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez, A.M., Martinelli, F., Reagan, R.L., Uratsu, S.L., Vo, A., Tinoco, M.A., Phu, M.L., Chen, Y., Rocke, D.M. and Dandekar, A.M. (2014) Transcriptome and metabolome analysis of citrus fruit to elucidate puffing disorder. Plant Sci. 217–218: 87–98. [DOI] [PubMed] [Google Scholar]
- Isuzugawa, K., Murayama, H. and Nishio, T. (2014) Characterization of a giant-fruit mutant exhibiting fruit-limited polyploidization in pear (Pyrus communis L.) Sci. Hortic. 170: 196–202. [Google Scholar]
- Jaillon, O., Aury, J.M., Noel, B., Policriti, A., Clepet, C., Casagrande, A., Choisne, N., Aubourg, S., Vitulo, N., Jubin, C.et al. (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449: 463–467. [DOI] [PubMed] [Google Scholar]
- Jiang, L., Zhang, L., Shi, Y., Lu, Z. and Yu, Z. (2014) Proteomic analysis of peach fruit during ripening upon post-harvest heat combined with 1-MCP treatment. J. Proteomics 98: 31–43. [DOI] [PubMed] [Google Scholar]
- Katz, E., Fon, M., Eigenheer, R.A., Phinney, B.S., Fass, J.N., Lin, D., Sadka, A. and Blumwald, E. (2010) A label-free differential quantitative mass spectrometry method for the characterization and identification of protein changes during citrus fruit development. Proteome Sci. 8: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, K., Sadamatsu, K. and Goto-Yamamoto, N. (2010) Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct. Integr. Genomics 10: 367–381. [DOI] [PubMed] [Google Scholar]
- Krost, C., Petersen, R. and Schmidt, E.R. (2012) The transcriptomes of columnar and standard type apple trees (Malus × domestica)—a comparative study. Gene 498: 223–230. [DOI] [PubMed] [Google Scholar]
- Krost, C., Petersen, R., Lokan, S., Brauksiepe, B., Braun, P. and Schmidt, E.R. (2013) Evaluation of the hormonal state of columnar apple trees (Malus × domestica) based on high throughput gene expression studies. Plant Mol. Biol. 81: 211–220. [DOI] [PubMed] [Google Scholar]
- Lauxmann, M.A., Brun, B., Borsani, J., Bustamante, C.A., Budde, C.O., Lara, M.V. and Drincovich, M.F. (2012) Transcriptomic profiling during the post-harvest of heat-treated Dixiland Prunus persica fruits: common and distinct response to heat and cold. PLoS ONE 7: e51052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauxmann, M.A., Borsani, J., Osorio, S., Lombardo, V.A., Budde, C.O., Bustamante, C.A., Monti, L.L., Andreo, C.S., Fernie, A.R., Drincovich, M.F.et al. (2014) Deciphering the metabolic pathways influencing heat and cold responses during post-harvest physiology of peach fruit. Plant Cell Environ. 37: 601–616. [DOI] [PubMed] [Google Scholar]
- Lawo, N.C., Weingart, G.J.F., Schuhmacher, R. and Forneck, A. (2011) The volatile metabolome of grapevine roots: first insights into the metabolic response upon phylloxera attack. Plant Physiol. Biochem. 49: 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisso, R., Buchanan, D., Lee, J., Mattheis, J. and Rudell, D. (2013) Cell wall, cell membrane, and volatile metabolism are altered by antioxidant treatment, temperature shifts, and peel necrosis during apple fruit storage. J. Agric. Food Chem. 61: 1373–1387. [DOI] [PubMed] [Google Scholar]
- Li, M., Xu, J., Qiu, Z., Zhang, J., Ma, F. and Zhang, J. (2014) Screening and identification of resistance related proteins from apple leaves inoculated with Marssonina coronaria (EII. & J. J. Davis). Proteome Sci. 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Liu, J., Tan, D., Allan, A.C., Jiang, Y., Xu, X., Han, Z. and Kong, J. (2013) A genome-wide expression profile of salt-responsive genes in the apple rootstock Malus zumi. Int. J. Mol. Sci. 14: 21053– 21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Korir, N.K., Liu, L., Shangguan, L., Wang, Y., Han, J., Chen, M. and Fang, J. (2012) Microarray analysis of differentially expressed genes engaged in fruit development between Prunus mume and Prunus armeniaca. J. Plant Physiol. 169: 1776–1788. [DOI] [PubMed] [Google Scholar]
- Li, X., Wu, J., Yin, L., Zhang, Y., Qu, J. and Lu, J. (2015) Comparative transcriptome analysis reveals defense-related genes and pathways against downy mildew in Vitis amurensis grapevine. Plant Physiol. Biochem. 95: 1–14. [DOI] [PubMed] [Google Scholar]
- Lijavetzky, D., Carbonell-Bejerano, P., Grimplet, J., Bravo, G., Flores, P., Fenoll, J., Hellín, P., Oliveros, J.C. and Martínez-Zapater, J.M. (2012) Berry flesh and skin ripening features in Vitis vinifera as assessed by transcriptional profiling. PLoS ONE 7: e39547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q., Wang, C., Dong, W., Jiang, Q., Wang, D., Li, S., Chen, M., Liu, C., Sun, C. and Chen, K. (2015) Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene 554: 64–74. [DOI] [PubMed] [Google Scholar]
- Liu, G., Li, W., Zheng, P., Xu, T., Chen, L., Liu, D., Hussain, S. and Teng, Y. (2012) Transcriptomic analysis of ‘Suli’ pear (Pyrus pyrifolia white pear group) buds during the dormancy by RNA-Seq. BMC Genomics 13: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Xue, C., Wu, T., Heng, W., Jia, B., Ye, Z., Liu, L. and Zhu, L. (2013) Molecular analysis of the processes of surface brown spot (SBS) formation in pear fruit (Pyrus bretschneideri Rehd. cv. Dangshansuli) by de novo transcriptome assembly. PLoS ONE 8: e74217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Zhai, R., Feng, W., Zhang, S., Wang, Z., Qiu, Z., Zhang, J., Ma, F. and Xu, L. (2014) Proteomic analysis of ‘Zaosu’ pear (Pyrus bretschneideri Rehd.) and its early-maturing bud sport. Plant Sci. 224: 120–135. [DOI] [PubMed] [Google Scholar]
- Lombardo, V.A., Osorio, S., Borsani, J., Lauxmann, M.A., Bustamante, C.A., Budde, C.O., Andreo, C.S., Lara, M.V., Fernie, A.R. and Drincovich, M.F. (2011) Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 157: 1696–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y.B., Yang, L.T., Qi, Y.P., Li, Y., Li, Z., Chen, Y.B., Huang, Z.R. and Chen, L.S. (2014) Identification of boron-deficiency-responsive microRNAs in Citrus sinensis roots by Illumina sequencing. BMC Plant Biol. 14: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra, V., Martins, P.K., Francisco, C.S., Ribeiro-Alves, M., Freitas-Astúa, J. and Machado, M.A. (2013) Candidatus Liberibacter americanus induces significant reprogramming of the transcriptome of the susceptible citrus genotype. BMC Genomics 14: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacarne, G., Vrhovsek, U., Zulini, L., Cestaro, A., Stefanini, M., Mattivi, F., Delledonne, M., Velasco, R. and Moser, C. (2011) Resistance to Plasmopara viticola in a grapevine segregating population is associated with stilbenoid accumulation and with specific host transcriptional responses. BMC Plant Biol. 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaria, P., Abbà, S. and Palmano, S. (2013) Novel aspects of grapevine response to phytoplasma infection investigated by a proteomic and phospho-proteomic approach with data integration into functional networks. BMC Genomics 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marondedze, C. and Thomas, L.A. (2012) Apple hypanthium firmness: new insights from comparative proteomics. Appl. Biochem. Biotechnol. 168: 306–326. [DOI] [PubMed] [Google Scholar]
- Martinelli, F., Uratsu, S.L., Albrecht, U., Reagan, R.L., Phu, M.L., Britton, M., Buffalo, V., Fass, J., Leicht, E., Zhao, W.et al. (2012) Transcriptome profiling of citrus fruit response to huanglongbing disease. PLoS ONE 7: e38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli, F., Reagan, R.L., Uratsu, S.L., Phu, M.L., Albrecht, U., Zhao, W., Davis, C.E., Bowman, K.D. and Dandekar, A.M. (2013) Gene regulatory networks elucidating huanglongbing disease mechanisms. PLoS ONE 8: e74256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserti, B.E., Del Carratore, R., Della Croce, C.M., Podda, A., Migheli, Q., Froelicher, Y., Luro, F., Morillon, R., Ollitrault, P., Talon, M.et al. (2011) Comparative analysis of proteome changes induced by the two spotted spider mite Tetranychus urticae and methyl jasmonate in citrus leaves. J. Plant Physiol. 168: 392–402. [DOI] [PubMed] [Google Scholar]
- Mellidou, I., Buts, K., Hatoum, D., Ho, Q.T., Johnston, J.W., Watkins, C.B., Schaffer, R.J., Gapper, N.E., Giovannoni, J.J., Rudell, D.R.et al. (2014) Transcriptomic events associated with internal browning of apple during postharvest storage. BMC Plant Biol. 14: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molassiotis, A., Tanou, G., Filippou, P. and Fotopoulos, V. (2013) Proteomics in the fruit tree science arena: new insights into fruit defense, development, and ripening. Proteomics 13: 1871–1884. [DOI] [PubMed] [Google Scholar]
- Monavarfeshani, A., Mirzaei, M., Sarhadi, E., Amirkhani, A., Nekouei, M.K., Haynes, P.A., Mardi, M. and Salekdeh, G.H. (2013) Shotgun proteomic analysis of the Mexican lime tree infected with ‘CandidatusPhytoplasma aurantifolia’. J. Proteome Res. 12: 785– 795. [DOI] [PubMed] [Google Scholar]
- Mousavi, S., Alisoltani, A., Shiran, B., Fallahi, H., Ebrahimie, E., Imani, A. and Houshmand, S. (2014) De novo transcriptome assembly and comparative analysis of differentially expressed genes in Prunus dulcis Mill. in response to freezing stress. PLoS ONE 9: e104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccilli, V., Licciardello, C., Fontanini, D., Cunsolo, V., Capocchi, A., Saletti, R., Torrisi, B. and Foti, S. (2013) Root protein profiles of two citrus rootstocks grown under iron sufficiency/deficiency conditions. Eur. J. Mass Spectrom. 19: 305–324. [DOI] [PubMed] [Google Scholar]
- Mulas, G., Galaffu, M.G., Pretti, L., Nieddu, G., Mercenaro, L., Tonelli, R. and Anedda, R. (2011) NMR analysis of seven selections of vermentino grape berry: metabolites composition and development. J. Agric. Food Chem. 59: 793–802. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fambuena, N., Mesejo, C., Agustí, M., Tárraga, S., Iglesias, D.J., Primo-Millo, E. and González-Mas, M.C. (2013a) Proteomic analysis of ‘Moncada’ mandarin leaves with contrasting fruit load. Plant Physiol. Biochem. 62: 95–106. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fambuena, N., Mesejo, C., Reig, C., Agustí, M., Tárraga, S., Lisón, P., Iglesias, D.J., Primo-Millo, E. and González-Mas, M.C. (2013b) Proteomic study of ‘Moncada’ mandarin buds from onversus off-crop trees. Plant Physiol. Biochem. 73: 41–55. [DOI] [PubMed] [Google Scholar]
- Nabity, P.D., Haus, M.J., Berenbaum, M.R. and DeLucia, E.H. (2013) Leaf-galling phylloxera on grapes reprograms host metabolism and morphology. Proc. Natl. Acad. Sci. USA 110: 16663–16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narduzzi, L., Stanstrup, J. and Mattivi, F. (2015) Comparing wild american grapes with Vitis vinifera: a metabolomics study of grape composition. J. Agric. Food Chem. 63: 6823–6834. [DOI] [PubMed] [Google Scholar]
- Nashima, K., Shimizu, T., Nishitani, C., Yamamoto, T., Takahashi, H., Nakazono, M., Itai, A., Isuzugawa, K., Hanada, T., Takashina, T.et al. (2013a) Microarray analysis of gene expression patterns during fruit development in European pear (Pyrus communis). Sci. Hortic. 164: 466–473. [Google Scholar]
- Nashima, K., Takahashi, H., Nakazono, M., Shimizu, T., Nishitani, C., Yamamoto, T., Itai, A., Isuzugawa, K., Hanada, T., Takashina, T.et al. (2013b) Transcriptome analysis of giant pear fruit with fruit-specific DNA reduplication on a mutant branch. J. Japan. Soc. Hort. Sci. 82: 301–311. [Google Scholar]
- Nashima, K., Terakami, S., Nishitani, C., Yamamoto, T., Habu, T., Takahashi, H., Nakazono, M., Isuzugawa, K., Hanada, T., Takashina, T.et al. (2014) Transcriptome analysis of flower receptacles of the European pear (Pyrus communis L.) ‘La France’ and its giant fruit sport using next-generation sequencing technology. J. Hortic. Sci. Biotechnol. 89: 293–300. [Google Scholar]
- Negri, A.S., Robotti, E., Prinsi, B., Espen, L. and Marengo, E. (2011) Proteins involved in biotic and abiotic stress responses as the most significant biomarkers in the ripening of Pinot Noir skins. Funct. Integr. Genomics 11: 341–355. [DOI] [PubMed] [Google Scholar]
- Nilo, P.R., Campos-Vargas, R. and Orellana, A. (2012) Assessment of Prunus persica fruit softening using a proteomics approach. J. Proteomics 75: 1618–1638. [DOI] [PubMed] [Google Scholar]
- Nilo-Poyanco, R., Olivares, D., Orellana, A., Hinrichsen, P. and Pinto, M. (2013) Proteomic analysis of grapevine (Vitis vinifera L.) leaf changes induced by transition to autotrophy and exposure to high light irradiance. J. Proteomics 91: 309–330. [DOI] [PubMed] [Google Scholar]
- Nishikawa, F., Endo, T., Shimada, T., Fujii, H., Shimizu, T., Kobayashi, Y., Araki, T. and Omura, M. (2010) Transcriptional changes in CiFT-introduced transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Tree Physiol. 30: 431–439. [DOI] [PubMed] [Google Scholar]
- Nishitani, C., Saito, T., Ubi, B.E., Shimizu, T., Itai, A., Saito, T., Yamamoto, T. and Moriguchi, T. (2012a) Transcriptome analysis of Pyrus pyrifolia leaf buds during transition from endodormancy to ecodormancy. Sci. Hortic. 147: 49–55. [Google Scholar]
- Nishitani, C., Yamaguchi-Nakamura, A., Hosaka, F., Terakami, S., Shimizu, T., Yano, K., Itai, A., Saito, T. and Yamamoto, T. (2012b) Parthenocarpic genetic resources and gene expression related to parthenocarpy among four species in pear (Pyrus spp.). Sci. Hortic. 136: 101–109. [Google Scholar]
- Niu, N., Cao, Y., Duan, W., Wu, B. and Li, S. (2013a) Proteomic analysis of grape berry skin responding to sunlight exclusion. J. Plant Physiol. 170: 748–757. [DOI] [PubMed] [Google Scholar]
- Niu, N., Wu, B., Yang, P. and Li, S. (2013b) Comparative analysis of the dynamic proteomic profiles in berry skin between red and white grapes (Vitis vinifera L.) during fruit coloration. Sci. Hortic. 164: 238–248. [Google Scholar]
- Nwafor, C.C., Gribaudo, I., Schneider, A., Wehrens, R., Grando, M.S. and Costantini, L. (2014) Transcriptome analysis during berry development provides insights into co-regulated and altered gene expression between a seeded wine grape variety and its seedless somatic variant. BMC Genomics 15: 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwugo, C.C., Lin, H., Duan, Y. and Civerolo, E.L. (2013) The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic profiles and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. BMC Plant Biol. 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa, A., Otsuka, T., Jikumaru, Y., Yamaguchi, S., Matsuda, F., Nakabayashi, R., Takashina, T., Isuzugawa, K., Saito, K. and Shiratake, K. (2011) Effects of freeze-drying of samples on metabolite levels in metabolome analyses. J. Sep. Sci. 34: 3561–3567. [DOI] [PubMed] [Google Scholar]
- Oikawa, A., Otsuka, T., Nakabayashi, R., Jikumaru, Y., Isuzugawa, K., Murayama, H., Saito, K. and Shiratake, K. (2015) Metabolic profiling of developing pear fruits reveals dynamic variation in primary and secondary metabolites, including plant hormones. PLoS ONE. 10: e0131408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, T.M., da Silva, F.R., Bonatto, D., Neves, D.M., Morillon, R., Maserti, B.E., Filho, M.A.C., Costa, M.G.C., Pirovani, C.P. and Gesteira, A.S. (2015) Comparative study of the protein profiles of Sunki mandarin and Rangpur lime plants in response to water deficit. BMC Plant Biol. 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico, S., D’Abrosca, B., Scognamiglio, M., Gallicchio, M., Potenza, N., Piccolella, S., Russo, A., Monaco, P. and Fiorentino, A. (2011) Metabolic profiling of strawberry grape (Vitis×labruscana cv. ‘Isabella’) components by nuclear magnetic resonance (NMR) and evaluation of their antioxidant and antiproliferative properties. J. Agric. Food Chem. 59: 7679–7687. [DOI] [PubMed] [Google Scholar]
- Palmieri, M.C., Perazzolli, M., Matafora, V., Moretto, M., Bachi, A. and Pertot, I. (2012) Proteomic analysis of grapevine resistance induced by Trichoderma harzianum T39 reveals specific defense pathways activated against downy mildew. J. Exp. Bot. 63: 6237–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, S.É., Parent, L.E., Egozcue, J.J., Rozane, D.E., Hernandes, A., Lapointe, L., Hébert-Gentile, V., Naess, K., Marchand, S., Lafond, J.et al. (2013a) The plant ionome revisited by the nutrient balance concept. Front. Plant Sci. 4: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, S.É., Parent, L.E., Rozane, D.E. and Natale, W. (2013b) Plant ionome diagnosis using sound balances: case study with mango (Mangifera Indica). Front. Plant Sci. 4: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore, C., Zenoni, S., Fasoli, M., Pezzotti, M., Tornielli, G.B. and Filippetti, I. (2013) Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 13: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M., Manvar, T., Apurwa, S., Ghosh, A., Tiwari, T. and Chikara, S.K. (2014) Comparative de novo transcriptome analysis and metabolic pathway studies of Citrus paradisi flavedo from naive stage to ripened stage. Mol. Biol. Rep. 41: 3071–3080. [DOI] [PubMed] [Google Scholar]
- Peng, H.Y., Qi, Y.P., Lee, J., Yang, L.T., Guo, P., Jiang, H.X. and Chen, L.S. (2015) Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genomics 16: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone, I., Pagliarani, C., Lovisolo, C., Chitarra, W., Roman, F. and Schubert, A. (2012) Recovery from water stress affects grape leaf petiole transcriptome. Planta 235: 1383–1396. [DOI] [PubMed] [Google Scholar]
- Podda, A., Checcucci, G., Mouhaya, W., Centeno, D., Rofidal, V., Del Carratore, R., Luro, F., Morillon, R., Ollitrault, P. and Maserti, B.E. (2013) Salt-stress induced changes in the leaf proteome of diploid and tetraploid mandarins with contrasting Na+ and Cl− accumulation behavior. J. Plant Physiol. 170: 1101–1112. [DOI] [PubMed] [Google Scholar]
- Pons, C., Martí, C., Forment, J., Crisosto, C.H., Dandekar, A.M. and Granell, A. (2014) A bulk segregant gene expression analysis of a peach population reveals components of the underlying mechanism of the fruit cold response. PLoS ONE 9: e90706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prassinos, C., Rigas, S., Kizis, D., Vlahou, A. and Hatzopoulos, P. (2011) Subtle proteome differences identified between post-dormant vegetative and floral peach buds. J. Proteomics 74: 607–619. [DOI] [PubMed] [Google Scholar]
- Puig, C.P., Dagar, A., Ibanez, C.M., Singh, V., Crisosto, C.H., Friedman, H., Lurie, S. and Granell, A. (2015) Pre-symptomatic transcriptome changes during cold storage of chilling sensitive and resistant peach cultivars to elucidate chilling injury mechanisms. BMC Genomics 16: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani, T.S. and Podile, A.R. (2014) Extracellular matrix-associated proteome changes during non-host resistance in citrus-Xanthomonas interactions. Physiol. Plant. 150: 565–579. [DOI] [PubMed] [Google Scholar]
- Rani, T.S., Durgeshwar, P. and Podile, A.R. (2015) Accumulation of transcription factors and cell signaling-related proteins in the nucleus during citrus-Xanthomonas interaction. J. Plant Physiol. 184: 20–27. [DOI] [PubMed] [Google Scholar]
- Reuscher, S., Isuzugawa, K., Kawachi, M., Oikawa, A. and Shiratake, K. (2014) Comprehensive elemental analysis of fruit flesh from European pear ‘La France’ and its giant fruit bud mutant indicates specific roles for B and Ca in fruit development. Sci. Hortic. 176: 255–260. [Google Scholar]
- Reuscher, S., Fukao, Y., Morimoto, R., Otagaki, S., Oikawa, A., Isuzugawa, K. and Shiratake, K. (2016) Quantitative proteomics based reconstruction and identification of metabolic pathways and membrane transport proteins related to sugar accumulation in developing fruits of pear (Pyrus communis). Plant Cell Physiol. doi: 10.1093/pcp/pcw004. [DOI] [PubMed] [Google Scholar]
- Risticevic, S., DeEll, J.R. and Pawliszyn, J. (2012) Solid phase microextraction coupled with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for high-resolution metabolite profiling in apples: implementation of structured separations for optimization of sample preparation. J. Chromatogr. A1251: 208–218. [DOI] [PubMed] [Google Scholar]
- Rocheta, M., Becker, J.D., Coito, J.L., Carvalho, L. and Amâncio, S. (2014) Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct. Integr. Genomics 14: 135–148. [DOI] [PubMed] [Google Scholar]
- Rodamilans, B., San León, D., Mühlberger, L., Candresse, T., Neumüller, M., Oliveros, J.C. and García, J.A. (2014) Transcriptomic analysis of Prunus domestica undergoing hypersensitive response to plum pox virus infection. PLoS ONE 9: e100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Celma, J., Lattanzio, G., Jiménez, S., Briat, J.F., Abadía, J., Abadía, A., Gogorcena, Y. and López-Millán, A.F. (2013) Changes induced by Fe deficiency and Fe resupply in the root protein profile of a peach-almond hybrid rootstock. J. Proteome Res. 12: 1162– 1172. [DOI] [PubMed] [Google Scholar]
- Rubio, M., Rodríguez-Moreno, L., Ballester, A.R., de Moura, M.C., Bonghi, C., Candresse, T. and Martínez-Gómez, P. (2015) Analysis of gene expression changes in peach leaves in response to plum pox virus infection using RNA-Seq. Mol. Plant Pathol. 16: 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, G., Besada, C., Badenes, M.L., Monforte, A.J. and Granell, A. (2012) A non-targeted approach unravels the volatile network in peach fruit. PLoS ONE 7: e38992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, G., Martínez, J., Romeu, J., García, J., Monforte, A.J., Badenes, M.L. and Granell, A. (2014) The peach volatilome modularity is reflected at the genetic and environmental response levels in a QTL mapping population. BMC Plant Biol. 14: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer, R.J., Ireland, H.S., Ross, J.J., Ling, T.J. and David, K.M. (2013) SEPALLATA1/2-suppressed mature apples have low ethylene, high auxin and reduced transcription of ripening-related genes. AoB Plants 5: pls047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom, L., Samuels, S., Zur, N., Shlizerman, L., Doron-Faigenboim, A., Blumwald, E. and Sadka, A. (2014) Fruit load induces changes in global gene expression and in abscisic acid (ABA) and indole acetic acid (IAA) homeostasis in citrus buds. J. Exp. Bot. 65: 3029– 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socquet-Juglard, D., Kamber, T., Pothier, J.F., Christen, D., Gessler, C., Duffy, B. and Patocchi, A. (2013) Comparative RNA-seq analysis of early-infected peach leaves by the invasive phytopathogen Xanthomonas arboricola pv. pruni. PLoS ONE 8: e54196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo, A., Magnin-Robert, M., Alayi, T.D., Cilindre, C., Mercier, L., Schaeffer-Reiss, C., Van Dorsselaer, A., Clément, C. and Fontaine, F. (2012) Physiological changes in green stems of Vitis vinifera L. cv. Chardonnay in response to esca proper and apoplexy revealed by proteomic and transcriptomic analyses. J. Proteome Res. 11: 461– 475. [DOI] [PubMed] [Google Scholar]
- Spagnolo, A., Larignon, P., Magnin-Robert, M., Hovasse, A., Cilindre, C., Van Dorsselaer, A., Clément, C., Schaeffer-Reiss, C. and Fontaine, F. (2014) Flowering as the most highly sensitive period of grapevine (Vitis vinifera L. cv Mourvèdre) to the Botryosphaeria dieback agents Neofusicoccum parvum and Diplodia seriata infection. Int. J. Mol. Sci. 15: 9644–9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L.M., Ai, X.Y., Li, W.Y., Guo, W.W., Deng, X.X., Hu, C.G. and Zhang, J.Z. (2012) Identification and comparative profiling of miRNAs in an early flowering mutant of trifoliate orange and its wild type by genome-wide deep sequencing. PLoS ONE 7: e43760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, R., He, F., Lan, Y., Xing, R., Liu, R., Pan, Q., Wang, J. and Duan, C. (2015) Transcriptome comparison of Cabernet Sauvignon grape berries from two regions with distinct climate. J. Plant Physiol. 178: 43–54. [DOI] [PubMed] [Google Scholar]
- Sun, X., Zhu, A., Liu, S., Sheng, L., Ma, Q., Zhang, L., Nishawy, E.M.E., Zeng, Y., Xu, J., Ma, Z.et al. (2013) Integration of metabolomics and subcellular organelle expression microarray to increase understanding the organic acid changes in post-harvest citrus fruit. J. Integr. Plant Biol. 55: 1038–1053. [DOI] [PubMed] [Google Scholar]
- Suzuki, M., Nakabayashi, R., Mori, T., Saito, K. and Shiratake, K. (2015a) The metabolic profile of grape berry skin and a comparison of metabolomes before veraison and at harvest. Plant Biotech. 32: 267– 272. [Google Scholar]
- Suzuki, M., Nakabayashi, R., Ogata, Y., Sakurai, N., Tokimatsu, T., Goto, S., Suzuki, M., Jasinski, M., Martinoia, E., Otagaki, S.et al. (2015b) Multiomics in grape berry skin revealed specific induction of the stilbene synthetic pathway by ultraviolet-C irradiation. Plant Physiol. 168: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman, C., Sadras, V.O., Hancock, R.D., Soole, K.L. and Ford, C.M. (2014) Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 65: 5975– 5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanou, G., Ziogas, V., Belghazi, M., Christou, A., Filippou, P., Job, D., Fotopoulos, V. and Molassiotis, A. (2014) Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 37: 864–885. [DOI] [PubMed] [Google Scholar]
- Teixeira, A., Martins, V., Noronha, H., Eiras-Dias, J. and Gerós, H. (2014) The first insight into the metabolite profiling of grapes from three Vitis vinifera L. cultivars of two controlled appellation (DOC) regions. Int. J. Mol. Sci. 15: 4237–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietel, Z., Feldmesser, E., Lewinsohn, E., Fallik, E. and Porat, R. (2011) Changes in the transcriptome of ‘Mor’ mandarin flesh during storage: emphasis on molecular regulation of fruit flavor deterioration. J. Agric. Food Chem. 59: 3819–3827. [DOI] [PubMed] [Google Scholar]
- Tsukaya, H., Sawada, Y., Oikawa, A., Shiratake, K., Isuzugawa, K., Saito, K. and Hirai-Yokota, M. (2015) Intraspecific comparative analyses of metabolites between diploid and tetraploid Arabidopsis thaliana and Pyrus communis. New Negat. Plant Sci. doi: 10.1016/j.neps.2015.06.001. [DOI] [Google Scholar]
- Vega, A., Gutiérrez, R.A., Peña-Neira, A., Cramer, G.R. and Arce-Johnson, P. (2011) Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 77: 261–274. [DOI] [PubMed] [Google Scholar]
- Vimolmangkang, S., Zheng, D., Han, Y., Khan, M.A., Soria-Guerra, R.E. and Korban, S.S. (2014) Transcriptome analysis of the exocarp of apple fruit identifies light-induced genes involved in red color pigmentation. Gene 534: 78–87. [DOI] [PubMed] [Google Scholar]
- Voo, S.S. and Lange, B.M. (2014) Sample preparation for single cell transcriptomics: essential oil glands in Citrus fruit peel as an example. Methods Mol. Biol. 1153: 203–212. [DOI] [PubMed] [Google Scholar]
- Wang, J., Zheng, R., Bai, S., Gao, X., Liu, M. and Yan, W. (2015) Mongolian almond (Prunus mongolica Maxim): the morpho-physiological, biochemical and transcriptomic response to drought stress. PLoS ONE 10: e0124442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Wang, Y., Zhao, Y.B., Chen, D.M., Han, Z.H. and Zhang, X.Z. (2014) Protein phosphorylation differs significantly among ontogenetic phases in Malus seedlings. Proteome Sci. 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H., Chen, X., Zong, X., Shu, H., Gao, D. and Liu, Q. (2015) Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the red and yellow fruits of sweet cherry (Prunus avium L.). PLoS ONE 10: e0121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, J., Xiong, Z., Nie, Z.L., Mao, L., Zhu, Y., Kan, X.Z., Ickert-Bond, S.M., Gerrath, J., Zimmer, E.A. and Fang, X.D. (2013) Transcriptome sequences resolve deep relationships of the grape family. PLoS ONE 8: e74394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, B.H., Cao, Y.G., Guan, L., Xin, H.P., Li, J.H. and Li, S.H. (2014a) Genome-wide transcriptional profiles of the berry skin of two red grape cultivars (Vitis vinifera) in which anthocyanin synthesis is sunlight-dependent or -independent. PLoS ONE 9: e105959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Xu, Z., Zhang, Y., Chai, L., Yi, H. and Deng, X. (2014b) An integrative analysis of the transcriptome and proteome of the pulp of a spontaneous late-ripening sweet orange mutant and its wild type improves our understanding of fruit ripening in citrus. J. Exp. Bot. 65: 1651–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T., Wang, Y., Zheng, Y., Fei, Z., Dandekar, A.M., Xu, K., Han, Z. and Cheng, L. (2015) Suppressing sorbitol synthesis substantially alters the global expression profile of stress response genes in apple (Malus domestica) leaves. Plant Cell Physiol. 56: 1748–1761. [DOI] [PubMed] [Google Scholar]
- Xie, M., Huang, Y., Zhang, Y., Wang, X., Yang, H., Yu, O., Dai, W. and Fang, C. (2013) Transcriptome profiling of fruit development and maturation in Chinese white pear (Pyrus bretschneideri Rehd). BMC Genomics 14: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, H., Zhu, W., Wang, L., Xiang, Y., Fang, L., Li, J., Sun, X., Wang, N., Londo, J.P. and Li, S. (2013) Genome wide transcriptional profile analysis of Vitis amurensis and Vitis vinifera in response to cold stress. PLoS ONE 8: e58740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C.Q., Liu, Y.Z., An, J.C., Li, S., Jin, L.F., Zhou, G.F., Wei, Q.J., Yan, H.Q., Wang, N.N., Fu, L.N.et al. (2013) Digital gene expression analysis of corky split vein caused by boron deficiency in ‘Newhall’ navel orange (Citrus sinensis Osbeck) for selecting differentially expressed genes related to vascular hypertrophy. PLoS ONE 8: e65737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y.A., Wang, J., Ma, X., Lutts, S., Sun, C., Ma, J., Yang, Y., Achal, V. and Xu, G. (2012) Proteomic analysis of Mn-induced resistance to powdery mildew in grapevine. J. Exp. Bot. 63: 5155–5170. [DOI] [PubMed] [Google Scholar]
- Yu, K., Xu, Q., Da, X., Guo, F., Ding, Y. and Deng, X. (2012) Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genomics 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni, A., Di Carli, M., Guzzo, F., Stocchero, M., Zenoni, S., Ferrarini, A., Tononi, P., Toffali, K., Desiderio, A., Lilley, K.S.et al. (2010) Identification of putative stage-specific grapevine berry biomarkers and omics data integration into networks. Plant Physiol. 154: 1439–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, G.J., Li, C.M., Zhang, X.Z., Han, Z.H., Yang, F.Q., Gao, Y., Chen, D.M., Zhao, Y.B., Wang, Y., Teng, Y.L.et al. (2010) Differential proteomic analysis during the vegetative phase change and the floral transition in Malus domestica. Dev. Growth Differ. 52: 635– 644. [DOI] [PubMed] [Google Scholar]
- Zeng, J., Gao, C., Deng, G., Jiang, B., Yi, G., Peng, X., Zhong, Y., Zhou, B. and Liu, K. (2012) Transcriptome analysis of fruit development of a citrus late-ripening mutant by microarray Sci. Hortic. 134: 32–39. [Google Scholar]
- Zeng, Y., Pan, Z., Ding, Y., Zhu, A., Cao, H., Xu, Q. and Deng, X. (2011) A proteomic analysis of the chromoplasts isolated from sweet orange fruits [Citrus sinensis (L.) Osbeck]. J. Exp. Bot. 62: 5297–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Y., Pan, Z., Wang, L., Ding, Y., Xu, Q., Xiao, S. and Deng, X. (2014) Phosphoproteomic analysis of chromoplasts from sweet orange during fruit ripening. Physiol. Plant. 150: 252–270. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Tian, Y. and Cong, P. (2015a) Proteome analysis of pathogen-responsive proteins from apple leaves induced by the alternaria blotch Alternaria alternata. PLoS ONE 10: e0122233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Dugé de Bernonville, T., Body, M., Glevarec, G., Reichelt, M., Unsicker, S., Bruneau, M., Renou, J.P., Huguet, E., Dubreuil, G.et al. (2015b) Leaf-mining by Phyllonorycter blancardella reprograms the host-leaf transcriptome to modulate phytohormones associated with nutrient mobilization and plant defense. J. Insect Physiol. doi: 10.1016/j.jinsphys.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang, J.Z., Ai, X.Y., Sun, L.M., Zhang, D.L., Guo, W.W., Deng, X.X. and Hu, C.G. (2011a) Transcriptome profile analysis of flowering molecular processes of early flowering trifoliate orange mutant and the wild-type [Poncirus trifoliata (L.) Raf.] by massively parallel signature sequencing. BMC Genomics 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J.Z., Zhao, K., Ai, X.Y. and Hu, C.G. (2014a) Involvements of PCD and changes in gene expression profile during self-pruning of spring shoots in sweet orange (Citrus sinensis). BMC Genomics 15: 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Yu, Z., Jiang, L., Jiang, J., Luo, H. and Fu, L. (2011b) Effect of post-harvest heat treatment on proteome change of peach fruit during ripening. J. Proteomics 74: 1135–1149. [DOI] [PubMed] [Google Scholar]
- Zhang, S., Ding, F., He, X., Luo, C., Huang, G. and Hu, Y. (2015c) Characterization of the ‘Xiangshui’ lemon transcriptome by de novo assembly to discover genes associated with self-incompatibility. Mol. Genet. Genomics 290: 365–375. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.J., Wang, X.J., Wu, J.X., Chen, S.Y., Chen, H., Chai, L.J. and Yi, H.L. (2014b) Comparative transcriptome analyses between a spontaneous late-ripening sweet orange mutant and its wild type suggest the functions of ABA, sucrose and JA during citrus fruit ripening PLoS ONE 9: e116056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, F., Chen, L., Perl, A., Chen, S. and Ma, H. (2011) Proteomic changes in grape embryogenic callus in response to Agrobacterium tumefaciens-mediated transformation. Plant Sci. 181: 485–495. [DOI] [PubMed] [Google Scholar]
- Zheng, B.B., Fang, Y.N., Pan, Z.Y., Sun, L., Deng, X.X., Grosser, J.W. and Guo, W.W. (2014) iTRAQ-based quantitative proteomics analysis revealed alterations of carbohydrate metabolism pathways and mitochondrial proteins in a male sterile cybrid pummelo. J. Proteome Res. 13: 2998–3015. [DOI] [PubMed] [Google Scholar]
- Zheng, Q., Song, J., Campbell-Palmer, L., Thompson, K., Li, L., Walker, B., Cui, Y. and Li, X. (2013) A proteomic investigation of apple fruit during ripening and in response to ethylene treatment. J. Proteomics 93: 276–294. [DOI] [PubMed] [Google Scholar]
- Zhong, Y., Cheng, C.Z., Jiang, N.H., Jiang, B., Zhang, Y.Y., Wu, B., Hu, M.L., Zeng, J.W., Yan, H.X., Yi, G.J.et al. (2015) Comparative transcriptome and iTRAQ proteome analyses of citrus root responses to Candidatus Liberibacter asiaticus infection. PLoS ONE 10: e0126973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S., Li, M., Guan, Q., Liu, F., Zhang, S., Chen, W., Yin, L., Qin, Y. and Ma, F. (2015) Physiological and proteome analysis suggest critical roles for the photosynthetic system for high water-use efficiency under drought stress in Malus. Plant Sci. 236: 44–60. [DOI] [PubMed] [Google Scholar]
- Zhu, H., Dardick, C.D., Beers, E.P., Callanhan, A.M., Xia, R. and Yuan, R. (2011) Transcriptomics of shading-induced and NAA-induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biol. 11: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Li, Y., Xin, D., Chen, W., Shao, X., Wang, Y. and Guo, W. (2015) RNA-Seq-based transcriptome analysis of dormant flower buds of Chinese cherry (Prunus pseudocerasus). Gene 555: 362–376. [DOI] [PubMed] [Google Scholar]
- Zhuang, W.B., Shi, T., Gao, Z.H., Zhang, Z. and Zhang, J.Y. (2013) Differential expression of proteins associated with seasonal bud dormancy at four critical stages in Japanese apricot. Plant Biol. 15: 233–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.